Published online Apr 14, 2020. doi: 10.3748/wjg.v26.i14.1554
Peer-review started: December 12, 2019
First decision: January 19, 2020
Revised: January 20, 2020
Accepted: March 9, 2020
Article in press: March 9, 2020
Published online: April 14, 2020
Processing time: 124 Days and 9.8 Hours
Infliximab (IFX), as a drug of first-line therapy, can alter the natural progression of Crohn’s disease (CD), promote mucosal healing and reduce complications, hospitalizations, and the incidence of surgery. Perianal fistulas are responsible for the refractoriness of CD and represent a more aggressive disease. IFX has been demonstrated as the most effective drug for the treatment of perianal fistulizing CD. Unfortunately, a significant proportion of patients only partially respond to IFX, and optimization of the therapeutic strategy may increase clinical remission. There is a significant association between serum drug concentrations and the rates of fistula healing. Higher IFX levels during induction are associated with a complete fistula response in these patients. Given the apparent relapse of perianal fistulizing CD, maintenance therapy with IFX over a longer period seems to be more beneficial. It appears that patients without deep remission are at an increased risk of relapse after stopping anti-tumor necrosis factor agents. Thus, only patients in prolonged clinical remission should be considered for withdrawal of IFX treatment when biomarker and endoscopic remission is demonstrated, especially when the hyperintense signals of fistulas on T2-weighed images have disappeared on magnetic resonance imaging. Fundamentally, the optimal timing of IFX use is highly individualized and should be determined by a multidisciplinary team.
Core tip: The long-term outcomes of infliximab in the treatment of perianal fistulizing Crohn’s disease are unfavorable, due to loss of response. The optimization of the therapeutic strategy may increase clinical remission. Higher infliximab concentrations during induction are associated with a complete fistula response. Only patients in prolonged clinical remission should be considered for withdrawal of infliximab when biomarker, endoscopic and radiological remission is demonstrated. Fundamentally, the optimal timing of infliximab use is highly individualized and should be determined by a multidisciplinary team.
- Citation: Sun XL, Chen SY, Tao SS, Qiao LC, Chen HJ, Yang BL. Optimized timing of using infliximab in perianal fistulizing Crohn's disease. World J Gastroenterol 2020; 26(14): 1554-1563
- URL: https://www.wjgnet.com/1007-9327/full/v26/i14/1554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i14.1554
Crohn's disease (CD) is a chronic, disabling and idiopathic inflammatory bowel disease that can involve any element of the gastrointestinal tract. Perianal fistulas are a common extraintestinal manifestation of CD, presenting in approximately 40% of patients before or at the time of diagnosis and in 24% after diagnosis[1]. The median interval between CD diagnosis and the first perianal fistula is 2.9-4.4 years[1,2]. Perianal fistulas correlated with CD are indicative of severe disease and a more aggressive course. The natural progression of perianal fistulizing CD (PFCD) is characterized by alternation of remission and relapse periods. The recurrence rate is up to 80% after a median follow-up of 10 years[2]. Repeated perianal symptoms, such as persistent purulent discharge, pain, and fecal incontinence, can cause fatigue, anxiety, and depression, which can be debilitating and negatively impact patients’ quality of life. As the disease progresses, fecal diversion may be necessary to achieve clinical remission in the advanced period. However, it is a fatal procedure because the success rate of restoring bowel continuity is only 16.6%[3]. Ultimately, proctectomy is performed in 41.6% of patients suffering from fecal diversion failure[3].
Emerging biologic agents have revolutionized the medical treatment of PFCD and achieved more promising outcomes than immunomodulators[4]. In the biological era, the treatment goal has changed from symptom relief to complete fistula healing, while also preventing relapse. Fistulizing CD was, together with steroid dependence or resistance, the first indication for biological therapy, after surgical drainage of any sepsis[5]. Infliximab (IFX) is the first anti-tumor necrosis factor (TNF) agent for the treatment of PFCD. The ACCENT trial showed that 68% of patients with fistulizing CD achieved symptom improvement following IFX monotherapy, whereas the closure rate of fistulas was only 36% at 54 wk[6,7]. This finding indicated that a substantial proportion of patients partially responded to IFX. Surgical interventions appear to be indispensable in assisting IFX to alter the natural course of PFCD, because the probability of perianal surgery does not significantly decrease after the emergence of biologic agents[8]. Anatomically, CD-related perianal fistulas can be categorized into two types: Simple and complex[9]. Fistulotomy achieves excellent outcomes in the treatment of symptomatic simple fistulas, with a recurrence rate of 3.4% during a mean follow-up of 1.6 years[10]. However, complex fistulas that are associated with an increased risk of fecal incontinence make up a larger proportion in PFCD. Although sphincter-preserving procedures, such as loose-seton and ligation of the intersphincteric fistula tract (LIFT), show promising outcomes in the treatment of PFCD, they might be restricted by concomitant proctitis in the early stage of the disease[11,12]. The optimal timing of IFX combined with perianal surgery is unclear due to a debate on the relationship between proctitis and surgical outcomes. Poor prognosis obliges the optimization of IFX therapy to induce a more complete response, alter the natural course of PFCD, and reduce complications, hospitalizations, and the incidence of major abdominal surgery.
To date, there is absence of a consensus on the optimal timing of IFX use. The purpose of this review is to examine the present state of knowledge regarding the use of IFX in PFCD patients in order to contribute to the better management of PFCD.
Owing to the clinical application of biologics, the healing rate of PFCD has improved. The capability of anti-TNF agents to modify the natural course of PFCD has been validated. The cumulative incidence of PFCD at 10 years has decreased from 24% in the prebiologic era to 12% in the biologic era; similarly the probability of proctectomy has decreased from 24% to 13%[1]. An increasing proportion of CD patients switch to biologics. Although IFX has been recommended as the first-line therapy for PFCD by current European Crohn's and Colitis Organisation consensus, there is still a divergence in the “top-down” strategy due to the hidden perils of overtreatment and severe adverse events[13].
Colombel et al[14] demonstrated that patients treated with IFX alone showed a higher healing rate of intestinal mucosa than those treated with azathioprine monotherapy. A real-life study showed that IFX as the first-line therapy was mainly applied in patients with risk factors, higher disease activity and lower quality of life scores[15]. PFCD patients who have a poorer prognosis may benefit from the early introduction of IFX. IFX immediately works to treat PFCD after its first infusion, while the effect-beginning time of adalimumab is over 4 wk after injection[16]. Moreover, the response rate of fistulizing CD to IFX is negatively related to the disease duration[17]. The “step-up” approach may potentially increase the loss of response due to a prolonged disease course and disease progression. Conversely, IFX used as the initial medication can rapidly induce clinical remission and prevent disease progression. Regarding adverse events, infection is the most common, accounting for 53.7% of CD patients treated with IFX[18]. However, the incidence rate of serious infections is only 2.15%[18]. Mortality and malignancy rates are similar between IFX-treated patients and patients with other treatments. Nonserious cerebrovascular accidents and pulmonary embolisms occur in less than 0.1% of the IFX-treated patients[18]. In light of the above evidence, a “top-down” strategy is better for the treatment of PFCD.
It is well known that surgical intervention is necessary for the drainage of septic complications before the initiation of IFX therapy. However, whether definitive surgery is needed is controversial since IFX can induce fistula closure in approximately 60% of PFCD patients[19]. Despite clinical closure, most fistula tracts can be visualized on pelvic magnetic resonance imaging (MRI). Perianal surgery can improve fistula response to IFX and promote deep remission. It is reported that IFX combined with surgery can improve clinical efficacy compared to monotherapy[20].
Active proctitis negatively affects the outcomes of PFCD, which determines the timing of IFX combined with surgery. Conventionally, surgical procedures are only considered after the elimination of proctitis by prior IFX therapy[21]. In a small sample size study, definitive surgeries, such as fistulotomy and advancement flap, were performed after proctitis was well controlled, with a median interval of 9 wk between the first infusion of IFX and surgery [22]. The healing rate of perianal fistulas was 80% with a median follow-up of 17.5 mo. Nonetheless, the failure of fistula closure may increase in patients with a partial response or primary nonresponse to IFX due to the increased aggression and complexity of perianal fistulas.
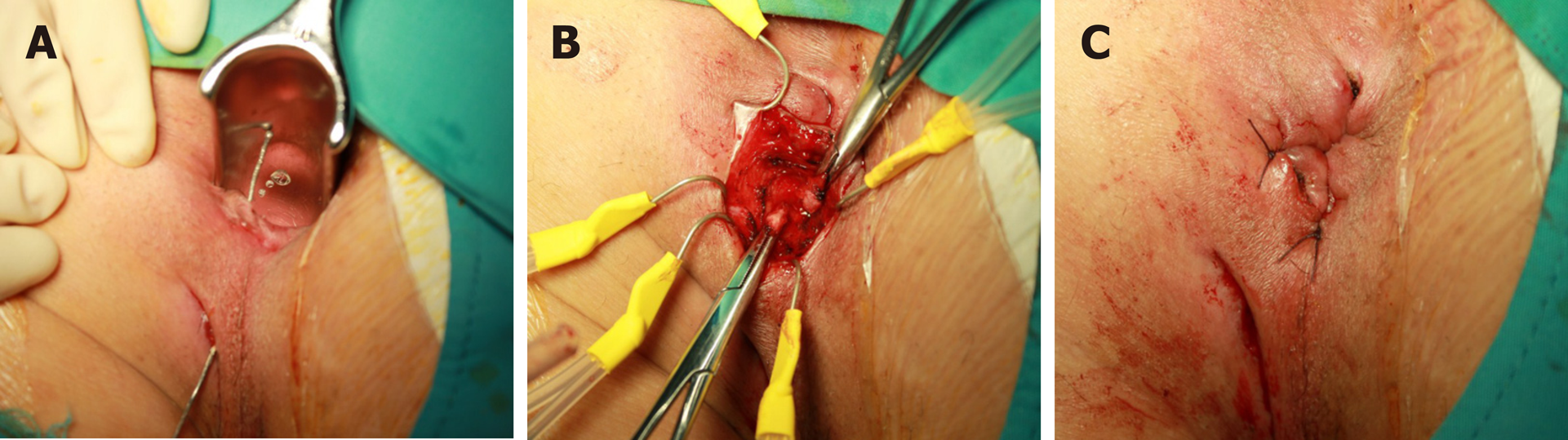
The authors performed loose-seton with the eradication of the internal opening within 1 wk before the first infusion of IFX. The clinical healing rate of perianal fistulas was 96.4% after a median follow-up of 26.4 mo[11]. Another study showed that proctitis was detected in 62.5% of patients who achieved improvement of PFCD following definitive surgery[23]. In a prospective study including 15 patients with PFCD, the healing rate of perianal fistulas following LIFT was 67%, with a follow-up duration of 12 mo and without fecal incontinence (Figure 1)[12]. This is comparable with the success rates in cryptoglandular anal fistulas[24,25]. In the cohort, 9 patients had active proctitis, but this finding was not closely related to LIFT failure. Pretreatment with biologic therapy did not improve the outcomes of LIFT[12,26]. A recent multicenter retrospective study demonstrated that multimodal treatment at the diagnosis of PFCD could reduce the probability of repeat surgery and proctectomy[27].
In addition, the issue of wound healing can be addressed by amelioration of immune inflammation, as the median response time of PFCD to IFX is only 9 d[28]. Early combination therapy without waiting for the disappearance of proctitis is viable and is of great importance, as it can alter the natural course of PFCD as soon as possible and improve the patients’ quality of life.
Perianal lesions predict an increased risk of loss of response. Better outcomes are associated with response monitoring and the timely optimization of the therapy regimen during the induction and maintenance of IFX. Nevertheless, the coexistence of luminal and perianal diseases makes the process monitoring and optimization complex and difficult.
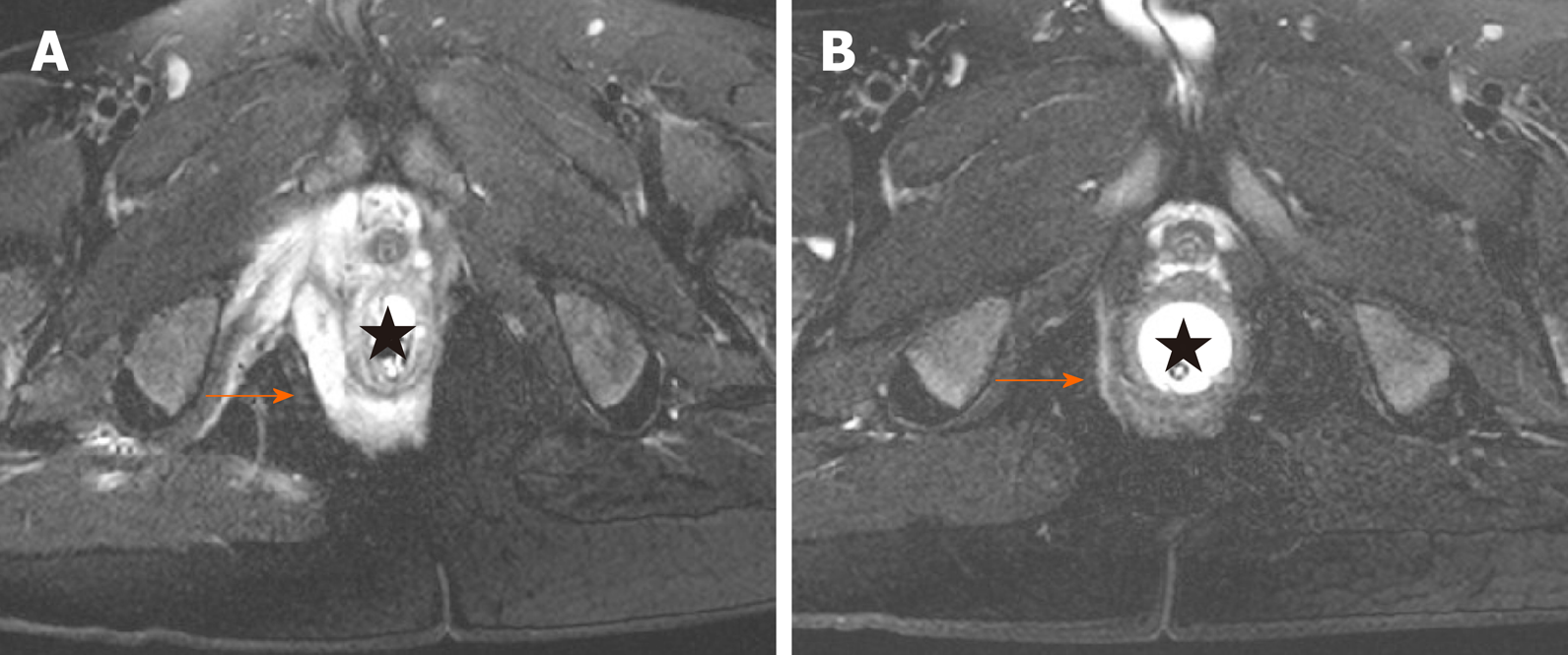
It is inadequate to assess the response of PFCD according to clinical symptoms, especially in terms of discriminating between a closed and healed fistula. The inaccurate assessment of the fistula healing process might misguide the adjustment of the IFX therapy regimen, resulting in worse therapeutic efficacy. Pelvic MRI has been suggested as the gold standard for the assessment of the anatomy and activity of CD-related perianal fistulas. Fistula healing is characterized by the disappearance of hyperintense signals on T2-weighted images and the absence of contrast enhancement after gadolinium injection on T1-weighted fat-suppression images (Figure 2)[29]. After anti-TNF therapy, healed fistulas confirmed by MRI account for 50%-61.5% of closed fistulas[30-32]. Persistent tracts indicate a large probability of recurrence and a prolonged duration of maintenance therapy.
Proctitis increases the risk for PFCD occurrence and recurrence. The formation of perianal fistulas occurs in 92% of CD patients with rectal involvement[33]. The absence or disappearance of rectal involvement plays a pivotal role in the deep remission of PFCD, which is defined as clinical remission associated with absence of anal canal ulcers and healing on MRI[31]. In the majority of studies, thickening of the rectal wall was considered an indicator of proctitis[31,32]. In addition, a recent study demonstrated that the size of the mesorectal lymph nodes, mural fat and creeping fat were also relevant to the evaluation of proctitis by pelvic MRI[34].
Changes in the signal intensity and morphology of fistulas and the rectum can indicate the healing or worsening of PFCD. Scheduled pelvic MRI examinations can provide objective evidence for the assessment of treatment efficacy and the optimization or modification of the therapeutic strategy. The monitoring timing varies. The probability of clinical remission is 5 times greater in PFCD patients with a clinical response to anti-TNF agents within 6 wk than in those responding longer than 6 wk[35]. Hence, the sixth week within induction period is a critical time point to evaluate the response of perianal fistulas and proctitis by pelvic MRI. Features of proctitis on MRI are significantly correlated with those on endoscopy during the maintenance therapy period[34]. Given that pelvic MRI is noninvasive and does not have radiation risk, it could be used to dynamically monitor the healing of PFCD at intervals of 8 wk, acquiring accurate information and providing personalized treatment. Radiological healing might lag behind clinical remission by 12 months, suggesting that MRI monitoring should be carried out for at least 1 year[35,36]. Prolonged treatment is often needed to observe the eradication of fistula tracts on MRI.
When the loss of response is observed on pelvic MRI, clinicians should check the serum trough levels of IFX. The exact mechanism of the loss of response is unclear, but may be associated with drug metabolism or the formation of antidrug antibodies. After exposure, specific antibodies secreted by clonally expanded lymphocytes form immune complexes with IFX. This process is also termed immunogenicity and may cause excessive drug clearance via the reticuloendothelial system. The levels of antibodies to IFX have been shown to be higher in patients with a loss of response than in those who maintained remission[37].
Increasing evidence suggests that low serum trough IFX levels are related to a lack or loss of response[38]. Although a cut-off level of 5.0 μg/mL is recommended as the target concentration for healing the intestinal mucosa, a specific level related to the complete response of PFCD has not been identified[39]. In a recent retrospective cross-sectional study including 29 PFCD patients receiving IFX, higher than 7.1 μg/mL was identified as the optimal threshold value for fistula healing (77.8% sensitivity and 100% specificity)[40]. The median trough concentrations in patients with healed fistulas were significantly higher than those without healed fistulas (8.1 μg/mL vs 3.2 μg/mL). Fistula healing was positively related with trough IFX levels. Another similar study with a larger sample size indicated that trough IFX levels above 10.1 μg/mL at 4 wk might provide better outcomes for PFCD[41]. Davidov et al[42] demonstrated that trough IFX levels of 9.25 μg/mL at week 2 (89% sensitivity and 90% specificity) and 7.25 μg/mL at week 6 (80% sensitivity and 83% specificity) were the best response predictors of perianal CD. The inconsistency of outcomes may be caused by the various assays and different testing time. Further studies are required to determine the optimal measurement time of drug concentrations and the target IFX levels for fistula healing. More attention should be paid in the induction phase, where multiple factors, such as tissue IFX levels, low albumin, and protein loss, affect the serum drug concentrations.
As mentioned above, adequate drug concentration is a crucial part of a treat-to-target strategy. The aim of therapeutic regimen optimization is to achieve a steady-state range of serum drug concentrations. Since a higher trough IFX level is necessary for fistula healing than that for mucosal healing, dose escalation should be primarily considered for PFCD patients who do not achieve a response or deep remission prior to switching therapy. Additionally, low drug concentrations can stimulate the germination of immunogenicity, which may be mitigated by early dose optimization. Preexisting antidrug antibodies may be spontaneously degraded in a portion of patients with the continuation of IFX treatment, which also supports the consideration of dose escalation following a loss of response[43]. A dose increase and/or a reduction in the infusion interval are mainly used for increasing serum IFX levels. After dose escalation, 84.8% and 62.3% of CD patients achieved a response, respectively, during the induction and maintenance periods[44]. In terms of safety, trough IFX levels above 7 μg/mL can provide better outcomes for CD patients without increasing the risk of infection[45].
At 54 wk after IFX treatment, antidrug antibodies that were responsible for a loss of response are detected in 62.1% of CD patients[46]. IFX combined with azathioprine is recommended to reduce immunogenicity and mitigate the development of antidrug antibodies. Concomitant therapy can increase serum trough levels of IFX and prolong the duration of fistula closure in CD patients[47,48]. However, early immunosuppressive administration has no effect in increasing clinical remission[49,50]. Furthermore, concomitant therapy does not show better efficacy than IFX monotherapy among CD patients with similar serum IFX levels[51]. Optimized IFX monotherapy leads to similar clinical efficacy as combination therapy[52]. As dose escalation is limited by the increased risk of serious adverse events and increases the consumption of IFX, azathioprine as an adjunct plays a role of dose-sparing by improving the pha-rmacokinetic features of IFX.
The positive rates of antibodies to IFX were 1.6% at 2 wk, 3.3% at 6 wk, and 17.2% at 14 wk[46]. This discrepancy suggests that a drug concentration below 7 μg/mL at 14 wk is an independent predictive factor for long-term nonresponse. Hence, dose escalation or the addition of immunomodulators within 14 wk can increase the clinical response and remission by elevating serum IFX levels. In addition, the benefits of concomitant therapy should be weighed against the increased risk of serious and opportunistic infections[53].
After IFX failure, it may be beneficial to switch to other biologic agents. Adalimumab (ADA) is another effective anti-TNF agent for the treatment of PFCD, which can maintain remission in 41% of patients naïve to anti-TNF drugs at 12 mo[54]. Moreover, ADA, as a second-line therapy, induced complete response in 50% of PFCD patients refractory to IFX[55]. Previous administration of IFX does not affect the efficacy of ADA induction of fistula closure[56]. Although certolizumab pegol, vedolizumab, and ustekinumab show potential benefits for PFCD patients who failed in IFX or ADA therapy, the dedicated efficacy needs further investigation with large sample size studies[57-59].
IFX withdrawal is an important question faced by patients and clinicians after disease remission, due to safety and cost-effectiveness concerns. It is well known that the cessation of IFX therapy after sustained clinical remission is responsible for the recurrence of CD. It has been shown that 29.4%-49.3% of patients with remission experienced relapse within 1-4 years after stopping anti-TNF therapy[60-62]. Overall, approximately 20% of patients never received retreatment with a biologic within a long-term follow-up[63,64]. Fortunately, clinical response can be successfully induced by retreatment with the same anti-TNF agents, primarily IFX, in 80%-94% of cases[60-62]. The high rate of secondary remission may counterbalance the high rate of relapse after withdrawal, suggesting that the discontinuation of IFX therapy and the establishment of a cyclic therapeutic strategy consisting of drug discontinuation and retreatment may be possible[65].
Currently, the decision to withdraw IFX treatment is based on the guidelines for luminal CD because of the absence of dedicated guidelines for PFCD[66]. Heterogeneity of disease phenotype and the absence of controlled trials make it difficult to draw decisive conclusions. Deep remission, defined as clinical remission associated with endoscopic and radiological remission, seems to be the criterion for IFX withdrawal. However, the outcomes are unfavorable, with a relapse rate of approximately 55%[35,63]. The risk factors for relapse after withdrawal included ileal localization at diagnosis, a persistent external opening, prior dose optimization, anemia and a white blood cell count above 5 × 109/L at the time of withdrawal[63,64]. Despite the elimination of risk factors, the optimal timing for withdrawal after deep remission is still unknown, which may affect disease progression. Given that after withdrawal, the relapse of disease is apparent while the clinical benefits, such as a reduction in infection or cancer risk, are theoretical because of the absence of controlled studies, maintenance IFX therapy over a longer period may be more beneficial for PFCD patients. IFX discontinuation as a part of a cyclic therapeutic strategy may be implemented in strictly selected patients. The definitive interruption time should be clarified in future studies.
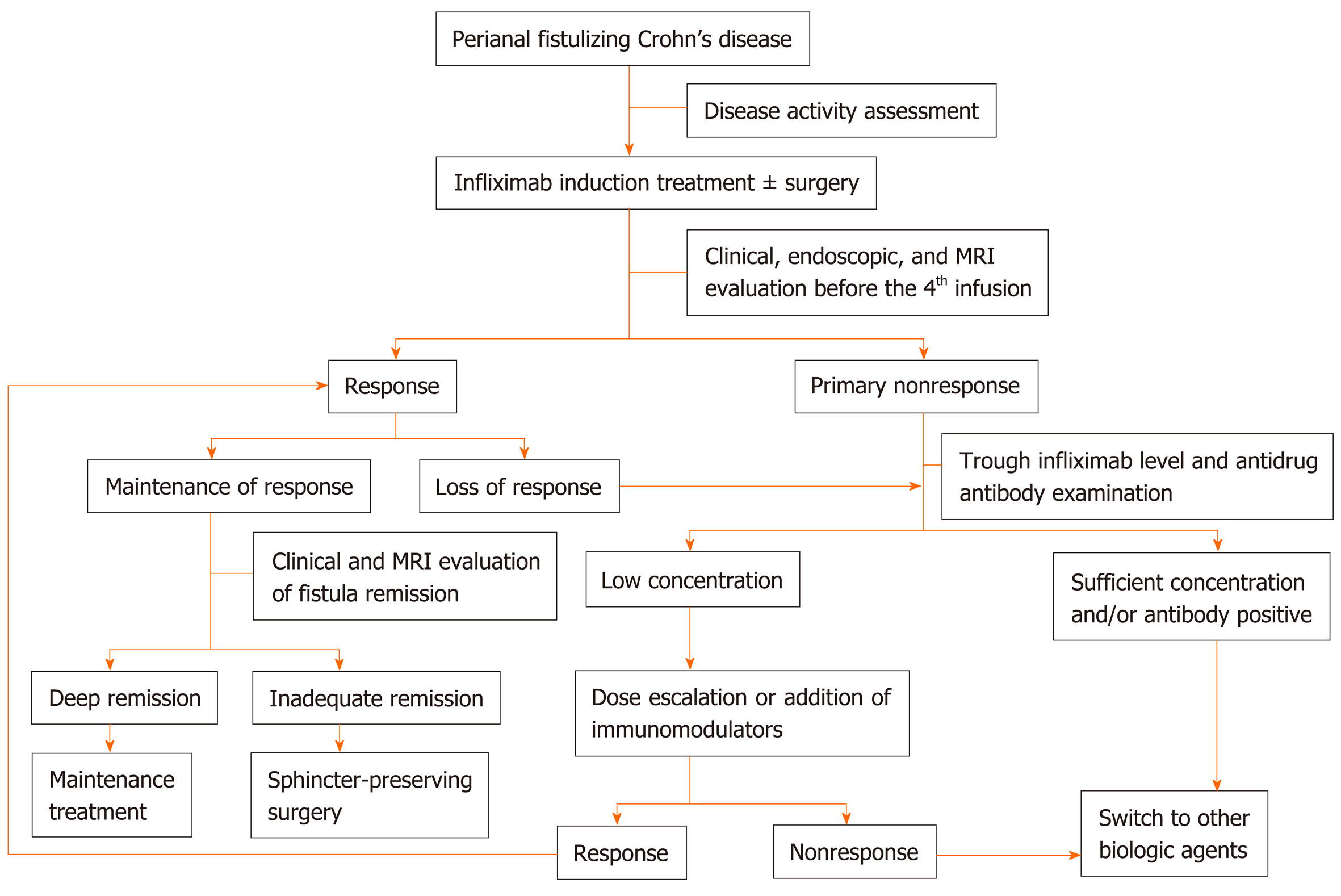
In general, no single treatment can successfully manage PFCD. Although IFX has been recommended as a first-line therapy, early combination with definitive surgery may rapidly lead to clinical remission. Monitoring drug concentrations plays a pivotal role in the optimization of the therapeutic regimen. Scheduled MRI scans can dynamically monitor remission of the internal tract in order to immediately adjust the treatment strategy (Figure 3). IFX withdrawal seems to be possible in the setting of deep remission but is not recommended. The optimal timing of IFX use is highly individualized and should be determined by a multidisciplinary team composed of gastroenterologists, colorectal surgeons, and radiologists.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ierardi E S-Editor:Gong ZM L-Editor: MedE-Ma JY E-Editor: Zhang YL
| 1. | Park SH, Aniwan S, Scott Harmsen W, Tremaine WJ, Lightner AL, Faubion WA, Loftus EV. Update on the Natural Course of Fistulizing Perianal Crohn's Disease in a Population-Based Cohort. Inflamm Bowel Dis. 2019;25:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Zhao M, Lo BZS, Vester-Andersen MK, Vind I, Bendtsen F, Burisch J. A 10-Year Follow-up Study of the Natural History of Perianal Crohn's Disease in a Danish Population-Based Inception Cohort. Inflamm Bowel Dis. 2019;25:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Singh S, Ding NS, Mathis KL, Dulai PS, Farrell AM, Pemberton JH, Hart AL, Sandborn WJ, Loftus EV. Systematic review with meta-analysis: faecal diversion for management of perianal Crohn's disease. Aliment Pharmacol Ther. 2015;42:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Hazlewood GS, Rezaie A, Borman M, Panaccione R, Ghosh S, Seow CH, Kuenzig E, Tomlinson G, Siegel CA, Melmed GY, Kaplan GG. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta-analysis. Gastroenterology. 2015;148:344-54.e5; quiz e14-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 5. | D'Haens GR, Panaccione R, Higgins PD, Vermeire S, Gassull M, Chowers Y, Hanauer SB, Herfarth H, Hommes DW, Kamm M, Löfberg R, Quary A, Sands B, Sood A, Watermeyer G, Lashner B, Lémann M, Plevy S, Reinisch W, Schreiber S, Siegel C, Targan S, Watanabe M, Feagan B, Sandborn WJ, Colombel JF, Travis S. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199-212; quiz 213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 301] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 6. | Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1969] [Cited by in RCA: 1837] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 7. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1581] [Cited by in RCA: 1548] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 8. | Chhaya V, Saxena S, Cecil E, Subramanian V, Curcin V, Majeed A, Pollok RC. Emerging trends and risk factors for perianal surgery in Crohn's disease: a 20-year national population-based cohort study. Eur J Gastroenterol Hepatol. 2016;28:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB; American Gastroenterological Association Clinical Practice Committee. AGA technical review on perianal Crohn's disease. Gastroenterology. 2003;125:1508-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 408] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 10. | Papaconstantinou I, Kontis E, Koutoulidis V, Mantzaris G, Vassiliou I. Surgical Management of Fistula-in-ano Among Patients With Crohn's Disease: Analysis of Outcomes After Fistulotomy or Seton Placement-Single-Center Experience. Scand J Surg. 2017;106:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Yang BL, Chen YG, Gu YF, Chen HJ, Sun GD, Zhu P, Shao WJ. Long-term outcome of infliximab combined with surgery for perianal fistulizing Crohn's disease. World J Gastroenterol. 2015;21:2475-2482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Gingold DS, Murrell ZA, Fleshner PR. A prospective evaluation of the ligation of the intersphincteric tract procedure for complex anal fistula in patients with Crohn's disease. Ann Surg. 2014;260:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, Lakatos PL, Adamina M, Ardizzone S, Buskens CJ, Sebastian S, Laureti S, Sampietro GM, Vucelic B, van der Woude CJ, Barreiro-de Acosta M, Maaser C, Portela F, Vavricka SR, Gomollón F; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis. 2017;11:135-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 529] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 14. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2362] [Article Influence: 157.5] [Reference Citation Analysis (1)] |
| 15. | Ghazi LJ, Patil SA, Rustgi A, Flasar MH, Razeghi S, Cross RK. Step up versus early biologic therapy for Crohn's disease in clinical practice. Inflamm Bowel Dis. 2013;19:1397-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Vasudevan A, Gibson PR, van Langenberg DR. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: What should the clinician expect, what should patients be told? World J Gastroenterol. 2017;23:6385-6402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 17. | Lionetti P, Bronzini F, Salvestrini C, Bascietto C, Canani RB, Dé Angelis GL, Guariso G, Martelossi S, Papadatou B, Barabino A. Response to infliximab is related to disease duration in paediatric Crohn's disease. Aliment Pharmacol Ther. 2003;18:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Safdi M, Popp JW, Langholff W, Sandborn WJ. Infliximab for Crohn's Disease: More Than 13 Years of Real-world Experience. Inflamm Bowel Dis. 2018;24:490-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Ardizzone S, Maconi G, Colombo E, Manzionna G, Bollani S, Bianchi Porro G. Perianal fistulae following infliximab treatment: clinical and endosonographic outcome. Inflamm Bowel Dis. 2004;10:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Yassin NA, Askari A, Warusavitarne J, Faiz OD, Athanasiou T, Phillips RK, Hart AL. Systematic review: the combined surgical and medical treatment of fistulising perianal Crohn's disease. Aliment Pharmacol Ther. 2014;40:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Gecse K, Khanna R, Stoker J, Jenkins JT, Gabe S, Hahnloser D, D'Haens G. Fistulizing Crohn's disease: Diagnosis and management. United European Gastroenterol J. 2013;1:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | van der Hagen SJ, Baeten CG, Soeters PB, Russel MG, Beets-Tan RG, van Gemert WG. Anti-TNF-alpha (infliximab) used as induction treatment in case of active proctitis in a multistep strategy followed by definitive surgery of complex anal fistulas in Crohn's disease: a preliminary report. Dis Colon Rectum. 2005;48:758-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | El-Gazzaz G, Hull T, Church JM. Biological immunomodulators improve the healing rate in surgically treated perianal Crohn's fistulas. Colorectal Dis. 2012;14:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Chen HJ, Sun GD, Zhu P, Zhou ZL, Chen YG, Yang BL. Effective and long-term outcome following ligation of the intersphincteric fistula tract (LIFT) for transsphincteric fistula. Int J Colorectal Dis. 2017;32:583-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Sun XL, Wen K, Chen YH, Xu ZZ, Wang XP. Long-term outcomes and quality of life following ligation of the intersphincteric fistula tract for high transsphincteric fistulas. Colorectal Dis. 2019;21:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Kamiński JP, Zaghiyan K, Fleshner P. Increasing experience of ligation of the intersphincteric fistula tract for patients with Crohn's disease: what have we learned? Colorectal Dis. 2017;19:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Sebastian S, Black C, Pugliese D, Armuzzi A, Sahnan K, Elkady SM, Katsanos KH, Christodoulou DK, Selinger C, Maconi G, Fearnhead NS, Kopylov U, Davidov Y, Bosca-Watts MM, Ellul P, Muscat M, Karmiris K, Hart AL, Danese S, Ben-Horin S, Fiorino G. The role of multimodal treatment in Crohn's disease patients with perianal fistula: a multicentre retrospective cohort study. Aliment Pharmacol Ther. 2018;48:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Cohen RD, Tsang JF, Hanauer SB. Infliximab in Crohn's disease: first anniversary clinical experience. Am J Gastroenterol. 2000;95:3469-3477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Sheedy SP, Bruining DH, Dozois EJ, Faubion WA, Fletcher JG. MR Imaging of Perianal Crohn Disease. Radiology. 2017;282:628-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Hermann J, Stajgis P, Kołodziejczak B, Eder P, Banasiewicz T. Treatment of Crohn's anal fistulas guided by magnetic resonance imaging. Prz Gastroenterol. 2019;14:55-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Thomassin L, Armengol-Debeir L, Charpentier C, Bridoux V, Koning E, Savoye G, Savoye-Collet C. Magnetic resonance imaging may predict deep remission in patients with perianal fistulizing Crohn's disease. World J Gastroenterol. 2017;23:4285-4292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Chambaz M, Verdalle-Cazes M, Desprez C, Thomassin L, Charpentier C, Grigioni S, Armengol-Debeir L, Bridoux V, Savoye G, Savoye-Collet C. Deep remission on magnetic resonance imaging impacts outcomes of perianal fistulizing Crohn's disease. Dig Liver Dis. 2019;51:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Panés J, Rimola J. Perianal fistulizing Crohn's disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:652-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 196] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 34. | Tutein Nolthenius CJ, Bipat S, Mearadji B, Spijkerboer AM, Ponsioen CY, Montauban van Swijndregt AD, Stoker J. MRI characteristics of proctitis in Crohn's disease on perianal MRI. Abdom Radiol (NY). 2016;41:1918-1930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Tozer P, Ng SC, Siddiqui MR, Plamondon S, Burling D, Gupta A, Swatton A, Tripoli S, Vaizey CJ, Kamm MA, Phillips R, Hart A. Long-term MRI-guided combined anti-TNF-α and thiopurine therapy for Crohn's perianal fistulas. Inflamm Bowel Dis. 2012;18:1825-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Karmiris K, Bielen D, Vanbeckevoort D, Vermeire S, Coremans G, Rutgeerts P, Van Assche G. Long-term monitoring of infliximab therapy for perianal fistulizing Crohn's disease by using magnetic resonance imaging. Clin Gastroenterol Hepatol. 2011;9:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Steenholdt C. Use of infliximab and anti-infliximab antibody measurements to evaluate and optimize efficacy and safety of infliximab maintenance therapy in Crohn's disease. Dan Med J. 2013;60:B4616. [PubMed] |
| 38. | Roda G, Jharap B, Neeraj N, Colombel JF. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol. 2016;7:e135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 516] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 39. | Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology. 2017;153:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 40. | Plevris N, Jenkinson PW, Arnott ID, Jones GR, Lees CW. Higher anti-tumor necrosis factor levels are associated with perianal fistula healing and fistula closure in Crohn's disease. Eur J Gastroenterol Hepatol. 2020;32:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 41. | Yarur AJ, Kanagala V, Stein DJ, Czul F, Quintero MA, Agrawal D, Patel A, Best K, Fox C, Idstein K, Abreu MT. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn's disease. Aliment Pharmacol Ther. 2017;45:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 42. | Davidov Y, Ungar B, Bar-Yoseph H, Carter D, Haj-Natour O, Yavzori M, Chowers Y, Eliakim R, Ben-Horin S, Kopylov U. Association of Induction Infliximab Levels With Clinical Response in Perianal Crohn's Disease. J Crohns Colitis. 2017;11:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Vande Casteele N, Gils A, Singh S, Ohrmund L, Hauenstein S, Rutgeerts P, Vermeire S. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 288] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 44. | Hendler SA, Cohen BL, Colombel JF, Sands BE, Mayer L, Agarwal S. High-dose infliximab therapy in Crohn's disease: clinical experience, safety, and efficacy. J Crohns Colitis. 2015;9:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Drobne D, Kurent T, Golob S, Svegl P, Rajar P, Terzic S, Kozelj M, Novak G, Smrekar N, Plut S, Sever N, Strnisa L, Hanzel J, Brecelj J, Urlep D, Osredkar J, Homan M, Orel R, Stabuc B, Ferkolj I, Smid A. Success and safety of high infliximab trough levels in inflammatory bowel disease. Scand J Gastroenterol. 2018;53:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, Thomas A, Nice R, Perry MH, Bouri S, Chanchlani N, Heerasing NM, Hendy P, Lin S, Gaya DR, Cummings JRF, Selinger CP, Lees CW, Hart AL, Parkes M, Sebastian S, Mansfield JC, Irving PM, Lindsay J, Russell RK, McDonald TJ, McGovern D, Goodhand JR, Ahmad T; UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 451] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 47. | Vermeire S, Noman M, Van Assche G, Baert F, D'Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. 2007;56:1226-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 467] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 48. | Ochsenkühn T, Göke B, Sackmann M. Combining infliximab with 6-mercaptopurine/azathioprine for fistula therapy in Crohn's disease. Am J Gastroenterol. 2002;97:2022-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Khanna R, Bressler B, Levesque BG, Zou G, Stitt LW, Greenberg GR, Panaccione R, Bitton A, Paré P, Vermeire S, D'Haens G, MacIntosh D, Sandborn WJ, Donner A, Vandervoort MK, Morris JC, Feagan BG; REACT Study Investigators. Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386:1825-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 348] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 50. | Hoekman DR, Stibbe JA, Baert FJ, Caenepeel P, Vergauwe P, De Vos M, Hommes DW, Benninga MA, Vermeire SA, D'Haens GR; BIRD (Belgian Inflammatory Bowel Disease Research and Development) Group; North-Holland Gut Club. Long-term Outcome of Early Combined Immunosuppression Versus Conventional Management in Newly Diagnosed Crohn's Disease. J Crohns Colitis. 2018;12:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Colombel JF, Adedokun OJ, Gasink C, Gao LL, Cornillie FJ, D'Haens GR, Rutgeerts PJ, Reinisch W, Sandborn WJ, Hanauer SB. Combination Therapy With Infliximab and Azathioprine Improves Infliximab Pharmacokinetic Features and Efficacy: A Post Hoc Analysis. Clin Gastroenterol Hepatol. 2019;17:1525-1532.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 52. | Drobne D, Kurent T, Golob S, Švegl P, Rajar P, Hanžel J, Koželj M, Novak G, Smrekar N, Ferkolj I, Štabuc B. Optimised infliximab monotherapy is as effective as optimised combination therapy, but is associated with higher drug consumption in inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:880-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of Serious and Opportunistic Infections Associated With Treatment of Inflammatory Bowel Diseases. Gastroenterology. 2018;155:337-346.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 412] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 54. | Castaño-Milla C, Chaparro M, Saro C, Barreiro-de Acosta M, García-Albert AM, Bujanda L, Martín-Arranz MD, Carpio D, Muñoz F, Manceñido N, García-Planella E, Piqueras M, Calvet X, Cabriada JL, Botella B, Bermejo F, Gisbert JP. Effectiveness of adalimumab in perianal fistulas in crohn's disease patients naive to anti-TNF therapy. J Clin Gastroenterol. 2015;49:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Echarri A, Castro J, Barreiro M, Carpio D, Pereira S, Lorenzo A. Evaluation of adalimumab therapy in multidisciplinary strategy for perianal Crohn's disease patients with infliximab failure. J Crohns Colitis. 2010;4:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Dewint P, Hansen BE, Verhey E, Oldenburg B, Hommes DW, Pierik M, Ponsioen CI, van Dullemen HM, Russel M, van Bodegraven AA, van der Woude CJ. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn's disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut. 2014;63:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 57. | Moon W, Pestana L, Becker B, Loftus EV, Hanson KA, Bruining DH, Tremaine WJ, Kane SV. Efficacy and safety of certolizumab pegol for Crohn's disease in clinical practice. Aliment Pharmacol Ther. 2015;42:428-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Dulai PS, Singh S, Jiang X, Peerani F, Narula N, Chaudrey K, Whitehead D, Hudesman D, Lukin D, Swaminath A, Shmidt E, Wang S, Boland BS, Chang JT, Kane S, Siegel CA, Loftus EV, Sandborn WJ, Sands BE, Colombel JF. The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn's Disease: Results From the US VICTORY Consortium. Am J Gastroenterol. 2016;111:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 59. | Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, Löwenberg M, Dijkstra G, Oldenburg B, de Boer NKH, van der Marel S, Bodelier AGL, Jansen JM, Haans JJL, Theeuwen R, de Jong D, Pierik MJ, Hoentjen F. Ustekinumab for Crohn's Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J Crohns Colitis. 2020;14:33-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 60. | Gisbert JP, Marín AC, Chaparro M. The Risk of Relapse after Anti-TNF Discontinuation in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Am J Gastroenterol. 2016;111:632-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 61. | Bots SJ, Kuin S, Ponsioen CY, Gecse KB, Duijvestein M, D'Haens GR, Löwenberg M. Relapse rates and predictors for relapse in a real-life cohort of IBD patients after discontinuation of anti-TNF therapy. Scand J Gastroenterol. 2019;54:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Molander P, Färkkilä M, Salminen K, Kemppainen H, Blomster T, Koskela R, Jussila A, Rautiainen H, Nissinen M, Haapamäki J, Arkkila P, Nieminen U, Kuisma J, Punkkinen J, Kolho KL, Mustonen H, Sipponen T. Outcome after discontinuation of TNFα-blocking therapy in patients with inflammatory bowel disease in deep remission. Inflamm Bowel Dis. 2014;20:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 63. | Legué C, Brochard C, Bessi G, Wallenhorst T, Dewitte M, Siproudhis L, Bouguen G. Outcomes of Perianal Fistulising Crohn's Disease Following Anti-TNFα Treatment Discontinuation. Inflamm Bowel Dis. 2018;24:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Reenaers C, Mary JY, Nachury M, Bouhnik Y, Laharie D, Allez M, Fumery M, Amiot A, Savoye G, Altwegg R, Devos M, Malamut G, Bourreille A, Flourie B, Marteau P, Vuitton L, Coffin B, Viennot S, Lambert J, Colombel JF, Louis E; Groupe d'Etude Therapeutique des Affections Inflammatoires du tube Digestif. Outcomes 7 Years After Infliximab Withdrawal for Patients With Crohn's Disease in Sustained Remission. Clin Gastroenterol Hepatol. 2018;16:234-243.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 65. | Louis E. Stopping Biologics in IBD-What Is the Evidence? Inflamm Bowel Dis. 2018;24:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Doherty G, Katsanos KH, Burisch J, Allez M, Papamichael K, Stallmach A, Mao R, Berset IP, Gisbert JP, Sebastian S, Kierkus J, Lopetuso L, Szymanska E, Louis E. European Crohn's and Colitis Organisation Topical Review on Treatment Withdrawal ['Exit Strategies'] in Inflammatory Bowel Disease. J Crohns Colitis. 2018;12:17-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |