Published online Apr 7, 2020. doi: 10.3748/wjg.v26.i13.1513
Peer-review started: December 8, 2019
First decision: January 9, 2020
Revised: March 6, 2020
Accepted: March 19, 2020
Article in press: March 19, 2020
Published online: April 7, 2020
Processing time: 120 Days and 22.4 Hours
177Lu peptide receptor radionuclide therapy (PRRT) is a recently approved therapy in Spain that has been demonstrated to be a well-tolerated therapy for positive somatostatin receptor advanced gastroenteropancreatic neuroendocrine tumors.
To determine the impact of PRRT on quality of life, radiologic and metabolic response, overall survival, prognostic factors and toxicity.
Thirty-six patients treated with 177Lu-PRRT from 2016 to 2019 were included. The most frequent location of the primary tumor was the gastrointestinal tract (52.8%), pancreas (27.8%), and nongastropancreatic neuroendocrine tumor (11.1%). The liver was the most common site of metastasis (91.7%), followed by distant nodes (50.0%), bone (27.8%), peritoneum (25.0%) and lung (11.1%). Toxicity was evaluated after the administration of each dose. Treatment efficacy was evaluated by two parameters: stable disease and disease progression in response evaluation criteria in solid tumors 1.1 criterion and prognostic factors were tested.
From 36 patients, 55.6% were men, with a median age of 61.1 ± 11.8 years. Regarding previous treatments, 55.6% of patients underwent surgery of the primary tumor, 100% of patients were treated with long-acting somatostatin analogues, 66.7% of patients were treated with everolimus, 27.8% of patients were treated with tyrosine kinase inhibitor, and 27.8% of patients were treated with interferon. One patient received radioembolization, three patients received chemoembolization, six patients received chemotherapy. Hematological toxicity was registered in 14 patients (G1-G2: 55.5% and G3: 3.1%). Other events presented were intestinal suboclusion in 4 cases, cholestasis in 2 cases and carcinoid crisis in 1 case. The median follow-up time was 3 years. Currently, 24 patients completed treatment. Nineteen are alive with stable disease, two have disease progression, eight have died, and nine are still receiving treatment. The median overall survival was 12.5 mo (95% confidence interval range: 9.8–15.2), being inversely proportional to toxicity in previous treatments (P < 0.02), tumor grade (P < 0.01) and the presence of bone lesions (P = 0.009) and directly proportional with matching lesion findings between Octreoscan and computed tomography pre-PRRT (P < 0.01), , primary tumor surgery (P = 0.03) and metastasis surgery (P = 0.045). In a multivariate Cox regression analysis, a high Ki67 index (P = 0.003), a mismatch in the lesion findings between Octreoscan and computed tomography pre-PRRT (P < 0.01) and a preceding toxicity in previous treatments (P < 0.05) were risk factors to overall survival.
Overall survival was inversely proportional to previous toxicity, tumor grade and the presence of bone metastasis and directly proportional to matching lesion findings between Octreoscan and computed tomography pre-PRRT and primary tumor and metastasis surgery.
Core tip: Peptide receptor radionuclide therapy has been used successfully in patients diagnosed with metastatic gastroenteropancreatic somatostatin receptor positive tumors when cytoreductive options are limited. In this study we found that overall survival was inversely proportional to toxicity to previous treatments, tumor grade, bone metastasis and directly proportional to matching lesion findings between Octreoscan and computed tomography pre-peptide receptor radionuclide therapy and primary tumor and metastasis surgery. Also, pseudo-progression in the middle of the treatment was a common finding that should be taken into consideration by clinicians in daily practice.
- Citation: Abou Jokh Casas E, Pubul Núñez V, Anido-Herranz U, del Carmen Mallón Araujo M, del Carmen Pombo Pasín M, Garrido Pumar M, Cabezas Agrícola JM, Cameselle-Teijeiro JM, Hilal A, Ruibal Morell Á. Evaluation of 177Lu-Dotatate treatment in patients with metastatic neuroendocrine tumors and prognostic factors. World J Gastroenterol 2020; 26(13): 1513-1524
- URL: https://www.wjgnet.com/1007-9327/full/v26/i13/1513.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i13.1513
Neuroendocrine tumors (NETs) form a heterogeneous group of neoplasms with predominantly neuroendocrine differentiation that can develop in any place of the human body and that have the ability to secrete peptides and neuroamines[1]. Physiologically the transcription factors that direct fate and cell proliferation during embryological development maintain a balance between proliferation, cellular differentiation and apoptosis. Therefore, its disturbance plays a key role in oncogenesis[1]. This can lead to a malignant transformation of cells of the neu-roendocrine system, which derives from the neural crest and endoderm and is characterized by its capacity to generate peptides that produce hormonal syn-dromes[1].
Although they can originate from any organ, gastroenteropancreatic endocrine tumors are the most numerous (67.5%) followed by bronchopulmonary tumors (25.3%)[2], and the remaining cases arise in other endocrine tissues. The most common site of gastroenteropancreatic endocrine tumors is the pancreas (30%-40%), the small intestine (15%-20%) and the rectum (5%-15%)[3].
NETs are an uncommon type of neoplasia; however, its prevalence is higher than other gastrointestinal cancers such as pancreatic, esophageal and hepatobiliary cancer, being exceeded only by colorectal neoplasms[2]. Its current incidence and prevalence increase are probably due to the extensive use of more developed routine radiological tests and endoscopic techniques[3]. According to the data from the Surveillance Epidemiology and End Results program of the National Cancer Institute, an increase in the incidence of gastric NETs in the latter was reported by 0.3 per 100000 in the last 30 years and that it is attributed to the routine use of endoscopic techniques. This increase in incidence was similar across sex and race[3]. According to the results of the national cancer registry in Spain, a substantial increase in the latest trends was reported, being the incidence of 2.5 to 5 cases per 100000 inhabitants in the Caucasian population[3].
An exclusive feature of well-differentiated gastropancreatic neuroendocrine tumor is the overexpression of somatostatin receptors, which is the basis for possible treatments with somatostatin analogues or with radionuclide-labeled peptides that bind to somatostatin receptors and for imaging tests[4,5]. In the past two decades, peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin analogues 177Lu-DOTA0-Tyr3-octreotate (177Lu-Dotatate) has been used successfully in patients diagnosed with metastatic gastroenteropancreatic somatostatin receptor positive tumors when cytoreductive options are limited[6].
177Lu-Dotatate is a radionuclide labeled peptide that binds to somatostatin receptors with high affinity for type 2 receptor and binds to the tumor cells that overexpress them. 177Lu is a radionuclide that disintegrates into stable hafnium (177Hf) by emission of β-particles. It also emits low energy gamma radiation with a half-life of 6.65 d and is indicated in adults for the treatment of gastroenteropancreatic endocrine tumors positive for somatostatin receptor, well differentiated (G1 and G2), progressive and unresectable or metastatic[7].
The aim of this study is to determine the impact of this treatment on the patient’s quality of life, radiological and metabolic response, overall survival and possible prognostic factors and its toxicity.
This is a retrospective longitudinal observational study in which impact on quality of life, radiological and metabolic response, overall survival, possible prognostic factors and toxicity were evaluated in patients diagnosed with advanced tumors expressing somatostatin receptors treated with PRRT. The information pertinent to this cohort of patients is collected through the clinical history, obtaining information about clinical data, treatment response and disease state. These data were treated confidentially and in an encrypted form for analysis. Written consent was obtained from all patients.
Patients diagnosed with advanced NET treated from May 2016 to November 2019 were included. All patients received 177Lu-Dotatate treatment in the Nuclear Medicine department of the University Hospital Santiago de Compostela in Spain. These patients come from all the autonomous community of Galicia and have been previously evaluated by a committee of endocrine tumors in their own hospital center. Also, this study was evaluated and approved by the ethical committee of our medical center.
To evaluate the impact of PRRT over quality life of each patient, three parameters were used by means of a questionnaire after each dose, including “overall im-provement,” “pain assessment” and “evaluation of hormonal secretion symptoms” that included the assessment of diarrhea, flushing, and abdominal pain.
The inclusion criteria were: Patients with advanced NET with a baseline tumor uptake on (111In-DTPA0) octreotide scintigraphy (Octreoscan®) in tumor cells at least as high as in normal liver tissue (Krenning score ≥ 2), tumor progression to previous treatments defined by response evaluation criteria in solid tumors (RECIST) in computed tomography (CT) or magnetic resonance imaging performed at least 3 mo before the treatment, a life expectancy of at least 6 mo, baseline serum hemoglobin ≥ 8 g/dL, white blood cell count > 3000/µL, platelet count ≥ 75.000/µL, creatinine clearance > 50 mL/min, bilirubin < 3 times the range limit, serum albumin > 30g/L (if the albumin < 30 g/L, then prothrombin time must be normal) and a Karnofsky performance status ≥ 60 or an ECOG < 2.
Patients were hospitalized for the administration of the treatment in which 30 min before the infusion of PRRT a capsule containing 300 mg of netupitant and 0.5 mg of palonosetron (Akynzeo®) was administrated orally. Later, an infusion of amino acids (2.5% arginine and 2.5% lysine, 1 L) was started 30 min before the administration of the radiopharmaceutical, lasting for 4-6 h. The radiopharmaceutical was co-administered using a second pump system. All cycle doses were 7.4 GBq (200 mCi) of 177Lu-Dotatate, injected over 30 min completing 4 cycles of treatment. The intended interval between treatments cycles was 6–10 wk.
Between treatment cycles patients underwent blood analysis 4 and 6 wk after the dose administration to detect possible side effects. These blood tests included a hemogram and renal and liver function parameters. A full body scintigraphy was performed 24 h after each dose in an Optima 640 gamma camera from General Electric as well as a whole body CT following the second cycle to evaluate RECIST criterion. The follow-up included CT or magnetic resonance imaging performed 3-6 mo after the last treatment and thereafter every 6 mo.
Statistical analyses were performed using IBM SPSS® version 23.0 with the data obtained. A descriptive analysis was carried out describing the continuous variables as means, medians and standard deviation while the categorical variables were described as proportions, including 95% confidence intervals (CIs). The radiological, clinical and metabolic response variable was calculated as the percentage of patients that responded. Progression-free survival was calculated from the initiation date treatment with PRRT until disease progression, assessed by RECIST criteria or death from any cause. Overall survival was calculated from the start date of treatment with PRRT until the date of death of the patient for any reason and was estimated using Kaplan-Meier. Cox regression was used to evaluate the association with independent variables.
The study was conducted following the Declaration of Helsinki of the World Medical Association (1964) and ratifications of the following assemblies on ethical principles for medical research in humans (RD 1090/2015, of December 24, of clinical trials, specifically the provisions of article 38 on good clinical practice and the Convention on Human Rights and Biomedicine), made in Oviedo on April 4, 1997 and successive updates.
The median age was 61.1 ± 11.8 years (age range: 38-85 years), and 20 were men (55.6%). Regarding medical history, seven patients were diabetic (19.4%), fifteen were hypertensive (41.7%), nine had smoking habits prior to the diagnosis (25%), five had cancer history (13.9%), of which 3 received chemo-radiotherapy treatments, and one patient was a carrier of a mutation in the MEN-1 gene.
Regarding chief complaints, 33.3% consulted for abdominal pain, 16.67% for gastrointestinal and hormonal related symptoms, 12.5% for weight loss and 11.1% had no symptoms prior the diagnosis. In 58.3% of patients, the diagnosis was casual by an imaging test performed for other reasons, 30.6% guided by clinical suspicion and 5.6% during a surgical intervention for uterine leiomyomatosis.
The most common primary tumor was in the gastrointestinal tract (52.8%), followed by the pancreas (27.8%). A nongastropancreatic NET was diagnosed 11.1% of patients, including two endobronchial NETs, one thymic and three with unknown origin of the primary tumor. The vast majority of the patients (91.7%) had liver metastases followed by metastasis in the peritoneum (25.0%), lymph nodes (50.0%), bone (27.8%), lung (11.1%) and other locations including a lesion in the heart, in the suprarenal gland, in the kidney and in the ovary. Baseline characteristics are presented in Table 1.
| Characteristics | Number of patients |
| Sex | |
| Male | 20 (55.6%) |
| Female | 16 (44.4%) |
| Comorbidities | |
| Hypertension | 15 (41.7%) |
| DM | 7 (19.4%) |
| Smoking habits | 9 (25.0%) |
| Cancer history | 5 (13.9%) |
| Symptoms prior to diagnosis | |
| Abdominal pain | 12 (33.3%) |
| Gastrointestinal and carcinoid symptoms | 6 (16.7%) |
| Weight loss | 5 (12.5%) |
| Asymptomatic | 4 (11.1%) |
| Primary tumor site | |
| Gastrointestinal tract | 19 (52.8%) |
| Pancreas | 10 (27.8%) |
| Nongastropancreatic NET | 4 (11.1%) |
| Endobronchial NETs | 2 |
| Thymic | 1 |
| Histologic grade | |
| Grade 1 | 15 (41.7%) |
| Grade 2 | 18 (50.0%) |
| Grade 3 (2 NEC + 1 TNE) | 3 (8.3%) |
| Site of metastasis | |
| Liver | 33 (91.7%) |
| Lymph nodes | 18 (50.0%) |
| Bone | 10 (27.8%) |
| Peritoneum | 9 (25.0%) |
| Lungs | 4 (11.1%) |
| Primary tumor resection | 20 (55.6%) |
| Metastasis resection | 7 (19.4%) |
| Primary treatment before PRRT | |
| SSA | 36 (100%) |
| Everolimus | 24 (66.7%) |
| Sunitinib | 10 (27.8%) |
| Interferon | 10 (27.8%) |
| Chemotherapy | 6 (16.7%) |
| Liver directed therapy | |
| Chemoembolization | 3 |
| Radioembolization | 1 |
Regarding anatomopathological characteristics, 41.7% were grade 1 (Ki67% ≤ 2), 50% were grade 2 (Ki67% = 3-20) and 8.3% were grade 3 (Ki67% > 20), of which two were poorly differentiated neuroendocrine carcinoma. Of the total number of patients, 63.9% completed treatment with PRRT, 25.0% patients are receiving treatment and 11.1% could not complete it due to death or complications of their underlying disease.
More than half of the patients had surgically removed the primary tumor (55.6%), and 19.4% underwent surgery of the metastases. All patients were treated with somatostatin analogues (SSA), 44.4% needed treatment with short-acting octreotide, 66.7% received everolimus, 27.8% received sunitinib, 27.8% received treatment with interferon, and 16.7% received chemotherapy. Four patients received liver directed therapy, three of which underwent chemoembolization and one underwent transarterial radioembolization of liver metastases.
Most patients (94.4%) had progression to previous treatments 12 mo after their start. Only 5.6% of patients had early progression of their disease according to RECIST criteria before 12 mo.
We assessed whether there was a matching coincidence between the lesions described in the CT evaluation and the Octreoscan® pre-PRRT. Twenty-six (72.2%) of the patients presented matching lesions in the two studies, 13.9% had lesions in the CT that did not express somatostatin receptors in Octreoscan®, and 13.9% presented metabolic lesions without anatomical correlate in the CT.
It was corroborated that 69.4% of the patients presented a greater number of lesions in the scintigraphy scan after the first dose of PRRT than in those described in the Octreoscan® pre-treatment. The most frequent localization was the liver (38.9%), bone (11.1%), lymph (5.6%), lung (2.8%) and spleen (2.8%).
At the end of the treatment, 23 patients completed the treatment. Of these patients, 11 (30.6%) presented a decrease in the number of lesions in the fourth post-dose scintigraphy compared to the first scan. The intensity of lesions decreased in 33.3% of patients, which changed the Krenning score from 4 to 3.
In the CT evaluation after the administration of the second dose, the radionuclide treatment was effective at achieving a radiological stability according to RECIST criteria in 20 patients (69%). However, there was an apparent radiological progression in nine cases (25%). Despite this, due to a significant clinical improvement and control of hormonal related symptoms, it was decided in a multidisciplinary committee to complete the treatment. Disease control was reached in the evaluation after the last dose in all cases. So far, nineteen patients (52.8%) are alive with stable disease, two (5.6%) have disease progression, eight (22.2%) have died and seven (25.0%) are still receiving treatment.
After the administration of the first and second doses, 75% of the patients presented an overall improvement that remained until the end of the treatment. Regarding the evaluation of pain, 15 patients (41.7%) reported a clear recovery after the first dose. Of these patients, ten (27%) were asymptomatic after the last dose. Of the 15 patients with hormonal secretion symptoms, 66.6% reported an amelioration of symptoms after the first dose, reaching control at the end of treatment in 26.6% of the patients.
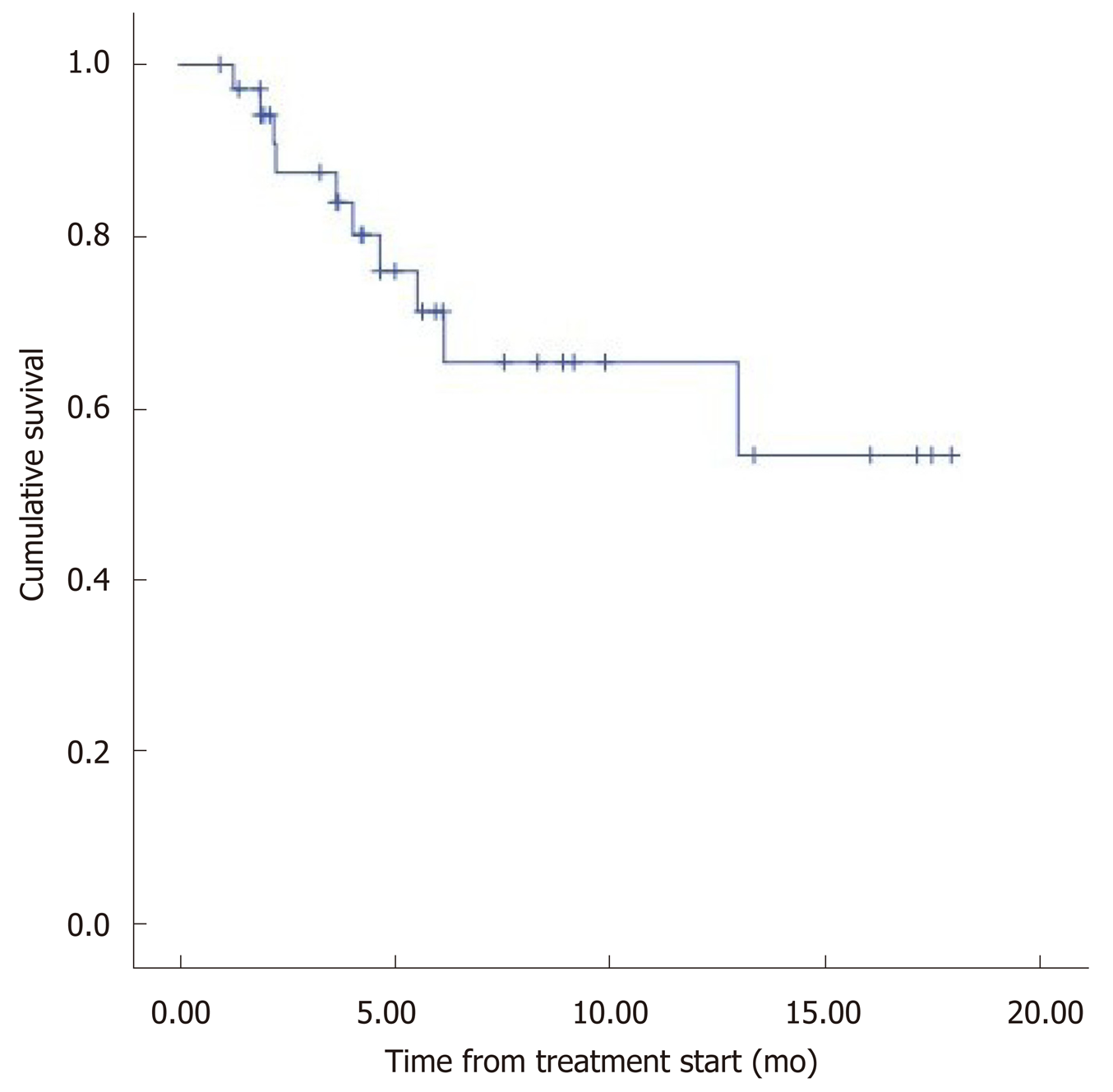
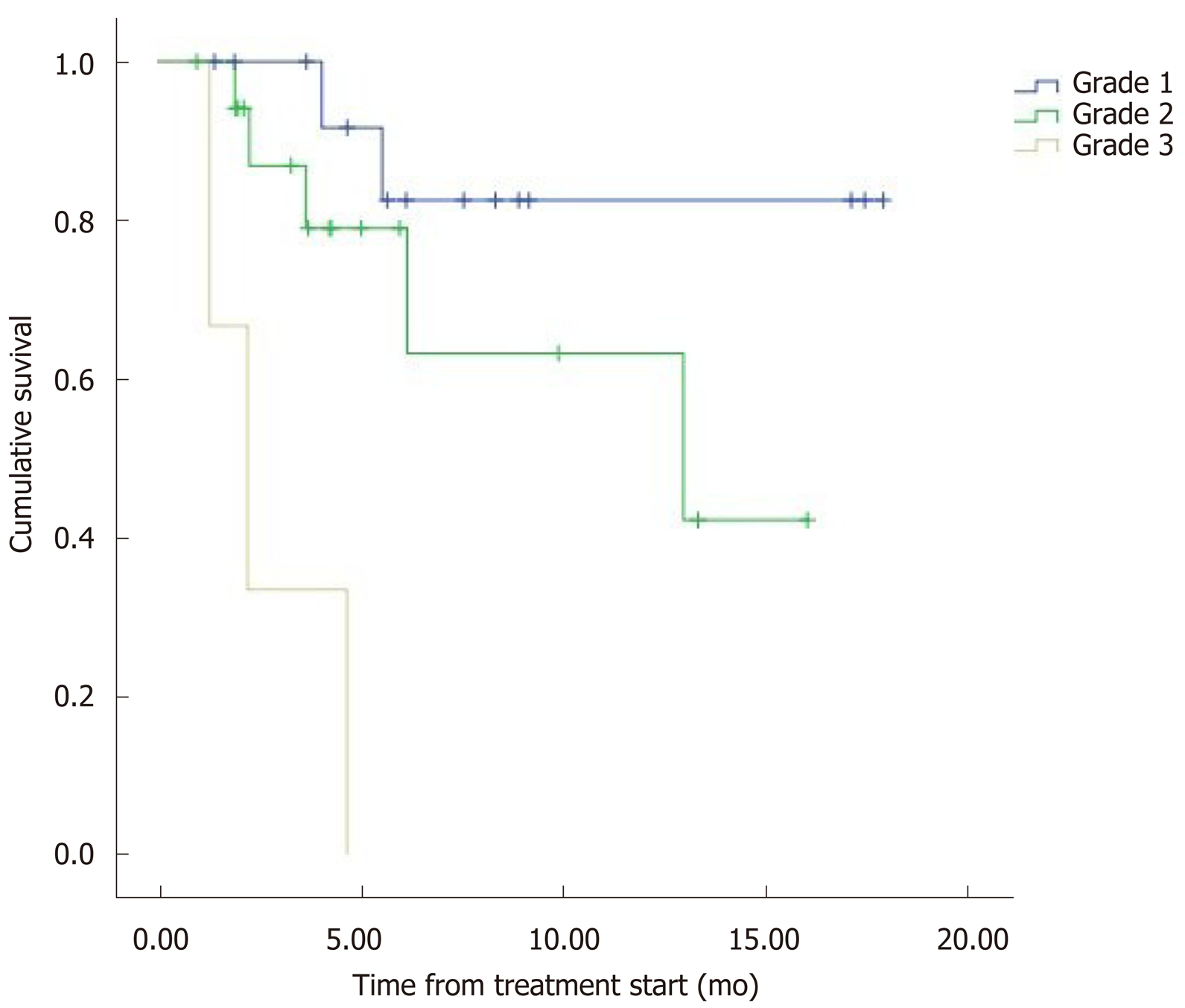
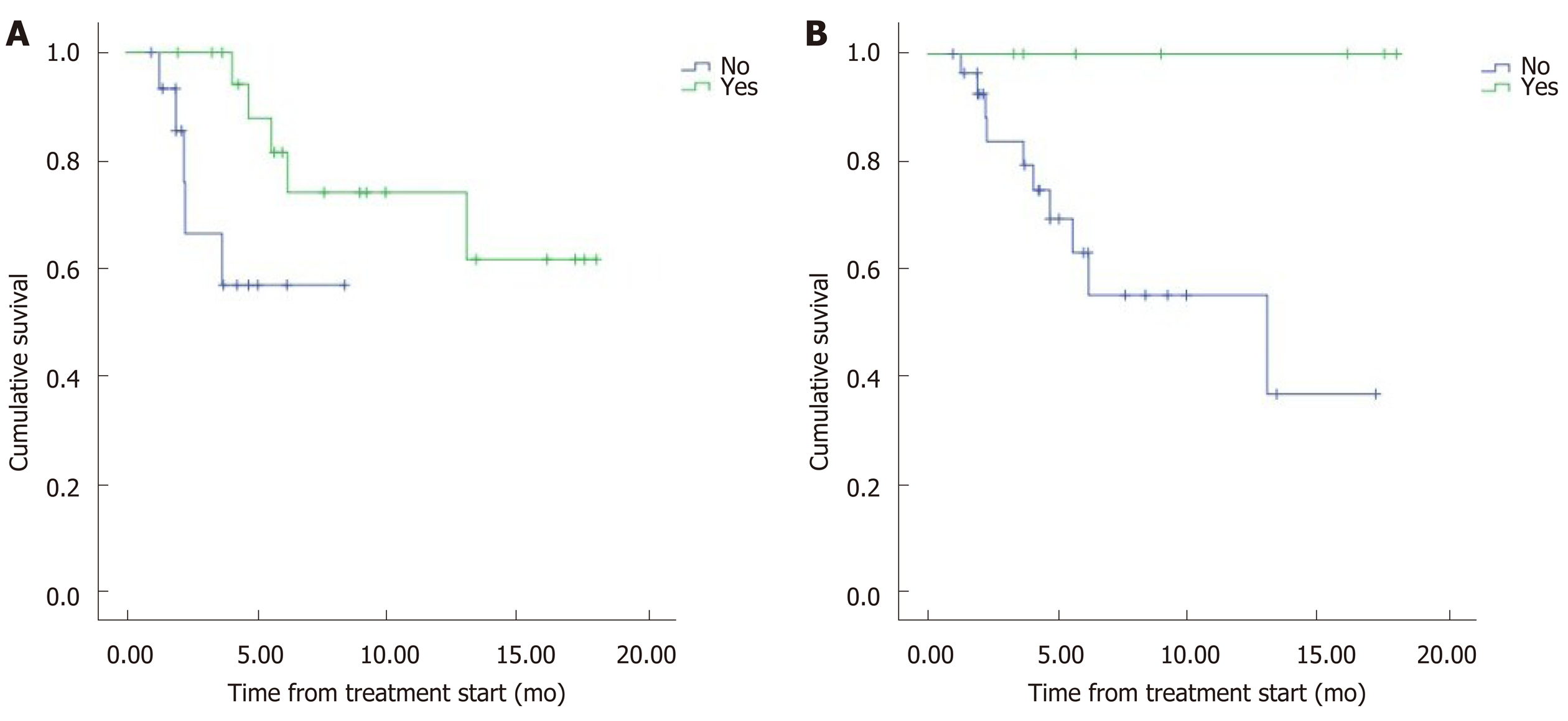
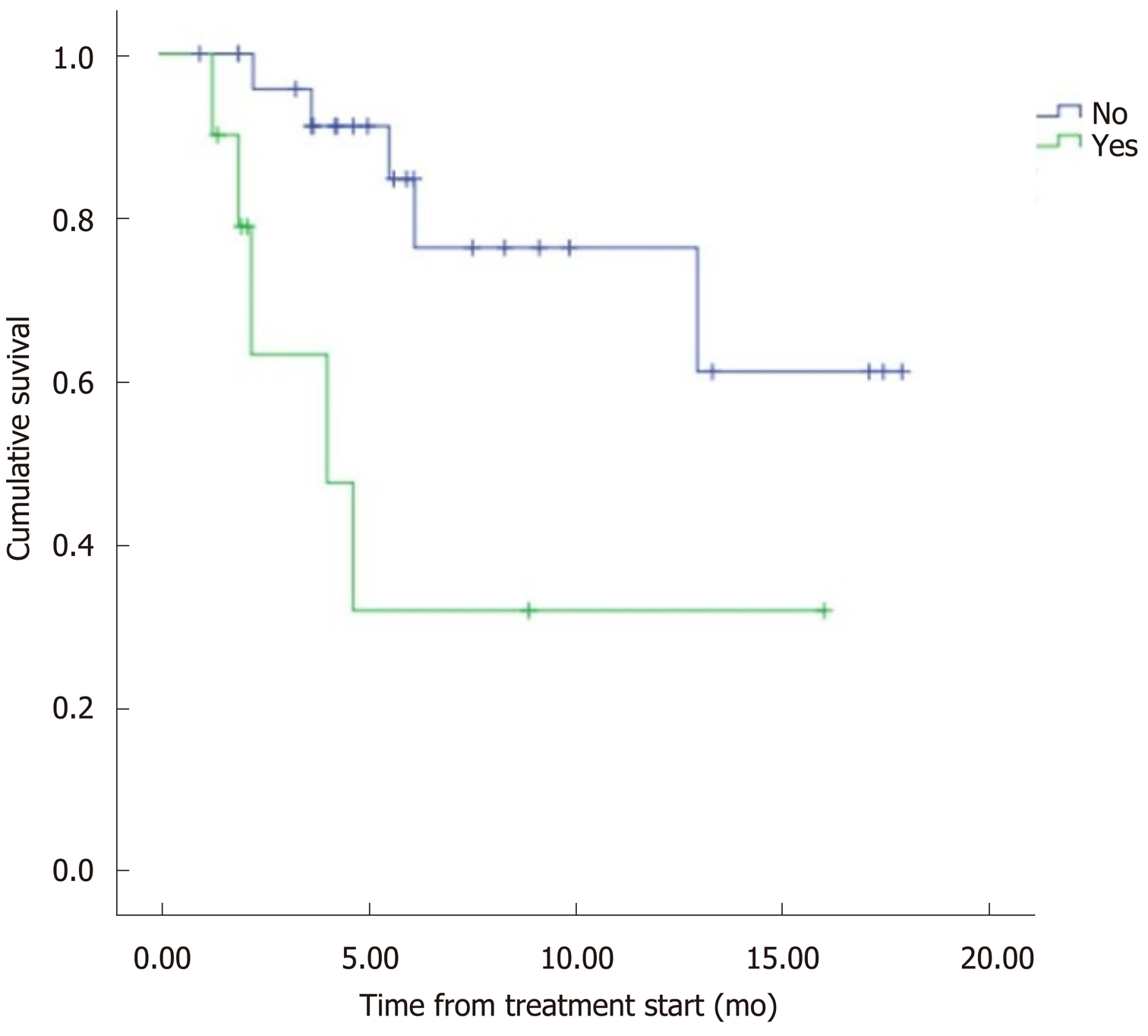
The median overall survival (OS) was 12.5 mo (95%CI: 9.8–15.2) (Figure 1). Eight patients (22.2%) died during follow-up. OS was inversely proportional to preceding toxicity in previous treatments (P < 0.02) with a hazard ratio (HR) of 0.09 (95%CI: 0.01–0.7; P = 0.02), tumor grade (P < 0.01, HR: 0.09, 95%CI: 0.014–0.676; P = 0.018) (Figure 2) and the presence of bone metastasis (BM) (P = 0.009, HR: 0.08, 95%CI: 0.015–0.446; P = 0.004) (Figure 4) and is directly proportional with matching lesion findings between Octreoscan and CT pre-PRRT (P < 0.01, HR: 0.36, 95%CI: 0.07–1.79; P = 0.21); primary tumor surgery (P = 0.03, HR: 1.7, 95%CI: 0.48–6.03; P = 0.4) and metastasis surgery (P = 0.045, HR: 26.2, 95%CI: 0.01–711; P = 0.41) (Figure 3). In a multivariate Cox regression analysis, a high Ki67 index (P = 0.003), a mismatch in the lesion findings between Octreoscan and CT pre-PRRT (P < 0.01) and a preceding toxicity in previous treatments (P < 0.05) were risk factors for OS.
OS was not significantly different by gender (P = 0.74, HR: 1.4, 95%CI: 0.39–5.47; P = 0.56); oncological history (P = 0.091, HR: 23.95, 95%CI: 0.00–316; P = 0.51); smoking (P = 0.34, HR: 5.07, 95%CI: 0.63–40.28; P = 0.12); treatment with SSA (P = 0.959, HR: 1.59, 95%CI: 0.32–7.71; P = 0.56); everolimus (P = 0.97, HR: 0.85, 95%CI: 0.21–3.35; P = 0.82); sunitinib (P = 0.993, HR: 0.85, 95%CI: 0.23–3.04; P = 0.80); interferon (P = 0.945, HR: 1.04, 95%CI: 0.21–5.17; P = 0.95); radioembolization (P = 0.425); qui-mioembolization (P = 0.972, HR: 0.25, 95%CI: 0.02–2.53; P = 0.24); and quimiotherapy (P = 0.06, HR: 0.42, 95%CI: 0.12–1.52; P = 0.18).
History of hypertension (P = 0.06), diabetes (P = 0.83), oncology diseases (P = 0.31), smoking (P = 0.08) as well as other factors such as the location of the primary tumor (P = 0.426), treatment with SSA (P = 0.56), everolimus (P = 0.82), sunitinib (P = 0.80) and interferon (P = 0.95) were not predictive risk factors.
In this study, 44.4% of the patients presented toxicity to previous treatments before PRRT, of which 36.1% were due to everolimus and 8.3% to chemotherapy. Toxicity grade 1-2 was present in 71.4% of patients, and grade 3-4 was present in 28.6% of patients
During PRRT, acute adverse effects (< 24 h) were more frequent after the administration of the first dose, which manifested in 33.3% of the patients with sickness (25%), abdominal discomfort or pain (2.8%) and extravasation (2.8%). A hormone-related crisis in one patient resulted in hospitalization within 5 d after the administration dose. All recovered after adequate care.
During treatment, 55.6% of the patients suffered complications or toxicity. Hematological toxicity was present in 38.8% with one case being severe (CTCAE v.4 o v.5.)[8] with thrombocytopenia. Four patients (11.1%) suffered intestinal suboclusion in which only one had to be surgically operated, and three patients (8%) presented with cholestasis that corrected spontaneously. One patient with extensive liver metastasis presented with serious delayed liver toxicity. Liver functions deteriorated in the weeks following the first administration, and the patient died of hepatic failure 5 wk later.
In this study, the median OS obtained was 12.5 mo after 3 years of follow-up, and death presented in 21.8% of patients. Thomas et al[9] in a retrospective review with 273 patients diagnosed with advanced gastrointestinal NET for a similar period of time (41 mo) recorded 28% mortality during follow-up.
In this cohort of patients, OS was inversely proportional with respect to toxicity in previous treatments (P < 0.05) tumor grade and the presence of bone lesions, while it was directly proportional to matching lesion findings between Octreoscan and CT pre-PRRT (P < 0.01) and surgery of the primary tumor or its metastasis. These findings are confirmed by conducting a multivariate Cox regression analysis. A high Ki67 index (P = 0.003), a mismatch in lesion findings between Octreoscan and CT pre-PRRT (P < 0.01) and toxicity in previous treatments (P < 0.05) were risk factors to OS. Other contributing factors to OS were tumor grade and the presence of bone lesions. It is increasingly recognized that Ki-67 index and tumor grade are powerful determinants of survival. A study with 74 patients demonstrated the favorable response and long-term outcome of patients with G1/G2 gastroenteropancreatic NET after PRRT being the most powerful predictive factor in OS[10].
Regarding BM, there is little published data on the general prognostic impact of bone metastasis in NET in the context of PRRT, but it is well known that this outcome is associated with pain and eventual decrease of bone marrow function[11]. It is difficult to evaluate the direct prognostic impact of BM in NET due to the incidence and the heterogeneity of these tumors as well as the frequent coexistence of multiple distant metastases. To the best of our knowledge, only retrospective studies and one systematic review have analyzed this topic. In the study by Strosberg et al[12], evaluating 146 cases of metastatic midgut NETs, BM represented a negative prognostic factor because patients with BMs (n = 35) had a median survival of 32 mo (95%CI: 28–35 mo) and a 5-year survival rate of 20%.
On the other hand, OS was similar and independent to other factors, such as the location of the primary tumor and the administration of previous treatments with SSA or others. However, a larger number of patients is needed to determine an accurate relationship between these variables.
Most patients (72.2%) presented with matching lesions identified in the CT evaluation and Octreoscan® pre-PRRT, while 13.9% had more lesions in the CT that did not express somatostatin receptors in Octreoscan® and 13.9% presented metabolic lesions without anatomical correlation in CT. Matching lesions between Octreoscan and CT pre-PRRT had a proportional relationship to OS, representing a prognostic factor (P < 0.05). This may be due to all lesions visualized on the CT expressing somatostatin receptors. Therefore, a greater number of tumor cells would be treated by the radionuclide and would respond well to treatment. However, in the case of a wide cellular heterogeneity and having lesions in the CT that are not visualized in the Octreoscan®, the scope of the radiopharmaceutical would be limited to the lesions with positive receptors, leaving the rest of the tumor cells untreated. This is the first study to our knowledge to report this important outcome. This result may help to evaluate individual prognostic factors to OS as well as for the need to conduct a 18F-FDG positron emission tomography/CT to predict treatment response in patients with cellular heterogeneity. In addition, they demonstrate the need for more research with a greater number of patients assessing the importance of this and other prognostic factors that are yet to be described in order to improve patient ma-nagement.
Another important result in this study was an apparent radiological progression in 20 cases (55.6%) in the CT evaluation after the second dose manifested as an increase in the lesion’s diameter. Despite this, due to a significant clinical improvement and symptom control, it was decided to complete the treatment. Disease control was reached after the last dose in all cases. This transient increase was probably due to inflammation causing localized edematous tissue at the site of the metastases and not based on progression. This radiogenic edema has been described previously as possible pitfalls by Brabander et al[13], in which the phenomenon was called pseudo-progression. These findings were previously described in brain tumors and external beam radiation, in cytokine studies, in monoclonal antibodies and in immunotherapy, where the increase in tumor size was probably related to infusion of lymphocytes and macrophages and was not always considered as disease progression. Therefore, in NET patients, clinicians should be aware and take into account in daily practice that an increase in tumor size is frequent due to radiation-induced inflammation and not always refers to tumor progression[13].
Currently, the only available curative treatment for NET is surgery, but for those patients who have an inoperable primary tumor, recurrent disease or metastatic disease, few therapeutic options are available. PRRT is commonly noncurative in patients diagnosed with NETs. Therefore, this systemic treatment should be focused on improving patient quality of life. Our results demonstrate an improvement in quality of life in 75% of our patients, better pain assessment in 41.7% and a better control of hormonal related symptoms in 66.6%, reaching full control at the end of the treatment in 26.6% of the population. Also, 41.6% of our patients were asymptomatic after the fourth dose. These results also prove the need for more research with a greater number of patients in assessing adverse events and effects on quality of life for NET therapies[14].
The NETTER-1 study showed a significant quality-of-life benefit for patients with midgut NETs who received 177Lu-DOTATATE vs those treated with high dose long-acting octreotide specifically for diarrhea, flushing and abdominal pain[15,16]. Likewise, Tellestar is a Phase III, multicenter, randomized, double-blind, placebo-controlled study in which clinical outcomes were assessed. The study suggested a sustained improvement in bowel movement frequency in patients with carcinoid syndrome and a long-term effect on patient´s well-being[17]. Additionally, Martini et al[18] studied the impact of health-related quality of life (HRQoL) from the first PRRT to the first restaging and compared the scores with general population norms. They observed improvements from baseline to the first restaging for diarrhea in small-intestine NET patients and a clinically relevant decrease in appetite loss (for female small-intestine NET patients only). Compared with HRQoL general population norms, patients had impairments consisting of diarrhea, fatigue, appetite loss, reduced physical, social, and role functioning and reduced global HRQoL. In conclusion their findings supported overall stable HRQoL under PRRT. However, significant HRQoL impairments compared with the general population and potentially specific subgroup patterns need to be considered.
Side effects in our group were either related to the administration of the amino acids or due to the radio-peptide itself[19,20]. During the follow-up, half of the patients suffered some kind of complication or toxicity, in order of frequency: Nausea, discomfort or abdominal pain and a carcinoid crisis triggered by the massive release of bioactive substances. Similarly, the evaluation of toxicity in a group of 504 patients who were given four cycles of treatment at intervals of 6 to 10 wk revealed that the most common symptoms in the first 24 h were nausea (25%), vomiting (10%) and pain (10%)[21]. In a similar study, carried out in 479 patients, it was determined that only 1% with hormonally active neuroendocrine tumors had a clinical crisis after ad-ministration[22].
The development of hematological toxicity was found in almost half of the patients (38.8%), being severe (grade 3) in one case (2.8%) with thrombocytopenia. A recent study conducted with 450 patients treated with PRRT in five different centers stipulated that serious adverse events were rare with leukopenia and throm-bocytopenia of grade 3 in 1.1% and 1.3% of patients respectively, and only one episode of grade 4 thrombocytopenia[23,24].
The most serious side effect in our study was observed in one patient with extensive liver metastasis who developed a severe deterioration of liver function. In patients with or without mild metastatic hepatic involvement, no significant liver toxicity has been reported. However, in patients with massive liver metastases and impaired hepatic function, hepatic toxicity may occur. This should be considered, along with pre-existing conditions affecting the liver, when deciding the appropriate dose[20].
This study has limitations. First, it is a retrospective observational study. The aim is to observe and describe and lacks an intervention in the natural course of patients. Therefore, one or more variables of interest could not be studied. As well, we are aware that the number of patients is scarce and limited, but this is the first study to our knowledge to describe that matching lesions in CT and Octreoscan® previous to PRRT treatment could be a prognostic factor that should be studied with a larger cohort of patients. If corroborated, this finding could be considered into treatment decisions and may result in major patient surveillance.
In this study we found that matching lesions in CT and Octreoscan® pre-PRRT, represent a prognostic factor to overall survival and that pseudo-progression is a common finding observed in the first stages of the treatment that should be taken into consideration by clinicians in daily practice.
The incidence and prevalence of neuroendocrine tumors are currently increasing, probably due to the extensive use of more developed routine radiological tests and endoscopic techniques. In patients with limited cytoreductive options, peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin analogues 177Lu-DOTA0-Tyr3-octreotate (177Lu-Dotatate) has been used successfully in patients diagnosed with metastatic gastroenteropancreatic somatostatin receptor positive tumors in the past two decades.
The aim of this study was to determine the impact of this treatment on patient’s quality of life, radiological and metabolic response, overall survival, prognostic factors and its toxicity.
The determination of prognostic factors that can modify the overall survival of these patients is of vital importance because it could allow a more specialized therapy and increase patient’s surveillance when required. This might be an interesting approach in future research.
This is a retrospective longitudinal observational study in which impact on quality of life, radiological and metabolic response, overall survival, prognostic factors and toxicity were evaluated in patients diagnosed with advanced tumors expressing somatostatin receptors treated with PRRT. The information pertinent to this cohort of patients was collected through the clinical history, obtaining information about clinical data, treatment response and disease state. These data were treated confidentially and in an encrypted form for analysis. Written consent was obtained from all patients.
In this cohort of patients, overall survival was inversely proportional with respect to toxicity in previous treatments (P < 0.05), tumor grade and the presence of bone lesions and was directly proportional to matching lesion findings between Octreoscan and computed tomography (CT) pre-PRRT (P < 0.01) andsurgery of the primary tumor or its metastasis. Also, we found that pseudo-progression is a common finding observed in the first stages of the treatment that should be taken into consideration by clinicians in daily practice. We consider that the matching lesions in CT and Octreoscan® before PRRT treatment could be a prognostic factor and should be studied with a greater cohort of patients. If corroborated, this finding could be considered in treatment decisions and may result in major patient surveillance.
Overall survival was inversely proportional with respect to toxicity in previous treatments (P < 0.05) and was directly proportional to matching lesion findings between Octreoscan and CT pre-PRRT. Matching lesion findings between Octreoscan and CT pre-PRRT should be taken into consideration when treating these patients.
This study reveals that prognostic factors should be taken into consideration because they modify the overall survival. Therefore, future research should focus on finding new prognostic factors that could allow specialized patient surveillance. In future studies, a larger number of patients should be included to extract more conclusive results that would allow the identification of new prognostic factors.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao BL, Khuroo MS, Yang ZH S-Editor: Zhang L L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Fernández-Rañada Shaw I, Arévalo MG, Martel IJ, Giménez DM, Valadés JIM, Martínez JM, Gárate CO, Salas NR, Sánchez JS. Guía Clínica de diagnóstico y tratamiento de Tumores Neuroendocrinos. OncoSur: Grupo de trabajo oncológico de centros hospitalarios del sur de Madrid 2011. Available from: https://cn.bing.com/search?q=Gu%C3%ADa+Cl%C3%ADnica+de+diagn%C3%B3stico+y+tratamiento+de+Tumores+Neuroendocrinos+2011&qs=n&form=QBRE&sp=-1&pq=&sc=8-0&sk=&cvid=DAC32997F9B64DFDBB72937861673A69. |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3239] [Article Influence: 190.5] [Reference Citation Analysis (0)] |
| 3. | Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Díaz-Pérez JA, Martínez Del Prado MP, Alonso Orduña V, Sevilla-García I, Villabona-Artero C, Beguiristain-Gómez A, Llanos-Muñoz M, Marazuela M, Alvarez-Escola C, Castellano D, Vilar E, Jiménez-Fonseca P, Teulé A, Sastre-Valera J, Benavent-Viñuelas M, Monleon A, Salazar R. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol. 2010;21:1794-1803. [PubMed] [DOI] [Full Text] |
| 4. | Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10:2259-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Maxwell JE, Howe JR. Imaging in neuroendocrine tumors: an update for the clinician. Int J Endocr Oncol. 2015;2:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Kendi AT, Halfdanarson TR, Packard A, Dundar A, Subramaniam RM. Therapy With 177Lu-DOTATATE: Clinical Implementation and Impact on Care of Patients With Neuroendocrine Tumors. AJR Am J Roentgenol. 2019;213:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Sanitarios agencia española de medicamentos. Informe de Posicionamiento Terapéutico de lutecio (177Lu) oxodotreotida (Lutathera®) en el tratamiento de tumores neuroendocrinos gastroenteropancreáticos bien diferenciados. Informe De Posicionamiento Terapéutico. Madrid: Ministerio de Sanidad 2019. Available from: https://cn.bing.com/search?q=Informe+de+Posicionamiento+Terap%C3%A9utico+de+lutecio+%28177Lu%29+oxodotreotida+%28Lutathera%C2%AE%29+en+el+tratamiento+de+tumores+neuroendocrinos+gastroenteropancre%C3%A1ticos+bien+diferenciados.&qs=n&form=QBLHCN&sp=-1&pq=&sc=8-0&sk=&cvid=78C09BCDE4C640F7B34FC5F3FD2A8138. |
| 8. | Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 3.0, Department of Health and Human Services US 2006. Available from: http://ctep.cancer.gov. |
| 9. | Thomas KEH, Voros BA, Boudreaux JP, Thiagarajan R, Woltering EA, Ramirez RA. Current Treatment Options in Gastroenteropancreatic Neuroendocrine Carcinoma. Oncologist. 2019;24:1076-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grünwald F, Guhlke S, Biersack HJ, Sabet A. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2014;55:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Ezziddin S, Sabet A, Heinemann F, Yong-Hing CJ, Ahmadzadehfar H, Guhlke S, Höller T, Willinek W, Boy C, Biersack HJ. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with (177)Lu-octreotate. J Nucl Med. 2011;52:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Strosberg J, Gardner N, Kvols, L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology. 2009;89:471–476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, de Herder WW, Feelders RA, Krenning EP, Kwekkeboom DJ. Pitfalls in the response evaluation after peptide receptor radionuclide therapy with [177Lu-DOTA0,Tyr3]octreotate. Endocr Relat Cancer. 2017;24:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Kaderli RM, Spanjol M, Kollár A, Bütikofer L, Gloy V, Dumont RA, Seiler CA, Christ ER, Radojewski P, Briel M, Walter MA. Therapeutic Options for Neuroendocrine Tumors: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2019;5:480-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Dash A, Chakraborty S, Pillai MR, Knapp FF. Peptide receptor radionuclide therapy: an overview. Cancer Biother Radiopharm. 2015;30:47-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2231] [Article Influence: 278.9] [Reference Citation Analysis (0)] |
| 17. | Cella D, Beaumont JL, Hudgens S, Marteau F, Feuilly M, Houchard A, Lapuerta P, Ramage J, Pavel M, Hörsch D, Kulke MH. Relationship Between Symptoms and Health-related Quality-of-life Benefits in Patients With Carcinoid Syndrome: Post Hoc Analyses From TELESTAR. Clin Ther. 2018;40:2006-2020.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Martini C, Buxbaum S, Rodrigues M, Nilica B, Scarpa L, Holzner B, Virgolini I, Gamper EM. Quality of Life in Patients with Metastatic Gastroenteropancreatic Neuroendocrine Tumors Receiving Peptide Receptor Radionuclide Therapy: Information from a Monitoring Program in Clinical Routine. J Nucl Med. 2018;59:1566-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Maqsood MH, Tameez Ud Din A, Khan AH. Neuroendocrine Tumor Therapy with Lutetium-177: A Literature Review. Cureus. 2019;11:e3986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O'Dorisio MS, O'Dorisio TM, Howe JR, Cremonesi M, Kwekkeboom DJ, Zaknun JJ. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 532] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 21. | Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO, Krenning EP. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1076] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 22. | de Keizer B, van Aken MO, Feelders RA, de Herder WW, Kam BL, van Essen M, Krenning EP, Kwekkeboom DJ. Hormonal crises following receptor radionuclide therapy with the radiolabeled somatostatin analogue [177Lu-DOTA0,Tyr3]octreotate. Eur J Nucl Med Mol Imaging. 2008;35:749-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R, Borbath I, Cwikla J, Toumpanakis C, Kaltsas G, Davies P, Hörsch D, Tiensuu Janson E, Ramage J; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology. 2017;105:295-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 24. | Hörsch D, Ezziddin S, Haug A, Gratz KF, Dunkelmann S, Miederer M, Schreckenberger M, Krause BJ, Bengel FM, Bartenstein P, Biersack HJ, Pöpperl G, Baum RP. Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: A multi-institutional registry study with prospective follow-up. Eur J Cancer. 2016;58:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |