Published online Apr 7, 2020. doi: 10.3748/wjg.v26.i13.1490
Peer-review started: December 12, 2019
First decision: January 19, 2020
Revised: February 14, 2020
Accepted: March 5, 2020
Article in press: March 5, 2020
Published online: April 7, 2020
Processing time: 117 Days and 7.5 Hours
Total laparoscopic distal gastrectomy (TLDG) is increasing due to some advantages over open surgery, which has generated interest in gastrointestinal surgeons. However, TLDG is technically demanding especially for lymphadenectomy and gastrointestinal reconstruction. During the course of training, trainee surgeons have less chances to perform open gastrectomy compared with that of senior surgeons.
To evaluate an appropriate, efficient and safe laparoscopic training procedures suitable for trainee surgeons.
Ninety-two consecutive patients with gastric cancer who underwent TLDG plus Billroth I reconstruction using an augmented rectangle technique and involving trainees were reviewed. The trainees were taught a laparoscopic view of surgical anatomy, standard operative procedures and practiced essential laparoscopic skills. The TLDG procedure was divided into regional lymph node dissections and gastrointestinal reconstruction for analyzing trainee skills. Early surgical outcomes were compared between trainees and trainers to clarify the feasibility and safety of TLDG performed by trainees. Learning curves were used to assess the utility of our training system.
Five trainees performed a total of 52 TLDGs (56.5%), while 40 TLDGs were conducted by two trainers (43.5%). Except for depth of invasion and pathologic stage, there were no differences in clinicopathological characteristics. Trainers performed more D2 gastrectomies than trainees. The total operation time was significantly longer in the trainee group. The time spent during the lesser curvature lymph node dissection and the Billroth I reconstruction were similar between the two groups. No difference was found in postoperative complications between the two groups. The learning curve of the trainees plateaued after five TLDG cases.
Preparing trainees with a laparoscopic view of surgical anatomy, standard operative procedures and practice in essential laparoscopic skills enabled trainees to perform TLDG safely and feasibly.
Core tip: The rapid expansion of total laparoscopic distal gastrectomy has led to concern about education for young surgeons. Laparoscopic training for young surgeons differs from training experienced previously due to fewer opportunities to perform open gastrectomy and higher technical demands. We introduced our laparoscopic training system and found that making a standard laparoscopic procedure and using the easy reconstruction method are useful in the success of the training system.
- Citation: Zhang S, Orita H, Egawa H, Matsui R, Yamauchi S, Yube Y, Kaji S, Takahashi T, Oka S, Inaki N, Fukunaga T. Effectiveness and safety of a laparoscopic training system combined with modified reconstruction techniques for total laparoscopic distal gastrectomy. World J Gastroenterol 2020; 26(13): 1490-1500
- URL: https://www.wjgnet.com/1007-9327/full/v26/i13/1490.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i13.1490
Laparoscopic assisted distal gastrectomy was first reported by Kitano et al[1] in 1991. Since then, the use of laparoscopic surgery has rapidly become popular due to improving patients’ quality of life and improving efficacy outcomes. The Japan Society of Endoscopic Surgery performs a national survey every 2 years that indicates that the number of laparoscopic procedures for gastric cancer is increasing. According to the 13th Japan Society of Endoscopic Surgery survey, laparoscopic distal gastrectomy accounted for the highest proportion of laparoscopic gastrectomies[2]. Nevertheless, laparoscopic distal gastrectomy involves technically complex elements and requires dedicated skills especially in the procedures of lymphadenectomy and gastrointestinal (GI) reconstruction. Adequate harvesting of lymph nodes (LNs) is necessary for the quality of gastrectomy and now is mentioned in most gastric cancer guidelines[3]. GI reconstructions were initially performed extracorporeally by laparoscopy assisted procedures. However, it is sometimes difficult in patients with a small remnant stomach or in obese patients with thick abdominal walls[4]. With the development of laparoscopic devices and improvement of the anastomosis method, the reconstruction procedures can be completed laparoscopically[5].
The rapid expansion of laparoscopic surgery has led to concern about education for young surgeons. Experienced surgeons learned, developed and introduced laparoscopic gastrectomy after mastering conventional open surgery. However, training and learning may differ for young surgeons who have less experience with open surgery[6]. The feasibility of laparoscopic gastrectomy operated by trainees is still debatable. To our knowledge, there are few studies describing the safety of laparoscopic assisted distal gastrectomy performed by trainee surgeons and even fewer studies on total laparoscopic distal gastrectomy (TLDG).
Our department was founded in May 2015 and mainly focuses on minimally invasive surgery. One experienced laparoscopic surgeon started performing laparoscopic gastrectomy in April 2004. About 100 cases were conducted yearly. TLDG is the standard procedure for distal gastrectomy. For those needing Billroth I reconstruction, the augmented rectangle technique (ART) is applied[7]. We established an education system for TLDG to help young surgeons master the technique quickly.
This study reports the technical feasibility and short-term surgical outcomes of TLDG combined with modified reconstruction techniques performed by trainee gastric surgeons using our training system
We retrospectively studied patients with gastric cancer, who underwent TLDG plus Billroth I reconstruction at Juntendo University Hospital, Tokyo, Japan from June 2016 to June 2019. Clinical, surgical and pathological data of these patients were collected and analyzed. The clinicopathological variables included age, gender, body mass index, American Society of Anesthesiologists physical status classification, medical history, pathological record and duration of postoperative hospital stay. The surgical variables included operation time, LN dissection time, estimated blood loss and number of harvested LNs. Histological results were described according to the Japanese Classification of Gastric Carcinoma[8]. Intraoperative and postoperative complications were stratified using the Clavien–Dindo classification system[9].
Laparoscopic gastrectomy was performed using a five trocar system. LN dissection was done according to the Japanese gastric cancer treatment guidelines[3]. Dissection was conducted in the following order: Infrapyloric LNs (No. 6), suprapyloric LNs (No. 5), great curvature LNs (No. 4 or plus 12a), suprapancreatic LNs (No. 8a, 7, 9 or plus 11p) and along lesser curvature LNs (No. 1 or 3). The operator stood on the left side of the patient for infrapyloric LN dissection and on the right side for other LN dissection. Concomitant cholecystectomy was performed during the operation for patients with symptomatic gallbladder stones. Concomitant appendectomy was performed for patients with recurrent appendicitis.
ART was applied for Billroth I reconstruction, and all the procedures were created laparoscopically. The operator performed this technique on the left side of the patient. Three automatic laparoscopic linear staplers were used to create the gastro-duodenostomy. The duodenum was transected from the greater curvature to the lesser curvature. Small incisions were made on the greater curvature side, for each of the duodenal stumps and the remnant stomach. One jaw of the stapler was pressed against the posterior wall of the stomach 2 cm away from the gastric resection margin, and then the remnant stomach was rotated clockwise to the duodenal side. The duodenal stump was inserted by another jaw of stapler and then rotated externally by 90°. After the initial suturing between the remnant stomach and the duodenum, the posterior wall and caudal wall formed a V-shape. A 30 mm linear stapler was then applied to close the insertion holes up to the closest side of the duodenal resection margin, creating the third side of a rectangle. After gastric and duodenal resection margins were ensured to be close together, the 60 mm linear stapler was used to transect the duodenal resection margin to create the fourth side of the rectangle. After the above steps, all the previous linear staplers were removed from duodenal resection margin, and the augmented rectangular gastroduodenal anastomosis was completed.
Seven operators were involved in this study. There were two trainers and five trainees. Two trainers were Endoscopic Surgical Skill Qualification System for gastric cancer accredited surgeons. Trainees had at least 7 years of experience as a surgeon after graduation. The surgical outcomes of five trainees who had performed more than five TLDG procedures were compared with the other two trainers.
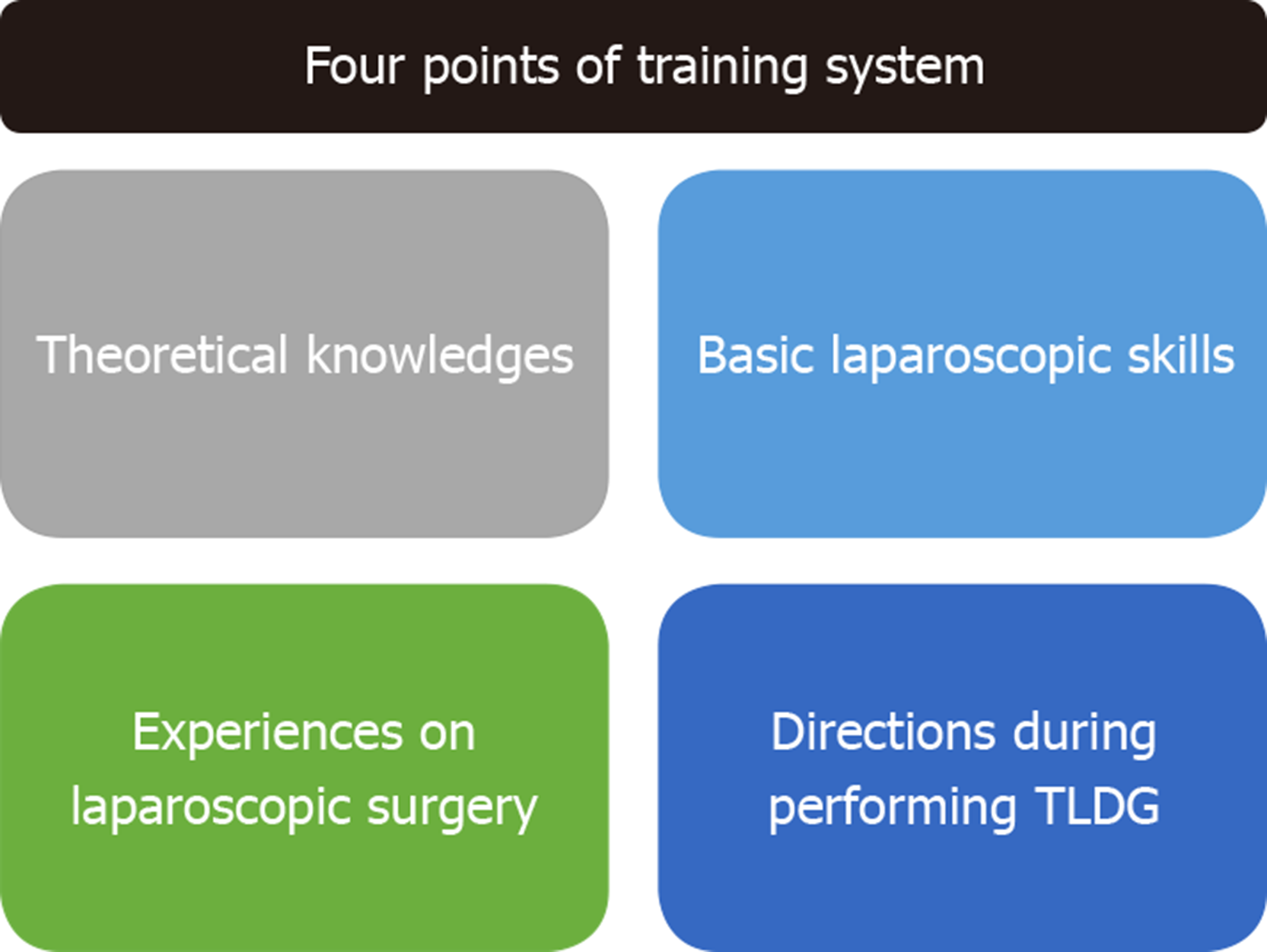
Trainees received systematic education about laparoscopic gastrectomy in four components (Figure 1).
The first component was understanding the anatomy and standard procedures of TLDG: (1) Study basic theoretical knowledge of vascular and lymphatic drainage anatomy especially in laparoscopy; and (2) Watch non-edited video from operations by the trainers as well as trainees repeatedly.
The second component was to master and improve the basic laparoscopic skills: (1) Develop hand-eye coordination, practice laparoscopic knot-tying and suturing techniques using training box; (2) Strengthen basic skills using computer simulator with programs for laparoscopic surgery; and (3) Participate in training sessions, such as hands-on training using porcine laboratory training organized by the Department of Minimally Invasive Surgery of Juntendo University Hospital and educational seminars organized by the Juntendo University Medical Technology and Simulation Center and other organizations.
The third component was experiences during laparoscopic surgery: (1) Complete simple laparoscopic surgery such as laparoscopic cholecystectomy and laparoscopic partial gastrectomy; and (2) Be a scope operator and then an assistant to understand the standard procedure of TLDG.
The fourth component was to receive direction during real TLDG. When a trainee performed the TLDG, the trainer surgeon was usually the first assistant to give guidance.
Two variables, operation time and intraoperative estimated blood loss, from patients who underwent TLDG by trainees were used to define the learning curve. Variables in each group were calculated as mean ± standard deviation and then compared with that of those performed by the trainer surgeons. Continuous curves were plotted for each variable to identify any plateau effect. Plateau was defined as variable with < 5% change. The patient number at which a < 5% change occurred within the variable gave the minimum number of cases needed to reach the learning curve for that variable.
Continuous data are presented as median and ranges. Independent-sample t test was used to analyze continuous data, and χ2 or Fisher’s exact tests was used to assess differences in categorical data. Statistical analysis was performed using the SPSS statistical software program (version 23). A P < 0.05 was considered significant.
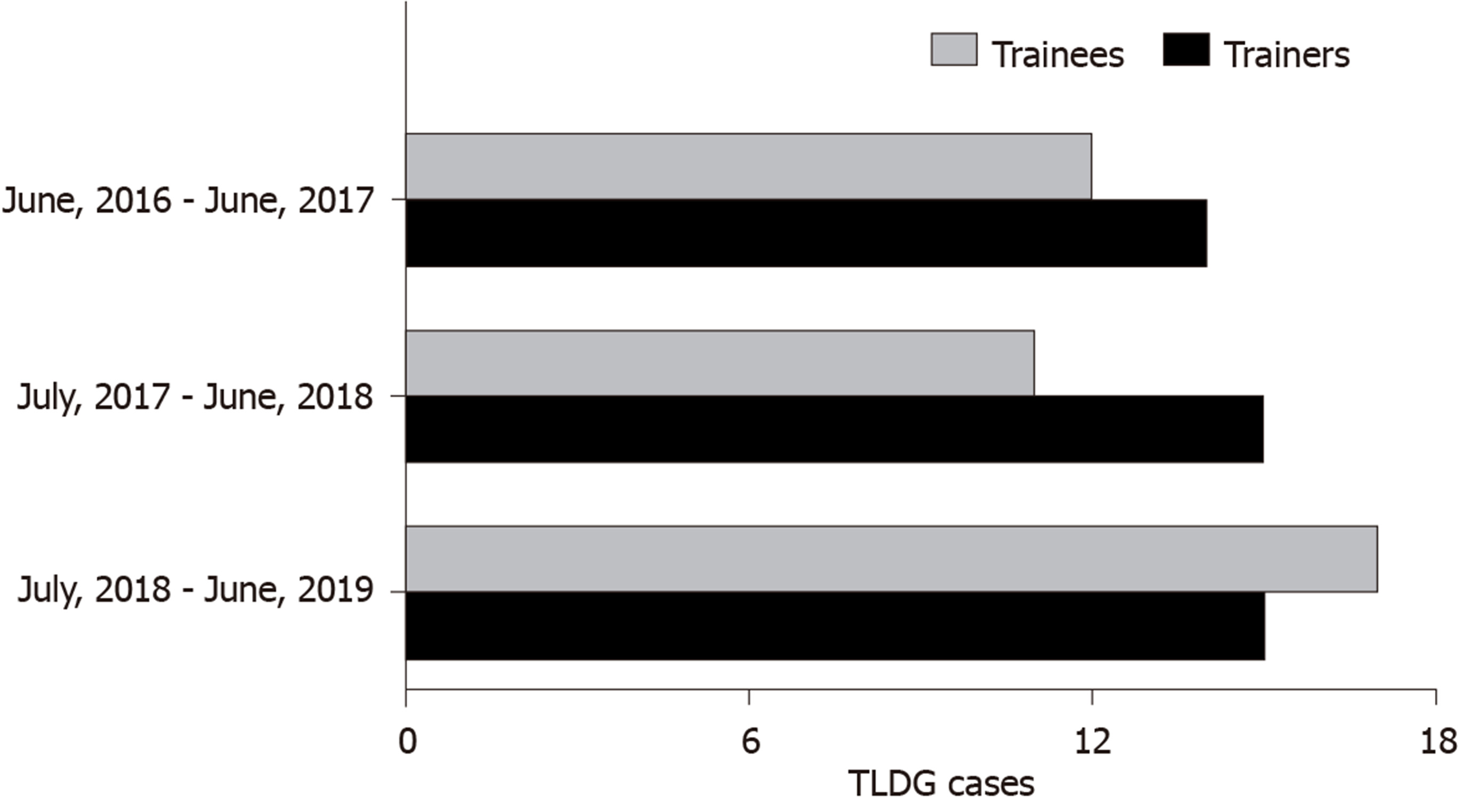
A total of 92 patients received TLDG with ART between June 2016 and June 2019. Among them, 52 patients were operated by the trainee group while the remaining 40 patients were operated by the trainer group (Figure 2). Compared with trainers, trainees performed more than 50% of the TLDG except for the first year.
Patient clinicopathological characteristics are summarized in Table 1. There were no significant differences between the two groups in patient characteristics, including age, sex, body mass index, American society of anesthesiologists status and pathology staging. The trainer group tended to perform operations for patients with higher depth of invasion (P = 0.004) and higher pathology stage (P = 0.017).
| Characteristics | Trainee surgeon | Trainer surgeon | P value |
| Age in yr | |||
| Median | 68.5 (37-83) | 69.6 (42-90) | 0.630 |
| < 80 | 44 (84.6%) | 31 (77.5%) | 0.794 |
| ≥ 80 | 8 (15.4%) | 9 (22.5%) | |
| Sex | |||
| Male | 32 (61.5%) | 23 (57.5%) | 0.830 |
| Female | 20 (38.5%) | 17 (42.5%) | |
| BMI in kg/m | |||
| Median | 22.01 (14.98-36.00) | 23.11 (18.67-32.56) | 0.145 |
| < 25 | 43 (82.7%) | 30 (75.0%) | 0.440 |
| ≥ 25 | 9 (17.3%) | 10 (25.0%) | |
| ASA | |||
| 1 | 20 (38.5%) | 13 (32.5%) | 0.793 |
| 2 | 29 (55.8%) | 24 (60.0%) | |
| 3 | 3 (5.7%) | 3 (7.5%) | |
| Previous abdominal surgery | |||
| Yes | 14 (26.9%) | 8 (20%) | 0.472 |
| No | 38 (73.1%) | 32 (80%) | |
| pT | |||
| T1 | 42 (80.8%) | 22 (55.0%) | 0.004 |
| T2 | 3 (5.8%) | 5 (12.5%) | |
| T3 | 6 (11.5%) | 4 (10%) | |
| T4 | 1 (1.9%) | 9 (22.5%) | |
| pStage | |||
| IA | 35 (67.3%) | 17 (42.5%) | 0.017 |
| IB | 7 (13.5%) | 6 (15%) | |
| IIA | 5 (9.6%) | 3 (7.5%) | |
| IIB | 4 (7.7%) | 3 (7.5%) | |
| IIIA | 1 (1.9%) | 8 (20%) | |
| IIIB | 0 | 1 (2.5%) | |
| IIIC | 0 | 2 (5%) |
The surgical outcomes, including intraoperative blood loss and harvested number of LNs, were not significantly different between the trainee and trainer groups (Table 2). The trainer group performed more D2 gastrectomies than the trainee group (P = 0.034). The operation time was significantly longer in the trainee group compared with the trainer group (P = 0.002). No patients required conversion to open gastrectomy in either group. The postoperative stay was almost equivalent. The results of lymphadenectomy and GI reconstruction time are shown in Table 3. There were significant differences between the groups in the infrapyloric, suprapyloric, greater curvature and suprapancreatic LN dissection times. The times for lesser curvature LN dissection and GI reconstruction were similar between the two groups.
| Items | Trainee surgeon | Trainer surgeon | P value |
| LN dissection | |||
| D1+ | 43 (82.7%) | 25 (62.5%) | 0.034 |
| D2 | 9 (17.3%) | 15 (37.5%) | |
| Combined organ resection | 4 (7.7%) | 2 (5%) | 0.568 |
| Cholecystectomy | 4 | 0 | |
| Appendicectomy | 0 | 1 | |
| Colectomy | 0 | 1 | |
| Blood loss | 26 (5-170) | 23 (3-125) | 0.566 |
| Conversion to open procedure | 0 | 0 | |
| Operation time in min | |||
| Median (range) | 270 (199-512) | 239 (154-375) | 0.002 |
| Harvested LNs, number | |||
| Median (range) | 39 (14-86) | 39 (14-70) | 0.989 |
| Postoperative hospital stays in d | |||
| Median (range) | 13.38 (7-60) | 12.70 (7-27) | 0.720 |
| Items | Trainee surgeon | Trainer surgeon | P value |
| Lymphadenectomy | |||
| Infrapyloric LNs | 58.8 (27-135) | 42.0 (19-85) | 0.004 |
| Suprapyloric LNs | 18.8 (4-40) | 10.6 (3-24) | 0.001 |
| Great Curvature LNs | 17.7 (8-34) | 12.3 (6-32) | 0.004 |
| Suprapancreatic LNs | 41.0 (23-82) | 28.4 (17-51) | 0.001 |
| Along lesser curvature LNs | 16.6 (7-36) | 14.1 (7-34) | 0.213 |
| GI reconstruction | 19.0 (11-37) | 18.9 (11-39) | 0.988 |
Four patients in the trainee group (7.7%) and two patients in the trainer group (5%) had complications (Table 4). The most frequent complication was intra-abdominal abscess (3.8%) in the trainee group. No complication needed surgical intervention. There was no mortality associated with surgery in either group.
| Items | Trainee surgeon | Trainer surgeon | P value |
| Anastomotic leakage | 0 | 0 | 1.000 |
| Anastomotic bleeding | 1 (1.9%) | 0 | 0.497 |
| Anastomotic stenosis | 0 | 0 | 1.000 |
| Intra-abdominal abscess | 2 (3.8%) | 1 (2.4%) | 0.683 |
| Pancreatic fistula | 1 (1.9%) | 1 (2.4%) | 1.000 |
| Ileus | 0 | 0 | 1.000 |
| Mortality | 0 | 0 | 1.000 |
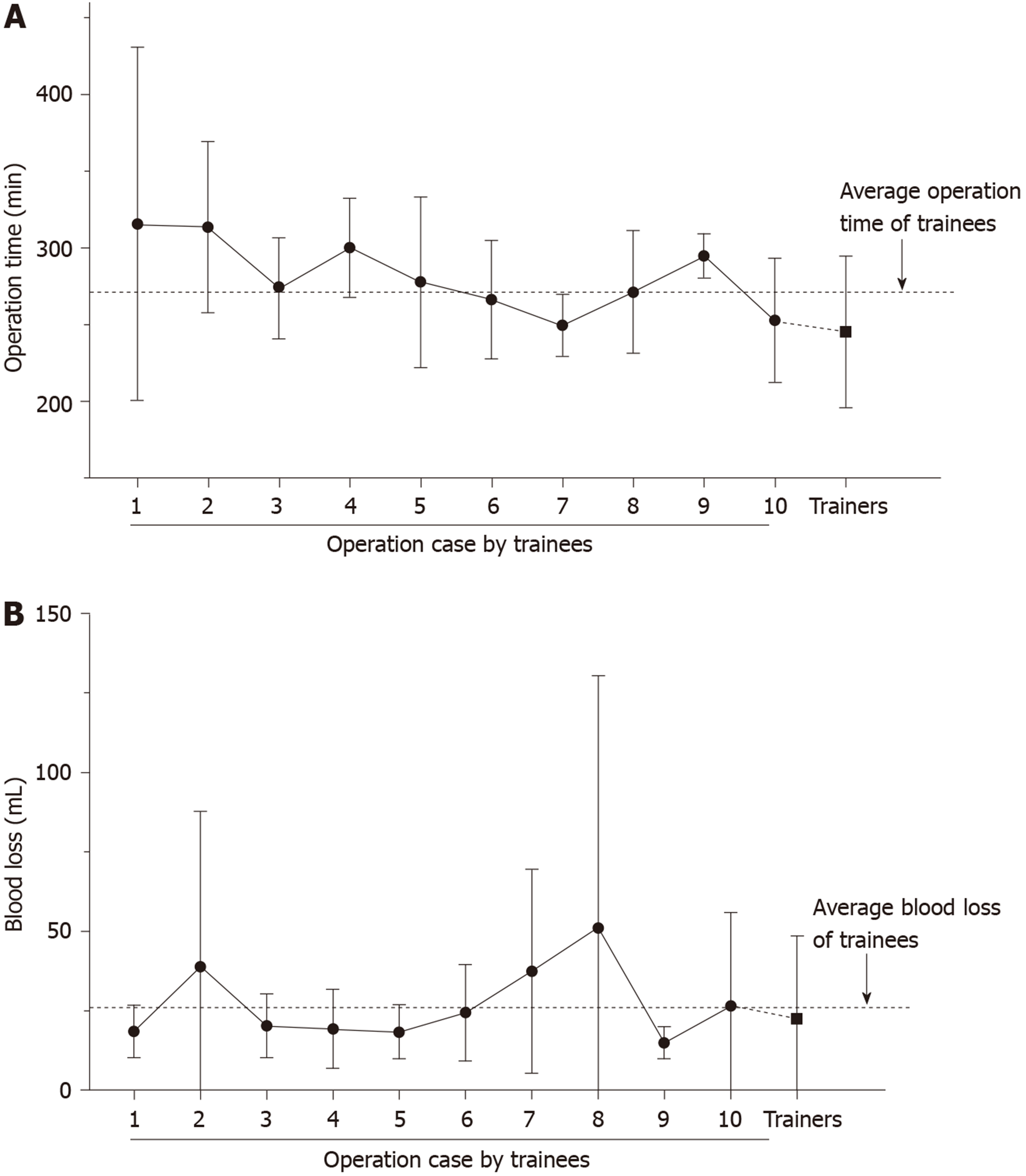
Among the 52 patients resected by trainees, the mean value of operation time is shown in Figure 3. The average operating time decreased from 315 min in cohort 1 to 253 min in cohort 10. The average operation time for the trainee group plateaued at around 260 min after five cases. There was less than 5% change in average operation time after cohort 5 up to cohort 10 but still a big gap compared with that of the trainer group. The average operative blood loss was similar for the two groups.
Gastric cancer ranks the fifth most common cancer and the third most common cause in cancer-related deaths worldwide with the highest incidence rate in Eastern Asia[10]. Radical resection is the only curative modality for patients with resectable gastric cancer. Introduction of laparoscopic gastrectomy has shown promising results in early gastric cancer[11] and even comparable outcomes in advanced gastric cancer[12,13] when compared with open surgery. Laparoscopic gastrectomy has therefore rapidly gained popularity in the world. With the developments in anastomosis devices and modification in anastomotic techniques[5,14], more and more cases can be performed by total laparoscopic gastrectomy[2]. Intracorporeal GI reconstruction showed some benefits especially in the setting of narrow spaces in obese patients and small remnant stomachs from high location of tumor.
Total laparoscopic gastrectomy has generated interest and desire not only in experienced surgeons but also in trainee surgeons. In this context, many efforts for research and education on laparoscopic surgery have been made. The Japan Society of Endoscopic Surgery established the Endoscopic Surgical Skill Qualification System and provides the educational environment for the training of qualified surgeons[15]. Some high-volume centers also reported their experiences of educating young surgeons on laparoscopic assisted distal gastrectomy[16-19]. However, most studies mixed different kinds of gastrectomy and even different reconstruction methods, which may cause bias of the results. In order to limit the influence of different techniques on results, we only focused on TLDG using the same surgical procedures and reconstruction methods for each patient in this study.
Our department was founded in 2015, and laparoscopic surgeries represent most of our surgeries. More than 90% of gastrectomies were performed by laparoscopy, and most GI reconstructions are done intracorporeally. The advantage of our volume is more opportunities for trainee surgeons to perform such surgeries. However, shortcomings of this are also evident in less opportunity to learn open surgery and higher technical skills. These characteristics make laparoscopic training procedures for young surgeons different from what experience surgeons experienced previously by placing higher educational and technical demands on residents[20]. Appropriate and efficient training systems suitable for the current situation need to be urgently established. One experienced surgeon in our department has performed laparoscopic surgery since 2004 and has been concentrating on laparoscopic training[16,21]. When starting laparoscopic gastrectomy in our newly founded department, an educational and training system for young surgeon was set up at the same time.
Our training system covers four parts: understanding anatomy and standard procedures, practicing basic laparoscopic skills, performing simple laparoscopic surgery and providing focal points during laparoscopic gastrectomy. It is useful to use a dry box to help trainees practice laparoscopic suturing techniques and improve hand-eye coordination[19,22]. However, the camera in the box is usually fixed to a particular point, which is different from practical surgery. In order to create a more realistic laparoscopic environment, we also use computer simulators to train young surgeons. Computer simulators with laparoscopic programs and magnetic feedback systems can strengthen basic skills and surgical training more than a traditional dry box.
Using a video recording system and online video websites, trainees can watch operative videos before operating and can analyze each step of their own surgery repeatedly after operation. Before becoming an operator, experiences gained from watching laparoscopic gastrectomy videos, performing simple laparoscopic surgeries, being a scope assistant, and being the first assistant helps trainees master laparoscopy-specific anatomical views, acquire skills of handling of laparoscopic energy devices and cooperating closely with other surgeons. Trainers play an important role, especially during the trainee’s actual operation. In our department, trainers usually worked as the first assistant when trainees performed TLDG. Trainers could not help the trainee’s procedure because the assistant’s hands were always occupied for exposing the operative field of vision[16]. But the first assistant trainers could directly provide direction, give confidence and control the quality of surgery.
The technically challenging component of TLDG for trainees mainly centers on lymphadenectomy and GI reconstruction. Trainees performed TLDG with longer time compared to trainers. After splitting the whole procedure of TLDG into lymphadenectomy for each station and GI reconstruction, we found that lymphadenectomy in the infrapyloric and suprapancreatic areas took longer in the trainee group. However, reconstruction time was similar between the two groups. Standardizing operative procedures is useful for surgical education[22-24]. Paying more attention to the details of lymphadenectomy could improve surgical efficiency, reduce unnecessary injury and result in less bleeding. In our department, D1 + LN dissection is normally performed in the following order: “No. 6 → No. 5 (plus 12a in D2) → No. 4sa, 4d → No. 8a, 7, 9 (plus 11p in D2) → No. 3, 1.” The total procedure is just like page-turning, which may make a good field of vision and avoid repeated clamping of the diseased gastric wall. Our results show there is a positive relationship between infrapyloric and suprapancreatic lymphadenectomy time. LN dissection in infrapyloric and suprapancreatic areas requires delicate manipulations especially in obese patients. More adipose tissue makes it difficult to identify the correct anatomical planes. We developed an intracorporeal reconstruction technique named ART, which is easily performed[7]. Two 60 mm and one 30 mm laparoscopic linear staplers are used to create a larger 4-sided anastomosis. Stay sutures are canceled and less (even no) intersection angle sutures are needed, which makes the technique easy and time-saving especially for trainees. Our results showed that the reconstruction time of trainees is similar to that of trainers. No anastomosis-related complications were found between the two groups. These results may indicate that this reconstruction method is easy and safe to perform for young surgeons.
In this study, we compared early outcomes of TLDG between trainees and trainers to clarify whether our training system was useful in maintaining the quality of trainee operations. The number of harvested LNs and intraoperative bleeding were not significantly different between the two groups. The incidence of postoperative complications was similar between the trainee and trainer groups. These results indicated that TLDG performed by trainees is safe and feasible. Some studies indicated pancreatic fistula occurred much more in trainee surgeries[17,23,24]. However, our results showed the incidence of pancreatic fistula was similar between the two groups. Much attention was paid to the dissection of the infrapyloric and suprapancreatic LNs. During operations we normally compress the adjacent tissues at the inferior border of the pancreas instead of directly touching the pancreas itself. The postoperative hospital stays are relatively long compared with other reported studies. One reason is that more older patients were included in our series[7].
The trainees in our department have less chance to perform open gastrectomies. However, the learning curve for TLDG showed that the average operative time of the trainees reached a plateau after five cases, especially in older and advanced stage patients, compared with other studies[17,23]. The learning curve showed no difference in blood loss in the two groups. All the results may support that our educational and training system may enable trainees to quickly learn to perform TLDG. The influence of patient selection on the learning curve should also be evaluated. In our department, trainees usually performed surgery for lower stage gastric cancer vs trainers. Careful patient selection for trainees might be one important factor for a successful initial experience with TLDG. We should take cognizance of the situation clearly that there is still a big gap between the two groups after trainees reach the plateau, which may be accounted for the technical complexity of lymphadenectomy needing a longer learning time.
There are some limitations in our study. Its retrospective nature may induce some bias. Because of the length of follow up, our study did not provide enough data to show conclusions about oncologic safety and long-term outcomes. LNs dissection number may be prognostic factor for survival of patients. In our study, 97.8% of patients had more than 15 LNs harvested. We still need further follow up on these patients. The number of patients in our study is limited, but the study interval was shorter than other studies and only focused on TLDG with the same procedure and reconstruction method. These may reduce the impacts of surgical techniques.
In conclusion, trainees can perform TLDG safely and feasibly after receiving systematic training. Making laparoscopic procedures standard and using an easy reconstruction method are useful in the success of the training system.
Total laparoscopic distal gastrectomy (TLDG), which involves technically complex elements and requires dedicated skills, has generated interest and desire not only in surgeon pioneers but also in trainee surgeons. The rapid expansion of TLDG has led to concern about education for young surgeons.
Fewer opportunities to perform open gastrectomy and higher technical demands has made laparoscopic training procedures for young surgeons differ from those of laparoscopic surgeon pioneers. Appropriate and efficient training systems suitable for the current situation need to be urgently established.
The patients underwent TLDG plus Billroth I reconstruction from June 2016 to June 2019. Clinical, surgical and pathological data of these patients were collected and analyzed.
This study assessed our laparoscopic training system for TLDG based on short-term surgical outcomes. We reviewed ninety-two consecutive patients with gastric cancer who underwent TLDG plus Billroth I reconstruction using the augmented rectangle technique. The trainees were required to receive systematic laparoscopic training. The total procedure of TLDG was divided into different regional lymph node dissection and gastrointestinal reconstruction for analyzing. Early surgical outcomes were compared between trainees and trainers to clarify the feasibility and safety of TLDG performed by trainees.
Five trainees performed a total of 52 TLDG (56.5%), while 40 TLDG were conducted by the two trainers (43.5%). Except for depth of invasion and pathology stage, there were no differences in patient clinicopathological characteristics. Trainers performed more D2 gastrectomies than trainees. The total operation time was significantly longer in the trainees. The time spent on less curvature lymph node dissection and Billroth I reconstruction was similar between the two groups. No difference was found in postoperative complications between the two groups. The learning curve of the trainees plateaued after five TLDG cases.
Preparing trainees with a laparoscopic view of surgical anatomy, standard operative procedures and practice in essential laparoscopic skills enabled trainees to perform TLDG safely and feasibly.
Making laparoscopic procedures standard and using an easy reconstruction method are useful in the success of the training system.
The authors thank Professor Mike Gibson (Vanderbilt University School of Medicine, United States) for his critical correction of the English language in the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fabozzi M S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Liu JH
| 1. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [PubMed] |
| 2. | Shiroshita H, Inomata M, Bandoh T, Uchida H, Akira S, Hashizume M, Yamaguchi S, Eguchi S, Wada N, Takiguchi S, Ieiri S, Endo S, Iwazaki M, Tamaki Y, Tabata M, Kanayama H, Mimata H, Hasegawa T, Onishi K, Yanaga K, Morikawa T, Terachi T, Matsumoto S, Yamashita Y, Kitano S, Watanabe M. Endoscopic surgery in Japan: The 13th national survey (2014-2015) by the Japan Society for Endoscopic Surgery. Asian J Endosc Surg. 2019;12:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1914] [Article Influence: 239.3] [Reference Citation Analysis (1)] |
| 4. | Kim MG, Kim KC, Kim BS, Kim TH, Kim HS, Yook JH, Kim BS. A totally laparoscopic distal gastrectomy can be an effective way of performing laparoscopic gastrectomy in obese patients (body mass index≥30). World J Surg. 2011;35:1327-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Zhang S, Fukunaga T. Current status of technique for Billroth-I anastomosis in totally laparoscopic distal gastrectomy for gastric cancer. Mini-invasive Surg. 2019;3:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Kano N, Takeshi A, Kusanagi H, Watarai Y, Mike M, Yamada S, Mishima O, Uwafuji S, Kitagawa M, Watanabe H, Kitahama S, Matsuda S, Endo S, Gremillion D. Current surgical training: simultaneous training in open and laparoscopic surgery. Surg Endosc. 2010;24:2927-2929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Fukunaga T, Ishibashi Y, Oka S, Kanda S, Yube Y, Kohira Y, Matsuo Y, Mori O, Mikami S, Enomoto T, Otsubo T. Augmented rectangle technique for Billroth I anastomosis in totally laparoscopic distal gastrectomy for gastric cancer. Surg Endosc. 2018;32:4011-4016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 9. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24842] [Article Influence: 1183.0] [Reference Citation Analysis (0)] |
| 10. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55828] [Article Influence: 7975.4] [Reference Citation Analysis (132)] |
| 11. | Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (1)] |
| 12. | Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Kim MC; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg. 2019;270:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 336] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 13. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 526] [Article Influence: 87.7] [Reference Citation Analysis (1)] |
| 14. | Shim JH, Yoo HM, Oh SI, Nam MJ, Jeon HM, Park CH, Song KY. Various types of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer. Gastric Cancer. 2013;16:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Tanigawa N, Lee SW, Kimura T, Mori T, Uyama I, Nomura E, Okuda J, Konishi F. The Endoscopic Surgical Skill Qualification System for gastric surgery in Japan. Asian J Endosc Surg. 2011;4:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Tokunaga M, Hiki N, Fukunaga T, Miki A, Nunobe S, Ohyama S, Seto Y, Yamaguchi T. Quality control and educational value of laparoscopy-assisted gastrectomy in a high-volume center. Surg Endosc. 2009;23:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Yamada T, Kumazu Y, Nakazono M, Hara K, Nagasawa S, Shimoda Y, Hayashi T, Rino Y, Masuda M, Shiozawa M, Morinaga S, Ogata T, Oshima T. Feasibility and safety of laparoscopy-assisted distal gastrectomy performed by trainees supervised by an experienced qualified surgeon. Surg Endosc. 2020;34:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Kuroda S, Kikuchi S, Hori N, Sakamoto S, Kagawa T, Watanabe M, Kubota T, Kuwada K, Ishida M, Kishimoto H, Uno F, Nishizaki M, Kagawa S, Fujiwara T. Training system for laparoscopy-assisted distal gastrectomy. Surg Today. 2017;47:802-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Kinoshita T, Kanehira E, Matsuda M, Okazumi S, Katoh R. Effectiveness of a team participation training course for laparoscopy-assisted gastrectomy. Surg Endosc. 2010;24:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Debes AJ, Aggarwal R, Balasundaram I, Jacobsen MB. A tale of two trainers: virtual reality versus a video trainer for acquisition of basic laparoscopic skills. Am J Surg. 2010;199:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Hiki N, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Ohyama S, Seto Y, Yoshiba H, Nohara K, Inoue H, Muto T. The benefits of standardizing the operative procedure for the assistant in laparoscopy-assisted gastrectomy for gastric cancer. Langenbecks Arch Surg. 2008;393:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Kaito A, Kinoshita T. Educational system of laparoscopic gastrectomy for trainee-how to teach, how to learn. J Vis Surg. 2017;3:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Nunobe S, Hiki N, Tanimura S, Nohara K, Sano T, Yamaguchi T. The clinical safety of performing laparoscopic gastrectomy for gastric cancer by trainees after sufficient experience in assisting. World J Surg. 2013;37:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Kameda C, Watanabe M, Suehara N, Watanabe Y, Nishihara K, Nakano T, Nakamura M. Safety of laparoscopic distal gastrectomy for gastric cancer when performed by trainee surgeons with little experience in performing open gastrectomy. Surg Today. 2018;48:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |