Published online Apr 7, 2020. doi: 10.3748/wjg.v26.i13.1474
Peer-review started: December 4, 2019
First decision: January 16, 2020
Revised: February 4, 2020
Accepted: March 9, 2020
Article in press: March 9, 2020
Published online: April 7, 2020
Processing time: 125 Days and 3.9 Hours
The incidence of colon cancer (CC) is currently high, and is mainly treated with chemotherapy. Oxaliplatin (L-OHP) is a commonly used drug in chemotherapy; however, long-term use can induce drug resistance and seriously affect the prognosis of patients. Therefore, this study investigated the mechanism of Opa-interacting protein 5 antisense RNA 1 (OIP5-AS1) on L-OHP resistance by determining the expression of OIP5-AS1 and microRNA-137 (miR-137) in CC cells and the effects on L-OHP resistance, with the goal of identifying new targets for the treatment of CC.
To study the effects of long non-coding RNA OIP5-AS1 on L-OHP resistance in CC cell lines and its regulation of miR-137.
A total of 114 CC patients admitted to China-Japan Union Hospital of Jilin University were enrolled, and the expression of miR-137 and OIP5-AS1 in tumor tissues and corresponding normal tumor-adjacent tissues was determined. The influence of OIP5-AS1 and miR-137 on the biological behavior of CC cells was evaluated. Resistance to L-OHP was induced in CC cells, and their activity was determined and evaluated using cell counting kit-8. Flow cytometry was used to analyze the apoptosis rate, Western blot to determine the levels of apoptosis-related proteins, and dual luciferase reporter assay combined with RNA-binding protein immunoprecipitation to analyze the relationship between OIP5-AS1 and miR-137.
OIP5-AS1 was up-regulated in CC tissues and cells, while miR-137 was down-regulated in CC tissues and cells. OIP5-AS1 was inversely correlated with miR-137 (P < 0.001). Silencing OIP5-AS1 expression significantly hindered the proliferation, invasion and migration abilities of CC cells and markedly increased the apoptosis rate. Up-regulation of miR-137 expression also suppressed these abilities in CC cells and increased the apoptosis rate. Moreover, silencing OIP5-AS1 and up-regulating miR-137 expression significantly intensified growth inhibition of drug-resistant CC cells and improved the sensitivity of CC cells to L-OHP. OIP5-AS1 targetedly inhibited miR-137 expression, and silencing OIP5-AS1 reversed the resistance of CC cells to L-OHP by promoting the expression of miR-137.
Highly expressed in CC, OIP5-AS1 can affect the biological behavior of CC cells, and can also regulate the resistance of CC cells to L-OHP by mediating miR-137 expression.
Core tip: Long non-coding RNA (lncRNA) has drug resistance in various diseases, which has become a research hotspot, and it can participate in the regulation of various biological functions in cells. In this study, the expression and regulation mechanism of lncRNA Opa-interacting protein 5 antisense RNA 1 (OIP5-AS1) in colon cancer were investigated, and it was found that OIP5-AS1 was up-regulated in colon cancer cells. Dual luciferase reporter gene and RNA-binding protein immunoprecipitation assays were carried out, and the relationship between microRNA-137 and OIP5-AS1 was determined. The results showed that OIP5-AS1 could mediate drug resistance to oxaliplatin in these cells by regulating microRNA-137.
- Citation: Liang J, Tian XF, Yang W. Effects of long non-coding RNA Opa-interacting protein 5 antisense RNA 1 on colon cancer cell resistance to oxaliplatin and its regulation of microRNA-137. World J Gastroenterol 2020; 26(13): 1474-1489
- URL: https://www.wjgnet.com/1007-9327/full/v26/i13/1474.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i13.1474
Colon cancer (CC) is a common cancer and one of the main causes of mortality worldwide, especially in people over 50 years old. According to the statistics, there are more than 1000000 new CC patients every year, and this number is expected to be 2200000 in 2030[1,2]. At present, surgery and chemotherapy are the two main treatments for CC[3]. Due to the availability of various chemotherapy regimens, the overall survival rate of CC patients has improved in the past few decades[4]. However, even though the current response rate to systemic chemotherapy can reach 50%, almost all CC patients have reportedly developed drug resistance, which limits the efficacy of anticarcinogens and eventually leads to chemotherapy failure[4]. Oxaliplatin (L-OHP), a third generation platinum drug, is usually used as a first-line chemotherapy agent for metastatic CC, and is often administered with leucovorin (FOLFOX) and 5-fluorouracil. It can induce intrachain and interchain bridges between guanines, thus inhibiting DNA replication and synthesis[5,6]. However, the long-term use of L-OHP results in drug resistance and ultimately limits its therapeutic effect[7]. Therefore, understanding the molecular mechanism of L-OHP resistance is of great significance in order to obtain better therapeutic response predictions in CC patients and to achieve better therapeutic decision-making.
Long non-coding RNA (lncRNA) a non-coding RNA with a length greater than 200 bp, was reported to be strongly linked to drug resistance of tumor cells in recent years[8,9]. The Opa-interacting protein 5 antisense RNA 1 (OIP5-AS1) gene is a lncRNA on human chromosome 15q15.1, which is up-regulated in various cancers, and promotes cancer development[10]. Kim et al[11] pointed out that OIP5-AS1 is a competitive endogenous RNA against HuR and can regulate the growth of cervical cancer (HeLa) cells. In addition, previous studies have found that OIP5-AS1 can affect cell apoptosis and proliferation in multiple myeloma by regulating various pathways[12]. MicroRNAs, a group of non-coding RNAs approximately 19-24 nucleotides long, are involved in drug resistance in various tumor cells[13]. Bi et al[14] concluded that microRNA-137 (miR-137) could suppress the migration, proliferation, and invasion of CC cell lines by targeting TCF4. One study demonstrated that miR-137 could suppress the development of lung carcinoma and enhance the sensitivity of cells to paclitaxel and cisplatin[15]. At present, there is no study on the role of OIP5-AS1 in CC, and the mechanism of OIP5-AS1 in regulating L-OHP resistance in CC requires further research.
The present study investigated the role of OIP5-AS1 and miR-137 in the biological behavior and L-OHP resistance in CC cells, and determined the mechanism of OIP5-AS1 on L-OHP resistance in these cells, with the goal of identifying new targets for the treatment of CC.
A total of 114 CC patients admitted to China-Japan Union Hospital of Jilin University from January 2017 to December 2018 were enrolled, including 63 males and 51 females, with an average age of 61.56 ± 7.09 years. CC tissue specimens (n = 114) and corresponding tumor-adjacent tissue specimens (n = 114) were obtained from the patients following their permission for later analysis. This study was carried out with permission from the Ethics Committee of China-Japan Union Hospital of Jilin University, and each subject signed an informed consent form after understanding the study in detail. The inclusion criteria were as follows: Patients diagnosed with CC based on pathology and imaging examination, patients with detailed clinical data, patients with good compliance, and those without a family history of mental diseases or other malignant tumors. The exclusion criteria were as follows: Patients not accompanied by their families at admission, patients with autoimmune diseases or severe liver or kidney dysfunction, and patients reluctant to receive treatment or cooperate during the study.
Human CC cell lines (HCT116, LOVO, HT29, and SW480), and a human normal colon epithelial cell line (FHC) obtained from Nanjing Cobioer Biosciences Co., Ltd. were cultured in RPMI 1640 containing 100 μg/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum under 5% CO2 and saturated humidity at 37°C. When the confluency of adherent cell growth reached 85%, 25% pancreatin was added to the cells for digestion, and the cells were continually cultured in the medium for passage after digestion. The lncRNA OIP5-AS1 and miR-137 expression in each cell line was subsequently determined. HCT116 and SW480 cells in logarithmic growth phase were then selected and transfected with blank control (Vector), targetedly inhibited OIP5-AS1 (si-OIP5-AS1), targetedly overexpressed OIP5-AS1 (sh-OIP5-AS1), miR-137-mimics (overexpressed sequence), miR negative control (miR-NC), and miR-137-inhibitor (inhibited sequence) using a Lipofectamine™ 2000 Kit (Invitrogen) in strict accordance with the kit instructions.
HCT116 and SW480 cells in the logarithmic growth phase with a cell density of 1 × 105 cells /mL were cultured for 48 h after the addition of L-OHP at the concentration of 1.6 μg/mL (Shanghai Yuanye Biotechnology Co., Ltd., China). After 48 h, the solution was discarded and the cells were continuously cultured in fresh solution without L-OHP. When the cells resumed normal growth, they were digested for passage. If the cells grew well, the above step was repeated once by increasing the concentration of L-OHP to 2.4 μg/mL. Drug-resistant cell lines (SW480/L-OHP and HCT116/L-OHP) were finally obtained by changing the solution and gradually increasing the concentration of L-OHP. L-OHP treatment of the cells obtained for future analysis was stopped one week before the experiment.
The cell counting kit-8 (Nanjing Enogene Biotech. Co., Ltd., China) was employed to analyze the inhibition rate of cells. Drug-resistant cell lines and parental cell lines in logarithmic growth phase with the concentration adjusted to 1 × 105 cells/mL were seeded into a 96-well plate at 1 × 104 cells/well. The plate included three replicates of each treatment, and each well was cultured for 48 h after the addition of L-OHP at different concentrations. The plate was cultured for another 2 h or 3 h after the addition of 10 μL of cell counting kit-8 solution to each well. The optical density of each well at the wavelength of 450 nm was then measured. The experiment was repeated three times, and the 50% inhibitory concentration (IC50) of the drugs in the cells from each group was calculated.
Total RNA was extracted from the tissues and cells using a Trizol Extraction Kit (Invitrogen, CA, United States), and its purity, concentration, and integrity were determined using an ultraviolet spectrophotometer and agarose gel electrophoresis. Reverse transcription was carried out to change RNA into cDNA according to the operating instructions of the reverse transcription kit. A 7500 real-time polymerase chain reaction (PCR) instrument (ABI Company) and SYBR Premix Ex Taq kit (TaKaRa) were employed. GAPD was taken as an internal reference for lncRNA OIP5-AS1, and U6 as an internal reference for miR-137 in the experiment. The specific sequences were as follows: OIP5-AS1: Upstream 5'-GGTCGTGAAACACCGTCG-3’ and downstream 5'-GTGGGGCATCCAGGGT-3’; GAPDH: Upstream 5'-CAGTCACTACTCAGCTGCCA-3’ and downstream 5'-GAGGGTGCTCC GGTAG-3; miR-137: Upstream 5'-GAAATCCGACAGCTTAAGGAGGTTTGA-3’ and downstream 5'-CATTGCACAGATAGGATTTGATTTACT-3’; and U6: Upstream 5'-CTCGCTTCGGCAGCACA-3’ and downstream 5'-AACGCTTCAC GAATTTGCGT-3’. PCR amplification was carried out at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 60°C for 30 s. Data were obtained after three repeated experiments, and the calculated results were expressed using the 2ΔΔct method.
Radio immunoprecipitation assay buffer (Thermo Scientific Company, United States) was adopted for lysis, and a Bicinchoninic Acid Kit (Thermo Scientific Company, United States) was adopted for protein concentration determination. The protein with an adjusted concentration of 4 μg/μL was transferred to a polyvinylidene fluoride membrane (Millipore Company) after being separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, the membrane was sealed with 5% skim milk for later immune response. The membrane was incubated with primary antibody (Santa Cruz Biotechnology, United States) (1: 1000) overnight at 4°C, and the primary antibody was then removed. The membrane and horseradish peroxidase labeled goat anti-rabbit secondary antibody (Abcam, United States) (1: 1000) were then incubated at 37°C for 1 h, and cleaned with phosphate buffered saline (PBS) 3 times, for 5 min each time. After incubation, the protein was developed and immobilized with an electrochemiluminescence agent. The image was obtained by the Quantity One infrared imaging system, and the relative protein expression level was recorded as the gray value of the band/gray value of the reference.
The transfected cells in each group were seeded into a 96-well plate at 5 × 103 cells/well, respectively. The cells were incubated under 5% CO2 at 37°C. At 0 h, 24 h, 48 h, and 72 h after incubation, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution and 150 μL of dimethyl sulfoxide were added to each well, respectively, and the plate was shaken for 5-10 min to completely dissolve the purple crystals. The optical density of each well at 450 nm wavelength was measured using the Multiskan™ GO microplate spectrophotometer (Thermo Fisher Scientific, China) to analyze cell proliferation, and then growth curves of the cells were drawn. The experiment was repeated three times. The MTT assay kit was purchased from Thermo Fisher Scientific (China).
An Annexin V-FITC/PI Apoptosis Assay Kit (Invitrogen Company, United States) was used to assess cell apoptosis. The cells were digested with trypsin, followed by washing twice with PBS. The 1 × 106 cells /mL cells were centrifuged for 5 min, and the supernatant discarded. Annexin-V-FITC labeling solution (20 μL) and PI reagent (20 μL) were successively added into 1 mL of buffer containing the cells, and incubated at room temperature in the dark for 5 min. A Beckman Coulter CytoFLEX LX Flow Cytometry System was employed to measure cell apoptosis. The experiment was repeated three times, and the data were averaged.
Both the scratch-wound healing assay and Transwell assay were performed to evaluate cell migration and invasion. The cells were scratched perpendicularly using sterilized 100 μL disposable pipette tips to ensure consistent width of each scratch wound as far as possible. The cell culture medium was removed, and the plate was washed three times with PBS to wash off cell debris generated by the scratches. The cells were placed in serum-free medium, and the scratch in each group was analyzed using a microscope at 0 h and 24 h after cell scratching, to evaluate cell migration. A Transwell Kit (Shanghai Fanke Biotechnology Co., Ltd., FK-lk019) was used for the invasion assay. First, 200 μL of RPMI 1640 culture medium was placed in the upper compartment, and 500 mL of RPMI 1640 with 10% FBS was placed in the lower compartment. After 48 h of culture at 37°C, the matrix gelatin and cells in the upper compartment were wiped off. The plate was cleaned three times with PBS, immobilized with paraformaldehyde for 10 min, cleaned three times with double distilled water, dried, followed by staining with 0.1% crystal violet, and cell invasion was assessed by microscopy.
StarBase 3.0 was used to predict lncRNA and miRNA target genes. PmirGLO reporter vectors carrying pmir-miR-137-3' untranslated region wild type (Wt) and pmir-miR-137-3' untranslated region mutant (Mut), sh-OIP5-AS1, and sh-NC were co-transfected into CC cells. After 48 h, luciferase activity was determined using the dual luciferase reporter gene determination kit (Solarbio, China).
A RNA-binding protein immunoprecipitation (RIP) kit (Beijing Biomars Technology Development Co., Ltd., China) was used to carry out the RIP assay following the manufacturer’s instructions. The cells were cleaned with pre-cooled PBS, and then the supernatant was discarded. An equal volume of RIP lysate was added to lyse the cells, and the whole cell protein extract was incubated with RIP washing buffer containing magnetic beads bound to Ago2 antibody or immunoglobulin G controls. The immunoprecipitated RNA was extracted from the samples after protein digestion by proteinase K. Finally, the purified RNA was analyzed by qRT-PCR to prove the existence of binding targets.
In this study, the collected data were analyzed statistically using SPSS20.0, and visualized by figures using GraphPad 7. Inter-group comparisons were conducted using the t test, and multi-group comparisons were performed using one-way ANOVA. In addition, post hoc pairwise comparison was carried out by the LSD-t test. Receiver operating characteristic curves were used to analyze the diagnostic value of OIP5-AS1 and miR-137 in CC, and Pearson’s correlation analysis was carried out to analyze the relationship between OIP5-AS1 and miR-137. P < 0.05 indicated statistically significant differences.
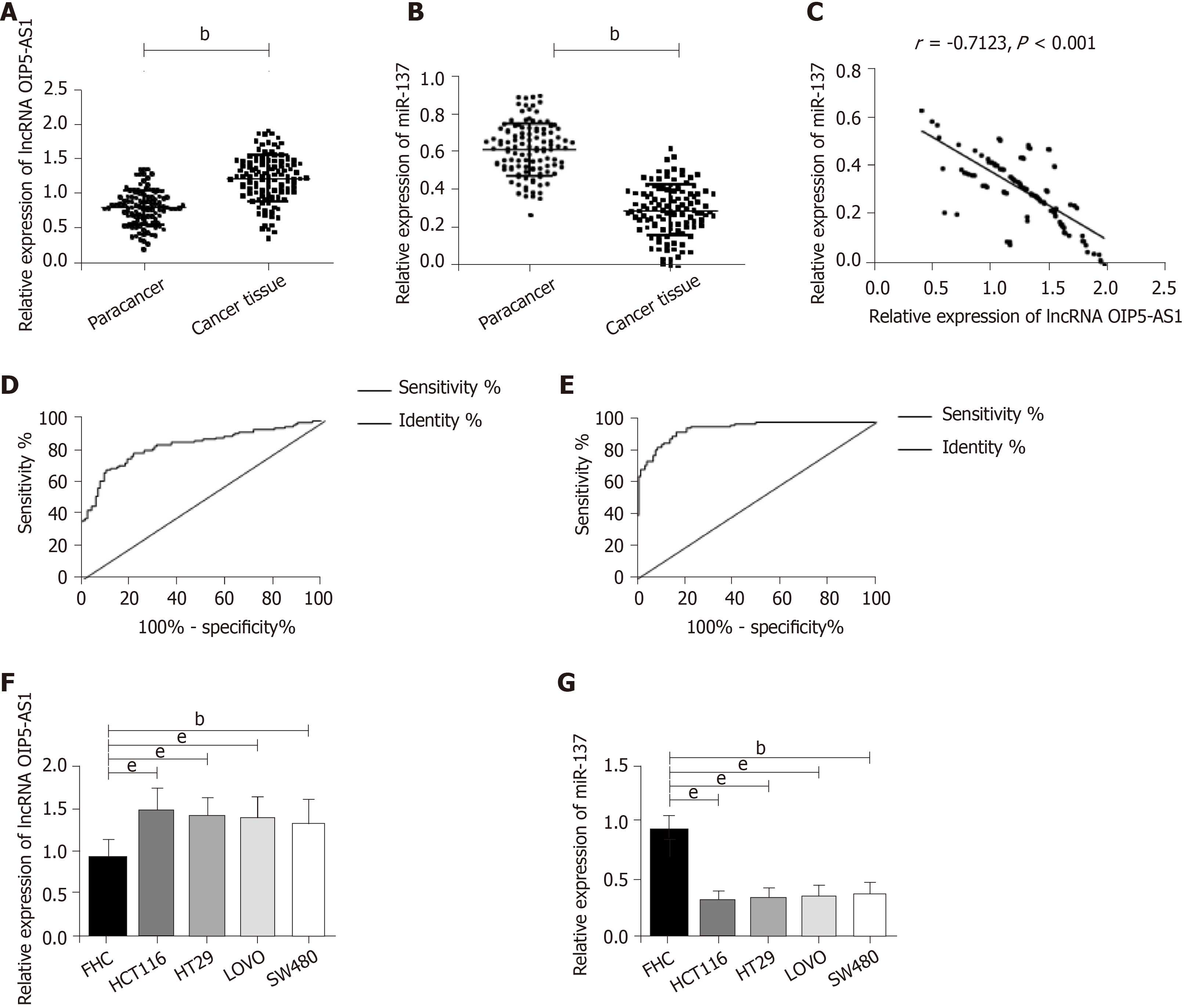
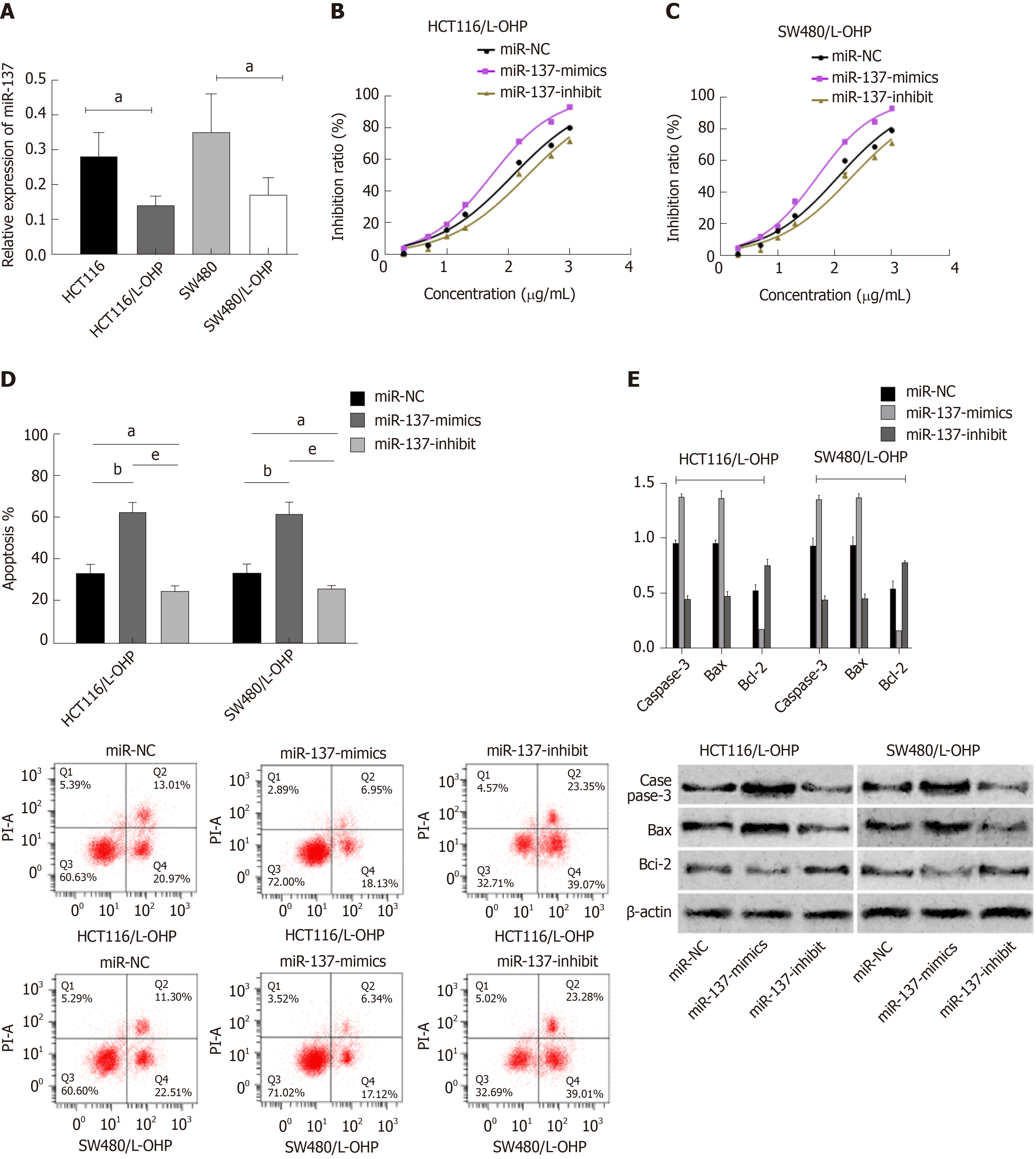
The results showed that OIP5-AS1 expression in CC tissues was significantly higher than that in corresponding tumor-adjacent tissues (P < 0.05) and miR-137 expression was low in cancer tissues (P < 0.05). In addition, correlation coefficient analysis revealed that there was a negative correlation between OIP5-AS1 expression and miR-137 expression in CC tissues (r = - 0.7123, P < 0.001) (Figure 1). Receiver operating characteristic curve results showed that OIP5-AS1 and miR-137 had high diagnostic value in CC patients. Further determination of the expression of these two factors in CC cells revealed that OIP5-AS1 expression in normal CC cell lines (HCT116, SW480, HT29, and LOVO) was significantly higher than that in the normal colon epithelial cell line (FHC), and miR-137expression was low in all CC cell lines.
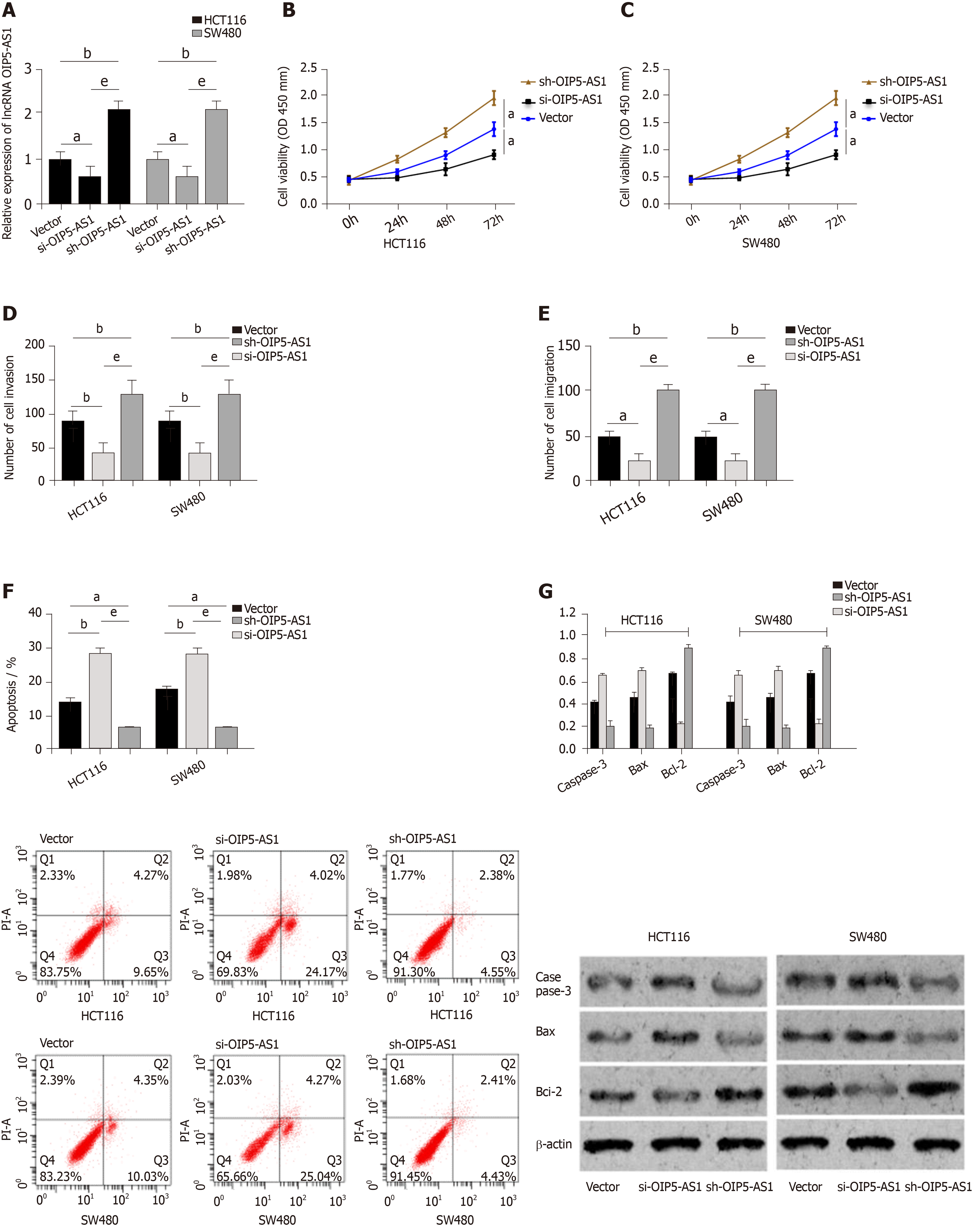
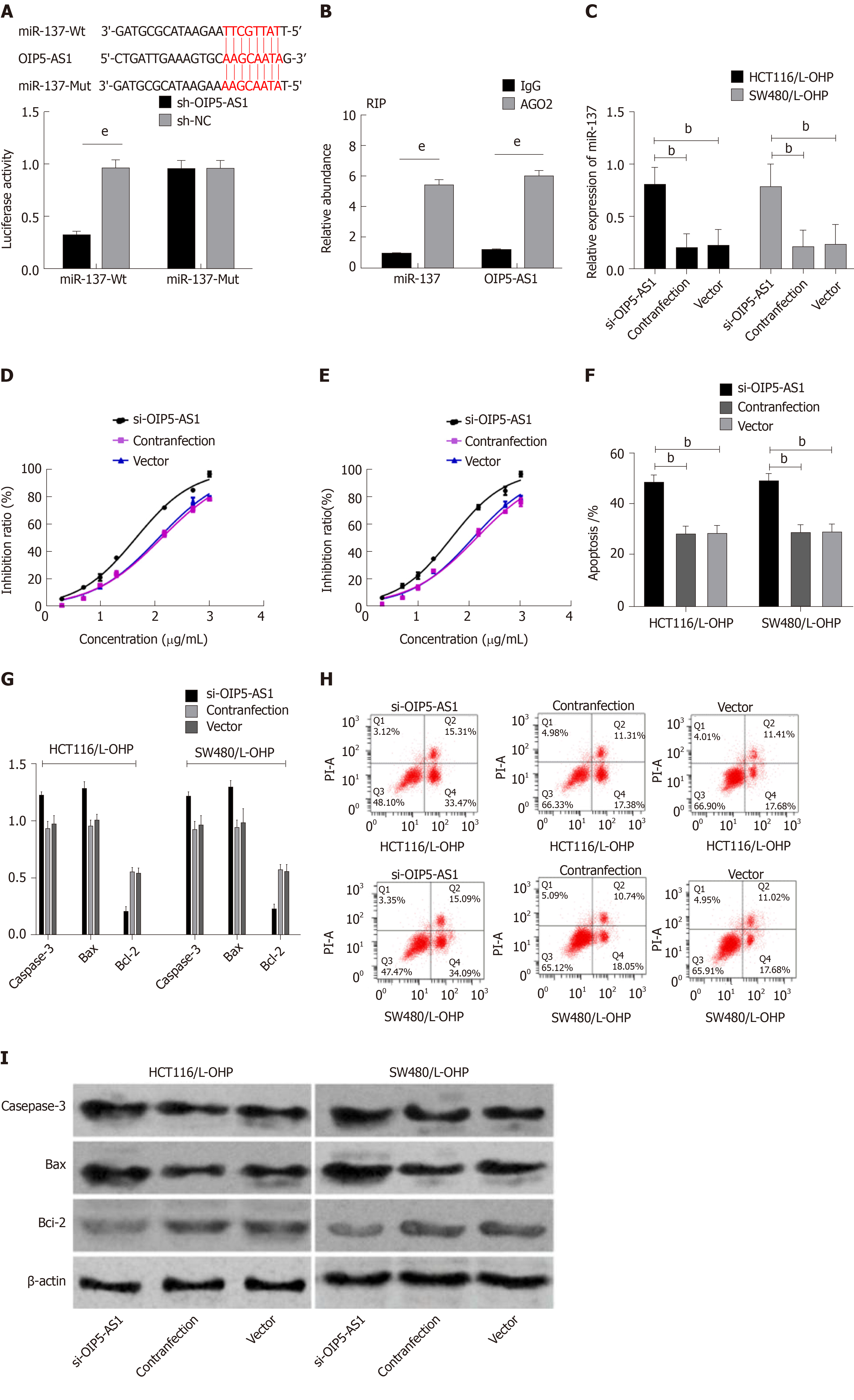
Vector, si-OIP5-AS1, and sh-OIP5-AS1 were transfected into CC cells (HCT116 and SW480), respectively. It was shown that compared with HCT116 and SW480 cells transfected with Vector, those transfected with sh-OIP5-AS1 showed significantly increased expression of OIP5-AS1, while those transfected with si-OIP5-AS1 showed significantly decreased OIP5-AS1 expression. Evaluation of the biological function of cells revealed that compared with the Vector group, HCT116 and SW480 cells transfected with si-OIP5-AS1 showed significantly suppressed proliferation, migration, and invasion abilities, and a significantly increased apoptosis rate (all P < 0.05), while those transfected with sh-OIP5-AS1 showed significantly enhanced proliferation, migration, and invasion abilities, and a significantly decreased apoptosis rate (all P < 0.05). In addition, compared with the Vector group, the si-OIP5-AS1 group showed significantly increased expression of apoptosis-related proteins (Bax and Caspase-3), and significantly decreased expression of Bcl-2 protein (all P < 0.05), while the sh-OIP5-AS1 group showed significantly decreased expression of apoptosis-related proteins (Bax and Caspase-3), and significantly increased expression of Bcl-2 protein (all P < 0.05) (Figure 2).
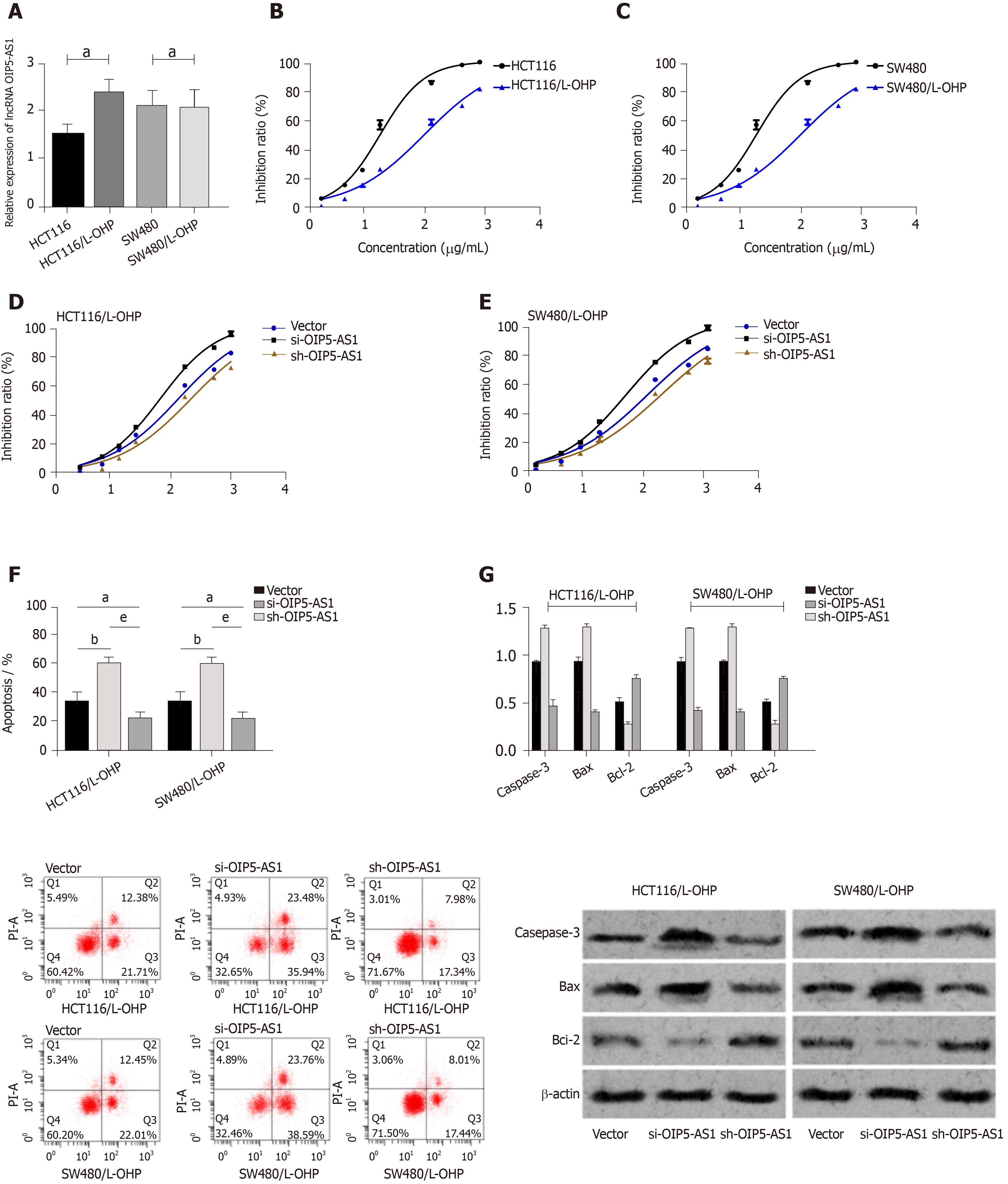
The OIP5-AS1 level in constructed drug-resistant cell lines was determined, and it was demonstrated that the OIP5-AS1 level in drug-resistant cell lines (HCT116/L-OHP and SW480/L-OHP) was significantly higher than that in the HCT116 and SW480 cell lines (P < 0.05). The inhibitory effect of L-OHP at different concentrations on cell growth was determined, and it was calculated that the IC50 of HCT116 and SW480 cells was 20.20 μg/mL and 19.40 μg/mL, respectively, while that of drug-resistant cell lines HCT116/L-OHP and SW480/L-OHP was 114.9 μg/mL and 109.8 μg/mL, respectively. Thus, after L-OHP treatment, the cell activity of drug-resistant cell lines was significantly higher than that of parent cell lines. The inhibitory effect of L-OHP at different concentrations on the growth of transfected drug-resistant cells was also determined, and it was found that the IC50 of HCT116/L-OHP cells transfected with Vector, those transfected with si-OIP5-AS1, and those transfected with sh-OIP5-AS1 was 116.5 μg/mL, 54.96 μg/mL, and 196.6 μg/mL, respectively, and the IC50 of SW480/L-OHP cells transfected with Vector, those transfected with si-OIP5-AS1, and those transfected with sh-OIP5-AS1 was 114.1 μg/mL, 52.33 μg/mL, and 186.7 μg/mL, respectively. Therefore, these findings indicated that silencing OIP5-AS1 expression significantly intensified the inhibition of drug-resistant cell growth. The IC50 of each drug-resistant cell line was used as the concentration of added L-OHP to observe the apoptosis of drug-resistant cells. It was found that compared with the cells transfected with Vector, those transfected with si-OIP5-AS1 showed significantly intensified apoptosis and significantly increased expression of apoptosis-related proteins (Bax and Caspase-3), and significantly decreased expression of Bcl-2 (all P < 0.05), while those transfected with sh-OIP5-AS1 showed the opposite trend (all P < 0.05) (Figure 3).
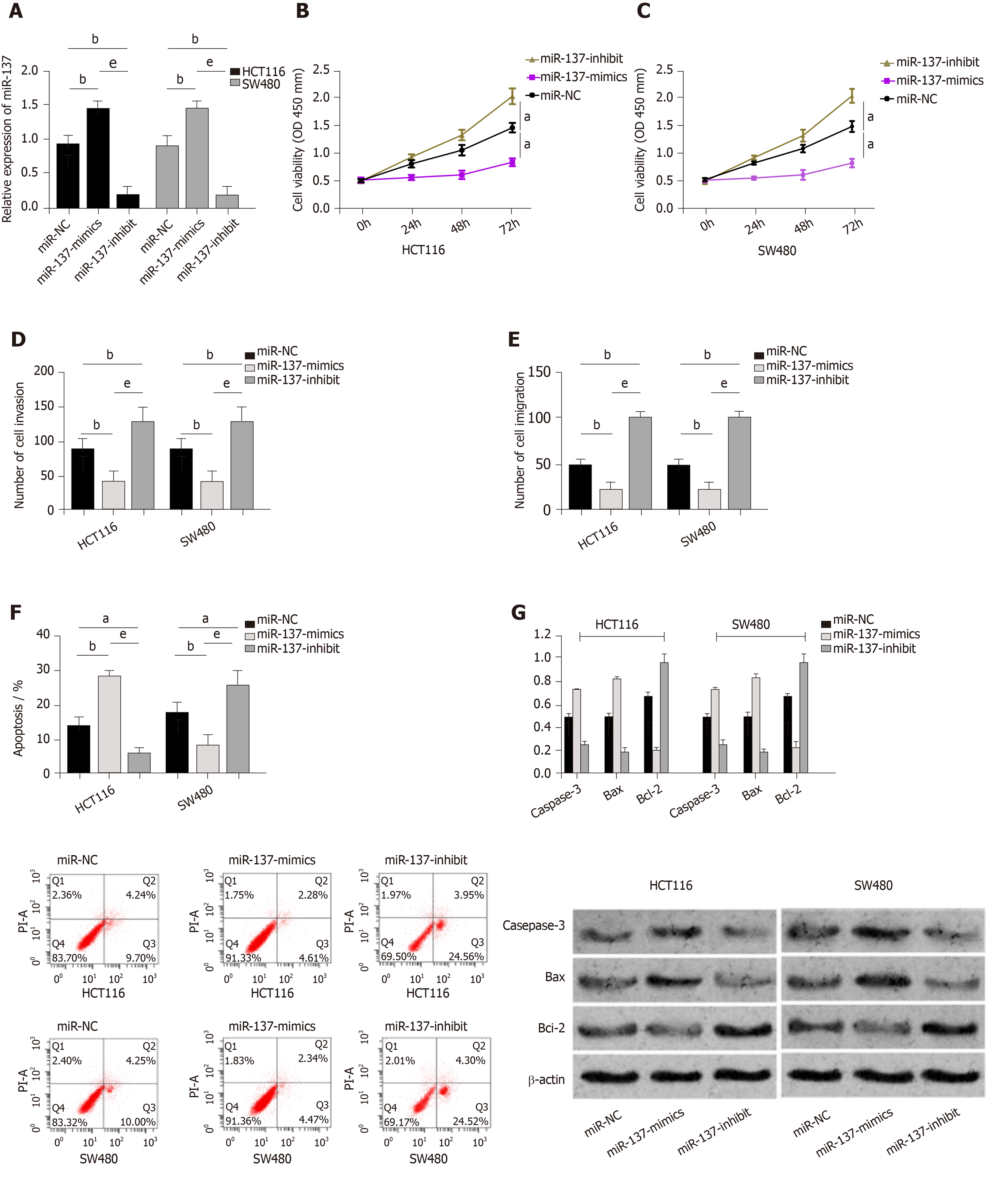
MiR-NC, miR-137-mimics, and miR-137-inhibitor were transfected into CC cells (HCT116 and SW48), respectively. It was shown that compared with HCT116 and SW480 cells transfected with miR-NC, those transfected with miR-137-mimics showed significantly up-regulated expression of miR-137, while those transfected with miR-137-inhibitor showed significantly decreased expression of miR-137. Assessment of the biological function of cells revealed that compared with the miR-NC group, HCT116 and SW480 cells transfected with miR-137-mimics showed significantly suppressed proliferation, migration, and invasion abilities, and significantly intensified apoptosis (all P < 0.05), while those transfected with miR-137-inhibitor showed the opposite trend (all P < 0.05). In addition, compared with the miR-NC group, the miR-137-mimics group showed significantly increased expression of apoptosis-related proteins (Bax and Caspase-3), and significantly decreased expression of Bcl-2 protein (all P < 0.05), while the miR-137-inhibitor group showed markedly down-regulated expression of apoptosis-related proteins (Bax and Caspase-3), and significantly up-regulated expression of Bcl-2 protein (all P < 0.05) (Figure 4).
The miR-137 level in constructed drug-resistant cell lines was determined, and it was found that the miR-137 level in drug-resistant cell lines (HCT116/L-OHP and SW480/L-OHP) was significantly lower than that in HCT116 and SW480 cell lines (all P < 0.05). The inhibition of L-OHP at different concentrations on the growth of transfected drug-resistant cells was determined, and the results showed that the IC50 of HCT116/L-OHP cells transfected with miR-NC, those transfected with miR-137-mimics, and those transfected with miR-137-inhibitor was 117.3 μg/mL, 52.87 μg/mL, and 202.0 μg/mL, respectively, and the IC50 of SW480/L-OHP cells transfected with miR-NC, those transfected with miR-137-mimics, and those transfected with miR-137-inhibitor was 112.5 μg/mL, 48.62 μg/mL, and 196.1 μg/mL, respectively. These findings indicated that overexpression of miR-137 could significantly intensify the growth inhibition of drug-resistant cells. The IC50 of each drug-resistant cell line was used as the concentration of added L-OHP to observe the apoptosis of drug-resistant cells. It was found that compared with the miR-NC group, the miR-137-mimics group showed significantly intensified apoptosis, significantly up-regulated apoptosis-related proteins (Bax and Caspase-3), and significantly down-regulated Bcl-2 protein, while the miR-137-inhibitor group showed the opposite trend (Figure 5).
Bioinformatics analysis revealed binding sites between miR-137 and OIP5-AS1. Therefore, RIP and dual luciferase assay were carried out, and showed that the fluorescence activity of miR-137-Wt decreased significantly. The RIP assay revealed that compared with immunoprecipitation with anti-IgG, the immunoprecipitation with anti-Ago2 antibody contributed to significantly increased enrichment content of miR-137 and OIP5-AS1. In order to further verify that OIP5-AS1 affects the resistance of CC cells by regulating miR-137, we co-transfected Si-OIP5-AS1 and miR-137-inhibitor into HCT116/L-OHP and SW480/L-OHP cells. It was found that Si-OIP5-AS1 could enhance the drug resistance sensitivity of HCT116/L-OHP and SW480/L-OHP cells to L-OHP, increase the apoptosis rate, significantly up-regulate the expression of Bax and Caspase-3 proteins, and significantly down-regulate the expression of Bcl-2 protein, but miR-137-inhibitor did not reverse this effect. The increased IC50 of L-OHP lowered the apoptosis rate, reduced the expression of Bax and Caspase-3 proteins in cells, and further increased the expression of Bcl-2 protein (Figure 6).
CC is a common malignant tumor in the gastrointestinal tract, its associated morbidity is rising year by year, and its incidence and mortality among men are higher than those among women[16-18]. At present, the most effective clinical treatment for CC is chemotherapy. With low renal toxicity, L-OHP has been widely used as a first-line chemotherapy drug in CC patients[19,20]. However, chemotherapy is often accompanied by drug resistance, which limits the clinical application of L-OHP and seriously compromises the effectiveness of platinum-containing chemotherapy regimens[21,22]. Therefore, finding a solution for L-OHP drug resistance has become the key to the treatment of tumors. At present, the correlation between cell resistance and specific genes during CC chemotherapy at the molecular biology level requires further clarification.
LncRNAs have gradually become key regulators of cellular processes and physiological and pathological processes[11]. OIP5-AS1 is highly expressed in various diseases and participates in the development and progression of diseases[11,12]. Earlier studies revealed that OIP5-AS1 can regulate the expression of miR-410 and can also regulate its target KLF10/PTEN/AKT to mediate the cellular behavior of multiple myeloma[12]. Wang et al[23] pointed out that OIP5-AS1, with low expression in lung cancer, could intensify the proliferation of lung cancer cells by targeting miR-378a-3p, resulting in poor prognosis. However, there are few studies on OIP5-AS1 in CC. In this study, OIP5-AS1 was up-regulated in CC tissues and cells, and silencing OIP5-AS1 expression significantly inhibited the invasion, proliferation, and migration abilities of CC cells and significantly increased the apoptosis rate. However, up-regulation of OIP5-AS1 resulted in the opposite effects. These results indicated that OIP5-AS1 promoted the development of CC, which was inconsistent with previous studies[11]. Subsequently, we constructed L-OHP resistant cells, and found that silencing the expression of OIP5-AS1 strongly intensified the growth inhibition of drug-resistant cells, and decreased the IC50 of L-OHP, significantly increased the apoptosis rate, up-regulated apoptosis-related proteins (Caspase-3 and Bax), and significantly down-regulated Bcl-2 protein. Similarly, up-regulation of the expression of OIP5-AS1 resulted in the opposite effects. This suggested that silencing OIP5-AS1 can improve the sensitivity of CC cells to L-OHP, and can also reverse the resistance of CC cells to L-OHP, thus improving chemotherapy efficacy. Recent studies have shown that silencing OIP5-AS1 inhibits the development of osteosarcoma cells in vitro and in vivo and promotes cell apoptosis, and it can also strengthen the drug resistance sensitivity of osteosarcoma cells to cisplatin[24]. These findings are similar to the results of this study. However, the mechanism of OIP5-AS1 in drug-resistant CC cells requires further study.
In recent years, more and more studies have found that a lncRNA can act as a miR response element and a competitive platform in a variety of tumors, and can also act as a miR molecular sponge by binding to miR, thus affecting miRNA expression and the regulation of cell function[25,26]. Some studies have revealed that lncRNA FOXD2-AS1 can promote drug resistance to gemcitabine in bladder cancer by regulating miR-143[27]. Moreover, one study concluded that lncRNA BLACAT1 could regulate ABCB1 through miR-361 to promote drug resistance to L-OHP in gastric cancer[28]. In this study, we found targeted binding sites between OIP5-AS1 and miR-137 through online tool analysis, and verified the results using dual luciferase reporter assay. We also found enrichment between OIP5-AS1 and miR-137 using the RIP assay. These findings further confirmed the competitive endogenous RNA relationship between OIP5-AS1 and miR-137. MiR-137 has low expression in various cancers and can participate in the regulation of cell biological behavior[29]. In this study, it was found that miR-137 expression was low in CC, and up-regulation of miR-137 expression could inhibit proliferation, invasion and migration of CC cells and increase the apoptosis rate, which was similar to the results of the study by Bi et al[14]. Previous studies revealed that down-regulation of miR-137 induced resistance to L-OHP in parent CC cells, while over-expression of miR-137 induced sensitivity to L-OHP in drug-resistant cells[30], which was similar to the results in this study and further proved the influence of miR-137 in drug-resistant cells. In this study, it was also found that the expression of OIP5-AS1 was negatively correlated with that of miR-137 in CC tissues. Finally, CC cells were co-transfected with Si-OIP5-AS1 and miR-137-inhibitor in this study, and it was found that the inhibitory effect on CC cells and the inhibitory effect on L-OHP drug resistance were offset. Therefore, the results of this study confirmed that OIP5-AS1 can mediate the expression of miR-137 to regulate drug resistance to L-OHP in CC cells.
This study investigated the effects of OIP5-AS1 and miR-137 on biological behaviors and L-OHP drug resistance in CC cells from many aspects. However, there are still some limitations in this study. No study has been conducted in nude mice, and whether OIP5-AS1 is involved in the prognosis of CC still requires further research and verification. Moreover, the regulatory network of OIP5-AS1 in CC remains unclear, and further research is needed to determine whether OIP5-AS1 can affect the development and progression of tumors in other ways. In future research, we will carry out more experiments to verify these results.
To sum up, OIP5-AS1 is highly expressed in CC and can affect the biological behaviors of CC cells, and can regulate drug resistance to L-OHP in CC cells by mediating miR-137 expression.
Recently, colon cancer (CC) has displayed a high incidence, and the main treatment of CC is chemotherapy. Oxaliplatin (L-OHP) is a common drug used in chemotherapy, but long-term use can result in drug resistance, seriously affecting the prognosis of patients.
Long non-coding RNA Opa-interacting protein 5 antisense RNA 1 (OIP5-AS1) appears to be up-regulated, which plays a tumor-promoting role in a number of cancers. It is speculated that miR-137 may be effective in mediating drug resistance in CC cells.
To determine the effect of long non-coding RNA OIP5-AS1 on drug resistance in CC cell lines and its role in regulating miR-137.
We not only analyzed the expression levels of OIP5-AS1 and miR-137 in surgical CC tissue samples, but also observed their effects on the biological behavior of CC cells as well as L-OHP resistance.
We noted high expression of OIP5-AS in CC tissues and cells and low expression of miR-137. In cytological studies, it was found that reducing OIP5-AS1 expression or increasing miR-137 expression controlled the proliferation, invasion and migration of CC cells, promoting the apoptosis rate of tumor cells by regulating the expression of apoptosis-related proteins.
OIP5-AS1 is highly expressed in CC, which contributes to regulation of the biological behavior of CC cells as well as drug resistance to L-OHP in CC cells via mediation of miR-137 expression.
This study reveals the mechanism of OIP5-AS1 in drug resistance to L-OHP in CC cells, which provides a new method to improve the sensitivity of CC cells to L-OHP.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: González-González R, Lieto E S-Editor: Tang JZ L-Editor: Webster JR E-Editor: Xing YX
| 1. | Gangireddy VGR, Coleman T, Kanneganti P, Talla S, Annapureddy AR, Amin R, Parikh S. Polypectomy versus surgery in early colon cancer: size and location of colon cancer affect long-term survival. Int J Colorectal Dis. 2018;33:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Lawler M, Alsina D, Adams RA, Anderson AS, Brown G, Fearnhead NS, Fenwick SW, Halloran SP, Hochhauser D, Hull MA, Koelzer VH, McNair AGK, Monahan KJ, Näthke I, Norton C, Novelli MR, Steele RJC, Thomas AL, Wilde LM, Wilson RH, Tomlinson I; Bowel Cancer UK Critical Research Gaps in Colorectal Cancer Initiative. Critical research gaps and recommendations to inform research prioritisation for more effective prevention and improved outcomes in colorectal cancer. Gut. 2018;67:179-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 3. | Stein A, Atanackovic D, Bokemeyer C. Current standards and new trends in the primary treatment of colorectal cancer. Eur J Cancer. 2011;47 Suppl 3:S312-S314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Hu T, Li Z, Gao CY, Cho CH. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J Gastroenterol. 2016;22:6876-6889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 287] [Cited by in RCA: 271] [Article Influence: 30.1] [Reference Citation Analysis (8)] |
| 5. | Brungs D, Aghmesheh M, de Souza P, Carolan M, Clingan P, Rose J, Ranson M. Safety and Efficacy of Oxaliplatin Doublet Adjuvant Chemotherapy in Elderly Patients With Stage III Colon Cancer. Clin Colorectal Cancer. 2018;17:e549-e555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Ray B, Gupta B, Mehrotra R. Binding of platinum derivative, oxaliplatin to deoxyribonucleic acid: structural insight into antitumor action. J Biomol Struct Dyn. 2019;37:3838-3847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Huang H, Aladelokun O, Ideta T, Giardina C, Ellis LM, Rosenberg DW. Inhibition of PGE2/EP4 receptor signaling enhances oxaliplatin efficacy in resistant colon cancer cells through modulation of oxidative stress. Sci Rep. 2019;9:4954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Majidinia M, Yousefi B. Long non-coding RNAs in cancer drug resistance development. DNA Repair (Amst). 2016;45:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Saeinasab M, Bahrami AR, González J, Marchese FP, Martinez D, Mowla SJ, Matin MM, Huarte M. SNHG15 is a bifunctional MYC-regulated noncoding locus encoding a lncRNA that promotes cell proliferation, invasion and drug resistance in colorectal cancer by interacting with AIF. J Exp Clin Cancer Res. 2019;38:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Sun WL, Kang T, Wang YY, Sun JP, Li C, Liu HJ, Yang Y, Jiao BH. Long noncoding RNA OIP5-AS1 targets Wnt-7b to affect glioma progression via modulation of miR-410. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Kim J, Abdelmohsen K, Yang X, De S, Grammatikakis I, Noh JH, Gorospe M. LncRNA OIP5-AS1/cyrano sponges RNA-binding protein HuR. Nucleic Acids Res. 2016;44:2378-2392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 12. | Yang N, Chen J, Zhang H, Wang X, Yao H, Peng Y, Zhang W. LncRNA OIP5-AS1 loss-induced microRNA-410 accumulation regulates cell proliferation and apoptosis by targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple myeloma. Cell Death Dis. 2017;8:e2975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 13. | Samuel P, Pink RC, Brooks SA, Carter DR. miRNAs and ovarian cancer: a miRiad of mechanisms to induce cisplatin drug resistance. Expert Rev Anticancer Ther. 2016;16:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Bi WP, Xia M, Wang XJ. miR-137 suppresses proliferation, migration and invasion of colon cancer cell lines by targeting TCF4. Oncol Lett. 2018;15:8744-8748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Shen H, Wang L, Ge X, Jiang CF, Shi ZM, Li DM, Liu WT, Yu X, Shu YQ. MicroRNA-137 inhibits tumor growth and sensitizes chemosensitivity to paclitaxel and cisplatin in lung cancer. Oncotarget. 2016;7:20728-20742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Cerdán Santacruz C, Frasson M, Flor-Lorente B, Ramos Rodríguez JL, Trallero Anoro M, Millán Scheiding M, Maseda Díaz O, Dujovne Lindenbaum P, Monzón Abad A, García-Granero Ximenez E; ANACO Study Group. Laparoscopy may decrease morbidity and length of stay after elective colon cancer resection, especially in frail patients: results from an observational real-life study. Surg Endosc. 2017;31:5032-5042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Eaglehouse YL, Georg MW, Richard P, Shriver CD, Zhu K. Costs for Colon Cancer Treatment Comparing Benefit Types and Care Sources in the US Military Health System. Mil Med. 2019;184:e847-e855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Manceau G, Mege D, Bridoux V, Lakkis Z, Venara A, Voron T, Sielezneff I, Karoui M; French Surgical Association Working Group. Emergency Surgery for Obstructive Colon Cancer in Elderly Patients: Results of a Multicentric Cohort of the French National Surgical Association. Dis Colon Rectum. 2019;62:941-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Witzig TE, Johnston PB, LaPlant BR, Kurtin PJ, Pederson LD, Moore DF, Nabbout NH, Nikcevich DA, Rowland KM, Grothey A. Long-term follow-up of chemoimmunotherapy with rituximab, oxaliplatin, cytosine arabinoside, dexamethasone (ROAD) in patients with relapsed CD20+ B-cell non-Hodgkin lymphoma: Results of a study of the Mayo Clinic Cancer Center Research Consortium (MCCRC) MC0485 now known as academic and community cancer research united (ACCRU). Am J Hematol. 2017;92:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Gallois C, Taieb J, Le Corre D, Le Malicot K, Tabernero J, Mulot C, Seitz JF, Aparicio T, Folprecht G, Lepage C, Mini E, Van Laethem JL, Emile JF, Laurent-Puig P; PETACC8 investigators. Prognostic Value of Methylator Phenotype in Stage III Colon Cancer Treated with Oxaliplatin-based Adjuvant Chemotherapy. Clin Cancer Res. 2018;24:4745-4753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Uppada SB, Gowrikumar S, Ahmad R, Kumar B, Szeglin B, Chen X, Smith JJ, Batra SK, Singh AB, Dhawan P. MASTL induces Colon Cancer progression and Chemoresistance by promoting Wnt/β-catenin signaling. Mol Cancer. 2018;17:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | El Khoury F, Corcos L, Durand S, Simon B, Le Jossic-Corcos C. Acquisition of anticancer drug resistance is partially associated with cancer stemness in human colon cancer cells. Int J Oncol. 2016;49:2558-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Wang M, Sun X, Yang Y, Jiao W. Long non-coding RNA OIP5-AS1 promotes proliferation of lung cancer cells and leads to poor prognosis by targeting miR-378a-3p. Thorac Cancer. 2018;9:939-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Song L, Zhou Z, Gan Y, Li P, Xu Y, Zhang Z, Luo F, Xu J, Zhou Q, Dai F. Long noncoding RNA OIP5-AS1 causes cisplatin resistance in osteosarcoma through inducing the LPAATβ/PI3K/AKT/mTOR signaling pathway by sponging the miR-340-5p. J Cell Biochem. 2019;120:9656-9666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 25. | Deguchi S, Katsushima K, Hatanaka A, Shinjo K, Ohka F, Wakabayashi T, Zong H, Natsume A, Kondo Y. Oncogenic effects of evolutionarily conserved noncoding RNA ECONEXIN on gliomagenesis. Oncogene. 2017;36:4629-4640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Liu T, Chi H, Chen J, Chen C, Huang Y, Xi H, Xue J, Si Y. Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene. 2017;631:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 27. | An Q, Zhou L, Xu N. Long noncoding RNA FOXD2-AS1 accelerates the gemcitabine-resistance of bladder cancer by sponging miR-143. Biomed Pharmacother. 2018;103:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Wu X, Zheng Y, Han B, Dong X. Long noncoding RNA BLACAT1 modulates ABCB1 to promote oxaliplatin resistance of gastric cancer via sponging miR-361. Biomed Pharmacother. 2018;99:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 29. | Wang M, Gao H, Qu H, Li J, Liu K, Han Z. MiR-137 suppresses tumor growth and metastasis in clear cell renal cell carcinoma. Pharmacol Rep. 2018;70:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Guo Y, Pang Y, Gao X, Zhao M, Zhang X, Zhang H, Xuan B, Wang Y. MicroRNA-137 chemosensitizes colon cancer cells to the chemotherapeutic drug oxaliplatin (OXA) by targeting YBX1. Cancer Biomark. 2017;18:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |