Published online Apr 7, 2020. doi: 10.3748/wjg.v26.i13.1450
Peer-review started: December 2, 2019
First decision: December 12, 2019
Revised: February 20, 2020
Accepted: March 9, 2020
Article in press: March 9, 2020
Published online: April 7, 2020
Processing time: 127 Days and 11.6 Hours
Calpain-2 is a Ca2+-dependent cysteine protease, and high calpain-2 activity can enhance apoptosis mediated by multiple triggers.
To investigate whether calpain-2 can modulate aberrant endoplasmic reticulum (ER) stress-related apoptosis in rat hepatocyte BRL-3A cells.
BRL-3A cells were treated with varying doses of dithiothreitol (DTT), and their viability and apoptosis were quantified by 3-[4, 5-dimethyl-2-thiazolyl]-2, 5-diphenyl-2-H-tetrazolium bromide and flow cytometry. The expression of ER stress- and apoptosis-related proteins was detected by Western blot analysis. The protease activity of calpain-2 was determined using a fluorescent substrate, N-succinyl-Leu-Leu-Val-Tyr-AMC. Intracellular Ca2+ content, and ER and calpain-2 co-localization were characterized by fluorescent microscopy. The impact of calpain-2 silencing by specific small interfering RNA on caspase-12 activation and apoptosis of BRL-3A cells was quantified.
DTT exhibited dose-dependent cytotoxicity against BRL-3A cells and treatment with 2 mmol/L DTT triggered BRL-3A cell apoptosis. DTT treatment significantly upregulated 78 kDa glucose-regulated protein, activating transcription factor 4, C/EBP-homologous protein expression by >2-fold, and enhanced PRKR-like ER kinase phosphorylation, caspase-12 and caspase-3 cleavage in BRL-3A cells in a trend of time-dependence. DTT treatment also significantly increased intracellular Ca2+ content, calpain-2 expression, and activity by >2-fold in BRL-3A cells. Furthermore, immunofluorescence revealed that DTT treatment promoted the ER accumulation of calpain-2. Moreover, calpain-2 silencing to decrease calpain-2 expression by 85% significantly mitigated DTT-enhanced calpain-2 expression, caspase-12 cleavage, and apoptosis in BRL-3A cells.
The data indicated that Ca2+-dependent calpain-2 activity promoted the aberrant ER stress-related apoptosis of rat hepatocytes by activating caspase-12 in the ER.
Core tip: Hepatocyte apoptosis is associated with many liver diseases. During the process of apoptosis, calpain-2 can cleave several apoptosis-related proteins. However, the regulatory mechanisms by which calpain-2 regulates the endoplasmic reticulum (ER) stress-mediated hepatocyte apoptosis remain unclear. In this study, the effect of calpain-2 on ER stress-mediated hepatocyte apoptosis and the underlying regulatory mechanisms was investigated. Our data indicate that calpain-2 is crucial for the aberrant ER stress-induced apoptosis of hepatocytes and may be a novel therapeutic target for liver diseases.
- Citation: Xie RJ, Hu XX, Zheng L, Cai S, Chen YS, Yang Y, Yang T, Han B, Yang Q. Calpain-2 activity promotes aberrant endoplasmic reticulum stress-related apoptosis in hepatocytes. World J Gastroenterol 2020; 26(13): 1450-1462
- URL: https://www.wjgnet.com/1007-9327/full/v26/i13/1450.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i13.1450
Hepatocyte apoptosis participates in the pathogenesis of many types of liver diseases. Actually, apoptotic hepatocytes are observed in the liver of patients with acute liver injury, non-alcoholic fatty liver diseases, hepatic fibrosis, and liver cirrhosis[1-5]. However, the regulatory mechanisms underlying hepatocyte apoptosis remains elusive. It is notable that aberrant endoplasmic reticulum (ER) stress can trigger hepatocyte apoptosis[6,7]. While the ER is physiologically responsible for the control of protein proper folding and function, many factors such as the unfolded protein response, ER overload response and others can disturb ER function, leading to ER stress[8,9]. During the process of ER stress, the ER chaperon glucose-regulated protein (GRP78) changes its binding from ER transmembrane protein PRKR-like endoplasmic reticulum kinase (PERK) to unfolded/misfolded proteins to release PERK[10]. The released PERK is subjected to self-phosphorylation, which promotes the expression of transcription factors of activating transcription factor 4 (ATF4) and C/EBP-homologous protein (CHOP) in cells[11]. The upregulated ATF4 and CHOP can enter the nucleus to regulate the transcription of related genes[12]. Such compensative responses may reduce the accumulation of unfolded proteins, leading to the re-establishment of cellular homeostasis. However, if the ER stress cannot be alleviated, aberrant ER stress can trigger cell apoptosis, particularly by activating caspase-12[13-15]. However, how aberrant ER stress induces caspase-12 activation to trigger cell apoptosis has not been clarified in hepatocytes.
Calpain-2 is a Ca2+-dependent cysteine protease that can cleave its protein substrates. Calpain-2 can regulate cell cycle, differentiation, and apoptosis[16]. Previous studies have revealed that calpains promote ER stress-related apoptosis by activating caspase-12[17-19]. Actually, our previous studies indicated that ER stress occurred in hepatocytes in a rat model of carbon tetrachloride-induced hepatic fibrosis, which was associated with increased calpain-2 and caspase-12 expression and hepatocyte apoptosis[20,21]. However, it is unclear whether calpain-2 can modulate caspase-12 activation and ER stress-related apoptosis in hepatocytes.
This study explored the importance of calpain-2 in regulating caspase-12 activation and dithiothreitol (DTT)-induced ER stress-related apoptosis in hepatocytes. The results indicated that calpain-2 activity was crucial for DTT-induced ER stress-related hepatocyte apoptosis by activating caspase-12 in vitro.
Special reagents included rat non-tumor BRL-3A cells (Number: KCB92013YJ, Kunming Cell Bank of Chinese Academy of Sciences, China), fetal bovine serum, Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, New York, NY, United States), Acrylamide, bisacrylamide, ammonium peroxydisulfate, glycine, Tri-hydroxymethyl aminomethane, 3-[4, 5-dimethyl-2-thiazolyl]-2, 5-diphenyl-2-H- tetrazolium bromide (MTT), Tween 20, DTT, dimethyl sulfoxide (Genview, League City, TX, United States), N-succinyl-Leu-Leu-Val-Tyr-AMC, antibodies against GRP78, PERK, ATF4, CHOP, and caspase-12 (Abcam, Cambridge, United Kingdom), antibodies against p-PERK (Affinity Biosciences, Cincinnati, OH, United States), calpain-2 and β-actin (Cell Signaling Technologies, Danvers, MA, United States), caspase-3 and secondary antibodies (Boster Biological Engineering, Wuhan, China), polyvinylidene difluoride membranes and enhanced chemiluminescence kit (Millipore, Burlington, MA, United States). Calpain-2-specific and control small interfering RNAs (siRNAs) and siRNA dilution buffer were produced by Santa Cruz Biotechnology (Santa Cruz, CA, United States), and the Annexin V-FITC Detection Kit, ER-tracker Red, and Fluo-3 AM were obtained from Beyotime Institute of Biotechnology (Nanjing, China).
Rat non-tumor hepatocyte BRL-3A cells were cultured in high-glucose DMEM supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2. When the cells reached 80% confluency, the cells were treated with DTT, an inhibitor of disulfide bond formation, to induce ER stress.
We examined the impact of DTT on the BRL-3A cell viability by MTT. Briefly, BRL-3A cells (5 × 103 cells/well) were treated in triplicate with 0-10 mmol/L of DTT for 24 h. During the last 4-h culture, the cells were treated with 0.5 mg/mL MTT reagent. The resulting formazan in individual wells was dissolved with 150 μL dimethyl sulfoxide, and the absorbance at 570 nmol/L in individual wells was measured using a microplate reader.
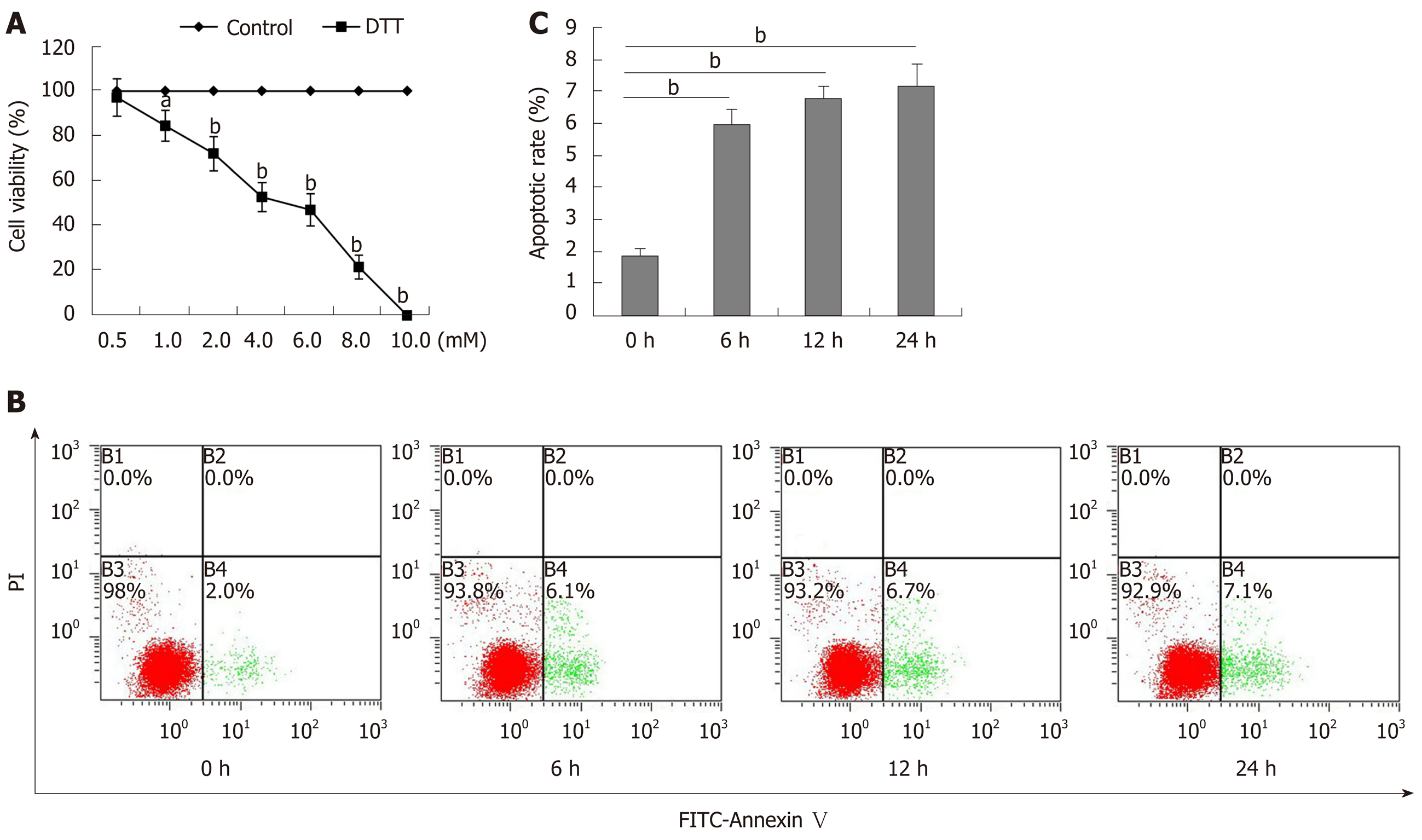
The effect of DTT on the apoptosis of BRL-3A cells was quantified by flow cytometry using the Annexin V/propidium (PI) kit per the manufacturer’s protocol. Briefly, BRL-3A cells (5 × 105 cells/flask) were treated in triplicate with vehicle or 2.0 mmol/L DTT, a sub-toxic dose, for varying time periods[22]. After being washed twice with phosphate-buffered saline, the cells were stained with FITC-Annexin-V and PI. The percentages of apoptotic cells in individual groups were quantified by flow cytometry in a FACSCalibur™ (BD Biosciences, Franklin Lakes, NJ, United States).
After treatment with 2.0 mmol/L DTT for varying time periods, BRL-3A cells were lysed in a RIPA buffer and centrifuged, followed by quantifying the protein concentrations. The cell lysates (50 μg/lane) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 10%-12% gels and transferred onto polyvinylidene difluoride membranes. After being blocked in 5% non-fat dry milk in Tris-buffered saline with Tween-20, the membranes were probed overnight at 4°C with primary antibodies against GRP78 (1:1500), PERK (1:1500), p-PERK (1:1500), ATF4 (1:1500), CHOP (1:1500), calpain-2 (1:1000), caspase-12 (1:1000), cleaved capase-3 (1:500), and β-actin (1:1000). The bound antibodies were detected with horseradish peroxidase-conjugated secondary antibodies and visualized using enhanced chemiluminescence reagents. The signal intensity was measured using Bio-Rad imaging system (Bio-Rad, Hercules, CA, United States) and analyzed by Quantity One software (Bio-Rad).
The intracellular Ca2+ content in BRL-3A cells was detected by microscopy after staining with a Ca2+-sensitive fluorescent dye, Fluo-3 AM[23]. In brief, BRL-3A cells were treated in triplicate with 2.0 mmol/L DTT for 0, 6, 12, and 24 h. After being washed with Hank's balanced salt solution, the cells were stained with Fluo-3 AM at 37 °C for 45 min. The morphology and fluorescent signals in individual wells of cells were examined under a light and fluorescent microscope (FV1000; Olympus, Tokyo, Japan).
We measured cellular calpain enzymatic activity using a fluorescence substrate N-succinyl-Leu-Leu-Val-Tyr-AMC, as previously described[24]. In brief, individual cell lysates were reacted at 37°C with 40 µmol/L N-succinyl-Leu-Leu-Val-Tyr-AMC for 1 h, and the fluorescent signals were measured using a fluorescence plate reader.
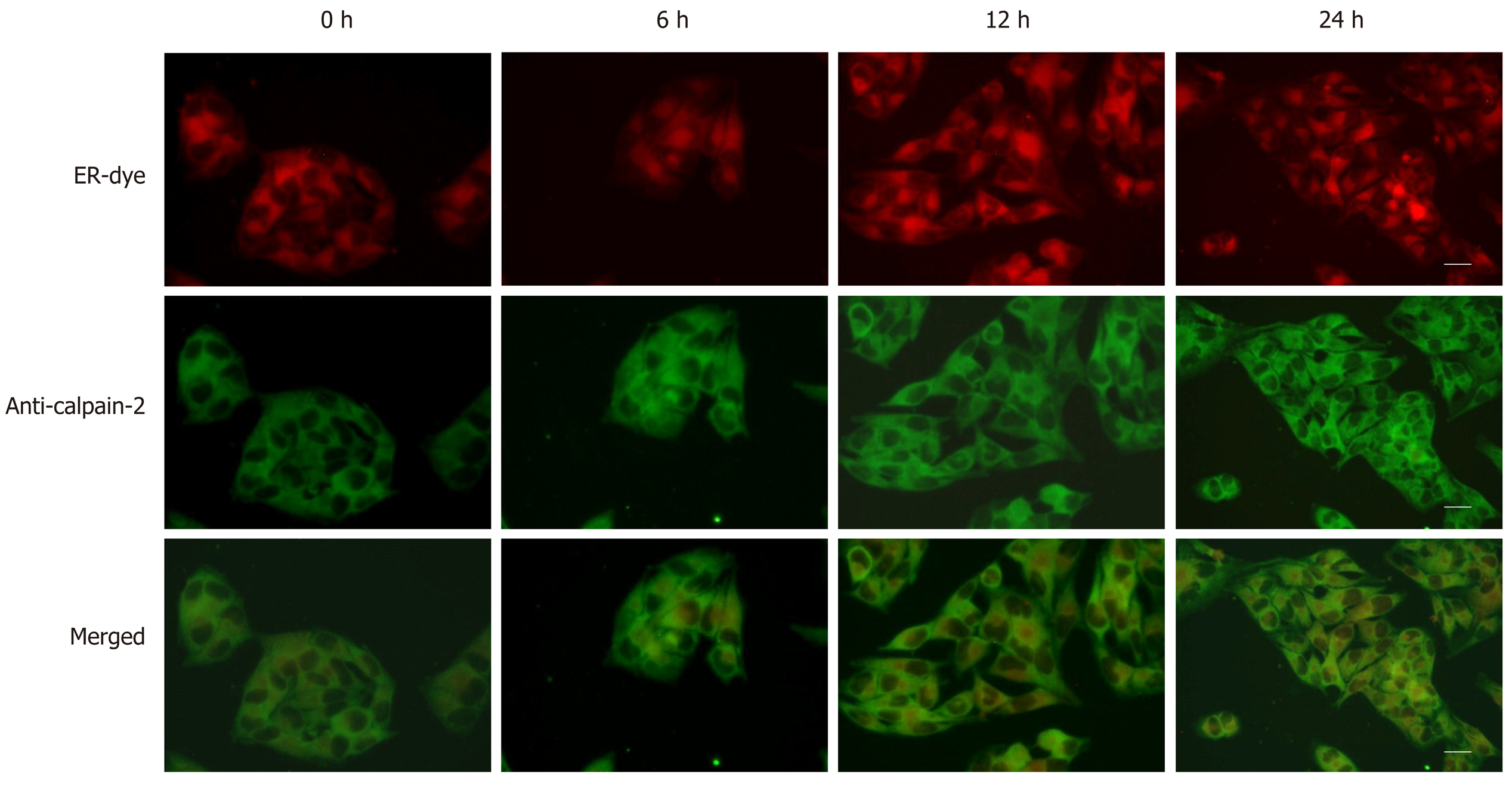
BRL-3A cells were cultured on coverslips overnight, and treated with 2.0 mmol/L DTT for 0, 6, 12, and 24 h. The cells were stained with ER-tracker Red (ER fluorescent dye)[25,26], fixed in 2% paraformaldehyde, and permeabilized with 0.2% Triton X-100. Subsequently, the cells were stained with anti-calpain-2 and Alexa Fluor 488-conjugated secondary antibody. We photoimaged the fluorescent signals under a fluorescence microscope (IX71; Olympus).
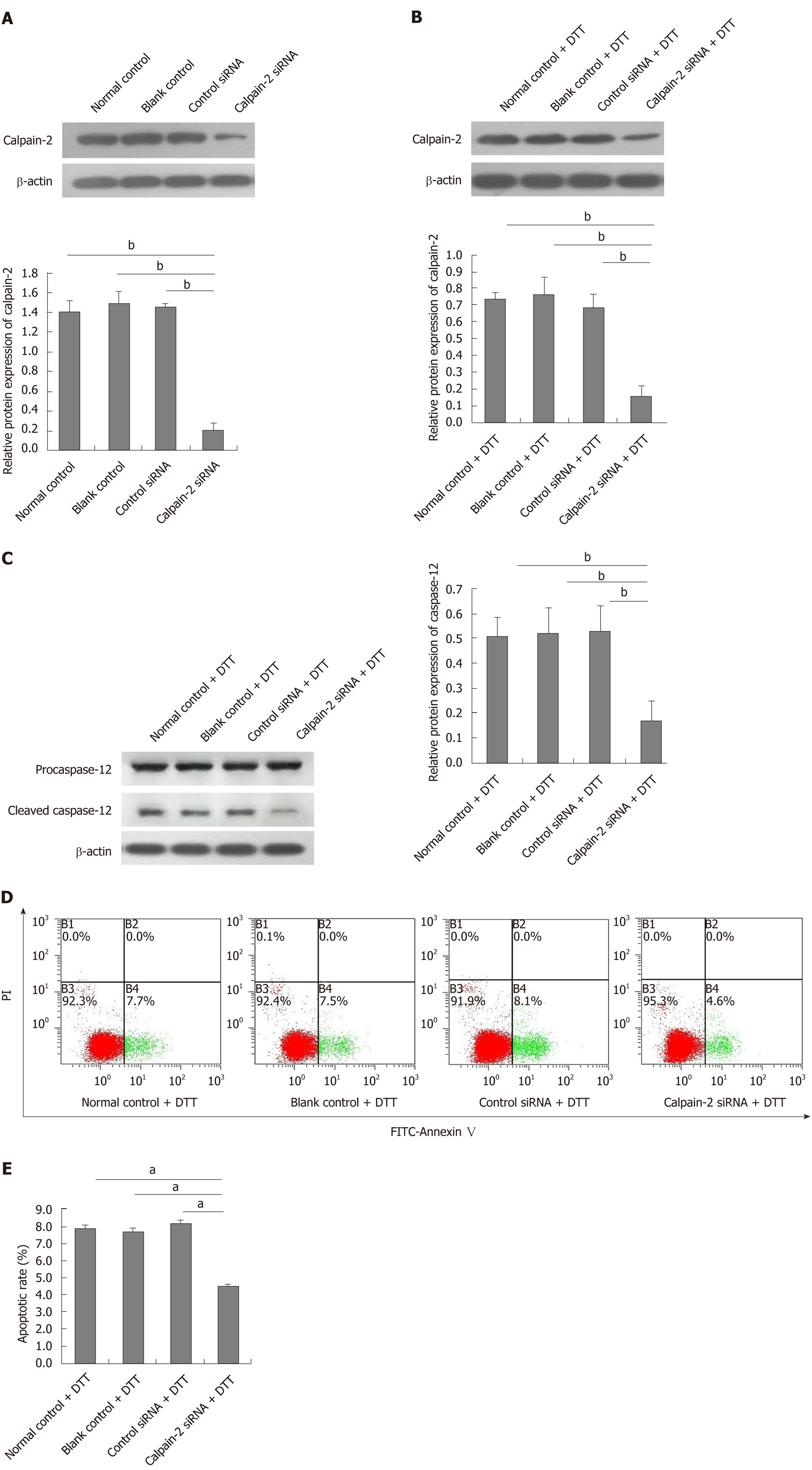
BRL-3A cells (5 × 105 cells/flask) were grown in antibiotic-free medium overnight and transfected with control or calpain-2 specific siRNA for 48 h. The efficacy of calpain-2 silencing was determined by Western blotting. Subsequently, the different groups of cells were treated in triplicate with 2.0 mmol/L DTT for 24 h and used for analysis of apoptosis, calpain-2, and cleaved caspase-12 expression.
Data are present as the mean ± SD. We compared the different groups of data by one-way analysis of variance and post hoc least significant difference test using SPSS 13.0 software. Statistical significance was defined at a P value < 0.05.
To determine the toxicity of DTT, BRL-3A cells were treated with 0-10 mmol/L DTT for 24 h and their viability was measured by MTT (Figure 1A). DTT had dose-dependent cytotoxicity against BRL-3A cells and the median lethal dose (LD50) value for BRL-3A cells was about 4 mmol/L in our experimental condition. Longitudinal analysis displayed that treatment with 2 mmol/L DTT for 6 h significantly increased the percentages of apoptotic BRL-3A cells by about 3-fold (P < 0.01), and treatment for a longer period did not significantly increase the frequency of apoptotic BRL-3A cells (Figure 1B and C). Hence, DTT exhibited dose-dependent cytotoxicity against BRL-3A cells by increasing their apoptosis in vitro.
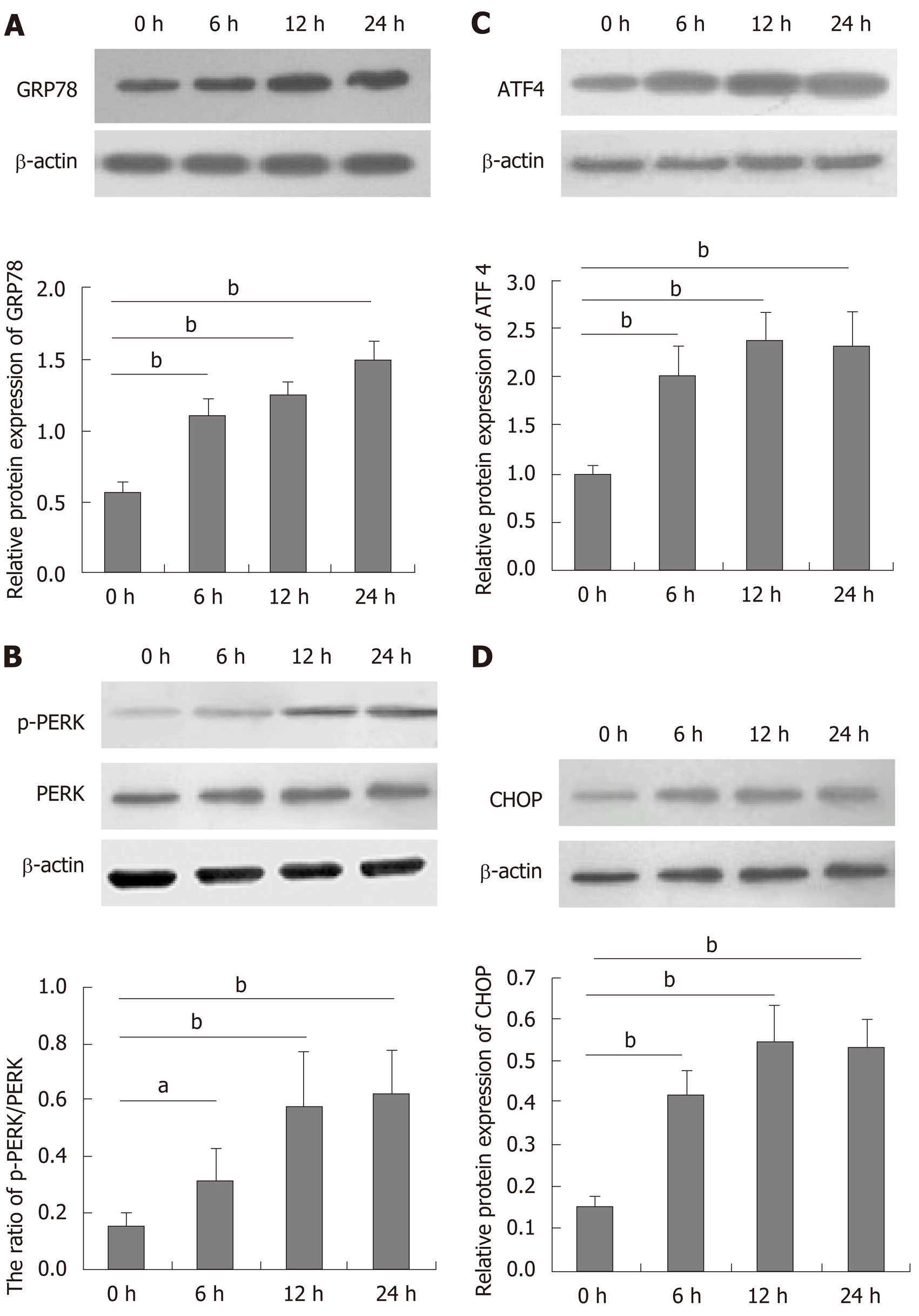
DTT can induce ER stress in many types of cells[27,28]. To understand the role of DTT in decreasing viability, BRL-3A cells were treated with 2.0 mmol/L DTT for varying time periods and their ER stress-related proteins were quantified by Western blot (Figure 2). DTT treatment for 6 h upregulated GRP78, ATF4, and CHOP expression and PERK phosphorylation by about 2-fold in BRL-3A cells and treatment with DTT for a longer period further increased its effects. The effects of DTT on upregulating GRP78 expression and PERK phosphorylation appeared to have a trend of time-dependence. Thus, DTT induced ER stress in BRL-3A cells in vitro.
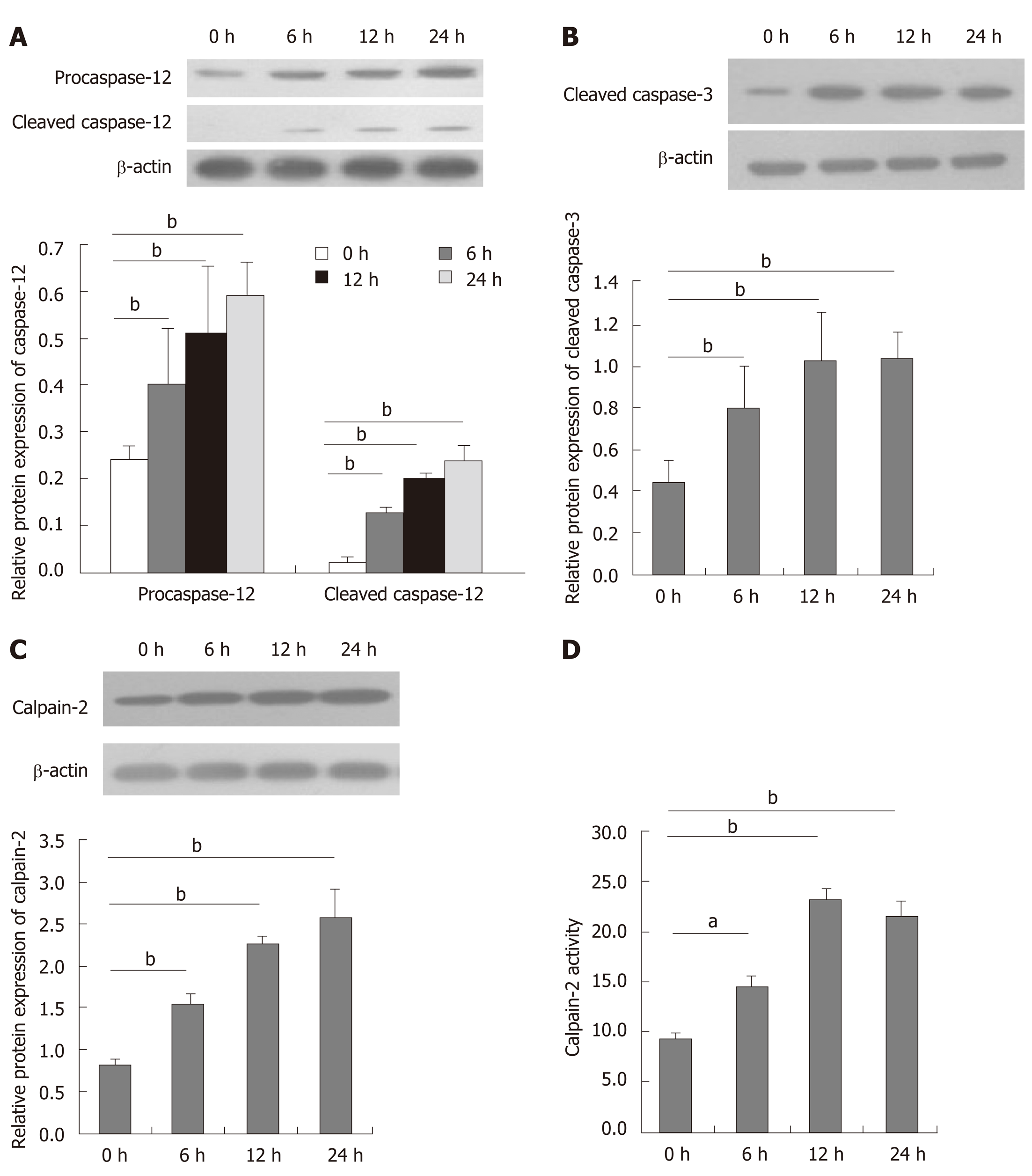
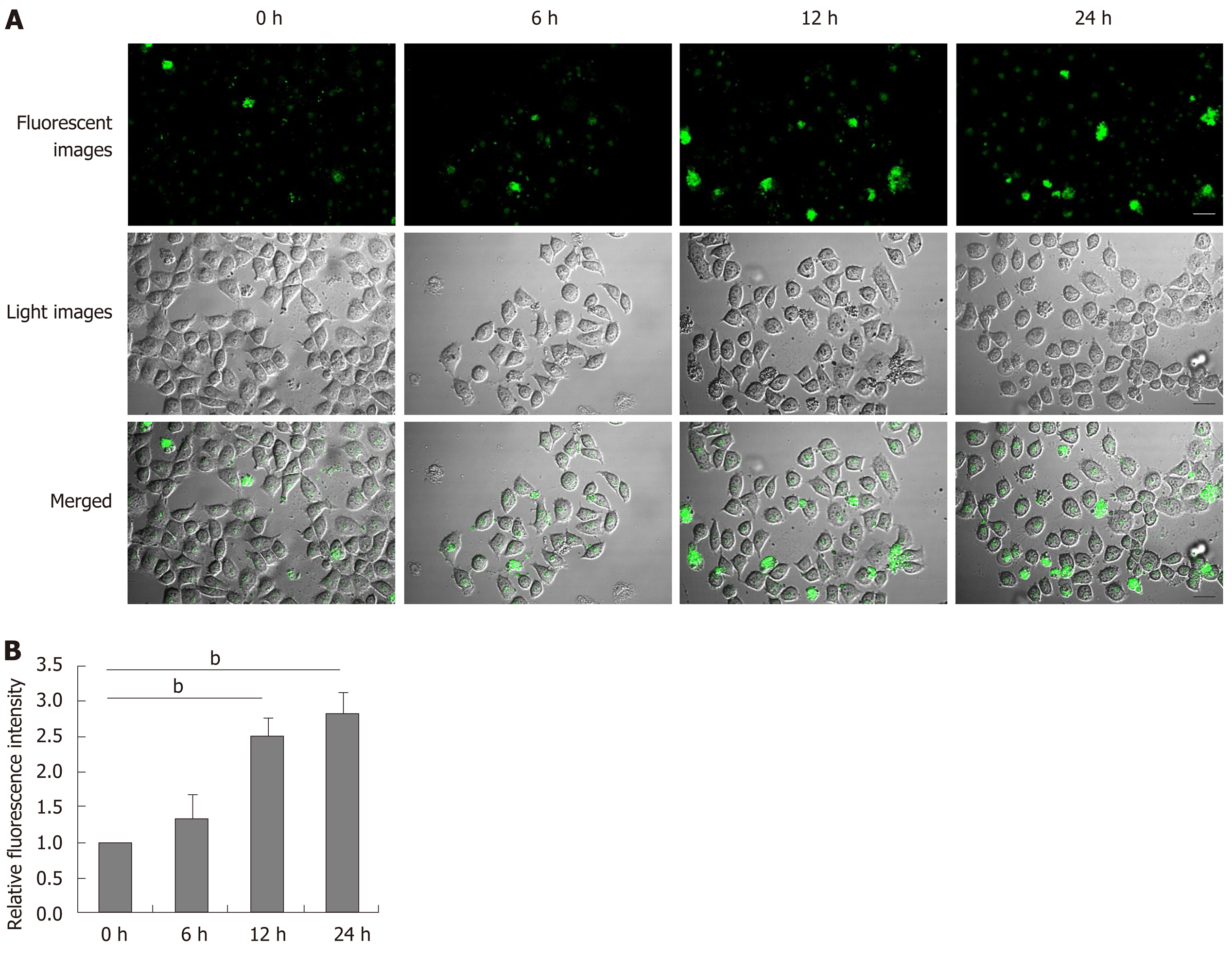
Aberrant ER stress can promote sensitive cell apoptosis by inducing caspase-12 and caspase-3 activation, and calpain-2 participates in the process of ER-stress-related apoptosis[18,19,25]. To understand the consequence of ER stress induced by DTT, the relative levels of caspase-3, and caspase-12 cleavage, and calpain-2 activity were quantified. Treatment with 2 mmol/L DTT significantly induced time-dependent caspase-12 cleavage and increased the levels of cleaved caspase-3 in BRL-3A cells by 2-2.5 fold (Figure 3A and B). Similarly, DTT treatment significantly upregulated calpain-2 expression by more than 2-fold and enhanced calpain-2 activity in a trend of time-dependence in BRL-3A cells (Figure 3C and D). Given that calpain-2 activity is Ca2+-dependent, we further quantified intracellular Ca2+ content in each group of cells by microscopy. After staining with Fluo-3 AM, we observed that DTT treatment time dependently increased the content of clustery Ca2+ by 2-3 fold in BRL-3A cells at 12 and 24 h post treatment (Figure 4). To identify additional evidence to demonstrate the importance of calpain-2 activity, we stained the different groups of cells with fluorescent anti-calpain-2 and ER-tracker Red, and observed the co-localization of red ER and green calpain-2 signals by fluorescent microscopy. As shown in Figure 5, there were obviously increased merged yellow signals in the DTT-treated cells, particularly at the later time point, while there was little merged signal in the cells without DTT treatment. Collectively, such data indicated that DTT-induced aberrant ER stress promoted the apoptosis of BRL-3A cells by activating caspase-12 and increasing calpain-2 activity.
Finally, we tested whether calpain-2 silencing could modulate the DTT-induced caspase-12 activation and apoptosis of BRL-3A cells. We found that transfection with calpain-2 specific siRNA, but not the control, significantly decreased calpain-2 expression by about 85%, demonstrating the efficacy of calpain-2 silencing (P < 0.01; Figure 6A). Calpain-2 silencing also significantly mitigated the DTT-upregulated calpain-2 expression near 80% (P < 0.01; Figure 6B). Although calpain-2 silencing did not alter the DTT-upregulated caspase-12 expression, it significantly reduced the caspase-12 cleavage near 63% in BRL-3A cells, relative to that in the control cells (P < 0.01; Figure 6C). More importantly, calpain-2 silencing significantly decreased the percentage of DTT-induced apoptosis of BRL-3A cells by 50% (P < 0.05; Figure 6D and E). Together, the significantly decreased caspase-12 activation and cell apoptosis indicated that calpain-2 activity was crucial for DTT-induced ER stress-related caspase-12 activation and apoptosis in BRL-3A cells.
Many factors can induce ER stress including chemical agents such as tunicamycin, thapsigargin, and DTT[27,29]. In this study, we used a sub-toxic 2.0 mmol/L DTT to induce ER stress in BRL-3A cells, based on a previous report[22]. It is well known that DTT can inhibit the formation of disulfide bonds and result in the accumulation of unfolded proteins in the ER, leading to ER stress.
Aberrant ER stress-induced apoptosis of hepatocytes participates in the pathogenesis of several types of liver diseases[30-32]. In this study, we found that DTT had strong cytotoxicity against rat hepatocyte BRL-3A cells and its cytotoxicity was dose-dependent. Furthermore, treatment with DTT induced ER stress in BRL-3A cells by significantly upregulating GRP78, ATF4, and CHOP expression and PERK phosphorylation. More importantly, DTT treatment significantly increased the frequency of apoptotic BRL-3A cells, supporting that aberrant ER stress promotes the apoptosis of hepatocytes[33,34]. Given that hepatocyte apoptosis participates in the pathogenesis of several types of liver diseases, inhibition of ER stress may be valuable for protecting hepatocytes from apoptosis.
ER stress-related apoptosis is independent of mitochondria and death receptors, and is thought to be mediated by activating caspase-12, an ER-anchored caspase[35,36]. A previous study has reported that the caspase-12 gene is mutated in most human hepatocyte lines[23], so we chose rat non-tumor hepatocyte BRL-3A cells as a cell model in this study. Similar to other caspases, procaspase-12 has to be activated by cleaving a short inhibitory peptide, and cleaved caspase-12 can activate the downstream effector caspase-3, leading to apoptosis[37]. Actually, caspase-12-/- cells are resistant to ER stress-induced apoptosis[38]. In this study, we found that DTT treatment significantly induced caspase-12 and caspase-3 cleavage in BRL-3A cells. Such data indicated that activated caspase-12 and caspase-3 contributed to the ER stress-related apoptosis of BRL-3A cells, although the precise mechanisms underlying caspase-12 activation in hepatocytes remain incompletely understood. A recent study revealed that enhanced calpain-2 activity is crucial for caspase-12 activation[39]. Calpain-2 is a Ca2+-dependent cysteine protease, and its activity and function depend on the concentrations of intracellular Ca2+[40]. As the ER holds the major pool of Ca2+, ER stress can promote Ca2+ efflux from the ER, which may be responsible for enhancing calpain-2 activity and its ER accumulation[41]. Interestingly, we found that DTT treatment significantly increased intracellular Ca2+ content and calpain-2 activity in BRL-3A cells. Furthermore, DTT treatment promoted the accumulation of calpain-2 in the ER of BRL-3A cells. More importantly, calpain-2 silencing not only significantly mitigated DTT-upregulated calpain-2 expression and caspase-12 activation, but also decreased the DTT-triggered apoptosis of BRL-3A cells. Hence, the DTT-induced ER stress increased intracellular Ca2+ content and calpain-2 expression, leading to calpain-2 activation, which cleaved caspase-12 to trigger apoptosis of BRL-3A cells. In our recent preliminary studies, we found that treatment with Z-LLY-fmk, a specific inhibitor of calpain, significantly mitigated the DTT-induced hepatocyte apoptosis by more than 90% (Chen et al[11], unpublished data). Such novel findings may provide new evidence to demonstrate that the Ca2+-dependent calpain-2 activity is crucial for promoting the ER stress-related apoptosis in hepatocytes.
In conclusion, our data indicated that DTT exhibited dose-dependent cytotoxicity against rat hepatocytes, and induced ER stress and apoptosis in BRL-3A cells. Evidently, DTT treatment significantly upregulated GRP78, ATF4, and CHOP expression and PERK phosphorylation; increased intracellular Ca2+ content and calpain-2 activity; and induced caspase-12 and caspase-3 activation in BRL-3A cells. Furthermore, calpain-2 silencing significantly mitigated DTT-upregulated calpain-2 expression and DTT-induced caspase-12 activation as well as apoptosis of BRL-3A cells. The results indicated that enhanced calpain-2 activity promoted aberrant ER stress-mediated apoptosis of hepatocytes. Our data suggest that ER stress may induce Ca2+ release from the ER and lead to the recruitment and activation of calpain-2 in the ER, where calpain-2 activates caspase-12 and caspase-3 and triggers apoptosis in hepatocytes. Therefore, ER stress may be a novel therapeutic target and our findings may provide new evidence to demonstrate the importance of Ca2+-dependent calpain-2 in caspase-12 activation and ER stress-related apoptosis in hepatocytes.
Endoplasmic reticulum (ER) stress-mediated hepatocyte apoptosis is associated with many liver diseases, however, the underlying mechanisms remain unknown. Calpain-2 is a Ca2+-dependent cysteine protease, which can cleave several apoptosis-related proteins and mediate the process of apoptosis. Our data indicate that calpain-2 is crucial for the aberrant ER stress-induced apoptosis of hepatocytes and may be a novel therapeutic target for liver diseases.
Calpain-2 protease is involved in multiple signaling pathways mediating apoptotic processes, including the mitochondrial pathway. However, the regulatory mechanisms by which calpain-2 regulates ER stress-mediated hepatocyte apoptosis remain unclear. Our study further assessed its potential as a therapeutic target for inhibiting hepatocyte apoptosis.
In this study, the effect of calpain-2 on ER stress-mediated hepatocyte apoptosis and the underlying regulatory mechanisms were investigated. Our data indicates that ER stress may be a novel therapeutic target, and our findings provide new evidence to demonstrate the importance of Ca2+-dependent calpain-2 in caspase-12 activation and ER stress-related apoptosis in hepatocytes.
Our study employed Western blotting, flow cytometry, confocal microscopy, and calpain-2 small interfering RNA to elucidate the mechanism of calpain-2-mediated activation of caspase-12, a key molecule in ER stress-related apoptosis. These research methods are relatively mature technically, thus ensuring the reliability of the results from this study.
The ER stress inducer dithiothreitol can significantly increase intracellular Ca2+content, calpain-2 expression and activity in hepatocytes and promote caspase-12 cleavage and apoptosis in hepatocytes. Moreover, calpain-2 silencing can mitigate dithiothreitol-enhanced calpain-2 expression, caspase-12 cleavage, and apoptosis in hepatocytes. These results indicated that enhanced calpain-2 activity promoted aberrant ER stress-mediated apoptosis of hepatocytes. In future studies, we will further investigate whether calpain-2 can mediate hepatocyte death through other mechanisms, such as regulating autophagy.
Ca2+-dependent calpain-2 activity promoted the aberrant ER stress-related apoptosis of rat hepatocytes by activating caspase-12 in the ER. ER stress may be a novel therapeutic target and our findings may provide new evidence to demonstrate the importance of Ca2+-dependent calpain-2 in caspase-12 activation and ER stress-related apoptosis in hepatocytes. ER stress may induce Ca2+ release from the ER and lead to the recruitment and activation of calpain-2 in the ER, where, calpain-2 activates caspase-12 and caspase-3, and triggers apoptosis in hepatocytes. Calpain-2 activity plays an important role in aberrant ER stress-related apoptosis in hepatocytes. Inhibition of calpain-2 expression and activity is expected to be an effective way to alleviate hepatocyte apoptosis and liver injury.
Because calpain-2 is a protease, in addition to focusing on its expression, studies should also focus on its activity. In our recent preliminary studies, we found that treatment with Z-LLY-fmk, a specific inhibitor of calpain activity, significantly mitigated DTT-induced hepatocyte apoptosis by more than 90%.
We thank Hua Pei and Jinxingyi Wang for their technical advice in using flow cytometry and microscope in the Basic Medical Science Research Center of Guizhou Medical University. We would like to thank Dr. Tengxiang Chen for his comments on this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aureliano M, Abdulnour-Nakhoul SM, El-Bendary M, Marickar F S-Editor: Zhang L L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Kanda T, Matsuoka S, Yamazaki M, Shibata T, Nirei K, Takahashi H, Kaneko T, Fujisawa M, Higuchi T, Nakamura H, Matsumoto N, Yamagami H, Ogawa M, Imazu H, Kuroda K, Moriyama M. Apoptosis and non-alcoholic fatty liver diseases. World J Gastroenterol. 2018;24:2661-2672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 229] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (2)] |
| 2. | Akazawa Y, Nakao K. To die or not to die: death signaling in nonalcoholic fatty liver disease. J Gastroenterol. 2018;53:893-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Iorga A, Dara L, Kaplowitz N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 4. | Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765-783.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 575] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 5. | Mehal W, Imaeda A. Cell death and fibrogenesis. Semin Liver Dis. 2010;30:226-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Tagawa R, Kawano Y, Minami A, Nishiumi S, Yano Y, Yoshida M, Kodama Y. β-hydroxybutyrate protects hepatocytes against endoplasmic reticulum stress in a sirtuin 1-independent manner. Arch Biochem Biophys. 2019;663:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Wang H, Chen L, Zhang X, Xu L, Xin B, Shi H, Duan Z, Zhang H, Ren F. Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed Pharmacother. 2019;111:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Mahfoudh-Boussaid A, Zaouali MA, Hauet T, Hadj-Ayed K, Miled AH, Ghoul-Mazgar S, Saidane-Mosbahi D, Rosello-Catafau J, Ben Abdennebi H. Attenuation of endoplasmic reticulum stress and mitochondrial injury in kidney with ischemic postconditioning application and trimetazidine treatment. J Biomed Sci. 2012;19:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002;21:2343-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Song S, Tan J, Miao Y, Li M, Zhang Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J Cell Physiol. 2017;232:2977-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 399] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 11. | Chen Y, Gui D, Chen J, He D, Luo Y, Wang N. Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside IV is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell Physiol Biochem. 2014;33:1975-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | B'chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41:7683-7699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 642] [Cited by in RCA: 875] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 13. | Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2547] [Cited by in RCA: 2610] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 14. | Zhang Q, Liu J, Chen S, Liu J, Liu L, Liu G, Wang F, Jiang W, Zhang C, Wang S, Yuan X. Caspase-12 is involved in stretch-induced apoptosis mediated endoplasmic reticulum stress. Apoptosis. 2016;21:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Zou X, Qu Z, Fang Y, Shi X, Ji Y. Endoplasmic reticulum stress mediates sulforaphane-induced apoptosis of HepG2 human hepatocellular carcinoma cells. Mol Med Rep. 2017;15:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Qiu K, Su Y, Block ER. Use of recombinant calpain-2 siRNA adenovirus to assess calpain-2 modulation of lung endothelial cell migration and proliferation. Mol Cell Biochem. 2006;292:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Martinez JA, Zhang Z, Svetlov SI, Hayes RL, Wang KK, Larner SF. Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis. 2010;15:1480-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Bajaj G, Sharma RK. TNF-alpha-mediated cardiomyocyte apoptosis involves caspase-12 and calpain. Biochem Biophys Res Commun. 2006;345:1558-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Cheng C, Dong W. Aloe-Emodin Induces Endoplasmic Reticulum Stress-Dependent Apoptosis in Colorectal Cancer Cells. Med Sci Monit. 2018;24:6331-6339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Xie RJ, Han B, Yang T, Yang Q. Activation of Caspase-12, a key molecule in endoplasmic reticulum stress related apoptosis pathway, induces apoptosis of hepatocytes in rats with hepatic fibrosis. World Chinese Journal of Digestology. 2016;24:2470-2477. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Xie RJ, Han B, Yang T, Wen JJ, Yang Q. Expression of calpain-2 and Bax in rat fibrotic liver tissues. Chinese Journal of Pathophysiology. 2013;29:1603-1608. [DOI] [Full Text] |
| 22. | Smith MD, Harley ME, Kemp AJ, Wills J, Lee M, Arends M, von Kriegsheim A, Behrends C, Wilkinson S. CCPG1 Is a Non-canonical Autophagy Cargo Receptor Essential for ER-Phagy and Pancreatic ER Proteostasis. Dev Cell. 2018;44:217-232.e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 23. | Zuo S, Kong D, Wang C, Liu J, Wang Y, Wan Q, Yan S, Zhang J, Tang J, Zhang Q, Lyu L, Li X, Shan Z, Qian L, Shen Y, Yu Y. CRTH2 promotes endoplasmic reticulum stress-induced cardiomyocyte apoptosis through m-calpain. EMBO Mol Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Luo X, Han B, Tian T, Yu L, Zheng L, Tang L, Yang T, Yang Q, Xin RJ, Huang JZ. Dithiothreitol induces of apoptosis of BRL-3A cells by activation of calpain-2/caspase-12 signaling pathway. Zhongguo Bingli Shengli Zazhi. 2018;34:1820-1826. [DOI] [Full Text] |
| 25. | Tan Y, Dourdin N, Wu C, De Veyra T, Elce JS, Greer PA. Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J Biol Chem. 2006;281:16016-16024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Phaniraj S, Gao Z, Rane D, Peterson BR. Hydrophobic resorufamine derivatives: potent and selective red fluorescent probes of the endoplasmic reticulum of mammalian cells. Dyes Pigm. 2016;135:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Xiang XY, Yang XC, Su J, Kang JS, Wu Y, Xue YN, Dong YT, Sun LK. Inhibition of autophagic flux by ROS promotes apoptosis during DTT-induced ER/oxidative stress in HeLa cells. Oncol Rep. 2016;35:3471-3479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Ren B, Wang Y, Wang H, Wu Y, Li J, Tian J. Comparative proteomics reveals the neurotoxicity mechanism of ER stressors tunicamycin and dithiothreitol. Neurotoxicology. 2018;68:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Deegan S, Saveljeva S, Logue SE, Pakos-Zebrucka K, Gupta S, Vandenabeele P, Bertrand MJ, Samali A. Deficiency in the mitochondrial apoptotic pathway reveals the toxic potential of autophagy under ER stress conditions. Autophagy. 2014;10:1921-1936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | He C, Qiu Y, Han P, Chen Y, Zhang L, Yuan Q, Zhang T, Cheng T, Yuan L, Huang C, Zhang S, Yin Z, Peng XE, Liang D, Lin X, Lin Y, Lin Z, Xia N. ER stress regulating protein phosphatase 2A-B56γ, targeted by hepatitis B virus X protein, induces cell cycle arrest and apoptosis of hepatocytes. Cell Death Dis. 2018;9:762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Wang N, Tan HY, Li S, Feng Y. Atg9b Deficiency Suppresses Autophagy and Potentiates Endoplasmic Reticulum Stress-Associated Hepatocyte Apoptosis in Hepatocarcinogenesis. Theranostics. 2017;7:2325-2338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Takahara I, Akazawa Y, Tabuchi M, Matsuda K, Miyaaki H, Kido Y, Kanda Y, Taura N, Ohnita K, Takeshima F, Sakai Y, Eguchi S, Nakashima M, Nakao K. Toyocamycin attenuates free fatty acid-induced hepatic steatosis and apoptosis in cultured hepatocytes and ameliorates nonalcoholic fatty liver disease in mice. PLoS One. 2017;12:e0170591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Miao L, Zhang H, Wu G, Zhang Z, Lv J. Chlorogenic acid against palmitic acid in endoplasmic reticulum stress-mediated apoptosis resulting in protective effect of primary rat hepatocytes. Lipids Health Dis. 2018;17:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Yang FW, Fu Y, Li Y, He YH, Mu MY, Liu QC, Long J, Lin SD. Prostaglandin E1 protects hepatocytes against endoplasmic reticulum stress-induced apoptosis via protein kinase A-dependent induction of glucose-regulated protein 78 expression. World J Gastroenterol. 2017;23:7253-7264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Sanges D, Marigo V. Cross-talk between two apoptotic pathways activated by endoplasmic reticulum stress: differential contribution of caspase-12 and AIF. Apoptosis. 2006;11:1629-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 378] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 37. | Yan Z, He JL, Guo L, Zhang HJ, Zhang SL, Zhang J, Wen YJ, Cao CZ, Wang J, Wang J, Zhang MS, Liang F. Activation of caspase-12 at early stage contributes to cardiomyocyte apoptosis in trauma-induced secondary cardiac injury. Sheng Li Xue Bao. 2017;69:367-377. [PubMed] |
| 38. | Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287-34294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 702] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 39. | Chan SL, Culmsee C, Haughey N, Klapper W, Mattson MP. Presenilin-1 mutations sensitize neurons to DNA damage-induced death by a mechanism involving perturbed calcium homeostasis and activation of calpains and caspase-12. Neurobiol Dis. 2002;11:2-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Chen B, Tang J, Guo YS, Li Y, Chen ZN, Jiang JL. Calpains are required for invasive and metastatic potentials of human HCC cells. Cell Biol Int. 2013;37:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Cui W, Ma J, Wang X, Yang W, Zhang J, Ji Q. Free fatty acid induces endoplasmic reticulum stress and apoptosis of β-cells by Ca2+/calpain-2 pathways. PLoS One. 2013;8:e59921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |