Published online Apr 7, 2020. doi: 10.3748/wjg.v26.i13.1382
Peer-review started: December 10, 2019
First decision: February 14, 2020
Revised: February 17, 2020
Accepted: March 9, 2020
Article in press: March 9, 2020
Published online: April 7, 2020
Processing time: 118 Days and 0.6 Hours
Gastric cancer remains one of the most lethal cancers. The incidence and mortality rates are quite similar. The main reason for the high mortality is diagnosis at advanced stages of disease, when treatment options are poor. One of the supposed strategies to overcome late-stage diagnosis is identifying people at high risk with the aim of establishing rigorous clinical control, including routine endoscopy and biopsies. Hereditary gastric cancer (HGC) syndromes, though representing a sizeable group to monitor for prevention or, at least, for early diagnosis, are apparently extremely rare. The low rate of HGC diagnosis might be related to the low rates of suspicion, insufficient familiarity about clinical diagnosis criteria, and the supposed conditional necessity of a molecular diagnosis. In this review, we will discuss simple measures to increase HGC diagnosis by applying three rules that might provide an opportunity for precision care to benefit the families affected by this disease.
Core tip: Although familial gastric cancer cases represent a potential source for the discovery of early-stage gastric cancer (and others) among patients’ relatives, this possibility has been little explored due to the low rate of suspicion of hereditary gastric cancer syndromes, which could be improved by applying simple rules for hereditary gastric cancer screening that are accessible for unskilled healthcare professionals.
- Citation: Assumpção P, Araújo T, Khayat A, Ishak G, Santos S, Barra W, Acioli JF, Rossi B, Assumpção P. Hereditary gastric cancer: Three rules to reduce missed diagnoses. World J Gastroenterol 2020; 26(13): 1382-1393
- URL: https://www.wjgnet.com/1007-9327/full/v26/i13/1382.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i13.1382
The main recognized causes of gastric adenocarcinoma are environmental, hereditary, and replicative DNA errors[1-4]. Measures to avoid environmental exposition are widely discussed, mainly Helicobacter pylori (H. pylori) prevention and treatment and changes in alimentary habits[5]. For replicative causes, there are no currently available alternatives for risk reduction[6,7].
Hereditary cancers, although representing a potential group for prevention and early diagnosis, seem to be disregarded, or even neglected, since their incidence is very uncommon, causing preventive measures to have low impact on gastric cancer burden and mortality.
The incidence of hereditary gastric cancer (HGC) is approximately 1%, and a degree of genetic influence seems to be present in over 10% of sporadic gastric cancer (GC) cases[8].
The discrepancy in HGC incidence might be explained by two different concepts: Molecular diagnosis; and diagnosis based on meeting clinical statements for hereditary cancers without a molecular diagnosis; patients diagnosed via the former also represent HGC cases[9,10].
Since molecular diagnosis is tricky and not widely available, a focus on the clinical criteria for hereditary gastric cancer cases should increase the number of recognized cases and allow an intensive search for early diagnosis, as well as select a larger number of cases for practicing molecular investigations in reference centers.
There are three robustly identified HGC syndromes: Hereditary diffuse gastric cancer (HDGC); gastric adenocarcinoma and proximal polyposis of stomach (GAPPS); and familial intestinal gastric cancer (FIGC). Additionally, GC may arise from other hereditary cancer syndromes, such as Lynch syndrome, Li-Fraumeni syndrome, Peutz-Jeghers syndrome, and ovarian and breast syndromes, among others[10-12].
The clinical criteria for each of these syndromes are not widely known, and as a consequence, the low rate of suspicion results in rare investigations and diagnoses of such syndromes.
Estimating the number of missed clinically diagnosable cases of HGC is extremely difficult. Nevertheless, missed diagnoses might represent a significant number of families that could benefit from such active searching and subsequent adoption of measures to allow for early diagnosis and efficient treatments.
Available initiatives to avoid sporadic GC are usually hard to implement since they include convincing the population to change alimentary habits, improving sanitary conditions in developing nations, and using large-scale antibiotics to treat H. pylori[6,7].
For HGC diagnosis, simple measures should be attempted as a first step to increase health professional suspicion and favor clinical diagnosis.
Three simple rules, accessible to every health-care professional, could improve the clinical diagnosis of HGC and allow referencing of such cases to specialized centers, either for patients to receive support at local units or, eventually, for patients to be transferred to those centers to benefit from complex measures.
Observation of the three rules might greatly increase the number of diagnosed cases of HGC, providing an opportunity for precision care to benefit these families.
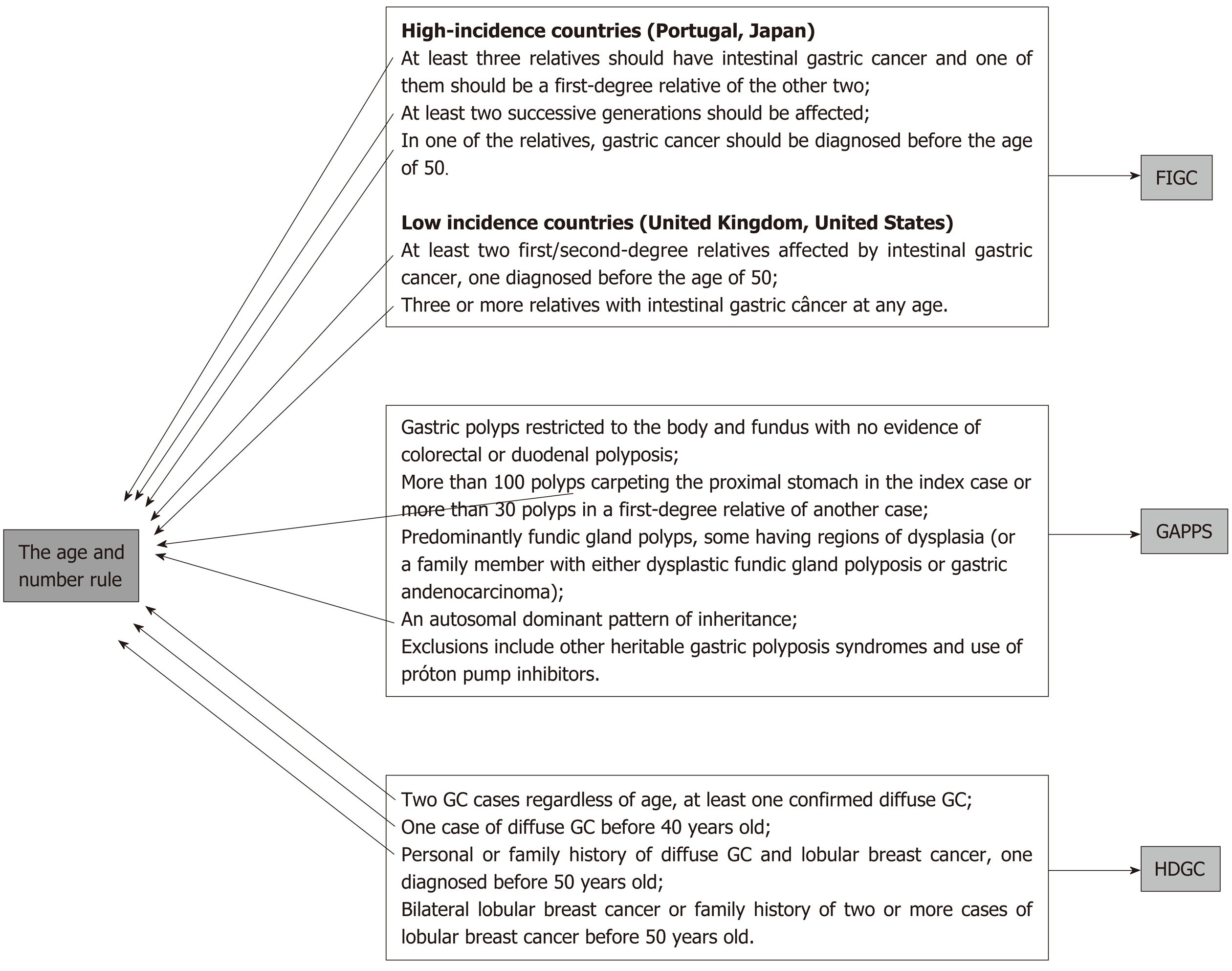
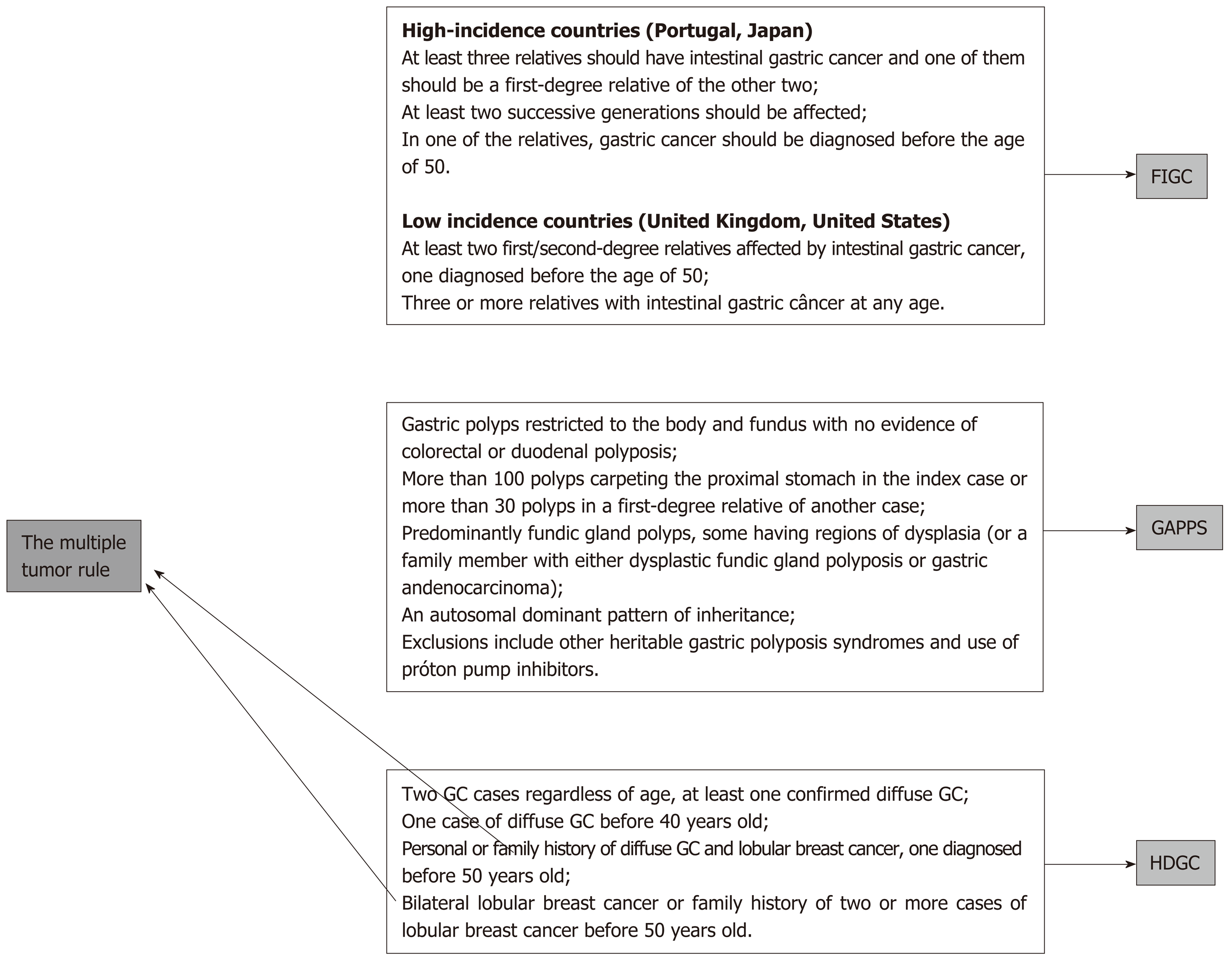
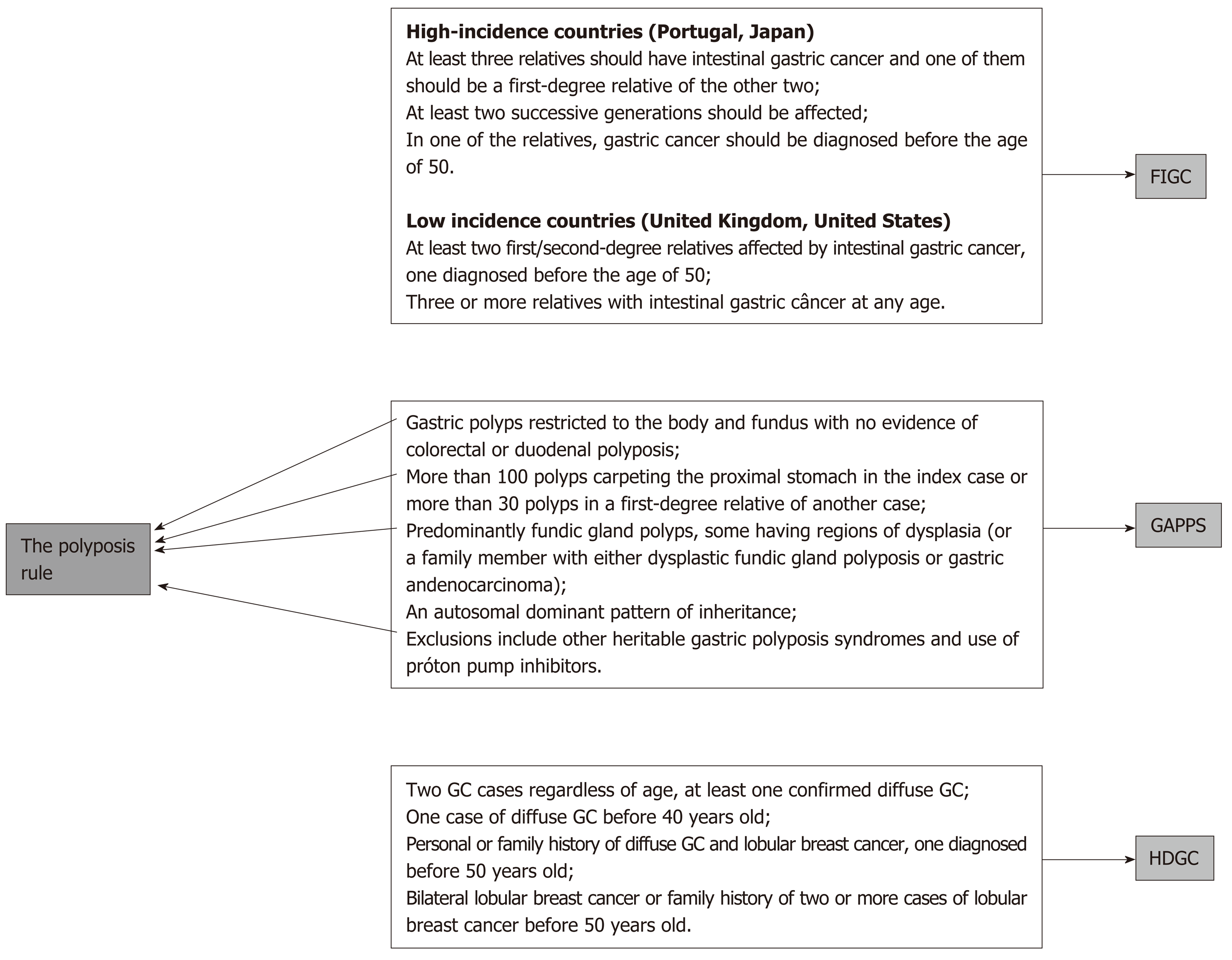
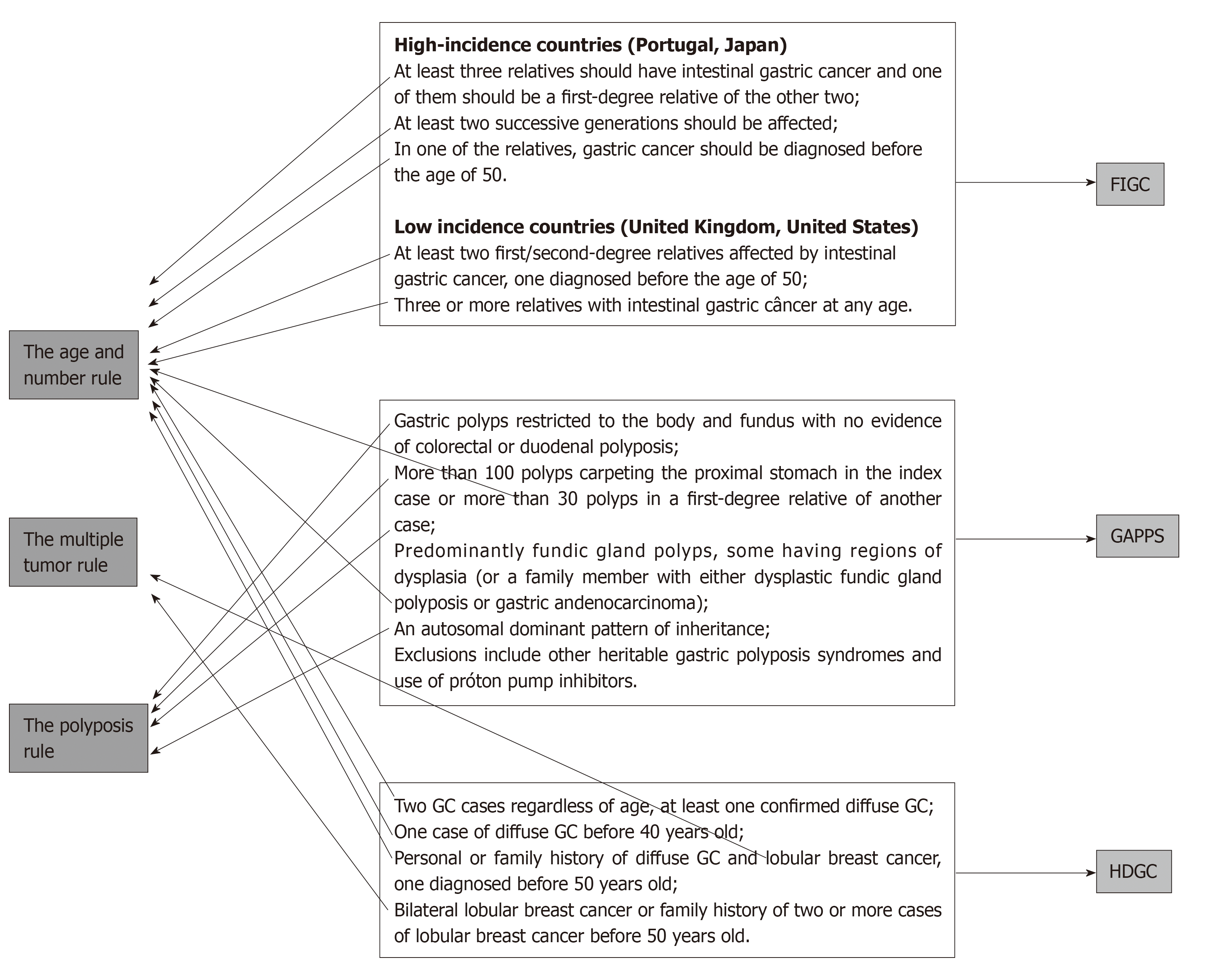
The suggested rules are the age and number rule, the multiple tumor rule, and the polyposis rule.
This is the number one rule and allows capture of the great majority of HGC cases (probably more than 95% of cases). Since age of occurrence and number of affected relatives are part of the clinical criteria for almost every hereditary GC syndrome, a simple check of the patient’s age and the occurrence of other cases among first- or second-degree relatives is sufficient to screen patients for a possible diagnosis of hereditary cancer[10,13,14] (Figure 1).
Patient age under 50, or the occurrence of at least one case of GC in relatives are enough to require further screening for hereditary GC syndromes and to implement protocols for family investigation and surveillance[15].
These can be done primarily at the site of the first consultation, but specialized remote support can be beneficial either for conducting the necessary investigation and procedures using local resources or for referring the case to a dedicated unit for ideal management.
The occurrence of additional cancers, both multiple GCs or cancers at other sites, such as breast, colon and others, might represent a potential for hereditary cancers[10,11] (Figure 2).
As discussed above, local and reference center investigations and management, according to available resources, are formally recommended.
Although it is very rare, GC-associated polyposis is another sign of hereditary cancers even if they are not caught by the two previous rules. Both HGC syndromes and other hereditary cancer syndromes may cause GC and stomach polyposis[10,11,13] (Figure 3).
Again, according to the availability of resources, those cases might be managed locally or referred to another center.
There are several criteria defining both HGC syndromes and other hereditary syndromes that might imply GC occurrence.
Considering only the three well-established HGC syndromes, at least 16 rules are currently published. Additionally, many of these rules are frequently updated, leading to a very complex net of possibilities to diagnose an HGC that, even for a specialist, let alone a generalist health worker, are very hard to remember[8,10,11,14].
An attempt to simplify these many criteria resulted in a three-rule method for the triage of patients for possible HGC formal diagnosis. None of the official criteria for diagnosis were questioned or modified. The aim of this simple method is to help clinicians and other health-care members remain alert for these possible diagnoses.
To demonstrate the simplicity and potential benefits of increasing HGC identification by applying the three rules strategy, some practical possibilities will be discussed.
All the criteria defined in recent meetings and conferences[8,13] are covered by the three rules, which can be used by any health-care professional, including nonspecialized ones, contributing to the avoidance of missed diagnoses (Figure 4).
Just by applying this rule, a great number of cases can be selected for a possible diagnosis of HGC. The following examples present the minimal requirements that can favor the discovery of new HGC cases and imply important investigational and therapeutic procedures that might change the outlooks for patients and their relatives.
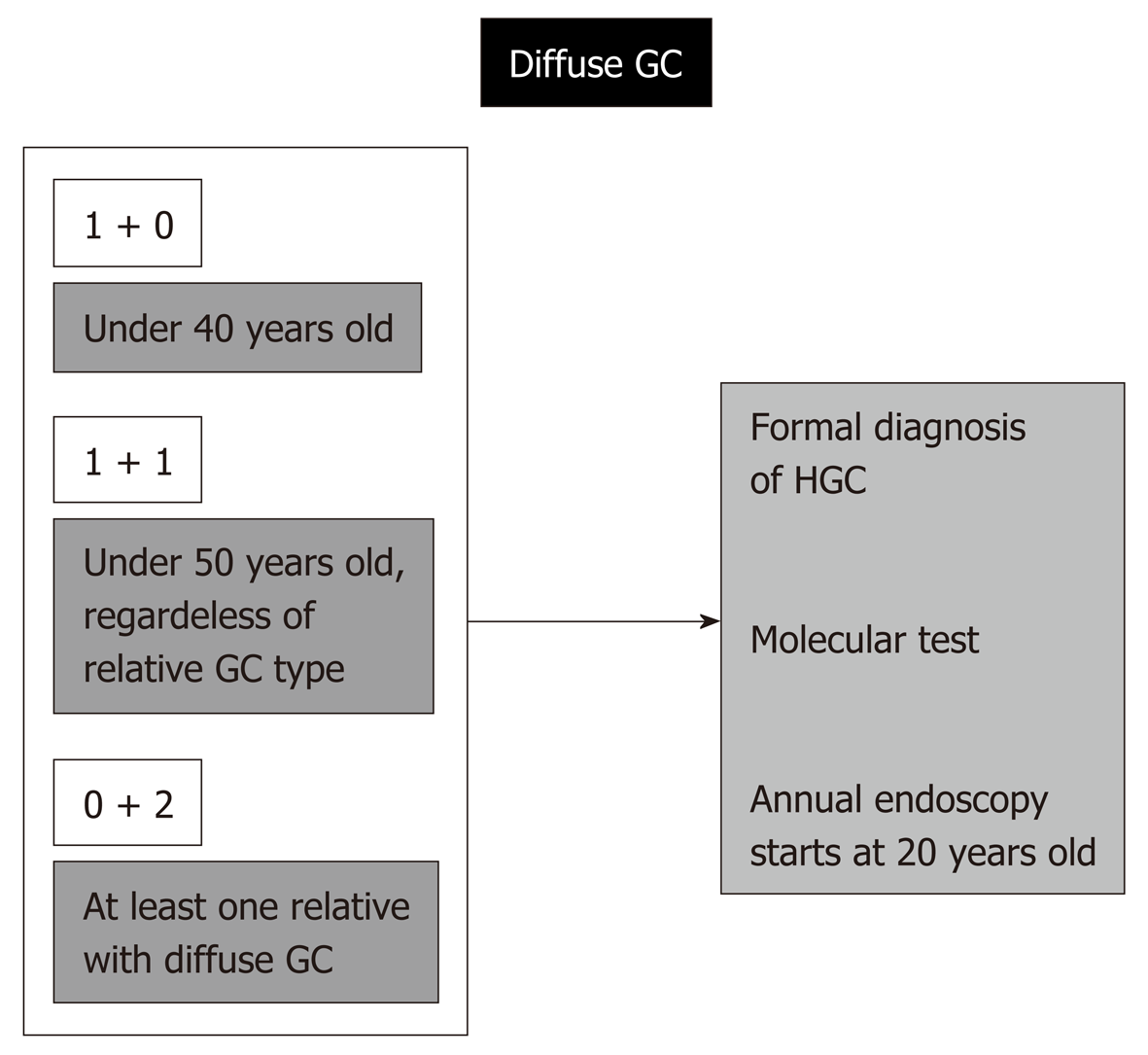
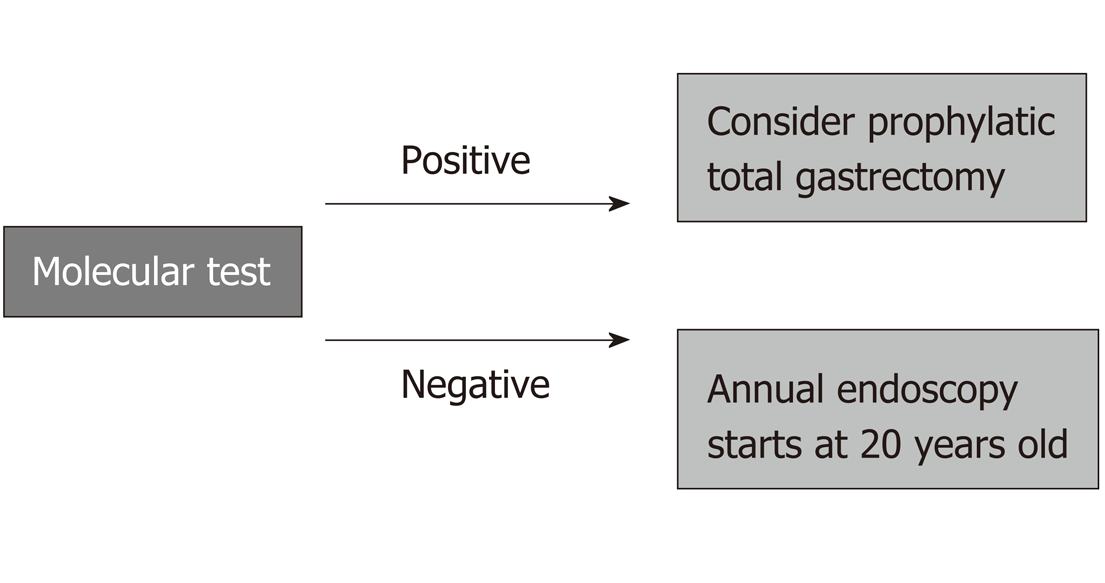
A case of diffuse-type GC in a patient under 50 years old and a case of GC among first- or second-degree relatives, regardless of the type of adenocarcinoma, are enough to establish a formal diagnosis of HGC, and this is very important, since it implies the potential for a molecular test (CDH1 mutation).
If positive, prophylactic total gastrectomy for the affected relatives might be necessary. Even with a negative genetic test, every first- and second-degree relative will need a dedicated endoscopy every year beginning at 20 years old. The standard number of first- and second-degree relatives older than 19 is over 20 individuals, and these are the people at high risk; in these individuals, rigorous surveillance may result in avoidance of late GC diagnosis and might change outcomes.
This not so alarming situation indeed also represents a formal diagnosis of HGC in the case of patients under 40 years old with diffuse GC. The same procedures described above are needed, including molecular investigation, possibility of prophylactic total gastrectomy, and engagement of the relatives in a rigorous program of follow-up beginning at 20 years old.
Regardless of the age of occurrence, if a patient has diffuse GC, independently of the relative gastric adenocarcinoma type, a formal diagnosis of HGC is again reached, with the same needs and consequences described above.
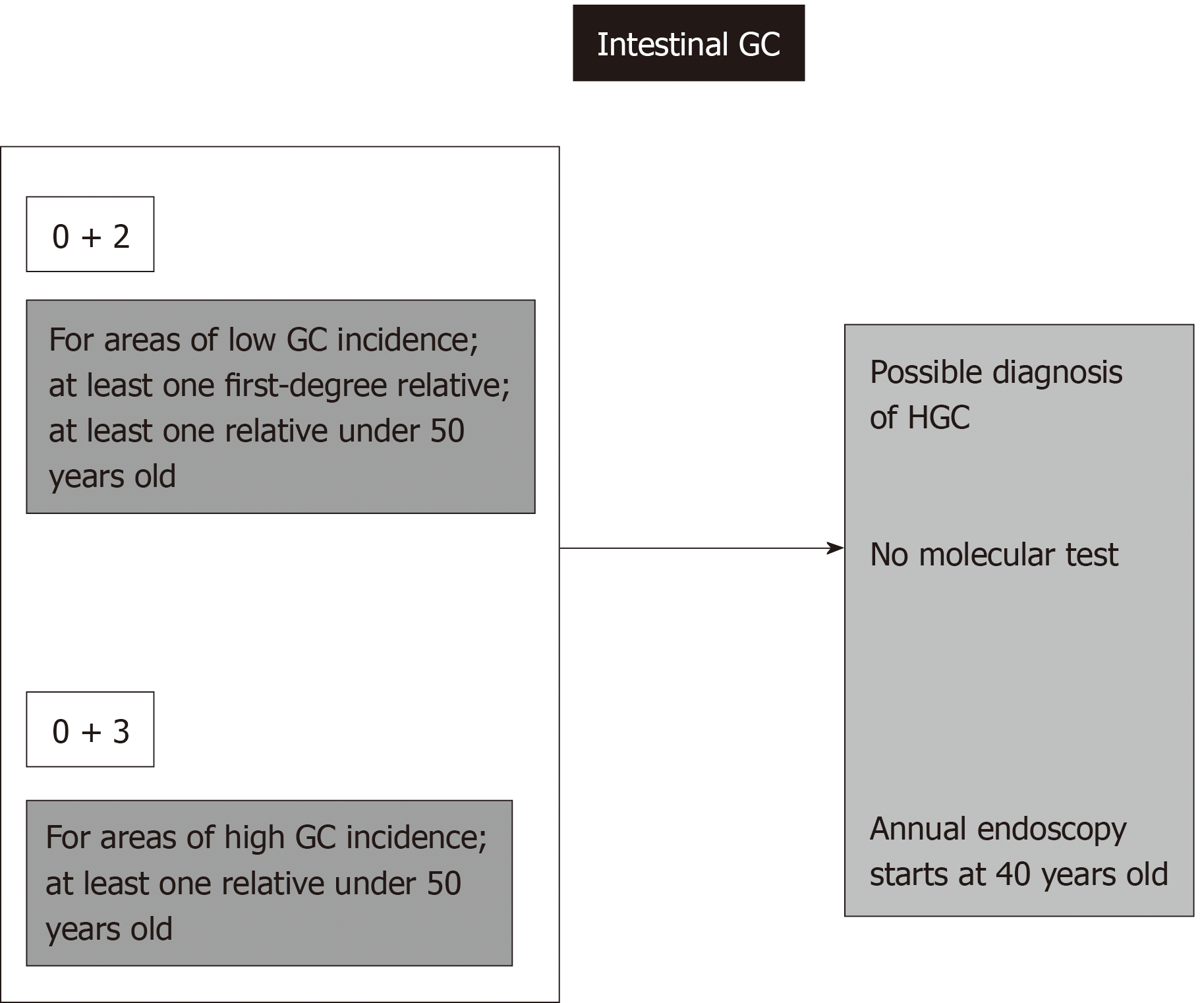
This situation also results in a formal diagnosis of HGC in cases of the intestinal type according to the familial intestinal gastric cancer syndrome criteria for low-risk areas. For this syndrome, there is no molecular test, and the recommendation includes annual endoscopies for first- and second-degree relatives beginning at 40 years old[9,11,16].
Regardless of age or histological type of gastric adenocarcinoma, three or more cases of GC among first- or second-degree relatives always signify an HGC syndrome diagnosis, even in high-incidence areas. If every case is of the intestinal type, FIGC syndrome guidelines require annual endoscopies beginning at 40 years old for first- and second-degree relatives[9,11].
When at least one diffuse GC case is present, a molecular test is necessary, and according to this result, total prophylactic gastrectomy or dedicated annual endoscopies starting at 20 years old will be necessary[9,11,16].
Figure 5 presents some of the possibilities for identifying hereditary diffuse tumors via the application of rule number 1.
The management of hereditary diffuse gastric cancer syndrome is presented in Figure 6. With a clinical diagnosis of HDGC, there is a formal indication for genetic investigation by sequencing of the CDH1 gene. If a functional mutation is discovered, every first- and second-degree relative will be investigated for that specific mutation by a much simpler and cheaper test, with no need for complete CDH1 gene sequencing[11,17].
Everyone who carries the functional mutation needs to be evaluated by a multiprofessional team including a cancer geneticist and accordingly may undergo a prophylactic total gastrectomy. In the case of a negative genetic test, or if the carrier of the mutation does not agree to undergo total gastrectomy, annual dedicated endoscopies are indicated, including multiple insufflation and deflation schemes and both random and directed biopsies carried out by a senior endoscopist[16,17].
Regarding intestinal-type gastric cancers, rule number 1 will not result in a genetic test, but the patient’s relatives identified by this rule will be included in a formal program of annual endoscopies beginning at 40 years old (Figure 7)[9,11,16].
Although extremely rare, the association of gastric polyposis and GC is a sign of HGC. GAPPS is very rare, with few affected families described. The main difference from other polyposis syndromes is the exclusive gastric involvement and the restricted localization of the polyps to the corpus and fundus. This syndrome is attributed to a mutation in the promoter region of the APC gene, the gene classically related to familial adenomatosis polyposis, which is characterized by colon polyposis and also carries a risk for associated GC[18-20].
In addition to FAP, juvenile polyposis and Peutz-Jeghers syndrome can also cause stomach polyposis and GC[11,21].
In the case of GC and polyposis, a specialized investigation is necessary, and the management will depend on the type of hereditary syndrome, potentially including investigational ant therapeutic procedures in other organs, such as the colon and rectum[11,21].
The presence of multiple tumors, both those restricted to the stomach and other primary tumors affecting distant sites, should alert suspicion for HGC. Although second primary cancers can occur regardless of a hereditary cause, this rule should always be applied to allow rare hereditary cancer discovery[11,22].
A patient with two or more synchronic GC tumors may be affected by HGC. Multiple diffuse GCs are frequently found in diffuse hereditary gastric cancer syndrome. Even if not caught by rule number 1, a patient with diffuse tumors of signet ring cells, although not fulfilling the formal clinical criteria for this syndrome, must undergo a CDH1 molecular investigation[11,22,23]. The recommendations for management according to the molecular diagnosis must be followed as described above.
Gastric stump cancers following gastrectomy due to GC are usually related to environmental causes, such as Epstein-Barr virus infection and alkaline reflux[24,25]. Additionally, the cancer field effect plays a role in this situation, since previous exposure to carcinogenic insults might have caused previous driver mutations in the remaining mucosa, which favor posterior development of a second primary tumor. Nevertheless, diffuse histology enhances suspiciousness for HGC[26-28].
The association of diffuse gastric cancer and lobular breast cancer is enough to secure an HDGC clinical diagnosis and requires molecular CDH1 investigation and additional investigational and therapeutic measures, as discussed. In addition to the already cited procedures, possible prophylactic bilateral mastectomy, according to genetic counsel, and mandatory annual breast MRIs are also recommended[17,27,29].
Typical breast cancer syndromes, in addition to bringing a high risk for breast and ovarian cancer, also carry GC risk, and those associations require specialized management[17,30].
Many studies have suggested that HDGC may be associated with colorectal carcinoma (CRC) because this type of cancer has been observed in some HDGC families[31-36]. However, despite studies demonstrating the correlation between CRC and the CDH1 mutation, there is insufficient evidence to propose that the risk of CRC in CDH1 mutation carriers is significantly elevated, and there are no recommendations in current clinical practice for CRC screening in CDH1 mutation carriers[8,37].
Although patients with rare tumors do not frequently harbor concomitant HGC, this suspicion should be maintained. Rare tumors usually result from hereditary cancer syndromes; thus, specialized care might be required.
Application of rule number 1 may bring additional benefits, favoring the early diagnosis of nonhereditary GCs.
Regarding environmentally caused GC, the environmental cancer-related factors that usually cause sporadic GC are also present in some of the patients’ relatives, such as H. pylori and alimentary habits[2,6,7]. Since the majority of people exposed to such carcinogens will never develop GC, the affected minority might have a peculiar genetic background, such as specific gene polymorphisms. These polymorphisms are not enough to cause cancer, but in cases of exposure to environmental carcinogens, they favor cancer development[38,39]. The genetic background necessary for the occurrence of sporadic environmental GC includes germline traits shared by most of the patient’s relatives. Therefore, investigating the relatives of GC patients caught by rule number 1, even if they do not reach a formal diagnosis of HGC, might result in finding additional sporadic cases or in subsequent fulfillment of the criteria for HGC.
The replicative cause of GC is due to random errors during the replication of stem cell DNA. Since these replicative errors might be more common in cases with an accumulation of DNA polymorphisms in replicative genes, as well as in DNA repair genes, these DNA polymorphisms might result in a major chance for “random” DNA replication errors to affect driver mutations in stem cells[40].
Accordingly, relatives sharing these genetic backgrounds might be identified by rule number 1.
To provide wide access to the benefits of the three rules, a mobile app is under development and will be available shortly free of charge.
Although there is potential for discovering GC at early stages among relatives of patients affected by HGC, this possibility is mostly underexplored due to the low suspicion of HGC syndromes. A significant increase in HGC diagnosis may be achieved by applying simple rules for HGC diagnosis triage that are accessible to nonspecialized health-care professionals both in nations with high and low incidence of gastric cancer.
We acknowledge Universidade Federal do Pará (PROPESP and Fadesp) for technical support and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for fellowship support.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eleftheriadis N, Tanabe S S-Editor: Gong ZM L-Editor: A E-Editor: Zhang YL
| 1. | Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355:1330-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 672] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 2. | Yusefi AR, Bagheri Lankarani K, Bastani P, Radinmanesh M, Kavosi Z. Risk Factors for Gastric Cancer: A Systematic Review. Asian Pac J Cancer Prev. 2018;19:591-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 161] [Reference Citation Analysis (0)] |
| 3. | Petrovchich I, Ford JM. Genetic predisposition to gastric cancer. Semin Oncol. 2016;43:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Shi J, Qu YP, Hou P. Pathogenetic mechanisms in gastric cancer. World J Gastroenterol. 2014;20:13804-13819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | de Assumpção PP, Araújo TMT, de Assumpção PB, Barra WF, Khayat AS, Assumpção CB, Ishak G, Nunes DN, Dias-Neto E, Coelho LGV. Suicide journey of H. pylori through gastric carcinogenesis: the role of non-H. pylori microbiome and potential consequences for clinical practice. Eur J Clin Microbiol Infect Dis. 2019;38:1591-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 724] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 7. | Tsukamoto T, Nakagawa M, Kiriyama Y, Toyoda T, Cao X. Prevention of Gastric Cancer: Eradication of Helicobacter Pylori and Beyond. Int J Mol Sci. 2017;18:pii: E1699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | van der Post RS, Oliveira C, Guilford P, Carneiro F. Hereditary gastric cancer: what's new? Update 2013-2018. Fam Cancer. 2019;18:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Boland CR, Yurgelun MB. Historical Perspective on Familial Gastric Cancer. Cell Mol Gastroenterol Hepatol. 2017;3:192-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW; American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223-62; quiz 263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1087] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 11. | Setia N, Clark JW, Duda DG, Hong TS, Kwak EL, Mullen JT, Lauwers GY. Familial Gastric Cancers. Oncologist. 2015;20:1365-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Chun N, Ford JM. Genetic testing by cancer site: stomach. Cancer J. 2012;18:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Vangala DB, Cauchin E, Balmaña J, Wyrwicz L, van Cutsem E, Güller U, Castells A, Carneiro F, Hammel P, Ducreux M, van Laethem JL, Matysiak-Budnik T, Schmiegel W. Screening and surveillance in hereditary gastrointestinal cancers: Recommendations from the European Society of Digestive Oncology (ESDO) expert discussion at the 20th European Society for Medical Oncology (ESMO)/World Congress on Gastrointestinal Cancer, Barcelona, June 2018. Eur J Cancer. 2018;104:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Polom K, Marrelli D, D'Ignazio A, Roviello F. Hereditary diffuse gastric cancer: how to look for and how to manage it. Updates Surg. 2018;70:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Kluijt I, Sijmons RH, Hoogerbrugge N, Plukker JT, de Jong D, van Krieken JH, van Hillegersberg R, Ligtenberg M, Bleiker E, Cats A; Dutch Working Group on Hereditary Gastric Cancer. Familial gastric cancer: guidelines for diagnosis, treatment and periodic surveillance. Fam Cancer. 2012;11:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Oliveira C, Pinheiro H, Figueiredo J, Seruca R, Carneiro F. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:e60-e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 17. | Kaurah P, Huntsman DG. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. Hereditary Diffuse Gastric Cancer. Seattle (WA): University of Washington, Seattle; 1993-2019. [PubMed] |
| 18. | Rudloff U. Gastric adenocarcinoma and proximal polyposis of the stomach: diagnosis and clinical perspectives. Clin Exp Gastroenterol. 2018;11:447-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Mitsui Y, Yokoyama R, Fujimoto S, Kagemoto K, Kitamura S, Okamoto K, Muguruma N, Bando Y, Eguchi H, Okazaki Y, Ishida H, Takayama T. First report of an Asian family with gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS) revealed with the germline mutation of the APC exon 1B promoter region. Gastric Cancer. 2018;21:1058-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Beer A, Streubel B, Asari R, Dejaco C, Oberhuber G. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS) - a rare recently described gastric polyposis syndrome - report of a case. Z Gastroenterol. 2017;55:1131-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Islam RS, Patel NC, Lam-Himlin D, Nguyen CC. Gastric polyps: a review of clinical, endoscopic, and histopathologic features and management decisions. Gastroenterol Hepatol (NY). 2013;9:640-651. [PubMed] |
| 22. | Cybulski C, Nazarali S, Narod SA. Multiple primary cancers as a guide to heritability. Int J Cancer. 2014;135:1756-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Luo W, Fedda F, Lynch P, Tan D. CDH1 Gene and Hereditary Diffuse Gastric Cancer Syndrome: Molecular and Histological Alterations and Implications for Diagnosis And Treatment. Front Pharmacol. 2018;9:1421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 719] [Article Influence: 102.7] [Reference Citation Analysis (1)] |
| 25. | Diogo Filho A, Botelho LF, Nishiyama A, Zumpano LE, Monte RC, Rosa SC. Gastric stump cancer after gastrectomy by gastroduodenal peptic ulcer. Arq Bras Cir Dig. 2016;29:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Assumpção MB, Moreira FC, Hamoy IG, Magalhães L, Vidal A, Pereira A, Burbano R, Khayat A, Silva A, Santos S, Demachki S, Ribeiro-Dos-Santos Â, Assumpção P. High-Throughput miRNA Sequencing Reveals a Field Effect in Gastric Cancer and Suggests an Epigenetic Network Mechanism. Bioinform Biol Insights. 2015;9:111-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Tan RY, Ngeow J. Hereditary diffuse gastric cancer: What the clinician should know. World J Gastrointest Oncol. 2015;7:153-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 28. | Dotto GP. Multifocal epithelial tumors and field cancerization: stroma as a primary determinant. J Clin Invest. 2014;124:1446-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Ford JM. Hereditary Gastric Cancer: An Update at 15 Years. JAMA Oncol. 2015;1:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Njoroge SW, Burgess KR, Cobleigh MA, Alnajar HH, Gattuso P, Usha L. Hereditary diffuse gastric cancer and lynch syndromes in a BRCA1/2 negative breast cancer patient. Breast Cancer Res Treat. 2017;166:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Lo W, Zhu B, Sabesan A, Wu HH, Powers A, Sorber RA, Ravichandran S, Chen I, McDuffie LA, Quadri HS, Beane JD, Calzone K, Miettinen MM, Hewitt SM, Koh C, Heller T, Wacholder S, Rudloff U. Associations of CDH1 germline variant location and cancer phenotype in families with hereditary diffuse gastric cancer (HDGC). J Med Genet. 2019;56:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Hamilton LE, Jones K, Church N, Medlicott S. Synchronous appendiceal and intramucosal gastric signet ring cell carcinomas in an individual with CDH1-associated hereditary diffuse gastric carcinoma: a case report of a novel association and review of the literature. BMC Gastroenterol. 2013;13:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Brooks-Wilson AR, Kaurah P, Suriano G, Leach S, Senz J, Grehan N, Butterfield YS, Jeyes J, Schinas J, Bacani J, Kelsey M, Ferreira P, MacGillivray B, MacLeod P, Micek M, Ford J, Foulkes W, Australie K, Greenberg C, LaPointe M, Gilpin C, Nikkel S, Gilchrist D, Hughes R, Jackson CE, Monaghan KG, Oliveira MJ, Seruca R, Gallinger S, Caldas C, Huntsman D. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004;41:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Oliveira C, Bordin MC, Grehan N, Huntsman D, Suriano G, Machado JC, Kiviluoto T, Aaltonen L, Jackson CE, Seruca R, Caldas C. Screening E-cadherin in gastric cancer families reveals germline mutations only in hereditary diffuse gastric cancer kindred. Hum Mutat. 2002;19:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Pharoah PD, Guilford P, Caldas C; International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 424] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 36. | Salahshor S, Hou H, Diep CB, Loukola A, Zhang H, Liu T, Chen J, Iselius L, Rubio C, Lothe RA, Aaltonen L, Sun XF, Lindmark G, Lindblom A. A germline E-cadherin mutation in a family with gastric and colon cancer. Int J Mol Med. 2001;8:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Figueiredo J, Melo S, Carneiro P, Moreira AM, Fernandes MS, Ribeiro AS, Guilford P, Paredes J, Seruca R. Clinical spectrum and pleiotropic nature of CDH1 germline mutations. J Med Genet. 2019;56:199-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Cai M, Dai S, Chen W, Xia C, Lu L, Dai S, Qi J, Wang M, Wang M, Zhou L, Lei F, Zuo T, Zeng H, Zhao X. Environmental factors, seven GWAS-identified susceptibility loci, and risk of gastric cancer and its precursors in a Chinese population. Cancer Med. 2017;6:708-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Peng Q, Chen Z, Lu Y, Lao X, Mo C, Li R, Qin X, Li S. Current evidences on XPC polymorphisms and gastric cancer susceptibility: a meta-analysis. Diagn Pathol. 2014;9:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1271] [Article Influence: 127.1] [Reference Citation Analysis (0)] |