Published online Jan 7, 2020. doi: 10.3748/wjg.v26.i1.11
Peer-review started: October 30, 2019
First decision: November 22, 2019
Revised: November 26, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: January 7, 2020
Processing time: 68 Days and 19.7 Hours
Hepatic hemangioma (HH) is the most common benign liver tumor and it is usually found incidentally during radiological studies. This tumor arises from a vascular malformation; however, the pathophysiology has not been clearly elucidated. Symptoms usually correlate with the size and location of the tumor. Less commonly the presence of a large HH may cause life-threatening conditions. The diagnosis can be established by the identification of HH hallmarks in several imaging studies. In patients that present with abdominal symptoms other etiologies should be excluded first before attributing HH as the cause. In asymptomatic patient’s treatment is not required and follow up is usually reserved for HH of more than 5 cm. Symptomatic patients can be managed surgically or with other non-surgical modalities such as transcatheter arterial embolization or radiofrequency ablation. Enucleation surgery has shown to have fewer complications as compared to hepatectomy or other surgical techniques. Progression of the tumor is seen in less than 40%. Hormone stimulation may play a role in HH growth; however, there are no contraindications for hormonal therapy in patients with HH due to the lack of concrete evidence. When clinicians encounter this condition, they should discern between observation and surgical or non-surgical management based on the clinical presentation.
Core tip: Hepatic hemangioma is the most common benign liver tumor and it is usually found incidentally during radiological studies. This tumor arises from a vascular malformation. Symptoms usually correlate with the size and location of the tumor. Symptomatic patients can be managed surgically or with other non-surgical modalities.
- Citation: Leon M, Chavez L, Surani S. Hepatic hemangioma: What internists need to know. World J Gastroenterol 2020; 26(1): 11-20
- URL: https://www.wjgnet.com/1007-9327/full/v26/i1/11.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i1.11
Hepatic hemangioma (HH) is a mesoderm-derived tumor consisting of a blood-filled space, fed by hepatic arterial circulation and lined by a single layer of flat endothelial cells[1]. It is the most common benign liver tumor, presenting as a well- circumscribed hypervascular lesion, more commonly found in women with a prevalence that ranges from 0.4% to 7.3% (based on autopsy findings) and an incidence of 0.4%-20% in the general population[1-5].
HH presents commonly as an incidental finding during radiological imaging and are describe as solitary or multiple lesions. They may be confined to one lobe (more in the right hepatic lobe) or extend throughout the entire liver. According to their dimension they can be small or giant (> 5 cm) and may range from 1 mm up to 50 cm[2,6]. HH are classified by their nature as cavernous, capillary and sclerosing hemangioma; the latter is characterized by degeneration and fibrous replacement and can be misdiagnosed as a malignant tumor[7,8].
The pathophysiology of HH is not completely understood, and in some cases, a genetic predisposition has been described[9]. HH arises from a vascular malformation with a growing pattern secondary to dilation rather than hypertrophy or hyperplasia.
One hypothesis suggest HH results from abnormal angiogenesis and an increase in pro-angiogenic factors[10].
Vascular endothelial growth factor (VEGF) is an important pro-angiogenic factor for endothelial cells. Mammalian target of rapamycin (mTOR) stimulates an autocrine loop of VEGF signaling and increase cell proliferation in vascular endotelial cells. TOR proteins are a group of serine/threanine kinases involved in ribosomal biogenesis, mRNA translation and cell mass growth and proliferation[11]. Zhang et al[12] found an increased expression of VEGF-A, pro-matrix metalloproteinase 2, and activated metalloproteinase 2 in HH cells compared to normal human liver endothelial cells.
Rapamycin inhibits mTOR and has been studied in mouse models and mouse cells as a possible treatment for vascular cell growths (mainly malignancies)[11].
Rapamycin is currently used as an antifungal, antineoplastic and antibacterial macrolide drug, but no human studies aimed to HH have been done.
Hormones such as estrogens play a role in HH growth, as they are seen more frequently among women and their size increase after hormone replacement therapy (HRT), oral contraceptive pills (OCPs), and pregnancy[13,14]. The direct mechanisms of hormone effects are unknown, as HH are negative for estrogen and progesterone receptors and current evidence does not support a contraindication of OCPs/HRT/anabolic steroids in patients with HH[15-18].
HH are usually asymptomatic, however symptoms may present when a HH is larger than > 5 cm[19]. Symptoms are nonspecific, patients usually describe abdominal pain, discomfort and fullness in the right upper quadrant, secondary to stretching and inflammation of the Glisson’s capsule. Tumors > 10 cm present with abdominal distention[19,20]. The location of the liver mass may cause pressure and compression of adjacent structures causing other symptoms such as nausea, early satiety, and postprandial bloating. Less commonly associated symptoms include fever, jaundice, dyspnea, high-output cardiac failure, and haemobilia[21-24].
Giant HH may cause a life-threatening coagulation disorder known as Kasabach-Merrit syndrome (thrombocytopenia, disseminated intravascular coagulation, and systemic bleeding) presenting with coagulopathy secondary to thrombocytopenia, anemia, hypofibrinogenimia, a decrease in prothrombin time, and increase in D-dimer. This syndrome has been reported with an incidence ranging from 0.3% of all HH to 26% in tumors > 15 cm[19,25].
Another serious complication is bleeding from spontaneous or traumatic rupture (in peripherally located and exophytic giant lesions), however the risk is extremely low (0.47%)[26].
The natural progression of HH varies, previously these lesions were considered to remain stable. However, multiple studies have shown progression and increase in size when followed throughout the years[27,28]. In a study of 236 patients with a median follow up of 48 mo (3-26), 61% experienced HH size increase with a peak growth rate when HH was 8-10 cm (0.80 ± 0.62 cm/year) and in patients less than 30 years of age[29].
In another study with 123 patients (163 HH) a 50.9% grew by any amount in absolute mean linear dimension with an annual growth rate of 0.03 cm for all lesions and 0.19 cm for those that grew > 5%. This study also found a correlation with increased annual growth in HH of 5 cm or more at initial size. They predicted an annual growth rate for all HH of 0.34 mm[5].
HH unique features by imaging are the presence of peripheral nodular enhancement and a progressive centripetal fill-in. Ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) are the most common imaging tests. Atypical lesions may require more than one imaging test.
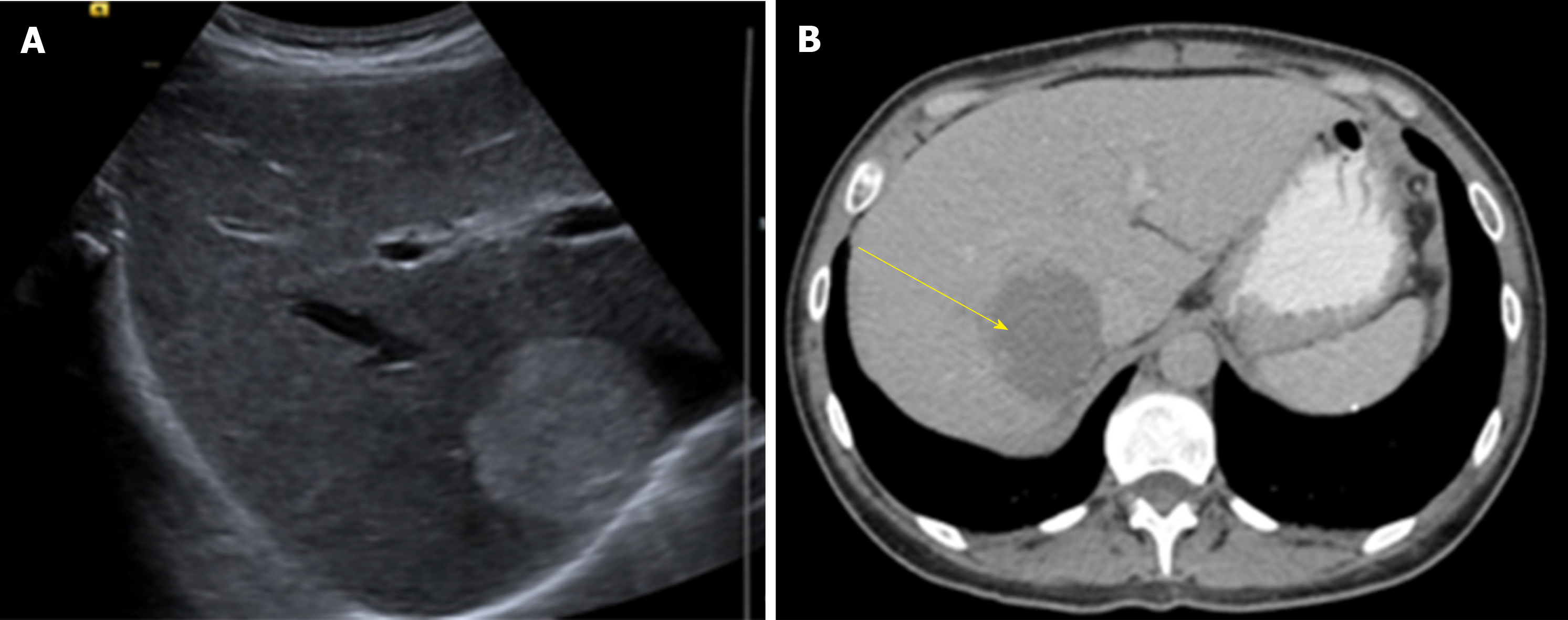
US is usually the first diagnostic imaging test due to its availability. HH appears as a well-defined, homogeneously hyper echoic mass with posterior acoustic enhancement (Figure 1). Color-Doppler US does not improve accuracy in diagnosis as it only shows blood flow in HH with an intra arterio-portal shunt[30,31].
US has a sensitivity of 96.9% and a specificity of 60.3%[32]. Some malignant hepatic lesions (Hepatocellular carcinoma and hepatic metastases) may produce similar acoustic patterns and other imaging modality must be used to confirm diagnosis.
Contrast-enhanced US (CEUS) uses gas-filled micro bubbles that delineate the signal produced by blood flow. HH shows a peripheral nodular contrast enhancement in the early phase (arterial) with centripetal filling in later phases.
Some studies have proven CEUS improves characterization and specificity for HH diagnosis[33,34].
CT has a sensitivity of 98.3% and a specificity of 55%[32]. HH are described as well-demarcated hypodense masses (Figure 2). When contrast is used, a peripheral nodular enhancement with centripetal homogeneous filling is expected, however small lesions and HH with cystic areas, fibrosis or thrombosis may show an atypical pattern[35].
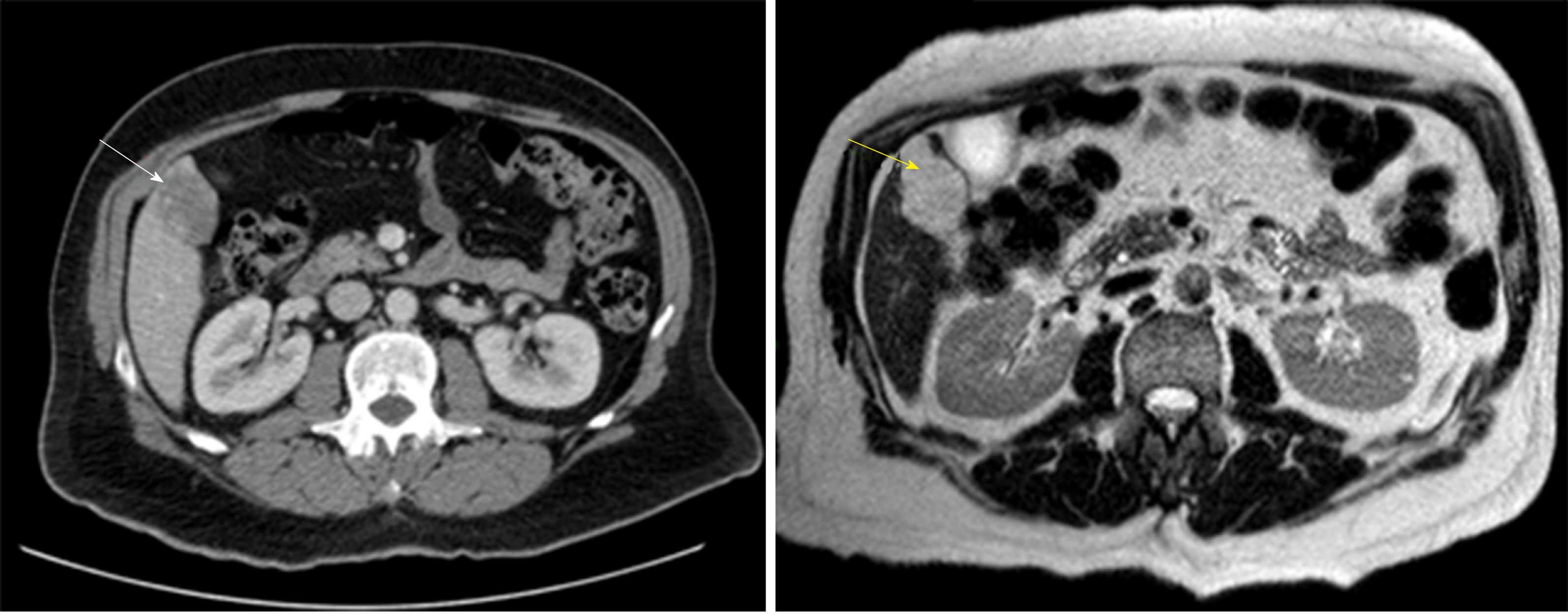
MRI shows a well-defined, smooth, homogenous lesion, hypointense on T1 and hyperintense on T2 weighted images (Figure 3). Some malignant lesions may show a similar hyperintensity on T2, to differentiate HH from solid neoplastic liver lesions the echo time is increased which causes signal from malignant lesions to decrease and signal from HH to increase. Gadolinium administration shows a peripheral enhancement on arterial phase and contrast retention on delayed phases, which allows differentiating from hypervascular tumors that usually have a contrast washout on delayed phase. MRI has been considered the best imaging method for HH with a sensitivity of 90%-100% and a specificity of 91%-99%[32,36].
Angiography is the best option for atypical HH that are difficult to diagnose with other imaging test. HH appears as a “snowy-tree” or “cotton wool” with a large feeding vessel and diffuse pooling of contrast that continues during delayed phase. Technetium-99m pertechnetate-labeled red blood cell pool scintigraphy, single photon emission computed tomography and positron emission tomography/CT are other imaging modalities available to diagnose HH in patients with atypical tumors, history of chronic liver disease or malignancy[37,38].
Needle aspiration biopsy is not recommended because of the high risk of hemorrhage and a low diagnostic yield[39-41].
Small, asymptomatic HH do not require treatment or follow up. Some authors suggest to follow-up in HH > 5 cm at 6-12 mo to asses for rapid growth with the same imaging test used at diagnosis[42].
Treatment should be restricted to symptomatic patients, with continuous mass growth, compression of adjacent organs (gastric outlet obstruction, Budd-Chiari syndrome) or complications such as rupture with intraperitoneal bleeding or Kasabach-Merrit syndrome.
Abdominal pain should be carefully evaluated in patients with HH and other possible causes should be kept in mind before definitive treatment is decided. Farges et al[43] diagnosed 87 patients with abdominal pain and HH, from these, 54% were found to have other condition responsible from the abdominal pain. Specific treatment for abdominal pain and HH was required in 14 patients and half of them remained symptomatic after treatment, suggesting another etiology causing the pain. In another study, the majority of patients with abdominal pain and HH were found to have symptoms attributable to different gastrointestinal diseases (Irritable bowel syndrome, gastroesophageal reflux disease, hepatitis, peptic ulcer, gallbladder disease) and in only 21.7% of symptomatic patients, abdominal pain was attributable to HH[44].
Surgery continues to be the most common treatment for HH. Surgical management includes liver resection, enucleation, hepatic artery ligation and liver transplantation. The most common procedures worldwide are liver resection and enucleation (open surgery, laparoscopy or robot)[45-48].
The first hepatic resection for HH was done in 1987 by Schwartz et al[49] and in 1988 Alper et al[50] reported the first nine patients treated with enucleation.
The choice of procedure depends on the size, number of lesions, location, surgeon experience, and institutional resources. Both techniques carry minimal postoperative morbidity.
In the last years several studies have evaluated enucleation vs hepatectomy and most have concluded that enucleation is associated with lower morbidity, shorter operation time, less blood loss and fewer complications[47,48,51]. However, when HH is larger than 10 cm, Zhang et al[52] found no difference in operation time, blood loss, complications or hospital stay between enucleation and resection.
Enucleation is technically easier in peripherally located HH, when done in centrally located HH the procedure causes a longer vascular inflow occlusion time, longer operating time and more blood loss[53]. Centrally located HH (Segments I, IV, V and VIII) are treated with extended right and left hepatectomy. This therapy may remove 60% to 80% of liver parenchyma, which convey a higher risk of postsurgical liver failure. Some lesions are suitable for a wedge resection[53].
Improvement in laparoscopic surgery has increased the cases treated with minimally invasive surgery for either resection or enucleation. Laparoscopic liver surgery is preferred in small, left lateral lesions with minor resections[32,54].
A recent retrospective study compared open versus laparoscopic liver surgery for HH; results favored laparoscopic therapy with less blood loss, lower complication rates, and a shorter postoperative hospital stay. However, baseline patient characteristics between the two groups were not equal as surgeons decided open or laparoscopic surgery based on tumor characteristics[54].
Liver transplantation for benign solid tumors is not considered a first line treatment due to morbidity and organ shortage. A study published in 2015 analyzed data from the United Network of Organ Sharing from 1988 to 2013 and found 147 (0.17%) liver transplants in US patients were performed for benign tumors of the liver, including 25 for HH[55].
Liver transplantation is reserved for unresectable giants HH causing severe symptoms (respiratory distress, abdominal pain), failure of previous interventions or life-threating complications such as Kasabach Merrit syndrome[56,57].
Transcatheter arterial embolization (TAE) is used to control acute bleeding or shrink HH prior to surgery with metallic coils, gelform particles, polyvinyl alcohol and liquid agents such as N-butyl-2-cyanoacrylate, bleomycin-lipiodol[58-61]. However, TAE as also been used as single treatment with acceptable results[62,63].
A mix of pingyangmycin/lipiodol was first studied as a single treatment for HH. Two studies reported good results with significant reduction of HH volume and relief of symptoms[64,65]. Pingyagmycin is only available in China, similar studies have been carried in other places with bleomycin as substitute for pingyagmycin[62,63].
A study with 23 patients (29 HH) managed with TAE with bleomycin-lipiodol concluded 73.9% of patients had > 50% volume regression of HH[62]. Bleomycin administration results in micro-thrombi formation, which leads to atrophy and fibrosis of the tumor. It also induces a non-specific inflammatory process around the HH and in the portal area. Acute liver failure, liver infarction, abscess, intrahepatic biloma, cholecystitis, splenic infarction, hepatic artery perforation, and sclerosing cholangitis have been reported as associated complications of TAE with Bleomycin[66].
Radiofrequency ablation (RFA) can be used percutaneously, laparoscopically or by open surgery. RFA induces a thermal damage to endothelial vascular structures and promotes thrombosis. RFA is usually performed under US guidance; CT guidance for percutaneous RFA is suitable for HH located deeply in liver parenchyma[67].
Laparoscopic RFA with US guidance is preferred for subcapsular HH[68]. Laparoscopic RFA compared with open resection is associated with shorter operative time, less pain, shorter hospital stay and the lower hospital cost[69,70].
Lengthy RFA is prone to cause hemolysis, hemoglobinuria and acute kidney injury, thus is not suitable for large HH[71]. Other complications of RFA include bleeding at the electrode entry site, rupture of HH and injury to adjacent organs by puncture or thermal injury.
The established indications for RFA in this population are maximum diameter of HH > 5 cm, tumor gaining enlargement > 1 cm within 2 years, persistent HH related abdominal pain with exclusion of other GI diseases. Contraindications include patients with severe bleeding tendency, malignant tumors, Kasabach-Merrit syndrome, infection (biliary system inflammation), low immune function, and severe organ failure[67].
The use of anti-VEGF such as sorafenib and bevacizumab have been reported in case reports to incidentally reduce HH size[72,73]. A retrospective study aimed to study HH size reduction with anti VEGF (bevacizumab or sunitinib) showed no significant volume reduction[10]. Metformin has also been reported in a case report to incidentally reduce HH size[74].
In the last years, the donor’s criteria for liver transplant has expanded to overcome organ shortage. Liver donors with the discarded partial liver resection from HH have proved to be a viable source for liver transplant with acceptable receptor outcomes and no growth of HH[75-77].
Most HH are diagnosed incidentally on imaging tests since most patients remain asymptomatic throughout their life. Patients who present with symptoms are usually due to larger lesions.
Since the natural history of HH is benign and an increase in size progression occurs in less than 40%, most patients can be reassured and only observed. When a patient is symptomatic, the first step is to exclude other causes of their symptoms. Once excluding other etiologies and HH is considered the cause of symptoms, treatment modalities are decided based on size, anatomy and comorbidities of the patient.
Over the last years, non-surgical minimal invasive procedures for tumor reduction and laparoscopic surgery have proven good results in selected patients.
Rarely HH present with life-threatening conditions such as an acute traumatic rupture or coagulation disorders. Only in these instances, emergent surgical management is warranted.
Clinicians should discern between observation and the best optimal management based on the clinical presentation. If treatment is needed, a minimal invasive approach should be pursued. Future research will help clinicians understand HH pathogenesis and guide management.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Pulmonary Associates (Fellow).
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boteon YL, Coelho J, Li W, Sawada K S-Editor: Tang JZ L-Editor: A E-Editor: Ma YJ
| 1. | Ishak KG, Rabin L. Benign tumors of the liver. Med Clin North Am. 1975;59:995-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 298] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Yang Z, Tan H, Liu X, Sun Y. Extremely Giant Liver Hemangioma (50 cm) with Kasabach-Merritt Syndrome. J Gastrointest Surg. 2017;21:1748-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Schumacker HB. Hemangioma of the liver: discussion of symptomatology and report of patient treated by operation. Surgery. 1942;11:209–222. |
| 4. | Reddy KR, Kligerman S, Levi J, Livingstone A, Molina E, Franceschi D, Badalamenti S, Jeffers L, Tzakis A, Schiff ER. Benign and solid tumors of the liver: relationship to sex, age, size of tumors, and outcome. Am Surg. 2001;67:173-178. [PubMed] |
| 5. | Hasan HY, Hinshaw JL, Borman EJ, Gegios A, Leverson G, Winslow ER. Assessing normal growth of hepatic hemangiomas during long-term follow-up. JAMA Surg. 2014;149:1266-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 6. | Adam YG, Huvos AG, Fortner JG. Giant hemangiomas of the liver. Ann Surg. 1970;172:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 147] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Mori H, Ikegami T, Imura S, Shimada M, Morine Y, Kanemura H, Arakawa Y, Kanamoto M, Hanaoka J, Sugimoto K, Tokunaga T. Sclerosed hemangioma of the liver: Report of a case and review of the literature. Hepatol Res. 2008;38:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol. 2016;65:386-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (2)] |
| 9. | Moser C, Hany A, Spiegel R. Familial giant hemangiomas of the liver. Study of a family and review of the literature. Praxis (Bern 1994). 1998;87:461-468. [PubMed] |
| 10. | Lee M, Choi JY, Lim JS, Park MS, Kim MJ, Kim H. Lack of anti-tumor activity by anti-VEGF treatments in hepatic hemangiomas. Angiogenesis. 2016;19:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Zheng N, Ding X, Jahan R. Low concentration of rapamycin inhibits hemangioma endothelial cell proliferation, migration, and vascular tumor formation in mice. Curr Ther Res Clin Exp. 2014;11:99-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Zhang WJ, Ye LY, Wu LQ, Xin YL, Gu F, Niu JX, Yang ZH, Zhu GJ, Grau GE, Lou JN. Morphologic, phenotypic and functional characteristics of endothelial cells derived from human hepatic cavernous hemangioma. J Vasc Res. 2006;43:522-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Glinkova V, Shevah O, Boaz M, Levine A, Shirin H. Hepatic haemangiomas: possible association with female sex hormones. Gut. 2004;53:1352-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Ozakyol A, Kebapci M. Enhanced growth of hepatic hemangiomatosis in two adults after postmenopausal estrogen replacement therapy. Tohoku J Exp Med. 2006;210:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Gemer O, Moscovici O, Ben-Horin CL, Linov L, Peled R, Segal S. Oral contraceptives and liver hemangioma: a case-control study. Acta Obstet Gynecol Scand. 2004;83:1199-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kim GE, Thung SN, Tsui WM, Ferrell LD. Hepatic cavernous hemangioma: underrecognized associated histologic features. Liver Int. 2006;26:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Saegusa T, Ito K, Oba N, Matsuda M, Kojima K, Tohyama K, Matsumoto M, Miura K, Suzuki H. Enlargement of multiple cavernous hemangioma of the liver in association with pregnancy. Intern Med. 1995;34:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Marrero JA, Ahn J, Rajender Reddy K; Americal College of Gastroenterology. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol. 2014;109:1328-47; quiz 1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 19. | Sakamoto Y, Kokudo N, Watadani T, Shibahara J, Yamamoto M, Yamaue H; Japanese Society of Hepato-Biliary-Pancreatic Surgery. Proposal of size-based surgical indication criteria for liver hemangioma based on a nationwide survey in Japan. J Hepatobiliary Pancreat Sci. 2017;24:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Luks FI, Yazbeck S, Brandt ML, Bensoussan AL, Brochu P, Blanchard H. Benign liver tumors in children: a 25-year experience. J Pediatr Surg. 1991;26:1326-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Liu X, Yang Z, Tan H, Zhou W, Su Y. Fever of Unknown Origin Caused by Giant Hepatic Hemangioma. J Gastrointest Surg. 2018;22:366-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 22. | Smith AA, Nelson M. High-Output Heart Failure from a Hepatic Hemangioma With Exertion-Induced Hypoxia. Am J Cardiol. 2016;117:157-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 23. | Mikami T, Hirata K, Oikawa I, Kimura M, Kimura H. Hemobilia caused by a giant benign hemangioma of the liver: report of a case. Surg Today. 1998;28:948-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Huang SA, Tu HM, Harney JW, Venihaki M, Butte AJ, Kozakewich HP, Fishman SJ, Larsen PR. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 308] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Miura JT, Amini A, Schmocker R, Nichols S, Sukato D, Winslow ER, Spolverato G, Ejaz A, Squires MH, Kooby DA, Maithel SK, Li A, Wu MC, Sarmiento JM, Bloomston M, Christians KK, Johnston FM, Tsai S, Turaga KK, Tsung A, Pawlik TM, Gamblin TC. Surgical management of hepatic hemangiomas: a multi-institutional experience. HPB (Oxford). 2014;16:924-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Mocchegiani F, Vincenzi P, Coletta M, Agostini A, Marzioni M, Baroni GS, Giovagnoni A, Guerrieri M, Marmorale C, Risaliti A, Vivarelli M. Prevalence and clinical outcome of hepatic haemangioma with specific reference to the risk of rupture: A large retrospective cross-sectional study. Dig Liver Dis. 2016;48:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Gibney RG, Hendin AP, Cooperberg PL. Sonographically detected hepatic hemangiomas: absence of change over time. AJR Am J Roentgenol. 1987;149:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Tait N, Richardson AJ, Muguti G, Little JM. Hepatic cavernous haemangioma: a 10 year review. Aust N Z J Surg. 1992;62:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Jing L, Liang H, Caifeng L, Jianjun Y, Feng X, Mengchao W, Yiqun Y. New recognition of the natural history and growth pattern of hepatic hemangioma in adults. Hepatol Res. 2016;46:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Perkins AB, Imam K, Smith WJ, Cronan JJ. Color and power Doppler sonography of liver hemangiomas: a dream unfulfilled? J Clin Ultrasound. 2000;28:159-165. [PubMed] [DOI] [Full Text] |
| 31. | Lim KJ, Kim KW, Jeong WK, Kim SY, Jang YJ, Yang S, Lee JJ. Colour Doppler sonography of hepatic haemangiomas with arterioportal shunts. Br J Radiol. 2012;85:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Toro A, Mahfouz AE, Ardiri A, Malaguarnera M, Malaguarnera G, Loria F, Bertino G, Di Carlo I. What is changing in indications and treatment of hepatic hemangiomas. A review. Ann Hepatol. 2014;13:327-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Dietrich CF, Mertens JC, Braden B, Schuessler G, Ott M, Ignee A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology. 2007;45:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 35. | Yamashita Y, Ogata I, Urata J, Takahashi M. Cavernous hemangioma of the liver: pathologic correlation with dynamic CT findings. Radiology. 1997;203:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | McFarland EG, Mayo-Smith WW, Saini S, Hahn PF, Goldberg MA, Lee MJ. Hepatic hemangiomas and malignant tumors: improved differentiation with heavily T2-weighted conventional spin-echo MR imaging. Radiology. 1994;193:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 140] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Caturelli E, Pompili M, Bartolucci F, Siena DA, Sperandeo M, Andriulli A, Bisceglia M. Hemangioma-like lesions in chronic liver disease: diagnostic evaluation in patients. Radiology. 2001;220:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Imperiale A, Greget M, Chabrier G, Keomany J, Rust E, Detour J, Pessaux P, Goichot B. Solitary hepatic metastasis from medullary thyroid carcinoma mimicking atypical hemangioma: insights from multimodality diagnostic approach by MRI, F-18 FDG and F-18 FDOPA PET/CT. Clin Nucl Med. 2010;35:434-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Terriff BA, Gibney RG, Scudamore CH. Fatality from fine-needle aspiration biopsy of a hepatic hemangioma. AJR Am J Roentgenol. 1990;154:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 40. | Davies R. Haemorrhage after fine-needle aspiration biopsy of an hepatic haemangioma. Med J Aust. 1993;158:364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 41. | Taavitsainen M, Airaksinen T, Kreula J, Päivänsalo M. Fine-needle aspiration biopsy of liver hemangioma. Acta Radiol. 1990;31:69-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Bajenaru N, Balaban V, Săvulescu F, Campeanu I, Patrascu T. Hepatic hemangioma -review-. J Med Life. 2015;8:4-11. [PubMed] |
| 43. | Farges O, Daradkeh S, Bismuth H. Cavernous hemangiomas of the liver: are there any indications for resection? World J Surg. 1995;19:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Etemadi A, Golozar A, Ghassabian A, Zarei M, Hashemi Taheri AP, Dawsey SM, Malekzadeh R. Cavernous hemangioma of the liver: factors affecting disease progression in general hepatology practice. Eur J Gastroenterol Hepatol. 2011;23:354-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Ozden I, Emre A, Alper A, Tunaci M, Acarli K, Bilge O, Tekant Y, Ariogul O. Long-term results of surgery for liver hemangiomas. Arch Surg. 2000;135:978-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Efthimiadis C, Ioannidis A, Grigoriou M, Kofina K, Lazaridis M, Kosmidis C. Robotic right segmental hepatectomy for the treatment of a giant hepatic hemangioma-a case report. J Surg Case Rep. 2017;2017:rjx118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Liu Y, Wei X, Wang K, Shan Q, Dai H, Xie H, Zhou L, Xu X, Zheng S. Enucleation versus Anatomic Resection for Giant Hepatic Hemangioma: A Meta-Analysis. Gastrointest Tumors. 2017;3:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Cheng WL, Qi YQ, Wang B, Tian L, Huang W, Chen Y. Enucleation versus hepatectomy for giant hepatic haemangiomas: a meta-analysis. Ann R Coll Surg Engl. 2017;99:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Schwartz SI, Husser WC. Cavernous hemangioma of the liver. A single institution report of 16 resections. Ann Surg. 1987;205:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Alper A, Ariogul O, Emre A, Uras A, Okten A. Treatment of liver hemangiomas by enucleation. Arch Surg. 1988;123:660-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Giuliante F, Ardito F, Vellone M, Giordano M, Ranucci G, Piccoli M, Giovannini I, Chiarla C, Nuzzo G. Reappraisal of surgical indications and approach for liver hemangioma: single-center experience on 74 patients. Am J Surg. 2011;201:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Zhang W, Huang ZY, Ke CS, Wu C, Zhang ZW, Zhang BX, Chen YF, Zhang WG, Zhu P, Chen XP. Surgical Treatment of Giant Liver Hemangioma Larger Than 10 cm: A Single Center's Experience With 86 Patients. Medicine (Baltimore). 2015;94:e1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 53. | Fu XH, Lai EC, Yao XP, Chu KJ, Cheng SQ, Shen F, Wu MC, Lau WY. Enucleation of liver hemangiomas: is there a difference in surgical outcomes for centrally or peripherally located lesions? Am J Surg. 2009;198:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Liu Q, Liu F, Ding J, Wei Y, Li B. Surgical outcomes and quality of life between laparoscopic and open approach for hepatic hemangioma: A propensity score matching analysis. Medicine (Baltimore). 2019;98:e14485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Sundar Alagusundaramoorthy S, Vilchez V, Zanni A, Sourianarayanane A, Maynard E, Shah M, Daily MF, Pena LR, Gedaly R. Role of transplantation in the treatment of benign solid tumors of the liver: a review of the United Network of Organ Sharing data set. JAMA Surg. 2015;150:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Prodromidou A, Machairas N, Garoufalia Z, Kostakis ID, Tsaparas P, Paspala A, Stamopoulos P, Sotiropoulos GC. Liver Transplantation for Giant Hepatic Hemangioma: A Systematic Review. Transplant Proc. 2019;51:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Longeville JH, de la Hall P, Dolan P, Holt AW, Lillie PE, Williams JA, Padbury RT. Treatment of a giant haemangioma of the liver with Kasabach-Merritt syndrome by orthotopic liver transplant a case report. HPB Surg. 1997;10:159-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Jain V, Ramachandran V, Garg R, Pal S, Gamanagatti SR, Srivastava DN. Spontaneous rupture of a giant hepatic hemangioma - sequential management with transcatheter arterial embolization and resection. Saudi J Gastroenterol. 2010;16:116-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Bailey J, Di Carlo S, Blackwell J, Gomez D. Same day arterial embolisation followed by hepatic resection for treatment of giant haemangioma. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Zhou JX, Huang JW, Wu H, Zeng Y. Successful liver resection in a giant hemangioma with intestinal obstruction after embolization. World J Gastroenterol. 2013;19:2974-2978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Igarashi G, Mikami K, Sawada N, Endo T, Sueyoshi N, Sato K, Tsushima F, Kakehata S, Ono S, Aoki M, Kurose A, Iwamura H, Fukuda S. Interventional Treatment for Giant Hepatic Hemangioma Accompanied by Arterio-portal Shunt with Ascites. Intern Med. 2018;57:2847-2851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Akhlaghpoor S, Torkian P, Golzarian J. Transarterial Bleomycin-Lipiodol Embolization (B/LE) for Symptomatic Giant Hepatic Hemangioma. Cardiovasc Intervent Radiol. 2018;41:1674-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Özden İ, Poyanlı A, Önal Y, Demir AA, Hoş G, Acunaş B. Superselective Transarterial Chemoembolization as an Alternative to Surgery in Symptomatic/Enlarging Liver Hemangiomas. World J Surg. 2017;41:2796-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 64. | Li Y, Jia Y, Li S, Wang W, Wang Z, Wang Y, Liu B, Wang W, Chang H, Li Z. Transarterial Chemoembolization of Giant Liver Haemangioma: A Multi-center Study with 836 Cases. Cell Biochem Biophys. 2015;73:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 65. | Sun JH, Nie CH, Zhang YL, Zhou GH, Ai J, Zhou TY, Zhu TY, Zhang AB, Wang WL, Zheng SS. Transcatheter Arterial Embolization Alone for Giant Hepatic Hemangioma. PLoS One. 2015;10:e0135158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Jin S, Shi XJ, Sun XD, Wang SY, Wang GY. Sclerosing cholangitis secondary to bleomycin-iodinated embolization for liver hemangioma. World J Gastroenterol. 2014;20:17680-17685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Gao J, Fan RF, Yang JY, Cui Y, Ji JS, Ma KS, Li XL, Zhang L, Xu CL, Kong XL, Ke S, Ding XM, Wang SH, Yang MM, Song JJ, Zhai B, Nin CM, Guo SG, Xin ZH, Lu J, Dong YH, Zhu HQ, Sun WB. Radiofrequency ablation for hepatic hemangiomas: A consensus from a Chinese panel of experts. World J Gastroenterol. 2017;23:7077-7086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Gao J, Ji JS, Ding XM, Ke S, Xin ZH, Ning CM, Guo SG, Li XL, Dong YH, Sun WB. Laparoscopic Radiofrequency Ablation for Large Subcapsular Hepatic Hemangiomas: Technical and Clinical Outcomes. PLoS One. 2016;11:e0149755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Zhang X, Yan L, Li B, Wen T, Wang W, Xu M, Wei Y, Yang J. Comparison of laparoscopic radiofrequency ablation versus open resection in the treatment of symptomatic-enlarging hepatic hemangiomas: a prospective study. Surg Endosc. 2016;30:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Chen L, Zhang L, Tian M, Hu Q, Zhao L, Xiong J. Safety and effective of laparoscopic microwave ablation for giant hepatic hemangioma: A retrospective cohort study. Ann Med Surg (Lond). 2019;39:29-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | van Tilborg AAJM, Dresselaars HF, Scheffer HJ, Nielsen K, Sietses C, van den Tol PM, Meijerink MR. RF Ablation of Giant Hemangiomas Inducing Acute Renal Failure: A Report of Two Cases. Cardiovasc Intervent Radiol. 2016;39:1644-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 72. | Yamashita S, Okita K, Harada K, Hirano A, Kimura T, Kato A, Okita K. Giant cavernous hepatic hemangioma shrunk by use of sorafenib. Clin J Gastroenterol. 2013;6:55-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Mahajan D, Miller C, Hirose K, McCullough A, Yerian L. Incidental reduction in the size of liver hemangioma following use of VEGF inhibitor bevacizumab. J Hepatol. 2008;49:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Ono M, Sawada K, Okumura T. A case of liver hemangioma with markedly reduced tumor size after metformin treatment: a case report. Clin J Gastroenterol. 2017;10:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 75. | Onishi Y, Kamei H, Imai H, Kurata N, Hori T, Ogura Y. Successful adult-to-adult living donor liver transplantation using liver allograft after the resection of hemangioma: A suggestive case for a further expansion of living donor pool. Int J Surg Case Rep. 2015;16:166-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Li G, Mu X, Huang X, Qian X, Qin J, Tan Z, Zhang W, Xu X, Tan S, Zhu Z, Li W, Wang X, Wang X, Sun B. Liver transplantation using the otherwise-discarded partial liver resection graft with hepatic benign tumor: Analysis of a preliminary experience on 15 consecutive cases. Medicine (Baltimore). 2017;96:e7295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Sun B, Mu X, Wang X. Successful adult-to-adult liver transplantation of an otherwise discarded partial liver allograft with a cavernous hemangioma: new strategy for expanding liver donor pool. Transpl Int. 2013;26:e79-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |