Published online Mar 7, 2019. doi: 10.3748/wjg.v25.i9.1100
Peer-review started: December 11, 2018
First decision: January 23, 2019
Revised: February 13, 2019
Accepted: February 15, 2019
Article in press: February 15, 2019
Published online: March 7, 2019
Processing time: 86 Days and 15.4 Hours
Axial and coronal reformations have been a widely used image post-processing protocol for the ordinary multidetector computed tomography (MDCT) examination of patients with small bowel obstruction (SBO) or other abdominal diseases. The diagnostic accuracy of MDCT for assessing SBO is expected to be further improved through the use of multiple post-processing techniques.
To systemically evaluate the diagnostic accuracy and efficiency of an optimized protocol using multiple post-processing techniques for MDCT assessment of SBO and secondary bowel ischemia.
This retrospective cross-sectional study included 106 patients with clinically suspected SBO. Two readers applied three protocols to image post-processing and interpretation of patients’ MDCT volume data. We compared the three protocols based on time spent, number of images, diagnostic self-confidence, agreement, detection rate, and accuracy of detection of SBO and secondary bowel ischemia.
Protocol 2 resulted in more time spent and number of images than protocols 1 and 3 (P < 0.01), but the results of the two readers using the same protocol were not different (P > 0.05). Using protocol 3, both readers added multiple post-processing techniques at frequencies of 29.2% and 34.9%, respectively, for obstruction cause, and 32.1% and 30.2%, respectively, for secondary bowel ischemia. Protocols 2 and 3 had higher total detection rates of obstruction cause and secondary bowel ischemia than protocol 1 (P < 0.01), but no difference was detected between protocols 2 and 3 (P > 0.05). The accuracy, sensitivity, specificity, positive predictive value and negative predictive value of protocols 2 and 3 were superior to those of protocol 1 for evaluating obstruction cause and secondary bowel ischemia.
Our optimized protocol of multiple post-processing techniques can both guarantee efficiency and improve diagnostic accuracy of MDCT for assessing SBO and secondary bowel ischemia.
Core tip: Axial and coronal reformations are inadequate for multidetector computed tomography (MDCT) used to identify some causes of small bowel obstruction (SBO) and secondary bowel ischemia. In our study, both integrated and optimized protocols of multiple post-processing techniques can improve the diagnostic accuracy of MDCT for assessing SBO, but the integrated protocol necessitates a longer time spent and increases the workload of radiologists. The optimized protocol can both guarantee the time efficiency and improve the diagnostic accuracy of MDCT for assessing SBO and secondary bowel ischemia. The optimized protocol can be considered as a routine image post-processing approach of MDCT for assessment of patients with SBO.
- Citation: Kuang LQ, Tang W, Li R, Cheng C, Tang SY, Wang Y. Optimized protocol of multiple post-processing techniques improves diagnostic accuracy of multidetector computed tomography in assessment of small bowel obstruction compared with conventional axial and coronal reformations. World J Gastroenterol 2019; 25(9): 1100-1115
- URL: https://www.wjgnet.com/1007-9327/full/v25/i9/1100.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i9.1100
Acute small bowel obstruction (SBO) is a common disease with high morbidity and mortality. Early, accurate, and comprehensive assessment of this disease is critical for clinicians to determine treatment plans. Multidetector computed tomography (MDCT) is becoming popular for clinical SBO assessment, and is showing promising results[1-4]. MDCT has shown great potential in identifying SBO and its severity, site, cause, and complications. However, the diagnostic accuracy of MDCT can be improved further.
MDCT uses volume scanning to produce isotropic images that have been widely used for axial, coronal, and sagittal reformations in clinical radiology. The axial and coronal reformation post-processing protocols have been the standard for MDCT examination of abdominal diseases[2-6]. MDCT has been equipped with many kinds of image post-processing techniques with a wide range of clinical applications in human vascular disease diagnosis[7-10]. For many non-vascular diseases, if the axial, coronal, and sagittal images provide poor resolution of the anatomic details of a lesion, radiologists could add one or more post-processing techniques to improve diagnostic accuracy[11,12].
However, comprehensive studies about combining MDCT multiple post-processing techniques for SBO assessment are lacking[13-15]. Therefore, we aimed to integrate and optimize the multiple post-processing techniques, and designed a retrospective cross-sectional study to systemically evaluate diagnostic accuracy and efficiency of the optimized protocol using multiple post-processing techniques on MDCT to assess SBO and secondary bowel ischemia.
This study was approved by the ethics committee of our hospital, and waivers of informed consent were obtained from the patients. The inclusion criteria were adults (≥ 18 years) with complete clinical and MDCT volume data and patients with clinical symptoms and signs of SBO. A total of 106 patients were selected between June 2015 and May 2018 to cover a range of causes. Ninety patients were confirmed by surgery and/or pathology to have SBO with causes including adhesions (20, 18.9%), neoplasms (11, 10.4%), intussusception [neoplastic (9, 8.6%) and nonneoplastic (5, 4.7%)], volvulus (8, 7.5%), internal hernias (6, 5.7%), external hernias (9, 8.6%), bezoars/stones (7, 6.6%), vascular lesions (8, 7.5%), and inflammatory lesions (7, 6.6%). The remaining 16 patients without SBO were identified by clinical and imaging studies.
Of the 106 patients, 49 (46.2%) were men and 57 (53.8%) were women. The mean age was 54.4 ± 14.9 years (range, 19-84 years). Their clinical manifestations included abdominal distension (91, 85.8%), nausea and vomiting (94, 88.7%), constipation (78, 73.6%), abdominal pain (97, 91.5%), weariness (54, 50.9%), abdominal mass (16, 15.1%), bloody stools (13, 12.3%), and hematemesis (2, 1.9%). All the 90 patients with SBO underwent surgical treatment, and 16 patients without SBO received other treatments for other diseases.
All patients underwent unenhanced and dual-phase enhanced (hepatic arterial and portal venous phases) CT scans from the diaphragm to the symphysis pubis on a 64-row MDCT system (LightSpeed VCT, GE Healthcare, Milwaukee, United States). Patients did not receive any oral contrast agent prior to the routine scan. A dual-head power injector was used to administer the contrast agent (Ultravist; Bayer Schering Pharma, Berlin, Germany) at 370 mg iodine/mL followed by 30 mL of saline, with an injection rate of 4 mL/s through an antecubital vein. Contrast agent volumes were delivered at 2 mL/kg body weight, and the upper dose limit was set to 120 mL for each patient.
The scanning parameters were set as follows: detector configuration of 64 mm × 0.625 mm, slice thickness and reconstruction interval of 0.625 mm, table speed of 64 mm per rotation, pitch of 0.984, matrix of 512 × 512, field of view of 180-240 mm, tube voltage of 120 kV, and tube current of 300 mA. Two identical workstations (Advantage Windows 4.3; GE Medical Systems) were used for image post-processing and detailed review.
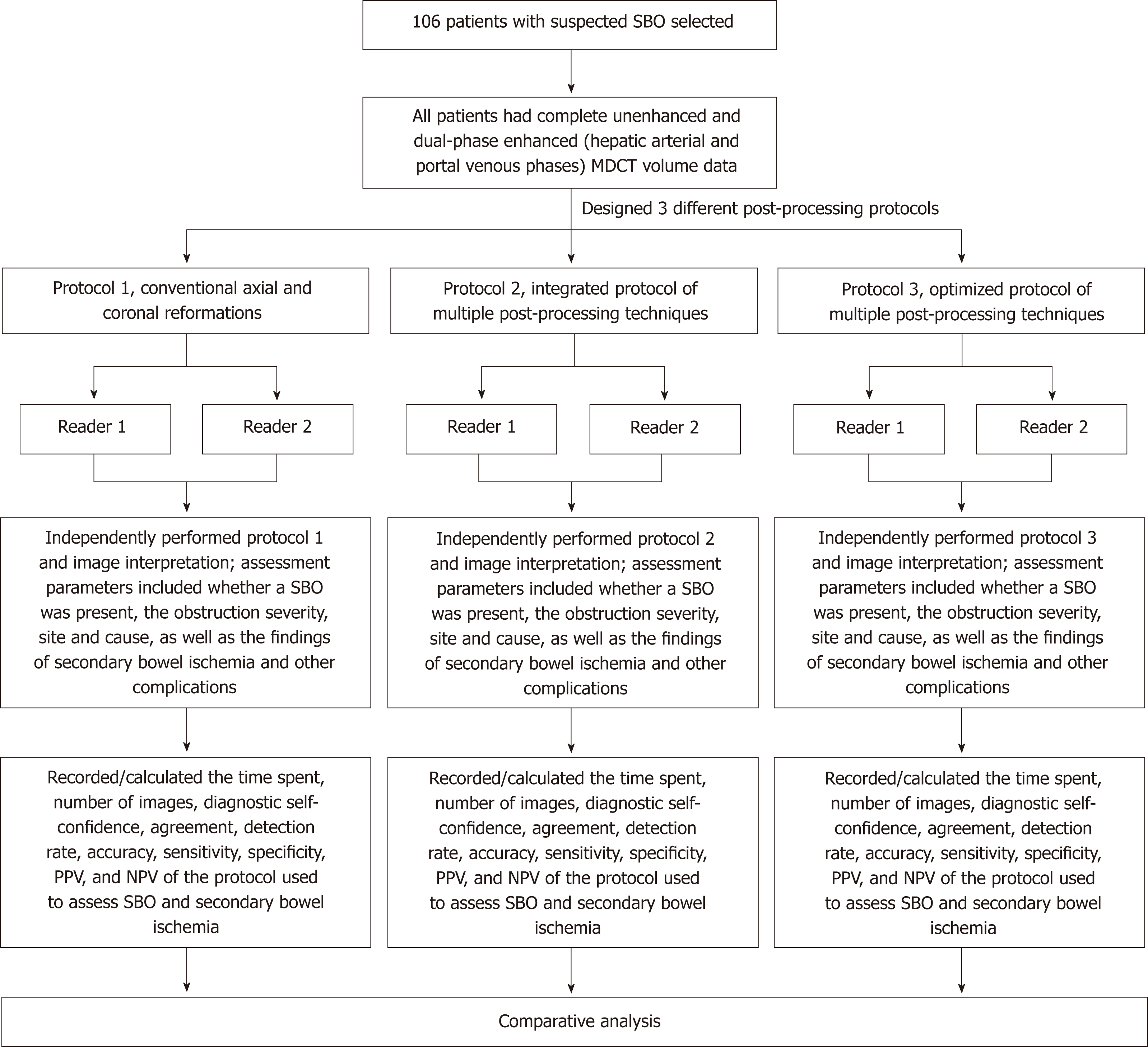
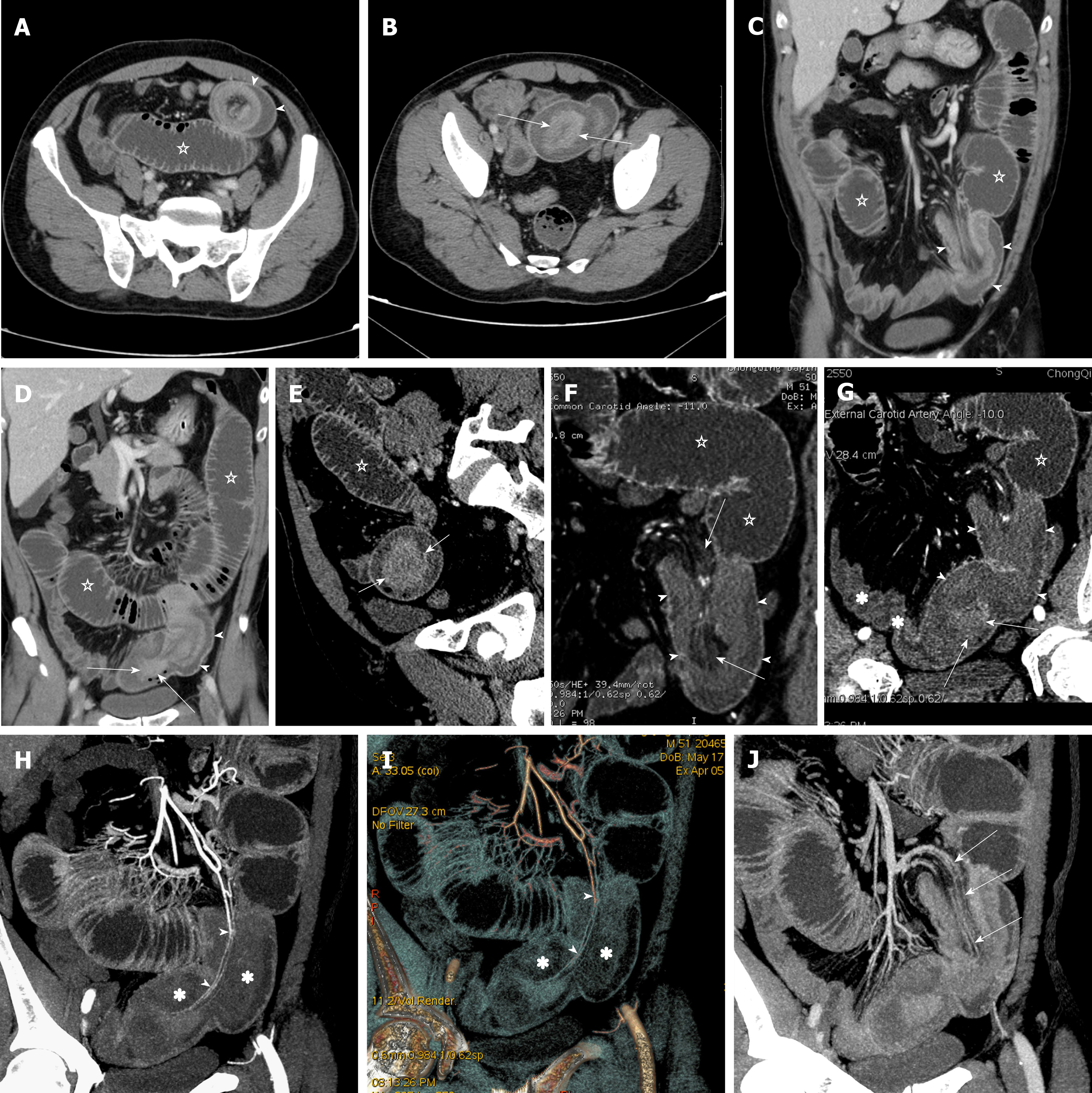
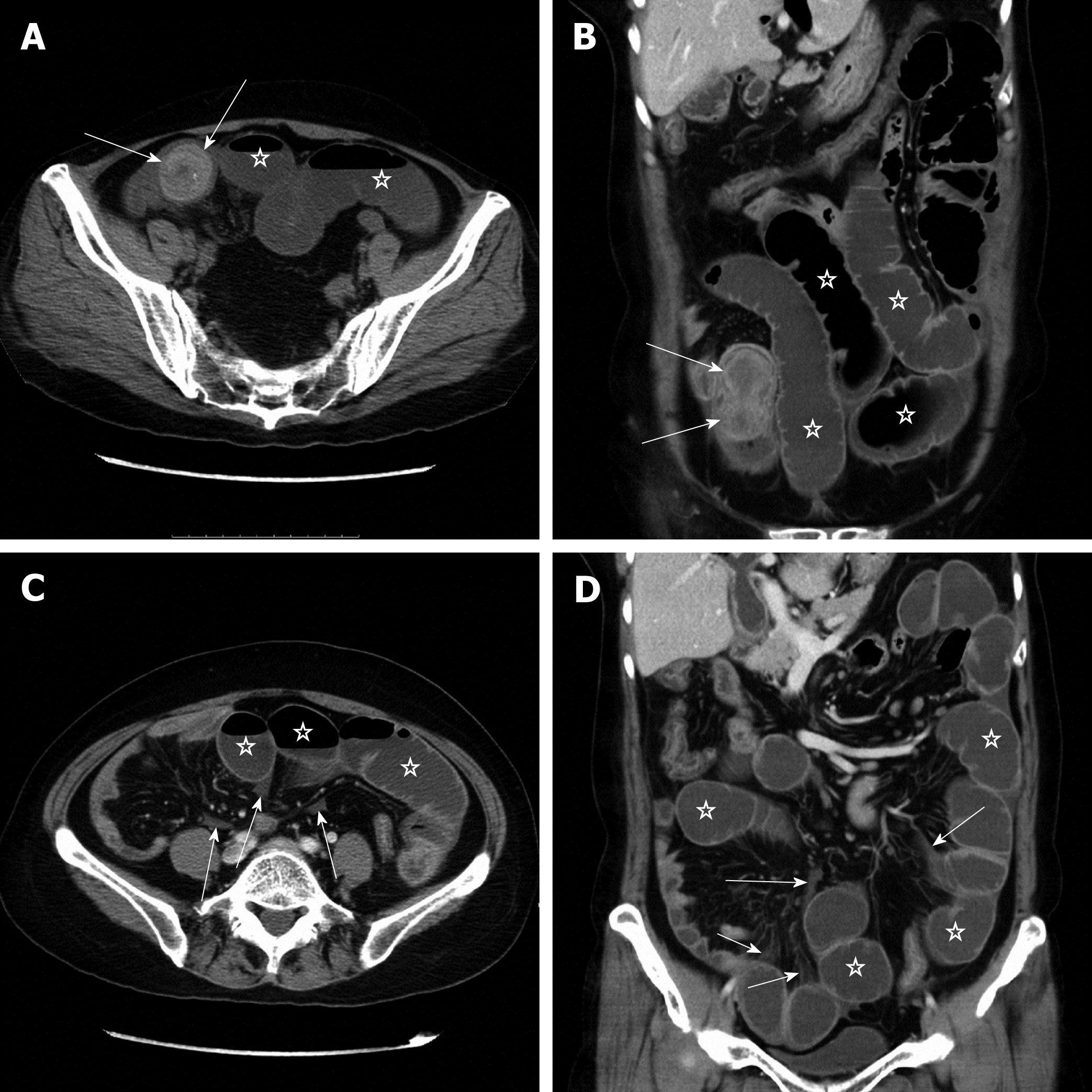
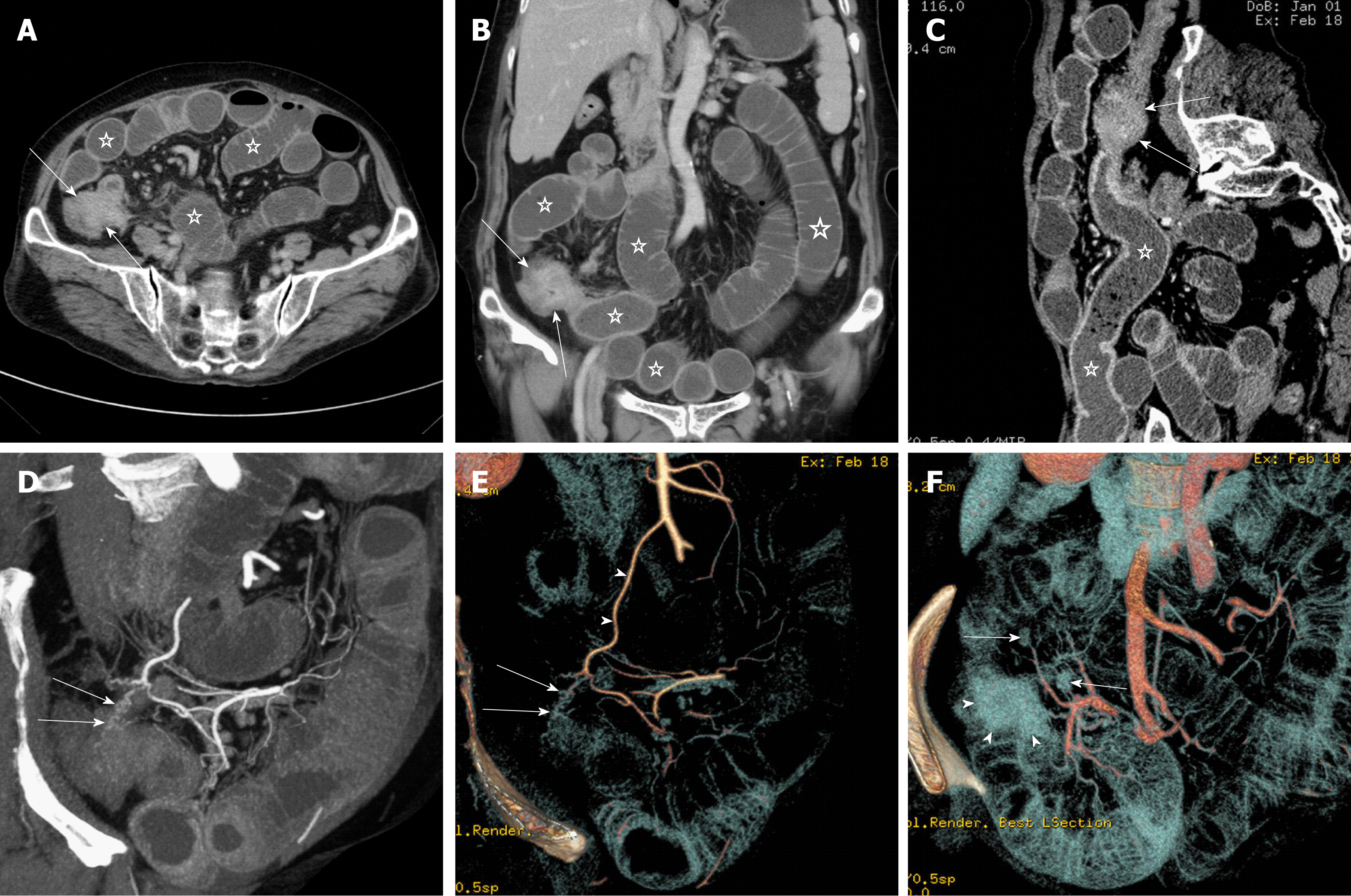
Figure 1 shows the flow chart of three protocols for disease assessment in patients with clinically suspected SBO. Protocol 1 consists of conventional axial (5-mm-thick sections at 5-mm intervals) and coronal (3-mm-thick sections at 3-mm intervals) reformations. Protocol 2 involves integration of multiple post-processing techniques, i.e., after conventional axial and coronal reformations, readers perform multi-planar reformations (MPR), curved planar reformations (CPR), maximum intensity projection (MIP), and volume rendering (VR) to assess SBO and secondary bowel ischemia (Figure 2). Protocol 3 is an optimization of the multiple post-processing techniques. The readers first perform the conventional axial and coronal reformations. If all assessment parameters can be evaluated with high self-confidence and satisfaction, the readers will not continue with the multiple post-processing techniques (Figure 3). If any of the assessment parameters are difficult to identify, the readers selectively add and perform MPR, CPR, MIP, or VR, until they are satisfied with the disease assessment (Figure 4).
Two radiologists with subspecialty training in abdominal imaging served as independent readers blinded to patient identification and clinical information. Training sessions were held for each reader including post-processing and review of five SBO patients on the workstation before the interpretation sessions, to allow them to become familiar with the three protocols and to freely perform the multiple post-processing techniques. Readers were first asked to independently perform protocol 1, then one month later to perform protocol 2, and again one month later to perform protocol 3 and image interpretation on a workstation (Figure 1).
Readers were asked to identify whether SBO was present, the obstruction site, severity, and cause, as well as secondary bowel ischemia based on a list of imaging findings on a worksheet (Table 1). The diagnostic self-confidence for each assessment parameter was determined using a continuous five-grade scoring system from 1 to 5 (1 = worst, 5 = best). The obstruction severity based on bowel distension was graded as none (< 2.5 cm), mild (2.5 to < 3 cm), moderate (3 to < 4 cm), or severe (≥ 4 cm). The obstruction site was categorized as proximal (duodenum to proximal jejunum), middle (distal jejunum to middle ileum), distal (distal ileum), or multi-segmental (involving more than one segment) according to the modified Cole’s method[16].
| Presence or absence of obstruction | External hernias |
| Presence (≥ 2.5 cm) | Bezoars/stones |
| Absence (< 2.5 cm) | Vascular lesions |
| Severity of obstruction | Inflammatory lesions |
| Mild (2.5 to < 3 cm) | Others (e.g., foreign body, abdominal cocoon, hematoma and so on) |
| Moderate (3 to < 4 cm) | Findings of secondary bowel ischemia |
| Severe (≥ 4 cm) | Main sings |
| Obstruction site | Increased bowel wall attenuation |
| Proximal (duodenum to proximal jejunum) | Decreased bowel wall enhancement |
| Middle (distal jejunum to mid-ileum) | Vascular embolus/thrombosis |
| Distal (distal ileum) | Vascular stenosis/occlusion |
| Multisegmental (more than one segment) | Pneumatosis/portomesenteric gas |
| Cause | Secondary sings |
| Adhesions | Bowel wall thickening |
| Neoplasms | Increased bowel wall enhancement |
| Intussusception | Mesenteric fluid/haziness |
| Neoplasms | Mesenteric vascular engorgement |
| Non-neoplasms | Small bowel feces sign |
| Volvulus | Ascites |
| Hernias | Others (solid organ infarction, free gas and so on) |
| Internal hernias | Other findings (fistula, leakage or perforation, pneumoperitoneum and so on) |
The diagnostic self-confidence, agreement, detection rate, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for the three protocols. The agreement was determined by using κ values ranging from 0 to 1 that were graded as follows: poor (< 0.40), moderate (0.40 to < 0.60), good (0.60 to < 0.80), or very good (0.80-1.00) agreement.
Results are given as the mean ± standard deviation. Continuous variables were tested for normality and equality of variances using the Kolmogorov-Smirnov-test and the Levene F test, respectively. The Student’s t-test, Pearson χ2 test, and Fisher exact test were used, as appropriate. The significance of the differences in all data among the three protocols was evaluated by either one-way or two-way analysis of variance. All statistical tests were performed with software (SPSS, version 20; SPSS, Chicago, IL, United States), and two-tailed P < 0.05 was considered to indicate a significant difference.
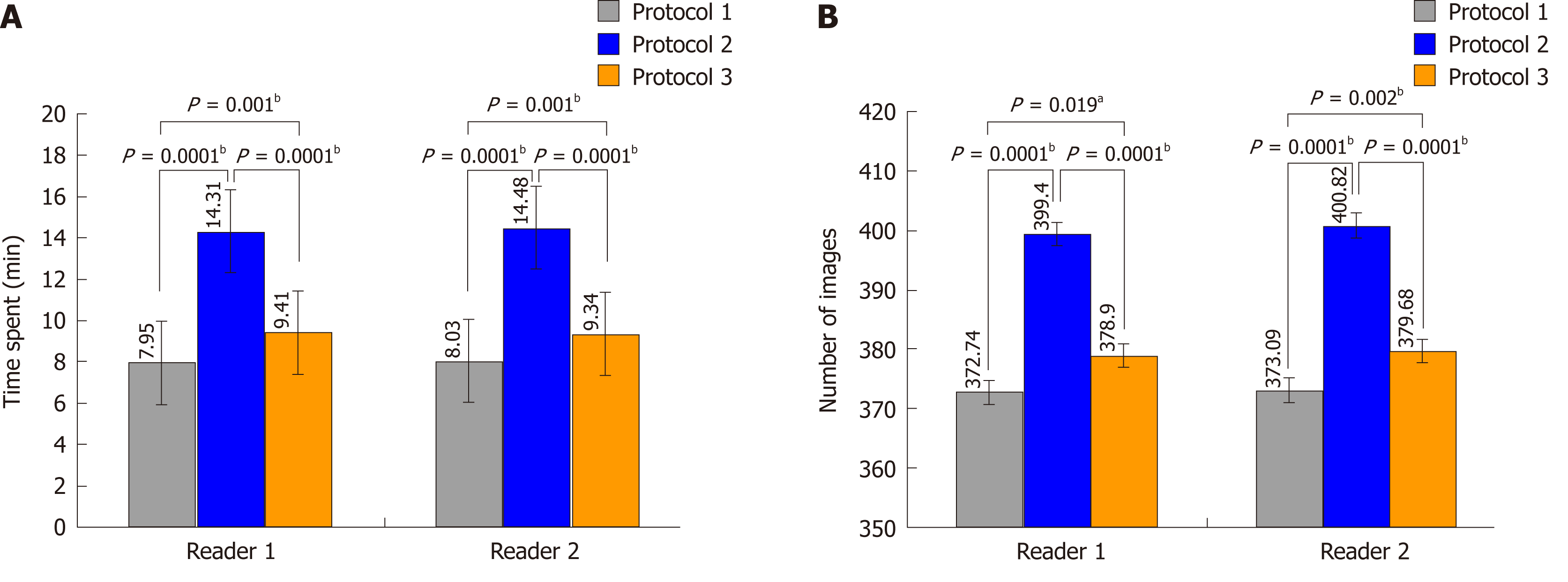
Protocol 2 took longer to perform than protocols 1 and 3 (P < 0.01) (Figure 5A). The two readers spent almost the same time using the same protocol (P > 0.05). Similarly, protocol 2 resulted in significantly more images than protocols 1 and 3 (P < 0.01) (Figure 5B), but the two readers produced an almost equal number of images for the same protocol (P > 0.05).
The frequencies at which the two readers used multiple post-processing techniques in protocol 3 are summarized in Table 2. The two readers seldom or never used multiple post-processing techniques to determine whether SBO was present or obstruction severity. The two readers added multiple post-processing techniques for 6 (5.7%) and 7 patients (6.6%), respectively, for obstruction site identification. Most of these focused on distal and middle obstructions. The two readers added multiple post-processing techniques when evaluating the causes in 31 (29.2%) and 37 patients (34.9%), respectively. These causes were mainly diagnosed as vascular lesions, internal hernias, volvulus, and neoplastic intussusception. Multiple post-processing techniques were added to evaluate secondary bowel ischemia by reader 1 in 34 (32.1%) patients and by reader 2 in 32 (30.2%). In these patients, they were mainly used to observe vascular stenosis, occlusion, embolus and thrombosis, and mesenteric vascular engorgement.
| Assessment parameter | Reader 1 | Reader 2 | P-value |
| Presence or absence of SBO (n = 106) | 1 (0.9) | 2 (1.9) | 1.000 |
| Severity (n = 106) | 0 | 0 | |
| Obstruction site | |||
| No (n = 16) | 1 (6.3) | 2 (12.5) | 1.000 |
| Proximal (n = 9) | 0 | 0 | |
| Middle (n = 37) | 2 (5.4) | 1 (2.7) | 0.556 |
| Distal (n = 44) | 3 (6.8) | 4 (9.1) | 0.694 |
| Total (n = 106) | 6 (5.7) | 7 (6.6) | 1.000 |
| Cause | |||
| No (n = 16) | 1 (6.3) | 2 (12.5) | 1.000 |
| Adhesions (n = 20) | 2 (10.0) | 1 (5.0) | 0.548 |
| Neoplasms (n = 11) | 1 (9.1) | 3 (27.3) | 0.269 |
| Intussusception | |||
| Neoplasms (n = 9) | 2 (22.2) | 2 (22.2) | 1.000 |
| Nonneoplasms (n = 5) | 3 (60.0) | 2 (40.0) | 0.527 |
| Volvulus (n = 8) | 6 (75.0) | 7 (87.5) | 0.522 |
| Hernias | |||
| Internal hernias (n = 6) | 6 (100) | 6 (100) | 1.000 |
| External hernias (n = 9) | 1(11.1) | 2 (22.2) | 0.527 |
| Bezoars/stones (n = 7) | 0 | 2 (28.6) | 0.127 |
| Vascular lesions (n = 8) | 8 (100) | 8 (100) | 1.000 |
| Inflammatory lesions (n = 7) | 1 (14.3) | 2 (28.6) | 0.515 |
| Total (n = 106) | 31 (29.2) | 37 (34.9) | 0.377 |
| Findings of secondary bowel ischemia | |||
| Increased bowel wall attenuation | 0 | 0 | |
| Decreased bowel wall enhancement | 0 | 0 | |
| Vascular embolus/thrombosis | 5 (4.7) | 5 (4.7) | 1.000 |
| Vascular stenosis/occlusion | 18 (17) | 15 (14.2) | 0.570 |
| Pneumatosis/portomesenteric gas | 1 (0.9) | 2 (1.9) | 1.000 |
| Bowel wall thickening | 0 | 0 | |
| Increased bowel wall enhancement | 0 | 0 | |
| Mesenteric haziness/fluid | 2 (1.9) | 0 | 0.477 |
| Mesenteric vascular engorgement | 8 (7.5) | 10 (9.4) | 0.622 |
| Small bowel feces sign | 0 | 0 | |
| Ascites | 0 | 0 | |
| Total (n = 106) | 34 (32.1) | 32 (30.2) | 0.767 |
Table 3 shows the readers’ self-confidence scores for each protocol. Overall, the two readers showed no difference in self-confidence scores for any assessment parameter within the same protocol (P > 0.05). However, for assessment parameters including middle-segmental SBO, adhesions, neoplasms, intussusception, volvulus, internal hernia and vascular diseases in the obstruction causes, vascular embolus/thrombosis, vascular stenosis/occlusion, mesenteric haziness/fluid, and mesenteric vascular engorgement in the findings of secondary bowel ischemia, there were differences in the self-confidence scores between the three protocols (P < 0.01 or 0.05). Protocols 2 and 3 had higher self-confidence scores than protocol 1, but there was no difference between protocols 2 and 3.
| Assessment parameter | Protocol 1 | Protocol 2 | Protocol 3 | P-value | ||||||
| Reader 1 | Reader 2 | P-value | Reader 1 | Reader 2 | P-value | Reader 1 | Reader 2 | P-value | ||
| Presence or absence of SBO | ||||||||||
| Presence | 4.8 ± 0.5 | 4.7 ± 0.6 | 0.070 | 4.8 ± 0.4 | 4.9 ± 0.3 | 0.103 | 4.9 ± 0.4 | 4.8 ± 0.4 | 0.184 | 0.110 |
| Absence | 4.6 ± 0.7 | 4.8 ± 0.6 | 0.270 | 4.9 ± 0.3 | 4.8 ± 0.4 | 0.164 | 4.7 ± 0.5 | 4.9 ± 0.3 | 0.188 | 0.341 |
| Severity of obstruction | ||||||||||
| No | 4.6 ± 0.7 | 4.8 ± 0.6 | 0.270 | 4.9 ± 0.3 | 4.8 ± 0.4 | 0.164 | 4.7 ± 0.5 | 4.9 ± 0.3 | 0.188 | 0.341 |
| Mild | 4.4 ± 0.7 | 4.5 ± 0.7 | 0.162 | 4.8 ± 0.5 | 4.7 ± 0.5 | 0.428 | 4.7 ± 0.6 | 4.6 ± 0.6 | 0.666 | 0.753 |
| Moderate | 4.8 ± 0.4 | 4.7 ± 0.4 | 0.323 | 4.8 ± 0.4 | 4.8 ± 0.4 | 0.710 | 4.7 ± 0.6 | 4.8 ± 0.4 | 0.133 | 0.418 |
| Severe | 4.9 ± 0.4 | 4.8 ± 0.4 | 0.326 | 4.9 ± 0.4 | 4.9 ± 0.4 | 1.000 | 4.9 ± 0.3 | 4.9 ± 0.4 | 0.663 | 0.872 |
| Obstruction site | ||||||||||
| Proximal | 4.4 ± 0.7 | 4.4 ± 0.5 | 1.000 | 4.8 ± 0.4 | 4.9 ± 0.3 | 0.594 | 4.7 ± 0.5 | 4.8 ± 0.4 | 0.681 | 0.323 |
| Middle | 4.2 ± 0.9 | 4.3 ± 0.8 | 0.054 | 4.6 ± 0.5 | 4.7 ± 0.5 | 0.058 | 4.4 ± 0.7 | 4.6 ± 0.6 | 0.710 | 0.004b |
| Distal | 4.4 ± 1.0 | 4.5 ± 0.9 | 0.083 | 4.7 ± 0.5 | 4.7 ± 0.5 | 0.570 | 4.6 ± 0.6 | 4.6 ± 0.6 | 0.660 | 0.221 |
| Cause | ||||||||||
| Adhesions | 3.6 ± 0.8 | 3.7 ± 0.8 | 0.083 | 4.7 ± 0.5 | 4.8 ± 0.4 | 0.428 | 4.5 ± 0.6 | 4.6 ± 0.6 | 0.163 | 0.001b |
| Neoplasms | 4.1 ± 0.7 | 4.2 ± 0.6 | 0.341 | 4.9 ± 0.3 | 4.8 ± 0.4 | 0.588 | 4.7 ± 0.5 | 4.6 ± 0.5 | 0.588 | 0.001b |
| Intussusception | ||||||||||
| Neoplasms | 4.1 ± 1.1 | 4.2 ± 1.0 | 0.594 | 4.9 ± 0.3 | 4.8 ± 0.4 | 0.594 | 4.7 ± 0.5 | 4.6 ± 0.5 | 0.594 | 0.019a |
| Nonneoplasms | 4.2 ± 0.8 | 4.0 ± 0.7 | 0.374 | 4.8 ± 0.4 | 4.8 ± 0.4 | 1.000 | 4.6 ± 0.5 | 4.8 ± 0.4 | 0.374 | 0.029a |
| Volvulus | 4.4 ± 0.7 | 4.5 ± 0.8 | 0.351 | 5.0 ± 0.0 | 4.9 ± 0.4 | 0.351 | 4.6 ± 0.5 | 4.8 ± 0.5 | 0.598 | 0.041a |
| Hernias | ||||||||||
| Internal hernias | 3.3 ± 1.4 | 3.5 ± 1.0 | 0.611 | 4.7 ± 0.5 | 4.8 ± 0.4 | 0.611 | 4.3 ± 0.8 | 4.5 ± 0.5 | 0.695 | 0.002b |
| External hernias | 4.7 ± 0.5 | 4.6 ± 0.7 | 0.347 | 4.9 ± 0.3 | 5.0 ± 0.0 | 0.347 | 4.9 ± 0.3 | 4.9 ± 0.3 | 1.000 | 0.054 |
| Bezoars/stones | 4.6 ± 0.5 | 4.7 ± 0.5 | 0.356 | 5.0 ± 0.0 | 4.9 ± 0.4 | 0.356 | 4.9 ± 0.4 | 4.7 ± 0.5 | 0.356 | 0.492 |
| Vascular lesions | 3.2 ± 1.3 | 3.3 ± 1.1 | 0.594 | 4.7 ± 0.5 | 4.6 ± 0.5 | 0.594 | 4.4 ± 0.5 | 4.3 ± 0.5 | 0.681 | 0.001b |
| Inflammatory lesions | 3.9 ± 1.3 | 4.1 ± 1.1 | 0.356 | 4.7 ± 0.5 | 4.4 ± 0.5 | 0.172 | 4.3 ± 1.0 | 4.4 ± 0.5 | 0.604 | 0.581 |
| Findings of secondary bowel ischemia | ||||||||||
| Increased bowel wall attenuation | 4.4 ± 0.5 | 4.5 ± 0.5 | 0.539 | 4.7 ± 0.5 | 4.7 ± 0.5 | 1.000 | 4.7 ± 0.5 | 4.6 ± 0.5 | 0.664 | 0.819 |
| Decreased bowel wall enhancement | 4.5 ± 0.5 | 4.4 ± 0.5 | 0.572 | 4.6 ± 0.5 | 4.7 ± 0.5 | 0.536 | 4.6 ± 0.5 | 4.7 ± 0.5 | 0.721 | 0.282 |
| Vascular embolus/thrombosis | 4.0 ± 0.9 | 4.1 ± 1.0 | 0.598 | 4.9 ± 0.4 | 4.8 ± 0.5 | 0.598 | 4.6 ± 0.5 | 4.5 ± 0.5 | 0.598 | 0.010a |
| Vascular stenosis/occlusion | 3.8 ± 0.8 | 3.9 ± 0.9 | 0.503 | 4.8 ± 0.4 | 4.7 ± 0.5 | 0.265 | 4.4 ± 0.5 | 4.5 ± 0.6 | 0.574 | 0.001b |
| Pneumatosis/portomesenteric gas | 4.1 ± 0.3 | 4.3 ± 0.7 | 0.447 | 4.4 ± 0.5 | 4.7 ± 0.4 | 0.081 | 4.6 ± 0.5 | 4.7 ± 0.5 | 0.681 | 0.814 |
| Bowel wall thickening | 4.5 ± 0.5 | 4.6 ± 0.5 | 0.321 | 4.6 ± 0.5 | 4.7 ± 0.5 | 0.109 | 4.6 ± 0.5 | 4.7 ± 0.5 | 0.254 | 0.059 |
| Increased bowel wall enhancement | 4.6 ± 0.5 | 4.8 ± 0.4 | 0.231 | 4.7 ± 0.5 | 4.8 ± 0.4 | 0.161 | 4.8 ± 0.4 | 4.7 ± 0.5 | 0.536 | 0.441 |
| Mesenteric haziness/fluid | 4.3 ± 0.6 | 4.3 ± 0.6 | 0.571 | 4.7 ± 0.5 | 4.8 ± 0.4 | 0.292 | 4.6 ± 0.5 | 4.7 ± 0.5 | 0.571 | 0.003b |
| Mesenteric vascular engorgement | 4.2 ± 0.6 | 4.1 ± 0.6 | 0.748 | 4.8 ± 0.4 | 4.9 ± 0.4 | 0.494 | 4.7 ± 0.5 | 4.8 ± 0.4 | 0.716 | 0.001b |
| Small-bowel feces sign | 4.4 ± 0.5 | 4.6 ± 0.6 | 0.329 | 4.7 ± 0.5 | 4.6 ± 0.5 | 0.576 | 4.5 ± 0.5 | 4.6 ± 0.6 | 0.428 | 0.748 |
| Ascites | 4.7 ± 0.5 | 4.6 ± 0.5 | 0.583 | 4.9 ± 0.4 | 4.8 ± 0.4 | 0.671 | 4.8 ± 0.4 | 4.7 ± 0.5 | 0.583 | 0.848 |
There was a very good agreement (κ range: 0.830-0.967) in the identification of SBO and obstruction severity and site among the three protocols by both readers. The overall inter- and intra-reader agreement was good or very good (κ range: 0.733-0.924) in the evaluation of cause. There was a moderate or good agreement (κ range: 0.572-0.696) between the two readers in protocol 1, and between protocols 1 and 2 and protocols 1 and 3 for each reader for evaluating secondary bowel ischemia. Also for secondary bowel ischemia evaluation, there was a very good agreement (κ range: 0.795-0.863) between the two readers in protocols 2 and between protocols 2 and 3 for each reader (Table 4).
| Assessment parameter | Inter-reader agreement | Intra-reader agreement | |||||||
| Protocol 1 | Protocol 2 | Protocol 3 | Reader 1 | Reader 2 | |||||
| Reader 1-2 | Reader 1-2 | Reader 1-2 | Protocol 1-2 | Protocol 1-3 | Protocol 2-3 | Protocol 1-2 | Protocol 1-3 | Protocol 2-3 | |
| Presence or absence of SBO (n = 106) | 0.902 | 0.897 | 0.966 | 0.866 | 0.902 | 0.964 | 0.933 | 0.897 | 0.967 |
| Severity of obstruction (n = 106) | 0.868 | 0.869 | 0.894 | 0.830 | 0.882 | 0.895 | 0.881 | 0.907 | 0.921 |
| Obstruction site (n = 106) | 0.873 | 0.926 | 0.925 | 0.855 | 0.890 | 0.925 | 0.908 | 0.870 | 0.963 |
| Cause | |||||||||
| No (n = 16) | 0.902 | 0.897 | 0.966 | 0.866 | 0.902 | 0.964 | 0.933 | 0.897 | 0.967 |
| Adhesions (n = 20) | 0.725 | 0.820 | 0.820 | 0.725 | 0.786 | 0.759 | 0.820 | 0.820 | 1.000 |
| Neoplasms (n = 11) | 0.946 | 1.000 | 0.946 | 0.946 | 0.946 | 1.000 | 1.000 | 0.946 | 0.946 |
| Intussusception | |||||||||
| Neoplasms (n = 9) | 0.927 | 0.935 | 0.935 | 0.863 | 0.927 | 0.935 | 0.863 | 0.935 | 0.935 |
| Nonneoplasms (n = 5) | 0.738 | 0.883 | 0.883 | 0.883 | 0.738 | 0.883 | 0.738 | 0.883 | 0.883 |
| Volvulus (n = 8) | 0.927 | 0.927 | 0.927 | 0.845 | 0.927 | 0.927 | 1.000 | 0.927 | 0.927 |
| Hernias | |||||||||
| Internal hernias (n = 6) | 0.554 | 0.903 | 0.788 | 0.789 | 0.649 | 0.903 | 0.739 | 0.739 | 1.000 |
| External hernias (n = 9) | 0.935 | 0.935 | 1.000 | 0.935 | 1.000 | 0.935 | 0.935 | 0.935 | 1.000 |
| Bezoars/stones (n = 7) | 0.917 | 0.917 | 1.000 | 0.917 | 1.000 | 0.917 | 0.917 | 0.917 | 1.000 |
| Vascular lesions (n = 8) | 0.554 | 0.927 | 0.751 | 0.522 | 0.419 | 0.845 | 0.518 | 0.711 | 0.845 |
| Inflammatory lesions (n = 7) | 0.371 | 0.586 | 0.637 | 0.444 | 0.411 | 0.637 | 0.363 | 0.333 | 0.751 |
| Total (n = 106) | 0.733 | 0.861 | 0.848 | 0.748 | 0.760 | 0.848 | 0.772 | 0.797 | 0.924 |
| Presence or absence of secondary bowel ischemia (n = 106) | 0.572 | 0.863 | 0.840 | 0.675 | 0.645 | 0.795 | 0.690 | 0.696 | 0.863 |
However, there were some differences among the inter- and intra-reader agreement results in determining a few of specific causes. For internal hernias, the agreement between the two readers in protocol 1 was only moderate, but a good or very good agreement was found between the two readers in protocols 2 and 3. Similarly, for vascular lesions, protocols 2 and 3 had better inter-reader agreement than protocol 1, and the intra-reader agreement between protocols 2 and 3 was also superior to that between protocols 1 and 2 and protocols 1 and 3. Especially for inflammatory lesions, a good agreement was only found between the two readers in protocol 3 and between protocols 2 and 3 for each reader (Table 4).
The detection rate for the three protocols used to assess SBO is presented in Table 5. Overall, for the same protocol, the detection rate was not different between the two readers for any assessment parameter (P > 0.05). However, comparison of the detection rate among the three protocols revealed a difference in the total detection rate for causes and secondary bowel ischemia (P < 0.01). Protocols 2 and 3 gave a higher detection rate than protocol 1, but no difference was seen between protocols 2 and 3 (P > 0.05). For each specific cause, protocols 2 and 3 had a higher detection rate than protocol 1 for evaluating internal hernia and vascular lesions (P < 0.01 or 0.05).
| Assessment parameter | Protocol 1 | Protocol 2 | Protocol 3 | P-value | ||||||
| Reader 1 | Reader 2 | P-value | Reader 1 | Reader 2 | P-value | Reader 1 | Reader 2 | P-value | ||
| Presence or absence of SBO | ||||||||||
| Presence (n = 90) | 85 (94.4) | 87 (96.7) | 0.469 | 89 (98.9) | 87 (96.7) | 0.312 | 88 (97.8) | 88 (97.8) | 1.000 | 0.378 |
| Absence (n = 16) | 15 (93.8) | 14 (87.5) | 0.544 | 15 (93.8) | 16 (100) | 0.310 | 15 (93.8) | 16 (100) | 0.310 | 0.430 |
| Obstruction site | ||||||||||
| No (n = 16) | 15 (93.8) | 14 (87.5) | 0.544 | 15 (93.8) | 16 (100) | 0.310 | 15 (93.8) | 16 (100) | 0.310 | 0.430 |
| Proximal (n = 9) | 8 (88.9) | 7 (77.8) | 0.527 | 9 (100) | 8 (88.9) | 0.303 | 8 (88.9) | 8 (88.9) | 1.000 | 0.570 |
| Middle (n = 37) | 34 (91.9) | 33 (89.2) | 0.691 | 34 (91.9) | 35 (94.6) | 0.643 | 35 (94.6) | 34 (91.9) | 0.643 | 0.775 |
| Distal (n = 44) | 40 (90.9) | 41 (93.2) | 0.694 | 43 (97.7) | 43 (97.7) | 1.000 | 42 (95.5) | 43 (97.7) | 0.557 | 0.160 |
| Total (n = 106) | 97 (91.5) | 95 (89.6) | 1.000 | 101 (95.3) | 102 (96.2) | 1.000 | 100 (94.3) | 101 (95.3) | 1.000 | 0.129 |
| Cause | ||||||||||
| No (n = 16) | 15 (93.8) | 14 (87.5) | 0.544 | 15 (93.8) | 16 (100) | 0.310 | 15 (93.8) | 16 (100) | 0.310 | 0.430 |
| Adhesions (n = 20) | 18 (90.0) | 19 (95.0) | 0.548 | 19 (95.0) | 20 (100) | 0.311 | 19 (95.0) | 20 (100) | 0.311 | 0.434 |
| Neoplasms (n = 11) | 10 (90.9) | 11 (100) | 0.306 | 11 (100) | 11 (100) | 1.000 | 11 (100) | 10 (90.9) | 0.306 | 0.597 |
| Intussusception | ||||||||||
| Neoplasms (n = 9) | 7 (77.8) | 8 (88.9) | 0.527 | 9 (100) | 8 (88.9) | 0.303 | 8 (88.9) | 9 (100) | 0.303 | 0.414 |
| Nonneoplasms (n = 5) | 4 (80.0) | 4 (80.0) | 1.000 | 5 (100) | 4 (80.0) | 0.292 | 4 (80.0) | 5 (100) | 0.292 | 0.749 |
| Volvulus (n = 8) | 7 (87.5) | 8 (100) | 0.302 | 7 (87.5) | 8 (100) | 0.302 | 8 (100) | 7 (87.5) | 0.302 | 1.000 |
| Hernias | ||||||||||
| Internal hernias (n = 6) | 4 (66.7) | 3 (50.0) | 0.558 | 6 (100) | 5 (83.3) | 0.296 | 5 (83.3) | 5 (83.3) | 1.000 | 0.045a |
| External hernias (n = 9) | 9 (100) | 8 (88.9) | 0.303 | 8 (88.9) | 9 (100) | 0.303 | 9 (100) | 9 (100) | 1.000 | 0.595 |
| Bezoars/stones (n = 7) | 7 (100) | 6 (85.7) | 0.299 | 6 (85.7) | 7 (100) | 0.299 | 7 (100) | 7 (100) | 1.000 | 0.592 |
| Vascular lesions (n = 8) | 3 (37.5) | 4 (50.0) | 0.614 | 8 (100) | 7 (87.5) | 0.302 | 6 (75.0) | 7 (87.5) | 0.522 | 0.004b |
| Inflammatory lesions (n = 7) | 6 (85.7) | 6 (85.7) | 1.000 | 6 (85.7) | 5 (71.4) | 0.515 | 6 (85.7) | 5 (71.4) | 0.515 | 0.857 |
| Total (n = 106) | 90 (84.9) | 91 (85.8) | 1.000 | 100 (94.3) | 100 (94.3) | 1.000 | 98 (92.5) | 100 (94.3) | 1.000 | 0.001b |
| Presence or absence of secondary bowel ischemia | ||||||||||
| Presence (n = 38) | 28 (73.7) | 27 (71.1) | 1.000 | 36 (94.7) | 35 (92.1) | 0.644 | 34 (89.5) | 36 (94.7) | 0.395 | 0.001b |
| Absence (n = 68) | 65 (95.6) | 67 (98.5) | 0.308 | 65 (95.6) | 66 (97.1) | 0.647 | 66 (97.1) | 65 (95.6) | 0.647 | 0.928 |
The sensitivity, specificity, PPV, NPV, and accuracy of the three protocols for assessment of SBO are shown in Table 6. Protocol 1 had high sensitivity, PPV, and accuracy for determining the presence or absence of SBO, whereas protocols 2 and 3 appeared to have slightly better specificity and NPV. Both protocols 2 and 3 improved the sensitivity, specificity, PPV, NPV, and accuracy in the identification of the obstruction sites, and had clearly superior specificity and NPV. Protocol 1 had high sensitivity for the evaluation of causes, whereas protocols 2 and 3 showed greater advantages in increasing specificity, PPV, NPV, and accuracy. Protocol 1 gave high specificity and PPV in evaluating secondary bowel ischemia, and the main advantages of protocols 2 and 3 were to substantially improve sensitivity, NPV, and accuracy. However, all the above parameter values were relatively close when comparing protocol 2 with protocol 3.
| Assessment parameter | Protocol 1 | Protocol 2 | Protocol 3 | |||
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| Presence or absence of SBO | ||||||
| Sensitivity | 94.4% | 96.7% | 98.9% | 96.7% | 97.8% | 97.8% |
| Specificity | 93.8% | 87.5% | 93.8% | 100% | 93.8% | 100% |
| PPV | 98.8% | 97.8% | 98.9% | 100% | 98.9% | 100% |
| NPV | 75.0% | 82.4% | 93.8% | 84.2% | 88.2% | 88.9% |
| Accuracy | 94.3% | 95.3% | 98.1% | 97.2% | 97.2% | 98.1% |
| Obstruction site | ||||||
| Sensitivity | 94.3% | 96.4% | 98.9% | 96.6% | 97.7% | 97.7% |
| Specificity | 78.9% | 63.6% | 78.9% | 94.1% | 78.9% | 84.2% |
| PPV | 95.3% | 91.0% | 95.6% | 98.9% | 95.5% | 96.6% |
| NPV | 75.0% | 82.4% | 93.8% | 84.2% | 88.2% | 88.9% |
| Accuracy | 91.5% | 89.6% | 95.3% | 96.2% | 94.3% | 95.3% |
| Cause | ||||||
| Sensitivity | 93.8% | 96.3% | 98.8% | 96.6% | 97.6% | 97.7% |
| Specificity | 57.7% | 53.8% | 75.0% | 84.2% | 71.4% | 80.0% |
| PPV | 87.2% | 86.5% | 94.4% | 96.6% | 93.3% | 95.5% |
| NPV | 75.0% | 82.4% | 93.8% | 84.2% | 88.2% | 88.9% |
| Accuracy | 84.9% | 85.8% | 94.3% | 94.2% | 92.5% | 94.3% |
| Secondary bowel ischemia | ||||||
| Sensitivity | 73.7% | 71.1% | 94.7% | 92.1% | 89.5% | 94.7% |
| Specificity | 95.6% | 98.5% | 95.6% | 97.1% | 97.1% | 95.6% |
| PPV | 90.3% | 96.4% | 92.3% | 94.6% | 94.4% | 92.3% |
| NPV | 86.7% | 85.9% | 97% | 95.7% | 94.3% | 97% |
| Accuracy | 87.7% | 88.7% | 95.3% | 95.3% | 94.3% | 95.3% |
Our study is the first to integrate and optimize the MDCT multiple post-processing techniques to develop a more accurate post-processing protocol for SBO assessment. The protocols mainly used MPR and CPR to show the obstruction site and its connected proximal and distal bowel segments[13,14,17-20]. MIP and VR were mainly used to reveal local mesenteric changes and mesenteric vascular diseases, as well as the obstruction site. We designed two new post-processing protocols: protocol 2, which integrates multiple post-processing techniques, and protocol 3, which optimizes the multiple post-processing techniques. By designing a retrospective cross-sectional study to compare their respective advantages and disadvantages with protocol 1, we systemically evaluated the diagnostic accuracy and efficiency of the optimized protocol to exploit the greatest potential of the MDCT post-processing techniques for assessment of SBO and secondary bowel ischemia.
We found that the main deficiency of protocol 2 was that it was inefficient. It took too long to complete and produced more images, which will inevitably increase the workload of radiologists. Therefore, it would be difficult to implement protocol 2 for routine use for assessing each patient in daily clinical practice. Protocol 3 only required slightly more time than protocol 1. Therefore, our findings suggest that protocol 3 is more suitable for the pre-treatment assessment of SBO.
Both readers seldom or never added multiple post-processing techniques when using protocol 3 to evaluate whether SBO was present and the obstruction severity and site. However, they added multiple post-processing techniques at frequencies of 29.2% and 34.9%, respectively, for causes, and 32.1% and 30.2%, respectively, for secondary bowel ischemia. This suggests that protocol 1 is inadequate for identifying secondary bowel ischemia and some causes, but it is not necessary to use the multiple post-processing techniques for all patients with SBO.
Several studies[13-15,20] have reported that MPR or/and CPR can improve diagnostic self-confidence in determining the transition zone of SBO, but fail to define in which specific segment of the small intestine the transition zone is located. In addition, the post-processing techniques used in these studies were incomplete compared with the multiple post-processing techniques in our study. Therefore, the results of these studies[13-15,20] may not truly reflect the value of multiple post-processing techniques in SBO assessment.
Furthermore, our study compared the diagnostic efficacy of our three protocols for each assessment parameter. For evaluating whether SBO was present and obstruction severity, there were no differences among the three protocols with respect to diagnostic self-confidence, agreement, detection rate, and accuracy. These results are consistent with previous findings[5,6,13,14,19,20] and suggest that the conventional axial and coronal reformations may be sufficient to evaluate whether SBO is present and the obstruction severity without the need for additional post-processing techniques.
In contrast with previous studies[13-15,20], our results showed significant differences among the three protocols for the diagnostic self-confidence in locating the obstruction site in middle-segmental SBO. Protocols 2 and 3 had better inter- and intra-reader agreement and improved the diagnostic accuracy. This difference is likely because the previous studies[13-15,20,21] failed to identify in which specific segment of the small intestine the transition zone was located, and when a transition zone was adjacent to the segment boundaries of the small intestine, on conventional post-processing images it may be difficult to determine whether it is in the proximal or middle and middle or distal segment. However, using the multiple post-processing techniques, locating the obstruction sites became relatively easy.
MDCT exhibits excellent diagnostic accuracy compared with plain abdominal radiography and is especially superior in ascertaining the obstruction cause[22-25]. The results of our study showed that both protocols 2 and 3 gave the reader greater self-confidence compared with protocol 1 in the diagnosis of causes, but there was no difference between protocols 2 and 3. Overall, there was also very good inter- and intra-reader agreement, detection rate, accuracy, sensitivity, specificity, PPV and NPV in protocols 2 and 3. With further analysis of each specific cause, it is clear that the main advantage of the multiple post-processing techniques is that they can significantly improve the diagnostic accuracy of both internal hernias and vascular lesions.
The diagnosis of secondary bowel ischemia in the presence of SBO, however, remains more challenging. Reported CT sensitivities are 75%-100% with specificities of 61%-100%[26-29]. Consistent with this, we found that protocol 1 had poor sensitivity and accuracy in the evaluation of secondary bowel ischemia. However, when the readers used protocols 2 and 3, the detection rate, diagnostic accuracy, sensitivity, and NPV were strongly improved to almost the same extent, which we attribute to the use of the multiple post-processing techniques.
The results of our study also showed that protocols 2 and 3 both increased self-confidence in evaluating vascular embolus/thrombosis, vascular stenosis/occlusion, mesenteric haziness/fluid, and mesenteric vascular engorgement as signs of secondary bowel ischemia, and had a very good overall inter- and intra-reader agreement. Therefore, protocols 2 and 3 are highly accurate in evaluating secondary bowel ischemia, which may be mainly because the multiple post-processing techniques increase the display rate of the above-mentioned signs.
Our study had several limitations, including the fact that it was performed retrospectively and thus failed to evaluate the improvement on the optimized protocol in guiding clinical treatment. Second, although our study had a large sample size, the causes of SBO included were limited and did not represent the MDCT characteristics of SBO for all causes. Third, all patients with SBO included in our study were patients who required surgery, which is inconsistent with the need for conservative treatment for most patients with SBO. Thus, the advantages of the optimized protocol relative to the conventional protocol should be considered with caution in clinical practice of MDCT assessment of SBO.
In conclusion, this is the first study that has established and evaluated an optimized protocol of multiple post-processing techniques on MDCT in SBO assessment. The optimized protocol can both guarantee efficiency and comprehensively improve diagnostic accuracy of MDCT for assessing SBO and secondary bowel ischemia, which are critical because these imaging parameters can guide patient care. The widespread use of this protocol should be supported by further prospective, larger-sample clinical trials including identification of its indications.
Acute small bowel obstruction (SBO) is a common clinical syndrome for which effective treatment depends on a rapid and accurate diagnosis. Despite advances in imaging and a better understanding of small bowel pathophysiology, SBO is often diagnosed late or misdiagnosed, resulting in significant morbidity and mortality. Nowadays, multidetector computed tomography (MDCT) with multiple post-processing techniques has shown great potential in assessment of SBO and related complications, and the accuracy and agreement are expected to be further improved.
On the applications of MDCT multiple post-processing techniques in the assessment of SBO, only a few studies on multi-planar reformations have been reported in the current literature. In the MDCT assessment of SBO, how to reasonably apply these post-processing techniques to further improve the diagnostic accuracy is an important issue worth exploring further to radiologists.
This study aimed to integrate and optimize MDCT multiple post-processing techniques, and designed a retrospective cross-sectional study to systemically evaluate diagnostic accuracy and efficiency of the optimized protocol using multiple post-processing techniques on MDCT to assess SBO and secondary bowel ischemia.
This retrospective cross-sectional study was conducted in a single center of China for evaluation of an optimized protocol on multiple post-processing techniques for MDCT assessment of SBO and secondary bowel ischemia. Two radiologists applied three protocols to image post-processing and interpretation for MDCT volume data of 106 patients with clinically suspected SBO. We compared the optimized protocol with the other two protocols based on time spent, number of images, diagnostic self-confidence, agreement, and accuracy of detection of SBO and secondary bowel ischemia.
Using the optimized protocol, two radiologists added multiple post-processing techniques at frequencies of 29.2% and 34.9%, respectively, for obstruction cause, and 32.1% and 30.2%, respectively, for secondary bowel ischemia. The integrated protocol resulted in more time spent and number of images than the conventional and optimized protocols (P < 0.01), for the optimized protocol, the time spent and the number of images were only slightly more than those for the conventional protocol. The integrated and optimized protocols had higher total detection rates of obstruction cause and secondary bowel ischemia than the conventional protocol (P < 0.01), but no difference was detected between the two (P > 0.05). The accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of the integrated and optimized protocols were superior to the conventional protocol for evaluating obstruction cause and secondary bowel ischemia, but these parameters between the two protocols were very close.
This is the first study to establish and evaluate an optimized protocol of the multiple post-processing techniques on MDCT used to assess SBO. The main deficiency of the integrated protocol was that it was inefficient. It took too long to complete and produced more images, which will inevitably increase the workload of radiologists. The optimized protocol can both guarantee the time efficiency and effectively control the number of images, and comprehensively improve the diagnostic self-confidence, agreement, accuracy of MDCT for determining the SBO severity, site and causes, and secondary bowel ischemia, which are critical because these imaging parameters can guide patient care.
Although the present study has several limitations, the optimized protocol can be considered for widespread recommendation in clinical practice of MDCT assessment of SBO and secondary bowel ischemia. Future studies should focus on applying this protocol to prospective, larger-sample clinical trials to further identify its advantages, disadvantages, and indications so that it can be continuously modified and improved.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kaya B, Kwon KA S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Paulson EK, Thompson WM. Review of small-bowel obstruction: the diagnosis and when to worry. Radiology. 2015;275:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Scrima A, Lubner MG, King S, Pankratz J, Kennedy G, Pickhardt PJ. Value of MDCT and Clinical and Laboratory Data for Predicting the Need for Surgical Intervention in Suspected Small-Bowel Obstruction. AJR Am J Roentgenol. 2017;208:785-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Millet I, Boutot D, Faget C, Pages-Bouic E, Molinari N, Zins M, Taourel P. Assessment of Strangulation in Adhesive Small Bowel Obstruction on the Basis of Combined CT Findings: Implications for Clinical Care. Radiology. 2017;285:798-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Chuong AM, Corno L, Beaussier H, Boulay-Coletta I, Millet I, Hodel J, Taourel P, Chatellier G, Zins M. Assessment of Bowel Wall Enhancement for the Diagnosis of Intestinal Ischemia in Patients with Small Bowel Obstruction: Value of Adding Unenhanced CT to Contrast-enhanced CT. Radiology. 2016;280:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Jaffe TA, Martin LC, Thomas J, Adamson AR, DeLong DM, Paulson EK. Small-bowel obstruction: coronal reformations from isotropic voxels at 16-section multi-detector row CT. Radiology. 2006;238:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Yaghmai V, Nikolaidis P, Hammond NA, Petrovic B, Gore RM, Miller FH. Multidetector-row computed tomography diagnosis of small bowel obstruction: can coronal reformations replace axial images? Emerg Radiol. 2006;13:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Dimitriu-Leen AC, van Rosendael AR, Smit JM, van Elst T, van Geloven N, Maaniitty T, Jukema JW, Delgado V, Scholte AJHA, Saraste A, Knuuti J, Bax JJ. Long-Term Prognosis of Patients With Intramural Course of Coronary Arteries Assessed With CT Angiography. JACC Cardiovasc Imaging. 2017;10:1451-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Taslakian B, Latson LA, Truong MT, Aaltonen E, Shiau MC, Girvin F, Alpert JB, Wickstrom M, Ko JP. CT pulmonary angiography of adult pulmonary vascular diseases: Technical considerations and interpretive pitfalls. Eur J Radiol. 2016;85:2049-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Chan FP, Rubin GD. MDCT angiography of pediatric vascular diseases of the abdomen, pelvis, and extremities. Pediatr Radiol. 2005;35:40-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Yu AY, Zerna C, Assis Z, Holodinsky JK, Randhawa PA, Najm M, Goyal M, Menon BK, Demchuk AM, Coutts SB, Hill MD. Multiphase CT angiography increases detection of anterior circulation intracranial occlusion. Neurology. 2016;87:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB. MDCT in Pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol. 2004;182:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Kim HC, Yang DM, Jin W, Park SJ. Added diagnostic value of multiplanar reformation of multidetector CT data in patients with suspected appendicitis. Radiographics. 2008;28:393-405; discussion 405-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Hodel J, Zins M, Desmottes L, Boulay-Coletta I, Jullès MC, Nakache JP, Rodallec M. Location of the transition zone in CT of small-bowel obstruction: added value of multiplanar reformations. Abdom Imaging. 2009;34:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Memon W, Khattak YJ, Alam T, Sconfienza LM, Awais M, Anwar SS. MDCT of Small Bowel Obstruction: How Reliable Are Oblique Reformatted Images in Localizing Point of Transition? Gastroenterol Res Pract. 2014;2014:815802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Aufort S, Charra L, Lesnik A, Bruel JM, Taourel P. Multidetector CT of bowel obstruction: value of post-processing. Eur Radiol. 2005;15:2323-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Morse RW, Cole LG. The anatomy of the normal small intestine as observed roentgenographically. Radiology. 1927;8:149-153. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Wang PY, Wang X, Zhang L, Li HF, Chen L, Wang X, Wang B. Bezoar-induced small bowel obstruction: Clinical characteristics and diagnostic value of multi-slice spiral computed tomography. World J Gastroenterol. 2015;21:9774-9784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Izuishi K, Sano T, Shiota A, Mori H, Ebara K. Small bowel obstruction caused by endometriosis in a postmenopausal woman. Asian J Endosc Surg. 2015;8:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kuang LQ, Zhao DW, Cheng C, Wang Y. Prediction of Small Bowel Obstruction Caused by Bezoars Using Risk Factor Categories on Multidetector Computed Tomographic Findings. Biomed Res Int. 2016;2016:6569103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Keoplung S, Teerasamit W, Suvannarerg V. Diagnosis of bowel obstruction: added value of multiplanar reformations from multidetector CT in comparison with axial planes alone. J Med Assoc Thai. 2013;96:1569-1577. [PubMed] |
| 21. | Colon MJ, Telem DA, Wong D, Divino CM. The relevance of transition zones on computed tomography in the management of small bowel obstruction. Surgery. 2010;147:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Thompson WM, Kilani RK, Smith BB, Thomas J, Jaffe TA, Delong DM, Paulson EK. Accuracy of abdominal radiography in acute small-bowel obstruction: does reviewer experience matter? AJR Am J Roentgenol. 2007;188:W233-W238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Pongpornsup S, Tarachat K, Srisajjakul S. Accuracy of 64 sliced multi-detector computed tomography in diagnosis of small bowel obstruction. J Med Assoc Thai. 2009;92:1651-1661. [PubMed] |
| 24. | Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics. 2009;29:423-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | O'Malley RG, Al-Hawary MM, Kaza RK, Wasnik AP, Platt JF, Francis IR. MDCT findings in small bowel obstruction: implications of the cause and presence of complications on treatment decisions. Abdom Imaging. 2015;40:2248-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Millet I, Taourel P, Ruyer A, Molinari N. Value of CT findings to predict surgical ischemia in small bowel obstruction: A systematic review and meta-analysis. Eur Radiol. 2015;25:1823-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Geffroy Y, Boulay-Coletta I, Jullès MC, Nakache S, Taourel P, Zins M. Increased unenhanced bowel-wall attenuation at multidetector CT is highly specific of ischemia complicating small-bowel obstruction. Radiology. 2014;270:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | He B, Gu J, Huang S, Gao X, Fan J, Sheng M, Wang L, Gong S. Diagnostic performance of multi-slice CT angiography combined with enterography for small bowel obstruction and intestinal ischaemia. J Med Imaging Radiat Oncol. 2017;61:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Nakashima K, Ishimaru H, Fujimoto T, Mizowaki T, Mitarai K, Nakashima K, Matsuoka Y, Uetani M. Diagnostic performance of CT findings for bowel ischemia and necrosis in closed-loop small-bowel obstruction. Abdom Imaging. 2015;40:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |