Published online Feb 14, 2019. doi: 10.3748/wjg.v25.i6.696
Peer-review started: December 8, 2018
First decision: December 28, 2018
Revised: January 14, 2018
Accepted: January 18, 2019
Article in press: January 18, 2019
Published online: February 14, 2019
Processing time: 69 Days and 15.9 Hours
Rectosigmoid endometriosis is an underdiagnosed disease responsible for abdominal pain, transit disorders and rectal bleeding. Two surgical approaches, rectosigmoid bowel resection (segmental or patch) or intramuscular layer dissection (shaving), are available.
To assess whether the lesion features observed via preoperative rectosigmoid endoscopic ultrasonography (RS-EUS) might predict the need for bowel resection.
This multicentric retrospective study was conducted on patients with rectosigmoid endometriosis who underwent a curative surgical procedure, evaluated by RS-EUS performed by two trained operators, between January 2012 and March 2018. A univariate statistical analysis was performed on nodules’ RS-EUS features (thickness, width, infiltration of the submucosae, presence of a bump into the digestive lumen and presence of multiple rectosigmoid localizations). A multivariate logistic regression was then performed on the significant results.
Of the 367 patients, 73 patients with rectosigmoid endometriosis were evaluated by RS-EUS and underwent rectosigmoid surgery. After the univariate analysis was completed, thickness, width and infiltration of the submucosae were identified as potential predictive factors for bowel resection. In a multivariate logistic regression model, only thickness appeared to be a significant [odds ratio (OR) = 1.49, 95% confidence interval (CI): 1.04-2.12, P = 0.028] predictive factor for bowel resection. Receiver operating characteristic analysis performed showed that a thickness over 5.20 mm might be used as cut-off with a sensitivity of 76%, a specificity of 81%, and an area under carve = 0.82. The cut-off values for 100% sensitivity and 100% specificity were 0.90 mm and 10.00 mm, respectively. A trend concerning width to predict the need for resection was also observed (OR 1.12, 95%CI: 1.00-1.26, P = 0.054)
The presence of a rectosigmoid nodule of endometriosis greater than 5.20 mm thick on RS-EUS might predict the need for bowel resection.
Core tip: Rectosigmoid endometriosis is an underdiagnosed disease responsible for abdominal pain, transit disorders and rectal bleeding. The treatment can be either medical or surgical. If a surgical resection of the bowel is needed, it must be performed by a multidisciplinary team. The aim of our study was to assess whether the lesion features observed via preoperative rectosigmoid endoscopic ultrasonography (RS-EUS), a key exam in this condition, might predict the need for bowel resection. We found that the presence of a rectosigmoid nodule of endometriosis greater than 5.20 mm thick on RS-EUS might predict the need for bowel resection.
- Citation: Desplats V, Vitte RL, du Cheyron J, Roseau G, Fauconnier A, Moryoussef F. Preoperative rectosigmoid endoscopic ultrasonography predicts the need for bowel resection in endometriosis. World J Gastroenterol 2019; 25(6): 696-706
- URL: https://www.wjgnet.com/1007-9327/full/v25/i6/696.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i6.696
Endometriosis is a benign estrogen-dependent disease associated with pelvic pain and infertility, affecting 10% to 15% of women of childbearing age[1]. Intestinal endometriosis is the most common extra-gynecologic localization[2], affecting 3% to 38% of women with endometriosis; this condition can be responsible for rectal bleeding, transit disorders and dyschezia, leading to an altered quality of life[3]. The decision on whether endometriosis requires surgical treatment depends on many factors, such as age, clinical findings, reproductive expectations and sites affected by the disease[4]. Although medical treatment is effective for the control of symptoms, it has no impact on organ lesions, and a surgical treatment might be needed.
Two surgical approaches are available for rectosigmoid endometriosis: intramuscular layer dissection (shaving) or rectosigmoid bowel resection (segmental or patch). Shaving is a conservative technique with some advantages: shorter operative time, shorter hospitalization time, and fewer peroperative complications[5]. Moreover, resection techniques may lead to more postoperative digestive disabilities compared with shaving, such as unsuccessful evacuatory attempts, feeling of incomplete evacuation, abdominal pain and painful evacuation effort[6]. Choice is led by clinical and radiological factors. The shaving technique is preferred for single, nonsymptomatic lesions, whereas the resection technique appears to be more adequate for multiple or symptomatic lesions[7]. According to local recommendations, the first-line exam involves pelvic ultrasonography. Magnetic resonance imaging (MRI) and preoperative rectosigmoid endoscopic ultrasonography (RS-EUS) are only considered as second- and third-line exams, respectively, for the evaluation of deep infiltrating lesions[8].
A recent study has shown that a pre-operative MRI-colonography might predict the need for bowel resection in recto-sigmoid endometriosis[9]. However, it has previously been demonstrated that RS-EUS exhibits better sensitivity and negative predictive value than MRI in the diagnosis of rectosigmoid endometriosis[10]. We therefore decided to conduct a study to assess whether the characteristics of endometriosis lesions evaluated on a preoperative rectosigmoid ultrasonography can predict the need for bowel resection in rectosigmoid endometriosis.
We conducted a multicentric retrospective study in a cohort of patients including 367 patients followed for endometriosis between January 2012 and March 2018. In this cohort, data were collected prospectively when patients were suspected of having endometriosis. Patients were suspected of having endometriosis when they experienced chronic pelvic pain for a greater than six-month duration. This pain could be a severe dysmenorrhea, a deep dyspareunia, a cyclic pelvic pain and painful defecation with or without infertility. In this cohort, patients could have been explored with other radiological exams, such as MRI or endovaginal ultrasonography, at the discretion of the clinician. Endometriosis was finally diagnosed according to surgical and histological criteria.
In our study, the inclusion criteria were patients aged over 18 with rectosigmoid endometriosis who underwent a surgery after a RS-EUS. Age, body mass index (BMI), pregnancy, parity and preoperative medical treatment were recorded from the patients’ files. In addition, the patients were asked to complete a questionnaire regarding their symptoms, including digestive symptoms. Each patient was asked to report whether the symptoms were never, sometimes, often, or always present. Patients were also asked whether these symptoms were exacerbated during their period. Symptoms that were not exacerbated during period were denoted as “none” to avoid confusion with other digestive conditions.
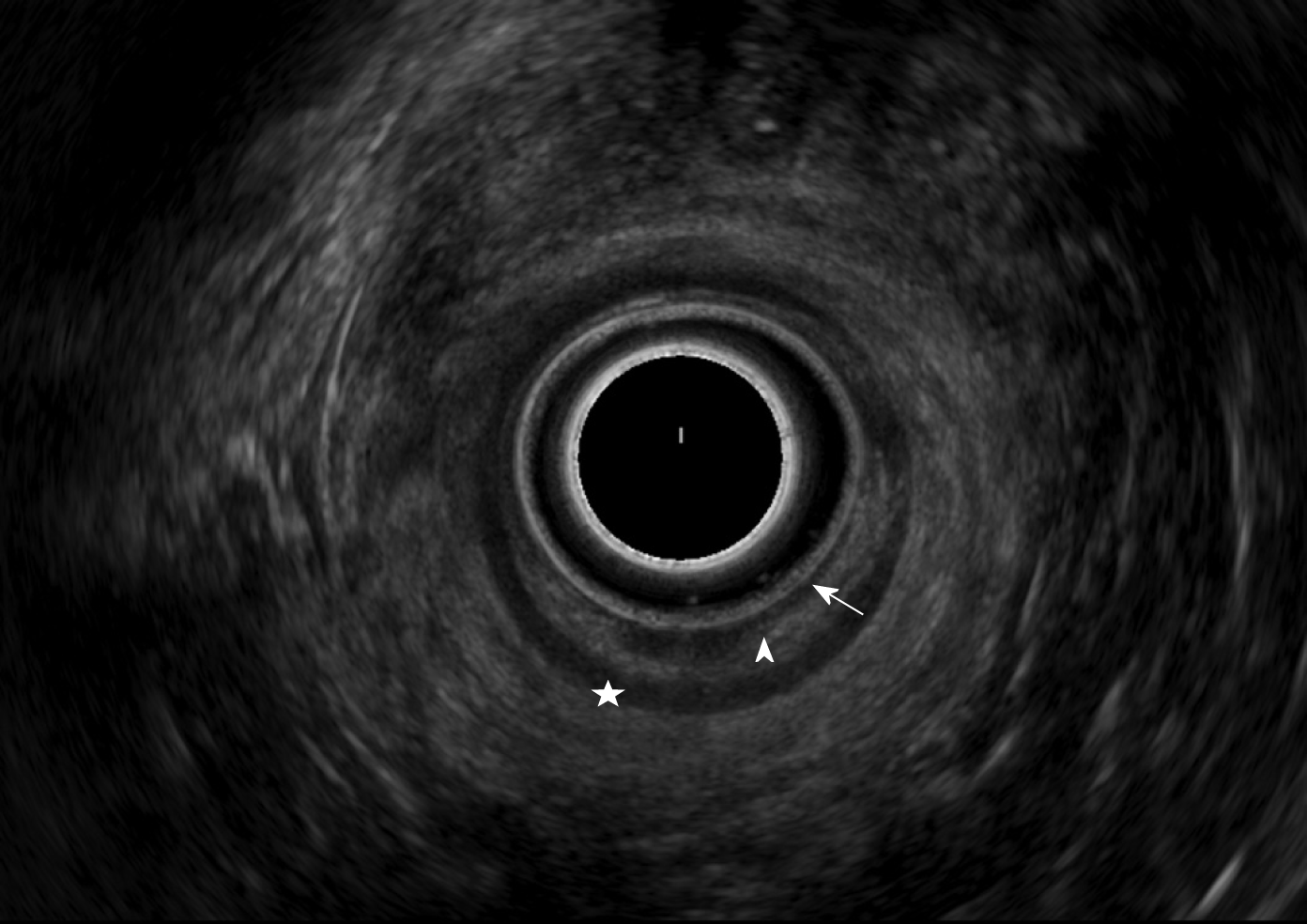
RS-EUS was performed by two well-trained ultrasonographists (RV and GR) in two different centers. The exam was divided into two different steps. The patients were in lateral decubitus position whenever it was possible. The first step involved endoscopic evaluation of the mucosae and the digestive lumen. The second step involved an ultrasonographic evaluation of the digestive wall. The device used was a flexible Pentax (Argenteuil, France) echoendoscope with radial probe. The normal rectosigmoid anatomy appears as follows on ultrasound 7.5 MHz (from the lumen to the serosa): Hypoechoic mucosa, hyperechoic submucosa, and hypoechoic muscular layer (Figure 1). Rectosigmoid endometriosis appears as a hypoechoic nodule infiltrating the muscular layer. Mucosal or submucosal invasion are characterized by an interruption of their hypoechogenic line (Figure 2). The evaluation criteria were thickness and width of the nodule, presence of a bump in the digestive lumen, presence of infiltration of the submucosae and presence of multiple lesions. The presence of a bump in the digestive lumen was evaluated during the endoscopic step of the exam and defined as a mucosal lesion or a narrowing of the digestive lumen.
All the interventions were performed by the same gynecologic surgeon (AF) who was well trained in endometriosis surgery. Interventions were performed via open laparoscopy. According to the recommendations, surgery on the digestive tract could involve a shaving (intramuscular layer dissection), a patch or a segmental digestive resection (subtotal proctectomy, sigmoidectomy or both). Patch and segmental resection surgeries were performed by a multidisciplinary team including digestive surgeons. A shaving, or intramuscular layer dissection of the lesions, involves the excision of the nodule on the digestive tract without digestive tract opening.
The choice of the surgical procedure was made by the surgeon, and shaving was preferred any time it was possible based on the nodule characteristics. This procedure was typically performed on unique and small lesions without submucosae involvement.
Patients were analyzed in two different groups: Shaving and digestive resection (patch or segmental resection). Univariate analysis was first performed to evaluate the relation between each criterion and the need for digestive resection. For qualitative variables, including the presence of a bump, infiltration of the submucosae and presence of multiple lesions, a Fisher exact test was performed when one or more of the theoretical frequencies were less than five. A chi-square test was performed when all of the theoretical frequencies were greater than or equal to five. For quantitative variables, Student t-tests were performed. Second, we performed multivariate logistic regression with significant variables after univariate analysis. Every test was performed with a two-tailed alpha risk of 5%. A P-value less than 0.05 was considered statistically significant. The statistical review of the study was performed by a biomedical statistician. All statistics were performed with XLSTAT version 20.1 (Addinsoft, 33000, Bordeaux, France).
All patients included in this retrospective analysis gave their prior informed consent for inclusion in the cohort. The cohort had the approval of the local ethic committee for protection of people (Comité de Protection des Personnes, CPP) on the 20th of June 2013.
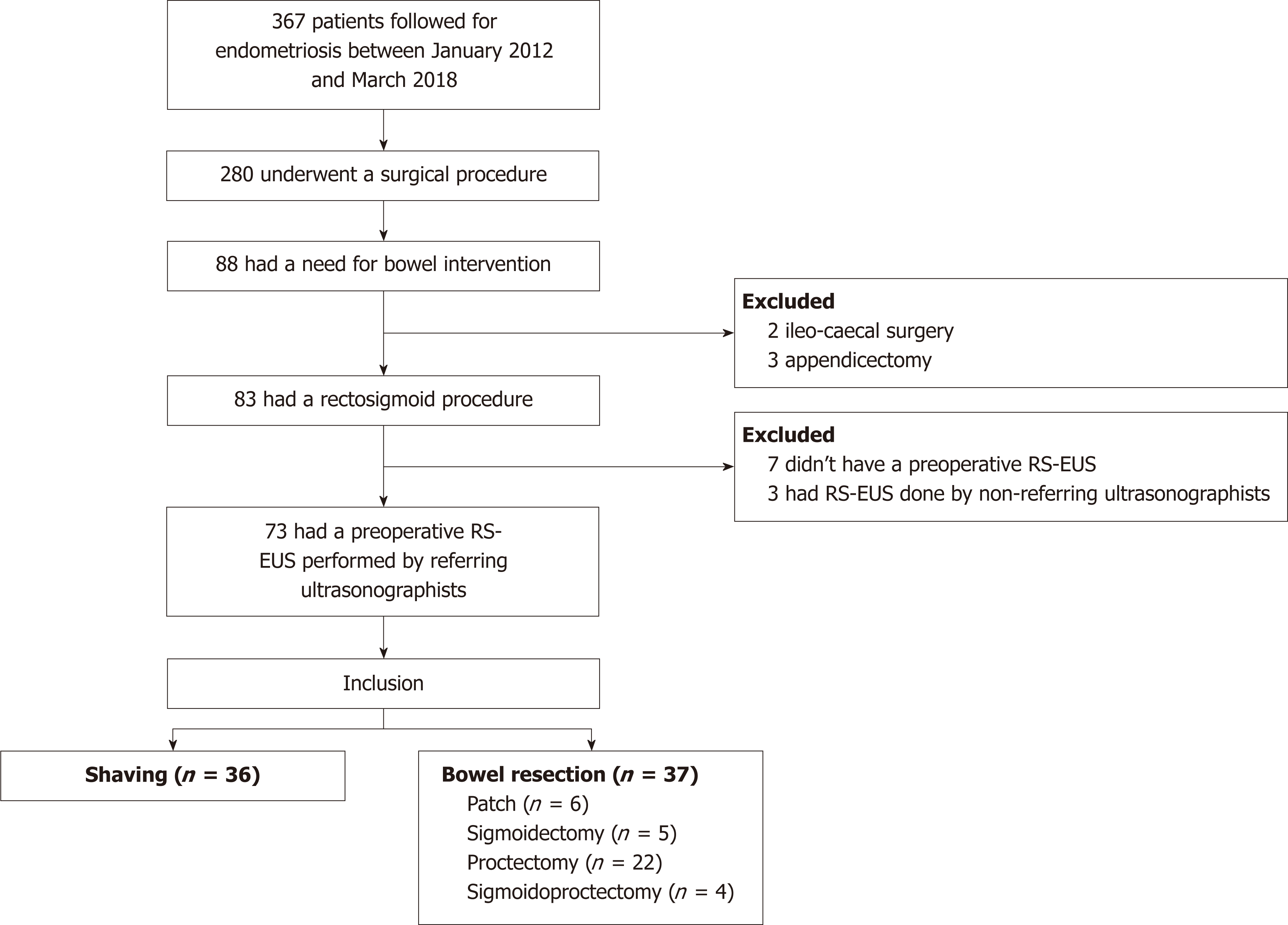
In total, 367 women were followed for endometriosis and included in the cohort between January 2012 and March 2018. Among them, 280 underwent a surgical procedure, 88 of whom had a need for bowel intervention. Two and three patients underwent an ileo-cecal surgery and appendectomy, respectively, and were therefore excluded from the analysis. An additional 10 women were excluded from the analysis because they did not undergo a preoperative RS-EUS (7 patients) or because the RS-EUS was not performed by well-trained ultrasonographists (3 patients). Finally, 73 patients were included in the analysis and separated in two different groups: shaving (36 patients) and bowel resection (37 patients) (Figure 3).
Concerning the study population, no differences in age, BMI, pregnancy, parity and preoperative medical treatment were noted between groups. No difference was found regarding digestive symptoms except dyschezia. Indeed, patients in the resection group exhibited significantly more severe dyschezia compared with the shaving group (P = 0,020) (Table 1).
| Shaving (n = 36) | Resection (n = 37) | P value | |
| Age | 0.4241 | ||
| n (%) | 36 (100) | 37 (100) | |
| Mean (SD) | 32.42 (6.57) | 33.54 (5.32) | |
| Body mass index | 0.6561 | ||
| n (%) | 34 (94) | 35 (95) | |
| Mean (SD) | 23.83 (3.96) | 24.31 (4.83) | |
| Pregnancy | 0.9143 | ||
| n (%) | 36 (100) | 35 (95) | |
| 0 (n) | 22 | 24 | |
| 1 (n) | 5 | 5 | |
| 2 (n) | 6 | 4 | |
| ≥ 3 (n) | 3 | 2 | |
| Parity | 0.5683 | ||
| n (%) | 36 (100) | 35 (95) | |
| 0 (n) | 25 | 26 | |
| 1 (n) | 4 | 6 | |
| 2 (n) | 6 | 3 | |
| ≥ 3 (n) | 1 | 0 | |
| Preoperative medical treatment | 0.7323 | ||
| n (%) | 36 (100) | 37 (100) | |
| None | 1 | 3 | |
| Progestin | 16 | 16 | |
| GnRH analog | 20 | 18 | |
| Estrogen | 3 | 6 | |
| ≥ 2 treatments | 4 | 6 | |
| Symptoms of rectosigmoid endometriosis | |||
| Dyschezia | 0.0202 | ||
| n (%) | 29 (81) | 34 (92) | |
| None to mild | 16 | 9 | |
| Moderate to severe | 13 | 25 | |
| Transit disorders | 0.8932 | ||
| n (%) | 29 (81) | 32 (86) | |
| None to mild | 14 | 16 | |
| Moderate to severe | 15 | 16 | |
| Vomiting | 1.003 | ||
| n (%) | 29 (81) | 31 (84) | |
| None to mild | 27 | 28 | |
| Moderate to severe | 2 | 3 | |
| Rectal Bleeding | 0.2373 | ||
| n (%) | 30 (83) | 29 (78) | |
| None to mild | 30 | 27 | |
| Moderate to Severe | 0 | 2 | |
| Abdominal pain | 0.9432 | ||
| n (%) | 32 (89) | 33 (89) | |
| None to mild | 8 | 8 | |
| Moderate to severe | 24 | 25 | |
In univariate analysis (Table 2), the mean thickness was 4.22 mm in the shaving group and 6.81 mm in the resection group, and the difference was statistically significant (P < 0.001). Regarding width, the mean measures were 13.43 and 19.34 mm in the shaving group and the resection group, respectively, and the difference was statistically significant (P < 0.001). Infiltration of the submucosae was observed in 5.6% (2/36) of the patients in the shaving group and 30% (11/35) in the resection group, leading to a significant difference (P = 0.007). No significant differences in the presence of a bump in the digestive lumen (5.6% in the shaving group vs 16% in the resection group, P = 0.261) and the presence of 2 or more lesions (2.8% in the shaving group vs 11% in the resection group, P = 0.358) were noted between the groups. No differences in classification in the shaving group and the resection group were noted between the two ultrasonographists (RV, GR) (P = 0.98) (See in Appendix).
| Shaving (n = 36) | Resection (n = 37) | P value | |
| Thickness | < 0.00011 | ||
| n (%) | 36(100) | 37(100) | |
| Mean (SD) | 4.22 (1.90) | 6.81 (2.61) | |
| Width | < 0.00011 | ||
| n (%) | 36 (100) | 36 (97) | |
| Mean (SD) | 13.70 (5.29) | 19.10 (5.53) | |
| Bump | 0.2613 | ||
| n (%) | 36 (100) | 37 (100) | |
| Presence (%) | 2 (5.6) | 6 (16) | |
| Submucosae infiltration | 0.0072 | ||
| n (%) | 36 (100) | 37 (100) | |
| Presence (%) | 2 (5.6) | 11 (30) | |
| Multiple lesions | 0.3583 | ||
| n (%) | 36 (100) | 37 (100) | |
| Presence (%) | 1 (2.8) | 4 (11) |
In the multivariate model, thickness, width and submucosae infiltration were included in the analysis. After logistic regression, only thickness appeared to be significant with an odds ratio (OR) = 1.49 [95% confidence interval (CI): 1.04–2.12, P = 0.028] (Table 3). Nevertheless, a sensible trend regarding width was identified. Specifically, width was increased in the resection group compared with the shaving group (OR 1.12, 95%CI : 1.00-1.26, P = 0.054).
| OR | 95%CI | P value | |
| Thickness | 1.49 | 1.04-2.12 | 0.028 |
| Width | 1.12 | 1.00-1.26 | 0.054 |
| Submucosae infiltration | 1.97 | 0.31-12.66 | 0.475 |
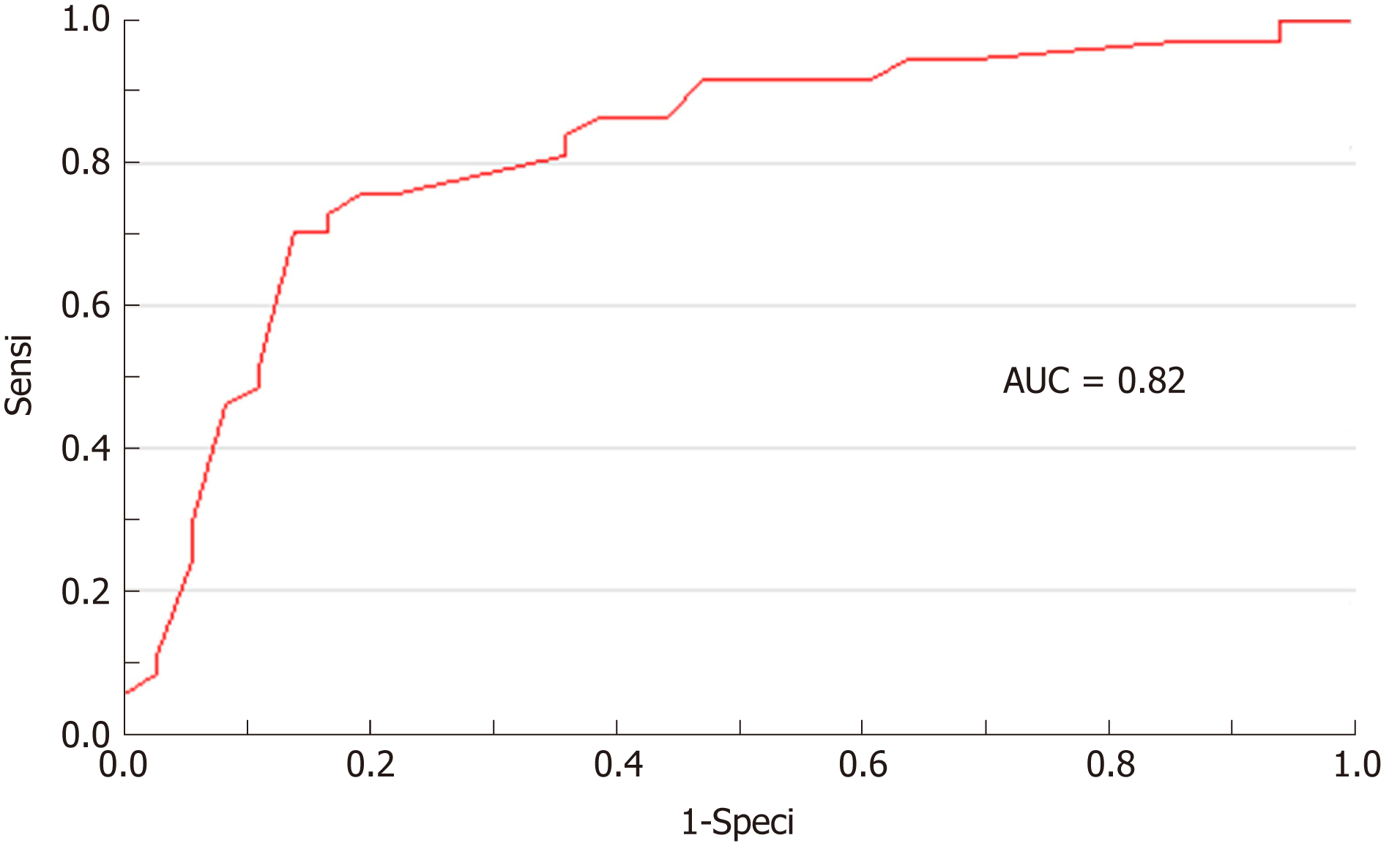
Based on receiver operating characteristic (ROC) analysis, a thickness greater than 5.20 mm was the best threshold to determine the need for digestive resection with a sensitivity of 76%, a specificity of 81%, and a positive predictive value (PPV) and negative predictive value (NPV) of 0.80 and 0.76, respectively. Cut-off values for 100% sensitivity and 100% specificity were 0.9 and 10.0 mm, respectively (Table 4). The area under curve of the ROC curve was 0.82 (Figure 4).
| Cut-off (mm) | Sensibility | Specificity | PPV | NPV | Overall accuracy |
| 0.9 | 1.00 | 0.03 | 0.51 | 1.00 | 0.52 |
| 5.2 | 0.76 | 0.81 | 0.80 | 0.76 | 0.78 |
| 10.0 | 0.05 | 1.00 | 1.00 | 0.51 | 0.52 |
We found that a rectosigmoid endometriosis nodule thickness greater than 5.20 mm measured by RS-EUS might be a predictive factor for the need for patch or segmental resection in rectosigmoid endometriosis with a sensitivity of 76% and a specificity of 81%. In addition, although the result was not significant, there was a trend for width to predict the need for resection. Further investigations with more patients should be conducted to confirm this finding, and width could appear as another independent predictive factor for resection.
It was previously demonstrated that RS-EUS exhibits good performance for detection and evaluation of rectosigmoid endometriosis with good sensitivity and negative predictive value[11]. However, RS-EUS remains a third-line exam, and its priority among examinations should be reevaluated. Other technics were also evaluated in detecting and characterizing deep infiltrating endometriosis nodules such as transvaginal ultrasonography, MRI and colonoscopy.
Nowadays, transvaginal ultrasonography has been well studied and experiences great performance in detecting and characterizing rectosigmoid endometriosis nodules and their digestive wall infiltration. In a study par Goncalves et al[4] this exam showed indeed sensitivity and a specificity of 97% and 100% respectively. Although, performance drops when detecting an infiltration of the submucosae with sensitivity between 62% and 83%[12]. This might explain why it has never been studied as a predictive factor for the surgery needed such as we did for RS-EUS.
Some new ultrasound techniques were also evaluated for the diagnosis of deep infiltrating endometriosis. Guerriero et al[13] compared the diagnostic accuracy of 2-Dimensions ultrasound (DUS) and 3-DUS in patients with deep infiltrating endometriosis confirmed surgically. They showed no significant difference regarding sensitivity, specificity, NPV and PPV between techniques for the intestinal location. Nevertheless, they used transvaginal ultrasound and, to our knowledge, there is no such study comparing the diagnostic accuracy of 2D and 3D RS-EUS. This could be the subject of further researches.
Another interesting study by Bergamini et al[14] compared the diagnostic accuracy of RS-EUS with Transvaginal Sonography with Water-Contrast in the Rectum (RWC-TVS) in the diagnosis of rectosigmoid endometriosis, confirmed by surgical and pathological findings. In this study RWC-TVS appeared to have better diagnostic performance than RS-EUS but the difference wasn’t significant with sensitivity, specificity, PPV and NPV of 96%, 90%, 98% and 81.8% and 88.2%, 80%, 95.7% and 57.1% respectively. However, this time again, they only studied the diagnostic accuracy of the exam and not its performance as a pretherapeutic test as we decided to do in our study. Similarly, this could be an interesting subject for further investigations.
Many other ultrasound techniques have also been studied in the diagnosis of intestinal endometriosis such as elastosonography[15], but none of them has been evaluated as a pretherapeutic test. Colonoscopy has also been studied but cannot be routinely performed for the diagnosis of intestinal endometriosis, giving poor outcomes to this exam with a sensitivity of 7% and a specificity of 85%, due to the paucity of lesions affecting the intestinal mucosa[16]. Regarding MRI, our study completes the recent findings concerning MRI measures of stenosis and long axis to predict the need for bowel resection In this study, a nodule’s short axis of 11 mm or more was a predictor for bowel resection with a sensitivity and a specificity of 93% and 99% respectively. Similarly, a bowel stenosis of 30% or more experienced a sensitivity of 95% and a specificity of 99% in predicting the need for a bowel resection[9]. Nevertheless, MRI colonography was a required parameter of this study, and this technique is only available in a limited number of centers. In comparison, RS-EUS is a common, easily accessible exam and is currently well evaluated in the staging of deep infiltrating endometriosis with better sensitivity and negative predictive value compared with MRI[17]; this finding indicates that MRI exams do not detect nodules that are detectable by RS-EUS. More precisely, RS-EUS experiences better sensitivity (79% vs 47%) and negative predictive value (71% vs 63%) than MRI when detecting a mucosal or submucosal involvement[18]. Therefore, RS-EUS appears to be more adequate in the preoperative evaluation of rectosigmoid endometriosis.
Another study demonstrated the interest of the presence of an infiltration of the muscular layer to determine whether bowel resection was needed[19]. In this study, lesion and infiltration characteristics were analyzed retrospectively on the surgical piece. In our study, information on rectosigmoid endometriosis nodules was collected prospectively before the surgical procedure. As mentioned above, the choice of the surgical procedure is actually based on clinical findings and local conditions. Nevertheless, the place for clinical findings in the decision path remains unclear. Indeed, another study by Wattiez et al[20] did not take into account the clinical manifestations of the disease to select the surgical procedure. However, our study brings to light the possibility of some specific symptoms, such as dyschezia, to aid in the selection of the surgical procedure, and other studies should be performed to evaluate the accuracy of the physical examination in this situation. Currently, RS-EUS is a third-line exam in the evaluation of deep infiltrating endometriosis[8]. However, our study suggests the possible use of RS-EUS as one of the first exams performed. Indeed, RS-EUS is an easy access exam with good diagnostic performance in endometriosis. It is also now clear that RS-EUS provides some important information on the extent of the disease and the local conditions to select the correct surgical procedure.
Our study has some strengths. First, this is the first study to evaluate the accuracy of RS-EUS before surgical procedure in rectosigmoid endometriosis. Second, RS-EUS was performed by two expert ultrasonographists working in two different centers, reducing the risk of evaluation bias. Similarly, no classification bias was noted between the operators, highlighting the reproducibility of RS-EUS in the evaluation of rectosigmoid endometriosis. Finally, all surgical procedures were realized by the same trained surgeon (AF) familiar with endometriosis procedures and preoperative management. This study has also some limitations. This is a retrospective study with a small patient sample. Prospective, multicentric studies should be performed to confirm our results. We decided to classify patients who did not experience worsening symptoms during periods as “none”. This system could have misclassified some patients with milder clinical manifestations and biased the results. Finally, our study has some implications as it reveals new interest in the realization of preoperative RS-EUS in deep infiltrating endometriosis. This information might help a surgeon to organize the procedure given that digestive resection must be performed by a multidisciplinary team. RS-EUS could also facilitate the transmission of appropriate information to the patient, defining the benefits and risks of the procedure.
In conclusion, in our retrospective study, we found that an endometriosis nodule greater than 5.20 mm thick might predict the need for bowel resection in rectosigmoid endometriosis. A trend concerning width to predict the need for resection was also observed. Further investigations, such as prospective studies, should be conducted to confirm these results.
Rectosigmoid endometriosis is an underdiagnosed disease responsible for digestive disorders. Two surgical approaches, rectosigmoid bowel resection (segmental or patch) or intramuscular layer dissection (shaving), are available. Nowadays, the choice is led by a conjunction of clinical and radiological findings without strict criteria. A recent study has shown that a pre-operative magnetic resonance imaging (MRI) colonography might predict the need for bowel resection in rectosigmoid endometriosis. However, it has previously been demonstrated that rectosigmoid endoscopic ultrasonography (RS-EUS) exhibits better sensitivity and negative predictive value than MRI in the diagnosis of rectosigmoid endometriosis.
To assess whether the characteristics of endometriosis lesions evaluated on a preoperative RS-EUS could predict the need for bowel resection in rectosigmoid endometriosis for a better optimization of the preoperative management of the patient.
To assess whether the lesion features observed via preoperative RS-EUS might predict the need for bowel resection.
This multicentric retrospective study was conducted on a cohort of patients with rectosigmoid endometriosis who underwent a curative surgical procedure, evaluated by RS-EUS performed by two trained operators, between January 2012 and March 2018. In this cohort, data were collected prospectively when patients were suspected of having endometriosis. RS-EUS features evaluated were thickness, width, infiltration of the submucosae, presence of a bump into the digestive lumen and presence of multiple rectosigmoid localizations All the surgical interventions were performed by one well trained gynecologic surgeon via open laparoscopy. Shaving was preferred any time it was possible based on the nodule characteristics. This procedure was typically performed on unique and small lesions without submucosae involvement. A univariate statistical analysis was performed on nodules’ RS-EUS features followed by multivariate logistic regression on significant results.
In total, 367 women were followed for endometriosis and included in the cohort between January 2012 and March 2018. 73 patients met the inclusion criteria and were included in the analysis, in two groups: Shaving (36 patients) and bowel resection (37 patients). In univariate analysis thickness (P < 0.001), width P < 0.001) and infiltration of the submucosae (P = 0.007) experienced significant results. After multivariate logistic regression only thickness appeared to be significant with an odds ratio (OR) = 1.49 [95% confidence interval (CI) 1.04-2.12, P = 0.028]. Nevertheless, a sensible trend regarding width was identified. Specifically, width was increased in the resection group compared with the shaving group (OR 1.12, CI95% 1.00-1.26, P = 0.054). Based on receiver operating characteristic analysis, a thickness greater than 5.20 mm was the best threshold to determine the need for digestive resection with a sensitivity of 76%, a specificity of 81%, and a positive predictive value and negative predictive value of 0.80 and 0.76, respectively.
We found that an endometriosis nodule greater than 5.20 mm thick might predict the need for bowel resection in rectosigmoid endometriosis. This is the first study to evaluate the accuracy of RS-EUS before surgical procedure in rectosigmoid endometriosis. It has some implications as it reveals new interest in the realization of preoperative RS-EUS in deep infiltrating endometriosis. This information might help surgeons to organize procedures in a multidisciplinary team. Although, our work has also some limitations, mainly because of its retrospective design with small patient samples.
Further investigations, such as prospective, multicentric studies, have to be conducted to confirm our results.
Manuscript source: Invited conference manuscripts
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jiang QP, Milone M, Richardson WS, Santoro GA S- Editor: Yan JP L- Editor: A E- Editor: Yin SY
| 1. | Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2295] [Cited by in RCA: 2445] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 2. | Williams TJ, Pratt JH. Endometriosis in 1,000 consecutive celiotomies: Incidence and management. Am J Obstet Gynecol. 1977;129:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 112] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Parasar P, Ozcan P, Terry KL. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr Obstet Gynecol Rep. 2017;6:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 421] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 4. | Goncalves MO, Podgaec S, Dias JA, Gonzalez M, Abrao MS. Transvaginal ultrasonography with bowel preparation is able to predict the number of lesions and rectosigmoid layers affected in cases of deep endometriosis, defining surgical strategy. Hum Reprod. 2010;25:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Canon B, Collinet P, Piessen G, Rubod C. Résection rectale segmentaire et shaving rectal laparoscopiques pour endométriose: Morbidité péri-opératoire. Gynécologie Obstétrique Fertil. 2013;41:275-281. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Roman H, Vassilieff M, Tuech JJ, Huet E, Savoye G, Marpeau L, Puscasiu L. Postoperative digestive function after radical versus conservative surgical philosophy for deep endometriosis infiltrating the rectum. Fertil Steril. 2013;99:1695-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Donnez O, Roman H. Choosing the right surgical technique for deep endometriosis: Shaving, disc excision, or bowel resection? Fertil Steril. 2017;108:931-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 8. | Prise en charge de l’endométriose - Recommandations HAS France. In: Haute Autorité de Santé [Internet]. Available from: https://www.has-sante.fr/portail/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=c_2820459. . |
| 9. | Scardapane A, Lorusso F, Francavilla M, Bettocchi S, Fascilla FD, Angelelli G, Scioscia M. Magnetic Resonance Colonography May Predict the Need for Bowel Resection in Colorectal Endometriosis. Biomed Res Int. 2017;2017:5981217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Alborzi S, Rasekhi A, Shomali Z, Madadi G, Alborzi M, Kazemi M, Hosseini Nohandani A. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine (Baltimore). 2018;97:e9536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Delpy R, Barthet M, Gasmi M, Berdah S, Shojai R, Desjeux A, Boubli L, Grimaud JC. Value of endorectal ultrasonography for diagnosing rectovaginal septal endometriosis infiltrating the rectum. Endoscopy. 2005;37:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Hudelist G, Tuttlies F, Rauter G, Pucher S, Keckstein J. Can transvaginal sonography predict infiltration depth in patients with deep infiltrating endometriosis of the rectum? Hum Reprod. 2009;24:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Guerriero S, Alcázar JL, Pascual MA, Ajossa S, Perniciano M, Piras A, Mais V, Piras B, Schirru F, Benedetto MG, Saba L. Deep Infiltrating Endometriosis: Comparison Between 2-Dimensional Ultrasonography (US), 3-Dimensional US, and Magnetic Resonance Imaging. J Ultrasound Med. 2018;37:1511-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Bergamini V, Ghezzi F, Scarperi S, Raffaelli R, Cromi A, Franchi M. Preoperative assessment of intestinal endometriosis: A comparison of transvaginal sonography with water-contrast in the rectum, transrectal sonography, and barium enema. Abdom Imaging. 2010;35:732-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Mezzi G, Ferrari S, Arcidiacono PG, Di Puppo F, Candiani M, Testoni PA. Endoscopic rectal ultrasound and elastosonography are useful in flow chart for the diagnosis of deep pelvic endometriosis with rectal involvement. J Obstet Gynaecol Res. 2011;37:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Milone M, Mollo A, Musella M, Maietta P, Sosa Fernandez LM, Shatalova O, Conforti A, Barone G, De Placido G, Milone F. Role of colonoscopy in the diagnostic work-up of bowel endometriosis. World J Gastroenterol. 2015;21:4997-5001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Roseau G. Recto-sigmoid endoscopic-ultrasonography in the staging of deep infiltrating endometriosis. World J Gastrointest Endosc. 2014;6:525-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kim A, Fernandez P, Martin B, Palazzo L, Ribeiro-Parenti L, Walker F, Bucau M, Collinot H, Luton D, Koskas M. Magnetic Resonance Imaging Compared with Rectal Endoscopic Sonography for the Prediction of Infiltration Depth in Colorectal Endometriosis. J Minim Invasive Gynecol. 2017;24:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Abrão MS, Podgaec S, Dias JA, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Wattiez A, Puga M, Albornoz J, Faller E. Surgical strategy in endometriosis. Best Pract Res Clin Obstet Gynaecol. 2013;27:381-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |