Published online Feb 14, 2019. doi: 10.3748/wjg.v25.i6.659
Peer-review started: November 1, 2018
First decision: November 29, 2018
Revised: January 10, 2019
Accepted: January 14, 2019
Article in press: January 15, 2019
Published online: February 14, 2019
Processing time: 109 Days and 4.6 Hours
Primary sclerosing cholangitis (PSC) is a rare cholestatic liver disease characterized by progressive fibroinflammatory destruction of the intra- and/or extrahepatic biliary ducts. While its features and disease course can be variable, most patients with PSC have concurrent inflammatory bowel disease and will eventually develop liver cirrhosis and end-stage liver disease, with liver transplantation representing the only potentially curative option. Importantly, PSC is associated with a significantly increased risk of malignancy compared to the general population, mainly cholangiocarcinoma, gallbladder carcinoma, hepatocellular carcinoma, and colorectal cancer, with nearly 50% of deaths in patients with PSC being due to cancer. Therefore, robust surveillance strategies are needed, though uncertainty remains regarding how to best do so. In this review, we discuss the epidemiology, prevention, and surveillance of cancers in patients with PSC. Where evidence is limited, we present pragmatic approaches based on currently available data and expert opinion.
Core tip: Primary sclerosing cholangitis is a rare cholestatic liver disease characterized by progressive fibroinflammatory destruction of the bile ducts. It is associated with a significantly increased risk of malignancy over the general population, with nearly 50% of deaths in patients with primary sclerosing cholangitis caused by cancer, thus necessitating robust surveillance strategies. In this article, we provide a synopsis of the epidemiology, prevention, and surveillance of cancers in patients with primary sclerosing cholangitis.
- Citation: Fung BM, Lindor KD, Tabibian JH. Cancer risk in primary sclerosing cholangitis: Epidemiology, prevention, and surveillance strategies. World J Gastroenterol 2019; 25(6): 659-671
- URL: https://www.wjgnet.com/1007-9327/full/v25/i6/659.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i6.659
Primary sclerosing cholangitis (PSC) is a rare, progressive cholestatic liver disease characterized by chronic inflammation and fibrosis of the intra- and extrahepatic bile ducts[1-3]. It is a heterogenous disease often presenting with an insidious onset and variable disease course, though ultimately leading to cirrhosis and end-stage liver disease in most cases[4-6]. Its incidence and prevalence appear to vary depending on geography, with estimated incidence ranging from 0-1.3 per 100000 and prevalence from 0-16.2 per 100000, both of which appear to be rising for unclear reasons[7]. The disease has a male predominance, though it can affect men and women of nearly all ages[8]. It also has a strong association with inflammatory bowel disease (IBD), with a majority of patients with PSC also having IBD[9]. Associations with other immune-mediated diseases such thyroid disease, psoriasis, and sarcoidosis have also been reported[10-12]. Furthermore, patients with PSC have a significantly increased risk of developing various abdominal malignancies, which in fact account for 40%-50% of the mortality in patients with PSC[13-16]. Nevertheless, predictors of cancer in PSC are still largely unclear, preventive measures are for the most part unproven if not non-existent, and much is still unknown regarding prevention and surveillance of cancer in PSC[8]. Furthermore, there is significant ambiguity regarding surveillance strategies, with limited recommendations provided by society guidelines. In this article, we discuss the epidemiology, prevention, and surveillance of cancer in patients with PSC. We review current recommendations and avenues for cancer surveillance based on currently available evidence and expert opinion.
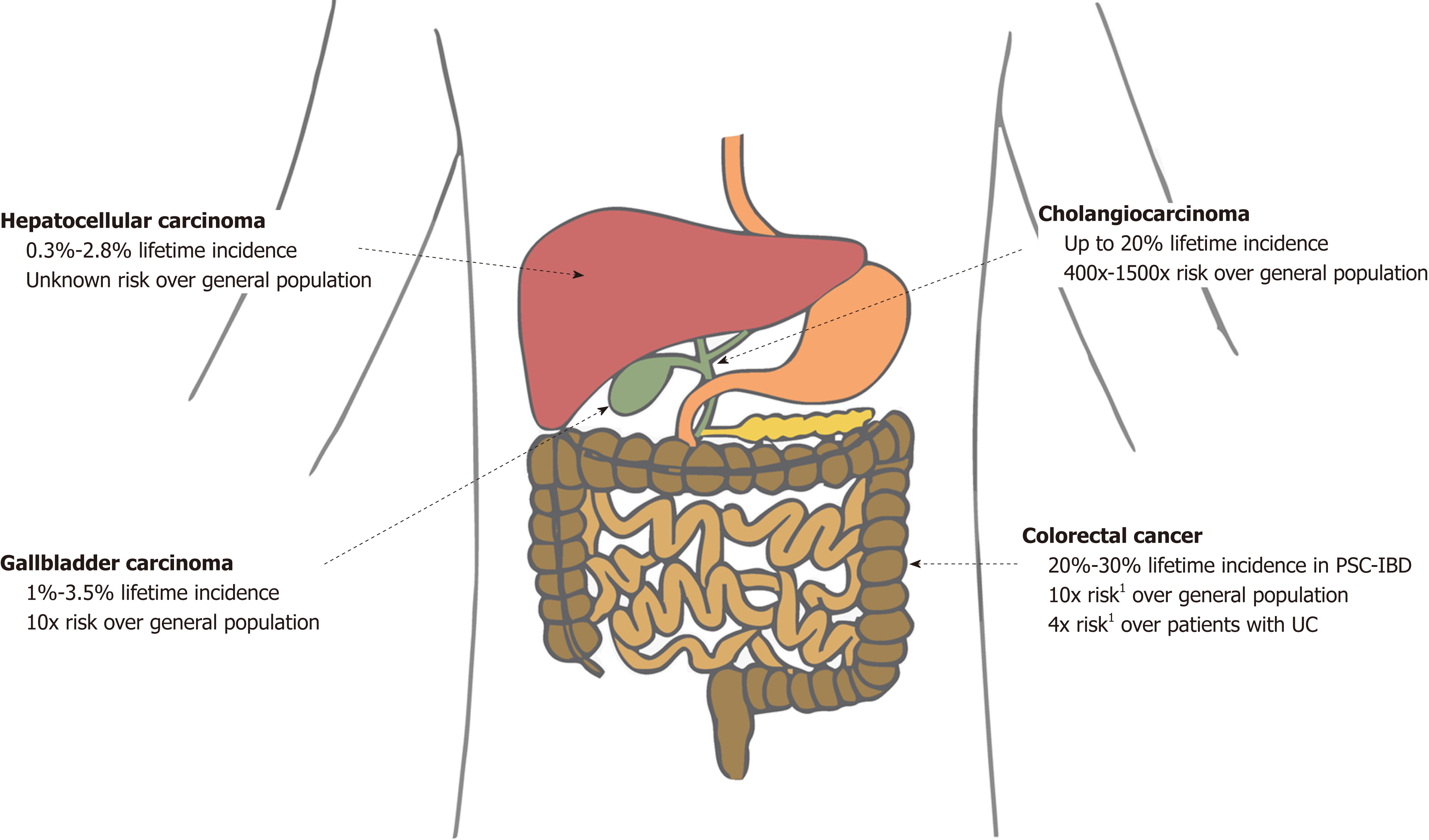
PSC is associated with a major lifetime risk of gastrointestinal cancers. Compared with the general population, patients with PSC have double the risk of cancer in general and 40 times the risk of a primary hepatobiliary cancer[17]. Various studies have shown that patients with PSC have a significantly increased risk of developing cholangiocarcinoma (CCA), gallbladder carcinoma (GBC), hepatocellular carcinoma (HCC), and colorectal carcinoma (CRC) (Figure 1)[15,16,18,19]. We discuss each of these in forthcoming sections.
Patients with PSC are at particularly increased risk for CCA. The annual incidence of CCA in patients with PSC is estimated to be 0.5%-1.5%, with a reported lifetime incidence of 20%[5,6,20]. Recently, a large international multi-centered cohort study (n = 7121 patients from 37 countries) estimated the prevalence of hepatobiliary cancer in PSC to be 10%, with CCA being the most common malignancy[21]. Compared to the general population, patients with PSC have a 400- to 1500- fold increased lifetime risk of CCA[5,22]. Though the predictors of CCA in PSC remain somewhat unclear, several factors seem to be associated with CCA risk. For example, prolonged duration of IBD in PSC-IBD patients seems to (further) increase the risk of developing CCA, as does a history of colonic dysplasia[14]. Notably, cirrhosis does not appear to significantly increase the risk of (or at least is not required for development of) CCA in patients with PSC, and many patients do not carry a diagnosis of cirrhosis at the time of CCA diagnosis[6,23].
To better characterize and manage CCA, tumors are often classified into one of three subtypes based on their location: Intrahepatic (located proximal to the secondary branches of the left and right hepatic ducts), perihilar (between the cystic duct confluence and the secondary branches of the left and right hepatic ducts), and distal (between the cystic duct and the hepatopancreatic ampulla)[24]. Based on the limited number of studies that have evaluated CCA by subtype, it appears that patients with PSC may have a higher proportion of intrahepatic tumors compared with patients without PSC[23,25,26]. However, studies are very heterogenous; one study even reported no cases of intrahepatic tumors[27]. Overall, studies evaluating the features and outcomes of CCA by location specifically in patients with PSC are very limited, with various studies using different classification schemes/definitions[27-29] or including other cancers (e.g., GBC) as CCA[30], thus limiting the ability to compare studies and draw meaningful conclusions in this regard.
Of note, though the median time between PSC diagnosis and CCA ranges from 4-6 years, as many as 50% of patients are diagnosed with CCA either at presentation or within 1 year of being diagnosed with PSC[6,20,22]. The presenting features of CCA are lamentably nonspecific, with extrahepatic CCA presenting with biliary obstruction symptoms [e.g., abdominal pain, jaundice, dark urine (choluria), pruritis, malaise, weight loss, pale stools (from acholia), and increases in serum alkaline phosphatase and bilirubin above PSC baseline] and intrahepatic CCA more frequently presenting with “cancer” symptoms (e.g., abdominal pain, fatigue, weight loss, diminished appetite, and night sweats; alkaline phosphatase is typically elevated above baseline, but bilirubin may remain unchanged)[31-33]. Unfortunately, CCA accounts for approximately a third of all-cause mortality in PSC[5], and up to 80% of patients who develop CCA die within 1 year[6]. Liver transplantation (LT) for PSC, though curative for many, does not preclude recurrence of PSC or PSC-associated CCA (though both occur in only a minority of patients). Moreover, CCA may develop post-LT, with at least several cases reporting de novo tumor growth in the remnant bile duct when the native bile duct was preserved[34-38]. For this reason, some have recommended avoiding choledochocholedochal anastomosis in patients with PSC[37]. Treatment of CCA and other PSC-associated malignancies has been recently reviewed and is discussed elsewhere[39,40].
Patients with PSC have an increased incidence of various gallbladder abnormalities, including gallstones, cholecystitis, gallbladder polyps, and GBC. It is important to note that although most gallbladder polyps are benign in the general population, patients with PSC have a high incidence of dysplastic or malignant polyps[41]. In a Swedish study of 286 patients with PSC, 6% had gallbladder masses, of which 56% harbored malignancy[19]. Similarly, in an American study of 102 patients with PSC undergoing cholecystectomy, 13.7% had a gallbladder mass, of which 57% had adenocarcinomas[42]. GBC can manifest on imaging as a mass replacing part or all of the gallbladder (seen in 45%-60%), wall thickening (20%-30%), or intraluminal polypoid lesion (15%-25%)[43]. Current societal guidelines recommend consideration of cholecystectomy in all patients with PSC with gallbladder polyps greater than 8 mm in size as well as gallbladder masses of any size due to the high risk of current or developing malignancy[19,44-46]. Smaller polyps, on the other hand, should be closely monitored, as cholecystectomy in patients with PSC may be associated with relatively high morbidity and should not be performed unless the benefit is believed to outweigh the potential risks[47]. The lifetime incidence of GBC in patients with PSC is estimated to be 3%-14%[48].
There is an increased risk of HCC in patients with PSC[49]. Though the incidence of HCC in PSC has not been well studied, limited data suggest a lifetime incidence of 0.3% to 2.8%[49,50]. In a recent study of 830 patients with PSC, 23 patients had HCC, all of which had cirrhotic-stage PSC, suggesting the increased risk for HCC in patients with PSC may be solely in the setting of cirrhosis, as with most other chronic liver diseases[51].
It is well known that patients with IBD are at increased risk for CRC. Although the exact mechanism is unclear, it is thought that chronic intestinal inflammation promotes pro-neoplastic changes, thus leading to dysplasia and IBD-associated cancer[52]. Most patients with PSC also have IBD, with an estimated prevalence of IBD in patients with PSC ranging from 50% to 80%, the majority of cases being of the ulcerative colitis (UC) subtype[53,54]. Remarkably, patients with PSC and IBD are at even higher risk for CRC than those with PSC alone or IBD alone. In one study, the reported 10-year and 20-year risks for CRC were 14% and 31%, respectively, compared to 2% and 2%, respectively, in patients with PSC alone[16,55]. Similarly, a large meta-analysis of 16844 patients revealed a 4-fold increased risk of CRC in patients with concurrent PSC and UC compared with patients with UC alone[56]. A more recent meta-analysis of 13379 IBD patients also came to the same conclusions[18]. Of note, although PSC has been found to be an independent risk factor for CRC in patients with UC, it is unclear whether PSC has the same influence in Crohn’s disease. The few studies on this topic in the literature have had differing results[57,58]. In general, however, it is believed that the risk of CRC in extensive Crohn’s colitis is similar to that of UC.
It should be mentioned that IBD co-existing with PSC (PSC-IBD) may represent a genetically and clinically distinct entity from IBD alone[59]. Intestinal disease in PSC-IBD is typically more quiescent and is often asymptomatic, thus only found on active screening with colonoscopy with biopsies[54,60]. Furthermore, the progression of colonic neoplasms from low grade dysplasia to advanced colorectal neoplasia occurs at a higher rate in patients with PSC-IBD (regardless of the apparent severity of PSC) compared with patients with IBD alone[61]. Patients with PSC-IBD also appear to have a younger age at onset of IBD symptoms (19 vs 29 years)[62], younger age at diagnosis of CRC (38 vs 48 years)[62], more extensive colitis[59], increased frequency of right sided cancers (67% vs 36%), and overall worse prognosis (5 year survival: 40% vs 75%)[63], compared to patients with IBD alone. Of note, unlike classical IBD where patients are considered to be at an increased risk of CRC after having IBD for a decade, patients with PSC-UC have an increased risk of CRC as soon as the initial diagnosis of both diseases is made[64,65]. In addition, for unclear reasons, the risk of CRC can increase after LT, thus routine surveillance for CRC is essential[66].
There is currently little data supporting an increased risk of pancreatic cancer in patients with PSC. A review of the literature reveals one study of 604 patients with PSC finding the risk of pancreatic cancer to be 14 times higher than the general population[15]. Subsequent studies, however, have not found such high rates of pancreatic cancer in patients with PSC. A study of 211 patients with PSC in the Netherlands found only 1 case of pancreatic cancer[16], and a study of 200 patients with PSC in Belgium found 5 cases of pancreatic cancer, though one of the cases could not be distinguished from distal CCA[67]. Given the paucity of data to date, specific surveillance for pancreatic cancer is not recommended at this time but is an area which merits further investigation.
Data regarding the prevention of cancer in PSC are scarce, largely due to the rarity of PSC and difficulty in amassing sufficient patient-years to power chemopreventive studies. There are currently no pharmacological agents that are routinely recommended for cancer prevention in patients with PSC. Multiple potential disease modifying agents and pharmacotherapeutics have been studied, including atorvastatin, azathioprine, colchicine, budesonide, docosahexaenoic acid, D-penicillamine, malotilate, methotrexate, metronidazole, minocycline, mycophenolate mofetil, nicotine, pentoxifylline, pirfenodone, prednisolone, tacrolimus, thalidomide, and silymarin, all without clear clinical benefit[46]. However, there are several pharmacological agents that may have potential benefit, thus necessitating further investigation.
Ursodeoxycholic acid (UDCA), a hydrophilic bile acid, has been one of the most studied pharmacological agents for PSC to date, with trials showing varying results. Although its mechanism for chemoprevention is still unclear, it is thought to act through alterations in colonic bile acid milieu (among other mechanisms), possibly lowering levels of carcinogenic compounds[68]. Several trials have shown that the use of UDCA reduces the risk of developing dysplasia or cancer in patients with PSC-IBD[68,69], while others show no benefit[70] or even deleterious effects when taken in high doses (28-30 mg/kg/day)[71]. In general, low- to intermediate-dose UDCA appears to have some chemopreventive benefit[69] while high-dose UDCA has been associated with an increased risk of adverse effects[72], including an increased risk of CRC[71], and thus should be avoided. The role of intermediate-dose UDCA in patients with PSC for chemopreventive purposes remains unclear at this time. The American Association for the Study of Liver Diseases (AASLD) and American College of Gastroenterology (ACG) both strongly recommend against the routine use of UDCA as chemoprevention for CRC in patients with PSC-IBD[44,46], while the European Association for Study of the Liver (EASL) does not provide specific recommendations for the general use of UDCA, but acknowledges consideration of UDCA in high-risk groups (e.g., those with strong family history of CRC, previous history of colorectal dysplasia and cancer, or longstanding extensive colitis)[45].
Oral vancomycin, an immunomodulating bacterial glycopeptide antibiotic, has been used to treat PSC and found to result in significant improvement in clinical symptoms and liver biochemistries in some PSC-IBD patients[73-75]. However, data regarding the impact of vancomycin on cancer prevention (or prevention of recurrent PSC post-LT) are not yet available. The use of oral vancomycin in PSC remains an area of active and exciting research[76].
Curcumin, a naturally-occurring phytoextract from the turmeric (Curcuma longa) rhizome is another compound of interest. Preclinical studies have suggested that curcumin has anti-inflammatory, anti-fibrotic, and anti-neoplastic effects, primarily with regard to HCC and CCA[77]. Although there are no published clinical trials to date on the effects of curcumin on hepatobiliary malignancies, a phase 1/2 study for the use of curcumin in the treatment of PSC is currently underway[78], and if therapeutic effects are found, would support further studies to investigate its chemopreventive potential.
In addition to pharmacological agents, prevention can involve minimizing modifiable risk factors for hepatobiliary malignancies. Several studies have found smoking and alcohol consumption to be associated with an increased risk of CCA[22,25,79]. However, there have not been any published studies evaluating whether cessation of smoking or alcohol consumption can reduce the risk of hepatobiliary malignancies or survival in patients with PSC.
In the health sciences, surveillance refers to the observation and monitoring of asymptomatic, but at-risk populations for the occurrence of an outcome of interest. When the outcome of interest is cancer, surveillance is performed to detect lesions before they become cancer and/or to detect cancers at an earlier stage, thereby increasing the chance of cure. For surveillance to be effective, the at-risk population must be identifiable, and tests used in the identification of patients with disease must be accurate, accessible, cost-effective, and with acceptable risks. Furthermore, the disease should be treatable with evidence-based modalities and should increase survival of the population under surveillance[48]. Strategies and recommendations for cancer surveillance in patients with PSC are discussed in the following sections.
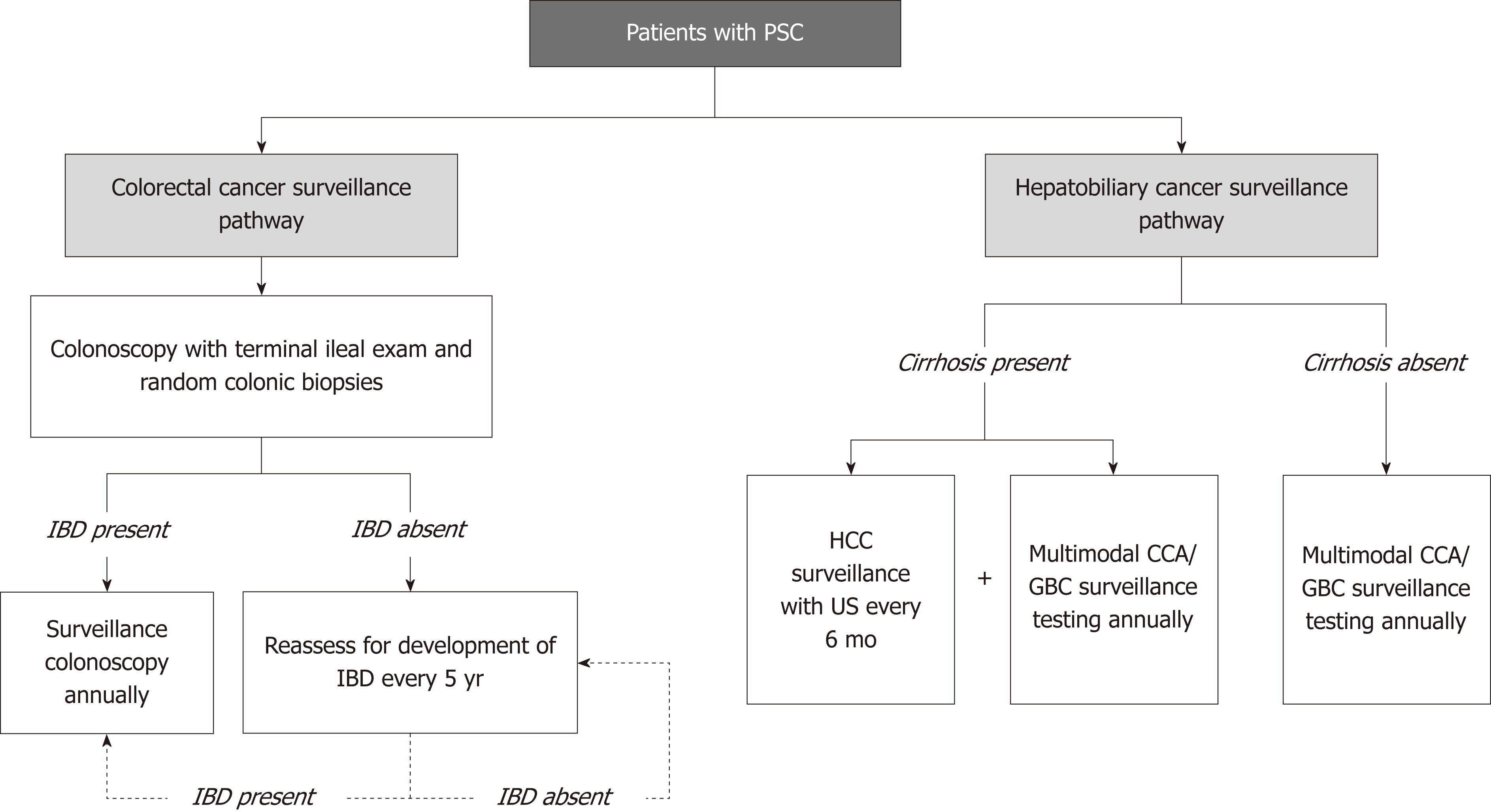
The development of surveillance guidelines for CCA has been challenging albeit an area of great interest. Patients with PSC are at risk for developing CCA, thus, a clear at-risk population can be identified for the purposes of developing an effective surveillance program. In addition, surveillance modalities are, for the most part, available and with acceptable risks to patients. However, early detection and tissue diagnosis of CCA have historically been challenging, limited treatment options are available if CCA is detected, and consequently, survival benefit of surveillance (until recently) has largely been unknown. Due to these limitations and others, unlike for GBC and CRC (Figure 2), there is currently no consensus, evidence-based societal guideline for CCA surveillance in PSC[32].
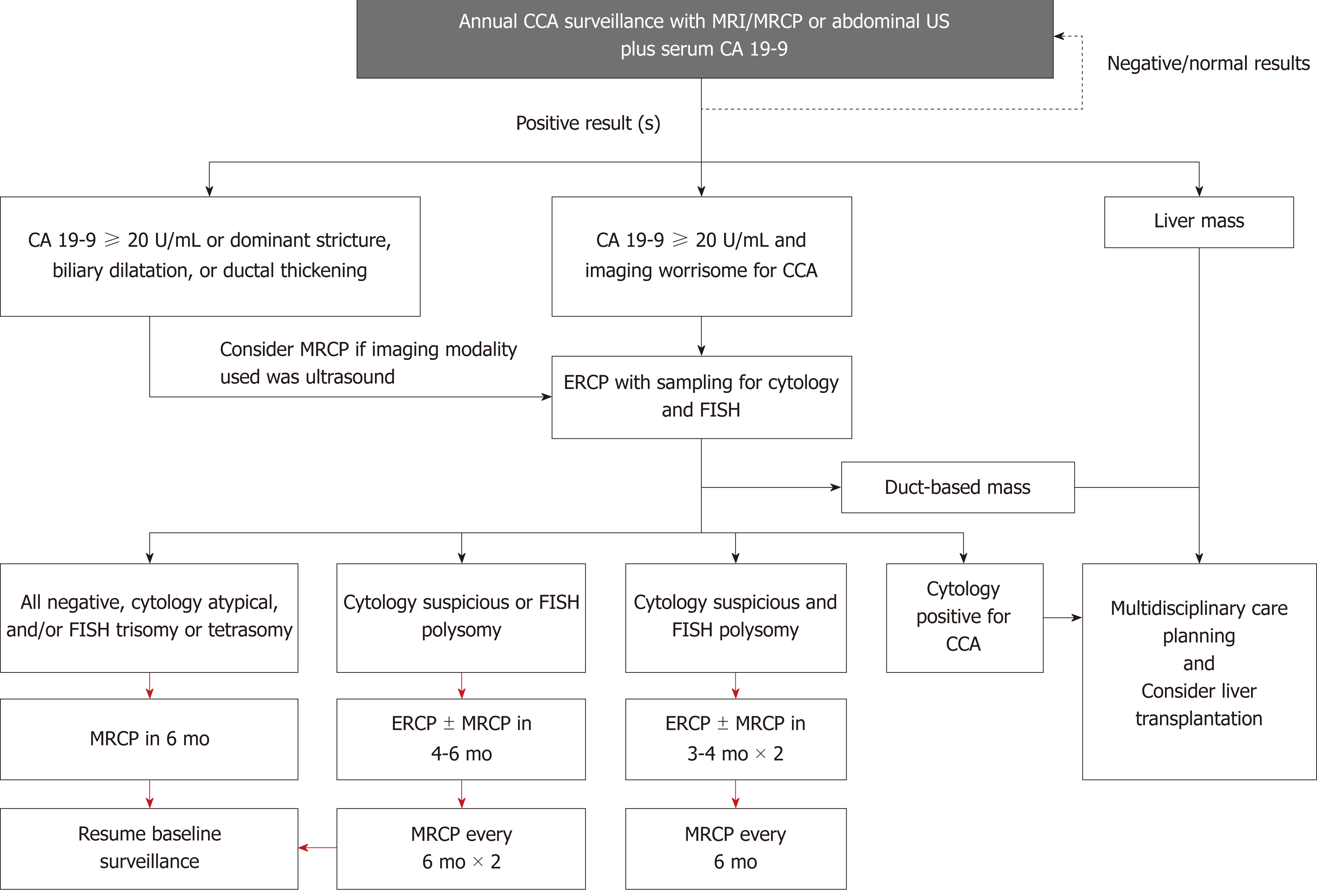
Despite the aforementioned shortcomings, many experts suggest the usage of regular imaging and measurement of the serum tumor marker carbohydrate antigen 19-9 (CA 19-9) for surveillance of CCA[13,46]. Indeed, the majority of large-volume centers perform yearly or biennial magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) for patients with PSC[80], which has a reported sensitivity and specificity of 89% and 75%, respectively[81]. Transabdominal ultrasound is another modality often considered given its lower cost, increased availability, and greater patient acceptability compared with MRI/MRCP; however, sensitivity is seemingly lower at 57% (while specificity is higher, 94%)[81]. Computed tomography has similar sensitivity and specificity to MRI/MRCP at 75% and 80%, respectively, but is not recommended due to the long-term risk of radiation and iodinated contrast and somewhat inferior diagnostic performance in some studies[13,51].
CA 19-9 is a serum biomarker that has been extensively studied for its role in the diagnosis of pancreatobiliary malignancy, including CCA. However, there is no agreement on cutoff for diagnosis, and its sensitivity and specificity is relatively low when used by itself without other diagnostic modalities (78% and 67%, respectively, when using a cutoff of 20 IU/mL)[81]. False positive results for CA 19-9 are frequently encountered, with one study finding approximately one third of patients with an increased CA 19-9 over 129 IU/mL not having CCA[82]. Nevertheless, CA 19-9 is still often used in clinical practice, and the combination of CA 19-9 levels greater than 20 IU/mL and suspicious findings on MRI/MRCP has been found to increase sensitivity of detecting CCA to near 100% (at the expense of decreased specificity of 38%)[81]. The use of CA 19-9 also increases the sensitivity of ultrasound to 91% (with specificity of 67%)[81]. Thus, we propose the use of annual abdominal imaging [ultrasound (US) or MRI/MRCP] combined with CA 19-9 for CCA surveillance (Figure 3). Of note, recent studies have explored other serum biomarkers (and even bile biomarkers) that may aid in the diagnosis of CCA, though more research is needed prior to their use in the clinical setting[82-84].
Endoscopic retrograde cholangiopancreatography (ERCP) with biliary sampling may be another surveillance strategy. When all definite, probable, and possible findings of CCA are included, the sensitivity of an abnormal ERCP is high (91%)[81]. However, the sensitivity of cytology for malignant lesions is low, with a systematic review and meta-analysis reporting a sensitivity of 43%[85]. Fluorescence in situ hybridization analysis has been found to improve this sensitivity[86,87], but the risks of post-ERCP complications, especially pancreatitis, cholangitis, and hospitalization rate of over 10% of patients with PSC make this an undesirable surveillance modality[88]. Thus, ERCP should be resorted to only when imaging and/or serum CA 19-9 are positive or indeterminate/insufficient.
New research has shown that surveillance for hepatobiliary cancers is associated with improved outcomes, including survival, in patients with PSC. A retrospective study of 830 patients with PSC found an association between hepatobiliary cancer surveillance and (1) earlier stage of cancer at diagnosis, (2) significantly lower 5-year risk of a cancer-related adverse event (32% vs 75%), and (3) significantly higher overall survival at 5 years (68% vs 20%) compared to patients that were not in a surveillance program[86]. Despite these promising results, care should still be exercised when implementing surveillance strategies, as diagnostic tools are still limited, and false positives are not uncommon. Furthermore, treatment options are limited, with curative surgical resection being appropriate in only a subset of patients and LT being offered only to selected patients with hilar CCA at highly specialized centers[89-92].
In the general population, GBC is often diagnosed at late stages because of the paucity of symptoms at early stages. By the time a diagnosis is made, patients often have metastatic cancer and a 5-year survival rate of less than 5%[93]. However, if detected incidentally or at an early stage (T1), 5-year survival after simple cholecystectomy is near 100%[94]. The same analogy can be made for patients with PSC; however, patients with PSC are much more likely to have gallbladder neoplasms (polyps, masses) than the general population, as mentioned earlier, and a high proportion of such lesions harbor malignancy in PSC[41]. Given these considerations, both the AASLD and EASL recommend yearly abdominal ultrasound in patients with PSC[44,45]. Although ultrasound is the preferred modality due to its high accuracy, availability, and cost-effectiveness, GBC surveillance can also be performed with MRI/MRCP (e.g., if used to concurrently surveil for CCA)[48]. As discussed earlier in this review, all patients with PSC with gallbladder polyps greater than 8 mm in size or gallbladder masses of any size should be evaluated for cholecystectomy[19,44-46]; while smaller lesions may be observed[47].
Historically, patients with PSC have been considered to have a relatively low risk for developing HCC, with an estimated lifetime incidence below 1.5%, a cut off established to justify regular HCC surveillance strategies based on cost-benefit analysis[95]. However, reports on the incidence of PSC have been limited and vary considerably. In a retrospective study of 119 patients with PSC and cirrhosis, no patients developed HCC[96]. Conversely, in a more recent study of 830 patients with PSC, 2.8% (n = 23) were found to have HCC, all of whom had underlying cirrhosis[51]. Therefore, it is unclear whether HCC surveillance is indicated for all patients with PSC[97]. At this time, the AASLD, EASL, and ACG do not provide specific recommendations on screening for HCC in patients with PSC, in part due to the fact that many patients with PSC do not (yet) have cirrhosis. Our practice is to conduct HCC surveillance with imaging every 6 mo for all patients with PSC-related cirrhosis, as is done for patients with cirrhosis due to other diseases[98,99]. For patients without cirrhosis, surveillance for HCC is effectively a byproduct of routine CCA surveillance.
Patients with PSC are at increased risk for CRC[16,18,55,57], and the risk is considered to be increased at time of initial PSC diagnosis[64]. Furthermore, the risk of CRC does not decrease after LT and can even increase further compared to pre-transplantation risk[66]. Thus, surveillance for CRC in patients with PSC pre- and post-transplantation is extremely important, with proven CRC-related survival benefit[5]. All leading societies recommend a full colonoscopy with biopsies in patients with a new diagnosis of PSC[44-46]. In patients with PSC and IBD, surveillance colonoscopy with biopsies should be performed at 1-2 year intervals from the time of diagnosis of PSC due to the high risk of CRC in patients with PSC-IBD[16,44,45] as well as the frequent lack of symptoms at diagnosis[54,60]. For patients without IBD, some experts advocate repeating a colonoscopy at 3-5 year intervals[46]. Of note, the use of chromoendoscopy with targeted biopsies has been recommended for surveillance of patients with IBD, though its value in unselected patients over high-definition colonoscopy is debatable, particularly considering the increased time and resources required for chromoendoscopy. Most providers thus tend to rely on high-definition colonoscopy with random colonic biopsies as first-line surveillance and reserve chromoendoscopy for patients known or believed to be at particularly increased risk for CRC (e.g., those with longstanding extensive colitis, family history of colon cancer, and concomitant PSC)[100-102].
PSC appears relatively infrequently in children compared to adults[17]. Pediatric PSC also presents differently than PSC in adults and has a variable natural history[44,46]. Given the rarity of CCA and GBC in children and the differences in pediatric compared to adult PSC, routine surveillance for these malignancies is not recommended[44,46]. However, similar to PSC in adults, IBD is frequently identified in pediatric PSC; therefore, it may be reasonable to consider colonoscopy with biopsies in children who are newly diagnosed with PSC[44].
Patients with PSC have a significantly increased risk of developing hepatobiliary and colorectal cancers, particularly the subset of patients with PSC-IBD. Currently, no proven pharmacological agents for prevention of carcinogenesis in patients with PSC exist. Leading national and international societies have published guidelines for CRC and GBC surveillance in patients with PSC, but surveillance strategies for CCA and HCC have not been well studied or data-proven. On the basis of a recent large study of patients with PSC, CCA surveillance appears to be associated with improved outcomes and should be performed once PSC has been diagnosed. The risk of HCC appears to be comparably lower and only present once PSC has progressed to cirrhosis. One common and (recent) evidence-based CCA surveillance strategy in patients with PSC is yearly cross-sectional imaging (US or MRI/MRCP) combined with serum tumor marker CA 19-9. Additional longitudinal, multicenter studies are needed to better evaluate the role, techniques, and impact of surveillance for CCA and other malignancies in patients with PSC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bove A, Kapoor S, Perini MV S- Editor: Yan JP L- Editor: A E- Editor: Yin SY
| 1. | Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med. 2016;375:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 2. | Tabibian JH, Lindor KD. Primary sclerosing cholangitis: A review and update on therapeutic developments. Expert Rev Gastroenterol Hepatol. 2013;7:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | O’Hara SP, Tabibian JH, Splinter PL, LaRusso NF. The dynamic biliary epithelia: Molecules, pathways, and disease. J Hepatol. 2013;58:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Porayko MK, Wiesner RH, LaRusso NF, Ludwig J, MacCarty RL, Steiner BL, Twomey CK, Zinsmeister AR. Patients with asymptomatic primary sclerosing cholangitis frequently have progressive disease. Gastroenterology. 1990;98:1594-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, van Nieuwkerk KM, Drenth JP, Witteman BJ, Tuynman HA, Naber AH, Kingma PJ, van Buuren HR, van Hoek B, Vleggaar FP, van Geloven N, Beuers U, Ponsioen CY; EpiPSCPBC Study Group. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 505] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 6. | Takakura WR, Tabibian JH, Bowlus CL. The evolution of natural history of primary sclerosing cholangitis. Curr Opin Gastroenterol. 2017;33:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: A systematic review. J Hepatol. 2012;56:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 441] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 8. | Molodecky NA, Kareemi H, Parab R, Barkema HW, Quan H, Myers RP, Kaplan GG. Incidence of primary sclerosing cholangitis: A systematic review and meta-analysis. Hepatology. 2011;53:1590-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Tsaitas C, Semertzidou A, Sinakos E. Update on inflammatory bowel disease in patients with primary sclerosing cholangitis. World J Hepatol. 2014;6:178-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Silveira MG, Mendes FD, Diehl NN, Enders FT, Lindor KD. Thyroid dysfunction in primary biliary cirrhosis, primary sclerosing cholangitis and non-alcoholic fatty liver disease. Liver Int. 2009;29:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Lamberts LE, Janse M, Haagsma EB, van den Berg AP, Weersma RK. Immune-mediated diseases in primary sclerosing cholangitis. Dig Liver Dis. 2011;43:802-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Rupp C, Mummelthei A, Sauer P, Weiss KH, Schirmacher P, Stiehl A, Stremmel W, Gotthardt DN. Non-IBD immunological diseases are a risk factor for reduced survival in PSC. Liver Int. 2013;33:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Folseraas T, Boberg KM. Cancer Risk and Surveillance in Primary Sclerosing Cholangitis. Clin Liver Dis. 2016;20:79-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Gulamhusein AF, Eaton JE, Tabibian JH, Atkinson EJ, Juran BD, Lazaridis KN. Duration of Inflammatory Bowel Disease Is Associated With Increased Risk of Cholangiocarcinoma in Patients With Primary Sclerosing Cholangitis and IBD. Am J Gastroenterol. 2016;111:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H, Almer S, Granath F, Broomé U. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 469] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 16. | Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 17. | Card TR, Solaymani-Dodaran M, West J. Incidence and mortality of primary sclerosing cholangitis in the UK: A population-based cohort study. J Hepatol. 2008;48:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Zheng HH, Jiang XL. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: A meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol. 2016;28:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Fevery J, Verslype C, Lai G, Aerts R, Van Steenbergen W. Incidence, diagnosis, and therapy of cholangiocarcinoma in patients with primary sclerosing cholangitis. Dig Dis Sci. 2007;52:3123-3135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Weismüller TJ, Trivedi PJ, Bergquist A, Imam M, Lenzen H, Ponsioen CY, Holm K, Gotthardt D, Färkkilä MA, Marschall HU, Thorburn D, Weersma RK, Fevery J, Mueller T, Chazouillères O, Schulze K, Lazaridis KN, Almer S, Pereira SP, Levy C, Mason A, Naess S, Bowlus CL, Floreani A, Halilbasic E, Yimam KK, Milkiewicz P, Beuers U, Huynh DK, Pares A, Manser CN, Dalekos GN, Eksteen B, Invernizzi P, Berg CP, Kirchner GI, Sarrazin C, Zimmer V, Fabris L, Braun F, Marzioni M, Juran BD, Said K, Rupp C, Jokelainen K, Benito de Valle M, Saffioti F, Cheung A, Trauner M, Schramm C, Chapman RW, Karlsen TH, Schrumpf E, Strassburg CP, Manns MP, Lindor KD, Hirschfield GM, Hansen BE, Boberg KM; International PSC Study Group. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology. 2017;152:1975-1984.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 364] [Article Influence: 45.5] [Reference Citation Analysis (1)] |
| 22. | Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 366] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 23. | Ahrendt SA, Pitt HA, Nakeeb A, Klein AS, Lillemoe KD, Kalloo AN, Cameron JL. Diagnosis and management of cholangiocarcinoma in primary sclerosing cholangitis. J Gastrointest Surg. 1999;3:357-67; discussion 367-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver. 2017;11:13-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 25. | Bergquist A, Glaumann H, Persson B, Broomé U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: A case-control study. Hepatology. 1998;27:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 178] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Ghouri YA, Mian I, Blechacz B. Cancer review: Cholangiocarcinoma. J Carcinog. 2015;14:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 27. | Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: A 25-year single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 1017] [Article Influence: 56.5] [Reference Citation Analysis (1)] |
| 29. | Lee WJ, Lim HK, Jang KM, Kim SH, Lee SJ, Lim JH, Choo IW. Radiologic spectrum of cholangiocarcinoma: emphasis on unusual manifestations and differential diagnoses. Radiographics. 2001;21 Spec No:S97-S116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Fevery J, Verslype C. An update on cholangiocarcinoma associated with primary sclerosing cholangitis. Curr Opin Gastroenterol. 2010;26:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 568] [Article Influence: 71.0] [Reference Citation Analysis (35)] |
| 32. | Hilscher MB, Tabibian JH, Carey EJ, Gostout CJ, Lindor KD. Dominant strictures in primary sclerosing cholangitis: A multicenter survey of clinical definitions and practices. Hepatol Commun. 2018;2:836-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Lazaridis KN, Gores GJ. Primary sclerosing cholangitis and cholangiocarcinoma. Semin Liver Dis. 2006;26:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Mouchli MA, Singh S, Loftus EV, Boardman L, Talwalkar J, Rosen CB, Heimbach JK, Wiesner RH, Hasan B, Poterucha JJ, Kymberly WD. Risk Factors and Outcomes of De Novo Cancers (Excluding Nonmelanoma Skin Cancer) After Liver Transplantation for Primary Sclerosing Cholangitis. Transplantation. 2017;101:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Khorsandi SE, Salvans S, Zen Y, Agarwal K, Jassem W, Heaton N. Cholangiocarcinoma complicating recurrent primary sclerosing cholangitis after liver transplantation. Transpl Int. 2011;24:e93-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Sanchez W, Gores GJ. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137:2010-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 37. | Landaverde C, Ng V, Sato A, Tabibian J, Durazo F, Busuttil R. De-novo cholangiocarcinoma in native common bile duct remnant following OLT for primary sclerosing cholangitis. Ann Hepatol. 2009;8:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Heneghan MA, Tuttle-Newhall JE, Suhocki PV, Muir AJ, Morse M, Bornstein JD, Sylvestre PB, Collins B, Kuo PC, Rockey DC. De-novo cholangiocarcinoma in the setting of recurrent primary sclerosing cholangitis following liver transplant. Am J Transplant. 2003;3:634-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Tabibian JH, Bowlus CL. Primary sclerosing cholangitis: A review and update. Liver Res. 2017;1:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Tabibian J, Lazaridis K, LaRusso N, Jarnagin W. Primary Sclerosing Cholangitis. Jarnagin W. Blumgart’s Surgery of the Liver, Biliary Tract and Pancreas. Philadelphia, PA, United States: Elsevier; 2017; 663-674. |
| 41. | Karlsen TH, Schrumpf E, Boberg KM. Gallbladder polyps in primary sclerosing cholangitis: Not so benign. Curr Opin Gastroenterol. 2008;24:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Buckles DC, Lindor KD, Larusso NF, Petrovic LM, Gores GJ. In primary sclerosing cholangitis, gallbladder polyps are frequently malignant. Am J Gastroenterol. 2002;97:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Sandrasegaran K, Menias CO. Imaging and Screening of Cancer of the Gallbladder and Bile Ducts. Radiol Clin North Am. 2017;55:1211-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 45. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1204] [Article Influence: 75.3] [Reference Citation Analysis (1)] |
| 46. | Lindor KD, Kowdley KV, Harrison ME; American College of Gastroenterology. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646-59; quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 338] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 47. | Eaton JE, Thackeray EW, Lindor KD. Likelihood of malignancy in gallbladder polyps and outcomes following cholecystectomy in primary sclerosing cholangitis. Am J Gastroenterol. 2012;107:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54:1842-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 49. | Harnois DM, Gores GJ, Ludwig J, Steers JL, LaRusso NF, Wiesner RH. Are patients with cirrhotic stage primary sclerosing cholangitis at risk for the development of hepatocellular cancer? J Hepatol. 1997;27:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Leidenius M, Höckersted K, Broomé U, Ericzon BG, Friman S, Olausson M, Schrumpf E. Hepatobiliary carcinoma in primary sclerosing cholangitis: A case control study. J Hepatol. 2001;34:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Ali AH, Tabibian JH, Nasser-Ghodsi N, Lennon RJ, DeLeon T, Borad MJ, Hilscher M, Silveira MG, Carey EJ, Lindor KD. Surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis. Hepatology. 2018;67:2338-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 52. | Francescone R, Hou V, Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 53. | de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21:1956-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 120] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 54. | Loftus EV, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: A unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 518] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 55. | Broomé U, Löfberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: Evidence for increased neoplastic potential. Hepatology. 1995;22:1404-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: A meta-analysis. Gastrointest Endosc. 2002;56:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 383] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 57. | Braden B, Halliday J, Aryasingha S, Sharifi Y, Checchin D, Warren BF, Kitiyakara T, Travis SP, Chapman RW. Risk for colorectal neoplasia in patients with colonic Crohn's disease and concomitant primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2012;10:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Lindström L, Lapidus A, Ost A, Bergquist A. Increased risk of colorectal cancer and dysplasia in patients with Crohn's colitis and primary sclerosing cholangitis. Dis Colon Rectum. 2011;54:1392-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Ricciuto A, Kamath BM, Griffiths AM. The IBD and PSC Phenotypes of PSC-IBD. Curr Gastroenterol Rep. 2018;20:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Broomé U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Shah SC, Ten Hove JR, Castaneda D, Palmela C, Mooiweer E, Colombel JF, Harpaz N, Ullman TA, van Bodegraven AA, Jansen JM, Mahmmod N, van der Meulen-de Jong AE, Ponsioen CY, van der Woude CJ, Oldenburg B, Itzkowitz SH, Torres J. High Risk of Advanced Colorectal Neoplasia in Patients With Primary Sclerosing Cholangitis Associated With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2018;16:1106-1113.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 62. | Brackmann S, Andersen SN, Aamodt G, Langmark F, Clausen OP, Aadland E, Fausa O, Rydning A, Vatn MH. Relationship between clinical parameters and the colitis-colorectal cancer interval in a cohort of patients with colorectal cancer in inflammatory bowel disease. Scand J Gastroenterol. 2009;44:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Claessen MM, Lutgens MW, van Buuren HR, Oldenburg B, Stokkers PC, van der Woude CJ, Hommes DW, de Jong DJ, Dijkstra G, van Bodegraven AA, Siersema PD, Vleggaar FP. More right-sided IBD-associated colorectal cancer in patients with primary sclerosing cholangitis. Inflamm Bowel Dis. 2009;15:1331-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Navaneethan U, Kochhar G, Venkatesh PG, Lewis B, Lashner BA, Remzi FH, Shen B, Kiran RP. Duration and severity of primary sclerosing cholangitis is not associated with risk of neoplastic changes in the colon in patients with ulcerative colitis. Gastrointest Endosc. 2012;75:1045-1054.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Thackeray EW, Charatcharoenwitthaya P, Elfaki D, Sinakos E, Lindor KD. Colon neoplasms develop early in the course of inflammatory bowel disease and primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2011;9:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Rao BB, Lashner B, Kowdley KV. Reviewing the Risk of Colorectal Cancer in Inflammatory Bowel Disease After Liver Transplantation for Primary Sclerosing Cholangitis. Inflamm Bowel Dis. 2018;24:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Fevery J, Henckaerts L, Van Oirbeek R, Vermeire S, Rutgeerts P, Nevens F, Van Steenbergen W. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: A long-term single-centre study. Liver Int. 2012;32:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 68. | Pardi DS, Loftus EV, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 356] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 69. | Tung BY, Emond MJ, Haggitt RC, Bronner MP, Kimmey MB, Kowdley KV, Brentnall TA. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 287] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 70. | Lindström L, Boberg KM, Wikman O, Friis-Liby I, Hultcrantz R, Prytz H, Sandberg-Gertzén H, Sangfelt P, Rydning A, Folvik G, Gangsøy-Kristiansen M, Danielsson A, Bergquist A. High dose ursodeoxycholic acid in primary sclerosing cholangitis does not prevent colorectal neoplasia. Aliment Pharmacol Ther. 2012;35:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Eaton JE, Silveira MG, Pardi DS, Sinakos E, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Lindor KD. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 72. | Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 491] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 73. | de Chambrun GP, Nachury M, Funakoshi N, Gerard R, Bismuth M, Valats JC, Panaro F, Navarro F, Desreumaux P, Pariente B, Blanc P. Oral vancomycin induces sustained deep remission in adult patients with ulcerative colitis and primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2018;30:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 74. | Tan LZ, Reilly CR, Steward-Harrison LC, Balouch F, Muir R, Lewindon PJ. Oral vancomycin induces clinical and mucosal remission of colitis in children with primary sclerosing cholangitis-ulcerative colitis. Gut. 2018;pii:gutjnl-2018-316599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Davies YK, Tsay CJ, Caccamo DV, Cox KM, Castillo RO, Cox KL. Successful treatment of recurrent primary sclerosing cholangitis after orthotopic liver transplantation with oral vancomycin. Case Rep Transplant. 2013;2013:314292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Lindor K. Vancomycin for Primary Sclerosing Cholangitis. [accessed 2018-10-22] In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. ClinicalTrials.gov Identifier: NCT03710122. Available from: https://clinicaltrials.gov/ct2/show/NCT03710122. |
| 77. | Hu RW, Carey EJ, Lindor KD, Tabibian JH. Curcumin in Hepatobiliary Disease: Pharmacotherapeutic Properties and Emerging Potential Clinical Applications. Ann Hepatol. 2017;16:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Eaton JE. A Study Evaluating the Safety and Efficacy of Curcumin in Patients With Primary Sclerosing Cholangitis. [accessed 2018-10-20] In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. ClinicalTrials.gov Identifier: NCT02978339. Available from: https://clinicaltrials.gov/ct2/show/NCT02978339. |
| 79. | Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, McCashland TM, Reddy KR, Zervos X, Anbari MA, Hoen H. Cholangiocarcinoma in patients with primary sclerosing cholangitis: A multicenter case-control study. Hepatology. 2000;31:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 208] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 80. | Schramm C, Eaton J, Ringe KI, Venkatesh S, Yamamura J, MRI working group of the IPSCSG. Recommendations on the use of magnetic resonance imaging in PSC-A position statement from the International PSC Study Group. Hepatology. 2017;66:1675-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 81. | Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 82. | Wannhoff A, Gotthardt DN. Recent developments in the research on biomarkers of cholangiocarcinoma in primary sclerosing cholangitis. Clin Res Hepatol Gastroenterol. 2018;pii:S2210-7401(18)30177-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Cuenco J, Wehnert N, Blyuss O, Kazarian A, Whitwell HJ, Menon U, Dawnay A, Manns MP, Pereira SP, Timms JF. Identification of a serum biomarker panel for the differential diagnosis of cholangiocarcinoma and primary sclerosing cholangitis. Oncotarget. 2018;9:17430-17442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 84. | Navaneethan U, Parsi MA, Lourdusamy V, Bhatt A, Gutierrez NG, Grove D, Sanaka MR, Hammel JP, Stevens T, Vargo JJ, Dweik RA. Volatile organic compounds in bile for early diagnosis of cholangiocarcinoma in patients with primary sclerosing cholangitis: A pilot study. Gastrointest Endosc. 2015;81:943-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: A systematic review and meta-analysis. Gastrointest Endosc. 2014;79:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 86. | Kipp BR, Stadheim LM, Halling SA, Pochron NL, Harmsen S, Nagorney DM, Sebo TJ, Therneau TM, Gores GJ, de Groen PC, Baron TH, Levy MJ, Halling KC, Roberts LR. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 255] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 87. | Bangarulingam SY, Bjornsson E, Enders F, Barr Fritcher EG, Gores G, Halling KC, Lindor KD. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology. 2010;51:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 88. | Bangarulingam SY, Gossard AA, Petersen BT, Ott BJ, Lindor KD. Complications of endoscopic retrograde cholangiopancreatography in primary sclerosing cholangitis. Am J Gastroenterol. 2009;104:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 89. | Zamora-Valdes D, Heimbach JK. Liver Transplant for Cholangiocarcinoma. Gastroenterol Clin North Am. 2018;47:267-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Stremitzer S, Jones RP, Quinn LM, Fenwick SW, Diaz-Nieto R, Poston GJ, Malik HZ. Clinical outcome after resection of early-stage hilar cholangiocarcinoma. Eur J Surg Oncol. 2018;pii:S0748-7983(18)31427-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, Burgart L, Gores GJ. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 239] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 92. | Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88-98.e3; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 93. | Vijayakumar A, Vijayakumar A, Patil V, Mallikarjuna MN, Shivaswamy BS. Early diagnosis of gallbladder carcinoma: An algorithm approach. ISRN Radiol. 2012;2013:239424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg. 1992;215:326-331. [PubMed] |
| 95. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 96. | Zenouzi R, Weismüller TJ, Hübener P, Schulze K, Bubenheim M, Pannicke N, Weiler-Normann C, Lenzen H, Manns MP, Lohse AW, Schramm C. Low risk of hepatocellular carcinoma in patients with primary sclerosing cholangitis with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1733-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 97. | Gossard AA, Lindor KD. Hepatocellular carcinoma: Low risk of HCC in patients who have PSC and cirrhosis. Nat Rev Gastroenterol Hepatol. 2014;11:276-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Khaderi SA, Sussman NL. Screening for malignancy in primary sclerosing cholangitis (PSC). Curr Gastroenterol Rep. 2015;17:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 99. | Ayuso C, Rimola J, Vilana R, Burrel M, Darnell A, García-Criado Á, Bianchi L, Belmonte E, Caparroz C, Barrufet M, Bruix J, Brú C. Diagnosis and staging of hepatocellular carcinoma (HCC): Current guidelines. Eur J Radiol. 2018;101:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 100. | Moussata D, Allez M, Cazals-Hatem D, Treton X, Laharie D, Reimund JM, Bertheau P, Bourreille A, Lavergne-Slove A, Brixi H, Branche J, Gornet JM, Stefanescu C, Moreau J, Marteau P, Pelletier AL, Carbonnel F, Seksik P, Simon M, Flé, jou JF, Colombel JF, Charlois AL, Roblin X, Nancey S, Bouhnik Y, Berger F, Flourié, B, the GETAID. Are random biopsies still useful for the detection of neoplasia in patients with IBD undergoing surveillance colonoscopy with chromoendoscopy? Gut. 2018;67:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 101. | van den Broek FJ, Stokkers PC, Reitsma JB, Boltjes RP, Ponsioen CY, Fockens P, Dekker E. Random biopsies taken during colonoscopic surveillance of patients with longstanding ulcerative colitis: Low yield and absence of clinical consequences. Am J Gastroenterol. 2014;109:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 102. | Navaneethan U, Kochhar G, Venkatesh PG, Bennett AE, Rizk M, Shen B, Kiran RP. Random biopsies during surveillance colonoscopy increase dysplasia detection in patients with primary sclerosing cholangitis and ulcerative colitis. J Crohns Colitis. 2013;7:974-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 103. | Tabibian JH, Ali AH, Lindor KD. Primary Sclerosing Cholangitis, Part 2: Cancer Risk, Prevention, and Surveillance. Gastroenterol Hepatol (NY). 2018;14:427-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |