Published online Feb 7, 2019. doi: 10.3748/wjg.v25.i5.608
Peer-review started: October 31, 2018
First decision: December 20, 2018
Revised: January 3, 2019
Accepted: January 14, 2019
Article in press: January 14, 2019
Published online: February 7, 2019
Processing time: 91 Days and 6.4 Hours
Intrahepatic sarcomatoid chonalgiocarcinoma (s-CCC) is an extremely rare disease, accounting for less than 1% of hepatobiliary system malignancies, and its pathophysiology is not well known. On the hypothesis that its clinical, serologic, or radiologic diagnosis are not fully understood and its prognosis is poor, we investigated the distinguishing features of s-CCC compared with those of intrahepatic bile duct adenocarcinoma [cholangiocellular carcinoma (CCC)] in patients from a single center.
To analyze the clinical, serologic, imaging, and histopathologic characteristics of intrahepatic s-CCC patients diagnosed in a single center.
The clinical, serologic, imaging, and histopathologic features of 227 patients diagnosed with intrahepatic cholangiocarcinoma (IHCC) in a single medical center during the last 17 years were analyzed. The characteristics of 11 patients with s-CCC were compared with those of 216 patients with CCC.
The number of patients with s-CCC who presented fever and abdominal pain and past history of chronic viral hepatitis or liver cirrhosis (LC) was higher than that of patients with CCC. In imaging studies, patients with s-CCC showed relatively aggressive features. However, no clear distinction was observed between s-CCC and CCC based on other clinical, serologic or radiologic examination results. An accurate diagnosis could be made only via a histopathologic examination through immunohistochemical staining. The clinical course of s-CCC was generally aggressive, and patients had a relatively poor prognosis.
In patients with s-CCC, early diagnosis through biopsy and aggressive treatment, including surgical resection, are important.
Core tip: Intrahepatic sarcomatoid cholangiocarcinoma is rare condition. Patients usually present with an advanced stage of the disease, and they have a poor prognosis. Diagnosis based on histopathologic examination is important because serologic and radiologic examinations cannot help in distinguishing such condition from intrahepatic bile duct adenocarcinoma or other intrahepatic masses. Thus, patients must be diagnosed as early as possible and should receive aggressive treatment, including surgical resection.
- Citation: Kim DK, Kim BR, Jeong JS, Baek YH. Analysis of intrahepatic sarcomatoid cholangiocarcinoma: Experience from 11 cases within 17 years. World J Gastroenterol 2019; 25(5): 608-621
- URL: https://www.wjgnet.com/1007-9327/full/v25/i5/608.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i5.608
Epithelial tumors with a sarcomatoid feature can develop in several organs, such as the skin, kidney, esophagus, stomach, gallbladder, thyroid, urinary bladder, uterus, and lungs. However, its pathologic mechanism has not been clearly elucidated[1]. Most malignant tumors in the intrahepatic bile duct are adenocarcinomas which are commonly referred to as cholangiocellular carcinoma (CCC). In contrast, intrahepatic sarcomatoid cholangiocarcinoma (s-CCC) is an extremely rare condition, accounting for less than 1% of hepatobiliary system malignancies, and its pathophysiology is not well known[2,3]. The most common primary hepatic malignancy with a sarcomatoid feature is hepatocellular carcinoma (HCC), and generally, it occurs due to secondary changes in tumor cells after embolization or chemotherapy. However, the relationship between the occurrence of s-CCC and previous treatment has not been clearly understood, and it is often considered as a change according to the natural history of CCC[4]. In some previous studies, the prognosis of s-CCC was reported worse than that of CCC, which was due to a more frequent metastasis to other organs or invasion to adjacent vasculatures, and only few established data about its clinical features, diagnosis, and treatment methods are available[5,6]. In this study, we hypothesized the pathogenesis of s-CCC, investigated the clinical, serologic, imaging, and histopathologic features of s-CCC, and compared with those of CCC in patients from a single center.
From January 2001 to June 30, 2018, a total of 228 patients with intrahepatic cholangiocarcinoma (IHCC) diagnosed via surgery or ultrasonography (US)-guided liver biopsy at Dong-A University Hospital were screened. Among them, 12 were diagnosed with s-CCC. One patient who was diagnosed with s-CCC via surgery in August 2001 was excluded from the analysis due to the lack of data about the clinical course of the disease and imaging study results. Two patients presented with mixed histopathologic features of HCC and IHCC were included in the study.
In 11 patients with s-CCC who were enrolled, the admission history, accompanying symptoms or signs, past history of the hepatobiliary system, and findings of serologic examinations, such as liver function tests and tumor marker analysis were assessed. In terms of imaging studies, such as US, computed tomography (CT) scan, and magnetic resonance imaging (MRI), the characteristic features, the size and number of primary masses, distant and lymph node (LN) metastases, and the TNM stage were investigated and compared with those of HCC, IHCC, and other hepatic tumors. Histopathologic examination and immunohistochemical staining of hepatic mass were also conducted. Finally, the types of treatment, consequent clinical course, follow-up results, and survival time were investigated.
Data were reported as means ± standard deviation and median value. Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) software version 20.0. Continuous variables were compared using the unpaired student t-test, Mann-Whitney U test, and Kruskal-Wallis test. Categorical variables were compared with the chi-square test and Fisher’s exact test, and they were reported as frequencies and percentages. P < 0.05 were considered statistically significant. Survival curves were obtained using the Kaplan-Meier method and compared using the log-rank test.
Among the 11 patients diagnosed with s-CCC, 9 (81.8%) were men and 2 (18.2%) were women. The median age of the patients was 61 (range: 45-68) years. During their first visit at the hospital, the patients mainly presented abdominal pain (n = 10, 90.9%), and fever (n = 4, 36.4%), which were more frequent in s-CCC patients compared with CCC patients. Past history of chronic viral hepatitis and liver cirrhosis (LC) were frequent in s-CCC patients: chronic hepatitis B (CHB), n = 3 (27.3%); chronic hepatitis C (CHC), n = 1 (9.1%); LC, n = 5 (45.5%). One of the patients was diagnosed with HCC at another hospital 1 mo before admission and was taking sorafenib. In addition, 1 (9.1%) patient had a previous history of Clonorchis sinensis infection and another (9.1%) patient presented with gallstones at the time of diagnosis. Meanwhile, 3 (27.3%) patients had a history of cholecystectomy. All 11 patients presented with stage IV disease by the TNM stage, of whom 4 (36.4%) had stage IVA and 7 (63.6%) had stage IVB (Tables 1 and 2).
| Case | Sex | Age | Chief complaint | Stone | CS | Chronic hepatitis | LC | HCC | Tumor size (cm) | Number of mass | Metastasis | Stage (TNM) |
| 1 | M | 45 | RUQ pain | - | - | CHB | + | + | 7.5 | 7 | LN, IH | IVB |
| 2 | M | 67 | Lt. flank pain | - | - | CHC | + | - | 2.5 | 1 | Bone, LN | IVB |
| 3 | M | 55 | RUQ pain, fever | CST | + | - | - | - | 6.5 | 2 | LN | IVA |
| 4 | M | 66 | RUQ pain, fever | GB stone | - | - | - | - | 10.0 | 1 | Lung, LN | IVB |
| 5 | M | 56 | RUQ pain, Fatigue | - | - | CHB | + | - | 8.0 | 1 | Thymus, LN | IVB |
| 6 | F | 66 | RUQ pain | CST | - | - | - | - | 7.5 | 1 | LN | IVB |
| 7 | M | 68 | Wt. loss, Fatigue | CST | - | - | + | - | 6.0 | 1 | Lung, Bone, LN | IVB |
| 8 | F | 55 | RUQ pain, fever | - | - | - | - | - | 8.5 | 3 | LN | IVA |
| 9 | M | 49 | LUQ pain, fever | - | - | CHB | + | - | 9.5 | 3 | LN | IVA |
| 10 | M | 65 | RUQ pain | - | - | - | - | 9.5 | 15 | LN, IH | IVA | |
| 11 | M | 61 | RUQ pain | - | - | - | - | 5.0 | 1 | Diaphragm, LN | IVB |
| Adenocarcinoma | Sarcomatoid | P value | ||
| Gender | Male | 155 (71.8) | 9 (81.8) | 0.732 |
| Female | 61 (28.2) | 2 (18.2) | ||
| Age | < 60 | 73 (33.8) | 5 (45.5) | 0.518 |
| ≥ 60 | 143(66.2) | 6 (54.5) | ||
| Abdominal pain | No | 106 (49.1) | 1 (9.1) | 0.011 |
| Pain | 110 (50.9) | 10 (90.9) | ||
| Fever | No | 195 (90.3) | 7 (63.6) | 0.022 |
| Fever | 21 (9.7) | 4 (36.4) | ||
| Jaundice | No | 199 (92.1) | 11 (100.0) | 1.000 |
| Jaundice | 17 (7.9) | 0 (0.0) | ||
| Weight loss | No | 201 (93.1) | 10 (90.9) | 0.561 |
| Weight loss | 15 (6.9) | 1 (9.1) | ||
| GB stone | No | 164 (75.9) | 8 (72.7) | 0.730 |
| GB stone | 52 (24.1) | 3 (27.3) | ||
| Hepatitis | No | 190 (88.0) | 7 (63.6) | 0.063 |
| CHB | 21 (9.7) | 3 (27.3) | ||
| CHC | 5 (2.3) | 1 (9.1) | ||
| LC | No | 183 (84.7) | 6 (54.5) | 0.022 |
| LC | 33 (15.3) | 5 (45.5) | ||
| Serum CEA | < 5 ng/mL | 123 (56.9) | 10 (90.9) | 0.029 |
| ≥ 5 ng/mL | 93 (43.1) | 1 (9.1) | ||
| Serum CA19-9 | < 37 U/mL | 64 (29.6) | 6 (54.5) | 0.098 |
| ≥ 37 U/mL | 152 (70.4) | 5 (45.5) | ||
| Main mass | Single | 145 (67.1) | 6 (54.5) | 0.513 |
| Multiple | 71 (32.9) | 5 (45.5) | ||
| Mass size | < 5 cm | 90 (41.7) | 1 (9.1) | 0.054 |
| ≥ 5 cm | 126 (58.3) | 10 (90.9) | ||
| LN metastasis | No | 84 (38.9) | 1 (9.1) | 0.057 |
| LN meta. | 132 (61.1) | 10 (90.9) | ||
| Distant metastasis | No | 140 (64.8) | 4 (36.4) | 0.104 |
| Distant meta. | 76 (35.2) | 7 (63.6) | ||
| TNM stage | I or II | 76 (35.2) | 0 (0.0) | 0.018 |
| III or IV | 140 (64.8) | 11 (100.0) | ||
The laboratory findings were as follows: serum aspartate aminotransferase (AST) level, 23-80 (median: 34, normal: < 40) U/L; alanine aminotransferase (ALT) level, 10-96 (median: 31, normal: <40) U/L; serum total bilirubin (TB) level, 0.3-1.1 (median: 0.7, normal: < 1.2) mg/dL; and direct bilirubin (DB) level, 0.1-0.8 (median: 0.4, normal: < 0.4) mg/dL. In all patients, the serum alkaline phosphatase (ALP) level was elevated at 252-1520 (median: 757, normal: < 120) U/L. The gamma-glutamyl transferase (GGT) level was measured in only 9 patients, ranging from 32 to 323 (median: 137, normal: < 64) U/L, and it was elevated above the normal limit in 7 patients.
Serum carcinoembryonic antigen (CEA) levels were elevated in only 1 (9.1%) patient, ranging from 0.1 to 12.7 (median: 1.8, normal: < 5) ng/mL. The carbohydrate antigen 19-9 (CA19-9) level ranged from 2.0 to 1809.57 (normal: < 37) U/mL, and it was elevated in 5 patients (45.5%). Alpha-fetoprotein (AFP) level was high in 2 (18.2%) patients (cases 1 and 2), of which 1 patient presented with CHB, LC, and HCC and treated with sorafenib, and the other patient had CHC and LC (Table 3).
| Case | CEA (ng/mL) | CA19-9 (U/mL) | AFP (ng/mL) | PIVKA-II (mAU/mL) | AST (U/L) | ALT (U/L) | TB (mg/dL) | DB (mg/dL) | ALP (U/L) | GGT (U/L) |
| 1 | 0.74 | > 1200.00 | 131.67 | 69 | 25 | 19 | 1.0 | 0.4 | 387 | 115 |
| 2 | 1.45 | 3.38 | 66.45 | 16 | 31 | 10 | 0.7 | 0.2 | 252 | 32 |
| 3 | 0.10 | 3.00 | 2.54 | 35 | 54 | 96 | 0.4 | 0.2 | 892 | 137 |
| 4 | 2.35 | 1809.57 | 1.73 | N/A | 42 | 30 | 0.9 | 0.6 | 1385 | N/A |
| 5 | 1.81 | 2.33 | 2.31 | 20 | 43 | 57 | 1.0 | 0.6 | 1520 | 203 |
| 6 | 12.70 | 710.38 | 3.92 | N/A | 23 | 39 | 0.7 | 0.3 | 1133 | 253 |
| 7 | 1.18 | 12.59 | 2.70 | 20 | 23 | 16 | 0.6 | 0.4 | 638 | 224 |
| 8 | 3.15 | > 1200.00 | 1.71 | N/A | 30 | 31 | 1.1 | 0.6 | 757 | 323 |
| 9 | 1.08 | < 2.00 | 1.52 | N/A | 80 | 30 | 0.7 | 0.5 | 476 | 98 |
| 10 | 3.56 | 599.14 | 1.02 | N/A | 37 | 47 | 1.1 | 0.8 | 804 | N/A |
| 11 | 1.81 | 5.77 | 3.02 | 14 | 34 | 36 | 0.3 | 0.1 | 369 | 35 |
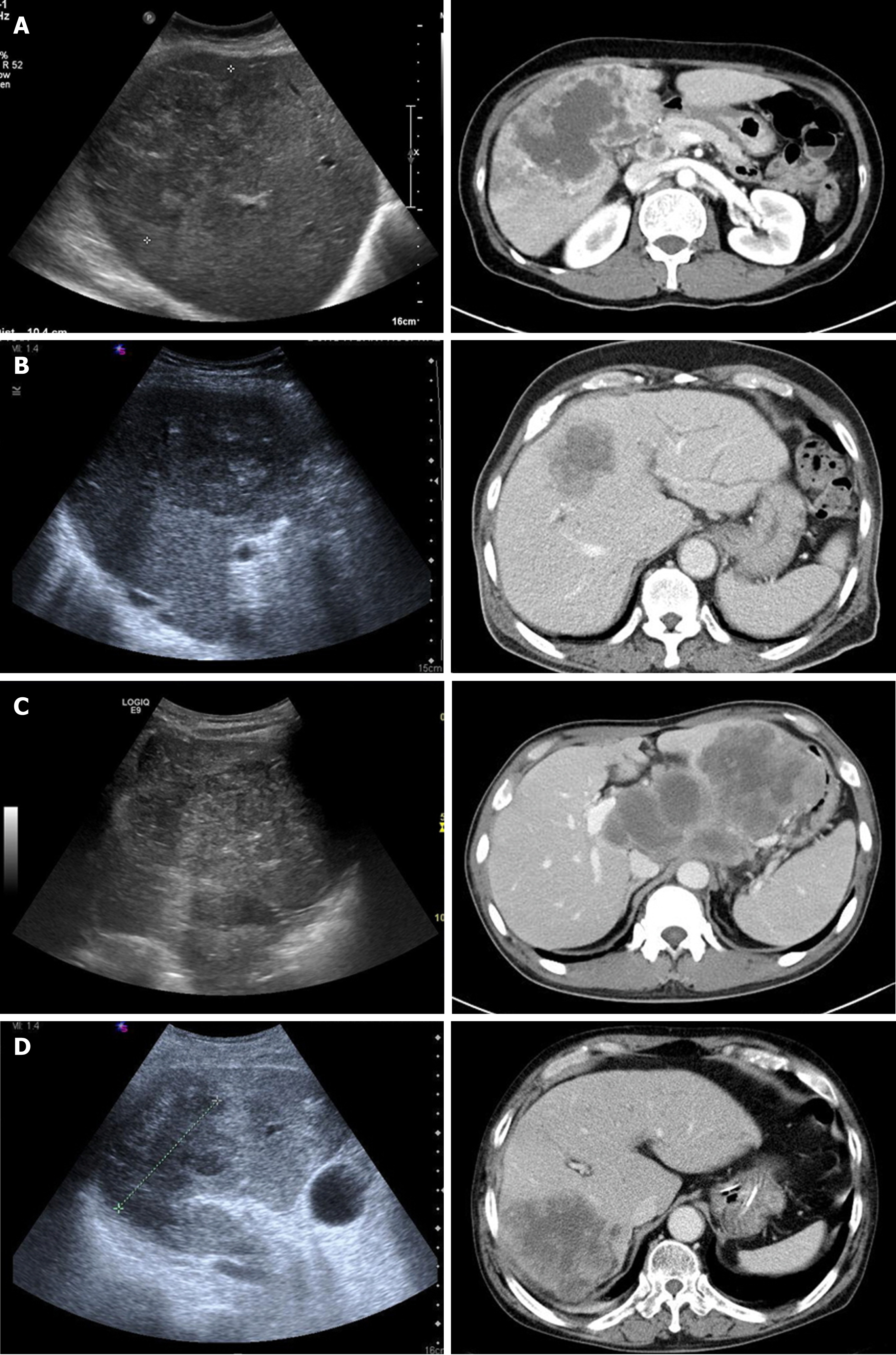
The imaging findings were newly reviewed by a radiologist. The initial radiologic impression obtained via abdominal US and CT scan was very variable as IHCC, HCC, lymphoma, and hepatic abscess. (Table 4, Figure 1). The size of the main tumor lesion on US, CT scan and MRI ranged from 2.5 to 10 (median: 7.5) cm, and the number of intrahepatic tumors was 1 in 6 (54.5%) patients, and multiple tumors were observed in the other 5 (45.5%) patients. All patients had stage IV disease by TNM stage. Multiple LN metastases were observed in all patients, and distant metastases to other organs were observed in 7 (63.6%) patients (Table 1).
| Case | US | CT Non-E | CT arterial | CT portal | CT delayed | Initial radiologic impression |
| 1 | Lobulated, heterogeneously hyperechoic | N/A | Thin rim enhancement | Gradual centripetal enhancement | Gradual centripetal enhancement | HCC |
| 2 | Lobulated hypoechoic | Ill-defined hypodense | Minimal to mild heterogeneous enhancement | Progressive heterogeneous enhancement | Progressive heterogeneous enhancement | HCC |
| 3 | Lobulated, heterogeneously hypoechoic internal hyperechoic | N/A | Non to minimal rim enhancement | Mild irregular rim enhancement | N/A | IHCC |
| 4 | Ill-defined hypoechoic | heterogeneously hypodense | Irregular rim enhancement | Gradual centripetal enhancement | N/A | Hepatic abscess |
| 5 | Lobulated, heterogenoeusly hypo- and hyper-echoic | N/A | Peripheral enhancement | Gradual centripetal enhancement | Gradual centripetal enhancement | HCC |
| 6 | Lobulated hyperechoic | N/A | Irregular rim enhancement | Irregular rim enhancement | N/A | IHCC |
| 7 | Lobulated, heterogeneously hypoechoic internal hyperechoic | Ill-defined hypodense | Irregular peripheral enhancement | Gradual centripetal enhancement | Gradual centripetal enhancement | HCC |
| 8 | Ill-defined heterogenoeusly hypo- and hyper-echoic | Ill-defined hypodense | Irregular peripheral enhancement | Gradual centripetal enhancement | N/A | IHCC |
| 9 | Lobulated, heterogenoeusly hypo- and hyper-echoic | N/A | Irregular peripheral enhancement | Gradual centripetal enhancement | N/A | Lymphoma |
| 10 | Lobulated, heterogeneously hyperecmoic | N/A | Minimal rim enhancement | Rim enhancement | N/A | IHCC |
| 11 | Well-defined, heterogeneously hypoechoic | Lobulated hypodense | Irregular peripheral enhancement | Irregular peripheral enhancement | N/A | IHCC |
All patients had lobulated heterogeneous mass lesions on abdominal US. Hypoechoic features were observed in 3 (27.3%) patients, hyperechoic features in 3 (27.3%) patients, and mixed echoic features in 5 (45.5%) patients. A hypodense mass with heterogeneous, ill-defined, or lobulated features was observed in the CT non-enhanced phase which was performed in only 5 patients. Enhanced CT scans were performed in all 11 patients. In the arterial phase, a hepatic mass presented with irregular rim enhancement (n = 5), peripheral enhancement (n = 5), or diffuse heterogeneous enhancement (n = 1). The CT portal phase showed gradual centripetal enhancement (n = 6), irregular rim enhancement (n = 3), and irregular peripheral enhancement (n = 1). In 1 patient with heterogeneous enhancement of the mass in the arterial phase, the enhancement was more prominent in the portal phase. CT scan in the delayed phase was performed in 4 patients and its findings were not significantly different from those in the portal phase (Table 4). MRI was performed in 3 patients with heterogeneous hyperintensity (n = 3) on the T2-weighted image, gradual centripetal (n = 2) or rim (n = 1) enhancement on the dynamic enhancement scan, and diffusion restriction (n = 3) on diffusion weighted image.
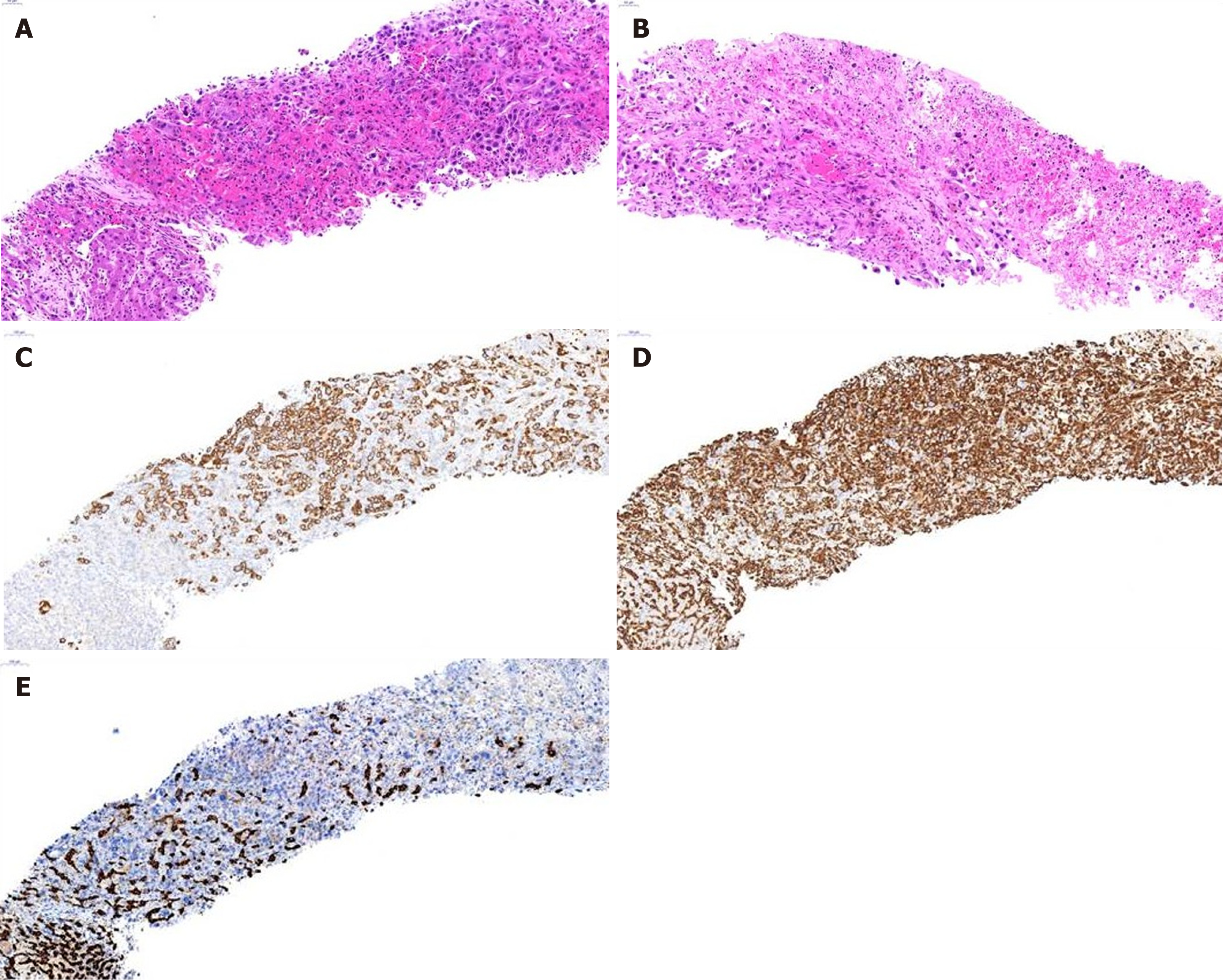
Figure 2 shows histopathological findings in case 9. In low-magnified microscopic findings, liver biopsy showed relatively well-demarcated tumor, characterized by poorly differentiated cancer cells without organoid structures, interspersed by stromal tissue, which is associated with necrosis (Figure 2A and B). Individual or clustered cancer cells consisted of enlarged epithelioid cells with pleomorphic and hyperchromatic nuclei and prominent nucleoli, and spindle cells with hyperchromatic and enlarged nuclei (Figure 2A). Occasionally, mucin-secreting cancer cells were observed. Inflammatory cell infiltration was present in the abundant stroma.
Immunohistochemical study had shown that cancer cells expressed cytokeratin 9 (CK8), cytokeratin 19 (CK19) (Figure 2C), and vimentin (Figure 2D), but not hepatocyte-specific antigen (HSA) (Figure 2E) and AFP. Based on the microscopic findings and immunohistochemical results, this liver tumor was diagnosed as s-CCC. In the remaining 10 patients, cells showing polymorphic and polygonal spindle features were observed on cytopathologic examination. Hepatocellular features were found in 2 patients. Immunochemical staining revealed positive vimentin expression in all patients and CK19 expression in 10 (90.9%) patients. HSA staining was performed in 9 patients, and all patients tested negative. Immunohistochemical findings are summarized in Table 5.
| Case | Specimen | Positive IHC results | Negative IHC results |
| 1 | Needle biopsy | CK19, vimentin | HSA, CD10 |
| 2 | Needle biopsy | CK, vimentin, CEA, AFP | CK7, CK19, HSA, c-kit, CD117 |
| 3 | Needle biopsy | CK, CK19, vimentin | CK8, Desmin, EMA, CEA, c-kit, S-100 |
| 4 | Needle biopsy | CK, CK8, CK19, vimentin, CEA, EMA | HSA, AFP, TTF-1 |
| 5 | Needle biopsy | CK, CK8, CK19, vimentin, SMA | HSA, CD5, CD68, HMW-CK |
| 6 | Needle biopsy | CK7, CK8, CK19, vimentin, CEA | HSA |
| 7 | Needle biopsy | CK7, CK8, CK19, vimentin, CD34 | HSA, CEA, HMW-CK |
| 8 | Needle biopsy | CK19, vimentin, CEA, p53 | CD31, CD34 |
| 9 | Needle biopsy | CK19, vimentin, CEA | CK7, Desmin, HSA, SMA, c-kit, S-100 |
| 10 | Needle biopsy | CK, CK19, vimentin, CEA | HSA, CD31 |
| 11 | Needle biopsy | CK7, CK19, vimentin, MUC1 | HSA, CD10 |
In this study, all s-CCC patients presented with stage IV disease at the time of diagnosis. Among them, 9 (81.8%) patients died due to disease progression, 1 (9.1%) patient was lost follow-up, and another (9.1%) patient survived. The mean follow-up period of all patients was 93.4 ± 106.3 (median: 47, 14-379) d. The mean survival time of 9 patients who died, from diagnosis to death, was 67.2 ± 53.4 (median: 47, 14-148) d (Table 6).
| Case | Sex | Age | Tumor size (cm) | Number of mass | Stage (TNM) | Treatment | Outcome | F/U duration (d) |
| 1 | M | 45 | 7.5 | 7 | IVB | Chemotherapy | Died | 47 |
| 2 | M | 67 | 2.5 | 1 | IVB | Chemotherapy | Died | 148 |
| 3 | M | 55 | 6.5 | 2 | IVA | Chemotherapy | Died | 129 |
| 4 | M | 66 | 10.0 | 1 | IVB | Supportive | Died | 20 |
| 5 | M | 56 | 8.0 | 1 | IVB | Chemotherapy | Died | 72 |
| 6 | F | 66 | 7.5 | 1 | IVB | Chemotherapy | Died | 125 |
| 7 | M | 68 | 6.0 | 1 | IVB | Supportive | Died | 19 |
| 8 | F | 55 | 8.5 | 3 | IVA | Chemotherapy | Died | 31 |
| 9 | M | 49 | 9.5 | 3 | IVA | Chemotherapy | F/U loss | 43 |
| 10 | M | 65 | 9.5 | 15 | IVA | Supportive | Died | 14 |
| 11 | M | 61 | 5.0 | 1 | IVB | viscum album | Survived | 379 |
None of the s-CCC patients could undergo surgical treatment. Seven (63.6%) of them underwent chemotherapy, all of whom were not treated with anticancer drugs for more than 4 cycles due to rapid disease progression, deterioration of systemic condition, or presence of adverse events. Among the 7 patients with chemotherapy, 1 patient was lost follow-up and the other 6 patients died within 6 months (mean survival time: 92 ± 48.5 d, range: 31-148 d). Three (27.3%) of 11 s-CCC patients who underwent conservative treatment died within 3 wk (mean survival time: 17 ± 3.2 d, 14-20 d) (Table 6). The survival of patients who received chemotherapy was significantly longer than that of patients who only received conservative treatment (P = 0.013). In addition, 1 patient (case 11) with a 5-cm sized single s-CCC with LN and diaphragm metastasis refused chemotherapy and was treated with ABNOBA VISCUM M® (viscum album) via subcutaneous injection as the second option. Despite the advanced stage, the treatment was well tolerated by the patient, and slow progression was observed. He went to regular follow-up for 379 d.
Compared with 216 patients with CCC, abdominal pain (P = 0.011), fever (P = 0.022), past history of LC (P = 0.022), advanced TNM stage (P = 0.018) were more common in patients with s-CCC. The incidence of chronic viral hepatitis, larger mass size, LN metastasis, and distant metastasis tended to be more common in s-CCC patients, although there was no statistical significance. Increased serum CEA (P = 0.029) and CA19-9 (P = 0.098) levels were more common in patients with CCC than in those with s-CCC (Table 2). The initial radiologic impressions were variable, however, there was no patient diagnosed with s-CCC by imaging studies (Table 4).
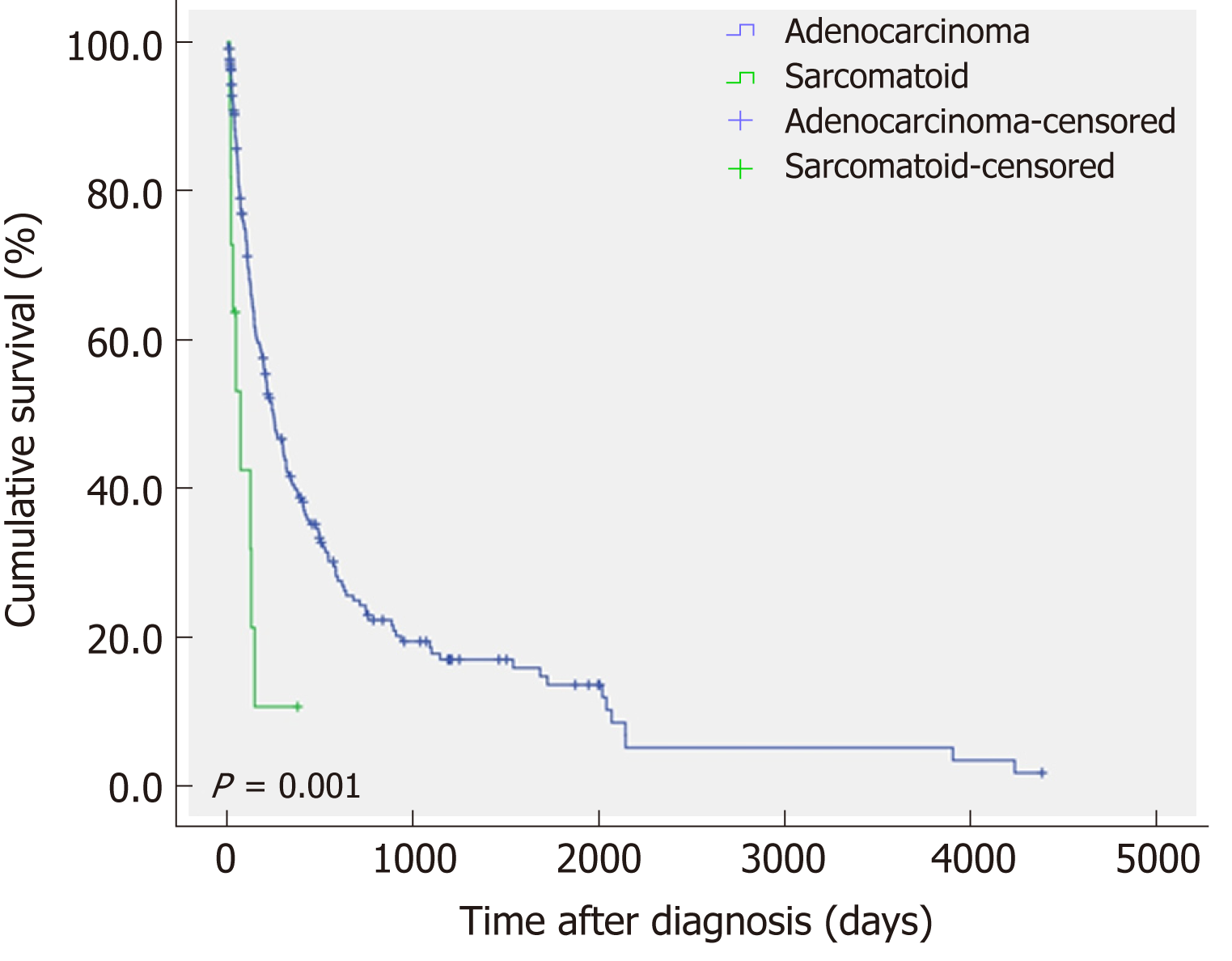
Thirty-three (15.3%) of the patients with CCC underwent surgical treatment, whereas all of the patients with s-CCC could not undergo surgery and received chemotherapy or conservative treatment. The mean survival time of patients with s-CCC was significantly shorter than that of patients with CCC (P = 0.001) (Figure 3).
Sarcomatoid tumor of the epithelial origin is commonly found in the bladder or lungs, whereas it is rarely observed in the liver or biliary tract and is referred to as “carcinosarcoma”, “sarcomatoid carcinoma”, “carcinoma with sarcomatoid change”, “pseudosarcoma”, “malignant mixed tumor” and “spindle cell carcinoma”[3,7,8]. HCC with sarcomatoid feature accounts for 1.8% of surgically resected HCC and 3.9%-9.4% of autopsy cases. According to some previous reports, it is known to occur due to a secondary change in tumor cells after previous treatment[4,8]. By contrast, s-CCC accounts for 4.5% of bile duct cancer, and has no definite relationship with previous anticancer therapy. Rather, sarcomatoid change is believed due to the natural progression of bile duct cancer[4,9,10].
The pathogenesis of sarcomatoid changes in cancers including cholangiocarcinoma, has not been elucidated yet, and the following possibilities have been suggested: First, sarcomatoid trans-differentiation or de-differentiation of primary carcinoma cells of epithelial origin, which is also explained as epithelial-mesenchymal transition (EMT) or metaplastic transformation. The EMT is an important mechanism in sarcomatoid changes, and this is a biologic process in which epithelial phenotypes are converted into mesenchymal phenotypes by biochemical changes, including enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, and increased production of extracellular matrix components[11]; Second, biphasic differentiation from pluripotent stem cells to carcinoma or sarcoma in various directions, which is divided into a combination and a collision depending on the type of each tumor growth; Finally, a sarcomatoid re-differentiation of cancer cells with multi-potent differentiation potency derived from carcinoma cells[6]. In this study, underlying CHB, CHC, and LC more common in patient with s-CCC, suggesting that these underlying diseases could have been the causative factors for de-differentiation or biphasic differentiation. This hypothesis about the mechanism of the sarcomatoid change is also an evidence supporting the poor prognosis of s-CCC.
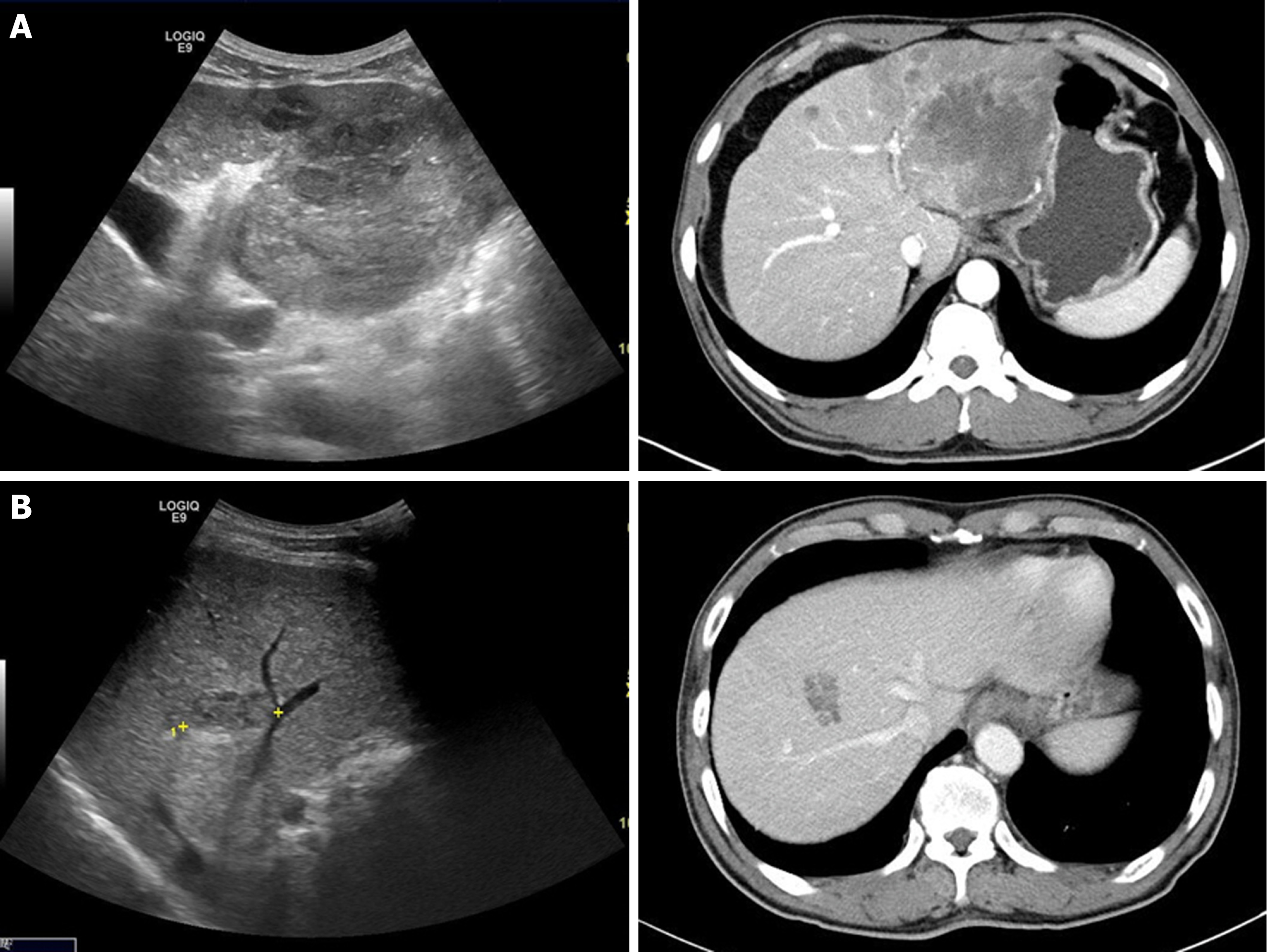
Sarcomatoid combined hepatocellular-cholangiocarcinoma is primary hepatic tumor in which hepatocellular carcinomas and cholangiocarcinomas coexist within the same hepatic tumor components showing distinct tumor pathologic features, and is observed in less than 1% of all patients with liver malignancies[12-14]. In this study, 2 (0.9%) patients presented with mixed histopathologic findings similar to sarcomatoid combined hepatocellular-cholangiocarcinoma (cases 1 and 2). These 2 patients had LC due to CHB and CHC, respectively, and they presented with increased serum AFP levels. At time of diagnoses of these 2 patients, it was difficult to distinguish HCC from IHCC, and the imaging diagnoses were even HCC (Figure 4). These 2 cases can be inferred due to the “sarcomatoid regeneration” of multipotent cancer cells derived from carcinoma cells, which is one of the hypotheses for the mechanism of sarcomatoid cholangiocarcinoma.
s-CCC could be asymptomatic, as is the case with other hepatic tumors, and it may be accompanied by non-specific symptoms or signs such as abdominal pain, nausea, fatigue, fever, and weight loss, of which abdominal pain and fever are more specific. In this study, 10 (90.9%) patients had abdominal pain and 4 (36.4%) patients had fever, which is similar to those reported in the previous articles[3,15]. It can be thought that these clinical features are more common in s-CCC patients because of the mass effect or tumor necrosis caused by relatively large mass size or rapid progression.
According to previously reported cases about s-CCC, serologic tumor markers, such as AFP, CEA, and CA19-9 levels, were negative or low[3,8,11,16-22]. Watanabe et al[23] investigated the clinical characteristics of operated sarcomatous intrahepatic cholangiocarcinoma and ordinary intrahepatic cholangiocarcinoma, and there was no significant difference in serum CEA and CA19-9 levels between the two groups. By comparison, in this study, CA19-9 level showed an increase in only 45.5% of s-CCC patients and CEA level increased more frequently in CCC patients with statistical significance. AFP levels were with normal range in most cases of s-CCC. Therefore, tumor markers were not helpful in the diagnosis of s-CCC. Additionally, as previously mentioned, two patients with elevated AFP levels were all suspected to be sarcomatoid combined hepatocellular-cholangiocarcinoma via histopathological examination.
A low echogenic liver mass on US, which shows hypo-attenuation and peripheral region enhancement after contrast injection on CT scan, is a commonly known imaging characteristic of s-CCC. In the present study, the hepatic masses showed various echoic, but mainly hypoechoic features on US. CT scan showed heterogeneous, lobulated hypodense hepatic masses with irregular rim or peripheral enhancement. These are similar to those reported in previous studies[3,24-26]. The imaging findings of 11 patients tended to show relatively aggressive features, which included large mass size (mean: 7.3 ± 2.2 cm, median: 7.5 cm), frequent LN and distant metastases, and extensive metastatic lymphadenopathy accompanied by invasion of portal vein or hepatic parenchyma (n = 2). In addition, portal vein thrombosis (n = 1), multiple intrahepatic metastasis (n = 2), and seeding metastasis (n = 1) were aslo observed. However, these findings are also observed in other mass-forming IHCC. Distal intrahepatic ductal dilatation (n = 5), capsular retraction (n = 8), satellite nodule (n = 3), peripheral enhancement (n = 10), and gradual centripetal enhancement on dynamic enhancement study are also well-known imaging findings of common mass-forming IHCC. Thus, even though the imaging findings of s-CCC show relatively aggressive features, these are not distinguishing from those of other hepatic tumors, especially IHCC. Indeed, none of the 11 patients were not clearly diagnosed with s-CCC during the first imaging study. Moreover, there were cases of underlying LC (n = 5), mimicking HCC (n = 4), and requirement for differentiation from lymphoma (n = 1), or hepatic abscess (n = 1) (Figure 1). In previous study, there was no meaningful difference in tumor size, number of tumors, and LN metastasis between sarcomatous intrahepatic cholangiocarcinoma and ordinary intrahepatic cholangiocarcinoma[23]. Therefore, it is nearly impossible to identify s-CCC only via imaging studies.
Biopsy is an indispensable test for the final diagnosis. In this study, polymorphic and polygonal cells exhibiting spindle features were commonly observed. In immunohistochemistry, all patients were positive for vimentin, and 10 (90.9%) patients were positive for CK19, and 9 patients who underwent HSA tested negative, which was considerably helpful for the final diagnosis of s-CCC. These results were similar to those of previous studies[9,27,28].
Although no definitive treatment for s-CCC is available, surgical resection is generally recommended first, and in patients undergoing surgical resection, the survival rate was significantly higher than that of patients who have not undergone surgery[3,23,29].
Adjuvant chemotherapy along with the combination of gemcitabine and cisplatin has been proposed as a treatment to improve survival[10,30]. Nevertheless, the prognosis of s-CCC is extremely poor compared with that of CCC, and there is no established treatment that can significantly prolong survival after surgery. This is due to the fact that LN or distant metastasis is common at time of diagnosis, and the rate of tumor growth is relatively fast. The tumor is also frequently accompanied by tumor thrombi, and the recurrence rate after treatment is high[3,6,10,30,31]. In addition, several studies have shown that survival prolongation was obtained with iniparib, cisplatin, ifosfamide, dacarbazine, doxorubicin, cyclophosphamide, taxol, thalidomide and paclitaxel treatment as postoperative adjuvant chemotherapy for carcinosarcoma in obstetrics and gynecology patients[2,32-34], however, there are also some reports on unsuccessful outcomes[35,36]. Among the 11 s-CCC patients of this study, no one could undergo surgical treatment and only seven patients underwent chemotherapy. In the remaining 3 patients with conservative treatment, shortened survival time may be due to the lack of the chemotherapy effect, but it is also thought to be due to the higher incidence of deteriorated general condition, old age, or poor performance status, which made the patients die earlier, regardless of cancer progression.
ABNOBA VISCUM M® (viscum album), also called mistletoe, is an extract of a plant that is hemiparasitic to various host plants. This is an immunomodulator and somewhat different from the cytotoxic drugs, target agents, or immune checkpoint inhibitors, and it is mainly, along with chemotherapy or radiation therapy, used to increase the therapeutic effect, decrease side effects, and enhance immunity. In some papers, various positive results regarding the use of viscum album as a palliative therapy in patients with terminal cancer who do not respond to conventional chemotherapy or for those with lung cancer or accompanying malignant pleural effusion and hematologic diseases[37-39]. However, there has been no report about the therapeutic effect of this drug alone for IHCC including s-CCC. Although the patient (case 11) of this study is an unusual case, whether the reason for the good prognosis in this patient is due to the effect of the viscum album or the slow progression of the tumor still remains unclear.
This study has some limitations. First, because only 11 patients with s-CCC were analyzed, some aspects of the comparison between s-CCC and CCC were not statistically significant. Second, a relatively large number of follow-up loss was observed; about 12% of the patients with CCC lost follow-up. This is believed that this study was conducted in a single center, and a large number of patients did not continue with aggressive treatment and wanted conservative treatment only, or to go to another hospital.
In conclusion, intrahepatic s-CCC of the liver is an extremely rare disease, and it is frequently characterized by abdominal pain, fever, and commonly diagnosed at an advanced stage. Its prognosis is extremely poor due to the rapid progression of the disease. Early diagnosis and appropriate treatment are important. Because clinical, serologic, and imaging findings are not helpful in distinguishing s-CCC from CCC, HCC, or other hepatic masses, biopsy should be performed to accurately diagnose and predict prognosis. Although there has been no fully established treatment thus far, surgical resection is usually prioritized, and in some cases, the survival rate can be improved through adjuvant chemotherapy. Further prospective, multicenter studies about the characteristic features, complementary treatment effect of various therapeutic options, and the effect of surgery on the survival rate must be conducted based on the pathogenesis of this disease.
Intrahepatic sarcomatoid chonalgiocarcinoma (s-CCC) is extremely rare, and its pathophysiology is not well known. Because of the poor prognosis, early diagnosis and aggressive treatment is important.
Clinical, serologic, and imaging findings are known to be not helpful to distinguish s-CCC from intrahepatic bile duct adenocarcinoma (CCC) or other hepatic tumors. There is no established treatment option to prolong survival, except surgery.
This study aimed to analyze the distinct characteristics of s-CCC patients for early diagnosis and appropriate treatment.
This retrospective study was conducted in a single center of South Korea for assessment of 11 patients with s-CCC diagnosed for 17 years. We analyzed the clinical, serologic, imaging, and histopathologic features of s-CCC patients and compared with those of CCC patients.
The patients with s-CCC tended to present abdominal pain or fever as the chief complaint and have past history of liver cirrhosis (LC) or chronic viral hepatitis more frequently, compared with the patients with CCC. In addition, s-CCC showed relatively aggressive features on imaging studies. However, no clear distinction in other clinical and serologic, or radiologic examination results between s-CCC and CCC patients. Only a histopathologic examination with immunohistochemical staining was helpful and essential for an accurate differential diagnosis of s-CCC. The clinical course of s-CCC was relatively aggressive, and patients had poor prognoses. Surgery is generally recommended first, however in this study, we could not obtain meaningful results of the surgical treatment or chemotherapy for s-CCC.
s-CCC is extremely rare disease which presents aggressive clinical course and poor prognosis. Clinical, serologic, and imaging studies are not helpful in diagnosis of s-CCC. In patients with s-CCC, early diagnosis through biopsy and aggressive treatment, including surgical resection, are important.
Although s-CCC has a poor prognosis, its pathogenesis and the effects of non-surgical treatment are not well established. In addition, there is no definite strategy for differential diagnosis except a histopathological examination. A large-scale, prospective, and multicenter study involving larger number of patients should be conducted in the future.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bramhall S, Karamouzis MV, Ramia JM, Xiao X S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
| 1. | Lu J, Zhang J, Xiong XZ, Li FY, Ye H, Cheng Y, Zhou RX, Lin YX, Cheng NS. Primary hepatic sarcomatoid carcinoma: clinical features and prognosis of 28 resected cases. J Cancer Res Clin Oncol. 2014;140:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Okabayashi T, Shima Y, Iwata J, Iiyama T, Sumiyoshi T, Kozuki A, Tokumaru T, Hata Y, Noda Y, Morita M. Surgical outcomes for 131 cases of carcinosarcoma of the hepatobiliary tract. J Gastroenterol. 2014;49:982-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Kaibori M, Kawaguchi Y, Yokoigawa N, Yanagida H, Takai S, Kwon AH, Uemura Y, Kamiyama Y. Intrahepatic sarcomatoid cholangiocarcinoma. J Gastroenterol. 2003;38:1097-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Chin S, Kim Z. Sarcomatoid combined hepatocellular-cholangiocarcinoma: a case report and review of literature. Int J Clin Exp Pathol. 2014;7:8290-8294. [PubMed] |
| 5. | Aishima S, Kuroda Y, Asayama Y, Taguchi K, Nishihara Y, Taketomi A, Tsuneyoshi M. Prognostic impact of cholangiocellular and sarcomatous components in combined hepatocellular and cholangiocarcinoma. Hum Pathol. 2006;37:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Lee DH, Han KH, Ahn SY, Kim SS, Shin HS, Bang KB, Choi JH, Kim SB, Lee WA, Song IH. Sarcomatoid intrahepatic cholangiocarcinoma: a rare case of primary liver cancer. J Liver Cancer. 2016;16:139-144. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Wang QB, Cui BK, Weng JM, Wu QL, Qiu JL, Lin XJ. Clinicopathological characteristics and outcome of primary sarcomatoid carcinoma and carcinosarcoma of the liver. J Gastrointest Surg. 2012;16:1715-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Jeong BJ, Hyun DH, Lee KW, Ryu ST, Lee JW, Lee JI, Jeong S, Lee DH, Kim PS, Kim HG, Kim YS, Kim JM. [A case of sarcomatoid combined hepatocellular-cholangiocarcinoma]. Korean J Gastroenterol. 2004;43:56-60. [PubMed] |
| 9. | Nakajima T, Tajima Y, Sugano I, Nagao K, Kondo Y, Wada K. Intrahepatic cholangiocarcinoma with sarcomatous change. Clinicopathologic and immunohistochemical evaluation of seven cases. Cancer. 1993;72:1872-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Malhotra S, Wood J, Mansy T, Singh R, Zaitoun A, Madhusudan S. Intrahepatic sarcomatoid cholangiocarcinoma. J Oncol. 2010;2010:701476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Kim HM, Kim H, Park YN. Sarcomatoid cholangiocarcinoma with osteoclast-like giant cells associated with hepatolithiasis: A case report. Clin Mol Hepatol. 2015;21:309-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Murata M, Miyoshi Y, Iwao K, Wada H, Shibata K, Tateishi H, Shimano T, Ohasawa M, Imai Y, Nishikawa M, Kobayashi T, Nakamura Y. Combined hepatocellular/cholangiocellular carcinoma with sarcomatoid features: genetic analysis for histogenesis. Hepatol Res. 2001;21:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 14. | Papotti M, Sambataro D, Marchesa P, Negro F. A combined hepatocellular/cholangiocellular carcinoma with sarcomatoid features. Liver. 1997;17:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Eriguchi N, Aoyagi S, Hara M, Okuda K, Fukuda S, Tamae T, Kanazawa N. Malignant sarcomatoid tumor of the liver: report of a case. Surg Today. 2001;31:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Jung GO, Park DE, Youn GJ. Huge subcapsular hematoma caused by intrahepatic sarcomatoid cholangiocarcinoma. Korean J Hepatobiliary Pancreat Surg. 2012;16:70-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Kim KS, Park JH, Bae BJ, Sohn KR, Shin DG. Sarcomatoid cholangiocarcinoma of the liver; a case study. Korean J Hepatobiliary Pancreat Surg. 2004;8:54-59. |
| 18. | Hong KS, Kim IK, Lee JK, Kim JW, Kim H, Hwang JH. A case of intrahepatic sarcomatoid cholangiocarcinoma with huge right ventricular tumor thrombus. Korean J Med. 2008;75:569-573. |
| 19. | Kim WS, Kim TH, Hwang JJ, Kim HJ, Jung WT, Lee OJ. A case of intrahepatic sarcomatoid cholangiocarcinoma with rhabdoid transformation. Korean J Med. 2011;80:453-457. |
| 20. | Lim JH, Kim JW, Heo SH, Jeong YY, Kang HK. Intrahepatic sarcomatoid cholangiocarcinoma with portal vein thrombosis: a case report. J Korean Soc Radiol. 2009;60:333-337. [DOI] [Full Text] |
| 21. | Kim MJ, Koo HL, Lee SK, Ro JY, Yu E. A case of combined hepatocellular and cholangiocarcinoma with neuroendocrine differentiation and sarcomatoid transformation: a case report. Korean J Pathol. 2005;39:125-129. |
| 22. | Lim BJ, Kim KS, Lim JS, Kim MJ, Park C, Park YN. Rhabdoid cholangiocarcinoma: a variant of cholangiocarcinoma with aggressive behavior. Yonsei Med J. 2004;45:543-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Watanabe G, Uchinami H, Yoshioka M, Nanjo H, Yamamoto Y. Prognosis analysis of sarcomatous intrahepatic cholangiocarcinoma from a review of the literature. Int J Clin Oncol. 2014;19:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Matsuo S, Shinozaki T, Yamaguchi S, Takami Y, Obata S, Tsuda N, Kanematsu T. Intrahepatic cholangiocarcinoma with extensive sarcomatous change: report of a case. Surg Today. 1999;29:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Imazu H, Ochiai M, Funabiki T. Intrahepatic sarcomatous cholangiocarcinoma. J Gastroenterol. 1995;30:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Sasaki M, Nakanuma Y, Nagai Y, Nonomura A. Intrahepatic cholangiocarcinoma with sarcomatous transformation: an autopsy case. J Clin Gastroenterol. 1991;13:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Sung CO, Choi H, Lee KW, Kim SH. Sarcomatoid carcinoma represents a complete phenotype with various pathways of epithelial mesenchymal transition. J Clin Pathol. 2013;66:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Leng Q, Xiang XI, Tang Y, Yang Y, Qiu LI. Primary hepatic sarcomatoid carcinoma: A case report. Exp Ther Med. 2015;10:1145-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Sumiyoshi S, Kikuyama M, Matsubayashi Y, Kageyama F, Ide Y, Kobayashi Y, Nakamura H. Carcinosarcoma of the liver with mesenchymal differentiation. World J Gastroenterol. 2007;13:809-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Damiano R, D'Armiento M, Cantiello F, Amorosi A, Tagliaferri P, Sacco R, Venuta S. Gemcitabine and cisplatin following surgical treatment of urinary bladder carcinosarcoma. Tumori. 2004;90:458-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Tsou YK, Wu RC, Hung CF, Lee CS. Intrahepatic sarcomatoid cholangiocarcinoma: clinical analysis of seven cases during a 15-year period. Chang Gung Med J. 2008;31:599-605. [PubMed] |
| 32. | Galaal K, van der Heijden E, Godfrey K, Naik R, Kucukmetin A, Bryant A, Das N, Lopes AD. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma. Cochrane Database Syst Rev. 2013;CD006812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Shylasree TS, Bryant A, Athavale R. Chemotherapy and/or radiotherapy in combination with surgery for ovarian carcinosarcoma. Cochrane Database Syst Rev. 2013;CD006246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Einstein MH, Klobocista M, Hou JY, Lee S, Mutyala S, Mehta K, Reimers LL, Kuo DY, Huang GS, Goldberg GL. Phase II trial of adjuvant pelvic radiation "sandwiched" between ifosfamide or ifosfamide plus cisplatin in women with uterine carcinosarcoma. Gynecol Oncol. 2012;124:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Aghajanian C, Sill MW, Secord AA, Powell MA, Steinhoff M. Iniparib plus paclitaxel and carboplatin as initial treatment of advanced or recurrent uterine carcinosarcoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2012;126:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | McMeekin DS, Sill MW, Darcy KM, Abulafia O, Hanjani P, Pearl ML, Rubin SC, Rose PG, Small L, Benbrook DM. A phase II trial of thalidomide in patients with refractory uterine carcinosarcoma and correlation with biomarkers of angiogenesis: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;127:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Thronicke A, Oei SL, Merkle A, Matthes H, Schad F. Clinical Safety of Combined Targeted and Viscum album L. Therapy in Oncological Patients. Medicines (Basel). 2018;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Schad F, Thronicke A, Steele ML, Merkle A, Matthes B, Grah C, Matthes H. Overall survival of stage IV non-small cell lung cancer patients treated with Viscum album L. in addition to chemotherapy, a real-world observational multicenter analysis. PLoS One. 2018;13:e0203058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Thronicke A, Steele ML, Grah C, Matthes B, Schad F. Clinical safety of combined therapy of immune checkpoint inhibitors and Viscum album L. therapy in patients with advanced or metastatic cancer. BMC Complement Altern Med. 2017;17:534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |