Published online Feb 7, 2019. doi: 10.3748/wjg.v25.i5.567
Peer-review started: September 10, 2018
First decision: October 11, 2018
Revised: December 2, 2018
Accepted: December 6, 2018
Article in press: December 6, 2018
Published online: February 7, 2019
Processing time: 143 Days and 20.9 Hours
To investigate the effect of adipose-derived mesenchymal stem cells (ADMSCs) and their conditioned media (CM) on hepatocellular carcinoma (HCC) cell tumorigenesis.
The proliferation rate of HepG2 and PLC-PRF-5 HCC cancer cells was measured using the trypan blue exclusion method and confirmed using the cell-counting kit 8 (commonly known as CCK-8) assay. Apoptosis was detected by flow cytometry using annexin V-FITC. Protein and mRNA expression was quantified by ELISA and real time PCR, respectively. Migration and invasion rates were performed by Transwell migration and invasion assays. Wound healing was examined to confirm the data obtained from the migration assays.
Our data demonstrated that when co-culturing HCC cell lines with ADMSCs or treating them with ADMSC CM, the HCC cell proliferation rate was significantly inhibited and the apoptosis rate increased. The decreased proliferation rate was accompanied by an upregulation of P53 and Retinoblastoma mRNA and a downregulation of c-Myc and hTERT mRNA levels. More notably, ADMSCs and their CM suppressed the expression of the two important markers of HCC carcinogenicity, alpha-fetoprotein and Des-gamma-carboxyprothrombin. In addition, the migration and invasion levels of HepG2 and PLC-PRF-5 cells significantly decreased, potentially through increased expression of the tissue inhibitor metalloproteinases TIMP-1, TIMP-2 and TIMP-3.
These findings shed new light on a protective and therapeutic role for ADMSCs and their CM in controlling HCC invasiveness and carcinogenesis.
Core tip: In this study, we report the in vitro effect of adipose derived mesenchymal stem cells (ADMSCs) on HepG2 and PLC-PRF-5 liver cell lines. It is the first study to demonstrate that ADMSCs and their respective conditioned media inhibited the expression of hepatocellular carcinoma markers alpha-fetoprotein and Des-gamma-carboxy-prothrombin and decreased cancer cell invasiveness by increasing the mRNA expression of tissue inhibitor metalloproteinases TIMP-1, TIMP-2 and TIMP-3. In addition, ADMSCs significantly reduced the proliferation rate, the invasiveness and the migration of the cancer cells while inducing their apoptosis.
- Citation: Serhal R, Saliba N, Hilal G, Moussa M, Hassan GS, El Atat O, Alaaeddine N. Effect of adipose-derived mesenchymal stem cells on hepatocellular carcinoma: In vitro inhibition of carcinogenesis. World J Gastroenterol 2019; 25(5): 567-583
- URL: https://www.wjgnet.com/1007-9327/full/v25/i5/567.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i5.567
Hepatocellular carcinoma (HCC) is the most common primary hepatic cancer that accounts for approximately 70%-80% of all primary liver cancers[1]. It is now considered the second cause of cancer related mortality worldwide[2]. HCC development results from an imbalance between excessive cell growth and apoptosis, which is mainly regulated by P53, a tumor suppressor gene. Alterations in the expression or activation of P53 have been extensively reported in HCC and are related to hepatocarcinogenesis[3,4].
Early detection of HCC is crucial but difficult due to the presence of inflammation and liver damage. Several markers, such as Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein (AFP) (AFP-L3), Des-gamma-carboxy-prothrombin (DCP), Dickkopf-1, Midkine and microRNA, have been suggested as biochemical indicators in the diagnosis of different phases of primary liver cancer[5]. However, AFP is used for monitoring liver cancer recurrence after treatment[6]. Late stages of HCC, more specifically HCC metastasis, is associated with upregulation of matrix metalloproteinases (MMPs)[7,8], as these proteins are implicated in matrix degradation that allows for malignant growth and cancer cell invasion.
HCC treatment entails liver transplantation and/or other palliative modalities such as liver resection, local ablation, transarterial chemoembolization, and systemic cytotoxic chemotherapy. These treatments are limited by their toxicity towards normal tissues, by multifocal development and tumor[9]. Hence, the development of new targeted therapies is necessary to prevent HCC in cirrhotic liver or to restrain metastasis and abolish cancer invasiveness.
Recent accomplishments in stem cell (SC) research provide a new prospective in cell-based therapy and tissue regeneration. Indeed, the interaction between mesenchymal SCs (MSCs) and cancer has been extensively studied. MSCs are adult, multipotent, non-hematopoietic cells that have auto-renewing capacity and a multilineage potential. MSCs can be isolated from different sources such as bone marrow[10], umbilical cord[11], peripheral blood[12], placenta[13], and adipose tissue[14]. Adipose tissue remains the most abundant source. SCs are called intrinsic drug stores, not only because of their differentiation capacity but because of their paracrine and trophic effects. Indeed, the exact role(s) that MSCs play in tumor modulation remains controversial. It has been reported that MSCs promote cancer via immune suppression[15,16], the promotion of vasculature or angiogenesis[16,17], the stimulation of epithelial-mesenchymal transition[18], and their contribution to the tumor microenvironment[19,20]. The use of bone marrow-derived MSCs in a model of Kaposi sarcoma has been shown to exert anti-tumorigenic and pro-apoptotic effects via the suppression of Akt activity upon direct cell-cell contact[21]. In addition, it has been demonstrated that co-culturing of glioma cancer cells with cord blood MSCs induced cancer cell apoptosis[22]. Emerging evidence has established that MSCs may serve as vehicles to deliver therapeutic agents, such as cytokines, apoptosis inducers and prodrugs, and that they can be genetically engineered to produce antitumor molecules such as interferon β (INF β) and tumor necrosis factor-related apoptosis inducing ligand (TRAIL)[23]. However, the antitumor properties of MSCs and their secretions are not yet clear. The role of MSCs on HCC remains controversial, and few reports have studied the effects of adipose-derived MSCs (ADMSCs) on HCC.
The present work aims to investigate the effect of human ADMSCs and their conditioned medium on HCC cell line carcinogenesis through the modulation of proliferation, apoptosis, tumor marker expression, migration and invasion.
The human HCC cell lines (HepG2/C3A/HB-8065, PLC-PRF-5/CRL-8024) were purchased from American Type Culture Collection (ATCC, Manassas, VA, United States) in 2015. All cells were cultured in cancer cell media as suggested by ATCC at 37 ˚C in low glucose DMEM media (1 g/L glucose) (Sigma Aldrich, Steinheim, Germany) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (PS) (Sigma Aldrich) in a humidified atmosphere containing 5% CO2 at passages 1 to 5.
Adipose tissues were obtained from healthy donors undergoing an elective liposuction procedure from abdominal, hip or thigh regions after consent in the Department of Plastic Surgery, Hotel Dieu De France Hospital, Beirut, Lebanon. Briefly, lipoaspirates were washed extensively with a saline solution then digested with type I collagenase solution (Sigma Aldrich) for 1-2 h at 37 ˚C. After centrifugation, the cell pellet was washed and filtered to remove debris. The synovial vascular fraction was plated into tissue culture flasks in DMEM nutrient mix F12 (Sigma Aldrich) containing 10% FBS (Sigma Aldrich) and 1% PS-amphotericin then incubated at 37 ˚C and 5% CO2. After 48 h, non-adherent cells were removed and fresh DMEM was added and replaced every 2-3 d. The isolated ADMSCs at passage 1 were used for subsequent experiments.
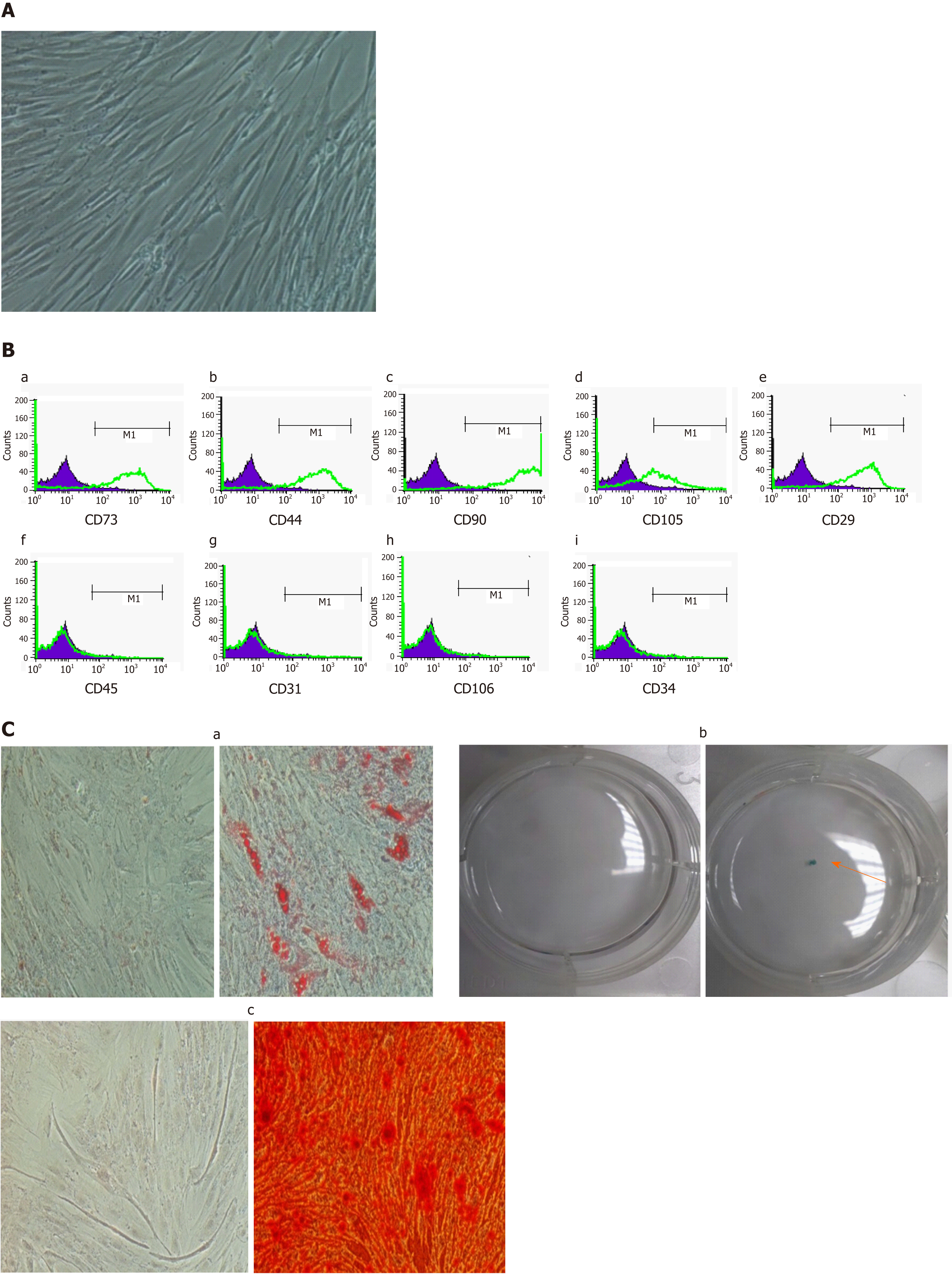
The morphology of ADMSCs and HCC cell lines before and after coculture was observed under an inverted microscope. Photos were taken at a magnification of 100 ×.
Morphology and immunophenotyping: ADMSCs at passage 1 had a fibroblast-like morphology and were characterized by immunophenotyping using flow cytometry analysis. The following PE-conjugated mAbs were used: anti CD73, anti CD29, anti CD44, anti CD45, anti CD31, anti CD106, anti CD34, anti CD90, and anti CD105 (BD Biosciences, San Jose, CA, United States). Appropriate isotype controls were used at the same concentrations to determine non- specific staining.
Multilineage differentiation of ADMSCs: ADMSCs showed a differentiation capacity to become adipocytes, osteocytes, and chondrocytes. Adipogenic differentiation, osteogenic differentiation and chondrogenic differentiation was performed as previously described[24].
To determine the effect of ADMSCs on HCC cell lines, the HepG2 and PLC-PRF-5 were cultured directly in six-well plates with ADMSCs and indirectly in an inverted Transwell cell culture insert for six-well plates (1-μm pore poly (ethylene terephthalate) (Corning, Corning, NY, United States) at 1:1 and 2:1. In the case of indirect coculture, ADMSCs were seeded in the apical compartment, and the cancer cells were seeded in the basal compartment. In the direct co-culture, the number of cells seeded are mentioned in each specific experiment. In all experiments, cells were grown in DMEM F12 supplemented with 10% FBS in a humidified atmosphere at 37 ˚C and 5% CO2. After 48 h, the media were removed and replaced with fresh DMEM F12. Finally, the supernatant was collected after 48 h and stored at -80 ˚C for subsequent ELISA analysis.
ADMSCs were grown in 75 cm2 flasks (Sarstedt, Newton, NC, United States) with 10% FBS DMEM F12 (Sigma Aldrich) at 37 ˚C with serum free media. After 24 h, the conditioned media (CM) was collected, centrifuged, filtered and conserved at -80 ˚C until used.
In all experiments where CM was used, the HepG2 and PLC-PRF-5 cancer cell lines were seeded in six-well plates in CCM as described earlier [low glucose DMEM media (1g/L glucose) supplemented with 10% FBS and 1% PS]. After adherence, the HepG2 and PLC-PRF-5 cell supernatants were removed and replaced with prepared ADMSC CM at different dilutions (1:1, 1:2, 1:4, 1:5, 1:10, 1:25, 1:50, 1:100, 1:200, 1:400) for 48 h. All dilutions were significant in respect to cancer markers and morphology. After 1:25 dilution, no differences in results were observed (data not shown). Thus, in all experiments, ADMSC CM was diluted at 1:1, 1:5 or 1:25.
HCC cells were harvested and counted with the trypan blue exclusion method using a hemocytometer.
The effect of ADMSCs and ADMSC CM on HCC cell proliferation was evaluated using a cell-counting kit 8 (CCK-8) (Sigma Aldrich) according to the manufacturer recommendations. The tetrazolium salt or WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2-H-tetrazolium, monosodium salt] is cleaved into formazan by succinate-tetrazolium reductase, an enzyme that exists only in the mitochondrial respiratory chain and is active only in viable cells. The formazan production is proportional to the number of living cells in the culture. Briefly, HCC cells were directly cocultured with ADMSCs in six-well plates, indirectly in six-well plates and 24-mm Transwells (Corning) or treated with ADMSC CM for 48 h. The WST-8 was then added into the wells after removal of Transwells in case of indirect coculture. Finally, the absorbance was measured in triplicates at 450 nm.
To study the effect of ADMSCs on inducing apoptosis of HCC cells, an annexin V-FITC kit (MiltenyiBiotec, Bergisch Gladbach, Germany) was used according to the manufacturer's instructions. Briefly, HCC cells were indirectly cocultured with ADMSCs in six-well plates and 24-mm Transwells (Corning) or were treated with ADMSC CM for 48 h. Next, the Transwell was removed and 106 of freshly obtained HCC cells were washed and resuspended in binding buffer. The cells were stained with annexin V-FITC, incubated in the dark for 15 min, then binding buffer and propidium iodide solution were added. For each sample, 106 cells were analyzed by flow cytometry using a MACSQuant analyzer device. Apoptosis was analyzed using the MACSQuant software, and the percentage of apoptosis was determined and plotted.
To test the level of AFP and DCP in cell supernatants, quantitative enzyme linked immunosorbent assay kit (HUMAN, Germany and CUSABIO, Houston, TX, United States, respectively) were performed as per manual instructions. The optical density was determined using an ELISA plate reader at 450 nm.
For monolayer wound healing assay, ADMSCs (negative control), HCC cells (positive control), and HCC cells directly cocultured with ADMSCs or treated with ADMSC CM were are seeded and cultured until > 90% confluence in 10% FBS DMEM F12 in six-well plates (Corning). By scratching the cells with a 20 μL plastic pipette tip, three wounds were stimulated per well. After gently washing the wells with PBS, the cells that migrated into the wounded areas were monitored and photographed at 0 h, 6 h, 12 h, 24 h and 48 h. The distance migrated was measured using image J 1.48v software (Wayne Rasband, National Institutes of Health, United States) by comparing the images from time 0 to the last time point. The relative migration distance of cells was measured by the distance of cell migration/the distance measured at 0 h.
The HepG2/C3A and PLC/PRF/5 cell migration assay was performed using a Boyden chamber in a 24-well plate designed by Cell Biolabs Inc. (San Diego, CA, United States) according to the manufacturer's recommendations. Briefly, for each condition, 106 cells were suspended in 1 mL serum-free DMEM. Then, 3 × 106 cells were added in the upper chamber of each well. The same medium supplemented with 10% serum was added to the lower chamber of each well as a chemo-attractant solution. After 24 h, the cells that migrated to the lower chamber of the wells were stained using crystal violet cell staining solution. The stain was instantly dissolved once the kit extraction solution was added. The solution was then transferred to a 96-well microtiter plate, and the absorbance was measured at 560 nm using a plate reader.
The invasion ability of HepG2/C3A and PLC/PRF/5 cells was assayed using a Boyden chamber in a 24-well plate designed by Cell Biolabs Inc. According to the manufacturer's recommendations, all cells were incubated in serum-free DMEM overnight. For each condition, 106 cells were suspended in 1 ml serum-free DMEM. Then, 3 × 106 cells were added in the upper chamber of each well. The same medium supplemented with 10% serum was added to the lower chamber of each well as a chemo-attractant solution. After 48 h, the cells that invaded the bottom of the membrane were stained using crystal violet cell staining solution. An extraction solution was then added, and the mixture was transferred to a 96-well microtiter plate. Finally, the absorbance was measured at 560 nm using a plate reader.
Total RNA from cell cultures was extracted using QIAamp RNA extraction kit (Qiagen, Valencia, CA, United States). cDNA was generated from 500 ng of total RNA with iScript Reverse Transcription Kit (Bio-Rad Laboratories, Hercules, CA, United States). Quantification of gene expression was conducted using iQ SYBR Green Supermix (Bio-Rad Laboratories). The reverse transcription (RT) product was used to measure the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a positive housekeeping gene, AFP, TIMP-1, TIMP-2, TIMP-3, P53, RB, hTERT and c-Myc using real time PCR (qPCR) and specific primer sequences (Table 1). The reaction conditions were as follows: pre-denaturation at 95 ˚C for 3 min; 40 cycles of denaturation at 94 ˚C for 20 s, annealing and elongation at 60 ˚C for 60 s. The threshold cycle (Ct) value for triplicate reactions was averaged, and the relative genomic expression was calculated by the 2-∆∆Ct method [∆Ct= Ct (gene) - Ct (GAPDH)][25]. Melting curves were performed to ensure that only a single product was amplified.
| Primer | Sequence |
| GAPDH F | 5'- GCACCACCAACTGCTTAGCA -3' |
| GAPDH R | 5'- CTTCCACGATACCAAAGTTGTCAT -3' |
| AFP F | 5'- CAGCCACTTGTTGCCAACTC -3' |
| AFP R | 5'- GGCCAACACCAGGGTTTACT -3' |
| TIMP-1 F | 5'- GACCAAGATGTATAAAGGGTTCCAA -3' |
| TIMP-1 R | 5'- GAAGTATCCGCAGACACTCTCCAT -3' |
| TIMP-2 F | 5'- AGGCGTTTTGCAATGCAGAT -3' |
| TIMP-2 R | 5'- TCCAGAGTCCACTTCCTTCTCACT -3' |
| TIMP-3 F | 5'- CAGGACGCCTTCTGCAACTC -3' |
| TIMP-3 R | 5'- AGCTTCTTCCCCACCACCTT -3' |
| P53 F | 5'- CAAGCAATGGATGATTTGATGCT -3' |
| P53 R | 5'- TGGGTCTTCAGTGAACCATTGT -3' |
| RB F | 5'- GCAAATTGGAAAGGACATGTGA -3' |
| RB R | 5'- GAAACTTTTAGCACCAATGCAGAA -3' |
| C-Myc F | 5'- CACCACCAGCAGCGACTCT -3' |
| C-Myc R | 5'- TTCCACAGAAACAACATCGATTTC -3' |
| hTERT F | 5'- GACGTAGTCCATGTTCACAATCG -3' |
| hTERT R | 5'- CGTCCAGACTCCGCTTCATC -3' |
For immunophenotyping experiments, the values are presented as the mean ± standard error mean of the mean (SEM). For other experiments, data were expressed as mean ± standard deviation (SD). The differences between the groups were analyzed by student’s t-test using GraphPad prism online software, and P < 0.05 was considered significant.
Isolated ADMSCs at passage 1 showed a fibroblast-like morphology (Figure 1A). Flow cytometry analysis showed a high expression of the fibroblast markers CD73 (82.02% ± 4.84%), CD29 (90.28% ± 2.24%), CD44 (88.3% ± 1.78%), CD90 (93.19% ± 1.65%), CD105 (59.63% ± 8.13%), and a lack of expression of the endothelial and hematopoietic markers CD34 (2.17% ± 0.34%), CD31 (2.27% ± 0.43%), CD45 (2.18% ± 0.31%), and CD106 (2.31% ± 0.11%) (Figure 1B). Furthermore, our cells were able to differentiate into adipocytes, chondrocytes and osteocytes (Figure 1C).
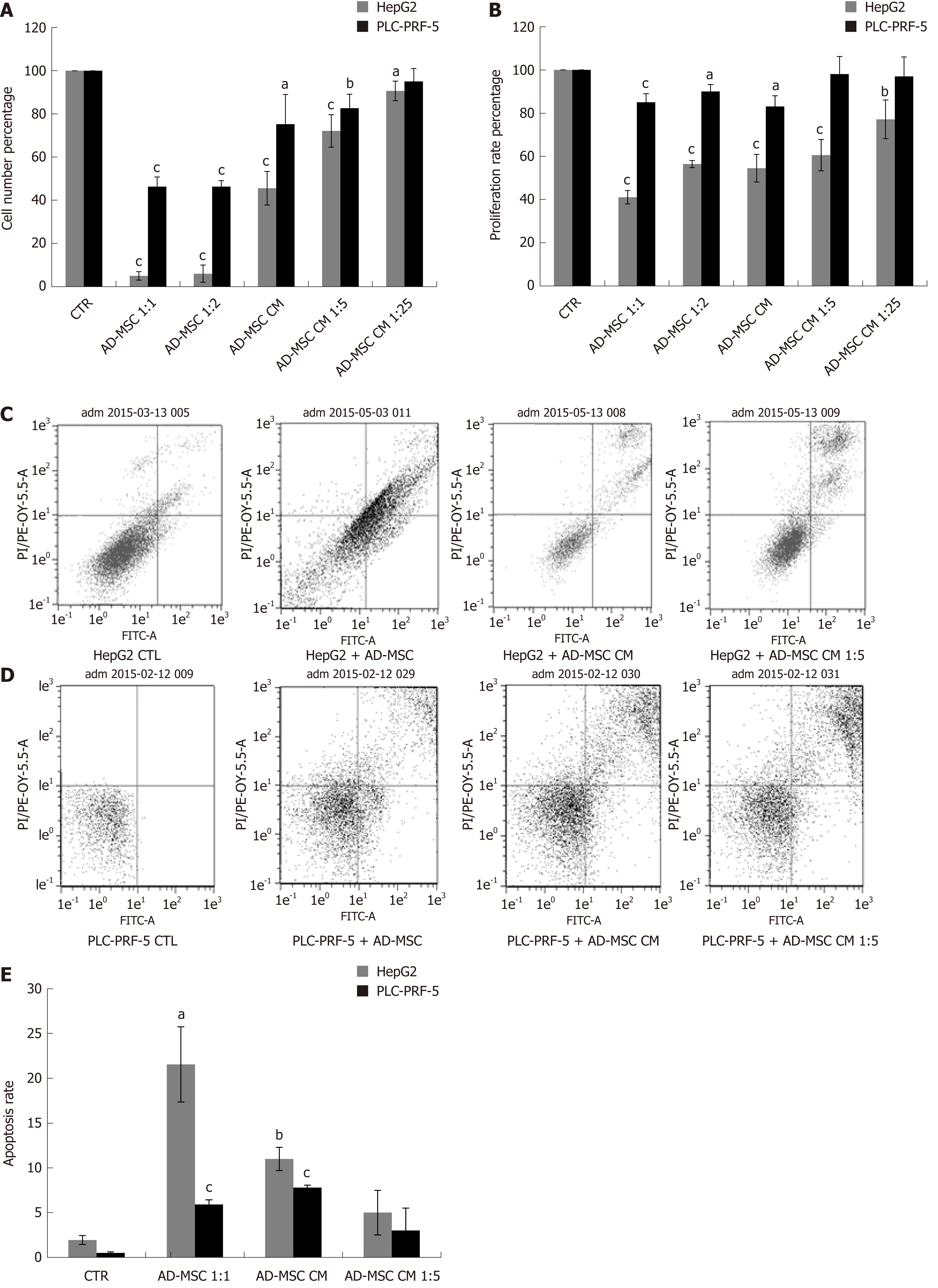
Unregulated cell proliferation is a fundamental abnormality in cancer development. Previous studies have been controversial and have demonstrated that MSCs either suppress or induce cell growth[26,27]. Here, we aimed to determine the effect of our ADMSCs and their secreted soluble factors on cancer cell proliferation and growth. Therefore, we cultured HCC cell lines under the following conditions: HepG2 and PLC-PRF-5 alone (control) or directly or indirectly with ADMSCs (at a ratio HCC: ADMSCs of 1:1 or 2:1) or treatment with different dilutions of ADMSC CM for 48 h. Morphological observation revealed a considerable inhibition in the cell numbers of the two cancer cell lines in the presence of ADMSCs, either in direct or indirect cocultures. This inhibition was less remarkable when cancer cells were treated only with ADMSC CM (Supplementary Figure 1). Using cell count assay and WST-8 proliferation tests, our results showed that ADMSCs in indirect coculture reduced the number of HCC cells (Figure 2A) and inhibited their proliferation (Figure 2B) compared to control cells (P < 0.001).
In direct coculture, we could not discriminate between the proliferation of HCC cells and ADMSCs (data not shown), knowing that the microscopic observation showed strong inhibition of cancer cell number (Supplementary Figure 1). Similarly, the ADMSC CM, undiluted or diluted 5 × or 25 ×, significantly reduced the number of HepG2 and PLC-PRF-5 cells (Figure 2A) and inhibited the proliferation of HepG2 cells (Figure 2B; P ≤ 0.001), while only the undiluted ADSMC CM was capable of reducing the proliferation of PLC-PRF-5 cells (Figure 2B; P = 0.001).
Resistance to cell death or apoptosis is a crucial process in malignant cells. It has been shown that bone marrow derived MSCs (BMSCs) induce apoptosis and cell cycle arrest in G0/G1 phase[27]. To elucidate the mechanism of growth suppression by ADMSCs, HepG2 and PLC-PRF-5 cells were cultured alone (control), indirectly co-cultured with ADMSCs or treated with ADMSC CM. Apoptosis was assessed by flow cytometry after removal of ADMSCs in the case of coculture. Our results showed that ADMSCs significantly increased the apoptotic rate of HepG2 (21.54% ± 4.1% vs control = 1.94% ± 0.3%, P < 0.05) and of PLC-PRF-5 (2.91% ± 0.2% vs control = 0.5% ± 0.1%, P < 0.001). As shown in Figure 2C-E, HepG2 and PLC-PRF-5 apoptosis was also significantly increased when treated with undiluted ADMSC CM compared to control cells (11% ± 0.5% vs control, P < 0.01 and 3.8% ± 0.15% vs control, P < 0.01, respectively).
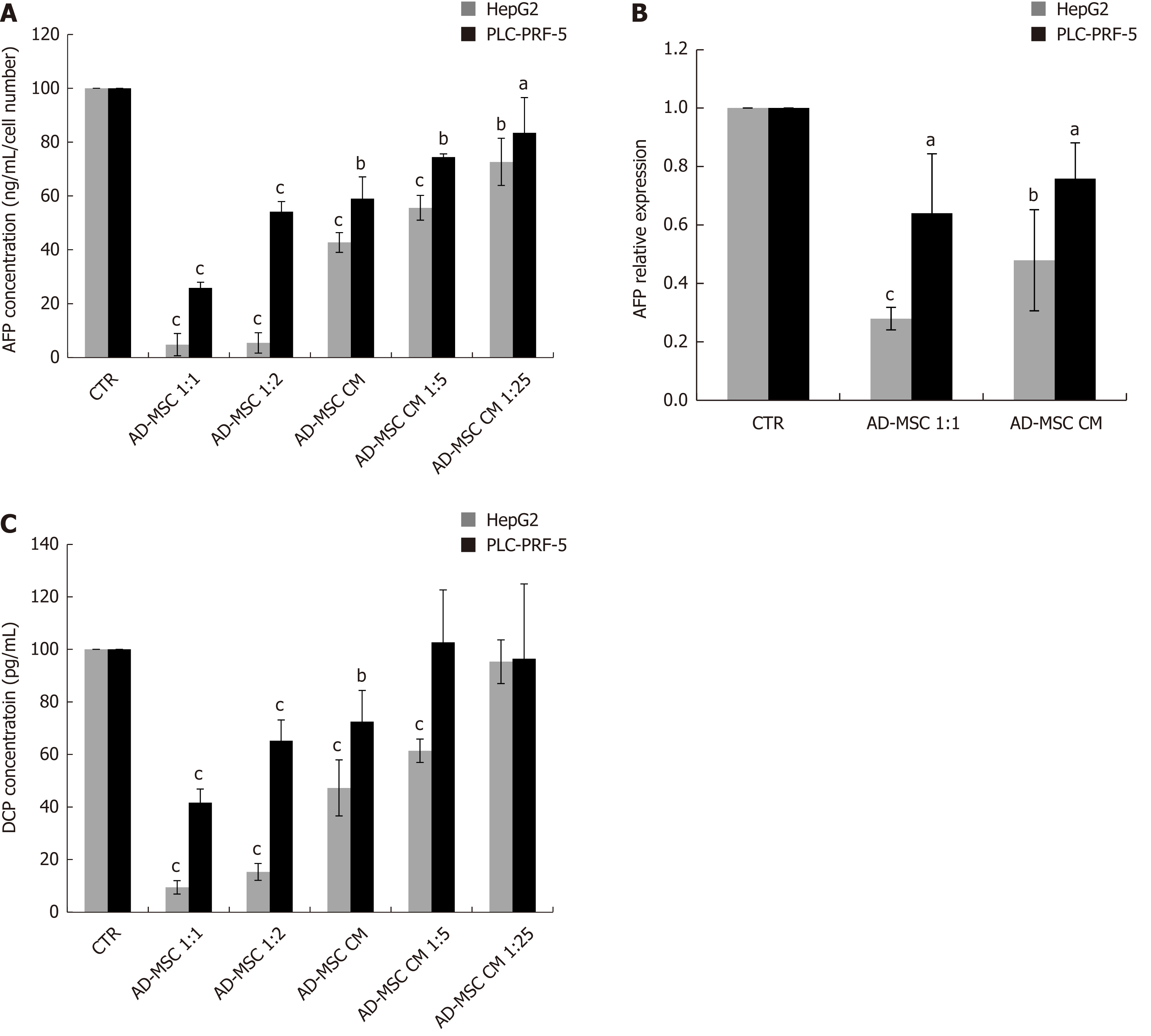
To monitor the malignant status of HCC cell lines, the levels of biochemical markers AFP and DCP were measured in the supernatant of ADMSCs (negative control), HCC cells (positive control), and HCC cells co-cultured directly with ADMSCs or treated with ADSMC CM. The ADMSCs in the negative control did not express AFP or DCP (data not shown). As illustrated in Figure 3, we found that AFP protein levels dramatically declined upon co-culturing HepG2 and PLC-PRF-5 cells with ADMSCs compared to control cells (P < 0.001). Similar results were obtained when HepG2 cells and PLC-PRF-5 cells were treated with different concentrations of ADMSC CM (P < 0.001 and P < 0.05, respectively, Figure 3A).
We next examined whether AFP was also repressed at the mRNA level. Our data show that ADMSCs and their CM significantly reduced AFP mRNA expression in HepG2 and PLC-PRF-5 cells (P ≤ 0.001 and P < 0.05 respectively, Figure 3B).
In addition to AFP, we also assessed DCP levels in HCC cells alone (control), co-cultured with ADMSCs or treated with ADMSC CM. We observed that ADMSCs significantly decreased DCP levels in HepG2 and PL-PRF-5 cells (P < 0.001). ADMSC CM, undiluted or diluted 1:5, significantly reduced DCP secretion by HepG2 cells (P < 0.001). In contrast, only the undiluted ADMSC CM significantly decreased DCP levels in PLC-PRF-5 cells (P < 0.001) (Figure 3C).
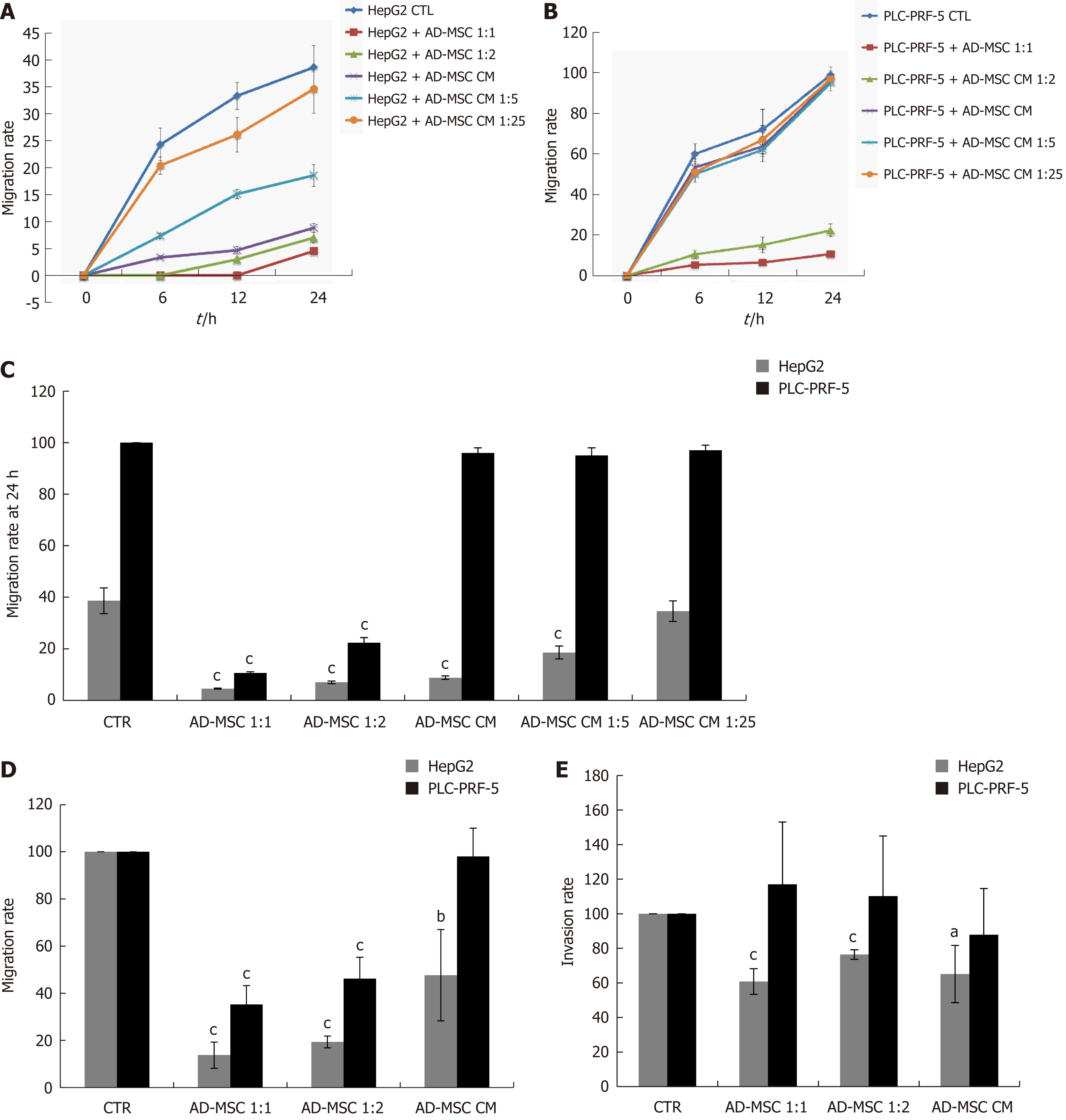
Cell migration and invasion are important processes in tumor development and metastasis. Bone marrow-derived MSCs have previously been found to promote microvascular HCC[28]. Conversely, they have also been shown to inhibit tumor invasion[26]. Thus, we tested whether ADMSCs altered HCC cell migration using the wound healing assay. The migratory rate of wounded cells was measured at different times (0 h, 6 h, 12 h, and 24 h). As shown in Figure 4A and B, the migration rate of HepG2 and PLC-PRF-5 cells was dramatically inhibited when directly co-cultured with ADMSCs for 24 h compared to when they were cultured alone (control) (P < 0.001). In addition, the ADMSC CM, undiluted or diluted 1:5, significantly reduced HepG2 cell migration rate (P < 0.001). However, the ADMSC CM had no effect on PLC-PRF-5 cell migration rates (Figure 4A-C).
The effect of ADMSCs on cell migration was confirmed using the Transwell migration technique. As shown in Figure 4D, the ADMSCs and their CM significantly decreased HepG2 cell migration rate (P < 0.01). ADMSCs also inhibited PLC-PRF-5 cell migration rate (P < 0.001, Figure 4D).
Using the Transwell invasion assay, our data show that HepG2 cell invasiveness was significantly reduced when co-cultured with ADMSCs at 1:1 and 1:2 ratios or ADMSC CM compared to control cells (P < 0.05). Conversely, ADMSCs had no significant effect on PLC-PRF-5 cell invasion (Figure 4E).
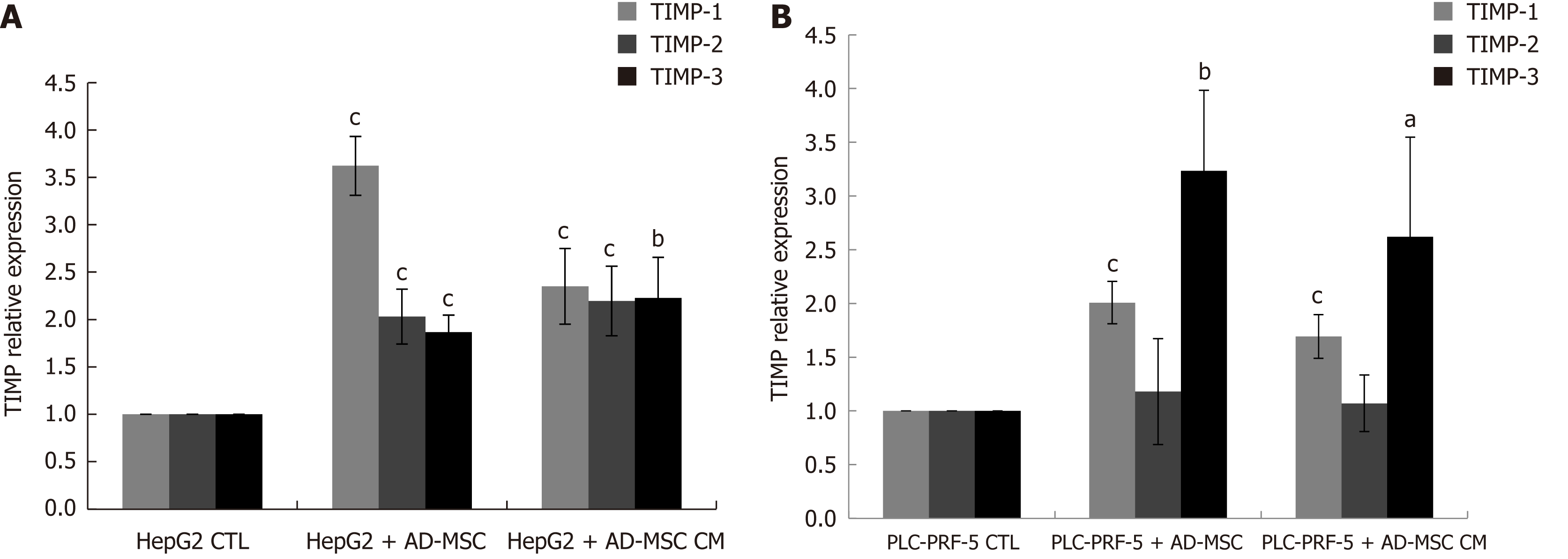
Migration and invasion are initially controlled by the dysregulated expression of MMPs and tissue inhibitor metalloproteinases (TIMPs). To examine whether TIMPs contribute to the decreased migration and invasion capacity of HepG2 and PLC-PRF-5 cells upon ADMSC or ADMSC CM coculture, we examined TIMP-1, -2 and -3 expression by real time PCR. We observed that ADMSCs and their CM significantly increased TIMP-1, -2 and -3 mRNA levels in HepG2 cells (Figure 5A), but only TIMP-1 and -3 in PLC-PRF-5 cells (P < 0.05, Figure 5).
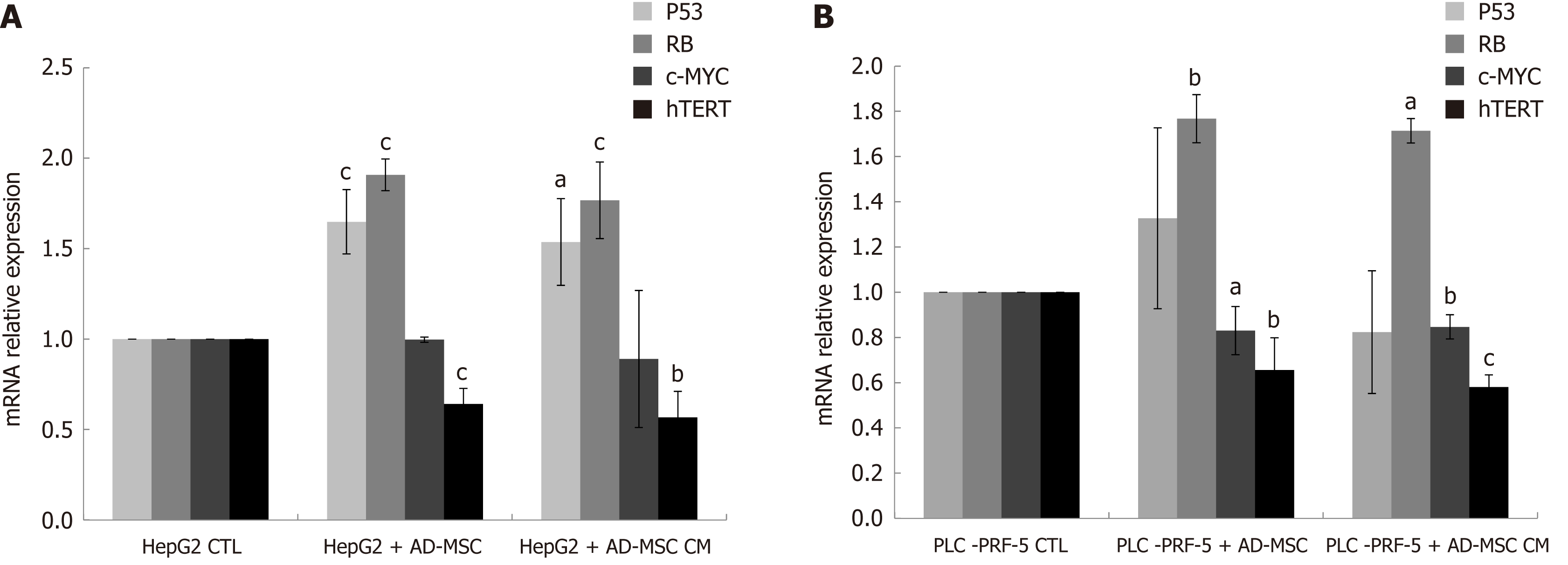
Excessive proliferation and resistance to cell death are regulated by deactivation of tumor suppressor genes and activation of oncogenes. P53 and RB are two tumor suppressor genes implicated in the regulation of apoptosis and cell cycle[29,30]. C-Myc, a proto-oncogene and growth regulator, is overexpressed in HCC[31]. Human telomerase reverse transcriptase (hTERT), the catalytic unit of telomerase, is highly expressed in HCC[32]. To assess the influence of ADMSCs and their CM on tumor suppressor genes and growth regulators, HepG2 and PLC-PRF-5 cells were cultured alone (control), cocultured with ADMSCs or treated with ADMSC CM, and the mRNA levels of P53, RB, c-Myc and hTERT were measured using RT-PCR. In HepG2 cell lines, we found that ADMSCs and their CM significantly induced RB and P53 expression while significantly decreasing hTERT expression (P < 0.05). c-Myc mRNA expression remained unchanged (Figure 6A). However, in PLC-PRF-5 cells, ADMSCs and their CM significantly upregulated of c-Myc and RB mRNA levels and downregulated hTERT mRNA expression (P < 0.05), while having no effect on P53 mRNA levels (Figure 6B).
HCC is a malignant condition with higher incidence and no effective treatment[33]. Adipose-derived SCs have been proven to have therapeutic efficacy in many diseases[34-37]. Their CM has been shown to inhibit HCC proliferation and increase apoptosis[38]. Adipose-derived SCs secrete anti-inflammatory cytokines and growth factors and have immunomodulatory effects. However, many controversies have been noted concerning their role in cancer. Therefore, the effect of ADMSCs and their CM on cancer is not clear. In our study, we investigated the role of ADMSCs and their CM in HCC by using two cell lines, HepG2 and PLC-PRF-5. ADMSCs inhibited cell proliferation, decreased the expression of the diagnostic cancer markers AFP and DCP and promoted apoptosis. In addition, ADMSCs decreased cancer cell migration and invasion by increasing TIMP expression. Thus, we suggest that ADMSCs might offer an alternative cell-based therapy for HCC patients.
There are several findings concerning the effect of MSCs on cancer cells, sometimes contradictory within the same type of cancer or between different types of cancer[39]. ADMSCs can be recruited by prostate cancer cells and stimulate tumor growth by increasing tumor vascularity[40]. It has been reported that the interaction of MSCs with tumor cells contributes to gastric carcinoma[41]. In addition, the co-culture or co-injection of MSCs with osteosarcoma cells enhanced tumor growth in mice and promoted osteocarcinoma proliferation. Furthermore, ADMSCs support breast tumor growth and progression[42] but can inhibit proliferation of pancreatic cancer cells in vitro and in vivo[40]. MSCs can also inhibit tumor growth in Kaposi’s sarcoma[43], colon cancer[44,45], hepatoma[46,47], prostate[48,49], pancreatic[50,51], lung cancer[47] and other tumor models[52]. Similar controversies were reported concerning the effect of the SC secretome. For example, bone marrow MSC CM has been shown to have anti-tumor effects on non-small lung cancer cells[53] and stimulatory effects on myeloma cells[54]. In contrast, ADMSC CM had no effect on human glioblastoma cancer SC subpopulations[55]. In addition, it has been demonstrated that human umbilical cord embryonic SC CM has anti-tumor effects on proliferation, apoptosis and tumor cell invasiveness[56]. These findings confirm that in certain types of cancer, MSCs could enhance tumor growth, but in others it can inhibit invasiveness and metastasis[26,57,58]. This might be explained by the complexity of the MSC source, the malignant cell type involved, and the interaction between the MSCs and the tumor cells. The MSC number and microenvironment might also influence tumor cell growth or inhibition. A recent review by Hill et al[59] focused primarily on the key mechanisms in which MSCs differentiate into tumor-associated MSCs and cancer-associated fibroblasts to promote pro-metastatic and growth states when in contact with the tumor microenvironment. Despite the described pro-metastatic role of MSCs when in contact with a tumor microenvironment, many other studies have reported that when LEAD MSCs are in contact with cancer cells, it might reduce tumorigenicity[60,61]. The paracrine effects of SCs, their trophic effects when in contact with a stimulatory environment, might provide them the potential to be anti-tumorigenic. We suggest that the type of cancer might dictate the secretion profile of the SCs. Therefore, the microenvironment, cell-cell interactions, and origin of MSCs contribute and direct MSCs to be tumorigenic or anti-tumorigenic.
There have not been any studies involving the direct effect of ADMSCs on HCC proliferation and apoptosis. In our study, ADMSCs inhibited proliferation and induced HepG2 and PLC-PRF-5 cancer cell death when co-cultured. Zhao W et al[38] reported that ADMSC CM inhibited HCC cell line proliferation and increased apoptosis. In our data, the inhibition was more significant when ADMSCs were co-cultured rather than using their CM. We hypothesize that this is due to a mechanism underlying cell-cell contact, suggesting that the interactions of receptors and ligands on ADMSCs and cancer cells contribute to the inhibition of proliferation, and thus, trigger cell death. This mechanism of cell-cell interaction should be further investigated. In addition, the upregulation of the tumor suppressor gene P53 and RB and downregulation of c-Myc and hTERT might also contribute to overall tumor suppression. However, tumor suppression is not confined only to inhibition of proliferation by cell-cell interactions and SC CM; it is much more complicated and liver cancer cells are primed for invasiveness and metastasis. Metalloproteinases play major roles in the progression and metastasis of numerous cancers including HCC[62]. This could suggest that inhibiting metalloproteinases is a possible way to decrease HCC invasiveness and metastasis.
In this study, we report the increase in the secretion of the tissue inhibitor metalloproteinases TIMP-1, -2, and -3 by ADMSCs, which may be partially responsible for the decreased HCC cell migration and invasion. The inhibition of invasion is also explained by the inhibition of TGF-β secretion (data not shown), which is well known to be associated with decreased metastasis and invasiveness[63] through feedback mechanisms. In the future, we aim to demonstrate the exact role of these factors, especially TIMPs, on cancer cell migration and invasion using specific siRNAs.
The increase of TIMP secretion in the presence of ADMSCs or ADMSC CM might be a mechanism that SCs use to restrain tumor invasion. Other important steps in the attempt of ADMSCs to protect against invasion is the decrease in AFP and DCP expression. AFP and DCP have been used as serum markers in HCC patients with and as detectors of tumor progression and malignant proliferation, respectively. When ADMSCs were co-cultured with HCC cell lines, both AFP and DCP were significantly decreased, which might be an indication of SCs attempt to halt proliferation and tumor progression. This is the first report to demonstrate a decrease in DCP in HCC cell lines. Our in vitro results will be subsequently confirmed in an in vivo study. The primary results of the pilot study confirm the effect of ADMSCs on tumor growth and AFP levels (data not shown).
To conclude our study, we report novel molecules contributing to the effect of adipose-derived SCs on HCC. The increase in TIMPs and in apoptosis, the inhibition of proliferation and invasiveness, and the decrease in AFP and DCP are a coordination attempt from SCs as strategic management to inhibit tumor cell proliferation, progression and invasiveness.
We would like to acknowledge the Faculty of Medicine and the research council of the Saint Joseph University for its support.
Hepatocellular carcinoma (HCC) is a malignant condition with a high incidence and no effective treatment. Mesenchymal stem cells (MSCs) secrete cytokines and growth factors known to have paracrine, trophic and immunomodulatory effects. Due to their paracrine and differentiation potential, adipose-derived SCs have proven therapeutic efficacy in many diseases. Their conditioned media (CM) has been shown to inhibit proliferation and increase apoptosis in HCC. However, many controversies have been noted concerning their role in cancer.
Many studies have demonstrated the effect of SCs or their CM on cancer, and some reports have shown that they suppress and inhibit tumor growth. Other studies have reported enhanced tumor growth and proliferation. There have not been any studies that reported the effect of adipose-derived MSCs (ADMSCs) on HCC proliferation and apoptosis. Thus, our aim was to investigate the therapeutic effects of adipose-derived SCs and their CM on HCC, specifically their effects on cancer cell marker expression, the proliferation and metastatic potential of cancer cells, and their effect on modulating cancer cell death.
By discovering that adipose-derived SCs and their CM modulate cancer marker expression and liver cancer cell proliferation and metastasis, we have opened a new path for research on the mechanism of action by which MSCs can affect cancer. If the results were to increase and stimulate cancer cells, then further investigations need to be pursued on two levels: (1) to study the behavior of SCs along with the factors contributing to the stimulatory effects; and (2) inhibition of the pathways leading to this progressive effect. In contrast, if the ADMSCs were to inhibit cancer and induce apoptosis, then ADMSCs could be a potential therapy for HCC, which currently has no cure. To achieve this goal, in vivo animal models and clinical studies need to be pursued.
In our study, the main objective was to investigate the role of ADMSCs and their CM in HCC using two cell lines, HepG2 and PLC-PRF-5. In particular, we wanted to study the effect of ADMSCs on alpha-fetoprotein (AFP) and Des-gamma-carboxyprothrombin (DCP) expression and the capacity of ADMSCs to modulate metastasis or proliferation of the above cancer cell lines. We studied TIMPs, P53, and RB. ADMSCs inhibited cell proliferation, decreased AFP and DCP expression and promoted apoptosis. In addition, ADMSCs decreased cancer cell migration and invasion by increasing TIMP expression. Our study has shed light on a novel apoptotic effect of MSCs on cancer. This will direct us and other researchers to further investigate the effect on other cell markers playing roles in cancer and the mechanisms by which ADMSCs exert their anti-cancer effects.
HCC cell lines purchased from ATCC were cultured in low glucose DMEM media. Adipose-derived MSCs isolated from lipoaspirates were cultured in DMEM nutrient mix F12. The isolation method of MSCs was modified and improved to obtain a high yield of living ADMSCs using a minimal quantity of fat and collagenases. Isolated ADMSCs were characterized to demonstrate their viability and capacity of multilineage differentiation.
The coculture conditions and treatment with ADMSC CM were extensively studied in order to determine the number of cells that should be used in all experiments. After co-culturing HCC cells with ADMSCs or stimulating with ADMSC CM, AFP and DCP protein and mRNA levels were detected using ELISA kits and real time PCR, respectively.
In addition, the proliferation level and apoptosis rate of HCC cells were measured using a WST-8 proliferation test and annexin V-FITC kit, respectively. Along with these tests, the mRNA levels of P53, RB, hTERT and c-Myc genes involved in the regulation of proliferation and apoptosis were quantified using real time PCR.
Furthermore, using wound healing assays and migration and invasion tests, we studied the effect of ADMSCs and their CM on HCC cell line metastasis. In parallel, TIMP mRNA levels were measured using real time PCR. TIMPs have been reported to play a major role in in inhibiting metastasis.
In all assays, the experiments were repeated at least three times in order to obtain statistically significant results.
In our study, ADMSCs inhibited cancer cell proliferation and increased cancer cell death when co-cultured with HepG2 and PLC-PRF-5. This effect was more significant in the case of direct co-culture, likely due to cell-cell interactions. The upregulation of the tumor suppressor genes P53 and RB and downregulation of c-Myc and hTERT might be the factors responsible for the mentioned findings. The mechanisms of these results should be further investigated.
We reported increased secretion of TIMP-1, -2, and -3, which may be partially responsible for the decreased HCC cell migration and invasion. Future studies should be performed to confirm this relation. In addition, further investigations are needed to study the involvement of the metalloproteinases MMP-2 and MMP-9 in the inhibition of metastasis. We also found that ADMSCs and ADMSC CM decreased HCC cell line migration and invasion.
We observed decreased AFP and DCP levels after coculturing HCC cells with ADMSCs or stimulating HCC cells with ADMSC CM. This might be an indication of an attempt by SCs to obliterate proliferation and tumor progression. These findings will be confirmed and used subsequently in an in vivo animal study.
This study reported many novel findings about the effects of ADMSCs on HCC. This is the first report to demonstrate a decrease in DCP expression in HCC cell lines. No other study has investigated the direct effect of ADMSCs on HCC proliferation and apoptosis. We reported novel molecules contributing to the effect of adipose-derived SCs on HCC, particularly TIMPs. We reported that coculture of ADMSCs with HepG2 or PLC-PRF-5 cell lines had an anti-cancer effect. This is explained by the inhibition of proliferation and cell death of the cancer cells. We also showed for the first time the effect of direct cell-cell interactions, which is a new mechanism by which ADMSCs might inhibit tumor cell proliferation. The indirect contact of ADMSCs with HCC cell lines inhibited their proliferation and metastasis and increased their apoptosis, though to a lesser extent than the direct coculture, suggesting that the paracrine effects of ADMSCs contribute to their antitumor effects.
These findings confirm that in certain types of cancer, MSCs could enhance tumor growth and in others, it can inhibit invasiveness and metastasis. This might be explained by the complexity of MSC sources, the malignant cell type involved, and the interaction between MSCs and tumor cells. The number of MSCs and the microenvironment might also influence tumor cell growth or inhibition.
Our findings will lead to many investigations: (1) the mechanism of cell-cell contact by which ADMSCs inhibit cancer cells (autophagy); (2) identification of the factors exerting these inhibitory effects. In particular, two candidates are STC-1 and DKK-1, which are known to have roles in suppressing the expression of genes involved in proliferation, migration and invasion and in the overexpression of apoptotic genes. These results should be demonstrated using WST-8 proliferation assays, apoptosis annexin/PI assays, and migration and invasion tests.
In vivo studies will be pursued to confirm our results, mainly the effect of ADMSCs and their CM on tumor growth, apoptosis and metastasis, as well as the paracrine effects of ADMSCs.
To understand and quantify the changes in hepatic cancer cell morphology when in direct contact with ADMSCs, a study will be conducted in collaboration with the Department of Physics at the American University of Beirut. This will also help us determine the mechanism by which ADMSCs induce HepG2 cell death.
In summary, ADMSCs are cells with complex mechanisms that have the capacity to interact with adjacent cells to exert trophic and paracrine effects, thus altering the microenvironment. Their role in each disease must be vigorously studied to elucidate their therapeutic effects. In this study, we determined the inhibitory effects of ADMSCs on cancer cell markers and on key factors known to play a major role in inflammation, invasion and metastasis. Our study has shed new light on the role of ADMSCs on HCC.
Dr Marwan Nasr at Hotel Dieu de France for his contribution to providing adipose tissues and Mr Charbil Khalil for his help in flow cytometry, Reviva Bsallim hospital.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Lebanon
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Pelagalli A S- Editor: Ma RY L- Editor: Filipodia E- Editor: Huang Y
| 1. | Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S206-S214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 2. | Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9:765-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 3. | Tannapfel A, Busse C, Weinans L, Benicke M, Katalinic A, Geissler F, Hauss J, Wittekind C. INK4a-ARF alterations and p53 mutations in hepatocellular carcinomas. Oncogene. 2001;20:7104-7109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Teufel A, Staib F, Kanzler S, Weinmann A, Schulze-Bergkamen H, Galle PR. Genetics of hepatocellular carcinoma. World J Gastroenterol. 2007;13:2271-2282. [PubMed] |
| 5. | Sengupta S, Parikh ND. Biomarker development for hepatocellular carcinoma early detection: current and future perspectives. Hepat Oncol. 2017;4:111-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Rich N, Singal AG. Hepatocellular carcinoma tumour markers: current role and expectations. Best Pract Res Clin Gastroenterol. 2014;28:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Sánchez-Lorencio MI, Saenz L, Ramirez P, Villalba-López F, de la Orden V, Mediero-Valeros B, Revilla Nuin B, Gonzalez MR, Cascales-Campos PA, Ferreras-Martínez D, Noguera-Velasco JA, Díaz-Rubio E, Parrilla P. Matrix Metalloproteinase 1 as a Novel Biomarker for Monitoring Hepatocellular Carcinoma in Liver Transplant Patients. Transplant Proc. 2018;50:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Naim A, Pan Q, Baig MS. Matrix Metalloproteinases (MMPs) in Liver Diseases. J Clin Exp Hepatol. 2017;7:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4516] [Article Influence: 347.4] [Reference Citation Analysis (2)] |
| 10. | Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403. [PubMed] |
| 11. | Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242. [PubMed] |
| 12. | Villaron EM, Almeida J, López-Holgado N, Alcoceba M, Sánchez-Abarca LI, Sanchez-Guijo FM, Alberca M, Pérez-Simon JA, San Miguel JF, Del Cañizo MC. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89:1421-1427. [PubMed] |
| 13. | Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 445] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5012] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 15. | Maby-El Hajjami H, Amé-Thomas P, Pangault C, Tribut O, DeVos J, Jean R, Bescher N, Monvoisin C, Dulong J, Lamy T, Fest T, Tarte K. Functional alteration of the lymphoma stromal cell niche by the cytokine context: role of indoleamine-2,3 dioxygenase. Cancer Res. 2009;69:3228-3237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1254] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 17. | Feng B, Chen L. Review of mesenchymal stem cells and tumors: executioner or coconspirator? Cancer Biother Radiopharm. 2009;24:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Lee DC, Fenton SE, Berkowitz EA, Hissong MA. Transforming growth factor alpha: expression, regulation, and biological activities. Pharmacol Rev. 1995;47:51-85. [PubMed] |
| 19. | Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C, Miller N, Hennessy E, Dockery P, Barry FP, O'Brien T, Kerin MJ. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat. 2010;124:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 857] [Cited by in RCA: 865] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 21. | Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 549] [Cited by in RCA: 587] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 22. | Dasari VR, Velpula KK, Kaur K, Fassett D, Klopfenstein JD, Dinh DH, Gujrati M, Rao JS. Cord blood stem cell-mediated induction of apoptosis in glioma downregulates X-linked inhibitor of apoptosis protein (XIAP). PLoS One. 2010;5:e11813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Li Z, Fan D, Xiong D. Mesenchymal stem cells as delivery vectors for anti-tumor therapy. Stem Cell Investig. 2015;2:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 24. | El Atat O, Antonios D, Hilal G, Hokayem N, Abou-Ghoch J, Hashim H, Serhal R, Hebbo C, Moussa M, Alaaeddine N. An Evaluation of the Stemness, Paracrine, and Tumorigenic Characteristics of Highly Expanded, Minimally Passaged Adipose-Derived Stem Cells. PLoS One. 2016;11:e0162332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101-1108. [PubMed] |
| 26. | Li GC, Ye QH, Xue YH, Sun HJ, Zhou HJ, Ren N, Jia HL, Shi J, Wu JC, Dai C, Dong QZ, Qin LX. Human mesenchymal stem cells inhibit metastasis of a hepatocellular carcinoma model using the MHCC97-H cell line. Cancer Sci. 2010;101:2546-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Lu YR, Yuan Y, Wang XJ, Wei LL, Chen YN, Cong C, Li SF, Long D, Tan WD, Mao YQ, Zhang J, Li YP, Cheng JQ. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 2008;7:245-251. [PubMed] |
| 28. | Gong P, Wang Y, Wang Y, Jin S, Luo H, Zhang J, Bao H, Wang Z. Effect of bone marrow mesenchymal stem cells on hepatocellular carcinoma in microcirculation. Tumour Biol. 2013;34:2161-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991;352:345-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1402] [Cited by in RCA: 1455] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 30. | Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220-5227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 869] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 31. | Yuen MF, Wu PC, Lai VC, Lau JY, Lai CL. Expression of c-Myc, c-Fos, and c-jun in hepatocellular carcinoma. Cancer. 2001;91:106-112. [PubMed] |
| 32. | Zhou XU, Lu J, Zhu H. Correlation between the expression of hTERT gene and the clinicopathological characteristics of hepatocellular carcinoma. Oncol Lett. 2016;11:111-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4077] [Article Influence: 582.4] [Reference Citation Analysis (6)] |
| 34. | Chung TN, Kim JH, Choi BY, Chung SP, Kwon SW, Suh SW. Adipose-derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood-brain barrier disruption and endothelial damage. Stem Cells Transl Med. 2015;4:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Saidi RF, Rajeshkumar B, Shariftabrizi A, Bogdanov AA, Zheng S, Dresser K, Walter O. Human adipose-derived mesenchymal stem cells attenuate liver ischemia-reperfusion injury and promote liver regeneration. Surgery. 2014;156:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Saleh F, Itani L, Calugi S, Grave RD, El Ghoch M. Adipose-derived Mesenchymal Stem Cells in the Treatment of Obesity: A Systematic Review of Longitudinal Studies on Preclinical Evidence. Curr Stem Cell Res Ther. 2018;13:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Brini AT, Amodeo G, Ferreira LM, Milani A, Niada S, Moschetti G, Franchi S, Borsani E, Rodella LF, Panerai AE, Sacerdote P. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci Rep. 2017;7:9904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 38. | Zhao W, Ren G, Zhang L, Zhang Z, Liu J, Kuang P, Yin Z, Wang X. Efficacy of mesenchymal stem cells derived from human adipose tissue in inhibition of hepatocellular carcinoma cells in vitro. Cancer Biother Radiopharm. 2012;27:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Hong IS, Lee HY, Kang KS. Mesenchymal stem cells and cancer: friends or enemies? Mutat Res. 2014;768:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Lin G, Yang R, Banie L, Wang G, Ning H, Li LC, Lue TF, Lin CS. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010;70:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem. 2008;283:19864-19871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Razmkhah M, Jaberipour M, Hosseini A, Safaei A, Khalatbari B, Ghaderi A. Expression profile of IL-8 and growth factors in breast cancer cells and adipose-derived stem cells (ASCs) isolated from breast carcinoma. Cell Immunol. 2010;265:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 43. | Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2013;22:758-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 44. | Kao TC, Lee HH, Higuchi A, Ling QD, Yu WC, Chou YH, Wang PY, Suresh Kumar S, Chang Y, Hung Chen Y, Chang Y, Chen DC, Hsu ST. Suppression of cancer-initiating cells and selection of adipose-derived stem cells cultured on biomaterials having specific nanosegments. J Biomed Mater Res B Appl Biomater. 2014;102:463-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Iplik ES, Ertugrul B, Kozanoglu I, Baran Y, Cakmakoglu B. An answer to colon cancer treatment by mesenchymal stem cell originated from adipose tissue. Iran J Basic Med Sci. 2018;21:465-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 46. | Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L, Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 47. | Yuan Y, Zhou C, Chen X, Tao C, Cheng H, Lu X. Suppression of tumor cell proliferation and migration by human umbilical cord mesenchymal stem cells: A possible role for apoptosis and Wnt signaling. Oncol Lett. 2018;15:8536-8544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 48. | Cavarretta IT, Altanerova V, Matuskova M, Kucerova L, Culig Z, Altaner C. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther. 2010;18:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 49. | Wu YJ, Wei Q, Nie M, Yin Y, Xi Y. [The Inhibitory Effect and Mechanism of Human Umbilical Cord Mesenchymal Stem Cells on Prostate Cancer Metastasis]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2017;48:543-548. [PubMed] |
| 50. | Omuro Y, Matsumoto G, Sasaki T, Tanaka Y, Maeda Y, Sakamaki H, Hiruma K, Tsuruta K, Takahashi T. Regression of an unresectable pancreatic tumor following nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. Bone Marrow Transplant. 2003;31:943-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Jing W, Chen Y, Lu L, Hu X, Shao C, Zhang Y, Zhou X, Zhou Y, Wu L, Liu R, Fan K, Jin G. Human umbilical cord blood-derived mesenchymal stem cells producing IL15 eradicate established pancreatic tumor in syngeneic mice. Mol Cancer Ther. 2014;13:2127-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304-6313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 315] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 53. | Attar-Schneider O, Zismanov V, Drucker L, Gottfried M. Secretome of human bone marrow mesenchymal stem cells: an emerging player in lung cancer progression and mechanisms of translation initiation. Tumour Biol. 2016;37:4755-4765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Marcus H, Attar-Schneider O, Dabbah M, Zismanov V, Tartakover-Matalon S, Lishner M, Drucker L. Mesenchymal stem cells secretomes' affect multiple myeloma translation initiation. Cell Signal. 2016;28:620-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Onzi GR, Ledur PF, Hainzenreder LD, Bertoni AP, Silva AO, Lenz G, Wink MR. Analysis of the safety of mesenchymal stromal cells secretome for glioblastoma treatment. Cytotherapy. 2016;18:828-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 871] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 57. | Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 620] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 58. | Fierro FA, Sierralta WD, Epuñan MJ, Minguell JJ. Marrow-derived mesenchymal stem cells: role in epithelial tumor cell determination. Clin Exp Metastasis. 2004;21:313-319. [PubMed] |
| 59. | Hill BS, Pelagalli A, Passaro N, Zannetti A. Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget. 2017;8:73296-73311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 60. | Melzer C, von der Ohe J, Hass R. MSC stimulate ovarian tumor growth during intercellular communication but reduce tumorigenicity after fusion with ovarian cancer cells. Cell Commun Signal. 2018;16:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 2018;7:1522236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 230] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 62. | Määttä M, Soini Y, Liakka A, Autio-Harmainen H. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumor progression and clinical prognosis. Clin Cancer Res. 2000;6:2726-2734. [PubMed] |
| 63. | Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, Wang SB, Wang YZ, Yang Y, Yang N, Zhou WP, Yang GS, Sun SH. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1274] [Article Influence: 115.8] [Reference Citation Analysis (0)] |