Published online Dec 21, 2019. doi: 10.3748/wjg.v25.i47.6866
Peer-review started: October 18, 2019
First decision: November 22, 2019
Revised: December 2, 2019
Accepted: December 13, 2019
Article in press: December 13, 2019
Published online: December 21, 2019
Processing time: 63 Days and 9.6 Hours
The worldwide epidemiology of inflammatory bowel disease (IBD) is rapidly changing. Increasing Crohn’s disease (CD) and ulcerative colitis (UC) incidence and prevalence have been recorded in developing regions such as Asia, Africa and Eastern Europe where it was previously thought to be uncommon. Whether this is also the case in South America is not well known. Demonstration that developing regions worldwide have increasing IBD incidence would indicate that environmental change plays a significant role in the development of IBD.
To report the incidence, prevalence and disease characteristics of CD and UC within the South American continent.
A systematic review was conducted by searching published studies in major international and regional databases (MEDLINE, EMBASE and Scopus) between January 1990 and December 2018. Outcomes considered were incidence, prevalence, phenotype, environmental and genetic factors, ethnicity and gender. A pair of independent reviewers screened and reviewed all identified articles.
One hundred and sixty two citations were initially retrieved with 18 studies included in this systematic review. The majority of included studies were from Brazil (n =13, 72%). The incidence of UC ranged from 4.3-5.3/100000 person-years whilst the incidence of CD ranged from 0.74-3.5/100000 person-years. Prevalence ranged from 15.0-24.1/100000 inhabitants for UC and from 2.4-14.1/100000 inhabitants for CD. The incidence and prevalence of both UC and CD has increased significantly in Brazil over the past 21 years. Pancolitis was the most common disease distribution in patients with UC whilst colonic involvement was the most common distribution in CD. People residing in urban areas were at higher risk of developing both CD and UC.
The IBD burden in South America is increasing at a rate possibly even greater than other developing regions around the world. There is a paucity of high-quality epidemiological studies and further robust and representative data are required to further explore modifiable risk factors and disease phenotypes.
Core tip: The worldwide epidemiology of inflammatory bowel disease (IBD) is rapidly changing with increasing disease incidence and prevalence noted in developing regions such as Asia, Africa and Eastern Europe where it was previously thought to be uncommon. Whether this is the case in South America was previously not well known. Our systematic review demonstrates that the IBD burden in South America is precipitously increasing, particularly in industrialised regions. With a total population exceeding 400 million, the South American continent is expected to carry a significant proportion of the future global IBD burden.
- Citation: Selvaratnam S, Gullino S, Shim L, Lee E, Lee A, Paramsothy S, Leong RW. Epidemiology of inflammatory bowel disease in South America: A systematic review. World J Gastroenterol 2019; 25(47): 6866-6875
- URL: https://www.wjgnet.com/1007-9327/full/v25/i47/6866.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i47.6866
Inflammatory bowel disease (IBD) refers to a chronic inflammatory disorder of the gastrointestinal tract which is thought to arise from an inappropriate inflammatory response to intestinal microbes in a genetically susceptible host[1]. The most common forms of IBD include ulcerative colitis (UC) and Crohn’s disease (CD). UC characteristically involves the rectum and colon whereas CD may involve any part of the gastrointestinal tract however differentiation between these two conditions is not always clear[1,2]. Symptomatic disease is characterised by abdominal pain, diarrhoea, rectal bleeding, fever and weight loss which has a significant impact on quality of life[1,2].
IBD was first recognised in Western Europe during the industrial revolution and has encountered a rising incidence in this population since this time[3]. Whilst previously regarded exclusively as a disease of western nations, the epidemiology of IBD is rapidly changing worldwide. Although IBD incidence in developed areas such as Western Europe and the United States have been relatively stable, recent epidemiological studies suggest a significant increase in IBD incidence and prevalence in areas such as Asia, Africa and Eastern Europe where it was previously thought to be uncommon[3-9]. This epidemiological shift, seen in newly industrialised countries as well as in immigrant populations in western countries, is comparable to the patterns noted in western countries more than 50 years ago which occurred during a period of rapid socioeconomic development[4].
An increase in IBD incidence in developing nations has substantial implications for the understanding of IBD pathogenesis and environmental triggers. A recent Asia-Pacific Crohn’s and Colitis epidemiology study demonstrated significant differences in risk factors and disease characteristics in IBD occurring in Asian vs Western populations[5]. Interestingly, in recent times the prevalence of CD appears to have caught up to the prevalence UC in the Asian population[5]. Furthermore, Asian patients tended to have a more severe CD phenotype at diagnosis[5]. Other notable findings included significant genetic heterogeneity between Asian and European patients as well as a protective effect of childhood antibiotic use in Asia for the development of IBD - a direct contrast to studies from Western nations[5].
Epidemiological data can provide valuable information about population-based disease characteristics and burden which can also be utilised to anticipate healthcare needs. This is particularly important with chronic and incurable diseases such as IBD for which the concept of personalised medicine is paramount.
Recent efforts have been made to describe IBD in some developing nations within the South American continent. Further information about IBD in this region will help provide a valuable insight into the emerging epidemic of this disease within this population which will therefore enable the delivery of high-quality patient-centred care.
We therefore sought to report the incidence, prevalence and disease characteristics of CD and UC within the South American population.
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines outlined in the PRISMA 2009 checklist[10].
Published studies in major international and regional databases (MEDLINE, EMBASE and Scopus) between January 1990 and December 2018 were searched. Search terms included Inflammatory Bowel Disease, Crohn, Crohn’s disease, ulcerative colitis AND epidemiology, incidence, prevalence AND South America, Argentina, Chile, Uruguay, Brazil, Paraguay, Bolivia, Peru, Ecuador, Colombia, Venezuela, Suriname, Guayana, French Guayana.
Randomised controlled trials, cohort studies, cross-sectional studies, case control studies and observational studies were included if they included an adult population (> 18 years of age). Outcomes considered were incidence, prevalence, phenotype, environmental and genetic factors, ethnicity and gender. Included studies were restricted to those published in the English language only.
A pair of independent reviewers screened titles and abstracts of all identified articles. Studies were initially sorted into one of the following categories: Excluded, low probability of inclusion, or high probability of inclusion. Studies deemed to be at low or high probability of inclusion were retrieved in their full-text version for further evaluation. Inclusion criteria was shown in Table 1.
| Inclusion criteria | |
| Population | Adult (> 18 yr old) |
| Language | English |
| Date range | January 1990 to December 2018 |
| Location | South America |
| Type of study | Randomised controlled trials, cohort studies, cross sectional studies, case series, and observational studies |
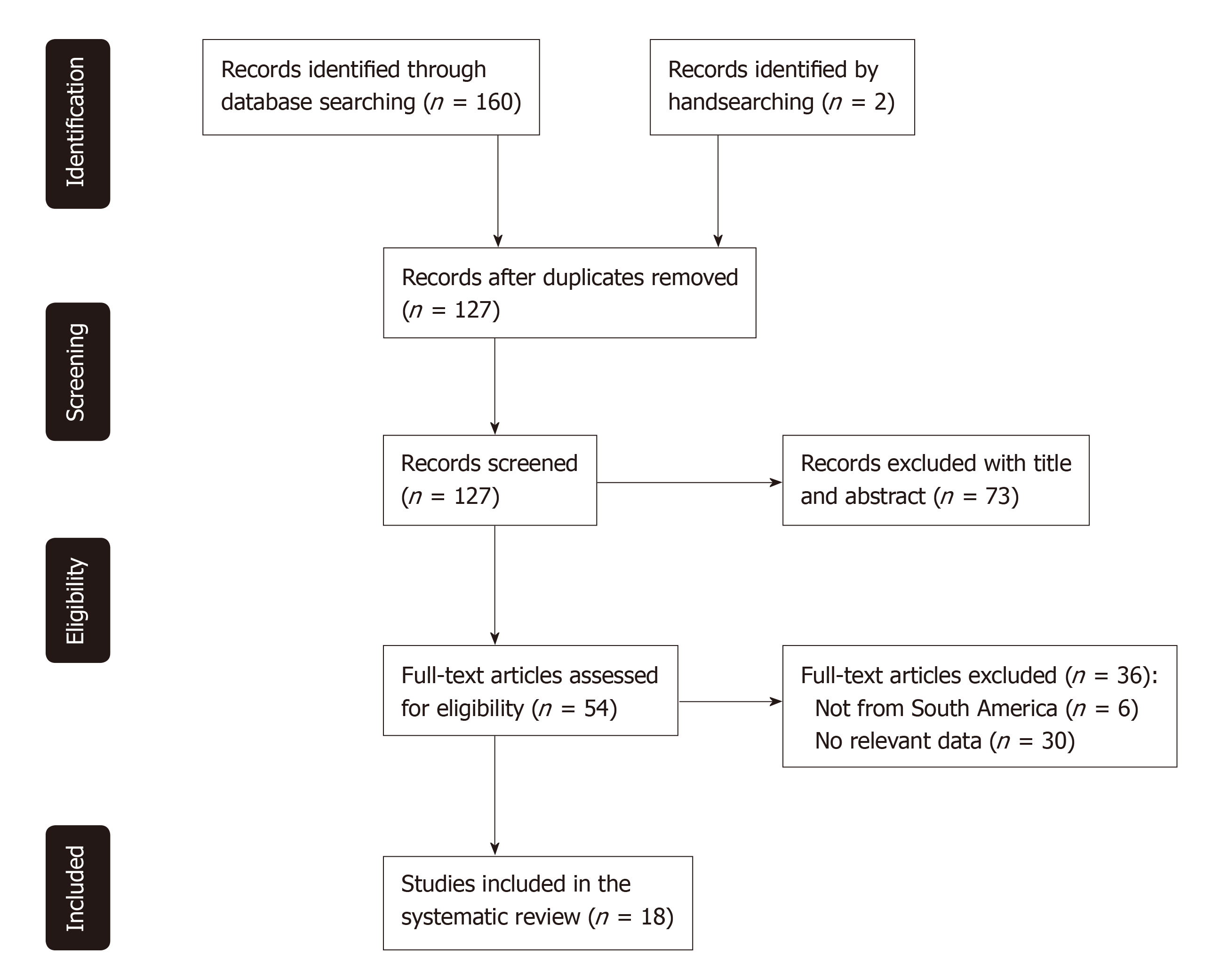
This search strategy retrieved 160 citations, of which 35 were duplicates. Two further citations were identified by handsearching and a total of 73 were subsequently excluded by title and abstract. Out of the 54 full-text manuscripts retrieved for detailed evaluation, 18 met the inclusion criteria and were included in the systematic review as outlined in Figure 1.
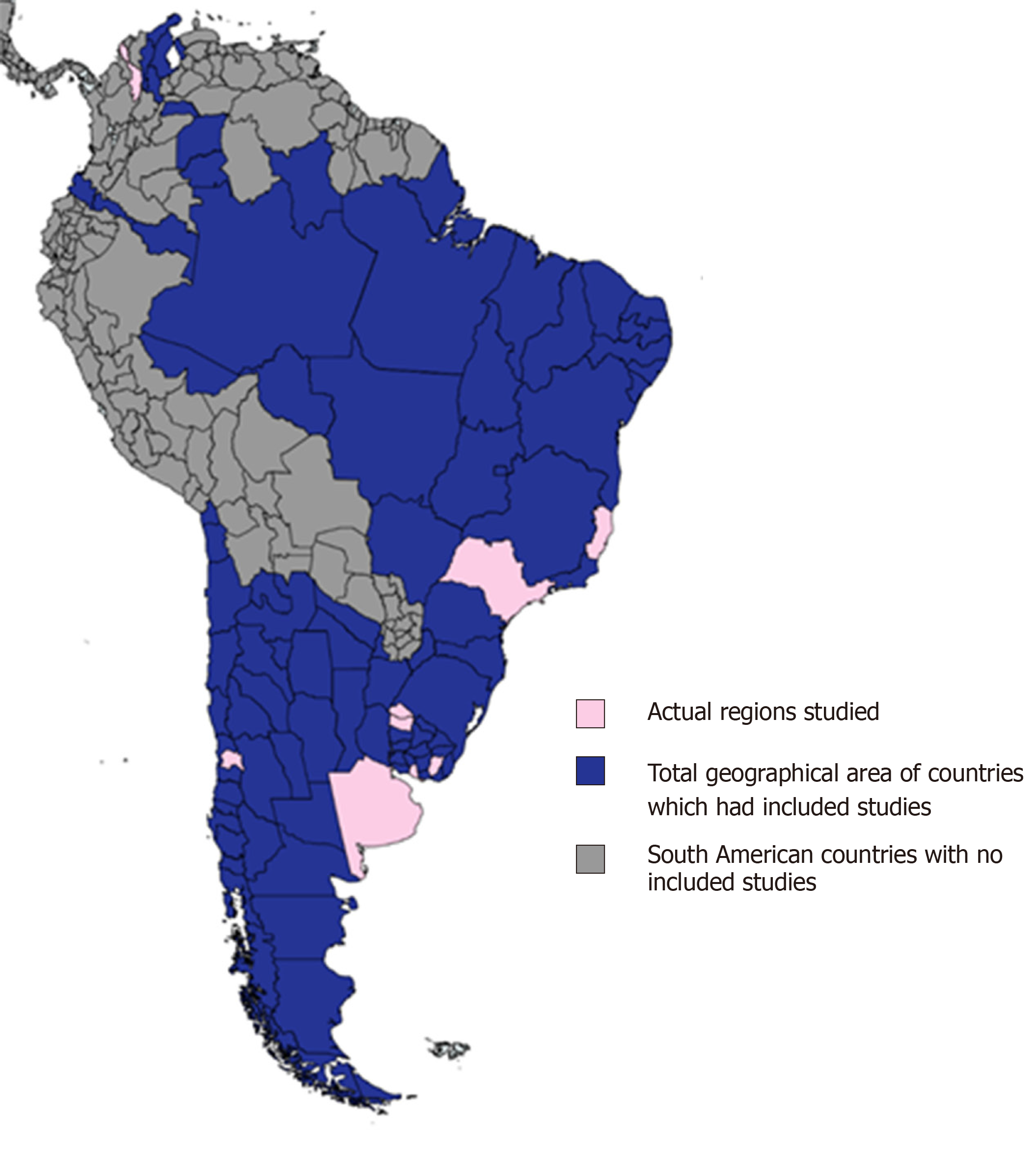
Included studies were conducted in five of twelve countries within the South American continent - the majority from Brazil (n = 13, 72%). All included studies were hospital-based. The geographic area of South America covered by the included studies is outlined in Figure 2. The years of publication of included studies ranged from 1999 to 2018, with most studies being published within the last ten years (n = 15, 83%). Most included studies included patients with both UC and CD (n = 10, 56%). The main characteristics of the included studies are outlined in Table 2.
| Ref. | Country | Type of study | Type of IBD | Year data set | n | Average patient age | Specific group |
| Linares et al[16] | Argentina | Registry | UC/CD | 1987-1993 | 39 | 38 | Multicentre |
| Salgado et al[18] | Brazil | Case-control | CD | 2017 | 145-163 | NR | Single Centre |
| Lima Martins et al[13] | Brazil | Registry | UC/CD | 2012-2014 | 1048 | NR | Multicentre |
| Queiroz et al[20] | Brazil | Case-control | UC/CD | 2017 | 85/541 | 40.0 | Single Centre |
| Santos et al[21] | Brazil | Registry | UC/CD | 2016 | 556 | 49.7 | Single Centre |
| da Silva et al[22] | Brazil | Cross-sectional | UC | 2011-2012 | 267 | 33.4 | Multicentre |
| Parente et al[12] | Brazil | Registry | UC/CD | 1988-2012 | 256 | 25.2 | Multicentre |
| Victoria et al[11] | Brazil | Registry | UC/CD | 1986-2005 | 115 | 38.0 | Multicentre |
| Santana et al[23] | Brazil | Cross-sectional | CD | 2006 | 65 | 37.3 | Single Centre |
| Torres Udos et al[24] | Brazil | Cross-sectional | CD | 1992-2007 | 90 | 33 | Single Centre |
| Cohen et al[25] | Brazil | Cross-sectional | UC/CD | 2008 | 50 | 42.2 | Single Centre |
| Hardt et al[26] | Brazil | Cross-sectional | CD | 2000-2012 | 175 | 35.5 | Multicentre |
| Santana et al[27] | Brazil | Cross-sectional | CD | 2005 | 47 | 38.5 | Single Centre |
| de Barros et al[28] | Brazil | Cross-sectional | UC/CD | 2012-2013 | 40 | 37.8 | Single Centre |
| Simian et al[17] | Chile | Registry | UC/CD | 2012-2015 | 716 | 36 | Single Centre |
| Alvarez-Lobos et al[19] | Chile | Case-control | CD | 2010-2012 | 94/90 | 35.5 | Single Centre |
| Barreto et al[15] | Colombia | Registry | UC/CD | 1991-2006 | 26 | 40 | Single Centre |
| Buenavida et al[16] | Uruguay | Registry | UC/CD | 2007-2008 | 34 | 40.7 | Multicentre |
Six studies described the incdence of IBD in South America. Three were Brazilian studies that reported data from hospital records during different study periods: 1986 to 2005, 1988 to 2012 and 2012 to 2014[11-13]. The other three studies were conducted in Argentina, Columbia and Uruguay[14-16].
Victoria et al[11] conducted a retrospective registry-based study to investigate the incidence and prevalance of UC and CD in a specific region in the mid-western zone of Sao Paulo, Brazil. This study was conducted in four, five-year blocks over a 20 year time period (1986-2005) to compare changes over time. Both the incidence and prevalence of UC and CD was seen to increase during this time period (expressed as number of cases per 100000 inhabitants); UC Prevalence 0.99 to 15.0, CD Prevalence 0.24 to 5.7, annual UC Incidence 0.74 to 4.5, annual CD Incidence 0.24 to 3.5[11].
Increasing IBD incidence and prevalence within Brazil was also noted in a 21-year retrospective study conducted by Parente et al[12] in the northeastern region of Brazil. This study, which included a total of 256 patients with IBD, demonstrated an increase in both combined IBD prevalence (1.2 to 21 per 100000 inhabitants) and combined annual IBD incidence (1.0 to 8.0 per 100000 inhabitants)[17]. Sub-analysis demonstrated that gradual increases occured in a similar pattern for both UC and CD during this time period[12].
As aforementioned, the incidence and prevalence of CD appears to be increasing over time, at least within the Brazilian population. A more recent retrospective study performed between 2012 and 2014 in the state of Espirto Santo, Brazil by Lima Martins et al[13] demonstrated a high incidence and prevalance of UC and CD (expressed as number of cases per 100000 inhabitants); annual UC Incidence 5.3, Prevalence 24.1 and annual CD Incidence 2.4, Prevalence 14.1. This higher than expected CD prevalence compared with other Brazilian studies, may potentially be explained by the high European immigrant population within this region[11,13]. Unfortunately, to date, there have been no studies to formally assess factors related to aetiopathogenesis within this specific region.
Similar prevalence data to that obtained in Brazil was also observed in a 2006 single-centred Columbian study by Barreto et al[14] which described UC and CD prevalences of 22 and 7 per 100000 inhabitants respectively within the city of Cartagena, Columbia. Interestingly, a prospective study by Buenavida et al[15] across five geographical areas of Uruguay between 2007 and 2008 also demonstrated a similar annual UC incidence to the Brazilian studies however CD appears to be less frequent with UC and CD incidences of 4.26 and 0.74 per 100000 inhabitants per year respectively.
All included studies demonstrated a significantly higher frequency of UC compared to CD within the South American population[11-28]. A single-centered prospective observational study conducted in Chile demonstrated a UC to CD ratio of 2.6:1 within 716 patients included in their study between 2012 and 2015[17]. In this study, pancolitis was the most common disease distribution in UC patients (n = 76, 50%) and colonic involvement was the most common distribution in CD (n = 44, 44%)[17]. In CD patients, the inflammatory subtype was most frequent (n = 80,80%) with perianal disease observed in 28% (n = 28)[17].
These phenotypic findings are also supported by the findings of Parente et al[12] in northeastern Brazil which demonstrated predominant CD features of; colonic disease location, nonstricturing and nonfistulizing disease behavior and a 25% frequency of perianal disease.
The distribution of patients with IBD according to residence was evaluated by both Victoria et al[11] and Parente et al[12] with both studies demonstrating that the majority of patients with IBD lived in urban districts. In the study conducted by Victoria et al[11], 104 patients (90.5%) lived in an urban area compared to 11 (9.5%) who lived in a rural area, P < 0.001. Similar findings were demonstrated by Parente et al[12]; total IBD [86.1% (n = 217) vs 13.9% (n = 35), P < 0.001), CD [93.0% (n = 93) vs 7.0% (n = 7) P < 0.001] and UC [81.6% (n = 124) vs 18.4% (n = 28), P < 0.001].
A case-control study conducted by Salgado et al[18] at a statewide tertiary referral centre in Rio de Janeiro sought to identify envionmental risk factors associated with the development of CD to re-assess the hygeine hypothesis in this unique population. In this study, 145 outpatients with CD were compared to 163 controls by means of a 94-item survey regarding perinatal and childhood circumstances, living conditions, smoking and familial socioeconomic status. Controls were were recruited from caregivers of patients seen in different outpatient clinics at the same hospital. On univariate analysis, predictive variables for CD included male gender [Odds ratio (OR) = 2.09, P = 0.003], age under 40 (OR = 2.71, P < 0.001), “white” race (OR = 2.32, P = 0.002), small family in childhood (OR = 2.34, P < 0.006) and adulthood (OR = 3.02, P = 0.002), exposure to enteric pathogens (OR = 2.23, P = 0.001 ), and history of cigarette smoking (OR = 2.83, P = 0.002)[18].
The role of Salmonella enterica exposure in Chilean CD patients was examined by Alvarez-Lobos et al[19]. This single-centre case-control study compared 94 adult CD patients with 88 healthy age and sex matched controls[19]. Participants were analysed for exposure to Salmonella enterica and for their NOD2/CARD15 gene status. Interestingly, no association between exposure to Salmonella enterica and CD was demonstrated in this study [16/94 (17%) vs 15/88 (17%), P = 0.8][19]. Seventeen CD patients (18%) had at least one mutation of the NOD2/CARD15 gene however NOD2/CARD15 gene status was not associated with Salmonella enterica exposure[19]. Queiroz et al[20] conducted the first (and only) South American study to exclusively examine associations among genetic polymorphisms with CD and UC. This landmark Brazilian study compared genetic polymorphisms in 43 patients with CD, 42 with UC and 541 controls. Data was analyzed in multivariate models adjusting for confounding factors. Queiroz et al[20] demonstrated positive associations between UC and proinflammatory polymorphisms at the 1L1RN [OR = 2.43, 95% confidence interval (CI): 1.50-3.90, P < 0.001] and TNFA-307 (OR = 1.70, 95%CI: 1.00-2.94, P < 0.001) loci as well as positive associations with CD and polymoprhisms in the NOD2 gene (G908R; OR = 6.83, 95%CI: 1.62-25.45, P = 0.02 and L1007fsinsC, OR = 20.00, 95%CI: 3.21-124.69, P < 0.001).
Ethnic background was also only explicitly evaluated within Brazil by the studies conducted by Victoria et al[11] and Parente et al[12]. Within the Sao Paulo region, Victoria et al[11] demonstrated a statistically significant predominence of combined IBD within Brazilians of European and Middle Eastern descent (“White” Brazilians) compared to Brazilians of African and Asiatic descent; 91.1% (n = 105) vs 8.0% (n = 9) vs 0.89% (n = 1), P < 0.001[2]. Parente et al[17] further classified ethncity into “Miscegenated”, “White”, “Black” and “Yellow” with the majority of CD, UC and combined IBD patients belonging to the miscegenated group; CD (64.0%, n = 64), UC (70.4%, 107), and combined IBD (67.9%, 171).
A non-statistically signficant female predominence for UC, CD and combined IBD was demonstrated in the majority of included studies from Brazil and all included studies from Argentina, Uruguay, Chile and Columbia[11-15,17-28]. The gender distribution in UC and CD specifically, was best presented by Lima Martins et al[13] in Espirto Santo, Brazil. Of 669 patients with UC and 357 with CD, 60.80% (n = 407) and 54.60% (n = 195) were female (P = 0.16).
The aforementioned 20-year study conducted by Victoria et al[11] in Sao Paulo, Brazil was the only study to demonstrate a statistically significant higher incidence of total IBD among females (Male:Female RR = 0.44, P < 0.001). Interestingly, female predominence appeared to also be increasing with time over the four five-year study intervals between 1986 and 2005[11].
Our systematic review demonstrates that IBD is an expanding problem within the South American continent. The rising disease burden of IBD in South America appears to mirror the recent epidemiological shift observed in other developing nations such as Africa, Asia and Eastern Europe. While the incidence and prevalence of IBD in South America currently remains lower than western nations such as America, Australia and the United Kingdom, data obtained from the studied regions in Brazil, Argentina, Columbia and Uruguay demonstrate a significantly higher burden of disease compared to Asian countries such as Mainland China, Hong Kong, Indonesia, Malaysia, Singapore, Sri Lanka and Thailand[4-6,29]. With a combined population of over 430 million people, it is anticipated that South America will have carry a significant future burden of IBD worldwide[4].
Rampant industrialisation, including increased urbanisation, has resulted in a transformation of lifestyle behaviours and exposures which promote the development of IBD. Higher rates of cigarette smoking, sedentary occupations and lower breast feeding rates - all risk factors for the development of IBD, have been associated with adopted lifestyles in concentrated urban cities[29]. The overall IBD incidence and prevalence was consistently higher in the study conducted in Sao Paulo, Brazil by Victoria et al[11] compared to the data obtained from Piuai, Brazil by Parente et al[12]. These findings are not entirely surprising given the unique population demographics of each region; with Sao Paulo known to be a vibrant industrialised region with a robust IBD service while Piuai generally consists of a population with lower socioeconomic status and poor living conditions. Whilst underreporting in Piuai may potentially be contributory to this discrepancy, it is interesting to note that both of these studies have independently demonstrated that people are more likely to develop IBD if they lived within an urban district[11,12].
Based on the studies included in this systematic review, the population of IBD patients in South America appear to have a unique disease phenotype compared to other parts of the world. As observed in Asia, UC was more common than CD in all included studies from South America - a direct contrast to western prevalence data[5,7,8,11-28]. Previous reports from Asia, Australia, the United Kington and United States of America demonstrate a predominance of small bowel CD and rectal UC however data from Santiago, Chile and Sao Paulo, Brazil suggest a predominance of colonic CD and pancolonic UC[3,5,7,11,27]. This interesting finding may be associated with differences in genetic polymorphisms or environmental exposures however the potential for confounding due to issues with reporting also need to be considered as colonic CD and pancolonic UC are generally very symptomatic and hence more likely to be reported compared to small bowel CD or more localised UC which is prone to underreporting or misdiagnosis particularly in underprivileged regions. Despite this, it is important to recognise that this high prevalence of colonic CD and pancolonic UC is likely to have important implications for bowel cancer incidence and the requirement of appropriate endoscopic surveillance in such patients within the South American continent.
The hygiene hypothesis postulates that improved hygiene and environmental conditions would reduce the incidence of infections and favour the development of immune-mediated diseases. In this hypothesis, it is thought that exposure to various microbial agents may confer a protective role in promoting immune system maturation by achieving a balance between a pro-inflammatory Th1 response and regulatory T cell tolerance[18,30-32]. This in turn would provide protection against subsequent exposure to antigens and allergens therefore decreasing the likelihood of developing autoimmune conditions such as IBD. Furthermore, the hygiene hypothesis suggests that improved sanitation and reduced exposure to enteric organisms during childhood might lead to inappropriate immunological responses later in life thus increasing the risk of developing IBD[31]. The case-control study conducted in Rio de Janiero by Salgado et al[18] was the only identified study from South America which explicitly examined the hygiene hypothesis in CD. The findings demonstrated by Salgado et al[18] suggest that most variables supporting the hygiene hypothesis were associated with CD but were not independent predictors of the diagnosis. Interestingly, this study controversially demonstrated that greater exposure to enteric pathogens was associated with a higher risk for the development of CD[18]. A 94-item survey was the basis of data collection in this study with no serological/ microbiological confirmation, therefore a plausible explanation for this unusual finding could be misdiagnosis of intestinal infection at CD onset.
Despite the rigorous methodology followed, this systematic review has several limitations. Firstly, the scarcity of high-quality epidemiological studies on IBD in South America conveyed significant variability between studies which precluded any meta-analysis from being undertaken. Heterogeneity between studies could be explained by methodological limitations, some of which include differences in population characteristics, study designs, access to health care and the variability of diagnostic modalities between countries. Perhaps most significantly, as outlined in Figure 2, the studies included in this review only represented a small geographical area of the South American continent. This limitation has obvious implications for generalisability and further highlights the need for high-quality population-based IBD epidemiological studies from the South American continent. Furthermore, studies were only included in this review if published in the English language which may have resulted in other relevant studies from being overlooked.
A concerted and collaborative South American approach is vital given the future propensity for this region to carry a significant proportion of the worldwide IBD burden. The establishment of central registries within individual countries would be a reasonable first step to overcome the considerable gap of evidence in this region by facilitating the collection of robust and representative data which may help identify aetiological factors and environmental triggers within this unique population. Further education of the primary care sector particularly in identifying risk factors which can be easily and cheaply modified (such as cigarette smoking and breast feeding) as well as differentiating IBD from enteric infection and irritable bowel syndrome will also help decrease morbidity, minimise misdiagnosis and improve patient care. Encouraging utilisation of non-invasive tests such as faecal calprotectin as well as ensuring equitable access to endoscopy will also be beneficial in this regard.
In conclusion, The IBD burden in South America appears to be increasing at a rate greater than other developing regions. Despite this, a paucity of high-quality epidemiological studies continues to exist within this region. The establishment of central registries will help facilitate the collection of robust and representative data to further explore modifiable risk factors and disease phenotypes within this unique population. This information could help facilitate the delivery of high-quality, patient-centred care for South American patients with IBD.
Inflammatory bowel disease (IBD) refers to a chronic inflammatory disorder of the gastrointestinal tract which is thought to arise from an inappropriate inflammatory response to intestinal microbes in a genetically susceptible host. The most common forms of IBD include ulcerative colitis (UC) and Crohn’s disease (CD). Whilst previously regarded predominantly as a disease of western nations, the worldwide epidemiology of IBD rapidly changed at the turn of the twenty-first century with studies demonstrating a plateauing of IBD incidence in western nations whilst IBD incidence and prevalence dramatically rose in developing countries from Asia, Africa and Eastern Europe.
Recent efforts have been made to describe IBD in some developing nations within the South American continent however limited collective data is available from this region. Further collective information about IBD within the South American continent will help provide a valuable insight into the emerging epidemic of this disease with the aim of improving the delivery of high-quality patient-centred care within this region.
To summarise the current literature on Inflammatory Bowel Disease in South America and report the incidence, prevalence and disease characteristics of CD and UC within this continent.
A systematic review using PRISMA guidelines was undertaken by searching published studies in major international and regional databases between January 1990 and December 2018. Outcomes considered were incidence, prevalence, phenotype, environmental and genetic factors, ethnicity and gender. A pair of independent reviewers screened and reviewed all identified articles.
One hundred and sixty two citations were initially retrieved with 18 studies included in this systematic review. The majority of included studies were from Brazil (n = 13, 72%). The incidence of UC ranged from 4.3-5.3/100000 person-years whilst the incidence of CD ranged from 0.74-3.5/100000 person-years. Prevalence ranged from 15.0-24.1/100000 inhabitants for UC and from 2.4-14.1/100000 inhabitants for CD. The incidence and prevalence of both UC and CD has increased significantly over the past 20 years. Pancolitis was the most common disease distribution in patients with UC whilst colonic involvement was the most common distribution in CD. People residing in urban areas were at higher risk of developing both CD and UC.
IBD is an expanding problem within the South American continent with disease burden increasing at a greater rate than other developing regions. Despite this, there remains a paucity of high-queality epidemiological studies from this region. With a total popuation exceeding 400 million, the South American continent is expected to carry a significant proportion of the future global IBD burden.
This represents the first systematic review to examine the epidemiology of Inflammatory Bowel Disease within South America. Given the current scarcity of high-quality IBD epidemiological studies from this region, a concerted and collaborative South American approach is vital. The establishment of central registries within individual countries would be a reasonable first step to overcome the considerable gap of evidence in this region by facilitating the collection of robust, representative and longitudinal data which may help identify aetiological factors, environmental triggers and modifiable risk factors within this unique population. Further education of the primary care sector, particularly in identifying risk factors which can be easily and cheaply modified (such as cigarette smoking and breast feeding) as well as differentiating IBD from enteric infection and Irritable Bowel Syndrome, will also help decrease morbidity, minimise misdiagnosis and improve patient care. This in turn will help facilitate the delivery of high-quality, patient-centred care for South American patients with IBD.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gheita T, Vradelis S S-Editor: Tang JZ L-Editor: A E-Editor: Ma YJ
| 1. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2202] [Article Influence: 137.6] [Reference Citation Analysis (6)] |
| 2. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1561] [Article Influence: 111.5] [Reference Citation Analysis (1)] |
| 3. | de Souza HSP. Etiopathogenesis of inflammatory bowel disease: today and tomorrow. Curr Opin Gastroenterol. 2017;33:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 5. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MNF, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJY, Chan FKL; Asia-Pacific Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 6. | Cui G, Yuan A. A Systematic Review of Epidemiology and Risk Factors Associated With Chinese Inflammatory Bowel Disease. Front Med (Lausanne). 2018;5:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Hou JK, El-Serag H, Thirumurthi S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: a systematic review. Am J Gastroenterol. 2009;104:2100-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Thia KT, Loftus EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167-3182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 408] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 9. | Kaplan GG, Ng SC. Globalisation of inflammatory bowel disease: perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol Hepatol. 2016;1:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 10. | Preferred Reporting Items for Systematic Reviews and Meta-Analyses. PRISMA statement. Available from: http://www.prisma-statement.org. |
| 11. | Victoria CR, Sassak LY, Nunes HR. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol. 2009;46:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Parente JM, Coy CS, Campelo V, Parente MP, Costa LA, da Silva RM, Stephan C, Zeitune JM. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World J Gastroenterol. 2015;21:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 13. | Lima Martins A, Volpato RA, Zago-Gomes MDP. The prevalence and phenotype in Brazilian patients with inflammatory bowel disease. BMC Gastroenterol. 2018;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Barreto I, Diaz F, Marin-Jimenez I. Prevalence and demographic characteristics of inflammatory bowel disease in Cartagena, Colombia. Reviw Col Gastroenterol. 2010;25:106-109. |
| 15. | Buenavida G, Casañias A, Vásquez C, De Souza M, Martínez L, Gardil I, Silveira A, Iade B; National Inflammatory Bowel Disease Registry. Incidence of inflammatory bowel disease in five geographical areas of Uruguay in the biennial 2007-2008. Acta Gastroenterol Latinoam. 2011;41:281-287. [PubMed] |
| 16. | Linares de la Cal JA, Cantón C, Hermida C, Pérez-Miranda M, Maté-Jiménez J. Estimated incidence of inflammatory bowel disease in Argentina and Panama (1987-1993). Rev Esp Enferm Dig. 1999;91:277-286. [PubMed] |
| 17. | Simian D, Fluxá D, Flores L, Lubascher J, Ibáñez P, Figueroa C, Kronberg U, Acuña R, Moreno M, Quera R. Inflammatory bowel disease: A descriptive study of 716 local Chilean patients. World J Gastroenterol. 2016;22:5267-5275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 18. | Salgado VCL, Luiz RR, Boechat N, Schorr BC, Leão IS, Nunes T, Zaltman C. Crohn's disease environmental factors in the developing world: A case-control study in a statewide catchment area in Brazil. World J Gastroenterol. 2017;23:5549-5556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Alvarez-Lobos M, Pizarro DP, Palavecino CE, Espinoza A, Sebastián VP, Alvarado JC, Ibañez P, Quintana C, Díaz O, Kalergis AM, Bueno SM. Role of Salmonella enterica exposure in Chilean Crohn's disease patients. World J Gastroenterol. 2013;19:5855-5862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Queiroz DM, Oliveira AG, Saraiva IE, Rocha GA, Rocha AM, das Graças Pimenta Sanna M, Guerra JB, Dani R, Ferrari Mde L, Castro LP. Immune response and gene polymorphism profiles in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2009;15:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Santos RMD, Carvalho ATP, Silva KDS, Sá SPC, Santos AHD, Sandinha MR. Inflammatory Bowel Disease: Outpatient Treatment Profile. Arq Gastroenterol. 2017;54:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | da Silva BC, Lyra AC, Mendes CM, Ribeiro CP, Lisboa SR, de Souza MT, Portela RC, Santana GO. The Demographic and Clinical Characteristics of Ulcerative Colitis in a Northeast Brazilian Population. Biomed Res Int. 2015;2015:359130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Santana GO, Lyra LG, Santana TC, Dos Reis LB, Guedes JC, Toralles MB, Lyra AC. Crohn's disease in one mixed-race population in Brazil. World J Gastroenterol. 2007;13:4489-4492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Torres Udos S, Rodrigues JO, Junqueira MS, Uezato S, Netinho JG. The Montreal classification for Crohn's disease: clinical application to a Brazilian single-center cohort of 90 consecutive patients. Arq Gastroenterol. 2010;47:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Cohen D, Bin CM, Fayh AP. Assessment of quality of life of patients with inflammatory bowel disease residing in Southern Brazil. Arq Gastroenterol. 2010;47:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Hardt MR KP, Teixeira FV, Ludvig JC, Malluta EF, Kleinubing Jr H, Miranda EF, Tonini WB, Olandoski M, Kotze LMS, Coy CSR. Epidemiological profile of 175 patients with Crohn's disease submitted to biological therapy. Journal of Coloproctology. 2012;32:395-401 [DOI 10.1590/S2237-93632012000400006]. |
| 27. | Santana GO, Souza LR, Azevedo M, Sá AC, Bastos CM, Lyra AC. Application of the Vienna classification for Crohn's disease to a single center from Brazil. Arq Gastroenterol. 2008;45:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | de Barros PAC dSA, Neto ML. The epidemiological profile of inflammatory bowel disease patients on biologic therapy at a public hospital in Alagoas. Journal of Coloproctology. 2014;34:131-135. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Zwi AB, Mills A. Health policy in less developed countries: past trends and future directions. J Int Dev. 1995;7:299-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Bernstein CN, Rawsthorne P, Cheang M, Blanchard JF. A population-based case control study of potential risk factors for IBD. Am J Gastroenterol. 2006;101:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Leong RW, Mitrev N, Ko Y. Hygiene Hypothesis: Is the Evidence the Same All Over the World? Dig Dis. 2016;34(1-2):35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Jakobsen C, Paerregaard A, Munkholm P, Wewer V. Environmental factors and risk of developing paediatric inflammatory bowel disease -- a population based study 2007-2009. J Crohns Colitis. 2013;7:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |