Published online Dec 21, 2019. doi: 10.3748/wjg.v25.i47.6835
Peer-review started: October 16, 2019
First decision: November 10, 2019
Revised: December 4, 2019
Accepted: December 14, 2019
Article in press: December 14, 2019
Published online: December 21, 2019
Processing time: 64 Days and 22.6 Hours
The management of proximal esophageal cancer differs from that of tumors located in the mid and lower part of the esophagus due to the close vicinity of vital structures. Non-surgical treatment options like radiotherapy and definitive chemoradiation (CRT) have been implemented. The trends in (non-)surgical treatment and its impact on overall survival (OS) in patients with proximal esophageal cancer are unclear, related to its rare disease status. To optimize treatment strategies and counseling of patients with proximal esophageal cancer, it is therefore essential to gain more insight through real-life studies.
To establish trends in treatment and OS in patients with proximal esophageal cancer.
In this population-based study, patients with proximal esophageal cancer diagnosed between 1989 and 2014 were identified in the Netherlands Cancer Registry. The proximal esophagus consists of the cervical esophagus and the upper thoracic section, extending to 24 cm from the incisors. Trends in radiotherapy, chemotherapy, and surgery, and OS were assessed. Analyses were stratified by presence of distant metastasis. Multivariable Cox proportional hazards regression analyses was performed to assess the effect of period of diagnosis on OS, adjusted for patient, tumor, and treatment characteristics.
In total, 2783 patients were included. Over the study period, the use of radiotherapy, resection, and CRT in non-metastatic disease changed from 53%, 23%, and 1% in 1989-1994 to 21%, 9%, and 49% in 2010-2014, respectively. In metastatic disease, the use of chemotherapy and radiotherapy increased over time. Median OS of the total population increased from 7.3 mo [95% confidence interval (CI): 6.4-8.1] in 1989-1994 to 9.5 mo (95%CI: 8.1-10.8) in 2010-2014 (logrank P < 0.001). In non-metastatic disease, 5-year OS rates improved from 5% (95%CI: 3%-7%) in 1989-1994 to 13% (95%CI: 9%-17%) in 2010-2014 (logrank P < 0.001). Multivariable regression analysis demonstrated a significant treatment effect over time on survival. In metastatic disease, median OS was 3.8 mo (95%CI: 2.5-5.1) in 1989-1994, and 5.1 mo (95%CI: 4.3-5.9) in 2010-2014 (logrank P = 0.26).
OS significantly improved in non-metastatic proximal esophageal cancer, likely to be associated with an increased use of CRT. Patterns in metastatic disease did not change significantly over time.
Core tip: Proximal esophageal cancer is a rare disease, accounting for only 10% of all esophageal cancer cases. Limited data on treatment and survival in this rare tumor have been published, restricting patient counseling. The present investigation is the largest population-based cohort study evaluating trends in treatment and survival in proximal esophageal cancer. This study represents daily clinical practice, showing improvement in overall survival in patients with non-metastatic proximal esophageal cancer, with a shift to non-surgical treatment.
- Citation: de Vos-Geelen J, Geurts SM, van Putten M, Valkenburg-van Iersel LB, Grabsch HI, Haj Mohammad N, Hoebers FJ, Hoge CV, Jeene PM, de Jong EJ, van Laarhoven HW, Rozema T, Slingerland M, Tjan-Heijnen VC, Nieuwenhuijzen GA, Lemmens VE. Trends in treatment and overall survival among patients with proximal esophageal cancer. World J Gastroenterol 2019; 25(47): 6835-6846
- URL: https://www.wjgnet.com/1007-9327/full/v25/i47/6835.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i47.6835
Esophageal cancer is the seventh most common cancer worldwide[1]. Although the absolute number of deaths has decreased, esophageal cancer is still the sixth leading cause of cancer-related mortality globally[1]. Surgical treatment of patients with esophageal cancer, and in particular treatment of cancer located in the proximal part of the esophagus, is challenging because of the close proximity to vital structures. The proximal part of the esophagus consists of the cervical and the upper thoracic segment. Proximal esophageal cancer is relatively uncommon, accounting for 10% of all esophageal cancer cases[2].
The management of proximal esophageal cancer differs from that of tumors located in the mid and lower part of the esophagus. Patients with proximal esophageal cancer often present with locally advanced disease, for which potentially curative surgery would require extensive mutilating resections, with a high risk of major complications and a significant impact on patients quality of life. To prolong survival and improve quality of life, non-surgical treatment options like radiotherapy and definitive chemoradiation (CRT) have been explored since the 1990s, following promising treatment results of cancers in the thoracic esophagus, hypopharynx, and non-small-cell lung cancer[3-6]. In a meta-analysis in 2006, Wong et al. showed that the addition of chemotherapy to radiotherapy for the definitive treatment of esophageal cancer significantly increased response and overall survival (OS) rates[7].
Therefore, definitive CRT is recommended as treatment modality for patients with non-metastatic proximal esophageal cancer[8,9]. However, only four of the 19 studies in the aforementioned meta-analysis incorporated patients with proximal esophageal cancers, limiting the extrapolation of these findings to the proximal esophagus.
Separate OS rates for patients with proximal esophageal cancer are largely lacking from clinical trials, due to exclusion of this subpopulation or related to its rare disease status. To optimize treatment strategies and counseling of patients with proximal esophageal cancer, it is therefore essential to gain more insight in patient characteristics, provided therapies and OS through real-life studies.
The aim of this population-based cohort study was to establish the trends in treatment and OS in patients diagnosed with non-metastatic or metastatic proximal esophageal cancer in a nationwide registry between 1989 and 2014.
All patients with a tumor located in the cervical or upper thoracic esophagus diagnosed between 1989 and 2014 were identified in the Netherlands Cancer Registry (NCR). The NCR is a population-based cancer registry of all residents of the Netherlands. The NCR is linked to the national automated pathological archive, which leads to the automatic inclusion of all newly diagnosed malignancies in the Netherlands. Additional data sources linked to the NCR are the national hospital discharge register and registers of radiotherapy institutions. Information on vital status was obtained through annual linkage with the Municipal Administrative Database, in which all deceased or emigrated individuals in the Netherlands are registered. This study was approved by the Privacy Review Board of the NCR and the need for a separate approval from an ethics committee in the Netherlands was waived.
Topography and histology were coded according to the International Classification of Diseases for Oncology (ICD-O)[10]. ICD-O histology codes were used to classify tumors as squamous cell carcinoma (SCC), adenocarcinoma, and other origin. Cancers of the proximal esophagus can be subdivided in cancers originating in the cervical esophagus (CEC, ICD-O C15.0), commencing at the lower border of the cricoid cartilage and ending at the thoracic inlet, approximately 18 cm from the incisors, and cancers in the upper thoracic section (UTEC, ICD-O C15.3), extending from the thoracic inlet to the level of the tracheal bifurcation, which is approximately 24 cm from the incisors[11].
Tumor staging was registered according to the Union for International Cancer Control TNM classification that was valid at the time of diagnosis. As the classification of tumor stage (cT) was reasonably comparable from the TNM-4 to -6, but changed with the introduction of the 7th edition in 2010, we converted all tumor and lymph node stages according to TNM-6th edition. Patients with a cM1a tumor according to TNM-6th edition, defined as cervical lymph node involvement, were categorized as having a positive lymph node status (cN+). Patients with unknown metastatic status (cMx) were included in the non-metastatic group.
All treatments for the primary disease stage were registered. Treatment categories included resection, neoadjuvant treatment and resection, radiotherapy, chemotherapy, radiotherapy and chemotherapy, other treatment, and no (anti-cancer) treatment. Resection included patients who received a surgical resection or an endoscopic excision (n = 20). The group of “neoadjuvant and resection” comprised patients who underwent a resection, preceded by radiotherapy, chemotherapy or with concurrent CRT. The group “radiotherapy and chemotherapy” included patients who were treated with sequential or concurrent radiotherapy and chemotherapy, without any resection. Other treatments were not otherwise specified (palliative) treatments. “Other treatment” and “no (anti-cancer) treatment” were summarized as “no localized treatment”. Type of surgical treatment and details on chemotherapy or radiotherapy were not collected by the data clerks of the NCR.
Five-year periods of diagnosis were defined: 1989-1994, 1995-1999, 2000-2004, 2005-2009, and 2010-2014.
OS was calculated by period of diagnosis using the Kaplan-Meier method and a comparison between groups was made using the log-rank test. OS was defined as the time from diagnosis to death from any cause, censored at last follow-up date or untill February 1, 2017. The median follow-up time was calculated using the reverse Kaplan Meier method (death censored). Multivariable Cox proportional hazards regression analyses were performed to assess the effect of period of diagnosis on OS, adjusted for age, histological type, tumor location, cT category, cN category, and treatment modality. Variance inflation factors were calculated to assess the degree of multicollinearity among the independent variables in the Cox proportional hazard model. Analyses were stratified by the presence of metastatic disease (cM0 vs cM1), tumor location (CEC vs UTEC), and histological type (SCC vs adenocarcinoma). As the interaction analysis did not show any difference in OS between tumor location, i.e., cervical or upper thoracic site, and histology, results are presented by presence or absence of metastatic disease.
The statistical review of the study was performed by two senior epidemiologists.
We identified 2783 patients diagnosed with proximal esophageal cancer in the Netherlands between 1989 and 2014 (Table 1). The median follow-up time of all patients was 103 mo [95% confidence interval (CI): 91-117 mo]. Fifty-six percent of patients were male, and 47% were between 60 and 74 years old at the time of diagnosis. In total, 81% of cancers were SCC. Two percent of the patients were diagnosed with clinical stage 1, 20% with stage 2, 28% with stage 3, 21% with stage 4, and 29% with unknown stage disease. The number of patients with unknown stage disease decreased over time. In 2010-2014, 27% of patients had been diagnosed with another malignancy prior to the diagnosis of proximal esophageal cancer (data not shown).
| Charactistics | Total (n = 2783) | 1989-1994 (n = 484) | 1995-1999 (n = 499) | 2000-2004 (n = 552) | 2005-2009 (n = 583) | 2010-2014 (n = 665) |
| Sex | ||||||
| Male | 1562 (56) | 259 (54) | 263 (53) | 308 (56) | 344 (59) | 388 (58) |
| Female | 1221 (44) | 225 (46) | 236 (47) | 244 (44) | 239 (41) | 277 (42) |
| Age (yr) | ||||||
| < 60 | 725 (26) | 140 (29) | 148 (30) | 178 (32) | 128 (22) | 131 (20) |
| 60-74 | 1304 (47) | 194 (40) | 219 (44) | 223 (40) | 301 (52) | 367 (55) |
| ≥ 75 | 754 (27) | 150 (31) | 132 (26) | 151 (27) | 154 (26) | 167 (25) |
| Histology | ||||||
| SCC | 2248 (81) | 382 (79) | 390(78) | 440 (80) | 480 (82) | 556 (84) |
| Adenocarcino-ma | 320 (11) | 62 (13) | 63 (13) | 70 (13) | 61 (10) | 64 (10) |
| Other | 215 (8) | 40 (8) | 46 (9) | 42 (8) | 42 (7) | 45 (7) |
| Tumor location | ||||||
| CEC | 648 (23) | 138 (29) | 138 (28) | 154 (28) | 126 (22) | 92 (14) |
| UTEC | 2135 (77) | 346 (71) | 361 (72) | 398 (72) | 457 (78) | 573 (86) |
| cT classification | ||||||
| cT1 | 81 (3) | 17 (4) | 16 (3) | 12 (2) | 16 (3) | 20 (3) |
| cT2 | 236 (8) | 12 (2) | 16 (3) | 36 (7) | 48 (8) | 124 (19) |
| cT3 | 447 (16) | 36 (7) | 39 (8) | 79 (14) | 109 (19) | 184 (28) |
| cT4 | 665 (24) | 115 (24) | 123 (25) | 161 (29) | 147 (25) | 119 (18) |
| cTx | 1354 (49) | 304 (63) | 305 (61) | 264 (48) | 263 (45) | 218 (33) |
| cN classification | ||||||
| cN0 | 892 (32) | 172 (36) | 173 (35) | 189 (34) | 157 (27) | 201 (30) |
| cN+ | 1193 (43) | 119 (25) | 158 (32) | 208 (38) | 313 (54) | 395 (59) |
| cNx | 698 (25) | 193 (40) | 168 (34) | 155 (28) | 113 (19) | 69 (10) |
| cM classification | ||||||
| cM0 | 1752 (63) | 311 (64) | 314 (63) | 316 (57) | 344 (59) | 467 (70) |
| cM1 | 589 (21) | 79 (16) | 88 (18) | 96 (17) | 135 (23) | 191 (29) |
| cMx | 442 (16) | 94 (19) | 97 (19) | 140 (25) | 104 (18) | 7 (1) |
| TNM stage | ||||||
| 1 | 64 (2) | 14 (3) | 14 (3) | 9 (2) | 14 (2) | 13 (2) |
| 2 | 565 (20) | 80 (17) | 72 (14) | 100 (18) | 125 (22) | 188 (28) |
| 3 | 763 (27) | 102 (21) | 126 (25) | 173 (31) | 174 (30) | 188 (28) |
| 4 | 589 (21) | 79 (16) | 88 (18) | 96 (17) | 135 (23) | 191 (29) |
| Unknown | 802 (29) | 209 (43) | 199 (40) | 174 (32) | 135 (23) | 85 (13) |
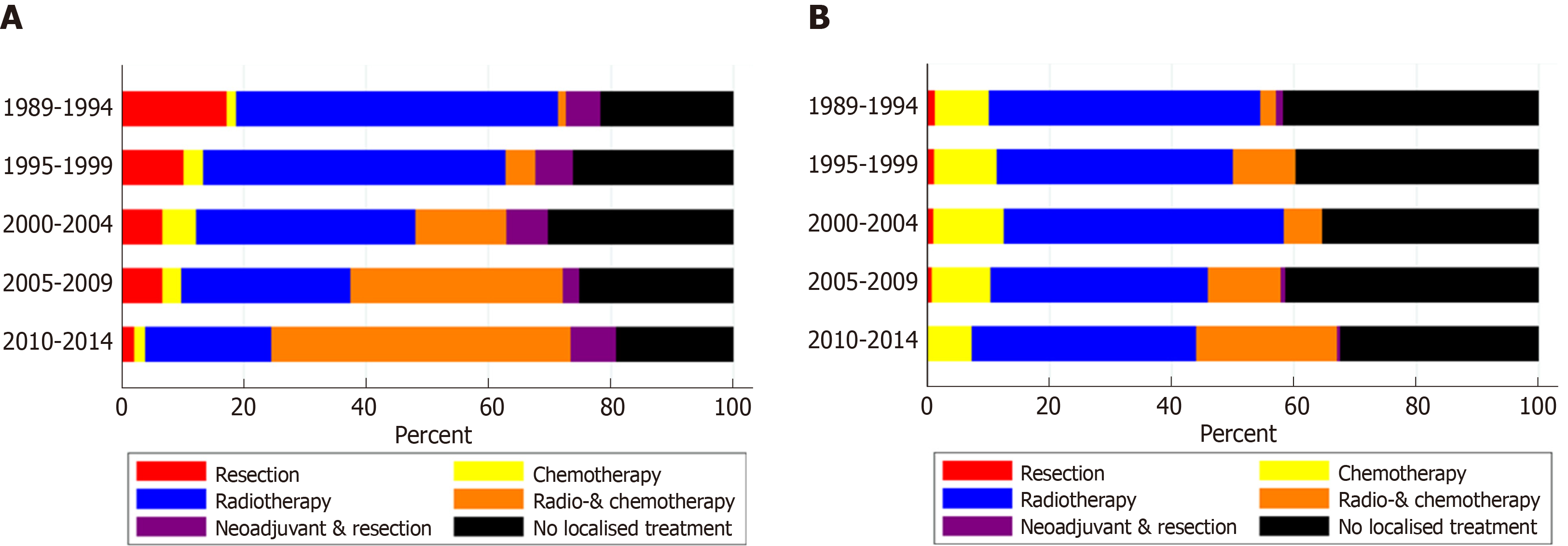
In patients with non-metastatic disease, the proportion of patients treated with CRT alone increased from 1% in 1989-1994 to 49% in 2010-2014 (Figure 1A). Resection without neoadjuvant treatment was performed in 17% of patients in 1989-1994 and in 2% of patients in 2010-2014. The proportion of patients treated with neoadjuvant therapy and resection was relatively constant over time, varying between 3% and 7%. The proportion of patients with non-metastatic proximal esophageal cancer that did not undergo any form of treatment varied between 15% and 22%, without a clear trend over time.
For patients with metastatic disease, only minor variations in treatment were observed (Figure 1B). Fourty-four percent of patients were treated with radiotherapy alone in 1989-1994, which slightly decreased to 37% in 2010-2014. Over time, multimodal treatment of chemotherapy and radiotherapy, concurrent or sequential, was administered more frequently: In 3% of patients in 1989-1994 and 23% of patients in 2010-2014. Chemotherapy alone was given to 7%-12% of patients in all time periods. The proportion of patients diagnosed with metastatic proximal esophageal cancer who did not undergo any form of anti-cancer treatment decreased from 33% in 1989-2004 to 24% in 2010-2014.
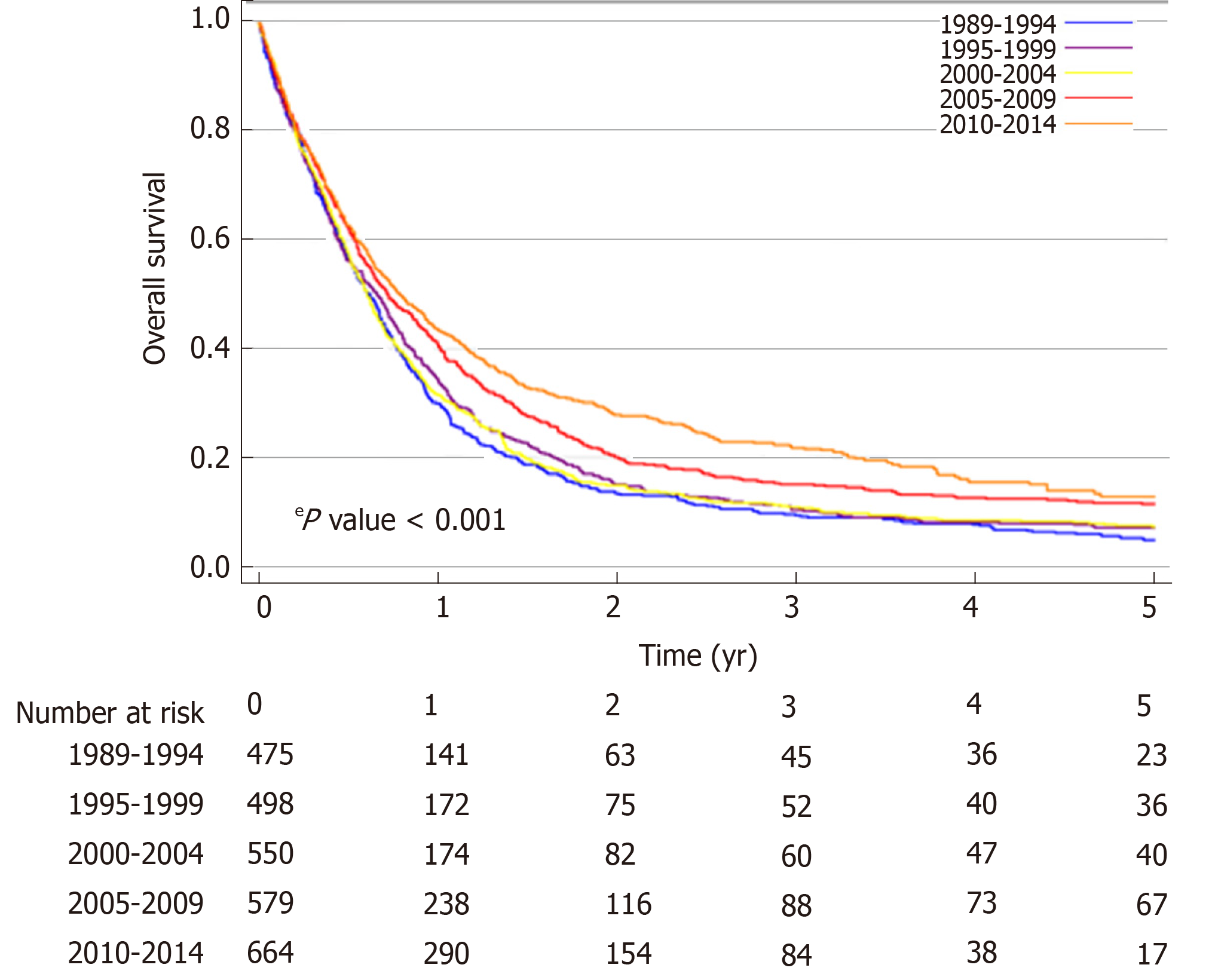
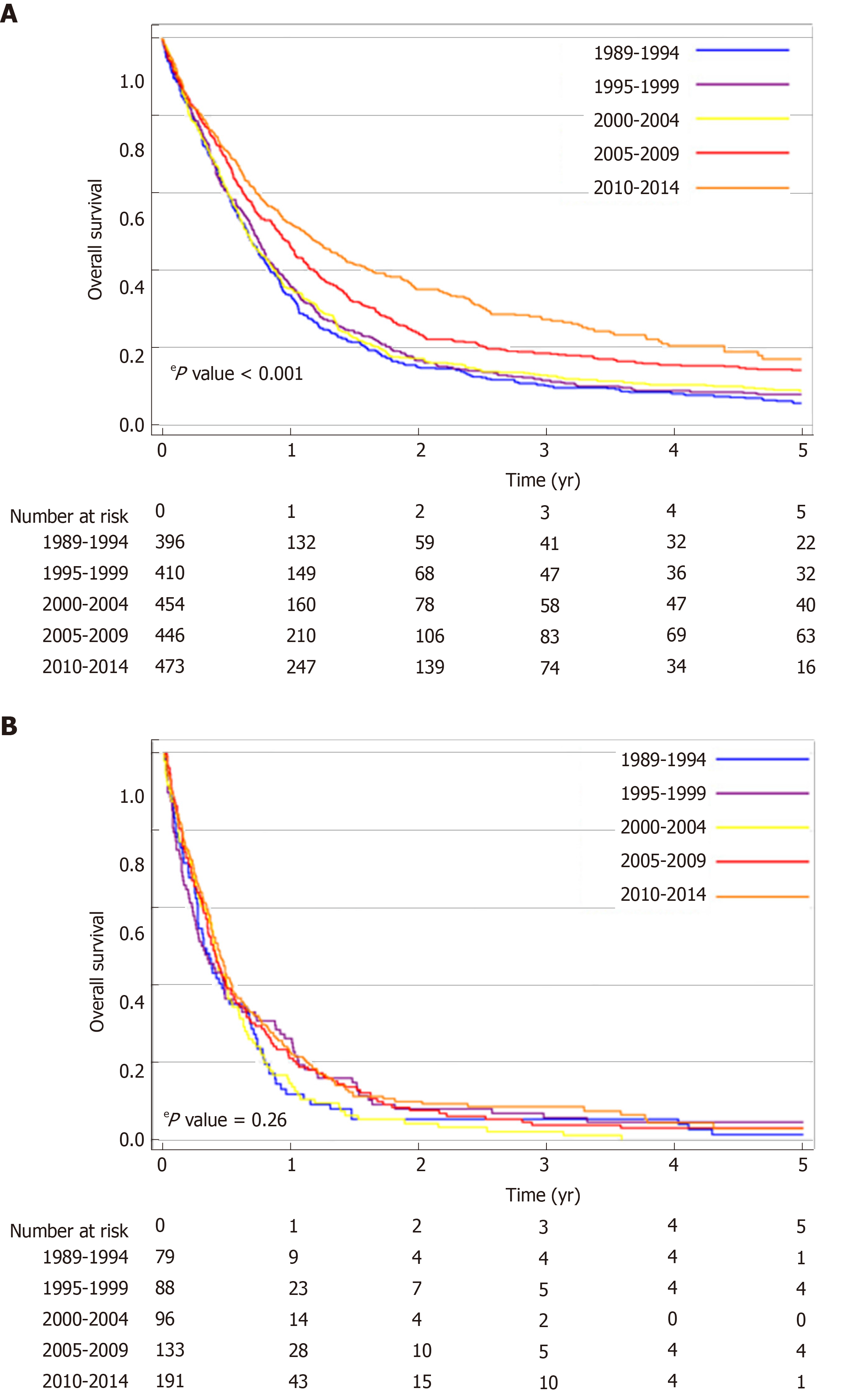
The median OS of the total population of patients with proximal esophageal cancer was 8.0 mo (95%CI: 7.6-8.5 mo). Median OS increased over the study period, from 7.3 mo (95%CI: 6.4-8.1 mo) in 1989-1994, to 9.5 mo (95%CI: 8.1-10.8 mo) in 2010-2014 (logrank P < 0.001) (Figure 2). In patients with non-metastatic proximal esophageal cancer, 1- and 5-year OS rates improved from 30% (95%CI: 26%-34%) and 5% (95%CI: 3%-7%) in 1989-1994, to 44% (95%CI: 40%-48%) and 13% (95%CI: 9%-17%) in 2010-2014, respectively (logrank P < 0.001) (Figure 3A). Median OS of patients with non-metastatic proximal esophageal cancer was 8.0 mo (95%CI: 7.0-8.9 mo) in 1989-1994 and 13.3 mo (95%CI: 11.1-15.5 mo) in 2010-2014. Patients with stage 1 disease showed the most favorable outcome with a 1- and 5-year OS rate of 70% (95%CI: 57%-80%) and 22% (95%CI 13%-34%), compared with 50% (95%CI: 46%-54%) and 15% (95%CI: 12%-18%) in stage 2, and 35% (95%CI: 32%-38%) and 10% (95%CI: 8%-13%) in stage 3 disease, respectively (logrank P < 0.001) (Supplementary Figure 1).
In patients with non-metastatic proximal esophageal cancer, univariable analysis showed that period of diagnosis, age, histological type, cT, cN, and treatment were all associated with OS (Table 2). OS was similar for patients diagnosed with CEC or UTEC. Multivariable Cox regression analysis adjusted for age, histological type, tumor location, cT, and cN demonstrated an OS benefit for patients diagnosed in 2005-2009 [Hazard ratio (HR) = 0.77, P < 0.001] or 2010-2014 (HR = 0.72, P < 0.001) when compared with patients diagnosed in 1989-1994. However, the time period effect disappeared after additional inclusion of treatment modality in the multivariable model. All treatment modalities had a statistically significant effect on OS compared with no localized treatment (P < 0.001). Patients with non-metastatic proximal esophageal cancer treated with surgery with or without neoadjuvant therapy or treated with definitive CRT showed 5-year OS rates of 31% (95%CI: 23%-40%), 21% (95%CI: 16%-28%), and 22% (95%CI: 19%-26%), respectively (logrank P = 0.32) (Supplementary Figure 2).
| Charactistics | n | Univariable analysis | Multivariable analysis | Multivariable analysis1 | |||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| Period | |||||||
| 1989-1994 | 405 | Ref. | Ref. | Ref. | |||
| 1995-1999 | 411 | 0.92 (0.80-1.05) | 0.21 | 0.91 (0.79-1.05) | 0.18 | 0.85 (0.74-0.98) | 0.03 |
| 2000-2004 | 456 | 0.92 (0.81-1.06) | 0.24 | 0.97 (0.85-1.12) | 0.71 | 0.94 (0.82-1.08) | 0.39 |
| 2005-2009 | 448 | 0.73 (0.63-0.83) | < 0.001 | 0.77 (0.67-0.89) | < 0.001 | 0.88 (0.76-1.02) | 0.09 |
| 2010-2014 | 474 | 0.59 (0.51-0.68) | < 0.001 | 0.72 (0.62-0.85) | < 0.001 | 0.94 (0.79-1.10) | 0.43 |
| Age | |||||||
| < 60 yr | 562 | Ref. | Ref. | Ref. | |||
| 60-74 yr | 1002 | 1.06 (0.95-1.18) | 0.30 | 1.11 (0.99-1.23) | 0.08 | 0.97 (0.86-1.08) | 0.53 |
| ≥ 75 yr | 630 | 1.50 (1.33-1.69) | < 0.001 | 1.51 (1.34-1.71) | < 0.001 | 1.00 (0.87-1.14) | 0.95 |
| Histology | |||||||
| SCC | 1797 | Ref. | Ref. | Ref. | |||
| AC | 242 | 1.07 (0.93-1.23) | 0.37 | 0.97 (0.84-1.12) | 0.64 | 0.88 (0.76-1.02) | 0.09 |
| Other | 155 | 1.47 (1.24-1.74) | < 0.001 | 1.22 (1.03-1.44) | 0.02 | 1.11 (0.93-1.31) | 0.25 |
| Tumor location | |||||||
| UTEC | 1672 | Ref. | Ref. | Ref. | |||
| CEC | 522 | 0.97 (0.88-1.08) | 0.59 | 0.89 (0.80-0.98) | 0.02 | 0.95 (0.86-1.06) | 0.37 |
| cT category | |||||||
| cT1-3 | 642 | Ref. | Ref. | Ref. | |||
| cT4 | 506 | 2.03 (1.79-2.30) | < 0.001 | 1.93 (1.69-2.19) | < 0.001 | 1.62 (1.42-1.85) | < 0.001 |
| cTx | 1046 | 1.75 (1.57-1.94) | < 0.001 | 1.50 (1.33-1.69) | < 0.001 | 1.25 (1.11-1.41) | < 0.001 |
| cN category | |||||||
| cN0 | 825 | Ref. | Ref. | Ref. | |||
| cN+ | 811 | 1.29 (1.16-1.43) | < 0.001 | 1.44 (1.29-1.60) | < 0.001 | 1.35 (1.21-1.50) | < 0.001 |
| cNx | 558 | 2.06 (1.84-2.30) | < 0.001 | 1.78 (1.59-2.00) | < 0.001 | 1.37 (1.22-1.55) | < 0.001 |
| Treatment | |||||||
| No localized treatment | 538 | Ref. | Ref. | ||||
| Resection | 183 | 0.19 (0.16-0.22) | < 0.001 | 0.22 (0.18-0.26) | < 0.001 | ||
| Neoadjuvant and resection | 126 | 0.15 (0.12-0.18) | < 0.001 | 0.17 (0.13-0.21) | < 0.001 | ||
| Radio- and chemotherapy | 480 | 0.17 (0.14-0.19) | < 0.001 | 0.19 (0.16-0.22) | < 0.001 | ||
| Chemotherapy | 67 | 0.38 (0.29-0.49) | < 0.001 | 0.39 (0.30-0.50) | < 0.001 | ||
| Radiotherapy | 800 | 0.38 (0.34-0.42) | < 0.001 | 0.40 (0.36-0.46) | < 0.001 | ||
In patients with metastatic disease, OS did not change significantly over time (logrank P = 0.26) (Figure 3B). Median OS was 3.8 mo (95%CI: 2.5-5.1 mo) in 1989-1994 and 5.1 mo (95%CI: 4.3-5.9 mo) in 2010-2014. One-year OS rate was 12% (95%CI: 6%-20%) in 1989-1994 and 23% (95%CI: 17%-29%) in 2010-2014.
In the Netherlands, median OS of patients with proximal esophageal cancer significantly increased by approximately two mo between 1989 and 2014. In patients with non-metastatic proximal esophageal cancer, 5-year OS almost tripled to 13% in 2010-2014, although the absolute longterm outcome remains poor. Multivariable analysis showed that improvements in treatment over time might have led to this survival benefit. The improvement is likely to be attributable to the implementation of CRT in the late nineties, accounting for almost 50% of treatment choices in non-metastatic proximal esophageal cancer nowadays. The proportion of patients who did not receive any anti-cancer treatment remained remarkably high, being one in five patients with non-metastatic and one in four patients with metastatic proximal esophageal cancer, which may be a reflection of the poor performance status of these patients.
We observed that in the patients with non-metastatic proximal esophageal cancer (n = 2194), the median OS improved from 8 mo in 1989-1994 to 13 mo in 2010-2014, with comparable OS between CEC and UTEC. Considering OS in patients with metastatic disease did not improve significantly over time, stage migration was not expected to be a major contributor to the improved survival in the non-metastatic group. A Surveillance, Epidemiology, and End Results (SEER) data-based study in 362 patients with non-metastatic CEC diagnosed between 1998 and 2008 showed a longer median OS, i.e., 14 mo[12]. The shorter median survival observed in our study may partly be explained by the inclusion of patients with a history of previous malignancies, whereas the SEER data-based study excluded these patients. In addition, we included patients with unknown metastatic status in the group of patients with non-metastatic disease, which could have lead to an underestimation of the OS in the non-metastatic patient group.
Our study showed a reduction of surgical approaches from 23% in the earliest time period to 10% in the most recent period. The aforementioned SEER population-based study showed similar results, where only 11% of patients with cervical esophageal cancer underwent surgery and 79% radiotherapy (chemotherapy data were not available)[12]. These findings confirm a different approach in the management of proximal esophageal cancer in specific as compared with cancers from all sites of the esophagus. In the latter group the proportion of patients treated with surgery remained relatively stable over time, from 25% between 1989 and 2004, to 29% between 2010 and 2014[2].
Considering bias by indication, we hypothesized that patients with resectable tumors, undergoing surgery, might show a superior outcome when compared with CRT. However, in the current population-based study, we observed a comparable OS in patients treated with surgery vs those treated with definitive CRT which is consistent with a recent observational study in 148 patients with cervical esophageal cancer[13]. The current study showed that period effect in the multivariable model disapeared after including treatment modality. These findings suggest that improvements in the (non-surgical) treatment had a substantial effect on the observed improvement in OS. However progress in OS may also have partly occurred due to advancements in the management of non-cancer related high mortality disorders, e.g., cardiovascular disease[14]. Figures from Statistics Netherlands show that the remaining life expectancy for, for example, an average 65 year old person was 17 years in 1989 and 20 years in 2014[15]. Whether this increase in life expectancy is also seen in the high-risk population presented in our study is unknown.
In patients with metastatic proximal esophageal cancer, we did not observe any significant improvements in OS over time. These findings are in contrast to previous population-based studies, observing an increased survival over the years in the total group of patients with metastatic esophageal cancer patients, including 10% of cancers originating from the proximal esophagus[16,17]. This difference in the trend in OS may be explained by the more prominent increased use of systemic therapy in metastatic adenocarcinomas[2], which are more common in the distal part of the esophagus[18]. For example, in patients with HER2 amplified adenocarcinomas of the distal esophagus, HER2 directed therapies have led to a survival benefit[19]. In metastatic SCC, palliative systemic therapy is scarcely applied[2]. A recent meta-analysis, however, showed that systemic therapy in patients with metastatic SCC improved OS and quality of life, and is considered standard of care[20]. The outcomes of patients with metastatic SCC is expected to improve in the coming decades, because the pace of development of cancer immunotherapies is accelerating. Recent studies show clinical evidence of efficacy of immune checkpoint inhibitors in SCC of the esophagus[21,22], and are expected to be approved for implementation in clinical practice.
Furthermore, since proximal esophageal cancer is extremely rare, development of high-volume expert centers is challenging. Centralization of surgery in esophageal cancer has led to an increased survival in resectable esophageal cancer[23]. A recent Dutch study showed that center volume of palliative systemic therapy for metastatic esophagogastric cancer was associated with improved survival, suggesting a volume-outcome relationship[24]. Giving the low incidence rate and the challenging performance status of these patients, this could be a plea for centralization of care for patients with proximal esophageal cancer.
The retrospective nature of this study is inherent with some limitations mainly attributable to the availability of information. Coding of the tumor was being performed on the basis of topography, extracted from the medical records depending on input of physicians and interpretation of administrators, posing a risk of misclassification. The NCR does not include information on treatment techniques, schedules, and its related toxicities, causing interpretation adversity. Furthermore, data regarding risk factors, e.g., smoking behaviour and alcohol consumption, comorbidity, performance status, and disease specific cause of death were not available, resulting in a risk of residual confounding. However, our multivariable model showed that the period effect almost completely dissapeared after including treatment modalities to the multivariable model, implicating that there are no major confounders missing.
The strength of our study is that it is a large population-based cohort. This nationwide cohort of patients with proximal esophageal cancer in the Netherlands represents daily clinical practice, reflecting real-life treatment and survival. Moreover, the follow-up period can be considered long, given the relatively short survival time of patients with proximal esophageal cancer.
In conclusion, this nationwide study in patients with proximal esophageal cancer showed an increasing use of definitive CRT over the study period, with improved survival in non-metastatic disease, although long-term result is still rather poor.
Proximal esophageal cancer is a rare disease, accounting for only 10% of all esophageal cancers. Nearby vital structures are involved in almost all proximal esophageal cancers at diagnosis, and as such surgical treatment is mutilating with major implications for quality of life of patients. Definitive chemoradiation (CRT) is an alternative treatment option, but survival data are scarce, restricting patient counseling.
To optimize treatment strategies and counseling of patients with proximal esophageal cancer, it is therefore essential to gain more insight in patient characteristics, provided therapies and outcome through real-life studies.
The aim of this population-based cohort study was to establish the trends in treatment and overall survival (OS) in patients diagnosed with non-metastatic or metastatic proximal esophageal cancer in a nationwide registry between 1989 and 2014.
All patients with a tumor located in the cervical or upper thoracic esophagus diagnosed between 1989 and 2014 were identified in the Netherlands Cancer Registry (NCR). The NCR is a population-based cancer registry of all residents of the Netherlands. Trends in radiotherapy, chemotherapy, and surgery, and OS were assessed. Analyses were stratified by presence of distant metastasis. Multivariable Cox proportional hazards regression analyses was performed to assess the effect f period of diagnosis on OS, adjusted for adjust for patient, tumor, and treatment characteristics.
Median OS of patients with proximal esophageal cancer significantly increased by approximately two mo between 1989 and 2014. In patients with non-metastatic proximal esophageal cancer, 5-year OS almost tripled to 13% in 2010-2014, although the absolute longterm outcome remains poor. Multivariable analysis showed that improvements in treatment over time have led to this survival benefit. The improvement is likely to be attributable to the implementation of CRT in the late nineties, accounting for almost 50% of treatment choices in non-metastatic proximal esophageal cancer nowadays, as shown in the current study. In metastatic disease, median OS did not change significantly between 1989 and 2014.
Surgical treatment for proximal esophageal cancer has been substituted by definitive CRT in the more recent years, and was likely to be associated with significant survival improvement of patients with non-metastatic proximal esophageal cancer. (Long-term) survival data of patients with (non-)metastatic proximal esophageal cancer are provided from a large national database, representing daily clinical practice.
Our findings give insights in real-life survival of patients with proximal esophageal cancer, providing crucial support for patient counseling. Future research should focuss on outcome between different CRT regimens, to optimize non-surgical treatment.
The authors would like to thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Netherlands
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hu B, Sanaei O, Shi H S-Editor: Tang JZ L-Editor: A E-Editor: Ma YJ
| 1. | Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 27 Feb 2019. Available from: https://gco.iarc.fr/today. |
| 2. | van Putten M, de Vos-Geelen J, Nieuwenhuijzen GAP, Siersema PD, Lemmens VEPP, Rosman C, van der Sangen MJC, Verhoeven RHA. Long-term survival improvement in oesophageal cancer in the Netherlands. Eur J Cancer. 2018;94:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1261] [Cited by in RCA: 1372] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 4. | al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle JS, Vaitkevicius VK, Cooper J, Byhardt R, Davis L, Emami B. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 464] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Pignon JP, Bourhis J, Domenge C, Designé L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1409] [Cited by in RCA: 1200] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 6. | Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A meta-analysis. Ann Intern Med. 1996;125:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 192] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Wong R, Malthaner R. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev. 2006;CD002092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D; ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50-v57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 665] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 9. | Ajani JA, D'Amico TA, Baggstrom M, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Leong S, Linn C, Ly QP, Matkowskyj KA, Mulcahy MF, Paluri RK, Perry KA, Pimiento J, Poultsides GA, Strong VE, Washingon MK, Weksler B, Wiesner G, Willett CG, Wright CD, Gurski L, McMillian N, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2018; 2018 [cited 2019 Dec 4]. Database: National Comprehensive Cancer Network [Internet]. Available from: https://www.nccn.org. |
| 10. | Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization. 2000;. |
| 11. | Greene FL, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual, 6th ed. New York: Springer-Verlag. AJCC Cancer Staging Manual, 6th ed. New York: Springer-Verlag 2002; 91-99. [DOI] [Full Text] |
| 12. | Grass GD, Cooper SL, Armeson K, Garrett-Mayer E, Sharma A. Cervical esophageal cancer: a population-based study. Head Neck. 2015;37:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Valmasoni M, Pierobon ES, Zanchettin G, Briscolini D, Moletta L, Ruol A, Salvador R, Merigliano S. Cervical Esophageal Cancer Treatment Strategies: A Cohort Study Appraising the Debated Role of Surgery. Ann Surg Oncol. 2018;25:2747-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5338] [Cited by in RCA: 4902] [Article Influence: 700.3] [Reference Citation Analysis (1)] |
| 15. | Statistics Netherlands. 2019 May 17 [cited 14 Nov 2019]. Available from: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/37360ned/table?ts=1573737810723. |
| 16. | Bernards N, Haj Mohammad N, Creemers GJ, Rozema T, Roukema JA, Nieuwenhuijzen GA, van Laarhoven HW, van der Sangen M, Lemmens VE. Improvement in survival for patients with synchronous metastatic esophageal cancer in the south of the Netherlands from 1994 to 2013. Acta Oncol. 2016;55:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Dikken JL, Lemmens VE, Wouters MW, Wijnhoven BP, Siersema PD, Nieuwenhuijzen GA, van Sandick JW, Cats A, Verheij M, Coebergh JW, van de Velde CJ. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur J Cancer. 2012;48:1624-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, Cunningham D. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 737] [Article Influence: 92.1] [Reference Citation Analysis (2)] |
| 19. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5324] [Article Influence: 354.9] [Reference Citation Analysis (3)] |
| 20. | Janmaat VT, Steyerberg EW, van der Gaast A, Mathijssen RH, Bruno MJ, Peppelenbosch MP, Kuipers EJ, Spaander MC. Palliative chemotherapy and targeted therapies for esophageal and gastroesophageal junction cancer. Cochrane Database Syst Rev. 2017;11:CD004063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, Hironaka S, Hara H, Satoh T, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Doki Y, Kitagawa Y. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 22. | Kojima T, Muro K, Francois E, Hsu CH, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Lin S, Qin SQ, Ferreira P, Doi T, Adenis A, Enzinger PC, Shah MA, Wang R, Bhagia P, Kang SP, Metges JP. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: The phase 3 KEYNOTE-181 study. 2019 Gastrointestinal Cancers Symposium; 2019 Jan 17-19; San Francisco, United States. American Society of Clinical Oncology. . |
| 23. | Dikken JL, Dassen AE, Lemmens VE, Putter H, Krijnen P, van der Geest L, Bosscha K, Verheij M, van de Velde CJ, Wouters MW. Effect of hospital volume on postoperative mortality and survival after oesophageal and gastric cancer surgery in the Netherlands between 1989 and 2009. Eur J Cancer. 2012;48:1004-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Haj Mohammad N, Bernards N, van Putten M, Lemmens VEPP, van Oijen MGH, van Laarhoven HWM. Volume-outcome relation in palliative systemic treatment of metastatic oesophagogastric cancer. Eur J Cancer. 2017;78:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |