Published online Dec 14, 2019. doi: 10.3748/wjg.v25.i46.6781
Peer-review started: October 1, 2019
First decision: November 4, 2019
Revised: November 11, 2019
Accepted: November 29, 2019
Article in press: November 29, 2019
Published online: December 14, 2019
Processing time: 74 Days and 9.5 Hours
A one-stage laparoscopic operation has recently been considered a favorable option for the management of patients with Hirschsprung's disease (HD) due to its superior cosmetic results. One-stage transanal endorectal pull-through for the treatment of rectosigmoid HD has been widely used in newborns without complications. However, enterostomy is required in some HD cases for enterocolitis and dilated colon. Our transumbilical enterostomy (TUE) and two-stage laparoscopy-assisted anorectoplasty were effective and achieved a similar cosmetic effect to one-stage laparoscopy on the abdominal wall in patients with anorectal malformation, but the effect in patients with HD is unclear.
To evaluate the safety, efficacy and cosmetic results of TUE in two-stage laparoscopy-assisted pull-through for HD.
From June 2013 to June 2018, 53 patients (40 boys, 13 girls; mean age at enterostomy: 5.5 ± 2.2 mo) who underwent enterostomy and two-stage laparoscopy-assisted pull-through for HD with stoma closure were reviewed at our institution. Two enterostomy approaches were used: TUE in 24 patients, and conventional abdominal enterostomy (CAE) in 29 patients. Eleven patients with rectosigmoid HD had severe preoperative enterocolitis or a dilated colon. 26 patients had long-segment HD, and 16 patients had total colonic aganglionosis (TCA). The patients with left-sided HD underwent the two-stage laparoscopic Soave procedure, and the patients with right-sided HD and TCA underwent the laparoscopic Duhamel procedure. Demographics, enterostomy operative time, complications and cosmetic results were respectively evaluated.
There were no differences between the groups with respect to gender, age at enterostomy, weight and clinical type (P > 0.05). No conversion to open technique was required. Two patients experienced episodes of stomal mucosal prolapse in the TUE group and 1 patient in the CAE group (8.33% vs 3.45%, P > 0.05). No parastomal hernia was observed in either of the two groups. Wound infection at the stoma was seen in 1 case in the TUE group, and 2 cases in the CAE group (4.17% vs 6.90%, P > 0.05). No obstruction was noted in any of the patients in the TUE group, whereas obstruction was found in 1 patient in the CAE group. Enterocolitis was observed in 3 and 5 patients in the TUE and CAE group, respectively (12.50% vs 17.24%, P > 0.05). There was no significant difference between the TUE group and CAE group in terms of the incidence of soiling and constipation (P > 0.05). The cosmetic result using the scar score in the TUE group was better than that in the CAE group (6.83 ± 0.96 vs 13.32 ± 1.57, P < 0.05).
TUE is a safe and feasible method for the treatment of HD, and the staged enterostomy and two-stage laparoscopy-assisted pull-through achieved a similar cosmetic effect to the one-stage laparoscopic procedure.
Core tip: This study describes a comparison between transumbilical enterostomy (TUE) and conventional abdominal enterostomy in a single-barreled fashion and two-stage laparoscopy-assisted pull-through in Hirschsprung's disease (HD) with stoma closure. TUE is a safe and feasible method for the treatment of HD, offering easier stoma care and better cosmetic results, and the staged enterostomy and two-stage laparoscopy-assisted pull-through achieved a similar cosmetic effect to the one-stage laparoscopic procedure.
- Citation: Xu PP, Chang XP, Zhang X, Chi SQ, Cao GQ, Li S, Yang DH, Li XY, Tang ST. Transumbilical enterostomy for Hirschsprung's disease with a two-stage laparoscopy-assisted pull-through procedure. World J Gastroenterol 2019; 25(46): 6781-6789
- URL: https://www.wjgnet.com/1007-9327/full/v25/i46/6781.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i46.6781
Hirschsprung’s disease (HD), also known as aganglionosis, is characterized by the absence of ganglion cells in the myenteric and submucosal plexuses of the colon[1]. In 1948, Swenson first reported a staged repair by a transabdominal pull-through operation, followed by closure of the colostomy of HD[2]. Since 1980, one-stage pull-through has been reported to be safe and effective in HD[3-5]. Primary laparoscopic pull-through for HD in infants and children was reported in mid 1990s[6, 7]. In 1998, De la Torre-Mondragon et al[8] first described single-stage transanal endorectal pull-through in the newborn, which has been widely used in the treatment of rectosigmoid HD without complications[9-12]. With the development of laparoscopy, laparoscopic procedures have been increasingly applied in various types of HD. Recently, many surgeons have accepted the one-stage laparoscopic procedure as a favorable option in the management of children with HD as it has a superior cosmetic result.
However, enterostomy is essential in some HD cases due to enterocolitis and dilated colon. The problem is that the cosmetic results of the laparoscopic approach have been reduced by the abdominal scar in some patients undergoing enterostomy. In the 1980s, Gordon et al[13] and Peter et al[14] reported the umbilical loop ostomy of HD. In 2005, Sauer et al[15] described their experience with the use of the umbilicus as a site for colostomy, biopsies and surgery in 6 infants with complicated HD. It was thought that the transumbilical operation was safe and effective. We previously reported that transumbilical colostomy in a modified double-barreled fashion and the two-stage laparoscopy-assisted anorectoplasty were safe and effective in patients with anorectal malformation[16], which achieved a similar cosmetic effect to the one-stage laparoscopy. Here, we describe a surgical technique for transumbilical enterostomy (TUE) in a single-barreled fashion and the two-stage laparoscopy-assisted pull-through of HD with stoma closure, and assess the safety, efficacy and cosmetic results of this procedure.
This study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The clinical data of 53 patients (40 boys, 13 girls; mean age at enterostomy: 5.5 ± 2.2 mo) who underwent two-stage laparoscopy-assisted pull-through for HD with stoma closure were included in this retrospective study. From June 2013 to June 2018, two enterostomy approaches were used: TUE in 24 patients, and conventional abdominal enterostomy (CAE) in 29 patients. Eleven patients (TUE 4, 16.67%; CAE 7, 24.14%) with rectosigmoid HD and severe enterocolitis or a dilated colon, 26 patients (TUE 11, 45.83%; CAE 15, 51.72%) with long-segment HD, and 16 patients (TUE 9, 37.50%; CAE 7, 24.14%) with total colonic aganglionosis (TCA) underwent enterostomy. Of these 53 cases, patients with left-sided HD underwent the two-stage laparoscopic Soave procedure, and patients with right-sided HD and TCA underwent the laparoscopic Duhamel procedure.
An intraoperative frozen-section biopsy and postoperative pathological examination in the definitive procedure were performed to determine the transition zone and confirm the diagnosis. The definitive surgical procedures were performed by the same surgical team at our institution.
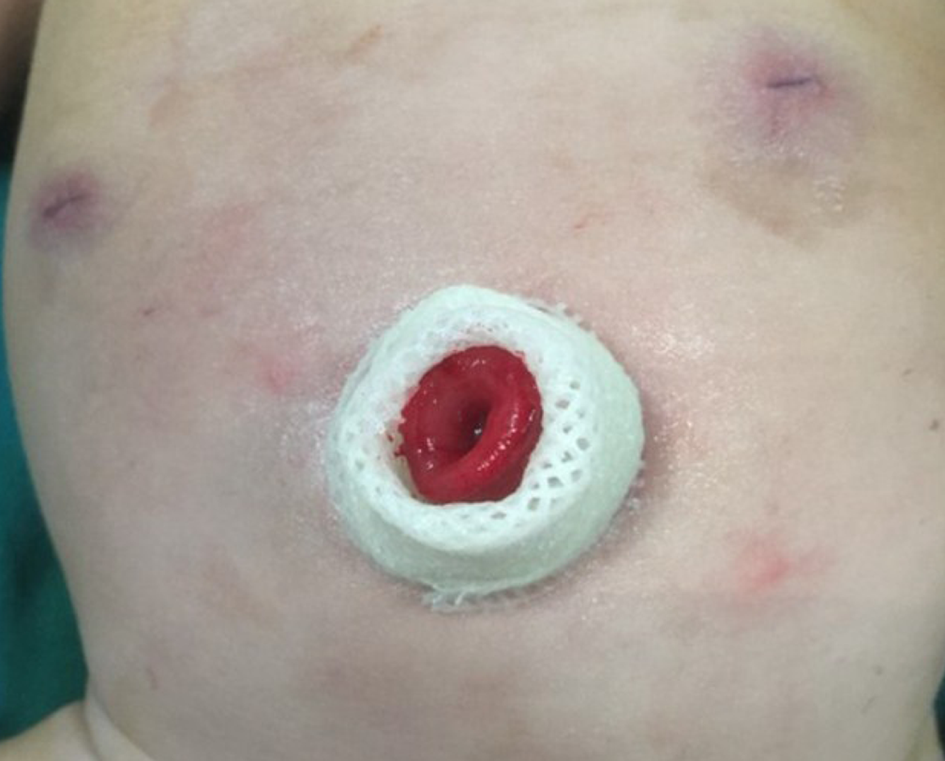
Under sufficient general anesthesia, a circumferential skin incision was made at the umbilicus. We vertically cored out the skin, subcutaneous tissue, and fascia. In neonates, we individually ligated the umbilical vessels and urachal remnant to the opening of the fascia. In other children, the umbilicus was directly cut longitudinally. After identification and exteriorization of a loop of the sigmoid colon through the opening in the fascia and peritoneum under laparoscopy, the transverse colon was directly identified through the umbilicus. In order to pull the intestine out without contamination, we pre-punctured the stomal site to reduce the intestine size due to the severely distended colon. An enterostomy was created in a single-barreled fashion with a chimney of the proximal end approximately 2 cm above the periumbilical skin. The distal end was closed and fixed to the proximal end in the abdominal cavity for easy finding next time (Figure 1).
Three to six months later, we closed the stoma through a peristomal skin incision. Our two-stage laparoscopic Soave procedure of left-sided HD was based on our previous reports[17,18]. We placed one 5 mm or 10 mm trocar as the working port through the stoma site, and two 3 mm trocars were respectively inserted in the right and left upper quadrant. Routinely, intraoperative frozen-section biopsy was performed before anastomosis[19]. We then used Lone Star retractors (Lone Star Medical Products, Stafford, TX, United States) to expose the anus, and electrocautery to make a circumferential incision in the rectal mucosa at 0.5-1.0 cm proximal to the dentate line. A long muscular rectal cuff was developed for > 5 cm up to the peritoneal reflection, which was then shortened to 1-2 cm. The rectal muscular cuff was partial dissected in a “V” shape at the posterior wall, and the necessary amount of the mobilized colon was dissected. A coloanal anastomosis was then performed.
The laparoscopic Duhamel procedure for right-sided HD and TCA was similar to that described previously[20]. By separating the stoma through a peristomal skin incision and mobilizing part of the bowel through the abdominal stoma opening, we were able to reach the splenic flexure. The positions of the trocars were the same as those for the laparoscopic Soave procedure for left-sided HD described above. The remaining intestines were mobilized by a laparoscopic ultrasound scalpel. In the patients with right-sided HD, the Delayer’s maneuver was used to transpose the colon 270° anticlockwise, and the appendix was cut at the same time. The colon was pulled down directly in TCA. An Endo-Cutting (Johnson-Johnson Co., Ltd) Stapler was then used to facilitate extra-anal division of the rectum perpendicular to the anus, to obtain a short 3.5-4.5 cm rectal stump. Single-layer 4-0 Vicryl was used to complete the colorectal or ileorectal end-to-side anastomosis and the Endo-Cutting Stapler was used to complete the colorectal or ileorectal side-to-side anastomosis.
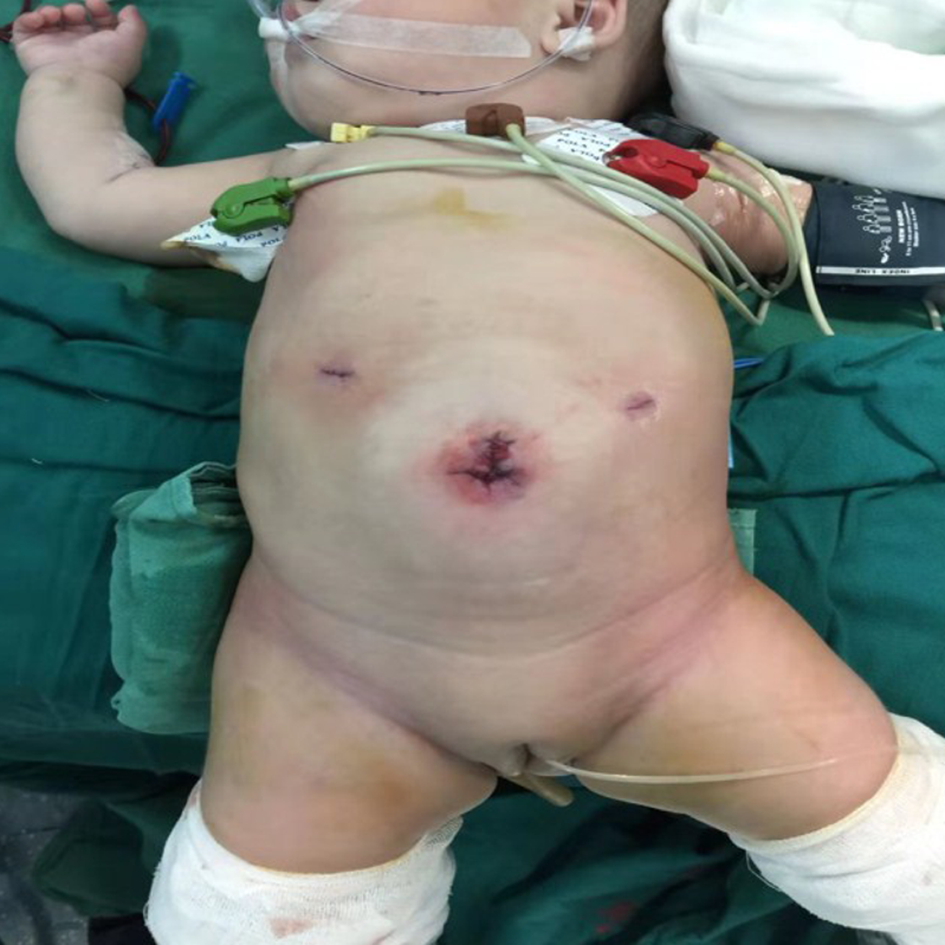
By using 0.5% iodine and warm 0.9% normal saline while suturing, the incision was washed repeatedly. After surgery, a tube was maintained in the anus. By leaving a central open area to create a deep circular scar resembling a normal umbilicus, the umbilical wound was reconstructed with a subcutaneous purse string suture of 4-0 absorbable sutures (Figure 2). Postoperatively, total parenteral nutrition and nasogastric decompression for 24-48 h were performed in all patients, and the peristomal area was kept clean and dry. When the patients were clinically stable, they were discharged.
According to the results of a routine digital rectal examination at 2 wk postoperatively, the process of anal dilatation was achieved with Hegar dilators for 2-3 mo[17].
SPSS software version 22.0 was used to analyze the data. The Student’s t test was used to compare the demographic data and the mean scar score. All results are reported as mean ± standard deviation and percentage. Values of P < 0.05 were considered statistically significant.
There were no statistically significant differences in demographics between the TUE and CAE groups, including gender, age at enterostomy, weight, and clinical type. No intraoperative complications were observed in either group. The mean operative time was not significantly different in the two groups (P = 0.381). There was no significant difference in mean blood loss and mean length of hospital stay between the two groups; no conversion to open technique was required. In 53 patients, a sigmoid colostomy (n = 11), a transverse colostomy (n = 23) or an ileostomy (n = 19) was performed (Table 1).
| Characteristic | TUE (n = 24) | CAE (n = 29) | P value |
| Age at enterostomy (mo) | 5.3 ± 2.6 | 5.6 ± 2.1 | 0.644 |
| Sex, male, n (%) | 17 (70.83) | 23 (79.31) | 0.694 |
| Weight (kg) | 4.9 ± 1.8 | 5.2 ± 1.2 | 0.472 |
| Clinical type, n (%) | 0.543 | ||
| Rectosigmoid HD | 4 (16.67) | 7 (24.14) | |
| Long-segment HD | 11 (45.83) | 15 (51.72) | |
| Total colonic HD | 9 (37.50) | 7 (24.14) | |
| Mean operative time (min) | 43.8 ± 9.5 | 41.6 ± 8.6 | 0.381 |
| Estimated blood loss (mL) | 10.3 ± 2.3 | 11.6 ± 2.5 | 0.056 |
| Hospital stay (d) | 7.3 ± 0.7 | 7.4 ± 0.9 | 0.659 |
| Cost of hospital (RMB) | 16582.8 ± 2354.3 | 17634.2 ± 2108.4 | 0.093 |
Two patients developed stomal mucosal prolapse in the TUE group and 1 patient in the CAE group (8.33% vs 3.45%, P > 0.05). During repair of the proximal intestine in 3 patients with stomal mucosal prolapse, 2 patients with transverse colostomy in the TUE group underwent successful reoperation for TUE, and 1 patient with terminal ileostomy in the CAE group underwent reoperation for CAE. The causes of stomal mucosal prolapse in these 3 patients were proximal intestinal redundancy and frequent crying. There was no case of parastomal hernia in the two groups. Wound infection at the stoma occurred in 1 patient in the TUE group, and 2 patients in the CAE group (4.17% vs 6.90%, P > 0.05). Dermatitis around the stoma was found in 4 patients in the TUE group and in 8 patients in the CAE group (16.67% vs 27.59%, P > 0.05). Meticulous nursing care and keeping the stoma clean decreased the pain and speeded up the recovery. Enterocolitis occurred in 8 patients, 3 patients in the TUE group and 5 patients in the CAE group (12.50% vs 17.24%, P > 0.05), and treatment consisted of intravenous fluid, antibiotics, parenteral nutrition, and enemas. In the TUE group, umbilical ring narrowing, obstructive symptoms, and fecal impaction were not observed; thus, the outcome after diverting the fecal stream was satisfactory. In the CAE group, we noted obstruction after enterostomy in 1 case. One child developed adhesive intestinal obstruction on postoperative day 16, but responded well to conservative management (Table 2).
| Group | TUE (n = 24) | CAE (n = 29) | P value |
| Stomal mucosal prolapse | 2 (8.33) | 1 (3.45) | 0.584 |
| Parastomal hernia | 0 | 0 | 1 |
| Wound infection | 1 (4.17) | 2 (6.90) | 1 |
| Incidence of dermatitis at stoma | 4 (16.67) | 8 (27.59) | 0.538 |
| Obstruction | 0 | 1 (3.45) | 1 |
| Enterocolitis | 3 (12.50) | 5 (17.24) | 0.715 |
Postoperative constipation was found in 3 patients in the TUE group and 2 patients in the CAE group (12.50% vs 6.90%, P > 0.05), which steadily improved after a laxative or enema. Seven patients (4 patients in the TUE group and 3 patients in the CAE group) experienced episodes of soiling (16.67% vs 10.34%, P > 0.05), which was effectively managed conservatively.
At the time of two-stage laparoscopy-assisted pull-through procedure, age, sex, preoperative enteritis and operative data were not significantly different between the TUE group and CAE group. The operative time for the two-stage laparoscopic procedure in the TUE group was shorter than that in the CAE group (144.8 ± 28.2 min vs 160.3 ± 27.6 min, P < 0.05) (Table 3).
| Group | TUE (n = 24) | CAE (n = 29) | P value |
| Age at operation (mo) | 8.0 ± 1.9 | 8.5 ± 2.2 | 0.386 |
| Sex (male), n (%) | 17 (70.83) | 23 (79.31) | 0.694 |
| Weight (kg) | 7.2 ± 2.0 | 7.9 ± 2.5 | 0.273 |
| Preoperative enteritis, n (%) | 4 (16.67) | 7 (24.14) | 0.735 |
| Mean operative time (min) | 144.8 ± 28.2 | 160.3 ± 27.6 | 0.049 |
| Hospital stay (d) | 9.8 ± 1.4 | 10.4 ± 1.8 | 0.189 |
| Cost of hospital (RMB) | 57325.5 ± 3607.9 | 55792.3 ± 3851.1 | 0.144 |
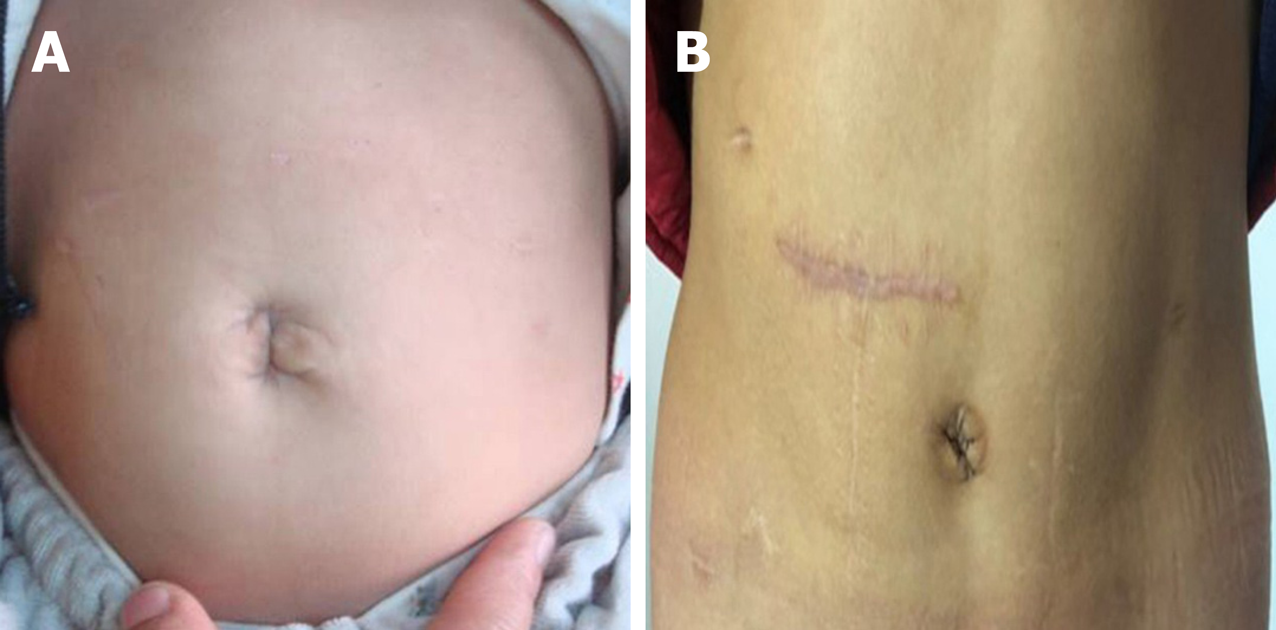
The laparoscopic-assisted two-stage pull-through procedure with simultaneous stoma closure was successfully performed in all patients, and no conversions to laparotomy were required. One year after surgery, a cosmetic assessment was conducted (Table 4). Assessment of the stoma revealed a scarless umbilicus in TUE patients (Figure 3A), and one visible abdominal scar in CAE patients (Figure 3B). The Manchester Scar Scale[18,21] was used to assess the scar score for TUE and CAE patients, and demonstrated a significant difference between the two groups (TUE: 6.83 ± 0.96 vs CAE: 13.32 ± 1.57, P < 0.05).
| Assessment | TUE (n = 24) | CAE (n = 29) | P value |
| Appearance of the umbilicus | Normal | Normal | - |
| Scar appearance on the abdomen | Near scarless | Visible | - |
| MSS score of visible scars on the abdomen | 6.83 ± 0.96 | 13.32 ± 1.57 | < 0.001 |
With the introduction of minimally invasive surgery, one-stage laparoscopic surgery has gradually replaced the traditional staging operation as the first choice for HD treatment[22,23]. This has the advantages of less postoperative pain and a better cosmetic result[7], but a diverting enterostomy in the normal proximal bowel is necessary in some patients with complicated HD as a life-saving procedure[24]. In recent years, the two-stage and the three-stage operation have been recommended. Although there is a debate regarding whether the two-stage operation is as safe as the three-stage operation, the two-stage operation has gained popularity in both parents and surgeons due to cost-effectiveness and no increase in postoperative complications[25]. The real issue is that the scar on the abdominal wall after CAE would have reduced cosmetic results compared with the laparoscopic approach.
Inspired by pyloroplasty through a small incision at the umbilicus, some pediatric surgeons reported that TUE was safe and effective in HD[13-15]. In anorectal malformation, transumbilical colostomy and laparoscopic anorectoplasty achieved a similar cosmetic effect to one-stage laparoscopy on the abdominal wall[16]. We then began to perform TUE and two-stage laparoscopy-assisted pull-through in HD. Patients and their families were advised of the advantages and disadvantages of TUE and CAE; thus, the enterostomy procedure could be freely chosen. A retrospective study of 24 patients with TUE in our hospital, found that TUE allowed diversion of the fecal stream and enteral decompression, and did not increase postoperative complications in patients with HD compared with CAE. In addition, there was no significant difference in the length of hospital stay and the total hospital cost between the two groups. These findings indicated that TUE was a safe and effective procedure without increasing the economic burden of patients.
TUE is an alternative method for patients who require enterostomy without increasing the difficulty of surgery or the incidence of postoperative complications. As reported in the literature, the ileocecum, ascending colon, transverse colon, the splenic flexure of the colon and biopsy could be easily mobilized through the umbilical incision after the umbilical stoma was separated, and converting laparoscopic total or right-sided colon resection into laparoscopic left-sided colon resection, shortened the anesthesia and operative time in TUE. However, the incision for CAE was either in the left abdomen or in the right abdomen. It is not convenient to perform these procedures in CAE. Therefore, the duration of the two-stage laparoscopic procedure for TUE was shorter than that for CAE.
This location for TUE is convenient as the stoma is on the apex of the abdominal wall, and the stoma bag is easily attached without side leakage. In the TUE group, the resulting scar after enterostomy closure closely resembled a normal umbilicus and had superior cosmetic results compared to the scar in the CAE group.
In conclusion, TUE is a safe and feasible treatment for HD. This method results in easier stoma care and better cosmetic results than CAE, and the staged laparoscopic enterostomy achieved a similar cosmetic effect to the one-stage laparoscopic procedure.
Since 1998, one-stage transanal endorectal pull-through for the treatment of rectosigmoid Hirschsprung's disease (HD) has been widely used in newborns without complications. Recently, the one-stage laparoscopic procedure has been considered a favorable option for the management of patients with HD due to its superior cosmetic results. However, enterostomy is required in some HD cases for enterocolitis and dilated colon. Our transumbilical enterostomy (TUE) and two-stage laparoscopy-assisted anorectoplasty were effective and achieved a similar cosmetic effect to the one-stage laparoscopy on the abdominal wall in patients with anorectal malformations, but the effect in patients with HD is unclear.
Our TUE and two-stage laparoscopy-assisted anorectoplasty were effective and achieved a similar cosmetic effect to the one-stage laparoscopy on the abdominal wall in anorectal malformations, but the effect in patients with HD is unclear.
This study aimed to evaluate the safety, efficacy and cosmetic results of TUE for the management of HD in a two-stage laparoscopy-assisted pull-through, and was retrospectively compared with conventional abdominal enterostomy (CAE).
From June 2013 to June 2018, 53 patients (40 boys, 13 girls; mean age at enterostomy: 5.5 ± 2.2 mo) who underwent enterostomy and two-stage laparoscopy-assisted pull-through for HD with stoma closure were reviewed at our institution. Two enterostomy approaches were used: TUE in 24 patients and CAE in 29 patients. Eleven patients with rectosigmoid HD had severe preoperative enterocolitis or a dilated colon. 26 patients had long-segment HD, and 16 patients had total colonic aganglionosis (TCA). Patients with left-sided HD underwent the two-stage laparoscopic Soave procedure, and patients with right-sided HD and TCA underwent the laparoscopic Duhamel procedure. Demographics, operation duration, complications and cosmetic results were respectively evaluated.
There were no differences between the groups with respect to gender, age at enterostomy, weight and clinical type (P > 0.05). No conversion to open technique was required. Two patients experienced stomal mucosal prolapse in the TUE group and 1 patient in the CAE group (8.33% vs 3.45%, P > 0.05). No parastomal hernia was observed in the two groups. Wound infection at the stoma was seen in 1 case in the TUE group, and 2 cases in the CAE group (4.17% vs 6.90%, P > 0.05). No obstruction was found in any of the patients in the TUE group, whereas obstruction was found in 1 patient in the CAE group. Enterocolitis was observed in 3 and 5 patients in the TUE and CAE group, respectively (12.50% vs 17.24%, P > 0.05). There was no significant difference between TUE group and CAE group in the incidence of soiling and constipation (P > 0.05). The cosmetic result in terms of the scar score in the TUE group was better than that in the CAE group (6.83 ± 0.96 vs 13.32 ± 1.57, P < 0.05).
TUE is a safe and feasible method for the treatment of HD, and the staged enterostomy and two-stage laparoscopy-assisted pull-through procedure achieved a similar cosmetic effect to the one-stage laparoscopic procedure.
TUE could help patients who require enterostomy to achieve good cosmetic results in the treatment of HD using the two-stage laparoscopy-assisted pull-through with stoma closure, which is expected to be favored by patients and their families in the future.
We are grateful to Professor Ping Yin for the statistical evaluation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciftci I, O'Donnell AM S-Editor: Tang JZ L-Editor: Webster JR E-Editor: Zhang YL
| 1. | Kenny SE, Tam PK, Garcia-Barcelo M. Hirschsprung's disease. Semin Pediatr Surg. 2010;19:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Swenson O, Bill AH. Resection of rectum and rectosigmoid with preservation of the sphincter for benign spastic lesions producing megacolon; an experimental study. Surgery. 1948;24:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | So HB, Schwartz DL, Becker JM, Daum F, Schneider KM. Endorectal "pull-through" without preliminary colostomy in neonates with Hirschsprung's disease. J Pediatr Surg. 1980;15:470-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 92] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Pierro A, Fasoli L, Kiely EM, Drake D, Spitz L. Staged pull-through for rectosigmoid Hirschsprung's disease is not safer than primary pull-through. J Pediatr Surg. 1997;32:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Calisti A, Molle PH, Vallasciani S. Congenital megacolon in neonates and infants: impact of early, one-stage repair on morbidity and surgical complications. Pediatr Med Chir. 2004;26:241-244. [PubMed] |
| 6. | Smith BM, Steiner RB, Lobe TE. Laparoscopic Duhamel pullthrough procedure for Hirschsprung's disease in childhood. J Laparoendosc Surg. 1994;4:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Georgeson KE, Fuenfer MM, Hardin WD. Primary laparoscopic pull-through for Hirschsprung's disease in infants and children. J Pediatr Surg. 1995;30:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 188] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | De la Torre-Mondragón L, Ortega-Salgado JA. Transanal endorectal pull-through for Hirschsprung's disease. J Pediatr Surg. 1998;33:1283-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 233] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Teeraratkul S. Transanal one-stage endorectal pull-through for Hirschsprung's disease in infants and children. J Pediatr Surg. 2003;38:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Elhalaby EA, Hashish A, Elbarbary MM, Soliman HA, Wishahy MK, Elkholy A, Abdelhay S, Elbehery M, Halawa N, Gobran T, Shehata S, Elkhouly N, Hamza AF. Transanal one-stage endorectal pull-through for Hirschsprung's disease: a multicenter study. J Pediatr Surg. 2004;39:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Hadidi A. Transanal endorectal pull-through for Hirschsprung's disease: experience with 68 patients. J Pediatr Surg. 2003;38:1337-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Dasgupta R, Langer JC. Transanal pull-through for Hirschsprung disease. Semin Pediatr Surg. 2005;14:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Cameron GS, Lau GY. The umbilicus as a site for temporary colostomy in infants. J Pediatr Surg. 1982;17:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Fitzgerald PG, Lau GY, Cameron GS. Use of the umbilical site for temporary ostomy: review of 47 cases. J Pediatr Surg. 1989;24:973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Sauer CJ, Langer JC, Wales PW. The versatility of the umbilical incision in the management of Hirschsprung's disease. J Pediatr Surg. 2005;40:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Yang L, Tang ST, Li S, Aubdoollah TH, Cao GQ, Lei HY, Wang XX. Two-stage laparoscopic approaches for high anorectal malformation: transumbilical colostomy and anorectoplasty. J Pediatr Surg. 2014;49:1631-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Tang ST, Wang GB, Cao GQ, Wang Y, Mao YZ, Li SW, Li S, Yang Y, Yang J, Yang L. 10 years of experience with laparoscopic-assisted endorectal Soave pull-through procedure for Hirschsprung's disease in China. J Laparoendosc Adv Surg Tech A. 2012;22:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Aubdoollah TH, Li K, Zhang X, Li S, Yang L, Lei HY, Dolo PR, Xiang XC, Cao GQ, Wang GB, Tang ST. Clinical outcomes and ergonomics analysis of three laparoscopic techniques for Hirschsprung's disease. World J Gastroenterol. 2015;21:8903-8911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Aubdoollah TH, Tang ST, Yang L, Li S, Lei HY, Zhang X. Hybrid Single-Incision Laparoscopic Approaches for Endorectal Pull-Through in Hirschsprung's Disease. J Laparoendosc. Adv Surg Tech A. 2015;25:595-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Yang L, Tang ST, Cao GQ, Li S, Jiang M, Xiong M, Yang DH, Chang XP, Li K, Ma YZ. Laparoscopic Duhamel Procedure with Ex-Anal Rectal Transection for Right-Sided Hirschsprung's Disease. J Laparoendosc Adv Surg Tech A. 2017;27:972-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43. [PubMed] |
| 22. | Zimmer J, Tomuschat C, Puri P. Long-term results of transanal pull-through for Hirschsprung's disease: a meta-analysis. Pediatr Surg Int. 2016;32:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Tomuschat C, Zimmer J, Puri P. Laparoscopic-assisted pull-through operation for Hirschsprung's disease: a systematic review and meta-analysis. Pediatr Surg Int. 2016;32:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Shah AA, Shah AV. Staged laparoscopic-assisted pull-through for Hirschsprung's disease. J Pediatr Surg. 2003;38:1667-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Li S, Liu Y, Chang XP, Li K, Yang DH, Zhang X, Yang L, Pu JR, Cao GQ, Tang ST. Two-Staged Versus Three-Staged Laparoscopic Anorectoplasty for Patients with Rectoprostatic and Bladder Neck Fistulas: A Comparative Study. J Laparoendosc Adv Surg Tech A. 2019;29:1486-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |