Published online Dec 7, 2019. doi: 10.3748/wjg.v25.i45.6693
Peer-review started: October 17, 2019
First decision: November 10, 2019
Revised: November 21, 2019
Accepted: November 23, 2019
Article in press: November 23, 2019
Published online: December 7, 2019
Processing time: 50 Days and 3.1 Hours
Inflammatory pseudotumor-like follicular dendritic cell (IPT-like FDC) tumors of the liver is an uncommon tumor with extremely low incidence. To date, the radiologic findings of this tumor in multiphase computed tomography (CT) and magnetic resonance imaging (MRI) imaging have not been described.
Patient 1 is a 31-year-old Chinese female, whose complaining incidentally coincided with the finding of multiple liver masses. In the local hospital, an abdominal enhanced CT found two hypo-dense solid lesions, with heterogeneous sustained hypoenhancement, in the upper segment of the liver’s right posterior lobe. In our hospital, enhanced magnetic resonance imaging (MRI) with hepatocyte-specific contrast agents showed a similar enhanced pattern of lesions with patchy hyperintensity in the hepatobiliary phase (HBP). The patient underwent surgery and recovered well. The final pathology confirmed an IPT-like FDC tumor. No recurrence was found on the regular re-examination. Patient 2 is a 48-year-old Chinese male admitted to our hospital for a huge unexpected hepatic lesion. A dynamic enhanced abdominal CT revealed a huge heterogeneous enhanced solid tumor in the right lobe of the liver with a size of 100 mm × 80 mm, which showed a heterogeneous sustained hypoenhancement. In addition, enlarged lymph nodes were found in the hilum of the liver. This patient underwent a hepatic lobectomy and lymph node dissection. The final pathology confirmed an IPT-like FDC tumor. No recurrence was found upon regular re-examination.
When a hepatic tumor shows heterogeneous sustained hypoenhancement with a patchy enhancement during HBP, an IPT-like FDC tumor should be considered in the differential diagnosis.
Core tip: Here we report two rare cases of inflammatory pseudotumor-like follicular dendritic cell tumors of the liver, and give the first description of multiphase computed tomography and magnetic resonance imaging features of these tumors. Radiologists should be alerted to this disease for the differential diagnosis of liver tumors.
- Citation: Li HL, Liu HP, Guo GWJ, Chen ZH, Zhou FQ, Liu P, Liu JB, Wan R, Mao ZQ. Imaging findings of inflammatory pseudotumor-like follicular dendritic cell tumors of the liver: Two case reports and literature review. World J Gastroenterol 2019; 25(45): 6693-6703
- URL: https://www.wjgnet.com/1007-9327/full/v25/i45/6693.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i45.6693
The inflammatory pseudotumor-like follicular dendritic cell (IPT-like FDC) tumor is a variant subset of a follicular dendritic cell (FDC) tumor[1]. FDC tumors most commonly occur in the cervical lymph nodes, which are extremely rare in the liver and represent < 0.1% of all primary hepatic tumors[2]. The IPT-like FDC tumors of the liver are exceptionally rare, and are different from FDC tumors by female predilection. They have nearly exclusive hepatic and splenic involvement, with low aggressivity and an association with the Epstein-Barr virus (EBV)[3]. As far as we know, only 26 cases of IPT-like FDC liver tumors have been reported in the English-language literature[4,5], but their radiologic findings have rarely been described. Herein, we report the imaging features of two histopathologically proven cases of IPT-like FDC tumors of the liver. This report is helpful for understanding hepatic IPT-like FDC tumors. The study was approved by the institutional review board of our hospital, which waived the need for informed consent.
Patient 1: A 31-year-old Chinese female was incidentally reported to have multiple liver masses.
Patient 2: A 48-year-old Chinese male was incidentally found to have a massive liver mass for 7 d during health examination.
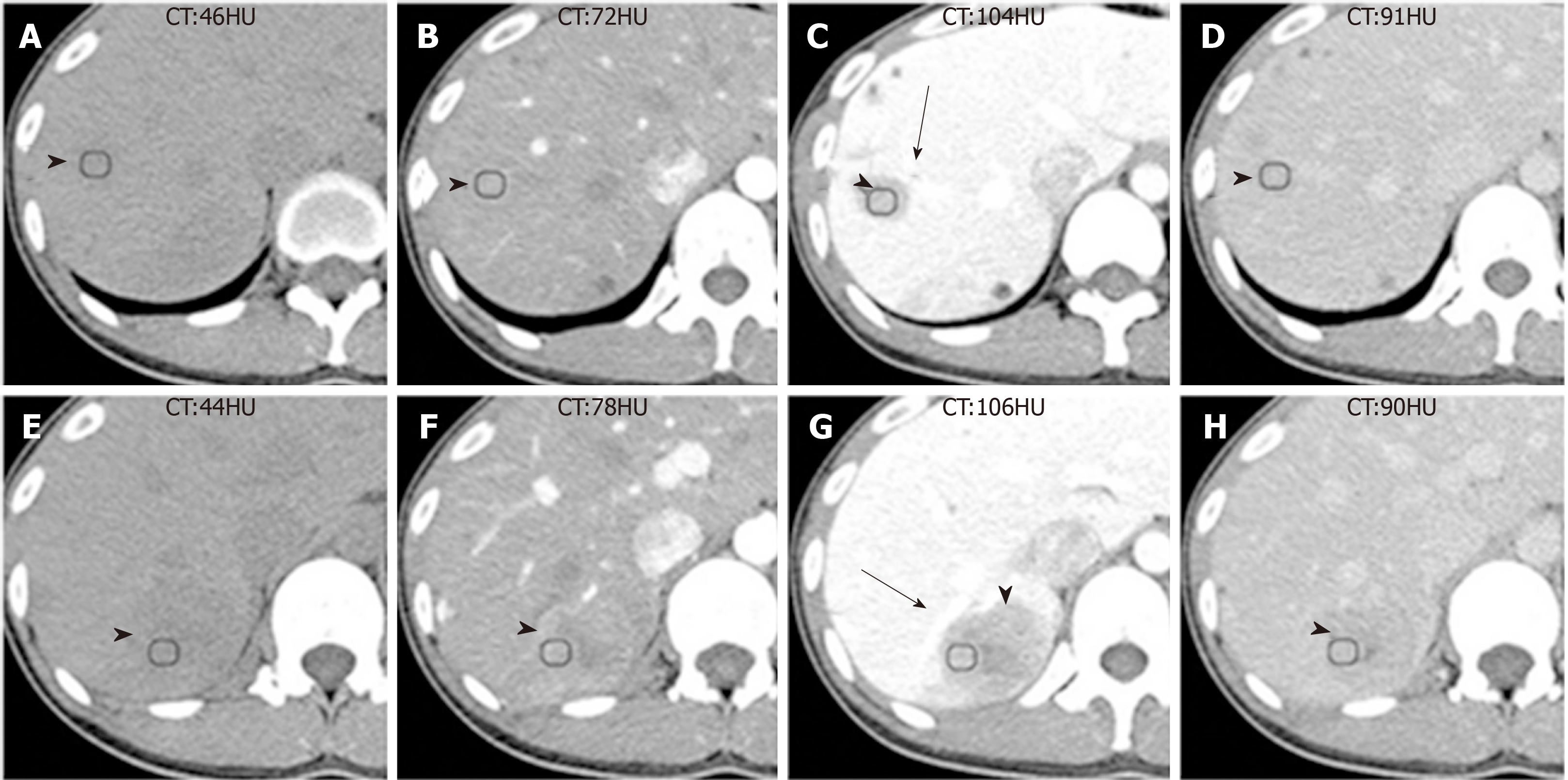
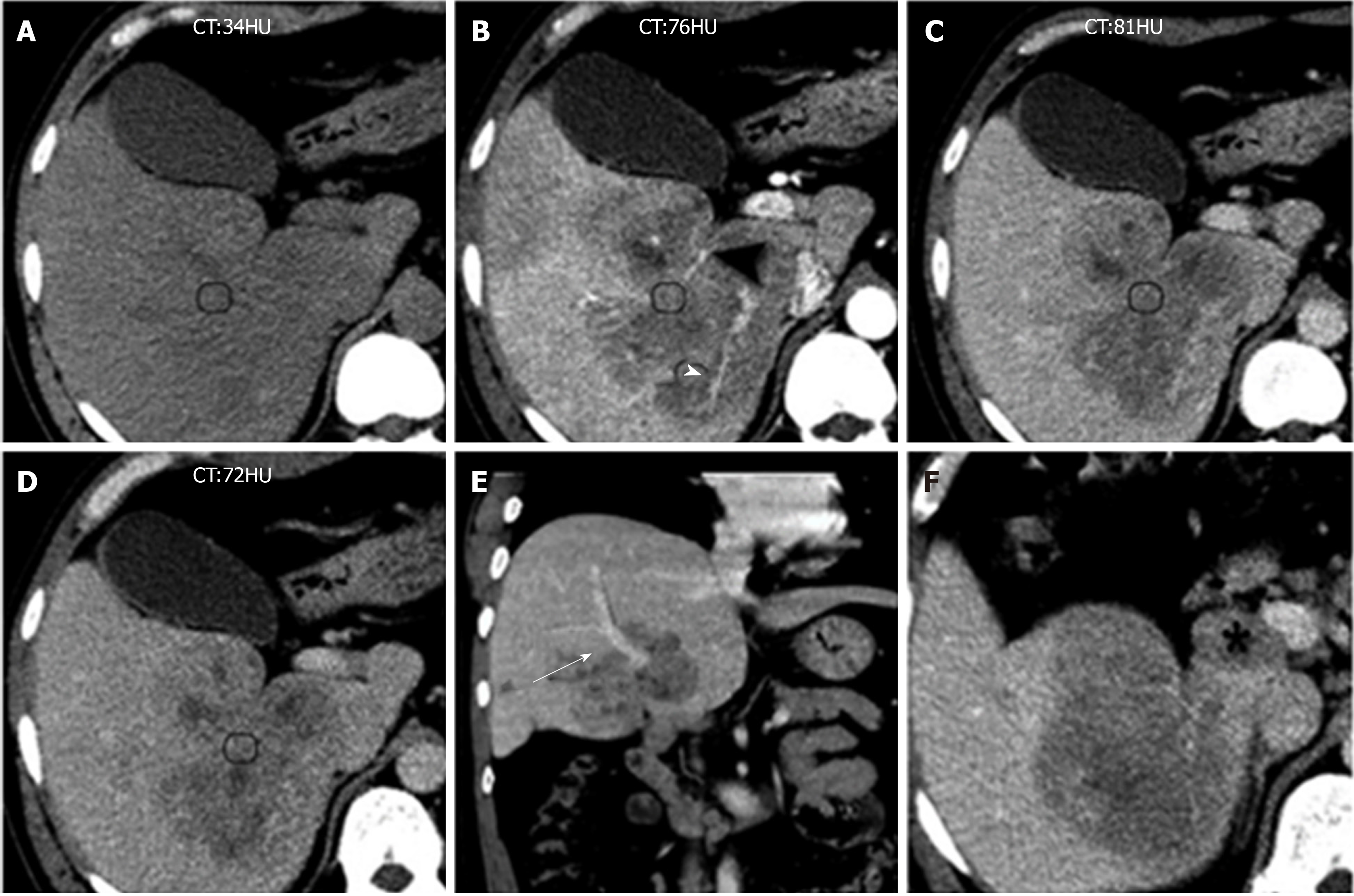
Patient 1: One month ago, the patient was found to have multiple hepatic lesions by chest computed tomography (CT), due to an upper respiratory tract infection in the local hospital. Two hypodense solid lesions, with sizes of 36 mm × 31 mm and 21 mm × 16 mm, respectively, were detected in the upper segment of the right posterior lobe (SVII) of the liver with enhanced abdominal CT (Figure 1, Table 1). The local hospital gave an imaging diagnosis of hepatocellular carcinoma or metastatic lesions, and recommended further MRI examination. The patient came to our hospital for further evaluation.
| Scan type | Case number | Number of lesions | No-contrast | Arterial phase | Portal venous phase | Equilibrium phase | Hepatobiliary phase | Dynamic enhancement pattern |
| MRI | 1 | 2 | Well-defined, hypointense on T1WI, hyperintense on T2WI | Hypoenhance-ment | Hypoenhance-ment | Hypoenhance-ment | Patchy hyperintense signal | Heterogeneous sustained enhancement |
| CT | 1 | 2 | Well-defined hypodensity | Isoenhance-ment | Hypoenhance-ment, reach the peak value | Hypoenhance-ment | Heterogeneous sustained enhancement | |
| 2 | 1 | Huge ill-defined heterogeneous hypodensity | Hypoenhance-ment, many distorted blood vessels | Hypoenhance-ment, reach the peak value | Hypoenhance-ment | Heterogeneous sustained enhancement |
Patient 2: The patient was admitted to the local hospital for a health examination, and a massive hepatic lesion was accidentally discovered by ultrasound 7 d ago. An enhanced abdominal CT showed a huge solid mass (100 mm × 80 mm) and suspected a hepatic carcinoma (HCC). The patient was admitted to our hospital for further evaluation and treatment.
Patient 1: The patient had a 10-year history of chronic hepatitis B virus infection, and had not received any antiviral therapy. The patient underwent two caesarean sections 7 years ago and 3 years ago.
Patient 2: Unremarkable.
Patient 1: Unremarkable.
Patient 2: Unremarkable.
Patient 1: Vital signs, such as temperature, respiration, heart rate, and blood pressure, were within normal limits. There were no positive signs upon abdominal examination.
Patient 2: Vital signs, such as temperature, respiration, heart rate, and blood pressure, were within normal limits. There were no positive signs upon abdominal examination.
Patient 1: Blood routine: neutrophil absolute counts and neutrophil percentages were low, at 1.53 × 109/L and 40.6%, respectively; the white blood cell counts, red blood cell counts, platelet counts, and hemoglobin assays were normal. Liver function tests, such as serum proteins, transaminases and bilirubin, were normal. Quantitative analysis of hepatitis B markers showed three small positives: Hepatitis B virus surface antigen (586.9 IU/mL), hepatitis B virus e antibody (0.07 COI), and hepatitis B virus core antibody (5.99 COI). The levels of tumor markers, including α-fetoprotein (AFP), carcinoembryonic antigen (CEA), cancer antigen (CA) 125 and CA19-9, were within normal ranges.
Patient 2: Blood routine examination: high white blood cell counts (12.27 × 109/L), high platelet counts (571 × 109/L), low hemoglobin levels (116 g/L), and low hematocrit levels (36.4%). Clinical biochemistry: low apolipoprotein A-1 and prealbumin were 0.76 g/L and 151 mg/L, respectively; high apolipoprotein B, 1.09 g/L; high lipoprotein (a), 1448 mg/L, total protein, 90.8 g/L, globulin, 47.70 g/L, alkaline phosphatase, 219 U/L and γ-glutamyltransferase, 109.0 U/L. A thyroid function test showed high total thyroxine (255.444 nmol/L), as well as normal triiodothyronine, thyroid stimulating hormone, free triiodothyronine and free thyroxine. The levels of tumor markers, including AFP, CEA, CA125, and CA19-9, were within normal ranges.
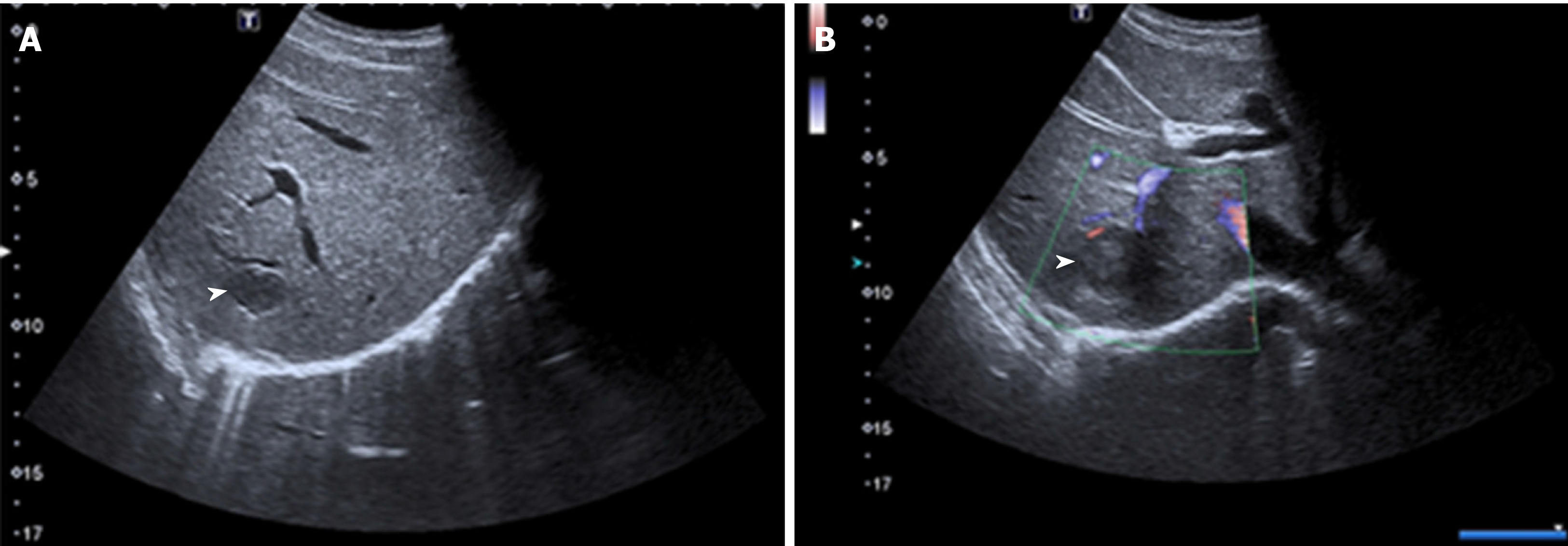
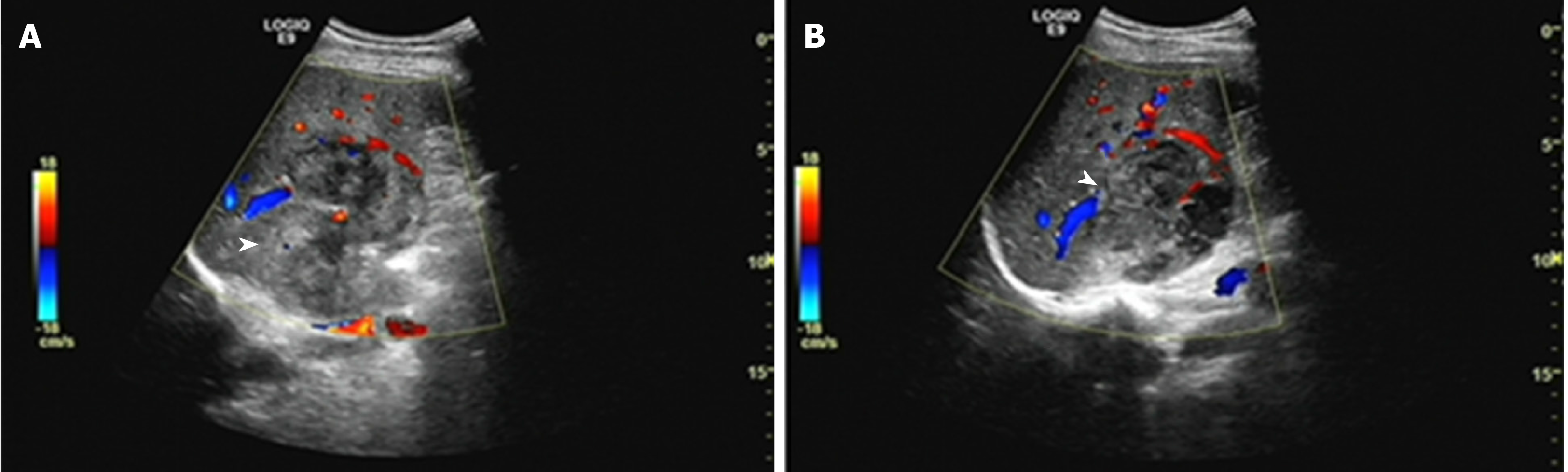
Patient 1: The unenhanced ultrasound (US) showed two heterogeneous hypoechoic lesions without detectable internal blood flow in the SVII of the liver, which was suspected for metastasis (Figure 2).
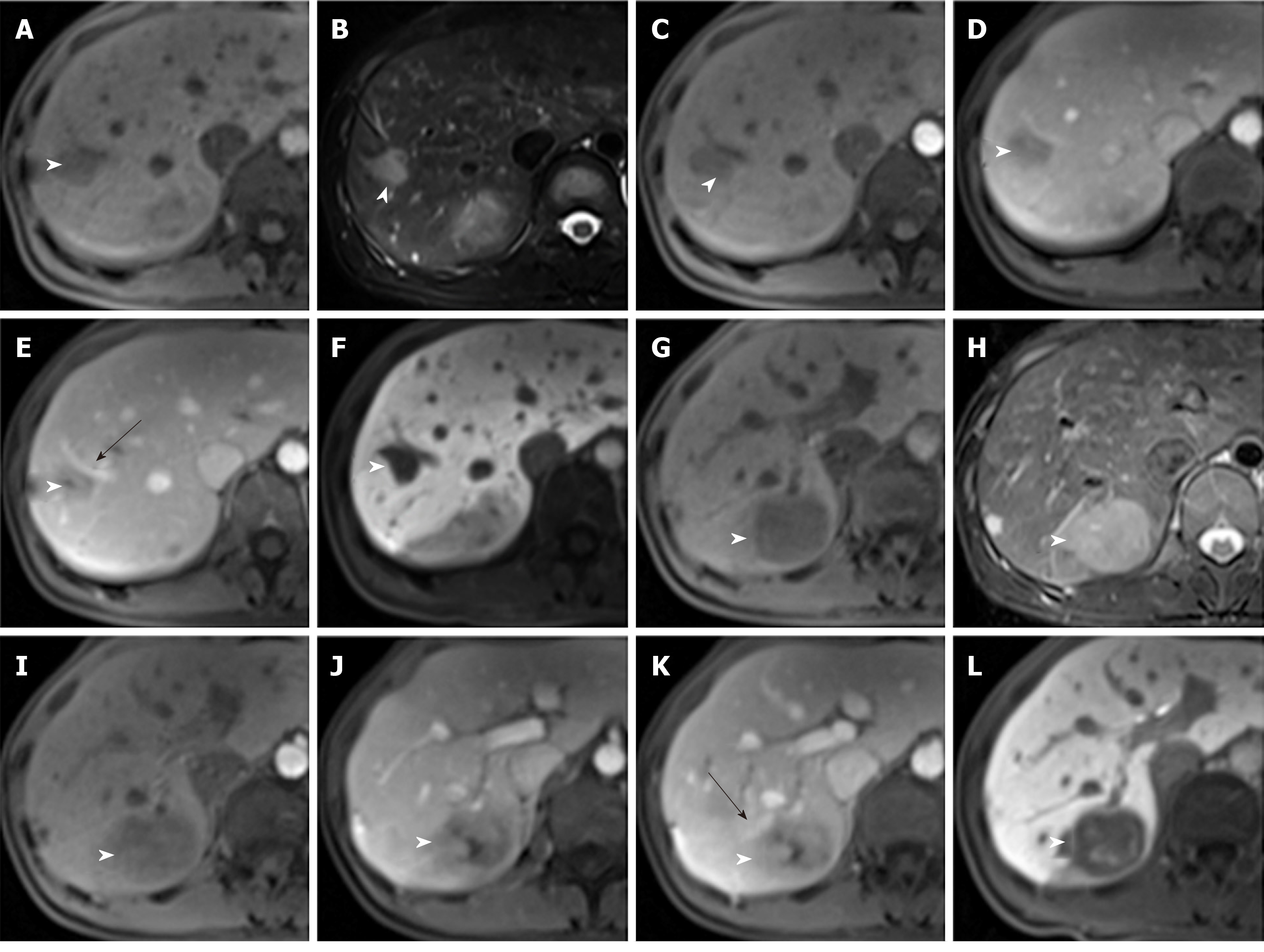
A gadoxetic acid [gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)]-enhanced liver MRI was performed using the Dutch Philips Achieva 3.0T superconducting MR scanner, with a 16-channel phase array coil. The no-contrast images, including axial and coronal T1- and T2-weighted with/without fat saturation images, were obtained in sequence[6]. Multiphase dynamic imaging was scanned after a rapid bolus of Gd-EOB-DTPA (0.025 mmol/kg) at a rate of 1.5 mL/s, immediately followed by a 30 mL saline flush using a power injector[7]. Arterial phase, portal venous phase, equilibrium phase and hepatobiliary phase (HBP) images were performed sequentially, and scanned at 20-30 s, 60-70 s, 120-150 s, and 20 min after the injection.
Enhanced MRI with hepatocyte-specific contrast agents showed two masses of 36 mm × 32 mm and 20 mm × 16 mm, respectively, in the SVII of the liver (Figure 3, Table 1). It was diagnosed as a metastatic tumor.
Patient 2: The US revealed one massive heterogeneous hypoechoic lesion with internal blood flow located in the right lobe of the liver, and was diagnosed as HCC (Figure 4).
The upper abdominal scan was performed by using Philips Brilliance 256-slices spiral CT. The patient was placed in the supine position. The scan ranged from the upper edge of the xiphoid to the lower pole of the kidney. The tube voltage was 120 kV, the tube current was 200 mA, and the section thickness was 5 mm. A non-ionic contrast agent (Omnipaque 350 mg I/mL) was injected at a dose of 0.9-1.5 mL/kg by using a power injector. A triphasic liver protocol was sequentially scanned at 30 s (arterial phase), 60 s (portal venous phase), and 120 s (equilibrium phase) after injection[8].
The enhanced CT revealed a huge heterogeneous enhancement solid tumor in the right lobe of the liver with a size of 100 mm × 80 mm. Multiple enlarged lymph nodes were seen in the hepatic hilar region (Figure 5, Table 1). The imaging diagnosis was a large HCC of the right lobe, with multiple lymph node metastasis in the hilar region.
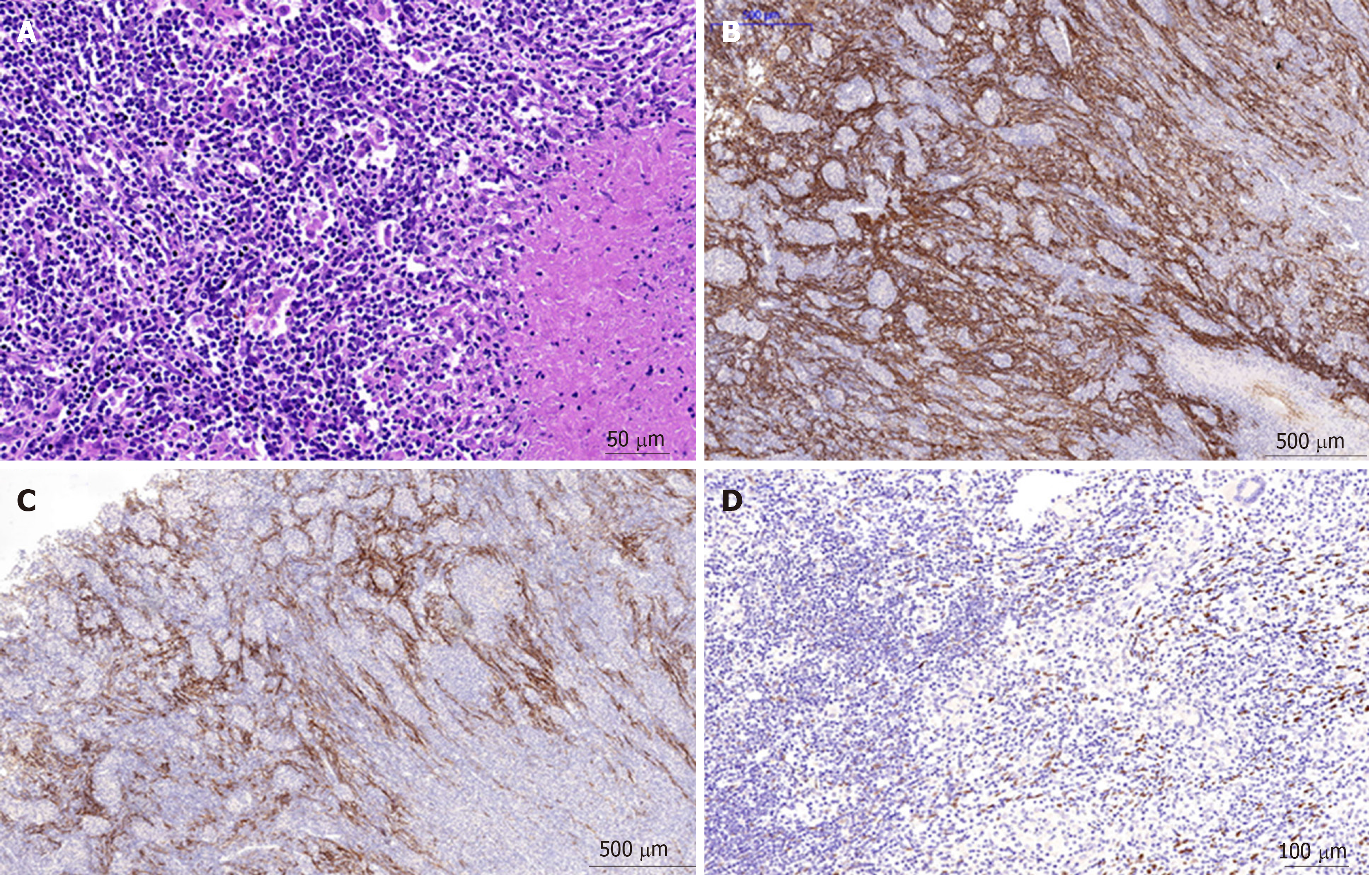
Microscopically, the tumors were composed of spindle cells, abundant small lymphocytes, eosinophils and plasmocytes (Figure 6), mixed with multifocal coagulative necrosis and granulomas. Molecular pathology results: Epstein-Barr-encoded-RNA (EBER)(+)(Figure 6). The immunohistochemical test results included the following: CD21(+), CD23(+) (Figure 6), CK (pan)(-), CD163(+), CD68(+), CD3(+), CD30(-), CD20(+), Ki-67 (20%+), S-100(-), CD138(+), CD2(+), CD7(+), CD5(+), CD56(-), Granzyme B(-), TIA-1(+), CD1a(-), and SMA (scattered +); Special staining results: Acid-fast(-), PAS(-). The primary IPT-like FDCS tumors of the liver were diagnosed pathologically after operation.
Microscopically, the tumors were composed of spindle cells, abundant small lymphocytes and plasmocytes, and scattered eosinophils mixed with multifocal coagulative necrosis. Molecular pathology results: Epstein-Barr-encoded-RNA(EBER)(+). The immunohistochemical test results included the following: CD21(+), CK (pan)(-), CD163(+), CD68(+), CD3(+), CD20(+), Ki-67 (20%+), S-100(-), CD138(+), CD38(+), kappa(+), lambda(+), IgG(+), IgG4(-), and PAS(-). Primary IPT-like FDCS tumors of the liver with lymph node involvement were diagnosed pathologically after operation. No tumor involvement was found in the liver surgical margin.
Considering that the primary cancer was not found in the chest and abdomen CT, and the patient was eager to remove the liver lesions, the right hepatectomy was planned to provide an optimized method for the complete resection of the two hepatic tumors. After operation, the patient was reviewed regularly.
Right hepatectomy and lymph node dissection were planned to provide an optimized method for the complete resection of the large mass. After operation, the patient accepted regular reexamination.
The surgery was successful and both tumors were completely removed. Postoperative regular review was performed until now, the patient recovered well, and no recurrence or metastasis was seen during the 28 mo follow-up.
The huge tumor and enlarged lymph nodes were completely removed without major intraoperative complications. After operation, the patient was followed-up until now, and no recurrence or metastasis appeared during the 10 mo follow-up.
The IPT-like FDC tumor is an extremely rare and low-grade malignant soft tissue sarcoma[1]. It presents with indolent clinical course, and is typically asymptomatic like in our cases, except for the side effects of weight loss and fever, and is associated with EBV[3]. In contrast to the female patient population predominance reported in the literature[1,3,9], the sex ratio of patients was equal in our series. This difference might be caused by the small number of cases. Previous studies have shown that the age range of the disease was 28-68 years[1]. In our series, the mean age at diagnosis is 39.5 years (range, 31-48 years). A strong relationship between FDC tumors and Castleman disease or autoimmune conditions have been reported in the literature[10,11]. This may be the reason why the case 2 patient developed a thyroid dysfunction. However, the exact mechanism remains unclear.
IPT-like FDC tumors are diagnosed based on variable amounts of spindle tumor cells with intense lymphoplasmacytic infiltrates, coupled with a heavy inflammatory background and supportive immunohistochemistry[1,12]. Moreover, the positive expression of EBV-encoded small RNA is helpful for diagnosis[1,3,12]. However, many IPT-like FDC tumors were previously diagnosed as IPT because specific immunohistochemical markers are not recognized to represent FDCs. The best available immunohistochemical markers for IPT-like FDC tumors are CD21, CD23 and CD35[1,12,13].
To the best of our knowledge, the radiologic findings of IPT-like FDC tumors of the liver have rarely been described. We only found two reports that briefly mention the imaging features of IPT-like FDC tumors of the liver (see Wu et al[14]), where the tumor appeared as a well-defined, heterogeneous arterial-enhanced mass without significant portovenous washout. However, this study did not provide the delay phase images; in another case report study[3], they only showed portal phase images without a summary of imaging appearances. Therefore, the enhancement patterns in the multiphase CT and MRI imaging findings of IPT-like FDC tumors have not been previously reported.
The imaging features of IPT-like FDC tumors of the liver aid in making correct diagnoses before treatment when a neoplasm is detected. In unenhanced US, the lesions showed well- or ill-defined heterogeneous hypoechoic tumors with or without detectable internal blood flow. Correlating to CT imaging, there is no calcification. The findings of unenhanced US imaging were non-specific and difficult to distinguish from other cystic and solid liver tumors[15-17]. In CT images (Table 1), all three tumors showed heterogeneous soft-tissue density with sustained hypoenhancement. This finding is consistent with the findings of Li et al[18] and Bui et al[19], who summarized the CT imaging findings of abdominal FDC sarcomas. Both of the cases we reported here had low-density areas after intravenous administration of contrast medium, but their pathological components were different. In case 1, the low-attenuating areas decreased during the multiphase images. This area histologically corresponded to the tumor’s spindle cells and fibrotic components, which showed centripetally persistent enhancement. These imaging features were also reported in IPT-like FDC tumors of the spleen[19,20]. However, in case 2, the hypodense parts were due to necrosis and hemorrhage in enhancement scanning, which might be related to the relatively large size[18]. On MRI (Table 1), with hepatocyte-specific contrast agents (Gd-EOB-DTPA) in case 1, the most characteristic feature was the central stellate enhancement during HBP. The mechanistic explanation for the retention of gadoxetic acid on HBP images remains elusive, but it may have something to do with the transporter’s located on either the sinusoidal (OATP1B1/3 and MRP3) or canalicular (MRP2) membranes of the cell[21]. Moreover, the stellate enhancement areas during HBP within the masses was T1 and T2 hypointense. Furthermore, the enhancement pattern in multiphase MRI was similar to that in CT.
Differential diagnosis of IPT-like FDC tumors of the liver should include many tumors, such as IPTs, HCCs, metastases, leiomyosarcomas, malignant fibrous histiocytomas, fibrosarcomas, extragastrointestinal stromal tumors, solitary fibrous tumors, and neurogenic tumors[18]. The imaging features of the above tumors overlap with IPT-like FDC tumors, and the first three are the main differential diagnosis. IPTs of the liver usually appears as heterogeneous enhancing tumors with uneven peripheral enhancements[22]. Suspicion should be high for IPT in patients with low grade fever, jaundice, hepatomegaly, weight loss or leukocytosis[23]. Typical HCC is characterized by portal phase washout, elevated AFP and threshold growth at follow-up imaging[22,24]. However, atypical HCC is sometimes difficult to distinguish from sarcomas and metastases[24,25]. Metastasis is most common in colon cancer[16], with typical peripheral rim enhancements and hypoavascular internal necrotic regions[15]. However, different primary tumors have different imaging characteristics upon metastasis to the liver[16,23]. Thus the differential diagnosis is very difficult. Given the rarity of IPT-like FDC tumors of the liver, preoperative imaging diagnosis of these tumors can be challenging; correct final diagnoses depend upon histopathological testing and immunohistochemical markers.
There is no standard treatment for IPT-like FDC tumors of the liver. If the lesion is confined to the liver, surgical excision is the treatment of choice[12]. There is no evidence to prove that adjuvant therapy is effective for this tumor[20]. However, given the potential of recurrence after hepatic lobectomy, surveillance is currently suggested after resection[1,19].
In conclusion, the IPT-like FDC tumor of the liver is an extremely rare tumor. When hepatic tumors show heterogeneous sustained hypoenhancement with patchy enhancement during HBP, IPT-like FDC tumors should be considered in the differential diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corvino A S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Chen Y, Shi H, Li H, Zhen T, Han A. Clinicopathological features of inflammatory pseudotumour-like follicular dendritic cell tumour of the abdomen. Histopathology. 2016;68:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Levi Sandri GB, Colasanti M, Vennarecci G, Ettorre GM. Paraneoplastic arthritis as first symptom of a liver inflammatory pseudotumor-like follicular dendritic cell sarcoma. Liver Int. 2016;36:1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Nguyen BD, Roarke MC, Yang M. Synchronous hepatic and splenic inflammatory pseudotumour-like follicular dendritic cell sarcomas. Liver Int. 2015;35:1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Ang WW, Bundele MM, Shelat VG. Follicular dendritic cell sarcoma: Rare presentation of incidental large hepatic mass. Ann Hepatobiliary Pancreat Surg. 2019;23:74-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Zhu C, Hu Y, Qin X. Hepatic inflammatory pseudotumour-like follicular dendritic cell tumor: A case report. Mol Clin Oncol. 2017;6:547-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Maurea S, Corvino A, Imbriaco M, Avitabile G, Mainenti P, Camera L, Galizia G, Salvatore M. Simultaneous non-functioning neuroendocrine carcinoma of the pancreas and extra-hepatic cholangiocarcinoma. A case of early diagnosis and favorable post-surgical outcome. JOP. 2011;12:255-258. [PubMed] |
| 7. | Kim R, Lee JM, Shin CI, Lee ES, Yoon JH, Joo I, Kim SH, Hwang I, Han JK, Choi BI. Differentiation of intrahepatic mass-forming cholangiocarcinoma from hepatocellular carcinoma on gadoxetic acid-enhanced liver MR imaging. Eur Radiol. 2016;26:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Corvino A, Corvino F, Radice L, Catalano O. Synchronous mucinous colonic adenocarcinoma and multiple small intestinal adenocarcinomas: report of a case and review of literature. Clin Imaging. 2015;39:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Horiguchi H, Matsui-Horiguchi M, Sakata H, Ichinose M, Yamamoto T, Fujiwara M, Ohse H. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen. Pathol Int. 2004;54:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Jain P, Milgrom SA, Patel KP, Nastoupil L, Fayad L, Wang M, Pinnix CC, Dabaja BS, Smith GL, Yu J, Hu S, Bueso Ramos CE, Kanagal-Shamanna R, Medeiros LJ, Oki Y, Fowler N. Characteristics, management, and outcomes of patients with follicular dendritic cell sarcoma. Br J Haematol. 2017;178:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Li L, Shi YH, Guo ZJ, Qiu T, Guo L, Yang HY, Zhang X, Zhao XM, Su Q. Clinicopathological features and prognosis assessment of extranodal follicular dendritic cell sarcoma. World J Gastroenterol. 2010;16:2504-2519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Li XQ, Cheuk W, Lam PW, Wang Z, Loong F, Yeong ML, Browett P, McCall J, Chan JK. Inflammatory pseudotumor-like follicular dendritic cell tumor of liver and spleen: granulomatous and eosinophil-rich variants mimicking inflammatory or infective lesions. Am J Surg Pathol. 2014;38:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Wu CH, Chiu NC, Yeh YC, Kuo Y, Yu SS, Weng CY, Liu CA, Chou YH, Chiou YY. Uncommon liver tumors: Case report and literature review. Medicine (Baltimore). 2016;95:e4952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Corvino A, Sandomenico F, Setola SV, Corvino F, Tafuri D, Catalano O. Morphological and dynamic evaluation of complex cystic focal liver lesions by contrast-enhanced ultrasound: current state of the art. J Ultrasound. 2019;22:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Harvey CJ, Albrecht T. Ultrasound of focal liver lesions. Eur Radiol. 2001;11:1578-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Corvino A, Catalano O, Corvino F, Petrillo A. Rectal melanoma presenting as a solitary complex cystic liver lesion: role of contrast-specific low-MI real-time ultrasound imaging. J Ultrasound. 2016;19:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Li J, Geng ZJ, Xie CM, Zhang XK, Chen RY, Cai PQ, Lv XF. Computer Tomography Imaging Findings of Abdominal Follicular Dendritic Cell Sarcoma: A Report of 5 Cases. Medicine (Baltimore). 2016;95:e2404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Bui PL, Vicens RA, Westin JR, Jensen CT. Multimodality imaging of Epstein-Barr virus-associated inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen: case report and literature review. Clin Imaging. 2015;39:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Kwon H. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen. Turk J Gastroenterol. 2018;29:128-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Ba-Ssalamah A, Antunes C, Feier D, Bastati N, Hodge JC, Stift J, Cipriano MA, Wrba F, Trauner M, Herold CJ, Caseiro-Alves F. Morphologic and Molecular Features of Hepatocellular Adenoma with Gadoxetic Acid-enhanced MR Imaging. Radiology. 2015;277:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Elsayes KM, Menias CO, Morshid AI, Shaaban AM, Fowler KJ, Tang A, Chernyak V, Szklaruk J, Bashir MR. Spectrum of Pitfalls, Pseudolesions, and Misdiagnoses in Noncirrhotic Liver. AJR Am J Roentgenol. 2018;211:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Guarino B, Catalano O, Corvino A, Corvino F, Amore A, Petrillo A. Hepatic inflammatory pseudotumor: educational value of an incorrect diagnosis at contrast-enhanced ultrasound. J Med Ultrason (2001). 2015;42:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Corvino A, Catalano O, Setola SV, Sandomenico F, Corvino F, Petrillo A. Contrast-enhanced ultrasound in the characterization of complex cystic focal liver lesions. Ultrasound Med Biol. 2015;41:1301-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Corvino A, Catalano O, Corvino F, Sandomenico F, Petrillo A. Diagnostic Performance and Confidence of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Cystic and Cysticlike Liver Lesions. AJR Am J Roentgenol. 2017;209:W119-W127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |