Published online Nov 7, 2019. doi: 10.3748/wjg.v25.i41.6238
Peer-review started: July 12, 2019
First decision: August 18, 2019
Revised: October 10, 2019
Accepted: October 17, 2019
Article in press: October 17, 2019
Published online: November 7, 2019
Processing time: 120 Days and 12.7 Hours
Jaundice or preoperative cholestasis (PC) are typical symptoms of pancreatic masses. Approximately 50% of patients undergo preoperative biliary drainage (PBD) placement. PBD is a common cause of bacterobilia (BB) and is a known surgical site infection risk factor. An adjustment of preoperative antibiotic prophylaxis (PAP) may be reasonable according to the profile of BB. For this, we examined the microbiological findings in routine series of patients.
To investigate the incidence and profile of biliary bacterial colonization in patients undergoing pancreatic head resections.
In the period from January 2009 to December 2015, 285 consecutive pancreatic head resections were performed. Indications for surgery were malignancy (71%), chronic pancreatitis (18%), and others (11%). A PBD was in 51% and PC was in 42%. The standard PAP was ampicillin/sulbactam. Intraoperatively, a smear was taken from the hepatic duct. An analysis of the isolated species and resistograms was performed. Patients were categorized according to the presence or absence of PC (PC+/PC-) and PBD (PBD+/PBD-) into four groups. Antibiotic efficiency was analyzed for standard PAP and possible alternatives.
BB was present in 150 patients (53%). BB was significantly more frequent in PBD+ (n =120) than in PBD- (n = 30), P < 0.01. BB was present both in patients with PC and without PC: (PBD-/PC-: 18%, PBD-/PC+: 30%, PBD+/PC-: 88%, PBD+/PC+: 80%). BB was more frequent in malignancy (56%) than in chronic pancreatitis (45%). PBD, however, was the only independent risk factor in multivariate analysis. In total, 357 pathogens (342 bacteria and 15 fungi) were detected. The five most common groups (n = 256, 74.8%) were Enterococcus spp. (28.4%), Streptococcus spp. (16.9%), Klebsiella spp. (12.6%), Escherichia coli (10.5%), and Enterobacter spp. (6.4%). A polymicrobial BB (PBD+: 77% vs PBD-: 40%, P < 0.01) and a more frequent detection of Enterococcus (P < 0.05) was significantly associated with PBD+. In PBD+, the efficiency of imipenem and piperacillin/tazobactam was significantly higher than that of the standard PAP (P < 0.01).
PBD-/PC- and PBD-/PC+ were associated with a low rate of BB, while PBD+ was always associated with a high rate of BB. In PBD+ patients, BB was polymicrobial and more often associated with Enterococcus. In PBD+, the spectrum of potential bacteria may not be covered by standard PAP. A more potent alternative for prophylactic application, however, was not found.
Core tip: The aim of our retrospective study was to analyze the microbial profile of bacteriobilia in patients undergoing pancreatic head resection (n = 285). Patients with preoperative biliary drainage (PBD+) had a significantly elevated risk of bacteriobilia. In those cases, the contamination was polymicrobiotic. Enterococcus was significantly more frequent in PBD+. Our standard preoperative antibiotic prophylaxis reaches an overall sensitivity rate of 60% and a resistance rate of 25% in 342 bacteria. The antibiotic efficiency of Imipenem and Piperacillin/Tazobactam is significantly higher in PBD+. The data on polymicrobial colonization of the biliary tract may be useful for the decision on antibiotic treatment in case of postoperative infection until the final results from the intraoperative smear are available. A more potent alternative for prophylactic application, however, was not found among the examined antibiotics.
- Citation: Krüger CM, Adam U, Adam T, Kramer A, Heidecke CD, Makowiec F, Riediger H. Bacterobilia in pancreatic surgery-conclusions for perioperative antibiotic prophylaxis. World J Gastroenterol 2019; 25(41): 6238-6247
- URL: https://www.wjgnet.com/1007-9327/full/v25/i41/6238.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i41.6238
With modern tomography techniques that yield cross-sectional images, a sufficiently predictive prognosis for the operability of pancreatic masses is now possible. A purely diagnostic endoscopic retrograde cholangiopancreatography (ERCP) is thus obsolete. In preoperative cholestasis (PC), the indication for preoperative biliary drainage (PBD) may exist. Often, however, the indication for ERCP is made even before operability has been determined. For example, an analysis of Medicare patients (1992-2007) showed that one in two patients already has a PBD at the time of the surgical consultation[1]. The disadvantage is that ERCP leads almost always to bacterobilia (BB)[2-4]. BB is a risk factor (up to factor 2) for surgical site infection[5-9].
According to the current guideline recommendations for pancreatic carcinoma, preoperative ERCP shall only be carried out if cholangitis is present or if surgery cannot be performed soon. A basic indication for preoperative antibiotic prophylaxis (PAP) in pancreatic resections is given regardless of the PBD status[8]. In the present study, the collected microbiological data are used to investigate whether a differentiated recommendation for PAP can be derived.
In the period from January 2009 to December 2015, 285 pancreatic head resections were performed at the Vivantes-Humboldt-hospital in Berlin. Pylorus preserving pancreatoduodenectomy was the standard procedure (n = 259; 91%) and Wipple was only performed in selected cases (n = 26; 9%). The morbidity was 59% (n = 169) and the mortalitiy was 3.8% (n = 11).
Intraoperatively, a standard aseptic smear of the bile fluid was obtained after the hepatocholedochal duct was severed on average 90 min after skin incision. It was then used for routine microbiological diagnostics.
During the study period, preoperative ERCP was performed in case of cholestasis due to evaluation of the initially treating gastroenterologist. Under surgical surveillance, it was always performed in patients with relevantly elevated bilirubin (> 8 mg/dL) and if a pancreas head resection could not be performed promptly. According to earlier data, serum bilirubin above 5 mg/dL already reduces liver function and thus a PBD is recommended[10].
During the study period, antibiotics were administered as follows: The standard PAP (ampicillin-sulbactam) was administered 30 min before the skin incision, with a second dose after 4 h of surgery. In the case of penicillin allergy, ciprofloxacin and metronidazole were used. If a postoperative infection was suspected, empirical treatment with piperacillin/tazobactam was carried out, which was adjusted after receiving the result of the smear.
The microbiological specimens were examined according to standard microbiological procedures. Sensitivity and resistance for the tested antibiotics were analyzed in this study. Intermediate sensitivity was considered as resistance according to clinical routine. The number of detected sensitivities and resistances was related to the number of bacteria in each group, even though not all antibiotics were tested for all genera. This procedure allowed comparability of the groups. The results are presented as "antimicrobial efficiency" (AE: Sensitivity in %/resistance in %).
All perioperative and microbiological data were collected in a local scientific database (IBM SPSS Statistics, IBM, version 22). For the significance calculation, both the Chi-squared and McNemar tests were used. An additional binary logistic regression analysis was performed. The graphs were created with the program "GraphPad Prism" (GraphPad Software). The workgroup affirms that the statistical review of the study was performed by a biomedical statistician.
The study defined the presence of a PC at a measured serum value above the bilirubin standard (1.2 mg/dL) in the final check prior to surgery. Some patients had a completely recovered cholestasis after placement of a biliary drainage. They were classified as PBD+ and PC-. Patient data were divided into 4 groups according to clinical criteria: PBD-/PC-, PBD-/PC+, PBD+/PC-, and PBD+/PC+.
The surgical treatment data are summarized in Table 1. BB was present in 150 patients (53%). In multivariate analysis of all factors from Table 1, only PBD was disclosed as an independent risk factor (P < 0.01).
| All patients, n = 285 (%) | With bacteriobilia (BB+), n = 150 (%) | Without bacteriobilia (BB-), n = 135 (%) | P value | |
| Indications | ||||
| Malignancy | n = 202 (71) | n = 114 (56)a | n = 88 (44) | < 0.05 |
| cP1 | n = 51 (18) | n =23 (45) | n =28 (55) | 0.23 |
| Others | n = 32 (11) | n =13 (41) | n =19 (59) | 0.15 |
| Biliary drainage | ||||
| PBD+ | n =144 (51) | n = 120 (83)b | n = 24 (17) | < 0.01 |
| PBD- | n =141 (49) | n = 30 (21)b | n = 111 (79) | |
| Cholestasis | ||||
| PC+ | n = 120 (42) | n = 73 (61)c | n = 47 (39) | 0.02 |
| PC- | n = 165 (58) | n = 77 (47)c | n = 88 (53) |
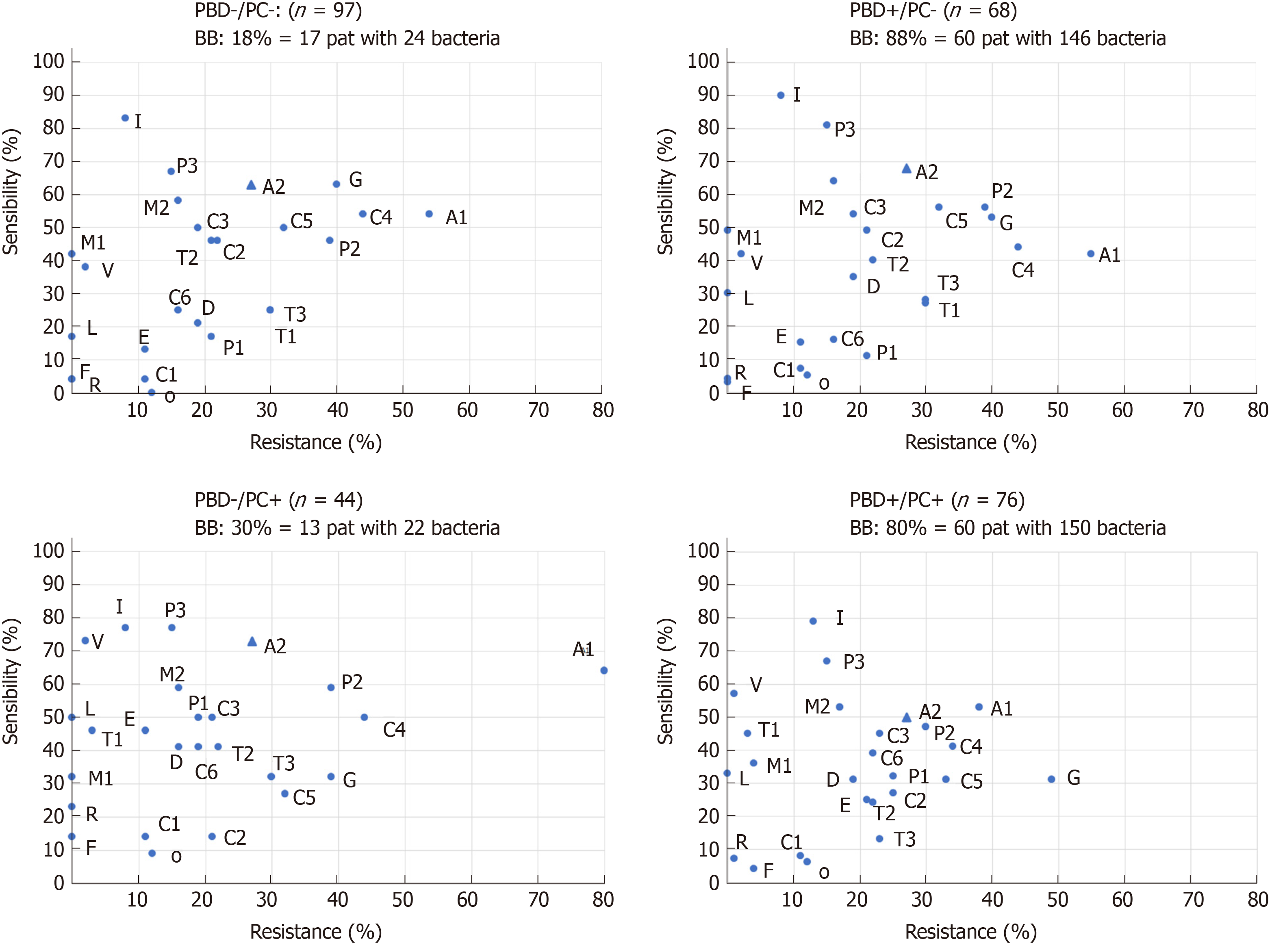
A total of 357 isolates were cultivated (342 bacteria, 15 fungi). With PBD+, BB was significantly more common than with PBD- (BB for PBD+: n = 120, 83.3% vs BB for PBD-: n = 30, 21.4%, P < 0.01). In the subgroup PBD+, the BB rate was high regardless of the presence of cholestasis (BB for PC+: 80% and with PC-: 88%). In the subgroup PC+ (n = 120), the BB rate with PBD+ was significantly higher compared to PBD- (PBD+/PC+: n = 60, 80% vs PBD-/PC+: n = 13; 30%, P < 0.01). In the four clinical groups, the incidence of BB with PBD-/PC- was 18%, with PBD-/PC+ 30%, with PBD+/PC- 88% and with PBD+/PC+ 80% (Table 2).
| Clinical group | Number of patients, (n) | Patients with bacterobilia, n (%) | Bacteria quantity, n | Fungi |
| PBD-/PC-No stent/no cholestasis | 97 | 17 (18) | 24 | 0 |
| PBD-/PC+No stent/cholestasis | 44 | 13 (30) | 22 | 2 |
| PBD+/PC-Stent/no cholestasis | 68 | 60 (88) | 146 | 4 |
| PBD+/PC+Stent/cholestase | 76 | 60 (80) | 150 | 9 |
| All groups | 285 | 150 (53) | 342 |
Of 342 bacteria, the five most commonly isolated (TOP5; n = 256, 74.8%) represented about three-quarters of all pathogens, and included the following genera: Enterococcus (n = 97), Streptococcus (n = 58), Klebsiella (n = 43), Escherichia (n = 36), and Enterobacter spp. (n = 22). In contrast, there were 13 different genera with a smaller number of cases (Varia: n = 86, 25.2%): Staphylococcus (n = 21), Citrobacter (n = 14), Bacteroides (n = 11), Prevotella (n = 8), Lactobacillus (n = 5), Pseudomonas (n = 4), Aeromonas (n = 3), Raoultella (n = 3), Morganella (n = 2), Clostridium (n = 2), Proteus (n = 2), Fusobacterium (n = 2), Hafnia (n = 2), Proteus (n = 2), MRSA (n = 2), Stenotrophomonas (n = 1), Achromobacter (n = 1), Lactococcus (n = 1), Gemella (n = 1), and Rothia spp. (n = 1).
Four species were multi-resistant bacteria (two MRSA and two triple-resistant Klebsiella pneumoniae). Anaerobes [Bacteroides (n = 11), Prevotella (n = 8), Clostridia (n = 2) and Fusobacteria (n = 2)] were rare (total: n = 23, 6.7%). A fungal colonization (total: n = 15, 4%) was significantly more frequent with PBD+ (n = 13, 9%) than with PBD- (n = 2, 1%) (P < 0.01) and was always associated with the occurrence of at least one bacterial genus. Out of 15 fungi, 13 cases (87%) consisted of Candida spp.
With PBD+, there was a higher rate (86%) and variety of bacteria. The distribution of bacteria in the groups PBD-/PC- and PBD-/PC+ consisted of only 8 genera per group, but in among the PBD+, 18 genera were found for PBD+/PC- and 16 for PBD+/PC+. The TOP5 were most common in both PBD+ and PBD- [PBD+: n = 222 (75%) vs PBD-: n = 34 (74%), P = 0.87] (Table 2). In contrast, the Varia/other were found almost exclusively with PBD+ (n = 74, 86%). In Subgroup analysis of the most frequent (TOP5) bacteria, only Enterococcus was significantly more frequent in PBD+. A broad spectrum of bacteria and a polymicrobial colonization was significantly characteristic of patients with PBD+ (Table 3).
| Patients with… | PBD-, n (%) | PBD+, n (%) | P value |
| 30 (20) | 120 (80) | ||
| Polymicrobial mixed flora (n = 104) | 12 (40)d | 92 (78) | < 0.01 |
| Enterococci (n = 82) | 9 (30)e | 73 (61) | < 0.05 |
| Streptococci (n = 41) | 6 (20) | 35 (29) | 0.32 |
| Klebsiella (n = 38) | 6 (20) | 32 (27) | 0.45 |
| Escherichia (n = 35) | 8 (27) | 27 (23) | 0.60 |
| Enterobacter (n = 21) | 1 (3) | 20 (17) | 0.06 |
| Multi-drug resistant bacteria (n = 4) | 1 (3) | 3 (3) | 0.80 |
The antimicrobial efficacy (AE) of the tested antibiotics in the whole group (n = 342) was 84%/11% for imipenem, 74%/16% for piperacillin-tazobactam, 60%/26% for ampicillin-sulbactam, 43%/33% for ciprofloxacin, 59%/17% for moxifloxacin, 50%/22% for ceftriaxone, and 44%/39% cefuroxime. The outstanding AE of imipenem is also evident in the graphical representation of all four clinical groups. Piperacillin-tazobactam was only significantly more effective with PBD+/PC+ and PBD+/PC- than PAP with ampicillin-sulbactam. This in turn also differed significantly from ciprofloxacin, cefuroxime, and ceftriaxone. The sensitivity rates of the other substances were lower than those of the PAP. Moxifloxacin has a good AE similar to that of the PAP used (Figure 1 and Table 4).
| Ampicillin Sulbactam | Imipenem | Piperacillin Tazobactam | Ciprofloxacin | Moxifloxacin | Ceftriaxon | Cefuroxim | ||
| PBD+ (n = 296) | Sensitivity | 174 (59) | 249 (84)e | 219 (74)e | 129 (44)e | 173 (58) | 147 (50)f | 126 (43)e |
| Resistance | 81 (27) | 31 (11)e | 45 (15)e | 96 (32) | 49 (17)e | 62 (21)f | 115 (39)e | |
| PBD- (n = 46) | Sensitivity | 31 (67) | 37 (80) | 33 (72) | 18 (39) + | 27 (59) | 23 (50)f | 24 (52) |
| Resistance | 8 (17) | 7 (15) | 8 (17) | 15 (33) | 8 (17) | 13 (28) | 18 (39)f |
Compared to the clinically relevant substances (ampicillin-sulbactam and piperacillin-tazobactam), there was no significant difference in AE in enterococci, streptococci and Klebsiella spp. (together: n = 198; 57%). However, in Escherichia and Enterobacter spp. as well as in the rarer genera (together: n = 144, 43%), a significant difference in the AE was found. The AE of PAP was lower in about half of the bacteria in direct comparison of the two named substances (Table 5).
| Sensitivity (%) | Signifi-cance | Resistance (%) | Significance | ||||
| AS | PT | P | AS | PT | P value | ||
| TOP5(n = 256) | Enterococci (n = 97) | 71 | 70 | NS | 27 | 29 | NS |
| Streptococci (n = 58) | 60g | 88 | < 0.01 | 0 | 0 | NS | |
| Klebsiella (n = 43) | 86 | 98 | NS | 14h | 0 | 0.03 | |
| Escherichia (n = 36) | 81g | 100 | < 0.01 | 19i | 0 | 0.02 | |
| Enterobacter (n = 22) | 5g | 91 | < 0.01 | 95g | 0 | < 0.01 | |
| Varia(n = 86) | Other genera | 40g | 80 | < 0.01 | 34g | 12 | < 0.01 |
BB often occurs after interventions on the bile duct system. A preoperative ERCP is the typical trigger[7,11-13]. Prophylactic use of antibiotics as part of ERCP cannot reliably affect BB or cholangitis. Again, even biliary excreted Ciprofloxacin does not provide uniform protection against post-ERCP cholangitis[2].
The suspended sphincter function of the papilla of Vater leads to colonization and a pathogen shift in the hepatobiliary system[1,14,15]. In only a few cases of our data, BB was detected without prior ERCP. However, a high BB rate is typical in patients with PBD+. This may have consequences for perioperative antibiotic prophylaxis (PAP). For pancreatic surgery, ampicillin-sulbactam is used for PAP in our clinic. It is not clear whether this strategy is sufficient for all patients prior to pancreatic resection. According to the recommendations of the guideline, no adaptation of the PAP depending on the PBD or PC status has been performed so far[8].
The literature describes a BB rate of 87% and 98%, respectively, with PBD+ vs 21% and 55%, respectively, for PBD- and the frequent detection of a mixed flora with PBD+[12,16], which is in line with our own data. The introduction of a drain represents a risk independent of the presence of a preoperative cholestasis[14]. As PBD leads to an increase in postoperative complications, it shall not be performed routinely but only in selected cases[9].
In a study of 91 ERCP patients, 15 different pathogen groups are identified, with Escherichia coli (28%) being the largest group[17]. In intraoperative smears of the hepatic duct, Klebsiella (18%), and enterococci spp. (13%) are the most frequent pathogens[18]. In patients with ERCP, enterococci spp. (31%) are most frequently detected with PBD+ and Escherichia coli (17%) with PBD-[12]. The literature also describes a shift to a more aggressive pathogen spectrum with an increase of E. cloacae and E. faecalis. At the same time, there is a lower sensitivity to ampicillin-sulbactam[19]. Therefore, it is recommended that an empirical PAP should take into account the more aggressive pathogen spectrum with PBD+[13]. A significant increase of the mentioned genera was not found in our total of 342 bacteria However, Enterococcus was significantly more frequent in PBD+ patients. The frequent detection of Enterobacter with PBD+ and the limited AE of ampicillin-sulbactam must be emphasized, too.
Of the antibiotics tested, imipenem is the most potent antibiotic substance. However, as it is an important reserve antibiotic, it should not be used for PAP. Our own data show a lower AE of ampicillin-sulbactam compared with piperacillin-tazobactam, depending on the implantation of a biliary drainage. The superiority of piperacillin-tazobactam is significantly detectable with PBD+. Same as Imipenem, this antibiotic has its place in treatment and not in prophylaxis. However, the AE of ampicillin-sulbactam stands out in comparison to the other substances examined and seems to be well suited to a PAP with PBD-.
Since anaerobes accounted for only 6% of the isolated genera, the administration of metronidazole in addition to the PAP does not seem to be necessary. In our data, the AE of moxifloxacin is comparable with that of the PAP. However, this substance has no indication with cholangitis.
In contrast to the guideline, adaptation of perioperative antibiotic therapy in the context of pancreatic surgery as a function of PBD status is recommended in the literature[8,20]. This is justified by an increase in infectious complications (especially wound infections) with PBD+[6,21]. However, severe complications or mortality are not affected by BB in this case[22].
In our own data, there is often only one species with PBD-. In addition, the pathogen spectrum is less aggressive (rarely Enterobacter spp.). We categorize the group PBD-/PC- as a low risk group due to the low BB probability. There is a slightly higher risk (30%) of BB for PBD-/PC+ as well as a relative accumulation of rare pathogens, so that we assess the risk of this group as intermediate. We classify PBD+/PC- and PBD+/PC+ as high-risk groups, because BB with polymicrobial mixed flora is almost always found.
Based on the findings of the resistogram, the AE of the PAP is sufficient when used in low clinical-risk situations. It should be noted, however, that depending on the regional resistance situation, other preferences for the PAP may arise. Therefore, continuous analysis and evaluation of the species isolated from the hepatocho-ledochus duct is recommended.
On the basis of our data, a risk-adapted intensification to PAP with piperacillin-tazobactam may be seriously considered. In the literature, even with high risk, the recommendation is a primary 5-d therapy with later adjustment to the microbiological findings[23]. However, in our opinion, our own microbiological data do not allow this clinical conclusion. This would require the detection of significantly increased postinterventional infections with pathogens sensitive to piperacillin-tazobactam in this patient group. It is necessary to further analyze our own clinical data in this respect. In case of postoperative infection, these data have to be reminded in the selection of antibiotic treatment until the final results from the intraoperative smear are available.
In conclusion, A smear should always be taken intraoperatively during a pancreas head resection. This allows targeted subsequent antibiotic therapy in the perioperative course. The data of the clinical groups yield the following consequences: Patients with PBD+ are at high risk for polymicrobial bacteriobilia. Standard PAP may not sufficiently cover all genera in those patients. A significantly better antibiotic efficiency exists only for Imipenem and Piperacillin/Tazobactam. Their administration, however, can not be recommended for prophylactic use. An adequate alternative to our standard PAP can not be derived from our data.
Preoperative biliary drainage (PBD) is a common cause of bacterobilia (BB) and is a known surgical site infection risk factor, especially in pancreatoduodenectomies.
An adjustment of preoperative antibiotic prophylaxis (PAP) may be reasonable according to the profile of BB. However, current guidelines do not recommend an adoption of the PAP according to the PBD status.
The objective of this study was to analyze the bacterial profile in routine patients undergoing pancreatic surgery and to find out, if our PAP is adequate for our patients. Antibiotic efficiency was analyzed for standard PAP and possible alternatives.
In the period from January 2009 to December 2015, 285 consecutive pancreatic head resections were performed. Indications for surgery were malignancy (71%), chronic pancreatitis (18%), and others (11%). A PBD was in 51% and preoperative cholestasis (PC) was in 42%. The standard PAP was ampicillin/sulbactam. Intraoperatively, a smear was taken from the hepatic duct. Patients were categorized according to the existence or lack of PC (PC+/PC-) and PBD (PBD+/PBD-).
BB was present in 150 patients (53%). BB was significantly more frequent in PBD+ (n = 120) than in PBD- (n = 30), P < 0.01. BB was more frequent in malignancy (56%) than in chronic pancreatitis (45%). PBD, however, was the only independent risk factor for BB in multivariate analysis (P < 0.01). The five most common groups (n = 256, 74.8%) were Enterococcus spp. (28.4%), Streptococcus spp. (16.9%), Klebsiella spp. (12.6%), Escherichia coli (10.5%), and Enterobacter spp. (6.4%). A polymicrobial BB (PBD+: 77% vs PBD-: 40%, P < 0.01) and a more frequent detection of Enterococcus (P < 0.05) was significantly associated with PBD+. In PBD+, the efficiency of imipenem and piperacillin/tazobactam was significantly higher than that of the standard PAP (P < 0.01).
PBD-/PC- and PBD-/PC+ were associated with a low rate of BB, while PBD+ was always associated with a high rate of BB. In PBD+ patients, BB was polymicrobial and more often associated with Enterococcus. In PBD+, the spectrum of potential bacteria may not be covered by standard PAP. A more potent alternative for prophylactic application, however, was not found.
The perspective of this study is to show more differentiated ways of perioperative antibiotic prophylaxis and to stratify patient groups according to PBD and PC status. As patients with PBD+ are not full covered by standard PAP, these patients have a well-known high risk for infectious complications. A more proper PAP is required. In these selected patients a primary antibiotic treatment adopted to the (suspected) resistogramm.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kitamura K, Ueno M S-Editor: Tang JZ L-Editor: A E-Editor: Ma YJ
| 1. | Jinkins LJ, Parmar AD, Han Y, Duncan CB, Sheffield KM, Brown KM, Riall TS. Current trends in preoperative biliary stenting in patients with pancreatic cancer. Surgery. 2013;154:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Ratanachu-ek T, Prajanphanit P, Leelawat K, Chantawibul S, Panpimanmas S, Subwongcharoen S, Wannaprasert J. Role of ciprofloxacin in patients with cholestasis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2007;13:276-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: a meta-analysis. Pancreas. 2009;38:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Ceyssens C, Frans JM, Christiaens PS, Van Steenbergen W, Peetermans WE. Recommendations for antibiotic prophylaxis before ERCP: can we come to workable conclusions after review of the literature? Acta Clin Belg. 2006;61:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Mezhir JJ, Brennan MF, Baser RE, D'Angelica MI, Fong Y, DeMatteo RP, Jarnagin WR, Allen PJ. A matched case-control study of preoperative biliary drainage in patients with pancreatic adenocarcinoma: routine drainage is not justified. J Gastrointest Surg. 2009;13:2163-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Sahora K, Morales-Oyarvide V, Ferrone C, Fong ZV, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Preoperative biliary drainage does not increase major complications in pancreaticoduodenectomy: a large single center experience from the Massachusetts General Hospital. J Hepatobiliary Pancreat Sci. 2016;23:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Nomura T, Shirai Y, Hatakeyama K. Enterococcal bactibilia in patients with malignant biliary obstruction. Dig Dis Sci. 2000;45:2183-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Adler G, Seufferlein T, Bischoff SC, Brambs HJ, Feuerbach S, Grabenbauer G, Hahn S, Heinemann V, Hohenberger W, Langrehr JM, Lutz MP, Micke O, Neuhaus H, Neuhaus P, Oettle H, Schlag PM, Schmid R, Schmiegel W, Schlottmann K, Werner J, Wiedenmann B, Kopp I. [S3-Guidelines "Exocrine pancreatic cancer" 2007]. Z Gastroenterol. 2007;45:487-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 9. | van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, Klinkenbijl JH, Nio CY, de Castro SM, Busch OR, van Gulik TM, Bossuyt PM, Gouma DJ. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 698] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 10. | Kawarada Y, Higashiguchi T, Yokoi H, Vaidya P, Mizumoto R. Preoperative biliary drainage in obstructive jaundice. Hepatogastroenterology. 1995;42:300-307. [PubMed] |
| 11. | Negm AA, Schott A, Vonberg RP, Weismueller TJ, Schneider AS, Kubicka S, Strassburg CP, Manns MP, Suerbaum S, Wedemeyer J, Lankisch TO. Routine bile collection for microbiological analysis during cholangiography and its impact on the management of cholangitis. Gastrointest Endosc. 2010;72:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 12. | Rerknimitr R, Fogel EL, Kalayci C, Esber E, Lehman GA, Sherman S. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis. Gastrointest Endosc. 2002;56:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Scheufele F, Aichinger L, Jäger C, Demir IE, Schorn S, Sargut M, Erkan M, Kleeff J, Friess H, Ceyhan GO. Effect of preoperative biliary drainage on bacterial flora in bile of patients with periampullary cancer. Br J Surg. 2017;104:e182-e188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Johnson RC, Ahrendt SA. The case against preoperative biliary drainage with pancreatic resection. HPB (Oxford). 2006;8:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Ertz-Archambault N, Keim P, Von Hoff D. Microbiome and pancreatic cancer: A comprehensive topic review of literature. World J Gastroenterol. 2017;23:1899-1908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Herzog T, Belyaev O, Akkuzu R, Hölling J, Uhl W, Chromik AM. The Impact of Bile Duct Cultures on Surgical Site Infections in Pancreatic Surgery. Surg Infect (Larchmt). 2015;16:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kaya M, Beştaş R, Bacalan F, Bacaksız F, Arslan EG, Kaplan MA. Microbial profile and antibiotic sensitivity pattern in bile cultures from endoscopic retrograde cholangiography patients. World J Gastroenterol. 2012;18:3585-3589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Fathi AH, Jackson T, Barati M, Eghbalieh B, Siegel KA, Siegel CT. Extended Perioperative Antibiotic Coverage in Conjunction with Intraoperative Bile Cultures Decreases Infectious Complications after Pancreaticoduodenectomy. HPB Surg. 2016;2016:3031749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Scheufele F, Schorn S, Demir IE, Sargut M, Tieftrunk E, Calavrezos L, Jäger C, Friess H, Ceyhan GO. Preoperative biliary stenting versus operation first in jaundiced patients due to malignant lesions in the pancreatic head: A meta-analysis of current literature. Surgery. 2017;161:939-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 20. | Sudo T, Murakami Y, Uemura K, Hashimoto Y, Kondo N, Nakagawa N, Ohge H, Sueda T. Perioperative antibiotics covering bile contamination prevent abdominal infectious complications after pancreatoduodenectomy in patients with preoperative biliary drainage. World J Surg. 2014;38:2952-2959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Mohammed S, Evans C, VanBuren G, Hodges SE, Silberfein E, Artinyan A, Mo Q, Issazadeh M, McElhany AL, Fisher WE. Treatment of bacteriobilia decreases wound infection rates after pancreaticoduodenectomy. HPB (Oxford). 2014;16:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Morris-Stiff G, Tamijmarane A, Tan YM, Shapey I, Bhati C, Mayer AD, Buckels JA, Bramhall SR, Mirza DF. Pre-operative stenting is associated with a higher prevalence of post-operative complications following pancreatoduodenectomy. Int J Surg. 2011;9:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Sourrouille I, Gaujoux S, Lacave G, Bert F, Dokmak S, Belghiti J, Paugam-Burtz C, Sauvanet A. Five days of postoperative antimicrobial therapy decreases infectious complications following pancreaticoduodenectomy in patients at risk for bile contamination. HPB (Oxford). 2013;15:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |