Published online Jan 28, 2019. doi: 10.3748/wjg.v25.i4.469
Peer-review started: October 29, 2018
First decision: November 29, 2018
Revised: December 31, 2018
Accepted: January 9, 2019
Article in press: January 10, 2019
Published online: January 28, 2019
Processing time: 92 Days and 2.2 Hours
Gastric ‘indefinite for neoplasm/dysplasia’ (IFND) is a borderline lesion that is difficult to diagnose as either regenerative or neoplastic. There is a need for guidance in the identification of a subset of patients, who have an IFND lesion with a higher risk of malignant potential, to enable risk stratification and optimal management.
To determine the clinical and pathologic factors for the accurate diagnosis of gastric IFND lesions.
In total, 461 gastric lesions diagnosed via biopsy as IFND lesions were retrospectively evaluated. Endoscopic resection (n = 134), surgery (n = 22), and follow-up endoscopic biopsy (n = 305) were performed to confirm the diagnosis. The time interval from initial biopsy to cancer diagnosis was measured, and diagnostic delays were categorized as > 2 wk, > 2 mo, > 6 mo, and > 1 year. The IFND lesions presenting as regenerating atypia (60%) or atypical epithelia (40%) at initial biopsy were adenocarcinomas in 22.6%, adenomas in 8.9%, and gastritis in 68.5% of the cases.
Four clinical factors [age ≥ 60 years (2.445, 95%CI: 1.305-4.580, P = 0.005), endoscopic size ≥ 10 mm (3.519, 95%CI: 1.891-6.548, P < 0.001), single lesion (5.702, 95%CI: 2.212-14.696, P < 0.001), and spontaneous bleeding (4.056, 95%CI: 1.792-9.180, P = 0.001)], and two pathologic factors [atypical epithelium (25.575, 95%CI: 11.537-56.695, P < 0.001], and repeated IFND diagnosis [6.022, 95%CI: 1.822-19.909, P = 0.003)] were independent risk factors for gastric cancer. With two or more clinical factors, the sensitivity and specificity for carcinoma were 91.3% and 54.9%, respectively. Ten undifferentiated carcinomas were initially diagnosed as IFND. In the subgroup analysis, fold change (5.594, 95%CI: 1.458-21.462, P = 0.012) predicted undifferentiated or invasive carcinoma in the submucosal layers or deeper. Diagnostic delays shorter than 1 year were not associated with worse prognoses. Extremely well-differentiated adenocarcinomas accounted for half of the repeated IFND cases and resulted in low diagnostic accuracy even on retrospective blinded review.
More than two clinical and pathologic factors each had significant cut-off values for gastric carcinoma diagnosis; in such cases, endoscopic resection should be considered.
Core tip: At initial biopsy, ‘indefinite for neoplasm/dysplasia’ (IFND) lesions proved to be adenocarcinomas (22.6%). Independent risk factors for gastric IFND cancer were age (≥ 60 years), endoscopic size (≥ 10 mm), single lesion, spontaneous bleeding, atypical epithelia, and repeated IFND diagnosis. Additionally, fold change predicted undifferentiated or invasive carcinoma in the submucosal layers or deeper. However, diagnostic delays shorter than 1 year were not associated with worse prognoses. In summary, for IFND lesions with these features, endoscopic resection may be a better option than repeated endoscopic biopsy. In the absence of associated risk factors, accurate diagnosis through follow-up within 1 year is recommended.
- Citation: Kwon MJ, Kang HS, Kim HT, Choo JW, Lee BH, Hong SE, Park KH, Jung DM, Lim H, Soh JS, Moon SH, Kim JH, Park HR, Min SK, Seo JW, Choe JY. Treatment for gastric ‘indefinite for neoplasm/dysplasia’ lesions based on predictive factors. World J Gastroenterol 2019; 25(4): 469-484
- URL: https://www.wjgnet.com/1007-9327/full/v25/i4/469.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i4.469
‘Indefinite for neoplasm/dysplasia’ (IFND) is a borderline lesion that is difficult to diagnose as either regenerative or neoplastic. Due to the diagnostic challenge following gastric forceps biopsy and as the biologic potential of these lesions is unknown, IFND lesions are classified as Category 2 according to the revised Vienna classification[1-4]. IFND is usually described in pathology reports either as regenerative atypia, atypical epithelia, or atypical gland/cells[2,5,6]. These indefinite terms are used by pathologists for cases displaying cellular architectural distortion and/or nuclear atypia that deviate from the normal but are not completely diagnostic of neoplasia. Pathologists fail to establish a definite diagnosis for reactive change, dysplasia, or carcinoma in part because of the lack of a sufficient quality and quantity of forceps biopsy specimens needed to ensure accurate diagnosis[5,7-9]. Thus, due to the possibility of dysplasia or carcinoma, follow-up evaluation according to the Vienna classification is recommended for gastric IFND cases[4,10,11].
Korea has one of the highest prevalence rates, globally, of gastric cancer[12-14]. Two previous Korean population-based studies showed that IFND lesions in the initial gastric biopsy proved to be gastric cancer in 37.6-62.5% of endoscopically resected specimens[8,9]. Although those patients were monitored for uncertain lesions endoscopically at the follow-up biopsy, difficulties in discriminating between reactive change and neoplasms even with repeated pathologic examinations remained. However, in clinical settings, no clear guideline exists that indicates the exact cut-off time for additional biopsy or endoscopic resection; additionally, there is no subsequent plan for endoscopists in dealing with more than two pathologic reports of IFND lesions at the follow-up biopsy[15]. Thus, there is a critical need for guidance in the identification of a subset of patients through forceps biopsy, who have an IFND lesion with a higher risk of malignant potential, to enable risk stratification and optimal management.
This study aimed to establish the correct diagnosis for gastric IFND lesions by evaluating a series of IFND lesions in detail and determining the key clinical and pathologic predictive factors for gastric cancer. The findings of this study may be useful in informing the decision to either perform biopsy repeatedly or resect gastric IFND lesions, and may be applicable to the prediction of gastric cancer from such lesions.
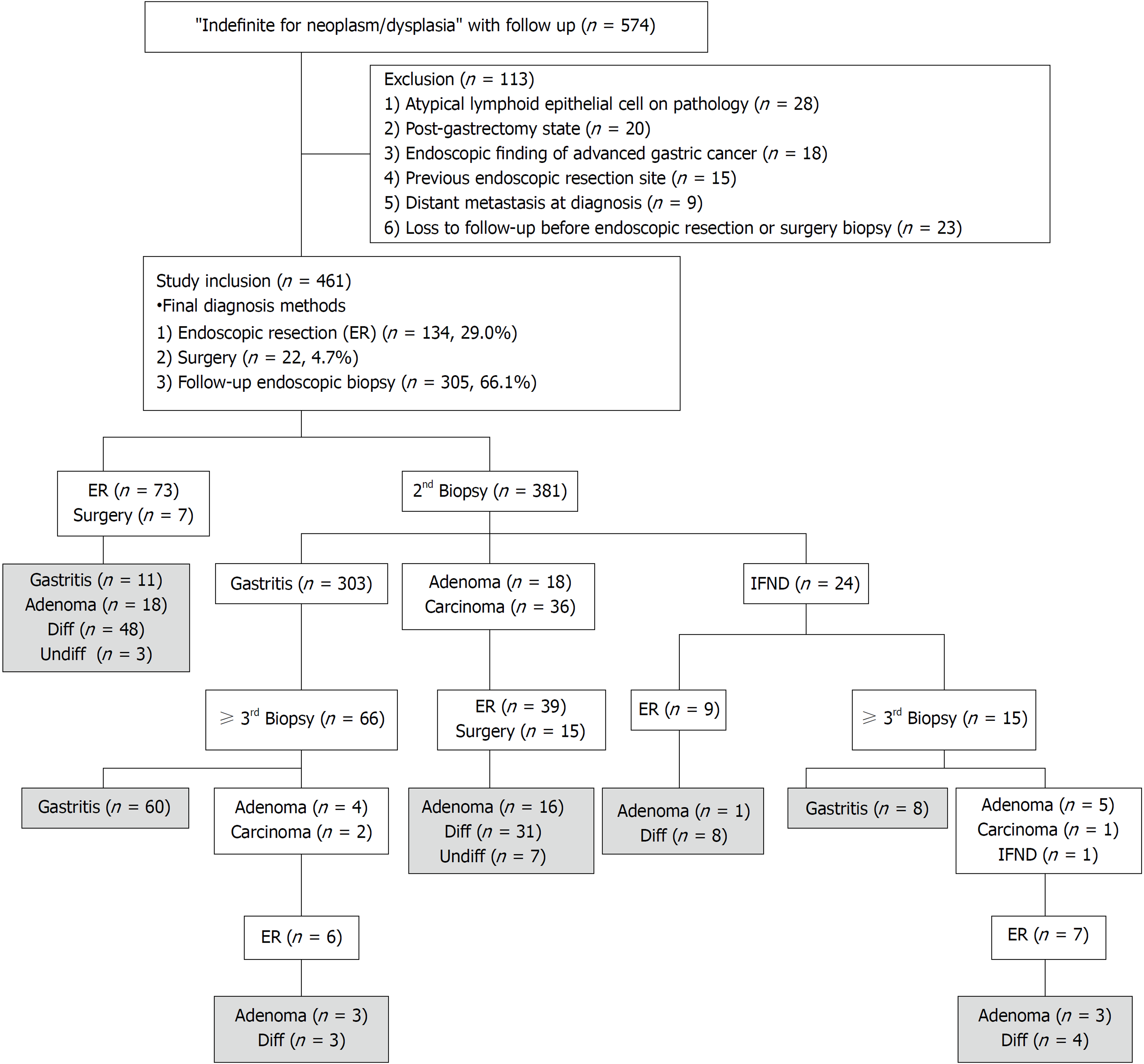
Medical records and pathologic reports of patients who underwent gastric endoscopic biopsy from January 2007 to December 2016 in Hallym University Sacred Heart Hospital were reviewed for the identification of pathologically-reported gastric IFND lesions. Inclusion criteria were the presence of any terms in patient records related to atypical cell/gland/epithelium or regenerative/regenerating atypia, without a confirmative mention of ‘consistent with, suggestive of, suspicious for, or favoring dysplasia or carcinoma’ at the initial biopsy. These inclusion criteria for IFND lesions were based on the Korean pathologic grading system for gastric epithelial proliferative disease and the classification of the Japanese Gastric Cancer Association[2,3]. Records of 574 patients with follow-up data were initially archived (Figure 1); these accounted for 1.04% (574/54781) of all patients who underwent gastric endoscopic biopsy, and during the same period, 1887 gastric cancer cases were diagnosed by endoscopy. Exclusion criteria were the presence of atypical lymphoid cells in pathologic reports (n = 28), previous receipt of subtotal gastrectomy before initial forceps biopsy (n = 20), presence of a definite feature of advanced gastric cancer on endoscopy findings (n = 18), identification of IFND at the previous endoscopic gastric neoplasm resection site (n = 15), diagnosis of distant metastasis at the time of biopsy (n = 9), or loss to follow-up before endoscopic resection or surgery (n = 23). Overall, 113 patients were excluded, and a total 461 patients were enrolled in this study.
In cases with endoscopic resection or surgery, the pathologic findings and clinical data of the final specimens were examined; in the absence of endoscopic resection or surgery, the most recent endoscopic follow-up biopsy finding was considered as the final diagnosis. The 461 pathologic final diagnoses were obtained through endoscopic follow-up biopsy (n = 305), endoscopic resection (n = 134), and surgical resection (n = 22).
We assessed the possible risk factors for diagnostic delays, and the potential effects of such delays on gastric carcinoma risk. The time interval from the onset of the first biopsy to the establishment of cancer diagnosis was also measured. Due to highly variable time intervals to cancer diagnosis, we dichotomized the early versus late diagnostic groups according to interval times (> 2 wk, > 2 mos, > 6 mo, > 1 year). This study was approved by the institutional review board of Hallym University Sacred Heart Hospital (IRB No. HALLYM 2018-01-003-001). The study was performed in accordance with the recommendations of the Declaration of Helsinki. As this was a retrospective study, the need for informed consent was waived.
The first endoscopic photographs before the initial biopsy were collected by five endoscopy specialists (JW, DM, SE, KH, BY). The location of the lesion[16], endoscopic size (mm), gross type[17], and presence of ulcerations, multiple lesions, color change, spontaneous bleeding and converging fold were determined by experienced endoscopic specialists (HS and JS) who performed a blinded review with no knowledge of the pathologic findings. In the case of disagreement between the specialists, a consensus was determined through discussion. The endoscopic size (longest diameter) of the lesion was measured during the procedure using open biopsy forceps (6 mm when fully opened). Ulceration was defined as the discontinuity of gastric mucosa with a crater with the longest diameter > 5 mm. Multiple lesions were defined as the presence of ≥ 2 features with similar gross types and color tones around the same location. Lesion color was defined as ‘red’ or ‘whitish discoloration’ in comparison with the surrounding mucosa, and if there was ulceration, the color of the ulcer base was excluded from the assessment. Spontaneous bleeding was defined as minor bleeding (bleeding from aeration or a weak touch) caused by friable mucosa.
Owing to the intradepartmental regulations of the study institute, all equivocal cases including IFND lesions in the endoscopic biopsies had been originally determined by a consensus of all of the pathologists’ histological evaluations using a multi-headed microscope. For this study, two gastrointestinal pathologists (MJ and JY) were blinded to patients’ clinical data and reviewed all the hematoxylin and eosin-stained tissue sections and confirmed all cases as IFND lesions according to standard guidelines[1-4,18]. To determine the accuracy of final diagnosis prediction, the slides of more than two pathologically-reported IFND lesions were independently interpreted by five experienced pathologists who were blinded to the report or case information; each had > 10 years’ experience in gastroendoscopic biopsy. The features that lead to IFND include moderately distorted architecture, nuclear atypia (variable nuclear size and shape, basally non-located nuclei, and increased nucleocytoplasmic ratio), dystrophic goblet cells, nuclear stratification, diminished or absent mucus production, increased basophilia, and increased mitoses; for all of these features, while changes are marked as negative, they are not sufficient for the diagnosis of dysplasia or carcinoma[2,3,19]. IFND lesions were divided into two subgroups: ‘atypical epithelia’ and ‘regenerating atypia[2,19,20]. The regenerating atypia was favored when the a few gland/epithelium had immature cells with basophilic cytoplasm and nuclear atypia (hyperchromatic nucleus, variable nuclear size and shape, basally non-located nuclei, increased nucleocytoplasmic ratio) showing pseudostratification, reduced or absent mucus secretion, and less maturation and differentiation toward the surface[2,19,20]. The atypical epithelium was favored when the above mentioned features were added with moderately distorted architecture (localized cellular crowding or irregular shaped glands) and haphazardly arranged dystrophic goblet cells with compressed nuclei showing loss of nuclear polarity[2,19,20]. In this study, gastritis and adenoma cases were categorized into the non-carcinoma group and adenocarcinoma cases into the carcinoma group. In the carcinoma group, carcinomas with undifferentiated features or those involving more than the submucosal layer were defined as ‘poor prognosis carcinomas’
Student’s t-tests, chi-square tests, and Fisher’s exact tests were used for comparisons between the non-carcinoma and carcinoma groups. The concordance rate with the kappa (κ) statistic was used to determine the concordance of repeated IFND diagnoses. Multivariate logistic regression analyses including significant predictors from the univariate analysis were performed, and the odds ratios (ORs) with their 95% confidence interval (CI) were determined. Survival differences between the individual groups were calculated using the Kaplan-Meier method with the log-rank test. P-values < 0.05 were considered significant. The predictive factors of gastric cancer were further evaluated using a receiver operating characteristic (ROC) curve. All statistical analyses were performed using SPSS (version 18; SPSS Inc., Chicago, IL, United States).
Overall, 461 patients [319 men; 142 women; median age, 59 (range 17-91) years] were enrolled (Table 1). Of these, the diagnoses of 104 (22.6%) were confirmed as carcinoma [by endoscopic resection (79.8%, 83/104) and gastrectomy (20.1%, 21/104)] while those of 357 (77.4%) were confirmed as non-carcinoma [including 316/357 (88.5%) gastritis and 41/357 (11.4%) dysplasia]. Carcinoma lesions were significantly associated with age ≥ 60 years (P < 0.001), endoscopic size ≥ 10 mm (P < 0.001), single lesion (P < 0.001), spontaneous bleeding (P < 0.001), atypical glands reported at initial biopsy (P < 0.001) rather than regenerating atypia (P < 0.001), and repeated IFND diagnosis in pathologic reports (P=0.002). The initial endoscopic biopsy numbers were significantly higher (P = 0.013) in the carcinoma cases (2.15 ± 1.37) than the non-carcinoma cases (1.77 ± 1.13). Clinicopathologic differences between atypical epithelia and regenerating atypia were also evaluated, as shown in Supplementary Table 1.
| Initial Dx | Total n = 461 | IFND | P value | |
| Final Dx | Non-carcinoma n = 357 | Carcinoma n = 104 | ||
| Sex | 0.816 | |||
| Male | 319 (69.2) | 248 (69.5) | 71 (68.3) | |
| Female | 142 (30.8) | 109 (30.5) | 33 (31.7) | |
| Age (yr) | < 0.001 | |||
| < 60 | 236 (51.2) | 204 (57.1) | 32 (30.8) | |
| ≥ 60 | 225 (48.8) | 153 (42.9) | 72 (69.2) | |
| Endoscopic size (mm) | < 0.001 | |||
| < 10 | 286 (62.0) | 251 (70.3) | 35 (33.7) | |
| ≥ 10 | 175 (38.0) | 106 (29.7) | 69 (66.3) | |
| Location I | 0.914 | |||
| Upper | 43 (9.3) | 34 (9.5) | 9 (8.7) | |
| Middle | 122 (26.5) | 93 (26.1) | 29 (27.9) | |
| Lower | 296 (64.2) | 230 (64.4 | 66 (63.5) | |
| Location II | 0.744 | |||
| Fundus | 9 (1.9) | 8 (2.2) | 1 (1.0) | |
| Body | 122 (26.5) | 93 (26.1) | 29 (27.9) | |
| Antrum | 277 (60.1) | 213 (59.7) | 64 (61.5) | |
| Angle | 53 (11.5) | 43 (12.0) | 10 (9.6) | |
| Location III | 0.267 | |||
| Anterior wall | 75 (16.3) | 55 (15.4) | 20 (19.2) | |
| Posterior wall | 76 (16.5) | 63 (17.6) | 13 (12.5) | |
| Greater curvature | 85 (18.4) | 61 (17.1) | 24 (23.1) | |
| Lesser curvature | 225 (48.8) | 178 (49.9) | 47 (45.2) | |
| Gross type | 0.585 | |||
| Elevated (type I and IIa) | 181 (39.3) | 136 (38.1) | 45 (43.3) | |
| Flat (type IIb) | 168 (36.4) | 134 (37.5) | 34 (32.7) | |
| Depressed (type IIc and III) | 112 (24.3) | 87 (24.4) | 25 (24.0) | |
| Color change | 0.140 | |||
| Redness | 314 (68.1) | 250 (70.0) | 64 (61.5) | |
| Whitish | 58 (12.6) | 45 (12.6) | 13 (12.5) | |
| No change | 89 (19.3) | 62 (17.4) | 27 (26.0) | |
| Single lesion | < 0.001 | |||
| No | 141 (30.6) | 134 (37.5) | 7 (6.7) | |
| Yes | 320 (69.4) | 223 (62.5) | 97 (93.3) | |
| Ulcer | 0.182 | |||
| No | 334 (72.5) | 264 (73.9) | 70 (67.3) | |
| Yes | 127 (27.5) | 93 (26.1) | 34 (32.7) | |
| Spontaneous bleeding | < 0.001 | |||
| No | 405 (87.9) | 332 (93.0) | 73 (70.2) | |
| Yes | 56 (12.1) | 25 (7.0) | 31 (29.8) | |
| Fold change | 0.206 | |||
| No | 418 (90.7) | 327 (91.6) | 91 (87.5) | |
| Yes | 43 (9.3) | 30 (8.4) | 13 (12.5) | |
| Initial Bx | < 0.001 | |||
| Regenerating atypia | 276 (59.9) | 267 (74.8) | 9 (8.7) | |
| Atypical epithelium | 185 (40.1) | 90 (25.2) | 95 (91.3) | |
| Repeated IFND | 0.002 | |||
| No | 436 (94.6) | 344 (96.4) | 92 (88.5) | |
| Yes | 25 (5.4) | 13 (3.6) | 12 (11.5) | |
| Bx numbers at initial endoscopic Bx | 1.77 ± 1.13 | 2.15 ± 1.37 | 0.013 | |
On multivariate logistic regression, the independent risk factors predictive of gastric carcinoma in the IFND lesions (Table 2) were age ≥ 60 years (P = 0.005, OR 2.445, 95%CI: 1.305-4.580), endoscopic size ≥ 10 mm (P < 0.001, OR 3.519, 95%CI: 1.891-6.548), single lesion (P < 0.001, OR 5.702, 95%CI: 2.212-14.696), spontaneous bleeding (P = 0.001, OR 4.056, 95%CI: 1.792-9.180), atypical epithelium described as IFND (P < 0.001, OR 25.575, 95%CI: 11.537-56.695), or repeated pathologic reports of IFND (P = 0.003, OR 6.022, 95%CI: 1.822-19.909). When evaluating the four clinical predictive factors (age, endoscopic size, single lesion, and spontaneous bleeding), the overall area under the ROC curve for the diagnosis of gastric carcinoma was 0.773 (95%CI: 0.722-0.825, P = 0.026) depending on the number of clinical predictive factors. When two or more clinical predictive factors were present, the sensitivity was 91.3% and the specificity 54.9% (Supplementary Table 2).
| Univariate | Multivariate | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) (< 60 vs ≥ 60) | 3.000 (1.882-4.782) | < 0.001 | 2.445 (1.305-4.580) | 0.005 |
| Endoscopic gross size (mm) (< 10 vs ≥ 10) | 4.631 (2.906-7.380) | < 0.001 | 3.519 (1.891-6.548) | < 0.001 |
| Multiple vs single lesion | 8.327 (3.755-18.465) | < 0.001 | 5.702 (2.212-14.696) | < 0.001 |
| Spontaneous bleeding (Absent vs Present) | 5.639 (3.143-10.119) | < 0.001 | 4.056 (1.792-9.180) | 0.001 |
| Initial Bx (Regenerating atypia vs Atypical epithelium) | 31.315 (15.180-64.599) | < 0.001 | 25.575 (11.537-56.695) | < 0.001 |
| Repeated IFND (No vs Yes) | 3.750 (1.631-8.622) | 0.002 | 6.022 (1.822-19.909) | 0.003 |
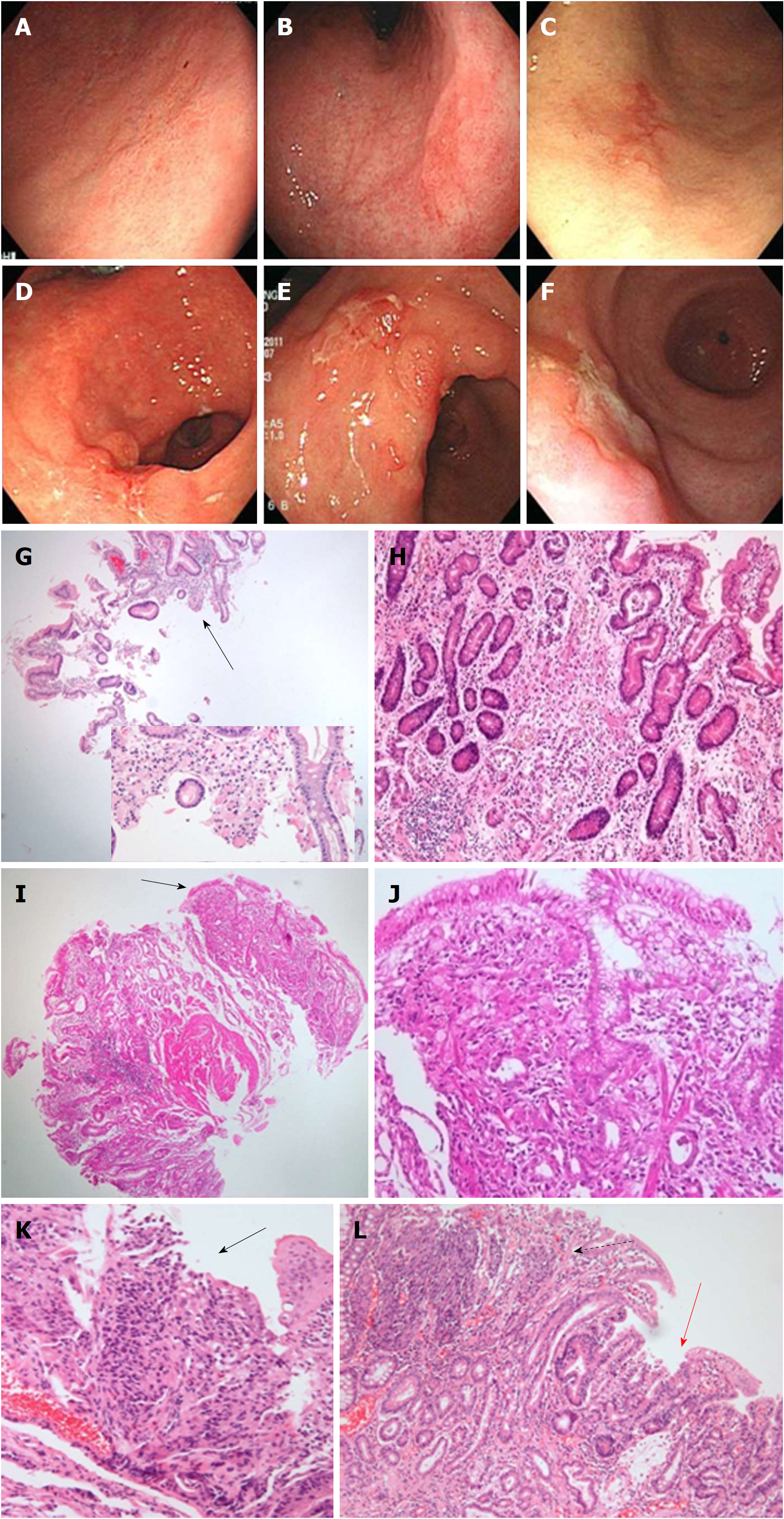
The 10 IFND lesion cases diagnosed as undifferentiated carcinomas (Figure 2) were associated with an endoscopic size ≥ 10 mm (P = 0.012), single lesion (P = 0.016), ulceration (P = 0.005), fold change (P < 0.001), spontaneous bleeding (P = 0.005), and atypical epithelia (P < 0.001) (Supplementary Table 3). In the histological review of the biopsied specimens, the 10 cases did not show a sufficient number of tumor cells to be confirmed as carcinoma; resected specimens of these cases showed poorly differentiated tubular adenocarcinomas mixed with signet ring cell components or well- to moderately-differentiated adenocarcinomas, within a single lesion.
The 29 IFND lesion cases were diagnosed as ‘poor prognosis carcinomas’ (undifferentiated or invasive carcinoma in the submucosal layer or deeper). In the subgroup analysis of the carcinoma group, poor prognosis carcinomas were significantly associated with the depressed gross type (P = 0.034), ulceration (P = 0.010), and fold change (P = 0.001) (Table 3). Fold change was the only independent risk factor (OR 5.594, 95%CI: 1.458-21.462; P = 0.012; Table 4) for the prediction of poor prognoses in carcinoma.
| Initial Dx | Total n = 104 | Carcinoma group | P value | |
| Final Dx | Non-poor prognostic carcinoma n = 75 | Poor prognostic carcinoma n = 29 | ||
| Sex | 0.924 | |||
| Male | 71 (68.3) | 51 (68.0) | 20 (69.0) | |
| Female | 33 (31.7) | 24 (32.0) | 9 (31.0) | |
| Age (yr) | 0.053 | |||
| < 60 | 32 (30.8) | 19 (25.3) | 13 (44.8) | |
| ≥ 60 | 72 (69.2) | 56 (74.7) | 16 (55.2) | |
| Endoscopic size (mm) | 0.082 | |||
| < 10 | 35 (33.7) | 29 (38.7) | 6 (20.7) | |
| ≥ 10 | 69 (66.3) | 46 (61.3) | 23 (79.3) | |
| Location I | 0.162 | |||
| Upper | 9 (8.7) | 7 (9.3) | 2 (6.9) | |
| Middle | 29 (27.9) | 17 (22.7) | 12 (41.4) | |
| Lower | 66 (63.5) | 51 (68.0) | 15 (51.7) | |
| Gross type | 0.034 | |||
| Elevated (type I and IIa) | 45 (43.3) | 36 (48.0) | 9 (31.0) | |
| Flat (type IIb) | 34 (32.7) | 26 (34.7) | 8 (27.6) | |
| Depressed (type IIc and III) | 25 (24.0) | 13 (17.3) | 12 (41.4) | |
| Color change | 0.745 | |||
| Redness | 64 (61.5) | 45 (60.0) | 19 (65.5) | |
| Whitish | 13 (12.5) | 9 (12.0) | 4 (13.8) | |
| No change | 27 (26.0) | 21 (28.0) | 6 (20.7) | |
| Single lesion | 0.186 | |||
| No | 7 (9.3) | 7 (6.7) | 0 (0) | |
| Yes | 97 (93.3) | 68 (90.7) | 29 (100) | |
| Ulcer | 0.010 | |||
| No | 70 (67.3) | 56 (74.7) | 14 (48.3) | |
| Yes | 34 (32.7) | 19 (25.3) | 15 (51.7) | |
| Spontaneous bleeding | 0.517 | |||
| No | 73 (70.2) | 54 (72.0) | 19 (65.5) | |
| Yes | 31 (29.8) | 21 (28.0) | 10 (34.5) | |
| Fold change | 0.001 | |||
| No | 91 (87.5) | 71 (94.7) | 20 (69.0) | |
| Yes | 13 (12.5) | 4 (5.3) | 9 (31.0) | |
| Initial Bx | 0.707 | |||
| Regenerating atypia | 9 (8.7) | 6 (8.0) | 3 (10.3) | |
| Atypical epithelium | 95 (91.3) | 69 (92.0) | 26 (89.7) | |
| Repeated IFND | 1.000 | |||
| No | 92 (88.5) | 66 (88.0) | 26 (89.7) | |
| Yes | 12 (11.5) | 9 (12.0) | 3 (10.3) | |
| Univariate | Multivariate | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Depressed Gross type (No vs Yes) | 3.367 (1.301-8.710) | 0.034 | 1.946 (0.621-6.104) | 0.254 |
| Ulceration (Absent vs Present) | 3.158 (1.290-7.729) | 0.010 | 1.746 (0.594-5.130) | 0.311 |
| Fold change (Absent vs Present) | 7.988 (2.225-28.672) | 0.001 | 5.594 (1.458-21.462) | 0.012 |
The mean time interval from initial biopsy to the final diagnosis of carcinoma was 116.70 ± 324.46 d (median 27 d). The follow-up period ranged from 2-127 (mean 36.1 ± 30.0) mo (Table 5). Except for the fact that the rates of additional surgical treatment (P = 0.033) and incomplete resection (P = 0.031) were significantly higher in the early diagnostic group within 2 weeks, there were no statistically significant differences in tumor differentiation, tumor pathologic size, invasion depth, lymphovascular invasion, complete resection status, additional surgery, recurrence, or endoscopic suspicion for carcinoma between the early and late diagnostic groups. Regenerating atypia lesions at initial biopsy were significantly associated with the late diagnostic group, while a larger number of atypical epithelium lesions were included in the early diagnostic group (> 2 mo and > 6 mo) (P < 0.001). The late diagnostic group had repeated pathologic results of IFND, while the early diagnostic group did not. Initial endoscopic suspicion for carcinoma was related to early diagnoses (≤ 2 wk and ≤ 2 mo, P < 0.001 and P = 0.016, respectively).
| Early | Delayed | P | Early | Delayed | P | Early | Delayed | P | Early | Delayed | P | |
| (≤ 2 wk) | (> 2 wk) | (≤ 2 mo) | (> 2 mo) | (≤ 6 mo) | (> 6 mo) | (≤ 1 yr) | (> 1 yr) | |||||
| n = 28 | n = 76 | n = 73 | n = 31 | n = 96 | n = 8 | n = 97 | n = 7 | |||||
| Initial Bx | 0.109 | < 0.001 | < 0.001 | 0.112 | ||||||||
| RegA | 0 (0) | 9 (11.8) | 1 (1.4) | 8 (25.8) | 1 (1.4) | 8 (25.8) | 7 (7.2) | 2 (28.6) | ||||
| AGL | 28 (100) | 67 (88.2) | 72 (98.6) | 23 (74.2) | 72 (98.6) | 23 (74.2) | 90 (92.8) | 5 (71.4) | ||||
| Repeated IFND | 0.033 | 0.001 | 0.048 | 0.032 | ||||||||
| No | 28 (100) | 64 (84.2) | 70 (95.9) | 22 (71.0) | 87 (90.6) | 5 (62.5) | 88 (90.7) | 4 (57.1) | ||||
| Yes | 0 (0) | 12 (15.8) | 3 (4.1) | 9 (29.0) | 9 (9.4) | 3 (37.5) | 9 (9.3) | 3 (42.9) | ||||
| Differentiation | 1.000 | 0.729 | 1.000 | 0.554 | ||||||||
| Differ | 25 (89.3) | 68 (89.5) | 66 (90.4) | 27 (87.1) | 86 (89.6) | 7 (87.5) | 87 (89.7) | 6 (85.7) | ||||
| Undiffer | 3 (10.7) | 8 (10.5) | 7 (9.6) | 4 (12.9) | 10 (10.4) | 1 (12.5) | 10 (10.3) | 1 (14.3) | ||||
| Tumor size (pathology) | 0.252 | 0.444 | 0.602 | 1.000 | ||||||||
| < 10 mm | 6 (24.0) | 27 (36.5) | 21 (30.9) | 12 (38.7) | 31 (34.1) | 2 (25.0) | 31 (33.7) | 2 (28.6) | ||||
| ≥ 10 mm | 19 (76.0) | 47 (63.5) | 47 (69.1) | 19 (61.3) | 60 (65.9) | 6 (75.0) | 61 (66.3) | 5 (71.4) | ||||
| Invasion depth | 0.103 | 0.395 | 0.197 | 0.341 | ||||||||
| ≤ Mucosa | 19 (67.9) | 62 (82.7) | 55 (76.4) | 26 (83.9) | 73 (76.8) | 8 (100) | 74 (77.1) | 7 (100) | ||||
| ≥ Submucosa | 9 (32.1) | 13 (17.3) | 17 (23.6) | 5 (16.1) | 22 (23.2) | 0 (0) | 22 (22.9) | 0 (0) | ||||
| LVI | 1.000 | 0.581 | 0.278 | 0.246 | ||||||||
| No | 27 (96.4) | 73 (96.1) | 71 (97.3) | 29 (93.5) | 93 (96.9) | 7 (87.5) | 94 (96.9) | 6 (85.7) | ||||
| Yes | 1 (3.6) | 3 (3.9) | 2 (2.7) | 2 (6.5) | 3 (3.1) | 1 (12.5) | 3 (3.1) | 1 (14.3) | ||||
| Complete resection | 0.033 | 1.000 | 1.000 | 1.000 | ||||||||
| No | 5 (17.9) | 3 (4.0) | 6 (8.3) | 2 (6.5) | 8 (8.4) | 0 (0) | 8 (8.3) | 0 (0) | ||||
| Yes | 23 (82.1) | 72 (96.0) | 66 (91.7) | 29 (93.5) | 87 (91.6) | 8 (100) | 88 (91.7) | 7 (100) | ||||
| Additional surgery | 0.031 | 1.000 | 1.000 | 1.000 | ||||||||
| No | 23 (82.1) | 73 (96.1) | 67 (91.8) | 29 (93.5) | 88 (91.7) | 8 (100) | 89 (91.8) | 7 (100) | ||||
| Yes | 5(17.9) | 3(3.9) | 6(8.2) | 2(6.5) | 8(8.3) | 0(0) | 8(8.2) | 0(0) | ||||
| Recurrence | 0.562 | 1.000 | 0.215 | 0.190 | ||||||||
| No | 28 (100) | 73 (96.1) | 71 (97.3) | 30 (96.8) | 94 (97.9) | 7 (87.5) | 95 (97.9) | 6 (85.7) | ||||
| Yes | 0 (0) | 3 (3.9) | 2 (2.7) | 1 (3.2) | 2 (2.1) | 1 (12.5) | 2 (2.1) | 1 (14.3) | ||||
| Endoscopic suspicion for carcinoma | 0.003 | 0.016 | 1.000 | 1.000 | ||||||||
| No | 10 (35.7) | 52 (68.4) | 38 (52.1) | 24 (77.4) | 57 (59.4) | 5 (62.5) | 58 (59.8) | 4 (57.1) | ||||
| Yes | 18 (64.3) | 24 (31.6) | 35 (47.9) | 7 (22.6) | 39 (40.6) | 3 (37.5) | 39 (40.2) | 3 (42.9) |
The recurrence-free survival rates were not statistically different between the early and late diagnostic groups with the criteria of 2 wk, 2 mo, 6 mo, or > 1 year (P = 0.280, P = 0.848, P = 0.283, and P = 0.283, respectively; Supplementary Figure 1).
IFND lesion diagnoses were observed more than twice in 24 (5.2%) of the 461 cases (Table 6). Of the 24, the final diagnosis for four cases (16.7%) was adenoma, while 12 cases (50.0%) were diagnosed as well-differentiated adenocarcinoma and the remaining eight (33.3%) diagnoses were of chronic gastritis with reactive change.
| Sex | Age (yr) | Bx N | Original report | Final Dx | Review and Dx rate | |||
| 1st Bx | 2nd Bx | 1st Bx | 2nd Bx | |||||
| 1 | M | 80 | 2 | AGL | AGL | W/D | 60% | 0% |
| 2 | F | 78 | 2 | RegA | RegA | Gastritis | 100% | 100% |
| 3 | M | 78 | 2 | AGL | AGL | W/D | 60% | 80% |
| 4 | F | 76 | 2 | AGL | RegA | W/D | 20% | 20% |
| 5 | M | 75 | 2 | AGL | AGL | W/D | 80% | 20% |
| 6 | M | 72 | 2 | RegA | AGL | W/D | 0% | 20% |
| 7 | M | 72 | 3 | RegA | AGL | W/D | 100% | 40% |
| 8 | M | 68 | 2 | AGL | AGL | W/D | 100% | 100% |
| 9 | F | 67 | 2 | RegA | AGL | Gastritis | 80% | 60% |
| 10 | M | 66 | 2 | RegA | RegA | Adenoma | 100% | 100% |
| 11 | M | 66 | 2 | AGL | AGL | Gastritis | 60% | 40% |
| 12 | M | 61 | 3 | AGL | AGL | Gastritis | 60% | 0% |
| 13 | M | 60 | 2 | AGL | AGL | W/D | 0% | 80% |
| 14 | F | 59 | 2 | AGL | AGL | W/D | 80% | 60% |
| 15 | M | 59 | 4 | RegA | RegA | Gastritis | 100% | 40% |
| 16 | M | 57 | 3 | RegA | RegA | Adenoma | 20% | 20% |
| 17 | M | 56 | 2 | AGL | AGL | Adenoma | 80% | 80% |
| 18 | M | 56 | 2 | AGL | AGL | W/D | 20% | 30% |
| 19 | M | 55 | 2 | RegA | RegA | Gastritis | 100% | 100% |
| 20 | F | 54 | 3 | AGL | RegA | W/D | 0% | 0% |
| 21 | M | 51 | 3 | AGL | RegA | W/D | 0% | 0% |
| 22 | M | 51 | 3 | RegA | RegA | Adenoma | 100% | 100% |
| 23 | M | 49 | 2 | RegA | RegA | Gastritis | 20% | 100% |
| 24 | M | 42 | 2 | RegA | RegA | Gastritis | 80% | 80% |
| Final Dx | 1st Bx (A) | 2nd Bx (A) | C | κ | P | |||
| Gastritis | n = 8 | 75.0% | 60.8% | 0.791 | 0.117 | 0.463 | ||
| Adenoma | n = 4 | 78.3% | 84.2% | 1.000 | 0.733 | 0.001 | ||
| Adenocarcinoma | n = 12 | 65.0% | 63.3% | 0.629 | 0.415 | 0.001 | ||
The first two biopsies and the final slides of the 24 cases were independently reviewed by five pathologists blinded to the case information. The diagnostic accuracy of the first biopsy was 75.0% for gastritis, 78.3% for adenoma, and 65.0% for adenocarcinoma. The accuracy of the second biopsy was 60.8% for gastritis, 84.2% for adenoma, and 63.3% for adenocarcinoma. The diagnostic concordance for adenoma and adenocarcinoma showed statistically substantial agreement between the first and second biopsies (κ = 0.733, P = 0.001; κ = 0.415, P = 0.001, respectively), whereas that of gastritis showed no statistically significant agreement between the first and second biopsies (κ = 0.117, P = 0.463). Therefore, overall concordances between pathologists in the review of IFND lesions were 50% for gastritis, 66.7% for gastric adenoma, and 50.7% for gastric cancer.
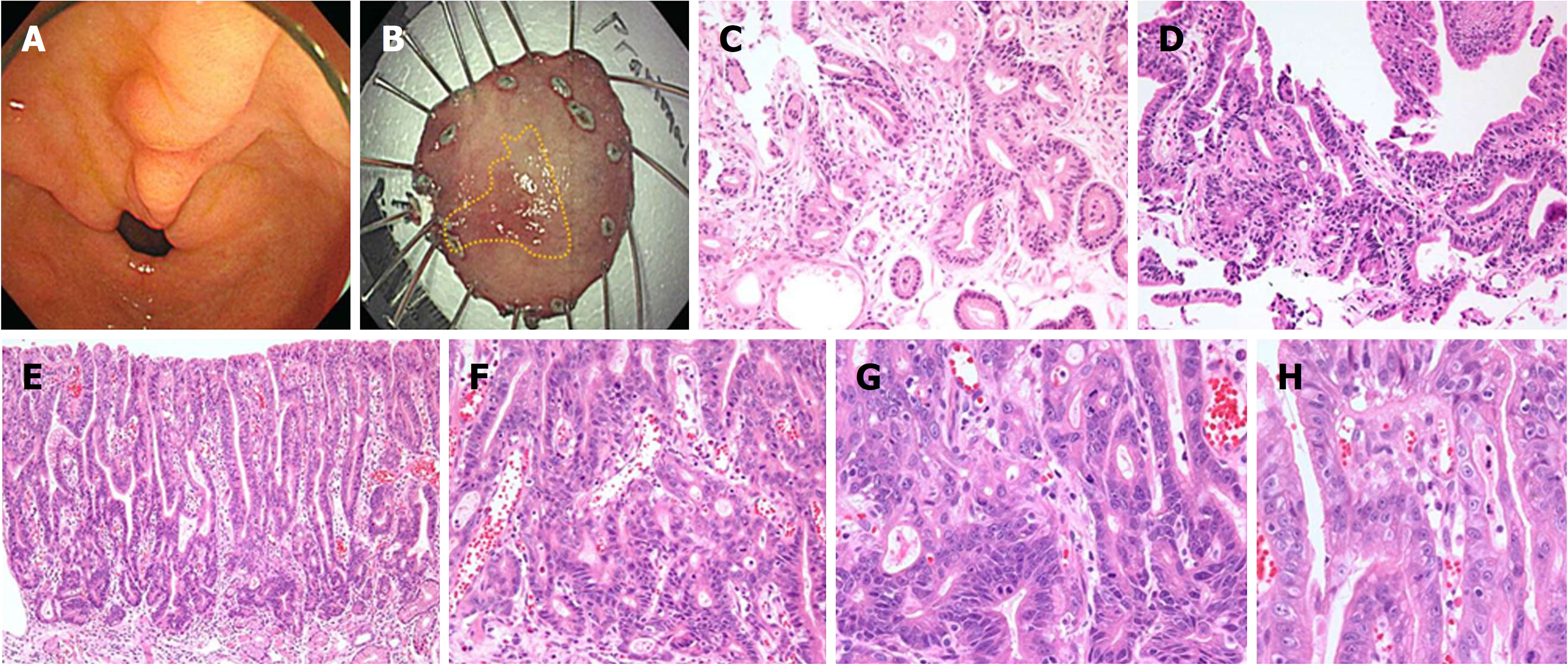
Two cases failed to be diagnosed as cancer in both the first and second biopsies by all five pathologists. In the biopsied specimens, focal glandular crowding and angulated glands were noted with glandular transition to the surrounding surface mucosa, characteristic of low cytologic atypia, but the structural atypia did not reach the diagnostic criteria of overt carcinoma. In the endoscopic submucosal dissection specimens, the histological geography of the entire lesion showed moderate-to-severe inflammatory infiltration, adenoma, and carcinoma, within a single lesion. The full epithelial layer view of the lesions confirmed well-differentiated adenocarcinoma (Figure 3).
In this study, approximately one-fourth of all IFND lesions at the initial biopsy were finally determined to be gastric cancers by endoscopic resection (79.8%) and gastrectomy (20.1%). Repeated IFND lesion diagnoses were uncommon (5.2%), with 50% cases exhibiting well-differentiated adenocarcinoma, and 16.7% and 33.3% confirmed as adenoma and gastritis, respectively. The carcinomas were usually diagnosed by resection, indicating that repeated biopsies did not detect the majority of cases. We found 24 repeated IFND lesions that displayed structural abnormality with mild cytologic atypia; half of these cases were extremely well-differentiated adenocarcinomas. Among them, two extremely well-differentiated adenocarcinomas could not be diagnosed at both the first and second biopsies in the blinded evaluation by all five pathologists. The high degree of differentiation and mild cellular atypia of these lesions resulted in frequent diagnostic difficulties, even at repeated biopsies, due to the surface maturation that mimicked intestinal metaplasia. These were frequently described at the endoscopic biopsies as ‘indeterminate for neoplasia’ (26%) or ‘reactive intestinal metaplasia’ (24%)[21]. Within this context, the diagnostic rate of adenocarcinoma, which was the lowest, did not increase despite repeated biopsies. Previous studies have also raised this concern, with 30-50% of the original diagnostic biopsies incorrectly interpreted[21-23]. Likewise in this study, the overall concordances between pathologists in IFND lesions were not satisfactory (50-67%). However, the problem of interpretation lies not in the ability of the endoscopist or pathologist, but in the structural and cytologic features of the lesion. Given the low diagnostic rate of highly differentiated gastric cancers, it is very important for clinicians to provide a comment in the pathology report indicating architectural atypia with mild nuclear atypia. This can act as a reminder for endoscopists to consider the possibility of malignancy.
The adenomas in this study showed the highest diagnostic rate and the most concordance in repeated biopsies; this indicates that repeated biopsies may be helpful in confirming gastric adenoma, in contrast to gastric cancer cases. However, we also found that gastritis with reactive change was correctly diagnosed despite the fact that repeat biopsies were inconsistent and showed the highest intra-observer and inter-observer variabilities. This indicates that regenerating epithelium is truly difficult to distinguish from carcinoma even in retrospective reviews by experienced pathologists. The overlapping histologic features of cribriform, villous/papillary structures, intraluminal papilla, thick irregular membrane, and vesicular nuclei considered to be beyond the upper spectrum of dysplasia were also observed in regenerating atypia cases[5].
Gastritis was diagnosed in 11/80 cases (7.2%) after immediate endoscopic resection; this is similar to the false-positive rate (7.5%) observed in a previous study[8]. Although immediate endoscopic resection is considered an excellent method for IFND diagnosis[9,24], this strategy should be carefully considered as a risk for false-positive reactive change. This is because the reactive changes after medical therapy showed an unequivocal non-dysplastic epithelium. Sampling bias and intratumoral heterogeneity, ranging from reactive change and adenoma to carcinoma, may be attributable to such discrepancies[9]. However, sampling errors or insufficient biopsy volumes due to biopsy alone may not fully explain these discrepancies[25,26]. Similar to our results, previous studies have shown that the number of initial biopsies is not positively correlated with the diagnosis of carcinoma in IFND cases[8,9]. Delayed diagnosis due to repeated atypia may be partially attributed to endoscopically-equivocal macroscopic findings. Therefore, it is of clinical importance for endoscopists to predict the possibility of carcinoma when IFND is encountered in pathologic reports.
Yu et al[8] reported that an endoscopic size ≥ 10 mm and the presence of depressed lesions are independent risk factors of gastric cancer in atypical epithelium lesions. Red discoloration and friability in IFND lesions are also associated with a final neoplastic diagnosis[24]. In contrast, some studies reported that endoscopic size, gross appearance, lesion location, number of biopsies, and Helicobacter pylori infection in gastric IFND are not predictive of gastric cancer following resection[9]. In this context, it is recommended that endoscopic resection be considered for cases with endoscopically worrisome findings (a single lesion size ≥ 10 mm and spontaneous bleeding). Additionally, in the subgroup analysis of carcinoma, fold change was observed to be a clinically independent predictive factor for undifferentiated or invasive carcinoma in the submucosal layer or deeper.
Limited attention has been paid to the validity of the follow-up strategy for this indefinite pathology[24], and to the association between diagnostic delays and worsened prognosis. In this study, we did not observe a prognostic impact of prolonged diagnostic delay on the final diagnosis of gastric cancer in either the early or delayed diagnostic groups (> 2 wk, >2 mo, > 6 mo, and > 1 year). The overall prognoses were favorable in both groups. This could be explained by the fact that those cases were finally confirmed as well-differentiated early gastric cancers. The delayed diagnosis for gastric cancer did not affect the increased tumor size, invasion depth, lymphovascular invasion, incomplete resection status, additional surgery, or recurrence in gastric cancers. Indeed, a delayed diagnosis after > 1 year of well-differentiated gastric adenocarcinoma is likely to have limited prognostic consequence because the lesions are slow-growing[21,22]. This is supported by the low Ki-67 labeling index and the lack of p53 overexpression[22,27]. Those lesions show minimal changes in size between the first examination and at resection and remain intramucosal cancers even after 84 months at endoscopic resection from the initial biopsy[21]. However, because the endoscopically-suspected gastric cancers in our retrospective study tended to lead to earlier diagnosis and resection than endoscopically-undetermined lesions; thus, less-aggressive gastric cancers may remain undiagnosed or have a delayed diagnosis, our results of no prognostic differences between the early and late diagnosis groups might be due to the possible selection bias. Therefore, our findings concerning the prognostic impact of prolonged diagnostic delays in indefinite lesions should not be considered conclusive and further prospective studies are necessary.
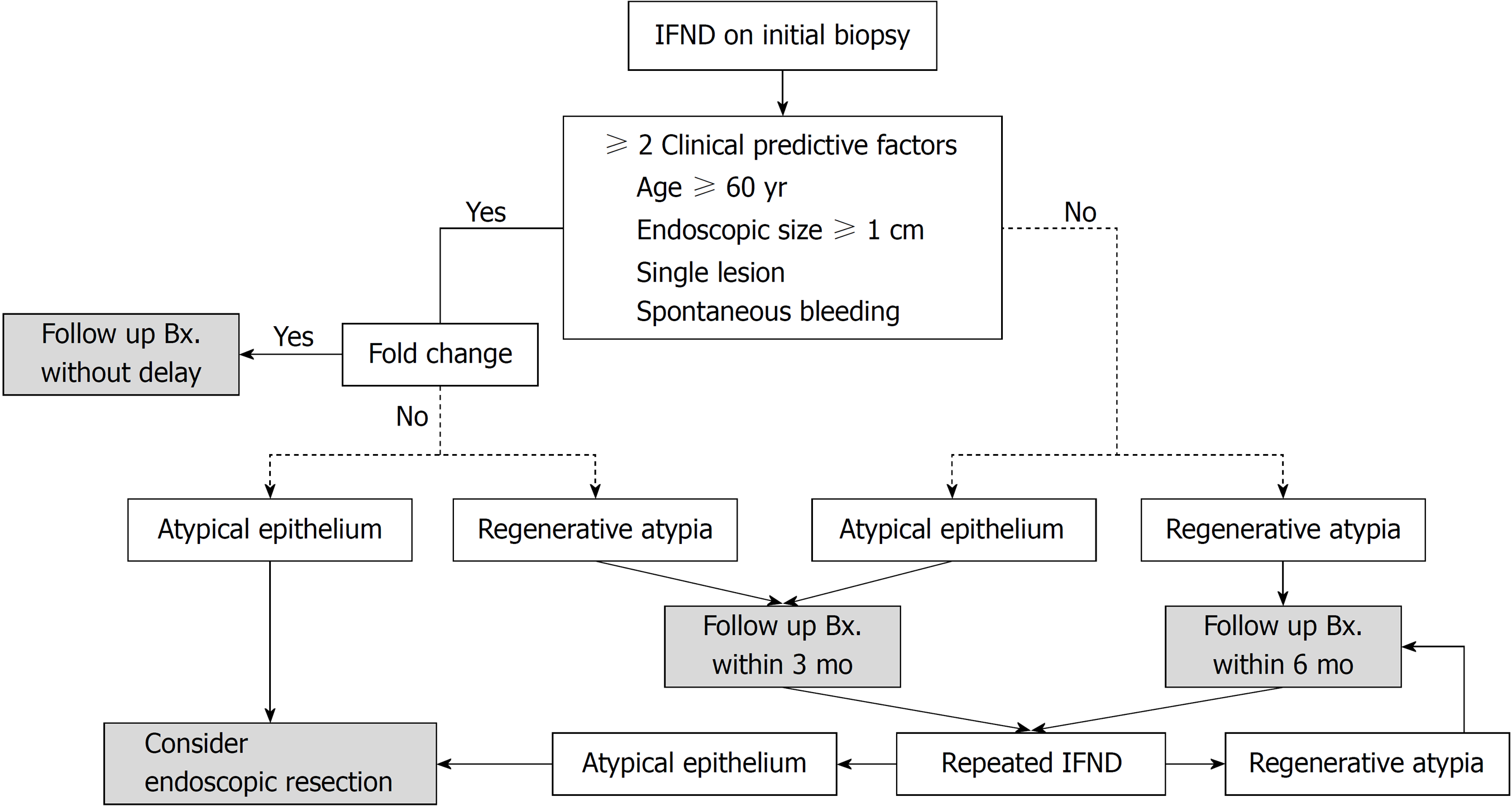
As a result of this study, our institute established a guideline for future prospective research (Figure 4). The guideline is based on the following: (1) two pathologic risk factors (atypical glands and repeated IFND) are strongly associated with gastric cancer, (2) more than two clinical risk factors (age ≥ 60, endoscopic size ≥ 10 mm, single lesions, and spontaneous bleeding) are determined to have a significant cut-off value for gastric carcinoma diagnosis, (3) fold change predicts undifferentiated or invasive carcinoma in the submucosal layer or deeper, and (4) considering that IFND lesions of Barrett’s esophagus are recommended for follow-up within 3-6 mo [28] and that diagnostic delays within 1 year had no effect on prognoses in our gastric IFND cases, follow-up biopsy for IFND should be performed within 6 mo.
Our study has some limitations. This was a retrospective, correlative study in a single center that was not based on a trial and lacked strictly regulated, periodical follow-up data or data on H. pylori infection and intestinal metaplasia. There may be a selection bias enrolled in a single, tertiary medical center located at the gastric cancer-endemic country. Nevertheless, our study has some strengths. This is among the rare reports describing the clinicopathologic features associated with repeated IFND diagnosis; the features of undifferentiated cancer initially diagnosed as IFND; and the correlation between diagnostic delays and IFND prognoses. These findings may enable the definition of high-risk malignant potential and therapeutic strategies. The prediction of gastric cancer may be reliable in ambiguous lesions in patients aged ≥ 60 years, with endoscopic lesion size ≥ 10 mm, with single lesions, with lesions that show spontaneous bleeding, and with atypical glands or repeated IFND diagnosis. In single lesions with these features, endoscopic resection would be a better option than repeated endoscopic biopsy. Otherwise, without any associated risk factors, accurate diagnosis through follow-up endoscopic biopsy within 1 year is recommended and will likely not worsen long-term outcomes.
Gastric ‘indefinite for neoplasm/dysplasia’ (IFND) is a borderline lesion that is difficult to diagnose as either regenerative or neoplastic. Thus, due to the possibility of dysplasia or carcinoma, follow-up evaluation according to the Vienna classification is recommended for gastric IFND cases. However, in clinical settings, no clear guideline exists that indicates the exact cut-off time for additional biopsy or endoscopic resection; additionally, there is no subsequent plan for endoscopists in dealing with more than two pathologic reports of IFND lesions at the follow-up biopsy.
There is a critical need for guidance in the identification of a subset of patients through forceps biopsy, who have an IFND lesion with a higher risk of malignant potential, to enable risk stratification and optimal management.
Our study aimed to establish the correct diagnosis for gastric IFND lesions by evaluating a series of IFND lesions in detail and determining the key clinical and pathologic predictive factors for gastric cancer. The findings of this study may be useful in informing the decision to either perform biopsy repeatedly or resect gastric IFND lesions.
Medical records and pathologic reports of patients who underwent gastric endoscopic biopsy from January 2007 to December 2016 were reviewed, and a total 461 IFND lesions were enrolled in this study. Two gastrointestinal pathologists confirmed all cases as IFND lesions according to standard guidelines (the Korean pathologic grading system for gastric epithelial proliferative disease, the classification of the Japanese Gastric Cancer Association, and the Vienna classification), and IFND lesions were divided into two subgroups: ‘atypical epithelia’ and ‘regenerating atypia. To assess the possible risk factors for diagnostic delays, the time interval from the onset of the first biopsy to the establishment of cancer diagnosis was measured.
At initial biopsy, ‘indefinite for neoplasm/dysplasia’ (IFND) lesions proved to be adenocarcinomas (22.6%). Independent risk factors for gastric IFND cancer were age (≥ 60 years), endoscopic size (≥ 10 mm), single lesion, spontaneous bleeding, atypical epithelia, and repeated IFND diagnosis. Additionally, fold change predicted undifferentiated or invasive carcinoma in the submucosal layers or deeper. Diagnostic delays shorter than 1 year were not associated with worse prognoses in our study. However, there may be a selection bias enrolled in a single, tertiary medical center located at the gastric cancer-endemic country.
More than two clinical and pathologic factors each had significant cut-off values for gastric carcinoma diagnosis; in such cases, endoscopic resection would be a better option than repeated endoscopic biopsy. Otherwise, without any associated risk factors, accurate diagnosis through follow-up endoscopic biopsy within 1 year is recommended and will likely not worsen long-term outcomes.
As a result of this study, our institute established a guideline. And we will use this guideline to conduct future prospective studies to reduce selection bias.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gonda T, Lazăr DC, Markic D S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
| 1. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 499] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 2. | Kim H, Jin SY, Jang JJ, Kim WH, Song SY, Kim KR, Yu ES, Shin HS, Kim HK, Sohn JH, Hong EK. Grading system for gastric epithelial proliferative diseases standardized guidelines proposed by Korean Study Group for Pathology of Digestive Diseases. Korean J Pathol. 1997;31:389-400. |
| 3. | Takao M, Kakushima N, Takizawa K, Tanaka M, Yamaguchi Y, Matsubayashi H, Kusafuka K, Ono H. Discrepancies in histologic diagnoses of early gastric cancer between biopsy and endoscopic mucosal resection specimens. Gastric Cancer. 2012;15:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1549] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 5. | Kim JM, Cho MY, Sohn JH, Kang DY, Park CK, Kim WH, Jin SY, Kim KM, Chang HK, Yu E, Jung ES, Chang MS, Joo JE, Joo M, Kim YW, Park DY, Kang YK, Park SH, Han HS, Kim YB, Park HS, Chae YS, Kwon KW, Chang HJ; Gastrointestinal Pathology Study Group of Korean Society of Pathologists. Diagnosis of gastric epithelial neoplasia: Dilemma for Korean pathologists. World J Gastroenterol. 2011;17:2602-2610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Jang JS. What Shall We Do for “Atypical Cells and Regenerative Atypia” on Forceps Biopsy Examination. Accessed 2014-03-30. Available from: URL: http://www.gie.or.kr/schedule/old_data/semina/semina/2014_50/seminar/50th_seminar_4.pdf. |
| 7. | Goldstein NS, Lewin KJ. Gastric epithelial dysplasia and adenoma: historical review and histological criteria for grading. Hum Pathol. 1997;28:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Yu CH, Jeon SW, Kim SK, Lee HS, Heo J, Kwon YH, Kim GY, Kim SZ, Bae HI. Endoscopic resection as a first therapy for gastric epithelial atypia: is it reasonable? Dig Dis Sci. 2014;59:3012-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Kim SI, Han HS, Kim JH, Lee KJ, Hong SN, Lee SY, Kim HU, Sung TS, Zheng H, Sung IK, Park HS, Shim CS. What is the next step for gastric atypical epithelium on histological findings of endoscopic forceps biopsy? Dig Liver Dis. 2013;45:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Sung JK. Diagnosis and management of gastric dysplasia. Korean J Intern Med. 2016;31:201-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 11. | Stolte M. The new Vienna classification of epithelial neoplasia of the gastrointestinal tract: advantages and disadvantages. Virchows Arch. 2003;442:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21373] [Article Influence: 2137.3] [Reference Citation Analysis (3)] |
| 13. | Jung KW, Won YJ, Kong HJ, Lee ES. Prediction of Cancer Incidence and Mortality in Korea, 2018. Cancer Res Treat. 2018;50:317-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J Gastric Cancer. 2016;16:131-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 15. | Yoshizawa N, Fujisaki J, Suzuki S, Taniguchi C, Horiuchi Y, Matsuo Y, Suganuma T, Ohmae M, Kubota M, Ishiyama A, Hirasawa T, Yamamoto Y, Tsuchida T, Igarashi M. A study on lesions diagnosed as atypical epithelium (new group classification; equivalent to Group 2) at our hospital by stomach biopsy. Prog Dig Endosc. 2012;80:42-46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2873] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 17. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1327] [Article Influence: 60.3] [Reference Citation Analysis (4)] |
| 18. | Yakirevich E, Resnick MB. Pathology of gastric cancer and its precursor lesions. Gastroenterol Clin North Am. 2013;42:261-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Rosai J. Rosai and Ackerman’s Surgical Pathology. 9th ed. Edinburgh: Mosby, 2004. . |
| 20. | Kim WH, Park CK, Kim YB, Kim YW, Kim HG, Bae HI, Song KS, Chang HK, Chang HJ, Chae YS. A standardized pathology report for gastric cancer. The Gastrointestinal Pathology Study Group of the Korean Society of Pathologists, Korea. Korean J Pathol. 2005;39:106-113. |
| 21. | Ushiku T, Arnason T, Ban S, Hishima T, Shimizu M, Fukayama M, Lauwers GY. Very well-differentiated gastric carcinoma of intestinal type: analysis of diagnostic criteria. Mod Pathol. 2013;26:1620-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Yao T, Utsunomiya T, Oya M, Nishiyama K, Tsuneyoshi M. Extremely well-differentiated adenocarcinoma of the stomach: clinicopathological and immunohistochemical features. World J Gastroenterol. 2006;12:2510-2516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Kang KJ, Kim KM, Kim JJ, Rhee PL, Lee JH, Min BH, Rhee JC, Kushima R, Lauwers GY. Gastric extremely well-differentiated intestinal-type adenocarcinoma: a challenging lesion to achieve complete endoscopic resection. Endoscopy. 2012;44:949-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Lee H, Kim H, Shin SK, Park JC, Lee SK, Lee YC, Kim H, Noh SH. The diagnostic role of endoscopic submucosal dissection for gastric lesions with indefinite pathology. Scand J Gastroenterol. 2012;47:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Jeon HK, Ryu HY, Cho MY, Kim HS, Kim JW, Park HJ, Kim MY, Baik SK, Kwon SO, Park SY, Won SH. A randomized trial to determine the diagnostic accuracy of conventional vs. jumbo forceps biopsy of gastric epithelial neoplasias before endoscopic submucosal dissection; open-label study. Gastric Cancer. 2014;17:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Kim YJ, Park JC, Kim JH, Shin SK, Lee SK, Lee YC, Chung JB. Histologic diagnosis based on forceps biopsy is not adequate for determining endoscopic treatment of gastric adenomatous lesions. Endoscopy. 2010;42:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Ito E, Saito K, Takizawa T, Koike M. Differential diagnosis of atypical epithelium of biopsied gastric mucosa using immunostaining of Ki-67, p53, hMLH1 and MDM2 expression. J Exp Clin Cancer Res. 2002;21:527-537. [PubMed] |
| 28. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1060] [Article Influence: 117.8] [Reference Citation Analysis (0)] |