Published online Jan 28, 2019. doi: 10.3748/wjg.v25.i4.457
Peer-review started: November 29, 2018
First decision: December 12, 2018
Revised: December 19, 2018
Accepted: January 9, 2019
Article in press: January 9, 2019
Published online: January 28, 2019
Processing time: 58 Days and 11.8 Hours
Endoscopic submucosal dissection (ESD) for gastric neoplasms during continuous low-dose aspirin (LDA) administration is generally acceptable according to recent guidelines. This retrospective study aimed to investigate the effect of continuous LDA on the postoperative bleeding after gastric ESD in patients receiving dual antiplatelet therapy (DAPT).
To investigate the feasibility of gastric ESD with continuous LDA in patients with DAPT.
A total of 597 patients with gastric neoplasms treated with ESD between January 2010 and June 2017 were enrolled. The patients were categorized according to type of antiplatelet therapy (APT).
The postoperative bleeding rate was 6.9% (41/597) in all patients. Patients were divided into the following two groups: no APT (n = 443) and APT (n = 154). APT included single-LDA (n = 95) and DAPT (LDA plus clopidogrel, n = 59) subgroups. In the single-LDA and DAPT subgroups, 56 and 39 patients were received continuous LDA, respectively. The bleeding rate with continuous single-LDA (10.7%) was similar to that with discontinuous single-LDA (10.3%) (P > 0.99). Although the bleeding rate with continuous LDA in patients receiving DAPT (23.1%) was higher than that with discontinuous LDA in patients receiving DAPT (5.0%), no significant difference was observed (P = 0.141).
The bleeding rate with continuous LDA in patients receiving DAPT was not statistically different from that with discontinuous LDA in patients receiving DAPT. Therefore, continuous LDA administration may be acceptable for ESD in patients receiving DAPT, although patients should be carefully monitored for possible bleeding.
Core tip: Antithrombotic therapy is considered as an independent risk factor for postoperative bleeding after gastric endoscopic submucosal dissection (ESD). In the latest guideline, low-dose aspirin (LDA) should be continued for patients with a high risk of thrombosis who undergo ESD. In this retrospective study, we aimed to investigate the effect of continuous LDA on postoperative bleeding after gastric ESD in patients receiving dual antiplatelet therapy.
- Citation: Harada H, Suehiro S, Murakami D, Nakahara R, Nagasaka T, Ujihara T, Sagami R, Katsuyama Y, Hayasaka K, Amano Y. Feasibility of gastric endoscopic submucosal dissection with continuous low-dose aspirin for patients receiving dual antiplatelet therapy. World J Gastroenterol 2019; 25(4): 457-468
- URL: https://www.wjgnet.com/1007-9327/full/v25/i4/457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i4.457
The safety of endoscopic submucosal dissection (ESD) for gastric neoplasms has been fully investigated, and postoperative bleeding after ESD has been proven to be a major complication. The rates of postoperative bleeding range from 0.9% to 15.6%[1-16], and the independent risk factors for postoperative bleeding have been identified as tumor location, tumor size, operation time, and the presence of ulcers[1,2,17].
Several reports have also presented antithrombotic therapy as an independent risk factor for postoperative bleeding after ESD[15,16,18]. Antiplatelet agents (APAs) are usually used for the prevention and treatment of cardiovascular diseases, such as acute coronary syndrome and after the placement of coronary stents, and aspirin is mandatory to prevent thromboembolic events after the placement of coronary stents[19]. Patients with drug-eluting stents (DES) have a higher risk of stent thrombosis and require dual antiplatelet therapy (DAPT; aspirin plus thienopyridine) for 12 mo after the placement of the DES[19-21]. Subsequently, monotherapy with APAs should be prescribed to those patients following DAPT.
According to the guidelines of both the American Society for Gastrointestinal Endoscopy (ASGE) and the Japan Gastroenterological Endoscopy Society (JGES), endoscopic procedures with a high risk of bleeding, including ESD, do not require the discontinuation of low-dose aspirin (LDA) for patients with a high risk of thrombosis[22-24]. In the latest guideline, the European Society for Gastrointestinal Endoscopy (ESGE) recommends that LDA should be continued for patients with a high risk of thrombosis who undergo high-risk endoscopic procedures including ESD[25]. Moreover, given that the prescribing physician allows cessation of thienopyridine administration, ESGE recommends the continuation of LDA for patients receiving DAPT.
Several studies have reported that continuous LDA is not an independent risk factor for postoperative bleeding after ESD[14,17,26,27]. On the other hand, only a few studies have reported that DAPT is a significant risk factor for postoperative bleeding after ESD[13,28,29]. However, several limitations have been found in these studies, such as small sample size and use of other types of thienopyridines, such as ticlopidine. In this study, we aimed to investigate the effect of continuous LDA on postoperative bleeding after gastric ESD in patients receiving DAPT (LDA plus clopidogrel).
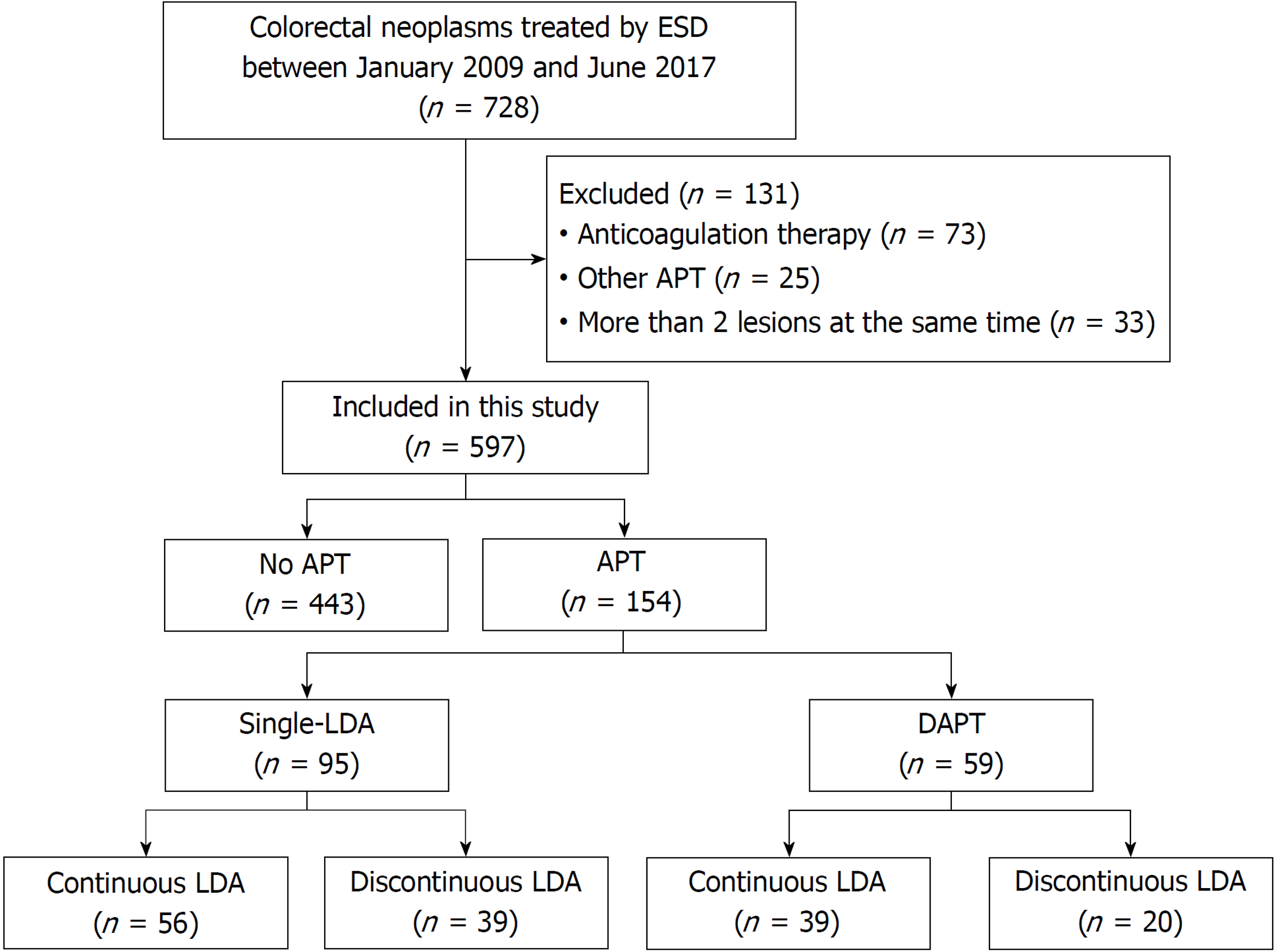
This retrospective study was conducted at New Tokyo Hospital. A total of 728 gastric neoplasms were treated with ESD between January 2010 and June 2017. The treatment indications for these patients were based on the guidelines proposed by the Japanese Gastric Cancer Association and included the expanded criteria proposed by Gotoda et al[30,31] as follows: (1) differentiated-type mucosal cancer or low-grade to high-grade dysplasia (adenoma), regardless of tumor size; (2) tumor size ≤ 3 cm if there was a differentiated-type mucosal cancer with ulcerative findings or minute submucosal invasion (< 500 micron from the muscularis mucosae); and (3) tumor size ≤ 2 cm if there was an undifferentiated-type mucosal cancer without ulcerative findings.
Antiplatelet therapy (APT) was defined as follows: oral administration of single-LDA [aspirin (100 mg/d)] or DAPT [aspirin (100 mg/d) plus clopidogrel (75 mg/d)]. A total of 131 lesions were excluded from this study: 73 lesions of anticoagulation therapy; 25 lesions of APT excluding single-LDA and DAPT: cilostazol, sarpogrelate, ticlopidine, and combined with these drugs, and 33 lesions of patients who underwent ESD for more than two lesions at the same time. Consequently, a total of 597 patients were enrolled in this study.
All patients provided written informed consent before undergoing ESD. We retrospectively reviewed their clinical records after obtaining approval from the institutional review board of New Tokyo Hospital (IRB No. NTH-0102).
For patients on single-LDA who were diagnosed as having a low risk of thrombosis, LDA treatment was discontinued more than five days before ESD. Patients on single-LDA who were diagnosed as having a high risk of thrombosis were treated with uninterrupted LDA. For patients on DAPT who were diagnosed as having a low risk of thrombosis, LDA and clopidogrel were discontinued more than five days before ESD. Patients on DAPT who were diagnosed as having a high risk of thrombosis were treated with continuous LDA, although clopidogrel was discontinued for more than five days before ESD. All APAs were recommenced on approximately 5 d after ESD. The high thrombosis risk was defined as the conditions based on ASGE and JGES guidelines[22-24]. We also consulted with the prescribing physician before the procedure to determine whether LDA and/or clopidogrel should be stopped or continued.
ESD was performed with either a single-channel or a 2-channel endoscope (GIF-Q260J, GIF-H290Z, and GIF-2TQ260M; Olympus, Tokyo, Japan), and with either a FlushKnife BT (DK2618JB; Fujifilm, Tokyo, Japan) or a DualKnife (KD-650L; Olympus, Tokyo, Japan) as the electrosurgical knife. ESD procedures were performed predominantly by four expert endoscopists who have performed more than 300 gastric ESDs. For the procedure, markings were made with the electrosurgical knife around the lesion. Sodium hyaluronate was injected into the submucosal layer, and a circumferential mucosal incision outside the markings was made with the electrosurgical knife. Subsequently, submucosal dissection of the lesion was performed, and pre-coagulation of visible vessels with hemostatic forceps (FD-230U or FD-410LR; Olympus, Tokyo, Japan) was performed during the submucosal dissection. After removal of the lesion, all visible vessels on the artificial ulcer bed were coagulated with hemostatic forceps. Neither endo-clip suturing and poly-glycolic acid sheet patching was done for post-ESD ulcer in this study.
Proton pump inhibitors were administered intravenously for two days following the ESD. All patients underwent a second-look endoscopy the day after ESD. If possible bleeding vessels were observed in the ulcer bed, prophylactic hemostasis was performed with a high-frequency soft coagulation using hemostatic forceps or hemostatic clips. A liquid diet concomitant with oral administration of proton pump inhibitors was started two days after ESD if no signs of postoperative bleeding were confirmed. All patients were also given oral proton pump inhibitors for eight weeks following ESD. All adverse events were recorded for 30 d after ESD.
Postoperative bleeding was defined as hematemesis/melena or a decrease in blood hemoglobin levels by greater than 2 mg/dL. Delayed postoperative bleeding was defined as bleeding after five days post ESD. Emergency EGD and endoscopic hemostasis were performed for patients with postoperative bleeding. A blood transfusion was usually administered when excessive bleeding with hemorrhagic shock and/or markedly decreased blood hemoglobin levels (less than 8 g/dL) were observed. An additional second-look endoscopy was carried out the day after the endoscopic hemostasis.
Included patients were categorized into the following two groups: with APT and without APT groups. The APT group included the single-LDA and the DAPT subgroups. To investigate the effect of the APT on postoperative bleeding, we performed a subgroup analysis among APT users. Furthermore, a subgroup analysis among continuous LDA users was performed to compare the effect of continuous single-LDA and continuous LDA on DAPT.
Baseline variables were expressed as the mean [standard deviation (SD)]. Categorical data were compared with the Fisher’s exact test or the chi-squared test, and differences in variables between groups were analyzed with the Student’s t-test or the Mann-Whitney U test. A P value of < 0.05 was considered statistically significant. All data analyses were conducted with SPSS version 24.0 (Armonk, NY: IBM Corp.).
Among the 597 patients analyzed, 154 (25.8%) patients were receiving APT to prevent thrombosis. There were 95 (15.9%) patients in the single-LDA group and 59 (9.9%) in the DAPT group. The number of patients receiving single-LDA with continuous LDA was 56 (9.4%), and the number of patients receiving DAPT with continuous LDA and discontinuation of clopidogrel was 39 (6.5%), as shown in Figure 1.
Baseline characteristics and therapeutic outcomes in all patients are shown in Table 1. The mean age of the patients was 72.3 years. The proportion of males was 69.3% (414/597). Coronary artery diseases were found in 23.8% (142/597) of the enrolled patients. The median of specimen size was 32.9 (IQR: 24-40) mm. The overall rate of postoperative bleeding after gastric ESD was 6.9% (41/597). The postoperative bleeding rate in patients with and without APT was 13.0% (20/154) and 4.7% (21/443), respectively (P = 0.001). Among the patients with postoperative bleeding, delayed bleeding (more than 5 d after ESD) was found in 2.7% (16/597) of all the enrolled patients. The delayed bleeding rate in patients with and without APT was 7.1% (11/154) and 1.1% (5/443), respectively. Among the patients with postoperative bleeding, the re-admission patients rate in those with and without APT was 3.2% (5/154) and 0.2% (1/443), respectively. Blood transfusions were required for 1.5% (9/597) of all the patients. Among them, the blood transfusion rates of patients with and without APT were 3.2% (5/154) and 0.9% (4/443), respectively.
| All patients (n = 597) | |
| Age, mean (SD), yr | 72.3 (8.815) |
| Sex, male, n (%) | 414 (69.3) |
| BMI, mean (SD), yr | 22.6 (3.424) |
| Comorbidity, n (%) | 379 (63.5) |
| Hypertension | 307 (51.4) |
| Diabetes mellitus | 114 (19.1) |
| Renal failure | 17 (2.8) |
| Coronary artery diseases | 142 (23.8) |
| Longitudinal location, n (%) | |
| Upper | 90 (15.1) |
| Middle | 250 (41.9) |
| Lower | 257 (43.0) |
| Macroscopic findings, n (%) | |
| Depressed | 275 (46.1) |
| Non-depressed | 322 (53.9) |
| Pathological findings, n (%)1 | |
| Differentiated | 578 (96.8) |
| Undifferentiated | 19 (3.2) |
| Depth of invasion, n (%) | |
| Mucosa | 530 (88.8) |
| Submucosa | 67 (11.2) |
| LDA intake, n (%) | 154 (25.8) |
| Continuous LDA | 95 (16.4) |
| Continuous single-LDA | 56 (9.3) |
| DAPT (LDA with clopidogrel), n (%) | 59 (9.9) |
| Continuous LDA on DAPT, n (%) | 39 (6.5) |
| Specimen size, median (IQR), mm | 32.9 (24-40) |
| Complete resection, n (%) | 557 (93.3) |
| Curative resection, n (%) | 524 (87.8) |
| Postoperative bleeding, n (%) | 41 (6.9) |
| Delayed bleeding | 16 (2.7) |
Characteristics are compared among patients receiving LDA in the continuous LDA group and the discontinuous LDA group in Table 2. There were no significant differences between groups in any of these characteristics. Therapeutic outcomes are compared among patients receiving LDA in the continuous LDA group and the discontinuous LDA group in Table 3. The postoperative bleeding rate in the continuous LDA group (15.8%) was numerically higher than that in the discontinuous LDA group (8.5%), although no significant difference was observed (P = 0.225).
| Continuous LDA (n = 95) | Discontinuous LDA (n = 59) | P value | |
| Age, mean (SD), yr | 75.5 (6.841) | 75.7 (7.684) | 0.856 |
| Sex, male, n (%) | 71 (74.7) | 46 (78.0) | 0.701 |
| BMI, mean (SD), yr | 22.7 (3.395) | 23.1 (3.079) | 0.382 |
| Comorbidity, n (%) | |||
| Hypertension | 70 (73.7) | 39 (66.1) | 0.364 |
| Diabetes mellitus | 32 (33.7) | 21 (35.6) | 0.862 |
| Renal failure | 7 (7.4) | 4 (6.8) | > 0.99 |
| Coronary artery diseases | 78 (82.1) | 42 (71.2) | 0.161 |
| Longitudinal location, n (%) | |||
| Upper | 18 (18.9) | 7 (11.9) | 0.429 |
| Middle | 40 (42.1) | 24 (40.7) | |
| Lower | 37 (39.0) | 28 (47.5) | |
| Macroscopic findings, n (%) | |||
| Depressed | 31 (32.6) | 21 (35.6) | 0.729 |
| Non-depressed | 64 (67.4) | 38 (64.4) |
| Continuous LDA (n = 95) | Discontinuous LDA (n = 59) | P value | |
| Pathological findings, n (%)1 | |||
| Differentiated | 94 (98.9) | 58 (98.3) | > 0.99 |
| Undifferentiated | 1 (1.1) | 1 (1.7) | |
| Depth of invasion, n (%) | |||
| Mucosa | 80 (84.2) | 52 (88.1) | 0.637 |
| Submucosa | 15 (15.8) | 7 (11.9) | |
| Specimen size, median (IQR), mm | 30 (25-39) | 33 (25-41.5) | 0.944 |
| Complete resection, n (%) | 91 (95.8) | 57 (96.6) | > 0.99 |
| Curative resection, n (%) | 85 (89.5) | 54 (91.5) | 0.785 |
| Postoperative bleeding, n (%) | 15 (15.8) | 5 (8.5) | 0.225 |
Characteristics are compared among patients receiving single-LDA in the continuous LDA group and the discontinuous LDA group are in Table 4. There were no significant differences between groups in any of these characteristics, except BMI. Therapeutic outcomes are compared among patients receiving single-LDA in the continuous LDA group and the discontinuous LDA group in Table 5. The postoperative bleeding rate in the continuous LDA group (10.7%) was numerically higher than that in the discontinuous LDA group (10.3%), although no significant difference was observed (P > 0.99).
| Continuous LDA (n = 56) | Discontinuous LDA (n = 39) | P value | |
| Age, mean (SD), yr | 76.5 (7.628) | 74.8 (5.961) | 0.255 |
| Sex, male, n (%) | 37 (66.1) | 32 (82.1) | 0.105 |
| BMI, mean (SD), yr | 22.2 (3.029) | 23.6 (2.958) | 0.025 |
| Comorbidity, n (%) | |||
| Hypertension | 41 (73.2) | 24 (61.5) | 0.266 |
| Diabetes mellitus | 13 (23.2) | 13 (33.3) | 0.351 |
| Renal failure | 3 (5.4) | 1 (2.6) | 0.642 |
| Coronary artery diseases | 41 (73.2) | 25 (64.1) | 0.372 |
| Longitudinal location, n (%) | |||
| Upper | 8 (14.3) | 4 (10.3) | 0.643 |
| Middle | 28 (50.0) | 17 (43.6) | |
| Lower | 20 (35.7) | 18 (46.2) | |
| Macroscopic findings, n (%) | |||
| Depressed | 20 (35.7) | 16 (41.0) | 0.669 |
| Non-depressed | 36 (64.3) | 23 (59.0) |
| Continuous LDA (n = 56) | Discontinuous LDA (n = 39) | P value | |
| Pathological findings, n (%)1 | |||
| Differentiated | 55 (98.2) | 39 (100) | > 0.99 |
| Undifferentiated | 1 (1.8) | 0 (0) | |
| Depth of invasion, n (%) | |||
| Mucosa | 50 (89.3) | 33 (84.6) | 0.542 |
| Submucosa | 6 (10.7) | 6 (15.4) | |
| Specimen size, median (IQR), mm | 28 (22-37.3) | 31 (25-43.5) | 0.440 |
| Complete resection, n (%) | 53 (94.6) | 37 (94.9) | > 0.99 |
| Curative resection, n (%) | 52 (92.9) | 34 (87.2) | 0.480 |
| Postoperative bleeding, n (%) | 6 (10.7) | 4 (10.3) | > 0.99 |
Characteristics are compared among patients receiving DAPT in the continuous LDA group and the discontinuous LDA group in Table 6. There were no significant differences between groups in any of these characteristics. Therapeutic outcomes are compared among patients receiving DAPT in the continuous LDA group and the discontinuous LDA group in Table 7. The postoperative bleeding rate in the continuous LDA group (23.1%) was numerically higher than that in the discontinuous LDA group (5.0%), although no significant difference was observed (P = 0.141).
| Continuous LDA (n = 39) | Discontinuous LDA (n = 20) | P value | |
| Age, mean (SD), yr | 74.7 (7.743) | 77.4 (7.665) | 0.216 |
| Sex, male, n (%) | 34 (87.2) | 14 (70.0) | 0.159 |
| BMI, mean (SD), yr | 23.5 (3.765) | 22.4 (3.232) | 0.273 |
| Comorbidity, n (%) | |||
| Hypertension | 29 (74.4) | 15 (75.0) | > 0.99 |
| Diabetes mellitus | 19 (48.7) | 8 (40.0) | 0.589 |
| Renal failure | 4 (10.3) | 3 (15.0) | 0.679 |
| Coronary artery diseases | 37 (94.9) | 17 (85.0) | 0.325 |
| Longitudinal location, n (%) | |||
| Upper | 10 (25.6) | 3 (15.0) | 0.726 |
| Middle | 12 (30.8) | 7 (35.0) | |
| Lower | 17 (43.6) | 10 (50.0) | |
| Macroscopic findings, n (%) | |||
| Depressed | 11 (28.2) | 5 (25.0) | > 0.99 |
| Non-depressed | 28 (71.8) | 15 (75.0) |
| Continuous LDA (n = 39) | Discontinuous LDA (n = 20) | P value | |
| Pathological findings, n (%)1 | |||
| Differentiated | 39 (100) | 19 (95.0) | 0.339 |
| Undifferentiated | 0 (0) | 1 (5.0) | |
| Depth of invasion, n (%) | |||
| Mucosa | 30 (76.9) | 19 (95.0) | 0.141 |
| Submucosa | 9 (23.1) | 1 (5.0) | |
| Specimen size, median (IQR), mm | 34 (29.5-40.0) | 35 (24.3-36.3) | 0.373 |
| Complete resection, n (%) | 38 (97.4) | 20 (100) | > 0.99 |
| Curative resection, n (%) | 33 (84.6) | 20 (100) | 0.09 |
| Postoperative bleeding, n (%) | 9 (23.1) | 1 (5.0) | 0.141 |
Treatment of patients with antithrombotic agents is common because of the increasing numbers of elderly patients and the consequent increases in the incidence of cardio- and cerebro-vascular diseases. We have evaluated the influence of anticoagulant therapy on gastric ESD in patients treated with continuous low-dose warfarin, and we reported that the postoperative bleeding rate with continuous low-dose warfarin was lower than that with heparin bridge therapy[32]. On the other hand, with regard to APA, rates of postoperative bleeding after gastric ESD in patients receiving continuous LDA have been reported to range from 3.6% to 21.1%[13-15,27]. However, nowadays, some dual antiplatelet therapies are applied for not a few cases with cardiovascular disease. Therefore, we suggest that further investigations of the effect of continuous LDA on postoperative bleeding in patients with dual antiplatelet therapies, such as the combination of LDA and clopidogrel, are needed.
In this study, first, it was proven that the postoperative bleeding rate of the continuous LDA group was not a significantly different from that of the discontinuous LDA group. Thus, we confirmed that continuous LDA is not a risk factor for postoperative bleeding as shown in previous reports[14,15,27]. Second, we assessed postoperative bleeding and clinical outcomes after ESD with continuous LDA in patients receiving DAPT, this being suggestive of the most important issue to be clarified by the present study. We found that the postoperative bleeding rate of this group was not significantly different from that with discontinuous LDA in patients receiving DAPT. ESGE recommend that DAPT should be replaced by monotherapy with continuous aspirin for patients undergoing ESD[25]. According to the ESGE guideline, we performed ESD with continuous LDA as a substitution for DAPT for patients with a high risk of thrombosis. However, few studies have assessed postoperative bleeding in patients receiving DAPT. It remains controversial whether there is an association between the risk of postoperative bleeding and DAPT administration[13,14,27,28]. In this study, the postoperative bleeding rate with continuous LDA in patients receiving DAPT was numerically higher than that with discontinuous LDA in patients receiving DAPT, although the difference was not significant. Therefore, continuous LDA administration may be acceptable for ESD in patients receiving DAPT. However, the postoperative bleeding rate of patients with continuous LDA receiving DAPT was higher compared that of those without APT [23.1% (9/39) vs 4.7% (21/443)]. Hence, meticulous observation of postoperative bleeding in patients receiving continuous LDA on DAPT must be still required.
Previous studies reported that most of the postoperative bleeding occurred within 72 h after gastric ESD[1,5,11,12,16,33,34]. In this study, the rate of delayed bleeding, after five days of gastric ESD in the APT group was more than twice those in the group without APT [55.0% (11/20) vs 23.8% (5/21), respectively]. Our results suggest that patients receiving APT should be carefully observed and managed with a longer follow-up period. Several studies reported that multiple APAs therapy was associated with post-ESD bleeding[27-29]. In our study, the incidence of postoperative bleeding in patients with discontinuous LDA on DAPT was only one patient of which bleeding occurred within 24 h. On the other hand, delayed bleeding was found in 6 out of 10 patients with postoperative bleeding on DAPT. Similarly, delayed bleeding was found in 6 out of 10 patients with postoperative bleeding on single-LDA. Therefore, the delayed bleeding might be associated with the reinitiating of LDA or clopidogrel rather than the number of APAs.
Delayed bleeding was found in 6 patients in the continuous LDA on DAPT group: 5 out of 6 patients after 10 d and the remaining one on 9th day after ESD. As clopidogrel is recommenced on approximately 5 d after ESD, the effect of clopidogrel might cause the delayed bleeding in the continuous LDA group on DAPT. The postoperative bleeding in patients on DAPT excluding the above 6 patients was observed on less than 48 h. On the other hand, in other patients on DAPT, the rate after 10 d in the continuous single-LDA group was almost similar to that in the discontinuous single-LDA group [33.3% (2/6) vs 25.0% (1/4)]. Hence, the present study suggests that the reinitiating of clopidogrel may be associated with the increasing risk of delayed bleeding after 5th day from the reinitiating. Patients receiving continuous LDA on DAPT may require extended hospitalization and/or need to be managed on an outpatient basis for a longer time.
This study has some limitations. First, this was a retrospective study done at a single institution. Second, the numbers of patients with postoperative bleeding might be comparatively small. The postoperative bleeding rate in the continuous single-LDA group was similar to that in the discontinuous single-LDA group. On the other hand, the postoperative bleeding rate in patients receiving continuous LDA on DAPT was four times higher than that in patients receiving discontinuous LDA on DAPT. This may be derived from the small number of cases receiving discontinuous LDA on DAPT. In the near future, it is mandatory to accumulate cases of gastric ESD on DAPT and to clarify the risks of the postoperative bleeding. However, patients receiving DAPT usually have a high risk of thrombosis via the discontinuation of APAs. Thus, it may be difficult to perform a randomized controlled study due to ethical issues.
In conclusion, the continuous LDA administration may be acceptable for gastric ESD in patients receiving DAPT, although patients should be carefully monitored for possible postoperative bleeding.
The postoperative bleeding after gastric endoscopic submucosal dissection (ESD) with continuous low dose aspirin (LDA) in patients with dual antiplatelet therapy (DAPT) is unclear.
Previous studies have had several limitations, such as small sample size and use of other types of thienopyridines, such as ticlopidine. We investigated the effect of only clopidogrel as thienopyridine.
Evaluate the postoperative bleeding for the effect of continuous LDA in patients with DAPT.
A total of 597 patients with gastric neoplasms treated with ESD between January 2010 and June 2017 were enrolled. Among them, we analyzed 59 patients receiving DAPT. The main outcome was the postoperative bleeding after ESD.
The bleeding rate with continuous LDA in patients receiving DAPT (23.1%) was higher than that with discontinuous LDA in patients receiving DAPT (5.0%).
The bleeding rate with continuous LDA in patients receiving DAPT was not statistically different from that with discontinuous LDA in patients receiving DAPT.
The continuous LDA administration may be acceptable for gastric ESD in patients receiving DAPT, although patients should be carefully monitored for possible postoperative bleeding.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Crino S, Jun C S- Editor: Ma RY L- Editor: A E- Editor: Yin SY
| 1. | Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 2. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, Kim HJ, Kim JJ, Ji SR, Seol SY. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 3. | Yamaguchi N, Isomoto H, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Ohnita K, Hayashi T, Nakao K, Kohno S, Shikuwa S. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Goto O, Fujishiro M, Kodashima S, Ono S, Niimi K, Hirano K, Yamamichi N, Koike K. A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary: a retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest Endosc. 2010;71:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, Matsuhashi N. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16:2913-2917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Mannen K, Tsunada S, Hara M, Yamaguchi K, Sakata Y, Fujise T, Noda T, Shimoda R, Sakata H, Ogata S, Iwakiri R, Fujimoto K. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Hirasawa K, Kokawa A, Oka H, Yahara S, Sasaki T, Nozawa A, Morimoto M, Numata K, Taguri M, Morita S, Maeda S, Tanaka K. Risk assessment chart for curability of early gastric cancer with endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Okada K, Yamamoto Y, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Chino A, Tsuchida T, Fujisaki J, Nakajima A, Hoshino E, Igarashi M. Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2011;25:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Higashiyama M, Oka S, Tanaka S, Sanomura Y, Imagawa H, Shishido T, Yoshida S, Chayama K. Risk factors for bleeding after endoscopic submucosal dissection of gastric epithelial neoplasm. Dig Endosc. 2011;23:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S, Morikawa T, Murakami T, Tomoda J. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Nakamura M, Nishikawa J, Hamabe K, Nishimura J, Satake M, Goto A, Kiyotoki S, Saito M, Fukagawa Y, Shirai Y, Okamoto T, Sakaida I. Risk factors for delayed bleeding from endoscopic submucosal dissection of gastric neoplasms. Scand J Gastroenterol. 2012;47:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Kim HH, Park SJ, Park MI, Moon W. Clinical impact of second-look endoscopy after endoscopic submucosal dissection of gastric neoplasms. Gut Liver. 2012;6:316-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Cho SJ, Choi IJ, Kim CG, Lee JY, Nam BH, Kwak MH, Kim HJ, Ryu KW, Lee JH, Kim YW. Aspirin use and bleeding risk after endoscopic submucosal dissection in patients with gastric neoplasms. Endoscopy. 2012;44:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Lim JH, Kim SG, Kim JW, Choi YJ, Kwon J, Kim JY, Lee YB, Choi J, Im JP, Kim JS, Jung HC, Song IS. Do antiplatelets increase the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms? Gastrointest Endosc. 2012;75:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Matsumura T, Arai M, Maruoka D, Okimoto K, Minemura S, Ishigami H, Saito K, Nakagawa T, Katsuno T, Yokosuka O. Risk factors for early and delayed post-operative bleeding after endoscopic submucosal dissection of gastric neoplasms, including patients with continued use of antithrombotic agents. BMC Gastroenterol. 2014;14:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Koh R, Hirasawa K, Yahara S, Oka H, Sugimori K, Morimoto M, Numata K, Kokawa A, Sasaki T, Nozawa A, Taguri M, Morita S, Maeda S, Tanaka K. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Uedo N, Takeuchi Y, Yamada T, Ishihara R, Ogiyama H, Yamamoto S, Kato M, Tatsumi K, Masuda E, Tamai C, Yamamoto S, Higashino K, Iishi H, Tatsuta M. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Takeuchi T, Ota K, Harada S, Edogawa S, Kojima Y, Tokioka S, Umegaki E, Higuchi K. The postoperative bleeding rate and its risk factors in patients on antithrombotic therapy who undergo gastric endoscopic submucosal dissection. BMC Gastroenterol. 2013;13:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2402] [Cited by in RCA: 2331] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 20. | Grines CL, Bonow RO, Casey DE Jr, Gardner TJ, Lockhart PB, Moliterno DJ, O’Gara P, Whitlow P; American Heart Association; American College of Cardiology; Society for Cardiovascular Angiography and Interventions; American College of Surgeons; American Dental Association; American College of Physicians. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation. 2007;115:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 551] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 21. | Kushner FG, Hand M, Smith SC Jr, King SB 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 926] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 22. | ASGE Standards of Practice Committee; Anderson MA, Ben-Menachem T, Gan SI, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N, Ikenberry SO, Jain R, Khan K, Krinsky MI, Lichtenstein DR, Maple JT, Shen B, Strohmeyer L, Baron T, Dominitz JA. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 352] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | ASGE Standards of Practice Committee; Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 458] [Article Influence: 50.9] [Reference Citation Analysis (1)] |
| 24. | Fujimoto K, Fujishiro M, Kato M, Higuchi K, Iwakiri R, Sakamoto C, Uchiyama S, Kashiwagi A, Ogawa H, Murakami K, Mine T, Yoshino J, Kinoshita Y, Ichinose M, Matsui T; Japan Gastroenterological Endoscopy Society. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 25. | Veitch AM, Vanbiervliet G, Gershlick AH, Boustiere C, Baglin TP, Smith LA, Radaelli F, Knight E, Gralnek IM, Hassan C, Dumonceau JM. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48:385-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Shindo Y, Matsumoto S, Miyatani H, Yoshida Y, Mashima H. Risk factors for postoperative bleeding after gastric endoscopic submucosal dissection in patients under antithrombotics. World J Gastrointest Endosc. 2016;8:349-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Sanomura Y, Oka S, Tanaka S, Numata N, Higashiyama M, Kanao H, Yoshida S, Ueno Y, Chayama K. Continued use of low-dose aspirin does not increase the risk of bleeding during or after endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. 2014;17:489-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Tounou S, Morita Y, Hosono T. Continuous aspirin use does not increase post-endoscopic dissection bleeding risk for gastric neoplasms in patients on antiplatelet therapy. Endosc Int Open. 2015;3:E31-E38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Ono S, Fujishiro M, Yoshida N, Doyama H, Kamoshida T, Hirai S, Kishihara T, Yamamoto Y, Sakae H, Imagawa A, Hirano M, Koike K. Thienopyridine derivatives as risk factors for bleeding following high risk endoscopic treatments: Safe Treatment on Antiplatelets (STRAP) study. Endoscopy. 2015;47:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1913] [Article Influence: 239.1] [Reference Citation Analysis (1)] |
| 31. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1326] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 32. | Harada H, Suehiro S, Murakami D, Shimizu T, Nakahara R, Katsuyama Y, Miyama Y, Tounou S, Hayasaka K. Continuous use of low-dose warfarin for gastric endoscopic submucosal dissection: a prospective study. Endosc Int Open. 2017;5:E348-E353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Ono S, Koike K. Endoscopists should keep up with the current trends of antithrombotic therapy in other fields. Dig Endosc. 2017;29:676-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Yoshio T, Tomida H, Iwasaki R, Horiuchi Y, Omae M, Ishiyama A, Hirasawa T, Yamamoto Y, Tsuchida T, Fujisaki J, Yamada T, Mita E, Ninomiya T, Michitaka K, Igarashi M. Effect of direct oral anticoagulants on the risk of delayed bleeding after gastric endoscopic submucosal dissection. Dig Endosc. 2017;29:686-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |