Published online Oct 21, 2019. doi: 10.3748/wjg.v25.i39.5936
Peer-review started: July 2, 2019
First decision: August 2, 2019
Revised: August 19, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 21, 2019
Processing time: 112 Days and 2 Hours
The use of methyl-tertiary butyl ether (MTBE) to dissolve gallstones has been limited due to concerns over its toxicity and the widespread recognition of the safety of laparoscopic cholecystectomy. The adverse effects of MTBE are largely attributed to its low boiling point, resulting in a tendency to evaporate. Therefore, if there is a material with a higher boiling point and similar or higher dissolubility than MTBE, it is expected to be an attractive alternative to MTBE.
To determine whether tert-amyl ethyl ether (TAEE), an MTBE analogue with a relatively higher boiling point (102 °C), could be used as an alternative to MTBE in terms of gallstone dissolubility and toxicity.
The in vitro dissolubility of MTBE and TAEE was determined by measuring the dry weights of human gallstones at predetermined time intervals after placing them in glass containers with either of the two solvents. The in vivo dissolubility was determined by comparing the weights of solvent-treated gallstones and control (dimethyl sulfoxide)-treated gallstones, after the direct infusion of each solvent into the gallbladder in both hamster models with cholesterol and pigmented gallstones.
The in vitro results demonstrated a 24 h TAEE-dissolubility of 76.7%, 56.5% and 38.75% for cholesterol, mixed, and pigmented gallstones, respectively, which represented a 1.2-, 1.4-, and 1.3-fold increase in dissolubility compared to that of MTBE. In the in vitro experiment, the 24 h-dissolubility of TAEE was 71.7% and 63.0% for cholesterol and pigmented gallstones, respectively, which represented a 1.4- and 1.9-fold increase in dissolubility compared to that of MTBE. In addition, the results of the cell viability assay and western blot analysis indicated that TAEE had a lower toxicity towards gallbladder epithelial cells than MTBE.
We demonstrated that TAEE has higher gallstone dissolubility properties and safety than those of MTBE. As such, TAEE could present an attractive alternative to MTBE if our findings regarding its efficacy and safety can be consistently reproduced in further subclinical and clinical studies.
Core tip: We developed a novel gallstone-dissolving agent, named tert-amyl ethyl ether (TAEE), a methyl-tertiary butyl ether (MTBE) analogue, with a relatively higher boiling point (102 °C). The in vitro results demonstrated a 24 h TAEE-dissolubility of 76.7%, 56.5% and 38.75% for cholesterol, mixed, and pigmented gallstones, respectively, which represented a 1.2-, 1.4-, and 1.3-fold increase in dissolubility compared to that of MTBE. In the in vivo experiment, TAEE showed a 1.4- and 1.9-fold higher dissolubility for cholesterol and pigmented gallstones than MTBE. As such, TAEE could present an attractive alternative to MTBE if further clinical studies validate its efficacy and safety.
- Citation: You DD, Cho SJ, Kim OH, Song JS, Hwang KS, Lee SC, Kim KH, Choi HJ, Hong HE, Seo H, Hong TH, Park JH, Lee TY, Ahn J, Jung JK, Jung KY, Kim SJ. Superior gallstone dissolubility and safety of tert-amyl ethyl ether over methyl-tertiary butyl ether. World J Gastroenterol 2019; 25(39): 5936-5952
- URL: https://www.wjgnet.com/1007-9327/full/v25/i39/5936.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i39.5936
Gallstone disease, or cholelithiasis, is a highly prevalent disease, particularly in developed countries. In the United States, adults with gallstones are estimated to account for over 14% of the population[1]. Presently, the definitive treatment modality for symptomatic cholelithiasis is a laparoscopic cholecystectomy. The prevalence of gallstones is significantly higher in the older population; it is estimated that approximately 30% of women develop gallstones by the age of 65 years, and 60% of both men and women develop gallstones by the age of 80 years[2]. Elderly patients also have a higher probability of having comorbid medical conditions that could complicate the use of general anesthesia for surgery. Moreover, some patients prefer nonsurgical options. To cope with these situations, investigators have developed various contact litholytic agents (CLAs) that cause chemical dissolution of gallstones by direct contact after entering the gallbladder through the percutaneous transhepatic route.
Methyl tert-butyl ether (MTBE) has been the first choice of CLA ever since its introduction in 1985[3-7]. A large survey in Europe, comprised of 803 patients from 21 institutions, investigated the effectiveness and side effects of MTBE[5]. The dissolubility of MTBE was 95.1%, and 43.1% of patients had post-treatment biliary sludge. The most severe complication was bile leakage, which had been related with the procedure. The stone recurrence rate was about 40% in solitary stones and about 70% in multiple stones over five years. MTBE has structural similarity with a representative anesthetic agent, diethyl ether. It, thus, has a relatively low boiling point (55 °C) and a higher evaporation rate, which could lead to the development of various side effects, including nausea, upper abdominal pain, duodenitis, mild-to-moderate anesthesia, and hemolysis[8-17].
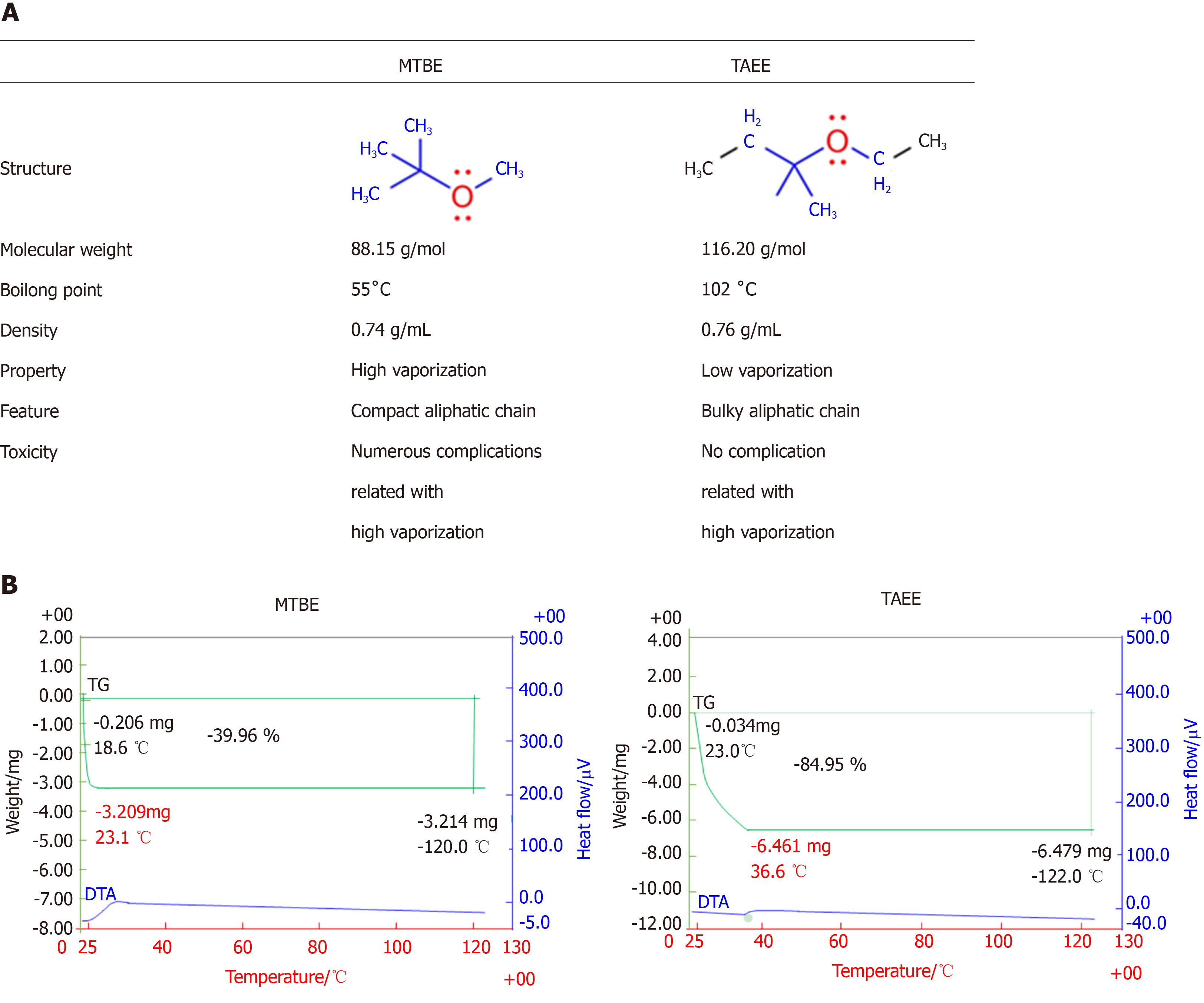
To overcome the toxicities of MTBE, we attempted to discover a novel CLA that has the higher, or at least similar, gallstone-dissolubility than MTBE while maintaining lesser toxicity. We discovered tert-amyl ethyl ether (TAEE) in the process of investigating numerous MTBE's analogues. TAEE has high boiling point of 102 °C that is higher than water and nearly twice as high as MTBE. Unlike the MTBE, TAEE also has a high molecular weight of 116.2 g/mol, but its density is like that of MTBE (0.76 g/mL). We think that these characteristics of TAEE could collectively enhance gallstone dissolubility, while maintaining lesser toxicity. In this study, our aim was to determine whether TAEE could be used as an alternative to MTBE by comparing the gallstone dissolubility and toxicity of these two compounds.
MTBE was obtained from Sigma-Aldrich (St Louis, MO, United States). TAEE was produced in the Korea Research Institute of Chemical Technology (KRICT, Daejeon, South Korea)
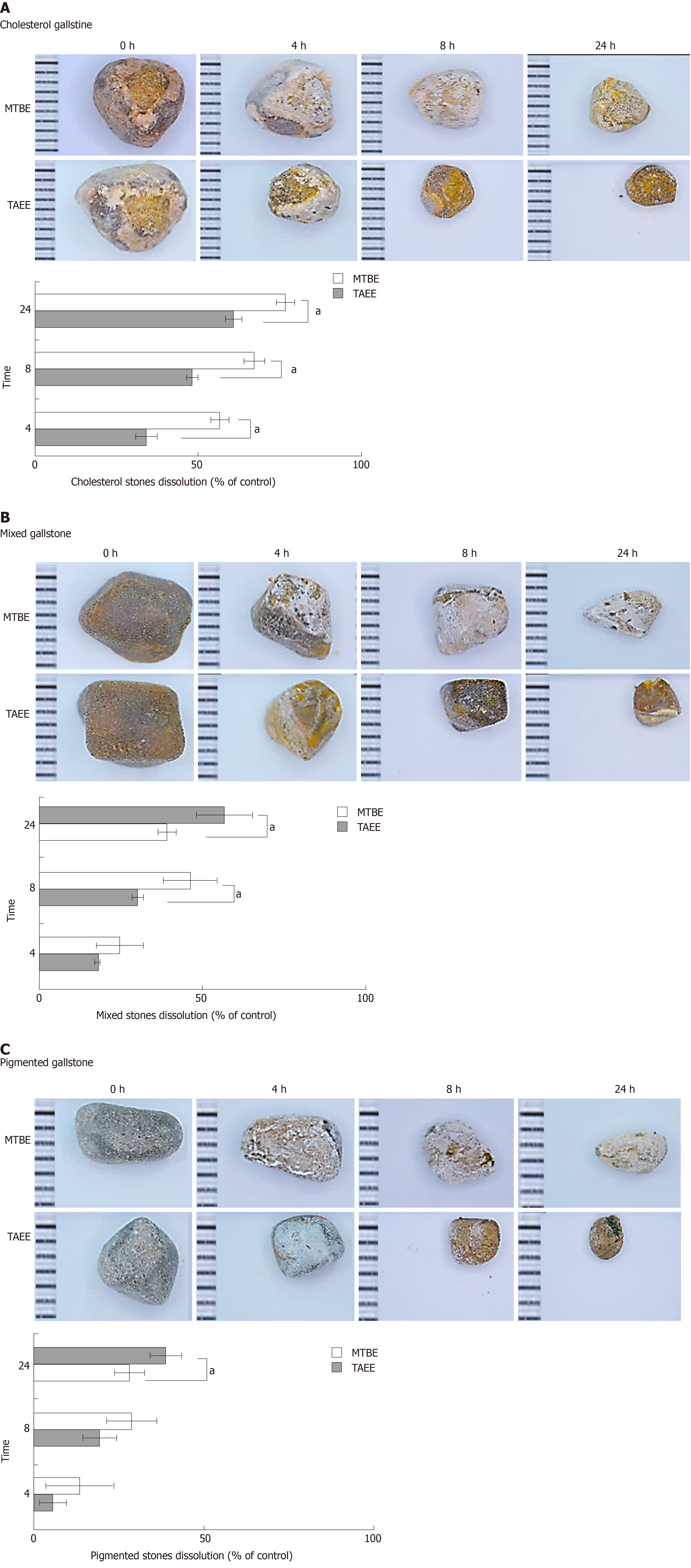
Gallstones were collected after cholecystectomy performed in Seoul St. Mary’s hospital. The study was approved by the Ethics Committee of Seoul St Mary’s hospital, the Catholic University of Korea (IRB code: KC18TESI0103). After being air-dried, weighed, and preserved in saline, the gallstones were matched for size, weight, and shape. Subsequently, the three types of gallstones were placed in separate glass containers, and 10 mL aliquots of MTBE or TAEE were added to the containers, respectively. The aliquots were aspirated and replaced every hour. The glass containers were gently stirred at 50 rpm on the reactor (VS-8480SF; Vision Co., Daejeon, Republic of Korea) at 37 °C for 24 h. Gallstone dissolubility was determined by measuring the dry weights of the gallstones at the determined intervals (4, 8, and 24 h).
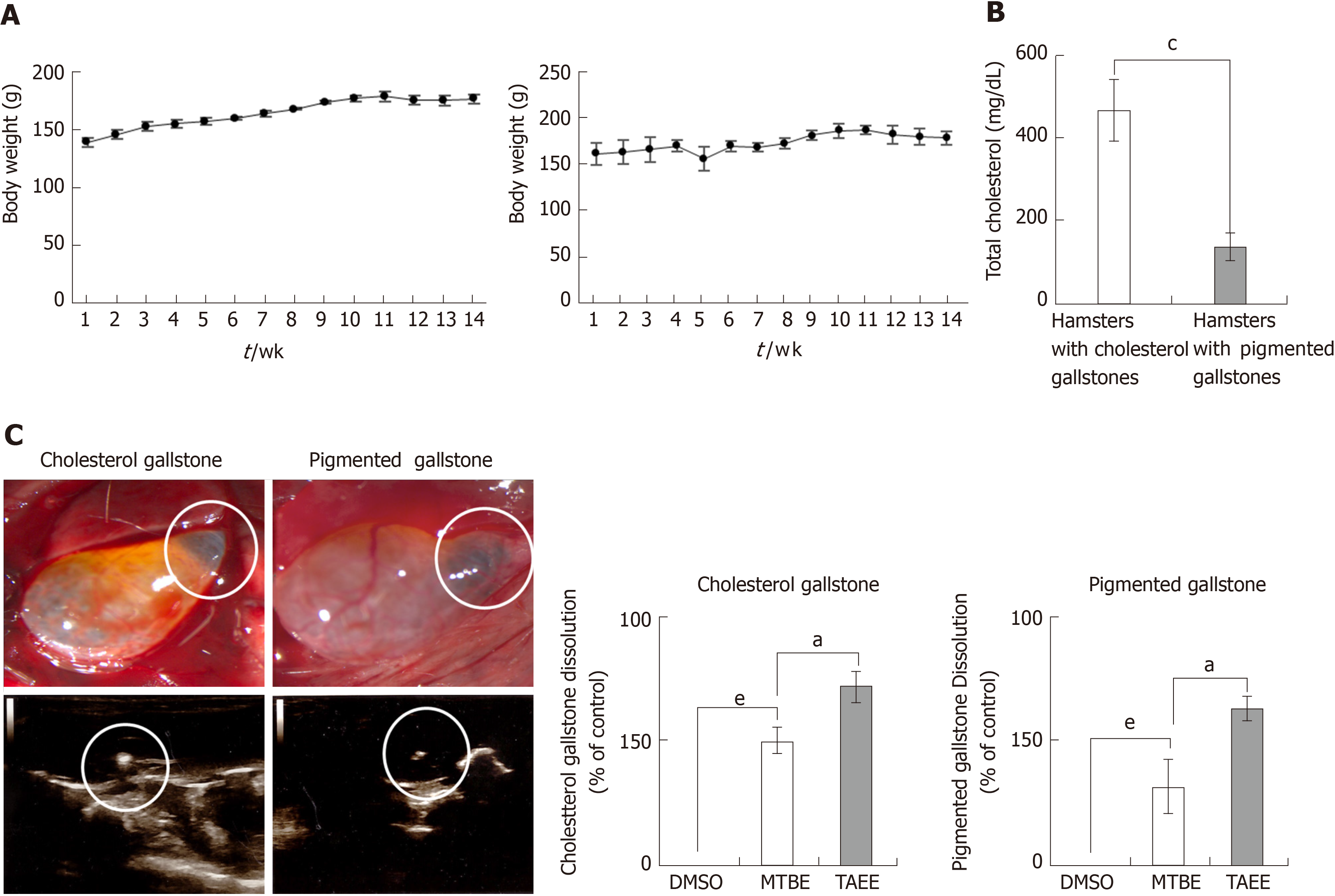
For in vivo validation of each solvent, we used 7-wk-old female Syrian golden hamsters (Mesocricetus auratus, Harlan Sprague Dawley Indianapolis, IN, United States). This animal study was approved by the Institutional Animal Care and Use Committee of the Clinical Research Institute at Daejeon St. Mary’s Hospital at the Catholic University of Korea (IRB No. CMCDJ-AP-2016-004). Hamsters were subdivided into three groups; control (n = 10), CG (hamsters with cholesterol gallstones, n = 17), and PG (hamsters with pigmented gallstones, n = 17) groups. Hamsters in each group were fed a different diet for 4 mo: Control group were fed a normal diet, CG group were fed a general rodent diet with 0.5% cholesterol, and PG group were fed a diet rich in carbohydrates, respectively. After 4 mo of diet, the hamsters with gallstones were selected for the subsequent experiments using abdominal ultrasonography. After laparotomy under general anesthesia, the gallbladder was identified, and the bile in the gallbladder was completely aspirated using a 30-gauge syringe. Subsequently, the gallbladder was cautiously filled with a volume (0.1 mL) of dimethyl sulfoxide (DMSO), MTBE, and TAEE, respectively. After 24 h, gallstone dissolubility of each solvent was determined by comparing the weights of solvent-treated gallstones and control (DMSO)-treated gallstones.
All data were presented as mean ± standard deviation (SD), and analyzed with SPSS 11.0 software (SPSS Inc., Chicago, IL, United Statae). Statistical comparisons between the groups were determined using the Kruskal–Wallis test followed by Dunnett’s test as the post hoc analysis. P < 0.05 was considered statistically significant.
Additional and more detailed information regarding the experimental procedures are fully described in the Supplementary Materials and Methods.
Figure 1A summarizes the comparisons between MTBE and TAEE in terms of their structure and basic characteristics. We first performed thermogravimetric analysis for determining the stability of each solvent according to temperature. In MTBE, we fail to attain TGA data of MTBE, because there was no analytical residue of MTBE due to its high volatility (Figure 1B, Left). However, TAEE was found to be vaporized slowly as the temperature increased (Figure 1B, Right).
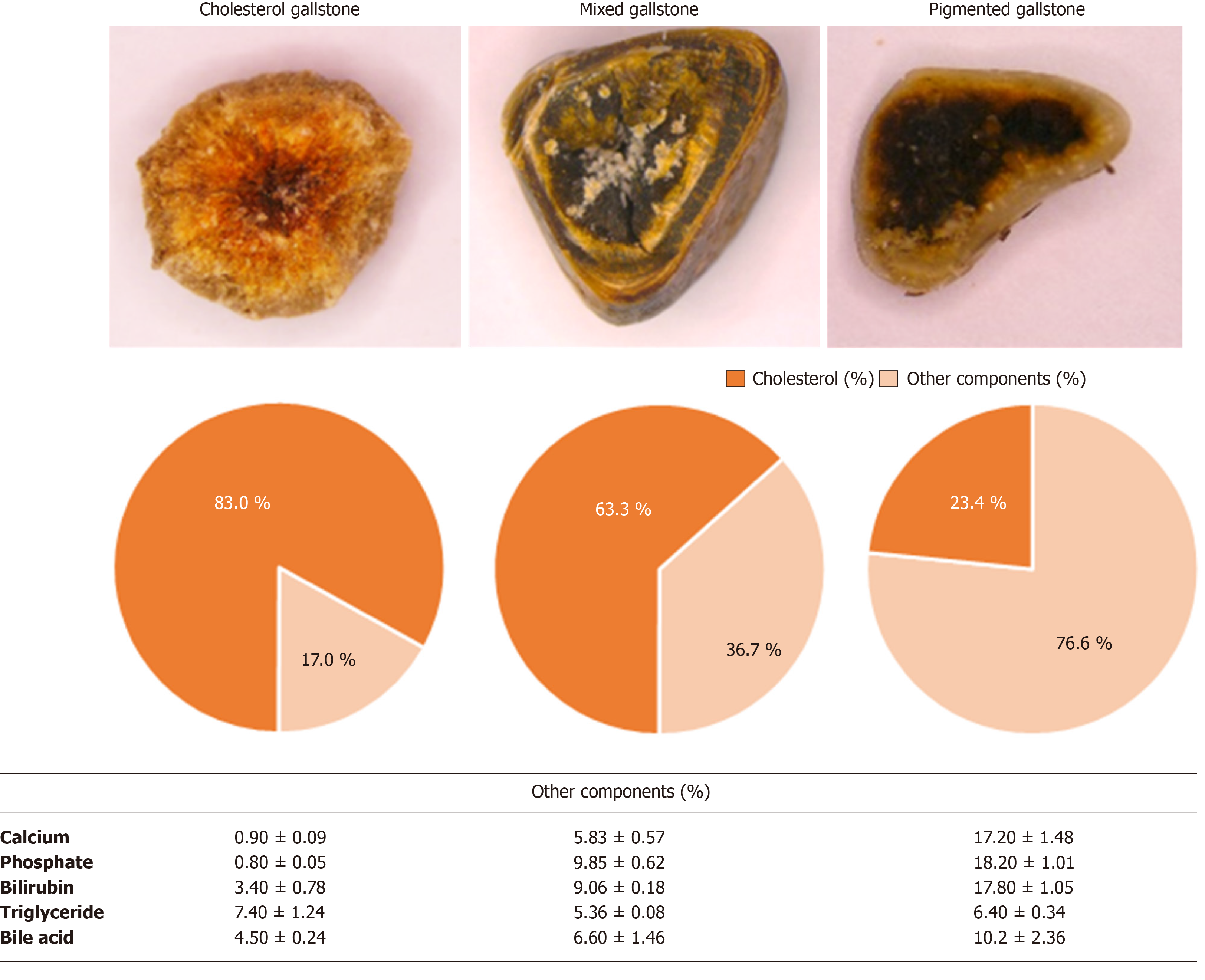
We next classified the gallstones for the purpose of determining the dissolubility of solvents with respect to the subtypes of gallstones. Gallstones were classified into cholesterol, mixed, and pigmented stones according to the cholesterol content (≥ 70%, 70%-30%, and < 30%), respectively[18]. The cholesterol contents of our tested cholesterol, mixed, and pigmented gallstones were 83.0 ± 3.3%, 63.3 ± 4.3%, 23.4 ± 3.9%, respectively (Figure 2). Besides, mixed and pigmented gallstones were found to have significantly higher concentrations of calcium and phosphate compared with cholesterol gallstones.
To determine the in vitro dissolubility, we compared the dry weights of human gallstones at three time-points (4, 8, and 24 h) following being directly treated with each solvent. The dissolubility of gallstones was measured as the difference in the weights of gallstones before and after treatment at determined time intervals. It was revealed that TAEE dissolubility was significantly higher than MTBE dissolubility at the all time-points in the all subtypes of gallstones (P < 0.05) (Figure 3A-C). Also, TAEE did not only have relatively higher dissolubility for cholesterol gallstone, but also had for mixed and pigmented gallstones than MTBE. Whereas MTBE was found to have 61.0%, 39.0% and 28.2% 24h-dissolubility for each subtype of gallstones (cholesterol, mixed, and pigmented stones), TIME exhibited 76.7%, 56.5% and 38.7% 24h-dissolubility, respectively (all P values < 0.05).
We generated the hamster models with cholesterol gallstones and pigmented gallstones using diet protocols for 3 mo, respectively, as described earlier[19]. During the period of feeding, the hamsters of each group exhibited consistent rises in body weights (Figure 4A). At the end of the feeding period, the serum level of total cholesterol was significantly higher in the hamsters with cholesterol gallstones than in those with pigmented gallstones (Figure 4B). Gallstones were found in 88% (15/17) in the hamsters with cholesterol gallstones (CG group), and 82% (14/17) in the hamsters with pigmented gallstones (PG group) (Figure 4C).
After laparotomy under general anesthesia, we directly infused DMSO (n = 4), MTBE (n =4), and TAEE (n = 7), respectively, into the gallbladder of the hamsters with cholesterol stones, respectively. Likewise, we infused DMSO (n = 4), MTBE (n = 4), and TAEE (n = 6), respectively, into the gallbladder of the hamsters with pigmented stones, respectively. DMSO was used as the control material. Thereafter, we measured the dissolubility of each solvent by comparing the weights of the residual gallstones at 24 h after infusion. TAEE infusion was found to have higher 24 h-dissolubility than MTBE infusion in both CG (76.7% vs 61.0%, P < 0.05) and PG (38.7% vs 28.2%, P < 0.05) groups (Figure 4D).
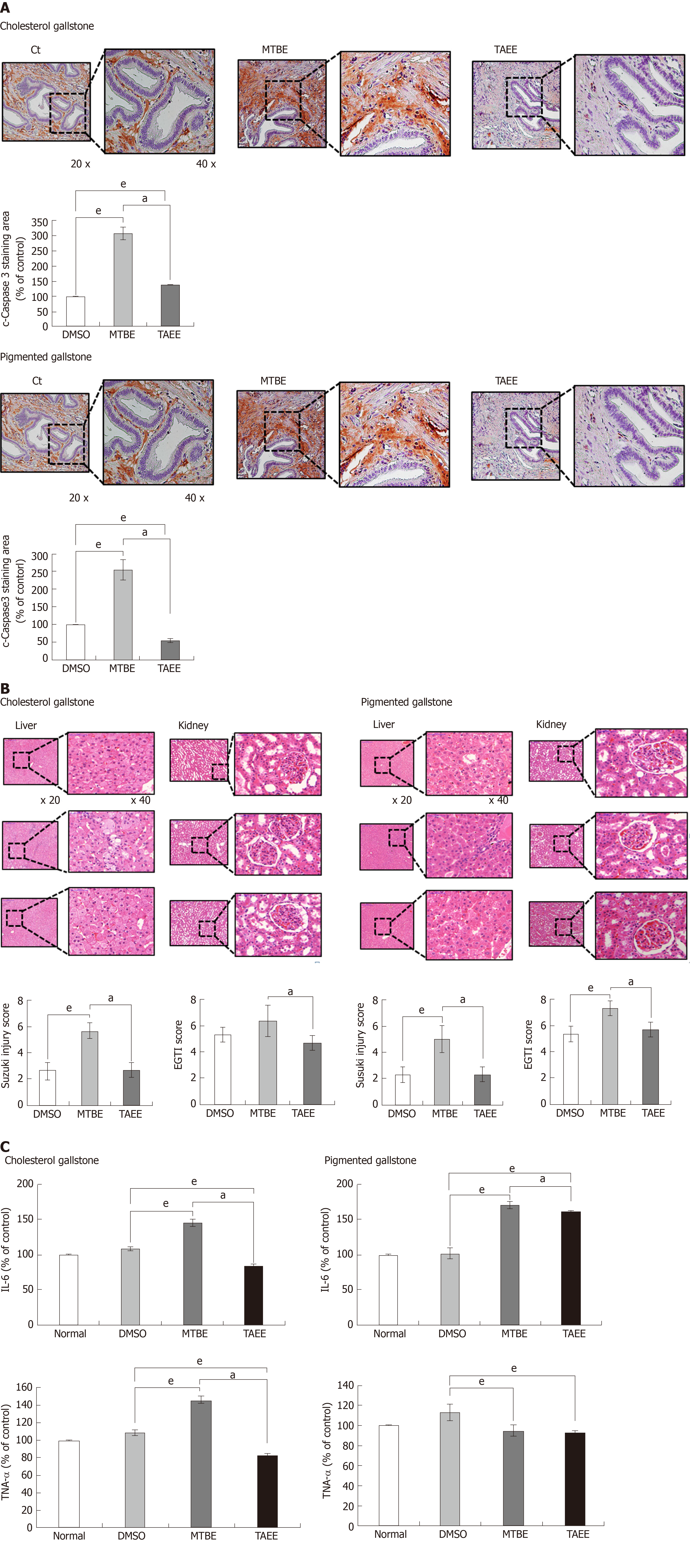
For determining the direct tissue toxicity of MTBE and TAEE, we performed immunohistochemistry (IHC) using the gallbladder specimens obtained from the hamsters treated with each solvent, respectively. We selected cleaved caspase-3 (a pro-apoptotic marker) as the IHC panel. In both hamsters with cholesterol and pigmented stones, TAEE infusion resulted in significantly lower expression of cleaved caspase-3 than did MTBE infusion (P < 0.05), suggesting a lower toxicity of TAEE compared with MTBE (Figure 5A).
Subsequently, for determining the toxic effects of MTBE and TAEE on the liver and kidney, we compared the histological findings of the liver and kidney specimens that had been attained from the hamsters at 24 h after infusion of each solvent into the gallbladder. The degree of injury of the liver and kidney were calculated as the Suzuki injury scores and EGTI scores, respectively. Contrasted by MTBE, TAEE was found not to significantly increase the injuries of these organs (Figure 5B).
Finally, for determining the effects of MTBE and TAEE on the systemic inflammation, we compared the serum levels of pro-inflammatory cytokines (IL-6 and TNF-α) by ELISA at 24 h after infusion. In CG group, whereas MTBE significantly increased the serum levels of these cytokines, TAEE rather decreased the serum levels of these cytokines (P < 0.05). In PG group, MTBE significantly increased the serum level of IL-6 (P < 0.05), and TAEE increased it without statistical significance. However, both treatment groups did not increase the serum levels of TNF-α in PG group (Figure 5C).
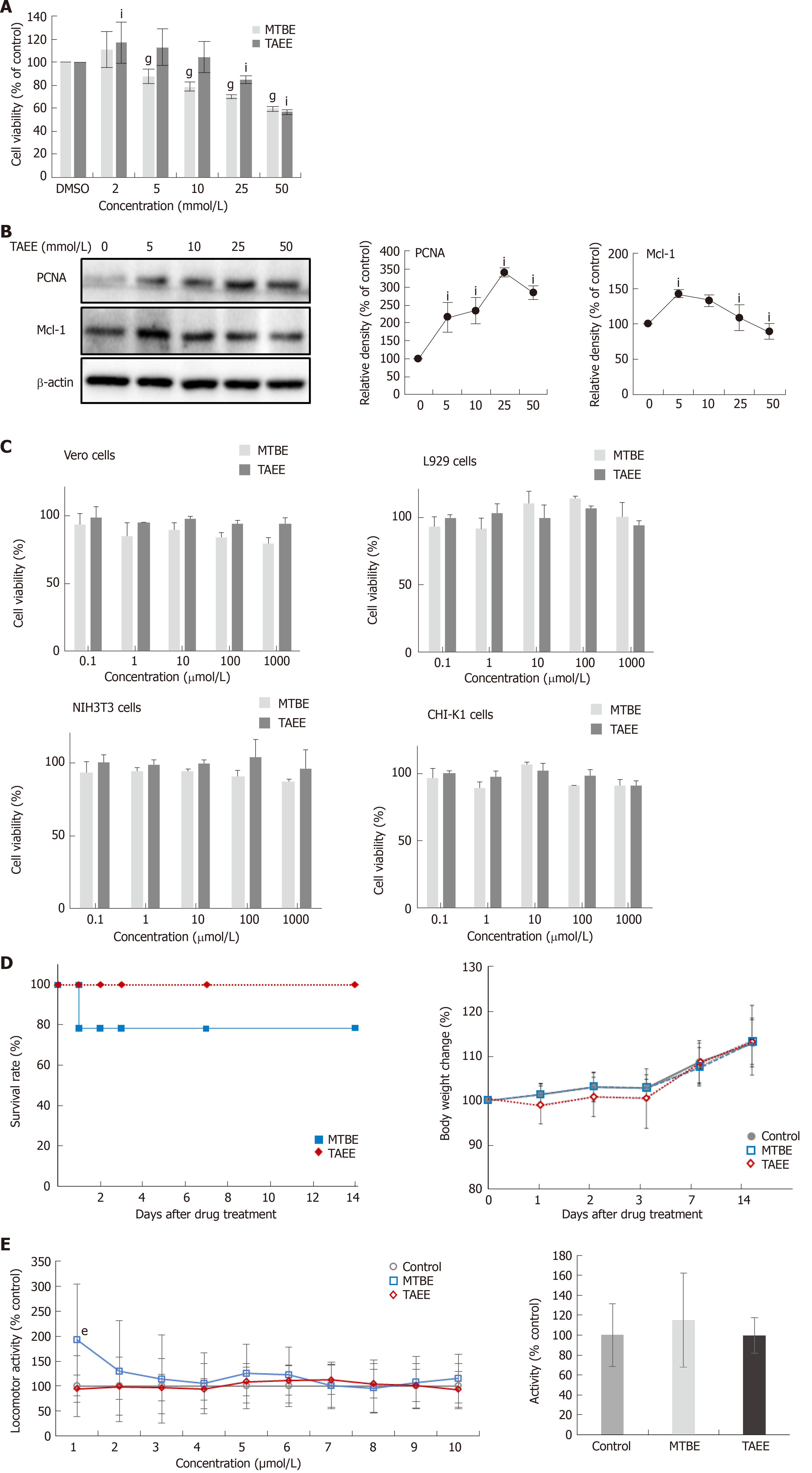
For determining the direct toxicities of MTBE and TAEE on gallbladder epithelial cells in vitro, we performed cell viability assay using human gallbladder epithelial cells (hGBECs) (Figure 6A). Cell viability assay showed that, overall, TAEE induced lesser significant reduction of the viability of hGBECs than did MTBE at the tested concentrations. Next, we investigated the effects of TAEE on the expression of the markers reflecting cell proliferation (PCNA) and anti-apoptosis (Mcl-1) in hGBECs (Figure 6B). Western blot analysis revealed that with rising TAEE concentrations, PCNA exhibited the tendency of progressively increasing, and Mcl-1 was exhibited the tendency of progressively decreasing after an initial increase.
To further determine the in vitro toxicities of MTBE and TAEE, we performed cell viability assay using several cells, including L929 cells, Vero cells, cells, NIH3T3, and CHI-K1 cells (Figure 6C). We found that both solvents generally did not significantly decrease cell viability of these cells, at least up to 1000 μmol/L concentration of the solvents.
Next, we intended to determine the in vivo acute toxicity resulting from a single exposure of each solvent. We monitored the body weight changes, mortality, and general behaviors of the mice during 14 d after oral administration of each solvent with higher concentration (2000 mg/kg). Whereas MTBE-treated mice showed 21.4% (3/14) of mortality, all of which were happened within first 24 h, TAEE-treated mice did not show any mortality. TAEE-treated mice were slightly underweight during the first 3 d and recovered thereafter. MTBE-treated mice showed prominent restlessness than vehicle controls on day 1, and thereafter, showed abnormal behaviors including tremors, ataxia, and wheezing for additional 2 d and progressively recovered thereafter. Although no mortality happened, TAEE-treated mice exhibited similar abnormal behavioral patterns as MTBE-treated mice for initial three days (Figure 6D).
Finally, we performed zebrafish locomotion test for the determination of the effects of MTBE and TAEE on the central nervous system (CNS) (Figure 6E). After placing larval zebrafish in individual wells of a 96-well plate contained with embryonic medium, locomotor activity of zebrafish was measured over 60 minutes after treating 100 μL of MTBE and TAEE to a final concentration of 1mM, respectively. During the overall period (60 min), both treatment groups did not significantly increase the locomotor activity of zebrafish than did control group, suggesting no significantly harmful effects of MTBE and TAEE on CNS.
In this study, we first validated the gallstone-dissolving capacity and toxicity of TAEE by comparing them with those of MTBE in both in vitro and in vivo models of cholesterol and pigmented gallstones. In both models, TAEE consistently exhibited a superior gallstone-dissolubility compared to MTBE. Specifically, in the in vitro experiment, TAEE showed a 1.2-, 1.4-, and 1.3-fold increase in dissolubility for the cholesterol, mixed, and pigmented gallstones, respectively, compared to that of MTBE. In the in vivo experiment, TAEE showed a 1.4- and 1.9-fold increase in dissolubility for the cholesterol and pigmented gallstones, respectively, compared to that of MTBE. Moreover, TAEE exhibited a toxicity similar to or lesser than that of MTBE. We postulated that the lower toxicity of TAEE could be partly attributed to its comparatively lower boiling point than that of MTBE, which results in less evaporation. Taken together, our results demonstrated that TAEE has the higher gallstone-dissolubility and safety compared with that of MTBE. If consistent efficacy and safety parameters can be reproduced in the further studies, incorporating large animals and human patients, TAEE is expected to present an attractive alternative to MTBE.
Currently, MTBE is the most widely used and the only clinically available CLA. Although MTBE does not exhibit a significant clinical toxicity, researchers have raised questions regarding the safety of MTBE. MTBE is categorized as a carcinogen, alongside benzene, vinyl chloride, and 1,3-butadiene, as exposure to doses of MTBE equal to those of these carcinogens could result in the development of cancers in several organs and tissues[20]. In particular, the inhalation of MTBE has been found to lead to a statistically significant increase in kidney tumors and liver tumors in rats[21], and oral exposure to MTBE was found to increase carcinomas, such as lymphoma, leukemia, and Leydig cell carcinoma of the testes in rats[22]. After being delivered into the gallbladder, MTBE is not only absorbed into the duodenum, but can also be systemically absorbed. Whereas absorption of MTBE into the duodenum can culminate in nausea, vomiting, or somnolence[8-12,14-17], systemic absorption can lead to hemolysis and kidney injuries[23].
TAEE has high boiling point of 102 °C that is higher than water and nearly twice as high as MTBE. Unlike the MTBE, TAEE also has a high molecular weight of 116.2 g/mol, but its density is similar to that of MTBE (0.76 g/mL). We herein demonstrated that TAEE has the higher gallstone-dissolubility and safety compared with that of MTBE. We thus think that TAEE fulfills lots of qualifications of being the ideal solvent for gallstone dissolution.
MTBE is classified into a typical aliphatic ether category with a methyl group and tertiary butyl group centering on oxygen atoms. MTBE is one of the compounds with low molecular weight (88.15 g/mol) and has a density of 0.74 g/mL, which is lower than that of water. Due to its low boiling point (55 °C), when it is used, MTBE is considerably volatilized as a vapor, provoking detrimental effects not only in patients but also in medical staffs. Basically, TAEE is an analogue of MTBE. In TAEE structure, another methyl moiety is added to the carbon of tert-butyl group of MTBE, while methoxy group of MTBE is substituted with ethoxy group. Although TAEE is an MTBE analogue, they have quite different chemical characteristics. TAEE has high boiling point of 102 °C that is higher than water and nearly twice as high as MTBE. In addition, TAEE also has relatively higher molecular weight of 116.2 g/mol, while maintaining its density like that of MTBE (0.76 g/mL). We think these differences might have led to the differences in the dissolubility and toxicities between these two CCAs. The ideal solvent must be able to dissolve cholesterol gallstones rapidly, should not cause injury to local tissue, should not have systemic toxicity, and should be widely available at a high purity[24]. Our results indicate that TAEE could fulfill the qualifications of the ideal solvent more preferably than MTBE.
Recently, the use of CLAs has fallen out of favor due to the recognition of an acceptable risk-benefit profile of laparoscopic cholecystectomy by both physicians and patients. However, we believe that CLAs could be considered in the selected patients with gallstones, if their effectiveness and safety are firmly established. Asymptomatic cholelithiasis accounts for 50%-70% of the total cholelithiasis population[2]. Later, approximately 10%-25% of asymptomatic cholelithiasis patients ultimately progress to symptomatic cholelithiasis[2]. Generally, patients with asymptomatic cholelithiasis do not require surgical intervention, except for in two reasons; suspicion of malignancy and increased risk of progression from asymptomatic to symptomatic disease. We believe that CLAs could reasonably replace operative intervention in patients with asymptomatic cholelithiasis of the latter reason of operative indication, which includes gallstones over 2 cm in diameter and calculi under 3 mm, as well as patent cystic ducts in patients with a life expectancy of more than 20 years[2].
In developed countries, most gallstones predominantly consist of cholesterol (> 85%)[25,26]. Fundamentally, MTBE and TAEE are solvents for cholesterol gallstones, not for pigmented gallstones. However, we found enhanced disssolubility of TAEE for mixed and pigmented gallstones. In our in vitro study, whereas MTBE showed 61.0%, 39.0%, and 28.2% 24 h-dissolubility, TAEE showed 76.7%, 56.5%, and 38.7% 24 h-dissolubility for cholesterol, mixed, and pigmented gallstones, respectively. Moreover, in the in vivo study, MTBE showed 50.0% and 32.0% 24 h-dissolubility, whereas TAEE showed 71.7% and 63.0% 24 h-dissolubility for cholesterol and pigmented gallstones, respectively. As TAEE preferably dissolves cholesterol gallstones, it is important to determine whether a patient has cholesterol-rich gallstones or not before carrying out the procedure. Currently, there are numerous tools that aid in the selection of patients with cholesterol-rich gallstones. Radiological evaluations, including ultrasound and computed tomography scans, can raise the predictive value of cholesterol gallstones up to 80%[27]. The predictability could also be enhanced by analyzing patient characteristics and epidemiology, such as gender, ethnicity, and body mass index[28].
Although its efficacy and safety have been established, we believe that laparoscopic cholecystectomy should be the last option for the treatment of patients with cholelithiasis. All patients undergoing laparoscopic cholecystectomy should be aware of the morbidity and mortality, albeit minimal, associated with the procedure and the use of general anesthesia. Despite improvements in the laparoscopic skills of surgeons, bile duct injuries are more prevalent than injuries following an open cholecystectomy, with a reported incidence of up to 0.6% for laparoscopic surgery compared to 0.1% for an open cholecystectomy[29-32]. Moreover, a functioning gallbladder should be preserved as much as possible, as the gallbladder has crucial functions, including as a reservoir for stored bile and for the coordinated release of bile in the small intestine[33]. Finally, several epidemiological studies and meta-analyses have found that a cholecystectomy may be a risk factor for gastrointestinal cancer[34-38]. In a nationwide Taiwanese cohort study comparing 15545 patients undergoing cholecystectomy with 62180 frequency-matched non-cholelithiasis patients, the hazard ratio for developing stomach and colorectal cancer was 1.81-fold and 1.56-fold higher in patients undergoing cholecystectomy, respectively[39]. Bile salts can act as carcinogens after persistent exposure due to the mechanisms of upregulation of reactive oxygen species, the stimulation of apoptosis, the induction of DNA damage, and resulting mutations[34]. After a cholecystectomy, the cells in the gastrointestinal tract have a higher probability of exposure to the deleterious effects of bile acids due to contact with the continuous secretion of biliary fluids, which can transform these cells into malignant cells[35,36,38,40,41]. Therefore, CLAs are worthwhile treatment to consider as a means of preserving gallbladder function as much as possible.
One of the limitations of CLAs is their inability to completely remove all the components of gallstones. A considerable proportion of the remaining debris, composed of calcium carbonate and bilirubinate, is insoluble to most CLAs[4]. Most of the debris do not usually cause any complications, as they readily pass through the bile ducts. However, debris larger than 3-4 mm can cause problems, and may act as a nidus for cholesterol recrystallization and gallstone formation[4]. This is thought to be the reason why there is the high rate of stone recurrence after successful gallstone dissolution by CLAs, which can be as high as 30%-50% in the 5 years after treatment[42]. To prevent stone recurrence, Edison et al. have recommended the use of adjuvant oral bile acid therapy after successful litholysis by CLAs[4]. The preservation of gallbladder function should be confirmed prior to chemolitholysis, as impaired gallbladder contractability predisposes the patient to stone recurrence after chemolitholysis.
In conclusion, our in vitro and in vivo experiments consistently indicated that TAEE, the derivative of MTBE, causes a similar or higher dissolubility of gallstones as that caused by MTBE. Specifically, in the in vitro experiment, TAEE showed a 1.2-, 1.4-, and 1.3-fold increase in dissolubility for the cholesterol, mixed, and pigmented gallstones, respectively, compared to that of MTBE. In the in vivo experiment, TAEE showed a 1.4- and 1.9-fold increase in dissolubility for the cholesterol and pigmented gallstones, respectively, compared to that of MTBE. We also found that TAEE exhibited a toxicity similar to or lower than that of MTBE. TAEE has a comparatively lower evaporation rate than MTBE, which is expected to significantly eliminate the MTBE’s toxicities related with lower boiling point, such as nausea, vomiting, and somnolence. We, thus, conclude that TAEE could present an attractive alternative to MTBE if its efficacy and safety can be consistently reproduced in further subclinical and clinical studies.
Methyl tert-butyl ether (MTBE) has been the first choice of contact litholytic angents ever since its introduction in 1985. However, the use of MTBE to dissolve gallstones was limited by concerns about toxicity and widespread awareness of the safety of laparoscopic gallbladder surgery. MTBE has a relatively low boiling point (55 °C) and a higher evaporation rate, which could lead to the development of various side effects, including nausea, upper abdominal pain, duodenitis, mild-to-moderate anesthesia, and hemolysis
As westernized diets increase, cholelithiasis is increasing. 20%-30% of patients with asymptomatic gallstones eventually progress to symptomatic gallstones requiring aggressive treatment. Although laparoscopic cholecystectomy is a safe and efficient treatment for symptomatic gallstones, the removal of the entire functioning gallbladder has an exaggerated aspect. These considerations have led us to perform this experiment.
We performed this experiment to determine whether tert-amyl ethyl ether (TAEE), an MTBE analogue with a relatively higher boiling point (102 °C), could be used as an alternative to MTBE in terms of gallstone dissolubility and toxicity.
To determine the dissolubility of TAEE, we compared the dissolubility of TAEE and MTBE in both in vitro and in vivo dissolubility tests, using human gallstones and hamster models with gallstones, respectively. Specifically, the in vitro dissolubility of each solvent was determined by measuring the dry weights of human gallstones at predetermined time intervals after placing them in glass containers with either of the two solvents. The in vivo dissolubility was determined by comparing the weights of solvent-treated gallstones and control (dimethyl sulfoxide)-treated gallstones, after the direct infusion of each solvent into the gallbladder of the hamsters with gallstones.
In both in vitro and in vivo models of gallstones, the TAEE consistently displayed better gallstone dissolubility than the MTBE. Specifically, in the in vitro experiments, TAEE showed a 1.2-, 1.4- and 1.3-fold higher dissolubility potentials for cholesterol, mixed, and pigmented gallstones, respectively, those of MTBE. In the in vivo tests, TAEE exhibited the 1.4 times and 1.9 times higher dissolubility potentials for cholesterol and pigment gallstones, respectively, than those of MTBE. In addition, TAEE had toxicities similar to or lesser than those of MTBE.
Our results showed that TAEE has the higher gallstone-dissolubility and safety compared with that of MTBE. It should be noted that MTBE showed significantly higher dissolubility for pigmented gallstones because there is no efficient dissolving agents for pigmented gallstones so far. We assume that the low toxicities of TAEE could be considerably attributed to its lower evaporation causing from a relatively higher boiling point (102 °C) than that of MTBE. If consistent efficacy and safety parameters can be reproduced in the further studies, incorporating large animals and human patients, TAEE is expected to present an attractive alternative to MTBE.
Currently, MTBE has been used for the purpose of dissolving gallstones worldwide, and its main indication is the patients who refuse surgery or are at high risk for surgery. However, if more effective and safe gallstone-dissolving agents are developed in the future, future therapeutic indications are expected to include the patients with asymptomatic gallstones, for a substantial proportion of them progress to have symptomatic gallstones.
We would like to thank the Francis Sahngun Nahm (a professional statistician) for his devoted assistance of statistical analysis. The authors thank Hye-Jung Kim and Ji-Hye Park for photoshop works and support for data processing.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan GK, Neri V S-Editor: Yan JP L-Editor: A E-Editor:Ma YJ
| 1. | Kim G, Malayaman SN, Green MS. Use of Methyl Tert-Butyl Ether for the Treatment of Refractory Intrahepatic Biliary Strictures and Bile Casts: A Modern Perspective. Case Rep Surg. 2015;2015:408175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Sakorafas GH, Milingos D, Peros G. Asymptomatic cholelithiasis: is cholecystectomy really needed? A critical reappraisal 15 years after the introduction of laparoscopic cholecystectomy. Dig Dis Sci. 2007;52:1313-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Allen MJ, Borody TJ, Bugliosi TF, May GR, LaRusso NF, Thistle JL. Rapid dissolution of gallstones by methyl tert-butyl ether. Preliminary observations. N Engl J Med. 1985;312:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 170] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Edison SA, Maier M, Kohler B, Schlauch D, Buttmann A, Gauer E, Riemann JF. Direct dissolution of gallstones with methyl tert-butyl ether by endoscopic cannulation of the gallbladder. Am J Gastroenterol. 1993;88:1242-1248. [PubMed] |
| 5. | Hellstern A, Leuschner U, Benjaminov A, Ackermann H, Heine T, Festi D, Orsini M, Roda E, Northfield TC, Jazrawi R, Kurtz W, Schmeck-Lindenau HJ, Stumpf J, Eidsvoll BE, Aadland E, Lux G, Boehnke E, Wurbs D, Delhaye M, Cremer M, Sinn I, Höring E, v Gaisberg U, Neubrand M, Paul F. Dissolution of gallbladder stones with methyl tert-butyl ether and stone recurrence: a European survey. Dig Dis Sci. 1998;43:911-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Leuschner U, Hellstern A, Schmidt K, Fischer H, Güldütuna S, Hübner K, Leuschner M. Gallstone dissolution with methyl tert-butyl ether in 120 patients--efficacy and safety. Dig Dis Sci. 1991;36:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Thistle JL, May GR, Bender CE, Williams HJ, LeRoy AJ, Nelson PE, Peine CJ, Petersen BT, McCullough JE. Dissolution of cholesterol gallbladder stones by methyl tert-butyl ether administered by percutaneous transhepatic catheter. N Engl J Med. 1989;320:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 147] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Allen MJ, Borody TJ, Bugliosi TF, May GR, LaRusso NF, Thistle JL. Cholelitholysis using methyl tertiary butyl ether. Gastroenterology. 1985;88:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 79] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Brandon JC, Teplick SK, Haskin PH, Sammon JK, Muhr WF, Hofmann AF, Gambescia RA, Zitomer N. Common bile duct calculi: updated experience with dissolution with methyl tertiary butyl ether. Radiology. 1988;166:665-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Di Padova C, Di Padova F, Montorsi W, Tritapepe R. Methyl tert-butyl ether fails to dissolve retained radiolucent common bile duct stones. Gastroenterology. 1986;91:1296-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Diaz D, Bories P, Ampelas M, Larrey D, Michel H. Methyl tert-butyl ether in the endoscopic treatment of common bile duct radiolucent stones in elderly patients with nasobiliary tube. Dig Dis Sci. 1992;37:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Foerster EC, Matek W, Domschke W. Endoscopic retrograde cannulation of the gallbladder: direct dissolution of gallstones. Gastrointest Endosc. 1990;36:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Kelly E, Williams JD, Organ CH. A history of the dissolution of retained choledocholithiasis. Am J Surg. 2000;180:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Neoptolemos JP, Hall C, O'Connor HJ, Murray WR, Carr-Locke DL. Methyl-tert-butyl-ether for treating bile duct stones: the British experience. Br J Surg. 1990;77:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Saraya A, Rai RR, Tandon RK. Experience with MTBE as a solvent for common bile duct stones in patients with T-tube in situ. J Gastroenterol Hepatol. 1990;5:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | vanSonnenberg E, Casola G, Zakko SF, Varney RR, Cox J, Wittich GR, Hofmann AF. Gallbladder and bile duct stones: percutaneous therapy with primary MTBE dissolution and mechanical methods. Radiology. 1988;169:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | vanSonnenberg E, Hofmann AF, Neoptolemus J, Wittich GR, Princenthal RA, Willson SW. Gallstone dissolution with methyl-tert-butyl ether via percutaneous cholecystostomy: success and caveats. AJR Am J Roentgenol. 1986;146:865-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kim IS, Myung SJ, Lee SS, Lee SK, Kim MH. Classification and nomenclature of gallstones revisited. Yonsei Med J. 2003;44:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Rege R, Ostrow J. Animal models of pigment and cholesterol gallstone disease. In: Muraca M. Methods in biliary research 1995; 203-243. |
| 20. | Bird MG, Burleigh-Flayer HD, Chun JS, Douglas JF, Kneiss JJ, Andrews LS. Oncogenicity studies of inhaled methyl tertiary-butyl ether (MTBE) in CD-1 mice and F-344 rats. J Appl Toxicol. 1997;17 Suppl 1:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Mehlman MA. Methyl-tertiary-butyl-ether (MTBE) misclassified. Am J Ind Med. 2001;39:505-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Mehlman MA. Dangerous and cancer-causing properties of products and chemicals in the oil-refining and petrochemical industry--Part XXII: Health hazards from exposure to gasoline containing methyl tertiary butyl ether: study of New Jersey residents. Toxicol Ind Health. 1996;12:613-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Ponchon T, Baroud J, Pujol B, Valette PJ, Perrot D. Renal failure during dissolution of gallstones by methyl-tert-butyl ether. Lancet. 1988;2:276-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Hofmann AF, Schteingart CD, vanSonnenberg E, Esch O, Zakko SF. Contact dissolution of cholesterol gallstones with organic solvents. Gastroenterol Clin North Am. 1991;20:183-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 25. | Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 312] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 26. | Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract Res Clin Gastroenterol. 2006;20:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 27. | Bell GD, Dowling RH, Whitney B, Sutor DJ. The value of radiology in predicting gallstone type when selecting patients for medical treatment. Gut. 1975;16:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Weerakoon HT, Ranasinghe JG, Navaratna A, Sivakanesan R, Galketiya KB, Rosairo S. Can the type of gallstones be predicted with known possible risk factors?: A comparison between mixed cholesterol and black pigment stones. BMC Gastroenterol. 2014;14:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 774] [Article Influence: 24.2] [Reference Citation Analysis (1)] |
| 30. | Ponsky JL. Complications of laparoscopic cholecystectomy. Am J Surg. 1991;161:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Rauws EA, Gouma DJ. Endoscopic and surgical management of bile duct injury after laparoscopic cholecystectomy. Best Pract Res Clin Gastroenterol. 2004;18:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 32. | Sicklick JK, Camp MS, Lillemoe KD, Melton GB, Yeo CJ, Campbell KA, Talamini MA, Pitt HA, Coleman J, Sauter PA, Cameron JL. Surgical management of bile duct injuries sustained during laparoscopic cholecystectomy: perioperative results in 200 patients. Ann Surg. 2005;241:786-92; discussion 793-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 294] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 33. | Jaunoo SS, Mohandas S, Almond LM. Postcholecystectomy syndrome (PCS). Int J Surg. 2010;8:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009;15:3329-3340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 196] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 35. | Ge Z, Zhao C, Wang Y, Qian J. Cholecystectomy and the risk of esophageal and gastric cancer. Saudi Med J. 2012;33:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Giovannucci E, Colditz GA, Stampfer MJ. A meta-analysis of cholecystectomy and risk of colorectal cancer. Gastroenterology. 1993;105:130-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Nogueira L, Freedman ND, Engels EA, Warren JL, Castro F, Koshiol J. Gallstones, cholecystectomy, and risk of digestive system cancers. Am J Epidemiol. 2014;179:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 38. | Reid FD, Mercer PM, harrison M, Bates T. Cholecystectomy as a risk factor for colorectal cancer: a meta-analysis. Scand J Gastroenterol. 1996;31:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Chen YK, Yeh JH, Lin CL, Peng CL, Sung FC, Hwang IM, Kao CH. Cancer risk in patients with cholelithiasis and after cholecystectomy: a nationwide cohort study. J Gastroenterol. 2014;49:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Ekbom A, Yuen J, Adami HO, McLaughlin JK, Chow WH, Persson I, Fraumeni JF. Cholecystectomy and colorectal cancer. Gastroenterology. 1993;105:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Gustavsson S, Adami HO, Meirik O, Nyrén O, Krusemo UB. Cholecystectomy as a risk factor for gastric cancer. A cohort study. Dig Dis Sci. 1984;29:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | O'Donnell LD, Heaton KW. Recurrence and re-recurrence of gall stones after medical dissolution: a longterm follow up. Gut. 1988;29:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 91] [Article Influence: 2.5] [Reference Citation Analysis (0)] |