Published online Oct 7, 2019. doi: 10.3748/wjg.v25.i37.5676
Peer-review started: June 13, 2019
First decision: July 21, 2019
Revised: July 30, 2019
Accepted: August 19, 2019
Article in press: August 19, 2019
Published online: October 7, 2019
Processing time: 109 Days and 6.8 Hours
Nonalcoholic fatty liver disease (NAFLD) is a frequently reported condition in patients with inflammatory bowel disease (IBD). Both intestinal inflammation and metabolic factors are believed to contribute to the pathogenesis of IBD-associated NAFLD.
To evaluate the prevalence of steatosis and liver fibrosis (LF) in a cohort of IBD patients and the identification of metabolic- and IBD-related risk factors for NAFLD and LF.
IBD patients were consecutively enrolled from December 2016 to January 2018. Demographic, anthropometric and biochemical data were collected so as eating habits. Abdominal ultrasound and transient elastography were performed to evaluate the presence of NAFLD and LF respectively.
A total of 178 consecutive patients were enrolled and included in the analysis (95 Ulcerative colitis, 83 Crohn’s disease). NAFLD was detected by imaging in 72 (40.4%) patients. Comparison between patients with and without NAFLD showed no significant differences in terms of IBD severity, disease duration, location/extension, use of IBD-related medications (i.e., steroids, anti-TNFs, and immunomodulators) and surgery. NAFLD was significantly associated with the presence of metabolic syndrome [MetS; odds ratio (OR): 4.13, P = 0.001] and obesity defined by body mass index (OR: 9.21, P = 0.0002). IBD patients with NAFLD showed higher caloric intake and lipid consumption than those without NAFLD, regardless disease activity. At the multivariate analysis, male sex, advanced age and high lipid consumption were independent risk factors for the development of NAFLD. An increased liver stiffness was detected in 21 patients (16%) and the presence of MetS was the only relevant factor associated to LF (OR: 3.40, P = 0.01).
In this study, we demonstrate that risk factors for NAFLD and LF in the IBD population do not differ from those in the general population.
Core tip: Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, frequently reported in patients with inflammatory bowel disease (IBD). The underlining causes and predisposing factors to NAFLD among IBD patients remain poorly investigated. We performed an observational study enrolling consecutive IBD patients to evaluate the prevalence of steatosis and liver fibrosis and their association with IBD-related risk factors. Our study confirms the epidemiological burden of NAFLD in IBD and demonstrates the absence of intestinal disease-specific risk factors associated to NAFLD, while confirming as risk factors those already identified in the general population. IBD care should not be limited to intestinal disease but should also include metabolic interventions by promoting healthy lifestyle and a correct dietary regimen in order to reduce the occurrence of chronic liver disease.
- Citation: Magrì S, Paduano D, Chicco F, Cingolani A, Farris C, Delogu G, Tumbarello F, Lai M, Melis A, Casula L, Fantini MC, Usai P. Nonalcoholic fatty liver disease in patients with inflammatory bowel disease: Beyond the natural history. World J Gastroenterol 2019; 25(37): 5676-5686
- URL: https://www.wjgnet.com/1007-9327/full/v25/i37/5676.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i37.5676
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, characterized by the presence of steatosis in > 5% of the hepatocytes[1], and whose prevalence is steeply increasing in parallel with obesity. The prevalence of NAFLD among adults is estimated to be 17%-46% in western countries, common risk factors are obesity-related metabolic disorders such as metabolic syndrome (MetS) and insulin resistance. The diagnosis of NAFLD requires the exclusion of both secondary causes of liver disease and daily alcohol consumption ≥ 30 g for men and ≥ 20 g for women[2]. NAFLD can progress from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH), liver fibrosis (LF), cirrhosis and hepatocellular carcinoma (HCC)[3].
Crohn’s disease (CD) and ulcerative colitis (UC), the two major forms of inflammatory bowel disease (IBD), are immune-mediated, chronic-relapsing inflammatory disorders affecting the gut and clinically characterized by abdominal pain, diarrhoea, bleeding, and weight loss, with a severely compromised quality of life[4]. Despite IBD is commonly considered a wasting disease characterized in some cases by malabsorption and severe weight loss, recent data indicate that the prevalence of NAFLD among IBD patients is increased as compared to the general population[5]. Though, the underlining causes and predisposing factors to NAFLD among IBD patients remain poorly investigated. In IBD patients, the increased risk to develop NAFLD might be related to intestinal disease-related factors such as inflammatory activity, disease duration, previous intestinal surgery, prolonged use of steroids, immunosuppressants and biologics[6]. On the other hand, the introduction of new therapies and the better control of the intestinal inflammation and symptoms have increased obesity and the MetS in IBD patients to rates comparable to those of the general population[7].
LF is the fearsome consequence of NAFLD. Although liver biopsy used to be the gold standard to stage LF, this procedure is invasive, expensive, not suitable as a screening tool and prone to sampling errors related to the limited amount of tissue analyzed. Transient elastography (TE) is a non-invasive and reproducible test, proved to be accurate in detecting significant fibrosis, advanced fibrosis and cirrhosis in patients with NAFLD[8]. Barbero et al[9] reported a frequency of LF between 6.4% and 10% in IBD patients, although few studies investigated the prevalence of LF in patients with IBD and NAFLD. The main aim of this observational study was to evaluate the prevalence of NAFLD and LF in a cohort of IBD patients. Furthermore, we investigated the association of IBD- and metabolic-related risk factors for NAFLD and LF in this population.
Between December 2016 and January 2018, consecutive IBD patients in regular follow-up at the Gastroenterology Clinic of the Department of Internal Medicine, Polyclinic University Hospital of Monserrato were evaluated through medical examination and complete blood tests. Endoscopy was performed according to the international guidelines for routinely follow-up[10]. At the time of enrollment, each patient underwent abdominal ultrasound to establish the presence and severity of NAFLD and a TE to evaluate LF. The research project has been approved by the Ethics Board (Prot. PG/2018/23). The study was conducted according to the Helsinki Declaration.
Patients aged 18 years or older with a diagnosis of IBD for at least 6 mo in active follow-up were enrolled. Exclusion criteria were inability to provide a valid consent, pregnancy, significant alcohol consumption (≥ 20 and 30 g per day in females and males, respectively) and any other known cause of chronic liver disease.
The diagnosis of IBD was based on a combination of imaging, clinical examination, endoscopy, chemical and histologic analyses according to international guidelines[11,12]. The disease activity was calculated according to the Crohn’s disease activity index and clinical MAYO score for CD and UC, respectively. Disease extension was evaluated by endoscopy and/or radiologic [computed tomography (CT) or magnetic resonance imaging] methods. The disease was conventionally referred as “extended” when inflammation involved beyond the rectum for UC, and when inflammation affected one bowel segment longer than 15 cm or more than one segment for CD.
Insuline resistance was detected by Homeostatic Model Assessment (HOMA) Index (fasting blood glucose x fasting serum insulin/22.5). MetS was defined by the presence of at least three of the following criteria: Waist circumference higher than 102 cm in men and 88 cm in women, hypertriglyceridemia, low HDL cholesterole, high blood pressure, and elevated fasting glucose[13]. An expert gastrointestinal radiologist performed an abdominal ultrasound to estimate the presence of NAFLD. Typical findings such as bright liver echo patterns, more echogenic respect to the renal cortex, and a loss of resolution of intrahepatic structures were evaluated. Steatosis was grated form 0 to 3 as previously described[14]. TE was performed on the same day of the abdominal ultrasound. Ten valid measurements were obtained for each patient, and the examination was considered successful only when valid measurements were > 75% and interquartile range was < 30%. The optimal cut-offs values for estimating the different stages of LF were: 7 kPa for fibrosis stage ≥ F2, 10 kPa for fibrosis stage ≥ F3, and 14 kPa for fibrosis stage F4[15]. The presence of other causes of chronic liver disease was excluded through laboratory tests (aminotransferases, colestatic indices, hepatotropic viruses markers, autoimmune markers, serum ferritin, transferrin saturation and ceruloplasmin) and, when necessary, through radiological exams (CT, magnetic resonance cholangiopanc-reatography).
All 178 patients underwent nutritional evaluation. Anthropometric parameters [body mass index (BMI), waist circumference, visceral fat, body fat, lean body mass] were measured using Body Composition Monitor (Omron Healthcare co., ltd.) to evaluate the presence and grade of obesity. Subsequently, a 24-h dietary recall was collected to estimate the usual dietary intake: patients were asked to describe the foods and drinks consumed in the previous 24 h. The nutritionist entered the anthropometric measurements and each 24-h dietary recall into the Dieto System Terapia Alimentare software for nutrient analysis.
Continuous variables were reported as mean (± SD) and categorical variables as number of cases and percentage. The non-parametric Mann-Withney U-test was used to compare continuous variables, the test of proportions or Chi-square test for categorical variables; P value level < 0.05 was considered significant. Univariate and, for variables that showed P value levels < 0.1, also a multivariate binary logistic regression analysis was performed using the absence/presence of NAFLD as dependent variable and considering IBD- and metabolic-related variables as predictors. A P value level < 0.05 was considered significant for all tests. Analyses were performed using STATA/SE, version 14, software (StataCorp LP, College Station, TX, United States) and SPSS v. 20 (IBM, Armonk, NY, United States).
Characteristics of the included population
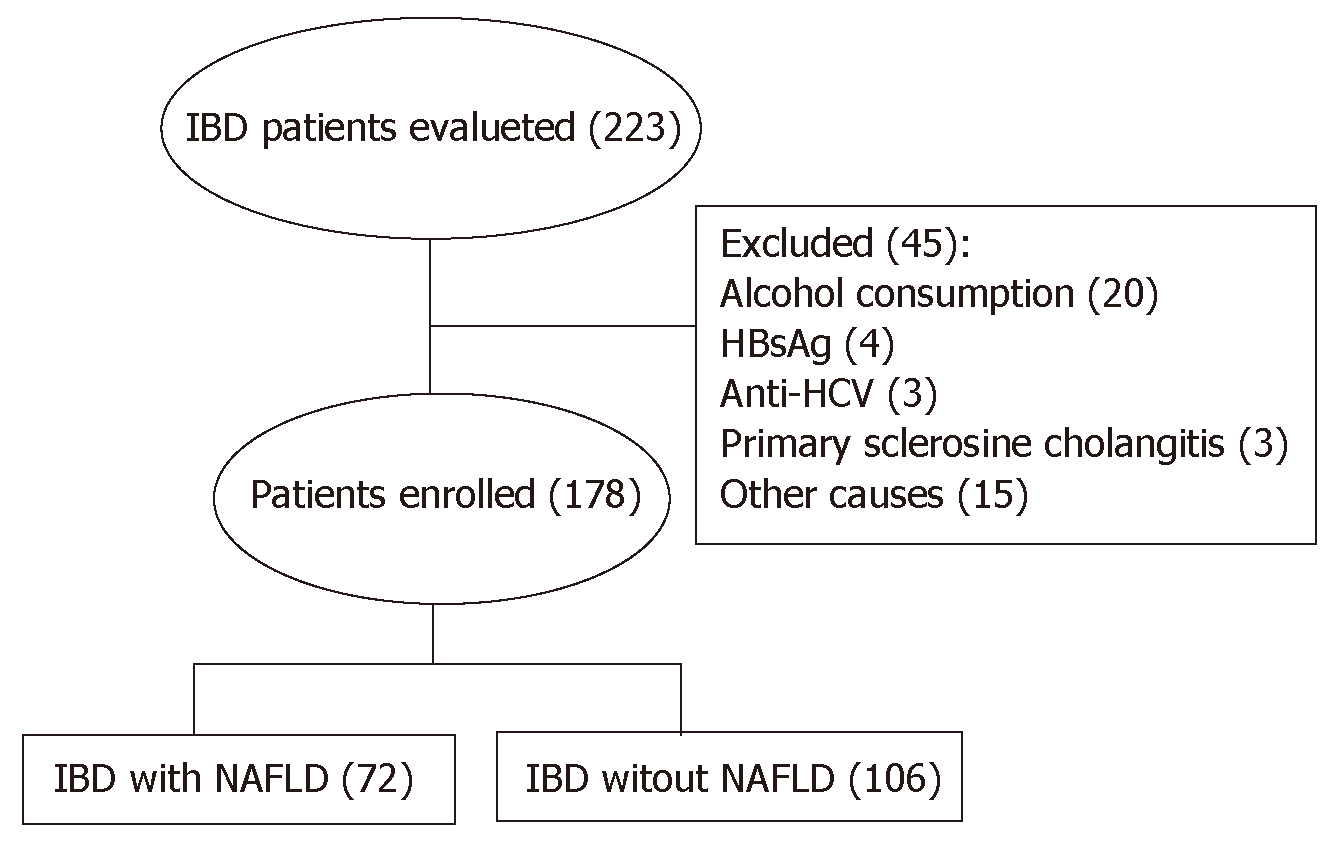
Two hundred and 23 patients have been evaluated for inclusion in the study. Of these, 45 were excluded because they did not meet the inclusion and exclusion criteria (Figure 1). Of the remaining 178 patients, 95 (53.4%) were identified as having UC and 83 patients (46.6%) had CD. 48 patients (27%) had active disease. When assessed separately, no significant differences were found between UC and CD patients. For this reason, both groups were considered together. The prevalence of NAFLD was determined in 72 patients (40.4%): grade 1, 2 and 3 steatosis was detected in 20 (11.2%), 30 (16.8%) and 22 (12.3%) patients respectively. Liver stiffness was evaluated in 162 (91%) patients and LF (F ≥ 2) was found in 21 patients (16%). MetS was diagnosed in 34 (19.1%) patients; 59 (33.1%) and 39 (21.9%) were classified as overweight according to BMI and waist circumference respectively, while, based on the same measurements, 22 (12.3%) and 39 (21.9%) were obese.
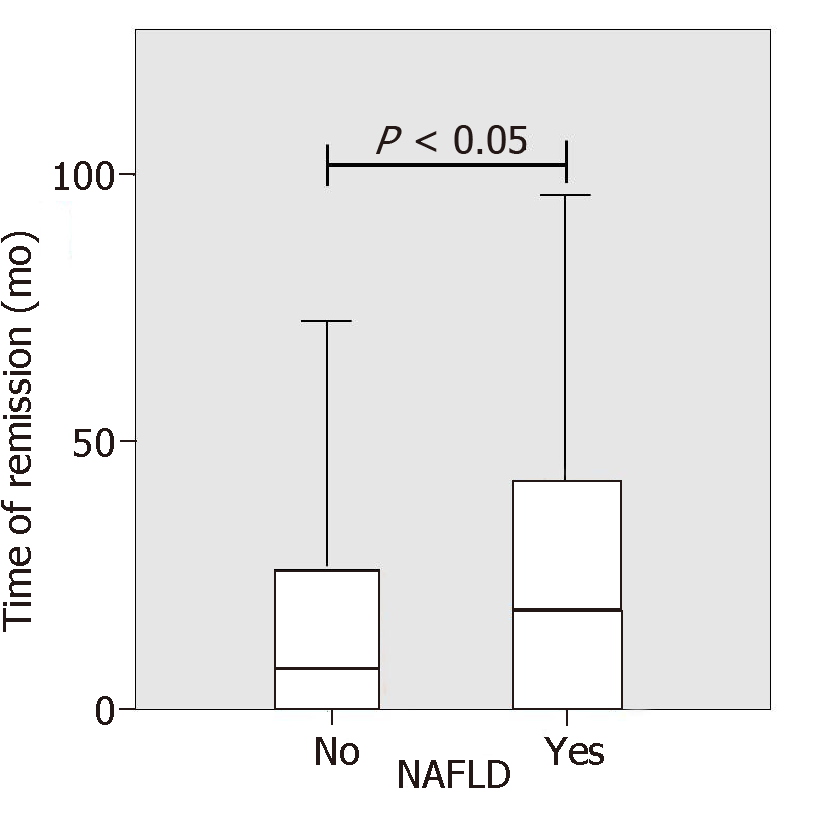
IBD patients with NAFLD were significantly older than patients without NAFLD (54 years vs 47 years, P = 0.001). No differences between IBD diagnosis (CD or UC) and disease duration were found but the duration of disease remission was significantly longer in NAFLD patients as compared to IBD patients without NAFLD (33.9 mo vs 21.14, P < 0.05, Figure 2). Laboratory tests of patients with and without NAFLD were compared. NAFLD patients showed increased levels of basal glucose (98.07 mg/dL NAFLD vs 89.79 mg/dL no-NAFLD, P = 0.03), basal insulin (11.47 mU/L NAFLD vs 6.99 mU/L no-NAFLD, P = 0.007) and higher HOMA index (2.49 NAFLD vs 1.59 no-NAFLD, P = 0.005). Aspartate aminotransferase, alanine aminotransferase (ALT), glutamyltransferase, triglycerides and cholesterol values were not statistically different between the two groups.
Next, we examined anthropometric measurements in both groups. As expected, IBD patients with NAFLD had higher mean BMI than the those without NAFLD; 26.54 kg/m² vs 23.88 kg/m² respectively (P < 0.0001). NAFLD patients also had higher mean body weight (73.53 kg NAFLD vs 64.24 kg no-NAFLD, P < 0.0001), waist circumference (93.09 cm NAFLD vs 85.52 cm no-NAFLD, P < 0.0001), arm circumference (28.31 cm NAFLD vs 26.83 cm no-NAFLD, P = 0.003) and visceral fat (10.52 NAFLD vs 6.87 no-NAFLD, P < 0.0001). No significant difference in lean body mass and body fat percentage was found between the two groups. When analyzing dietary habits, patients with NAFLD had higher caloric intake and lipid consumption than patients without NAFLD (1970.27 kcal vs 1752.35 kcal, P = 0.07 and 75.18 g/day vs 64.88 g/day, P = 0.005). This difference did not change when dietary habits of patients were analyzed in patients with active disease. Finally, although numerically different, the mean values of liver stiffness between IBD patients with and without NAFLD were not statistically different (5.5 kPa vs 4.6 kPa, P = 0.09, Table 1).
| Variables, mean (SD) | NAFLD | No-NAFLD | P value |
| Age (yr) | 53.9 (12.9) | 46.9 (13.5) | 0.0007 |
| Disease duration (yr) | 10.7 (7.6) | 11.5 (8.7) | 0.51 |
| Time of remission (mo) | 33.9 (38.2) | 21.1 (30.6) | < 0.05 |
| Antropmetric measurements | |||
| Weight (kg) | 73.5 (14.5) | 64.2 (12.0) | < 0.0001 |
| BMI (kg/m²) | 26.5 (4.7) | 23.9 (3.7) | < 0.0001 |
| Waist circumference (cm) | 93.1 (11.5) | 85.5 (10.0) | < 0.0001 |
| Visceral fat (%) | 10.5 (5.2) | 6.8 (3.3) | < 0.0001 |
| Body fat (%) | 29.9 (12.6) | 27.9 (10.7) | 0.23 |
| Lean body mass (%) | 31.4 (6.1) | 31.1 (7.4) | 0.75 |
| Diet daily intake | |||
| Kcal | 1970.27 (810.42) | 1752.35 (689.48) | 0.07 |
| Protein (g/day) | 90.31 (33.59) | 82.69 (33.75) | 0.16 |
| Lipid (g/day) | 75.18 (34.01) | 61.88 (24.77) | 0.005 |
| Carbohydrates (g/day) | 241.31 (110.81) | 225.18 (101.13) | 0.35 |
| Laboratory findings | |||
| AST (UI/L) | 21.1 (6.1) | 21.3 (7.5) | 0.84 |
| AST (UI/L) | 21.1 (6.1) | 21.3 (7.5) | 0.84 |
| ALT (UI/L) | 22.6 (10.3) | 20.0 (10.5) | 0.09 |
| GGT (UI/L) | 24.6 (11.5) | 22.1 (28.0) | 0.43 |
| Glycemia (mg/dL) | 98.1 (28.0) | 89.8 (12.2) | 0.03 |
| Serum insulin (μUI/mL) | 11.5 (14.1) | 7.0 (4.1) | 0.007 |
| HOMA index | 2.49 (2.42) | 1.59 (1.10) | 0.005 |
| Cholesterol (mg/dL) | 192.7 (41.4) | 191.9 (39.7) | 0.90 |
| Triglycerides (mg/dL) | 105.1 (49.4) | 93.6 (49.7) | 0.15 |
| LDL (mg/dL) | 119.8 (36.5) | 113.3 (34.1) | 0.28 |
| HDL (mg/dL) | 56.9 (17.7) | 60.9 (17.6) | 0.16 |
| Liver stiffness (kPa) | 5.5 (2.7) | 4.6 (3.5) | 0.09 |
At the univariate analysis, male sex was associated to an increased risk to develop NAFLD (odds ratio (OR): 3.17, 95% confidence interval (CI) 1.67-5.99, P = 0.0005). As shown in Table 2, no significant associations were found between NAFLD and IBD-related factors. Indeed, NAFLD was not associated to disease activity, evaluated separately in both UC (OR: 0.62, P = 0.20) and CD (OR: 1.70, P = 0.31), presence of extra-intestinal manifestations (OR :0.97, P = 0.94), long disease duration (OR: 1.17, P = 0.35), disease extension (OR: 1.14, P = 0.68), use of steroids (OR: 0.49, P = 0.57), thiopurines (OR: 1.11, P = 0.80), biologic therapy (OR: 0.78, P = 0.41), prior surgery (OR: 0.63, P = 0.24) and inflammatory markers (i.e., fecal calprotectin, OR: 1.53, 95%CI 0.75-3.12, P = 0.24; c-reactive protein, OR: 0.72, 95%CI 0.39-1.33, P = 0.18). Even by stratyfing patients according to their steatosis grade, no significant association between disease activity and NAFLD was found. As for the metabolic profile, MetS (OR: 4.13, 95%CI 1.85-9.24, P = 0.001) and obesity, measured either by BMI (OR: 9.21, 95%CI 3.06-27.70, P = 0.0002), waist circumference (OR: 2.69, 95%CI 1.22-5.90, P = 0.001) or visceral fat (OR: 3.82, 95%CI 1.91-7.64, P = 0.001) but not diabetes were associated to NAFLD (Table 3). At the multivariate analysis, only advanced age (OR: 1.04, 95%CI 1.01-1.07, P = 0.006), male sex (OR: 4.25, 95%CI 1.95-9.26, P < 0.0001), obesity by BMI (OR: 1.17, 95%CI 1.06-1.28, P = 0.001) and high lipid consumption were associated to NAFLD (OR: 1.02, 95%CI 1.01-1.03, P = 0.004, Table 4).
| Variables, n (%) | NAFLD | Univariate analysis | ||
| Yes | No | Odds ratio (95%CI) | P value | |
| Sex (Male) | 51 (52) | 46 (48) | 3.17 (1.67-5.99) | 0.0005 |
| Smoke | 12 (35) | 23 (65) | 0.69 (0.32-1.51) | 0.36 |
| Disease related factors | ||||
| Disease activity | ||||
| MAYO | 11 (30) | 24 (70) | 0.62 (0.26-1.50) | 0.20 |
| CDAI | 6 (54) | 5 (46) | 1.70 (0.47-6.09) | 0.31 |
| Extraintestinal manifestations | 20 (40) | 31 (60) | 0.97(0.50-1.90) | 0.94 |
| Extended disease | 47 (41) | 66 (59) | 1.14 (0.61-2.13) | 0.68 |
| Disease duration | ||||
| > 10 yr | 35 (42) | 48 (58) | 1.00 Rif. | |
| < 10 yr | 36 (38) | 58 (62) | 1.17 (0.64-2.14) | 0.35 |
| Steroids | 12 (27) | 31 (73) | 0.49 (0.23-1.03) | 0.06 |
| Immunomodulators | 11 (43) | 15 (53) | 1.11 (0.48-2.58) | 0.80 |
| Biologic therapy | 28 (37) | 49 (63) | 0.78 (0.42-1.43) | 0.41 |
| Surgery | 11 (31) | 24 (69) | 0.63 (0.28-1.38) | 0.24 |
| Fecal calprotectin (mg/kg) | ||||
| > 200 | 29 (40) | 44 (60) | 1.00 Rif. | |
| < 200 | 19 (20) | 44 (45) | 1.53 (0.75-3.12) | 0.24 |
| CRP (mg/L, high levels) | 30 (36) | 53 (64) | 0.72 (0.39-1.33) | 0.18 |
| Metabolic profile, n (%) | NAFLD | Univariate Analysis | P-value | |
| Yes | No | Odds ratio (95%CI) | ||
| BMI (kg/m²) | ||||
| Normal | 24 (27) | 65 (73) | 1.00 Rif. | Normal |
| Overweight | 29 (49) | 30 (51) | 2.62 (1.31-5.23) | 0.005 |
| Obese | 17 (77) | 5 (23) | 9.21 (3.06-27.70) | 0.0002 |
| Waist circumference (cm) | ||||
| Normal | 26 (33) | 54 (67) | 1.00 Rif. | |
| Overweight | 14 (39) | 22 (61) | 1.30 (0.57-2.94) | 0.34 |
| Obese | 22 (56) | 17 (44) | 2.69 (1.22-5.90) | 0.001 |
| Visceral fat (> 8) | 43 (55) | 35 (45) | 3.82 (1.91-7.64) | 0.001 |
| Diabetes | 7 (58) | 5 (42) | 2.40 (0.72-7.87) | 0.14 |
| MetS | 22 (66) | 11 (34) | 4.13 (1.85-9.24) | 0.001 |
| Variables | Multivariate analysis | P-value |
| Odds ratio (95%CI) | ||
| Advanced age | 1.04 (1.01-1.07) | 0.006 |
| Sex (male) | 4.25 (1.95-9.26) | < 0.00001 |
| Obese (BMI) | 1.17 (1.06-1.28) | 0.001 |
| High lipidic diet | 1.02 (1.01-1.03) | 0.004 |
No IBD-related risk factors were found to be associated to LF at the univariate analysis. As for the metabolic factors, the presence of MetS was the only factor significantly associated with LF (OR: 3.40, 95%CI 1.26-9.20, P = 0.01).
NAFLD is the most common hepatic disease in Western countries[16] and it is recognized as an increasingly relevant health issue. Indeed, NAFLD is candidate to become the first cause of liver transplant in the next years. In our IBD cohort, 46.6% of the patients were diagnosed with NAFLD. Obesity, evaluated by BMI, waist circumference and visceral fat, was significantly correlated with the presence of hepatic steatosis. Accordingly, we observed a significant correlation between high-caloric, high-fat diet and NAFLD. Patients with NAFLD tend to follow a Western rather than a Mediterranean diet[17]. Indeed, we observed a reduced intake of complex carbohydrates, fruit and vegetables and an increased intake of lipids and proteins among IBD patients. This dietary habit might be due to the fact that IBD patients associate symptoms such as abdominal distention and diarrhea to fiber and complex carbohydrates intake, thus limiting their daily consumption, preferring proteic and fat foods instead[18]. Furthermore, a long period of well-being, favored by the therapy-induced improvement of the general conditions and of the intestinal symptoms, in particular diarrhea, may be the reasons for the increased food intake. We also observed a positive correlation between high basal glucose and serum insulin levels, as indicated by the high HOMA index value, and NAFLD, thus suggesting a common pathogenic mechanism. Indeed, insulin resistance in the target organ, further exacerbates hepatic steatosis.
Older age was also independently associated with NAFLD and this finding could be related to the progressive increase of the metabolic risk factors with aging. Conflicting data have been reported on the relationship between natural history of IBD and the presence and grade of steatosis. Bessissow et al[6] showed the association between duration of IBD, disease activity and prior surgery with NAFLD development, and these findings have been replicated by others[19,20]. In contrast with these data, but in line with our findings, Saroli et al[5] failed to demonstrate the association between IBD-related factors including medications (steroids, thiopurine, and TNF inhibitors) and the risk to develop NALFD.
In our view, the lack of association between IBD-related risk factors and NAFLD and the presence of an unbalanced diet in both active and inactive disease, emphasizes the importance of the dietary habits and the metabolic profile rather than the intestinal inflammation in the stratification risk for liver steatosis. Accordingly, we found that the duration of remission was strongly associated to NAFLD, suggesting that patients in clinical remission tend to follow a more unbalanced diet and to develop more NAFLD.
NAFLD confers increased risk of cardiovascular-related mortality and HCC, therefore therapeutic options for NAFLD targeting either obesity or hepatic inflammation and fibrosis is advisable. The first approach includes weight loss through caloric restriction and increased physical activity[1]. There are currently no recommended routine screening strategies for NAFLD in patients with IBD. However, we believe that the assessment of hepatic steatosis is always necessary, in particular in patients with high-risk features, including obesity, overweight, MetS and advanced age, regardless of the presence of elevated serum transaminases or clinical signs of liver disease. Indeed, NAFLD has been shown to develop in the absence of serum transaminase elevation. Accordingly, only 3.3% of patients in our cohort of IBD patients had elevated serum transaminases.
NAFLD can progress to NASH and LF. TE test performed in our cohort to evaluate liver stiffness, showed LF in 16% of patients. The only significant fibrosis-related factor was MetS, and no significant association between hepatic transaminases and LF was found. Since elevated ALT was not predictive of NAFLD or LF in our cohort, it would be necessary to implement the management of IBD patients with a metabolic and liver profile evaluation in order to prevent the onset of liver complications.
Our results suggest that IBD patients should be screened for NAFLD-associated risk factors in order to prevent the development of liver disease. Moreover, the early detection of fatty liver disease and fibrosis by ultrasonography and TE might have a relevant impact on liver disease evolution though the correction of the dietary habits and the improvement of patients’ metabolic profile. A practical approach might be to encourage the use of complex carbohydrates together with fruit and vegetables and to reduce the intake of fats and proteins thus decreasing the state of hypeinsulinism and insulin resistance.
In conclusion, our study confirms the epidemiological burden of NAFLD in IBD. In addition, it demonstrates the absence of intestinal disease-specific risk factors associated to NAFLD, while confirming as risk factors those already identified in the general population. Moreover, these data suggest that IBD care should not be limited to intestinal disease but should also include metabolic interventions by promoting healthy lifestyle and a correct dietary regimen in order to reduce the occurrence of chronic liver disease.
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease. It can progress from simple hepatic steatosis to nonalcoholic steatohepatitis, liver fibrosis (LF), cirrhosis and hepatocellular carcinoma. NAFLD is a frequently reported condition in patients with inflammatory bowel disease (IBD).
The underlining causes and predisposing factors to NAFLD among IBD patients remain poorly investigated. Both intestinal inflammation and metabolic factors are believed to contribute to the pathogenesis of IBD-associated NAFLD.
The aim of the study was to evaluate the prevalence of steatosis and LF in a cohort of IBD patients and the identification of metabolic- and IBD-related risk factors for NAFLD and LF. The results of the present project could provide new diagnostic tools for the estimation of individual risk of evolution allowing to set up new tools for prevention, diagnosis and prognosis with a personalized approach to the patient.
We conduct an observational study enrolling consecutive IBD patients in regular follow-up. At the time of enrollment each patient was evaluated through medical examination, complete blood tests and a nutritional evaluation where were obtained anthropometric parameters [body mass index (BMI), waist circumference, visceral fat, body fat, lean body mass] and, by asking the patients to describe the foods and drinks consumed in the previous 24 h (24-h dietary recall), we collected the usual dietary intake. The same day they underwent abdominal ultrasound to establish the presence and severity of NAFLD and a transient elastography to evaluate LF.
Of 178 consecutive IBD patients were enrolled in our study (95 ulcerative colitis and 83 Crohn’s disease). The prevalence of NAFLD was found in 72 patients (40.4 %). Comparison between patients with and without NAFLD showed no significant differences in terms of IBD severity, disease duration, location/extension, use of IBD-related medications (i.e., steroids, anti-TNFs, and immunomodulators) and surgery. NAFLD was significantly associated with the presence of metabolic syndrome (MetS, OR: 4.13, P = 0.001) and obesity defined by BMI (OR: 9.21, P = 0.0002), waist circumference (OR: 2.69, P = 0.001) and visceral fat (OR: 3.82, P = 0.001). IBD patients with NAFLD showed higher caloric intake and lipid consumption than those without NAFLD, regardless disease activity. At the multivariate analysis, male sex, advanced age and high lipid consumption were independent risk factors for the development of NAFLD. An increased liver stiffness was detected in 21 patients (16%) and the presence of MetS was the only relevant factor associated to LF (OR: 3.40, P = 0.01).
Our study confirms the epidemiological burden of NAFLD in IBD. It failed to demonstrate the association between IBD-related factors including medications (steroids, thiopurine, and TNF inhibitors) and the risk to develop NALFD, while confirming as risk factors the same of the general population, including obesity, overweight, unbalanced high lipidic diet, MetS and advanced age. The recent improvements in IBD therapy induce patients with long periods of well-being, bringing them to increase food intake and unbalanced diet. Furthmore, patients with IBD could tend to associate symptoms such as abdominal distention and diarrhea to fiber and complex carbohydrates intake, thus limiting their daily consumption, preferring proteic and fat foods. This emphasizes the importance of the dietary habits and the metabolic profile rather than the intestinal inflammation in the stratification risk for liver steatosis. It appears necessary that IBD care should also include nutritional and metabolic interventions, with the objective to maintain the intestinal welfare of patients, avoiding the development of metabolic complications associated with an unbalanced diet. A pratical approach could be to promote a healthy life style and encourage the use of complex carbohydrates together with fruit and vegetables and to reduce the intake of fats and proteins.
There are currently no recommended routine screening strategies for NAFLD in patients with IBD. However, our results suggest that IBD patients should be screened for NAFLD-associated risk factors in order to prevent the development of liver disease. Considering the fearsome consequence of NAFLD, data demonstrates that IBD care should not be limited to intestinal therapy, but should include metabolic interventions, by promoting healthy life-style and a correct dietary intake. However further perspective studies are still necessary to determinate the impact of the natural history of IBD with the presence of NAFLD and their evolution.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hua J, Yu CH, Zhu HF S-Editor: Yan JP L-Editor: A E-Editor: Zhou BX
| 1. | European Association for the Study of the Liver (EASL. European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO); EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3176] [Article Influence: 352.9] [Reference Citation Analysis (4)] |
| 2. | Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 789] [Article Influence: 52.6] [Reference Citation Analysis (1)] |
| 3. | Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2292] [Article Influence: 163.7] [Reference Citation Analysis (0)] |
| 4. | Kirsner JB, Shorter RG. Recent developments in "nonspecific" inflammatory bowel disease (first of two parts). N Engl J Med. 1982;306:775-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 159] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Saroli Palumbo C, Restellini S, Chao CY, Aruljothy A, Lemieux C, Wild G, Afif W, Lakatos PL, Bitton A, Cocciolillo S, Ghali P, Bessissow T, Sebastiani G. Screening for Nonalcoholic Fatty Liver Disease in Inflammatory Bowel Diseases: A Cohort Study Using Transient Elastography. Inflamm Bowel Dis. 2019;25:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Bessissow T, Le NH, Rollet K, Afif W, Bitton A, Sebastiani G. Incidence and Predictors of Nonalcoholic Fatty Liver Disease by Serum Biomarkers in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:1937-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 7. | Nagahori M, Hyun SB, Totsuka T, Okamoto R, Kuwahara E, Takebayashi T, Naganuma M, Watanabe M. Prevalence of metabolic syndrome is comparable between inflammatory bowel disease patients and the general population. J Gastroenterol. 2010;45:1008-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 8. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 9. | Barbero-Villares A, Mendoza Jimé, nez-Ridruejo J, Taxonera C, López-Sanromán A, Pajares R, Bermejo F, Pérez-Calle JL, Mendoza JL, Algaba A, Moreno-Otero R, Maté J, Gisbert JP. Madrid Group for the Study of Inflammatory Bowel Disease ENICMAD.Evaluation of liver fibrosis by transient elastography (Fibroscan®) in patients with inflammatory bowel disease treated with methotrexate: A multicentric trial. Scand J Gastroenterol. 2012;47:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 10. | Bharadwaj S, Tandon P, Kulkarni G, Rivas J, Charles R. The role of endoscopy in inflammatory bowel disease. J Dig Dis. 2015;16:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, Lakatos PL, Adamina M, Ardizzone S, Buskens CJ, Sebastian S, Laureti S, Sampietro GM, Vucelic B, van der Woude CJ, Barreiro-de Acosta M, Maaser C, Portela F, Vavricka SR, Gomollón F; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis. 2017;11:135-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 530] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 12. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F. European Crohnâs and Colitis Organisation [ECCO].Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1292] [Article Influence: 161.5] [Reference Citation Analysis (0)] |
| 13. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 14. | van Werven JR, Marsman HA, Nederveen AJ, Smits NJ, ten Kate FJ, van Gulik TM, Stoker J. Assessment of hepatic steatosis in patients undergoing liver resection: Comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010;256:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Carr RM, Oranu A, Khungar V. Nonalcoholic Fatty Liver Disease: Pathophysiology and Management. Gastroenterol Clin North Am. 2016;45:639-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 17. | Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: A systematic review. J Gastroenterol Hepatol. 2012;27:1266-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 18. | Levenstein S, Prantera C, Luzi C, D'Ubaldi A. Low residue or normal diet in Crohn's disease: A prospective controlled study in Italian patients. Gut. 1985;26:989-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Sartini A, Gitto S, Bianchini M, Verga MC, Di Girolamo M, Bertani A, Del Buono M, Schepis F, Lei B, De Maria N, Villa E. Non-alcoholic fatty liver disease phenotypes in patients with inflammatory bowel disease. Cell Death Dis. 2018;9:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Sourianarayanane A, Garg G, Smith TH, Butt MI, McCullough AJ, Shen B. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:e279-e285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (2)] |