Published online Sep 28, 2019. doi: 10.3748/wjg.v25.i36.5494
Peer-review started: May 31, 2019
First decision: July 21, 2019
Revised: August 8, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: September 28, 2019
Processing time: 121 Days and 1.9 Hours
Laparoscopy-assisted pylorus-preserving gastrectomy (LAPPG) was known to have benefits of function-preserving surgery compared to laparoscopy-assisted distal gastrectomy (LADG). However, in clinical settings, delayed gastric emptying and esophageal reflux following LAPPG can be serious issues, making surgeons reluctant to perform LAPPG. It is unclear that LAPPG had better long-term functional outcomes and quality of life compared to LADG.
To evaluate the long-term functional outcomes and patient-reported quality of life of LAPPG compared to those of LADG.
We reviewed the clinicopathological data of 195 patients who underwent LADG with Billroth II anastomosis and 101 patients who underwent LAPPG for cT1N0 gastric cancer in the middle third of the stomach between 2012 and 2015. Postoperative complications, nutritional parameters, and survey results of the European Organization for Research and Treatment of Cancer Questionnaire C30 and STO22 questionnaire were compared between the two groups.
The serum hemoglobin level was significantly higher in the LAPPG group than in the LADG group (P < 0.001). In the endoscopic findings, incidence of bile reflux was lower (P < 0.001); however, the incidence of residual food was higher in the LAPPG group than in the LADG group (P < 0.001). Regarding the quality of life score, the LAPPG group had a better physical functioning score (86.7 vs 90.0, P = 0.032) but also greater pain and reflux when compared to the LADG group [8.3 vs 16.7 in pain, 11.1 (interquartile range, 0, 22.2) vs 11.1 (interquartile range, 11.1, 33.3) in reflux, P = 0.034 and 0.001, respectively].
LAPPG is beneficial to recovery of anemia and to bile reflux, however, it might be unfavorable in terms of pain and reflux symptoms compared to LADG with Billroth II anastomosis.
Core tip: Laparoscopy-assisted pylorus-preserving gastrectomy (LAPPG) was known to have benefits of function-preserving surgery compared to laparoscopy-assisted distal gastrectomy (LADG). However, in clinical settings, delayed gastric emptying and esophageal reflux following LAPPG can be serious issues, making surgeons reluctant to perform LAPPG. In this study, we evaluated long-term quality of life and functional outcomes of LAPPG compared to those of LADG with Billroth II anastomosis. LAPPG is beneficial to recovery of postoperative anemia and bile reflux, however, it might be unfavorable in terms of long-term pain and reflux symptoms compared to LADG with Billroth II anastomosis.
- Citation: Eom BW, Park B, Yoon HM, Ryu KW, Kim YW. Laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer: A retrospective study of long-term functional outcomes and quality of life. World J Gastroenterol 2019; 25(36): 5494-5504
- URL: https://www.wjgnet.com/1007-9327/full/v25/i36/5494.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i36.5494
Pylorus-preserving gastrectomy (PPG) was first proposed by Maki et al[1] in 1967 to treat peptic ulcers, and its use was expanded to include middle-third early gastric cancer in 1991[1,2]. By preserving the pyloric ring and its functionality, PPG was expected to reduce the risk of postgastrectomy syndrome while improving quality of life (QOL). According to recent meta-analyses, the advantages of PPG include lower incidence rates of dumping syndrome, remnant gastritis, and bile reflux in addition to a better postoperative nutritional status when compared to distal gastrectomy[3,4]. However, the incidence of delayed gastric emptying was higher with PPG.
In the 2000s, the feasibility and safety of minimally invasive approaches were widely evaluated, and the laparoscopy-assisted PPG (LAPPG) was also introduced and evaluated during this time[5-8]. When compared to conventional PPG, LAPPG had several benefits of a minimally invasive approach, including less blood loss, less postoperative pain, and a faster recovery of bowel function[9-11]. Moreover, LAPPG resulted in a lower risk of postgastrectomy syndrome and better nutritional outcomes when compared to laparoscopy-assisted distal gastrectomy (LADG)[12-14]. Because of this combination of advantages of a minimally invasive approach and a function-preserving surgery, LAPPG is considered to be an excellent option for middle-third early gastric cancer.
However, in clinical settings, instances of delayed gastric emptying and esophageal reflux following LAPPG can be serious issues, making surgeons reluctant to perform LAPPG. While postgastrectomy syndrome is transient and does not require hospitalization or medication in most cases, delayed gastric emptying increases the length of the hospital stay, and reflux symptoms requires medication, such as proton pump inhibitors. Moreover, only a few previous studies have evaluated the QOL after LAPPG, and these studies only included a small number of patients[13,14]. Therefore, it remains unclear whether LAPPG is superior to LADG in terms of functional outcomes, including QOL. The aim of this study is to evaluate the long-term functional outcomes and QOL of LAPPG when compared to those of LADG with Billroth II anastomosis.
This study was conducted in accordance with the principles of the Declaration of Helsinki, and was approved by the Institutional Review Board of the National Cancer Center (No. NCC 2017-0183).
From January 2012 to December 2015, 720 patients underwent LADG with Billroth II anastosmosis or LAPPG for cT1N0 gastric cancer in the National Cancer Center, Korea. Among these patients, the following were excluded in the analysis; 309 patients whose primary tumor was located less than 5 cm from the pylorus, 9 patients who had multiple gastric cancers, 92 patients who received adjuvant chemotherapy because their postoperative pathological stages were stage II or III; 13 patients who had some other history of cancer before and after the gastrectomy; and 1 patient due to follow-up loss.
LAPPG was performed by four surgeons that were experienced in LADG (EBW, YHM, RKW, and KYW). Infrapyloric vessels were routinely preserved to maintain blood supply to the pyloric cuff, and the right gastric artery with lymph node station No. 5 was also preserved and dissected distally to the first branch of the right gastric artery. The hepatic and pyloric branches of the vagus nerves were preserved, while the celiac branch was generally not preserved. The extent of lymph node dissection was D1+ or more according to the treatment guidelines of the Japanese Gastric Cancer Association[15]. D1+ in the context of PPG indicates the lymph node station numbers 1, 3, 4sb, 4d, 6, 7, 8a, and 9.
After resection was completed, a short 4- to 5-cm midline incision was made, and the tumor location was checked by palpation of the proximal and distal clips. A 3- to 5-cm pyloric cuff remained after the resection of the distal portion, and the gastrogastrostomy was performed by a hand-sewn suture. In LADG, the laparoscopic procedures were performed the same as those used in LAPPG, except for the dissection of the No. 5 and 6 lymph nodes and the vagus nerve. After the procedures, a gastrojejunal anastomosis (Billroth II) was performed extracorporeally through the same incision that was used for LAPPG.
Comorbidity was evaluated using the American Society of Anesthesiologists (ASA) physical status classification system[16]. Histological types were classified according to the 2010 World Health Organization classification[17]. When a tumor consisted of components of two or more histological types, the quantitative predominance was recorded as the histological type. The pathological stage was categorized according to the 8th American Joint Committee on Tumor-Node-Metastasis classification system[18]. Postoperative symptom complications were evaluated using the Clavien-Dindo classification system within 1 month after surgery[19].
After surgery, the patients visited the outpatient clinic every 6 mo. Body weight, serum hemoglobin, protein, and albumin were checked during this visit and analyzed as nutritional outcomes. Patients with anemia were provided with iron supplementation at the outpatient clinic depending on patient symptoms, hemoglobin levels, and the discretion of the physician. In general, oral iron was administered by 80 mg of ferrous sulfate once or twice per day for a period of 3 or 6 mo. Intravenous iron was typically administered as a single dose of ferric carboxymaltose (500 mg). An esophagogastroduodenoscopy was performed annually, and the patient was evaluated for the presence of bile reflux and the grade of residual food according to the following criteria; no food residue, liquid only, soft diet residue, and nearly normal diet residue.
Postoperative QOL was estimated using the European Organization for Research and Treatment of Cancer (EORTC) QOL Questionnaire (QLQ) -C30 and the gastric cancer-specific module, STO22[20]. The EORTC QLQ-C30 is composed of a global health status/QOL scale, 5 functional scales and 9 symptom scales. The EORTC QLQ-STO22 is composed of 9 symptom scales. Each scale is represented by a score ranging from 0 to 100. A higher score indicates a better QOL as defined by the global health status and functional scales of the EORTC QLQ-C30, while a higher score indicates a poorer QOL on the symptom scale of the EORTC QLQ-C30 and STO22[21].
The QOL survey was performed between October 2016 and December 2018 for patients who underwent surgeries at least 2 years prior and visited the outpatient clinic. The period between surgery and the day that the questionnaire was administered varied, ranging from 2 to 5 years postoperatively.
The continuous variables are shown as the means ± standard deviations or the medians with interquartile ranges, and the categorical variables are presented as proportions. Differences between these groups were tested using a t-test or the Wilcoxon rank-sum test for continuous variables and the chi –square test or Fisher’s exact test for categorical variables. A mixed effect model was performed to analyze changes of the nutritional outcomes between the two groups. In the QOL analysis, each subscale or item is presented as the median and the interquartile range. The distribution of QOL scores did not follow a normal distribution. Therefore, nonparametric statistics (i.e., the Willcoxon rank-sum test) were used to evaluate their statistical significance. Data analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, United States). P values less than 0.05 were considered significant.
Among the 720 patients, 195 patients with LADG and 101 patients with LAPPG were ultimately included in this study (Supplementary Figures 1 ). There were no significant differences in baseline demographics, including age, sex, body mass index, and ASA classification (Table 1). The pathological results showed that the distal margin was shorter, and there were less harvested lymph nodes in the LAPPG group when compared to the LADG groups (P < 0.001 and P = 0.046, respectively). The operative time was longer in the LAPPG group (median, 150 min vs 210 min, P < 0.001), while the estimated blood loss and the length of hospital stay were not significantly different between the two groups.
| Factors | Subgroup | LADG | LAPPG | P value |
| (n = 195) (%) | (n = 101) (%) | |||
| Age (mean ± SD) (yr) | 56.5 ± 11.8 | 58.3 ± 12.0 | 0.218 | |
| Sex | Male | 114 (58.5) | 54 (53.5) | 0.458 |
| Female | 81 (41.5) | 47 (46.5) | ||
| BMI (mean ± SD) (kg/m2) | 24.0 ± 3.1 | 24.1 ± 3.1 | 0.617 | |
| ASA | I | 79 (40.5) | 35 (34.7) | 0.441 |
| II | 109 (55.9) | 64 (63.4) | ||
| III | 7 (3.6) | 2 (2.0) | ||
| Tumor size (mean ± SD) (cm) | 2.8 ± 1.5 | 2.5 ± 1.4 | 0.078 | |
| Histological type | WD | 30 (15.4) | 15 (14.9) | 0.716 |
| MD | 45 (23.1) | 20 (19.8) | ||
| PD | 47 (24.1) | 23 (22.8) | ||
| SRC | 70 (35.9) | 43 (42.6) | ||
| Others | 3 (1.5) | 0 (0) | ||
| Proximal margin (mean ± SD) (cm) | 2.7 ± 2.0 | 2.5 ± 2.2 | 0.388 | |
| Distal margin (mean ± SD) (cm) | 8.0 ± 2.5 | 3.6 ± 2.6 | < 0.001 | |
| Number of positive LN (mean ± SD) | 0.1 ± 0.3 | 0.1 ± 0.4 | 0.364 | |
| Number of Harvested LN (mean ± SD) | 33.5 ± 12.2 | 30.5 ± 11.6 | 0.046 | |
| pT | 1 | 188 (96.4) | 96 (95.0) | 0.551 |
| 2 | 7 (3.6) | 5 (5.0) | ||
| pN | 0 | 182 (93.3) | 92 (91.1) | 0.641 |
| 1 | 13 (6.7) | 9 (8.9) | ||
| Operating time (median, IQR) (min) | 150.0 (130.0, 185.0) | 210.0 (185.0, 235.0) | < 0.001 | |
| Estimated blood loss (median, IQR) (cc) | 100.0 (12.5, 200.0) | 100.0 (50.0, 200.0) | 0.235 | |
| Hospital stay (median, IQR) (d) | 7.0 (6.0, 7.0) | 7.0 (5.5, 7.0) | 0.940 |
The incidence of overall early complications was not significantly different between the LADG and LAPPG groups (Table 2). There were also no significant differences in any of the severity grades of the Clavien-Dindo classification. However, the incidence of delayed gastric emptying was higher in the LAPPG group (P = 0.001). Four patients with grade III delayed gastric emptying were treated with endoscopic Botox injections, after which they improved[22].
| Factors | Complications | LADG | LAPPG | P value |
| (n = 195) (%) | (n = 101) (%) | |||
| Grade I | Wound | 2 | 0 | 0.519 |
| Ileus | 3 | 1 | ||
| Delayed gastric emptying | 0 | 3 | ||
| Urologic problem | 1 | 1 | ||
| Grade II | Ileus | 2 | 0 | 0.378 |
| Delayed gastric emptying | 2 | 2 | ||
| Intra-abdominal abscess | 0 | 1 | ||
| Intra-abdominal bleeding | 1 | 0 | ||
| Acute cholecystitis | 1 | 0 | ||
| Pulmonary problem | 1 | 2 | ||
| Urologic problem | 0 | 1 | ||
| Grade III | Wound | 2 | 0 | 0.282 |
| Delayed gastric emptying | 0 | 4 | ||
| Anastomotic leakage | 1 | 1 | ||
| Anastomosis bleeding | 1 | 0 | ||
| Grade IV | Septic shock | 1 | 0 | 0.999 |
| Overall Complications | Absent | 179 (91.8) | 86 (85.1) | 0.108 |
| Present | 16 (8.2) | 15 (14.9) | ||
| Delayed gastric emptying | Absent | 193 (99.0) | 92 (91.1) | 0.001 |
| Present | 2 (1.0) | 9 (8.9) |
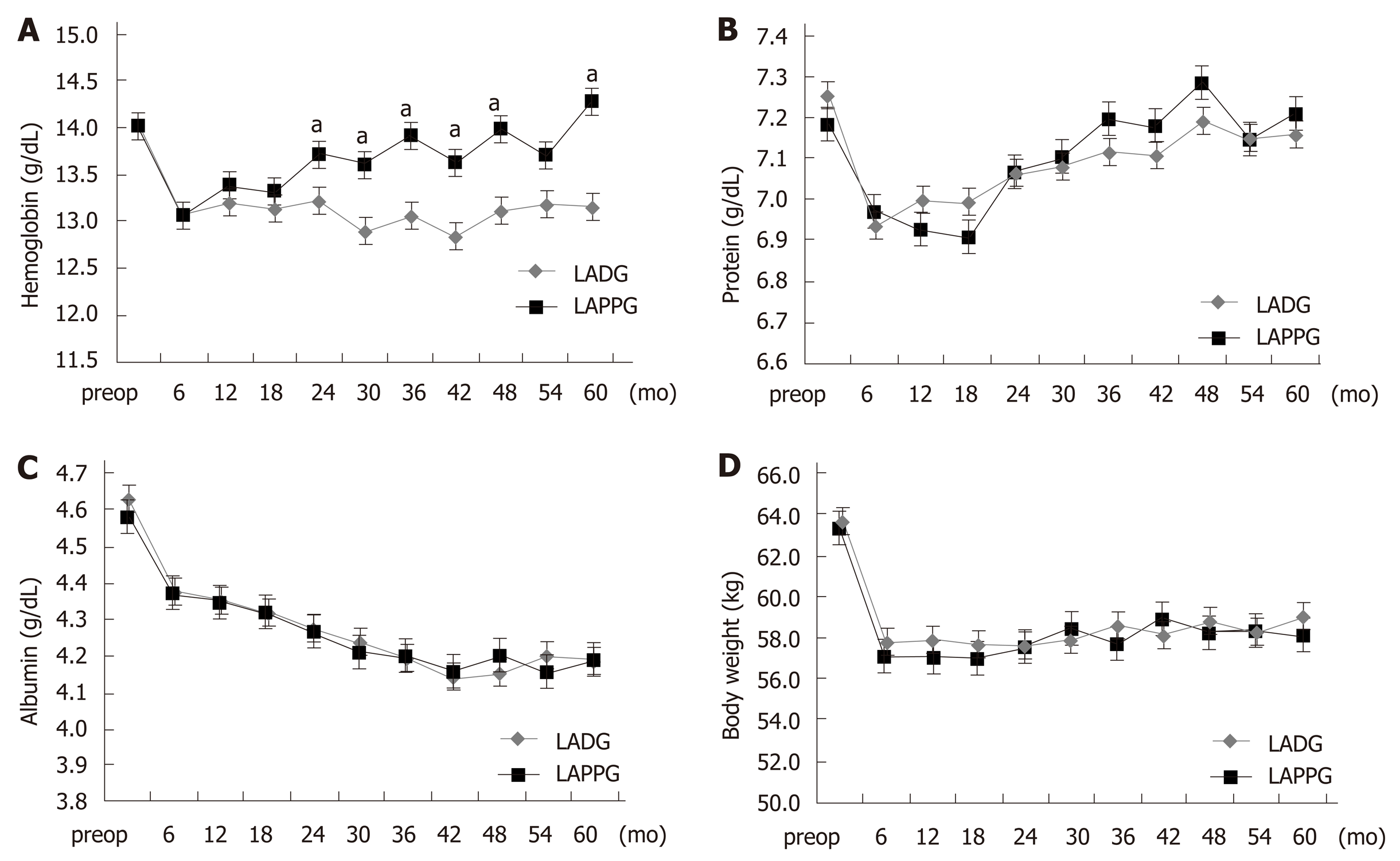
Figure 1 shows the comparison of long-term nutritional outcomes between the LADG and LAPPG groups. In the LAPPG group, hemoglobin levels gradually increased and had almost recovered by postoperative year 5. However, decreased hemoglobin levels persisted in the LADG group. Hemoglobin levels were significantly higher in the LAPPG group than in the LADG group (P < 0.001). Iron supplementation in the outpatient clinic was also evaluated; the proportions of patients who were administered any iron supplement were similar between the two groups (22.1% vs 19.8%, P = 0.765) (Table S1).
Serum protein decreased after surgery but increased after 1-2 years postoperatively in both groups. Serum albumin continuously decreased after surgery. There were no significant differences in the changes of serum protein and albumin level between the two groups (P = 0.083 and 0.304, respectively). After surgery, a loss of body weight of approximately 5-6 kg was observed in both groups, and the decreased body weight persisted in the follow-up period. There were no significant differences in the changes in body weight between the two groups (P = 0.092).
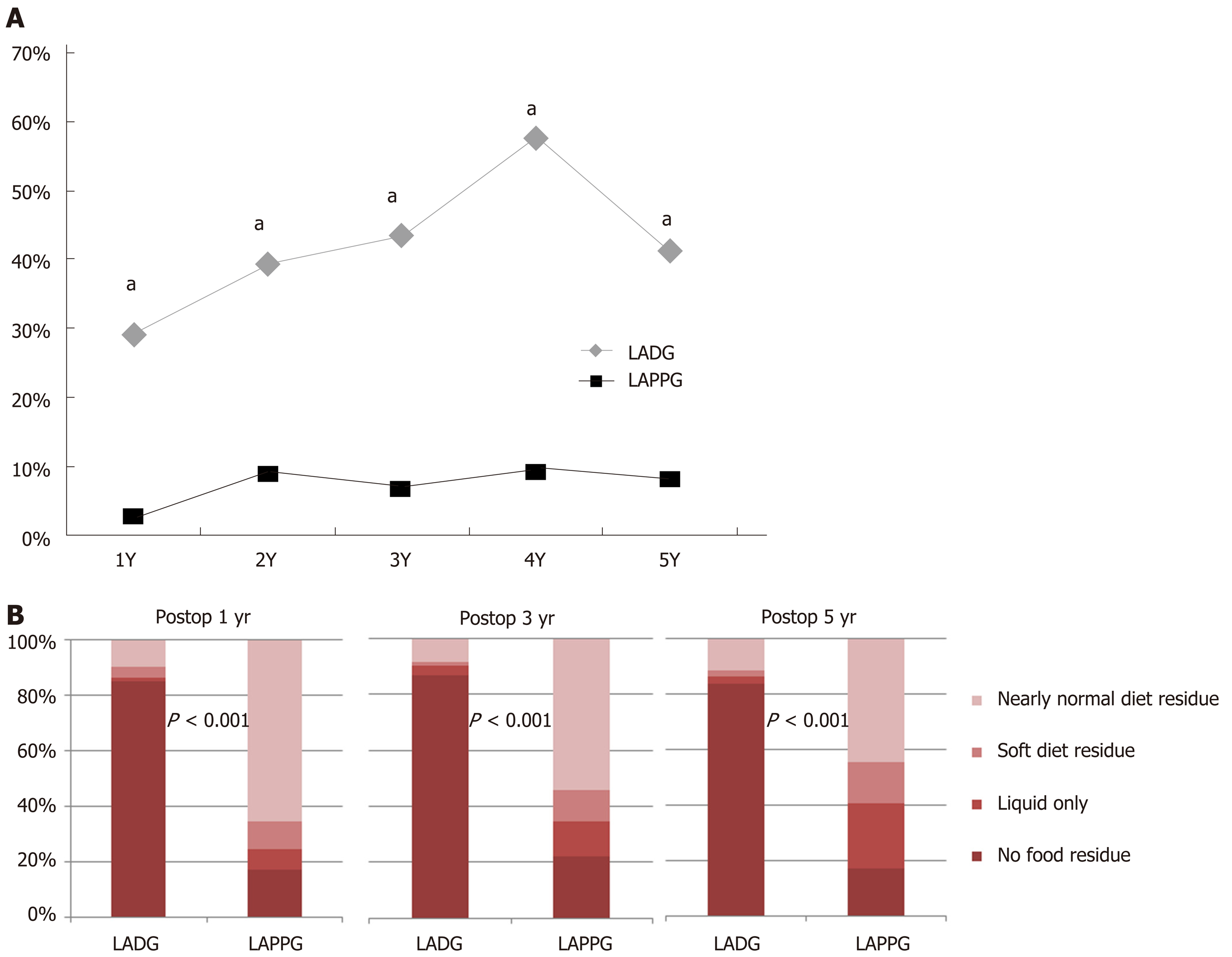
Figure 2 shows the proportions of the presence of bile reflux and residual food. After surgery, bile reflux was observed in 30%-60% of the LADG group, and in less than 10% of the LAPPG group (P < 0.001 at every time point). In the LAPPG group, 65% of patients had a nearly normal diet residue at 1 year postoperatively, and the proportion decreased slightly to 45% at 5 years postoperatively. In contrast, less than 10% of the LADG group had a nearly normal diet residue postoperatively. The proportions of residual food were significantly different between the two groups at every time point (P < 0.001).
A total of 108 (55.3%) and 61 (60.4%) patients in the LADG and LAPPG groups answered the QOL questionnaire, respectively, and the time points for the responses to the QOL questionnaire ranged from 2 years to 5 years (Table S2). For the EORTC QLQ-C30, the LAPPG group had better physical function when compared to the LADG group (86.7 vs 90.0, P = 0.032) (Table 3). For the EORTC QLQ-STO22, the pain and reflux score were significantly higher in the LAPPG group than in the LADG group (8.3 vs 16.7 in pain, 11.1 (interquartile range, 0, 22.2) vs 11.1 (interquartile range, 11.1, 33.3) in reflux, P = 0.034 and 0.001, respectively). Other functional and symptom scales/items did not show any differences.
| LADG (n = 108) | LAPPG (n = 61) | P value | |
| Median (interquartile) | Median (interquartile) | ||
| EORTC QLQ C-30 | |||
| Global health status/QoL | 66.7 (50.0, 83.3) | 66.7 (50.0, 83.3) | 0.466 |
| Functional scales | |||
| Physical functioning | 86.7 (80.0, 93.3) | 90.0 (86.0, 100.0) | 0.032 |
| Role functioning | 100.0 (66.7, 100.0) | 100.0 (83.3, 100.0) | 0.07 |
| Emotional functioning | 83.3 (66.7, 100.0) | 83.3 (75.0, 100.0) | 0.757 |
| Cognitive functioning | 83.3 (83.3, 100.0) | 83.3 (83.3, 100.0) | 0.690 |
| Social functioning | 83.3 (83.3, 100.0) | 83.3 (83.3, 100.0) | 0.776 |
| Symptom scales/items | |||
| Fatigue | 22.2 (11.1, 33.3) | 22.2 (11.1, 33.3) | 0.845 |
| Nausea and vomiting | 0 (0, 16.7) | 0 (0, 16.7) | 0.916 |
| Pain | 0 (0, 16.7) | 0 (0, 16.7) | 0.779 |
| Dyspnea | 0 (0, 33.3) | 0 (0, 33.3) | 0.977 |
| Insomnia | 0 (0, 33.3) | 0 (0, 33.3) | 0.387 |
| Appetite loss | 0 (0, 0) | 0 (0, 33.3) | 0.070 |
| Constipation | 0 (0, 33.3) | 0 (0, 33.3) | 0.073 |
| Diarrhea | 16.7 (0, 33.3) | 33.3 (0, 33.3) | 0.718 |
| Financial difficulties | 0 (0, 33.3) | 0 (0, 16.7) | 0.809 |
| EORTC QLQ STO-22 | |||
| Dysphagia | 0 (0, 11.1) | 0 (0, 11.1) | 0.722 |
| Pain | 8.3 (0, 25.0) | 16.7 (8.3, 33.3) | 0.034 |
| Reflux | 11.1 (0, 22.2) | 11.1 (11.1, 33.3) | 0.001 |
| Eating restrictions | 8.3 (0, 16.7) | 8.3 (0, 25.0) | 0.599 |
| Anxiety | 22.2 (11.1, 33.3) | 22.2 (11.1, 38.9) | 0.949 |
| Dry mouth | 33.3 (0, 33.3) | 0 (0, 33.3) | 0.204 |
| Taste | 0 (0, 0) | 0 (0, 0) | 0.091 |
| Body image | 0 (0, 33.3) | 33.3 (0, 33.3) | 0.847 |
| Hair loss | 33.3 (0, 66.7) | 33.3 (16.7, 33.3) | 0.875 |
In this study, we evaluated the long-term functional outcomes and the QOL of patients who underwent LAPPG for early gastric cancers that were located in the middle third of the stomach. When compared to the LADG group, the LAPPG group had the advantage of long-term hemoglobin recovery and physical function. However, the LAPPG group also experienced delayed gastric emptying at a higher frequency and a higher reflux and pain scores on the EORTC QLQ-STO22 questionnaire.
To date, only a few studies have evaluated the functional outcomes following LAPPG[12-14,23]. One of these reports utilized a single-arm study design that could not determine the functional superiority of LAPPG to LADG[23]. The other study demonstrated the nutritional advantages of LAPPG when compared to LADG, but only short-term outcomes (within 1 postoperative year) were described[12]. Two studies reported QOL outcomes but encountered issues with either very small numbers of participants employing questionnaires that were not validated in other ethnic populations[13,14]. Therefore, further studies that would evaluate long-term functional results including QOL are required.
In the present study, (1) A moderate number of patients (approximately 300) were included; (2) All patients were followed-up on for more than 3 year after surgery; (3) Both functional and QOL outcomes were reported; and (4) QOL was assessed using a validated questionnaire (the EORTC QLQ C-30 and the STO-22). Based on these points, this study aims to provide a more comprehensive and reliable understanding of patients who undergo LAPPG.
In this study, hemoglobin recovery was better in the LAPPG group than in the LADG group. Iron is mainly absorbed in the duodenum, and gastric acidity promotes the intestinal absorption of nonheme iron. After LAPPG, food passes through the duodenum and gastric acid secretion is less decreased due to postoperatively enhanced G cell function along with a smaller decrease in gastrin levels[24,25]. In contrast, there were considerable reductions in the secretion of gastrin and acid, and the absorption of dietary iron is limited when food bypasses the duodenum after LADG with Billroth II anastomosis. For these reasons, there is a greater potential for improvements in postoperative anemia after LAPPG when compared to LADG with Billroth II anastomosis[26]. In our study, physical and role functions were significantly or marginally worse in the LADG group (P = 0.032, 0.07), and the lower functional scores might be associated with the lower hemoglobin levels.
Regarding endoscopic findings, less than 10% of the patients experienced bile reflux, and more than half of the patients experienced food residue after LAPPG. The low proportion of bile reflux and the high proportion of food residue after LAPPG that were shown in this study were also observed in a previous study that reported proportions of approximately 1% bile reflux and 48% residual food, as shown by endoscopic evaluations performed 12 months after LAPPG[27].
The QOL results showed that abdominal pain and reflux symptom scores were significantly higher in the LAPPG than in the LADG group, which might be related to gastric stasis. Patients with a high degree of residual food are more likely to experience gastric fullness and reflux. The pain scores of the EORTC QLQ STO-22 indicate high levels of discomfort when eating, severe pain in the stomach area, and a bloated feeling in the abdomen (Q34-37), and all of these symptoms could result from gastric stasis.
The QOL data were collected from patients who visited the outpatient clinic no earlier than October 2016, and the time points for answering the questionnaire varied between patients. To reduce this time point variability, we excluded the QOL data of patients who underwent surgery within 2 years because the early portion of the QOL data displayed a wide range of fluctuation. The long-term QOL data (two years or more after the operation) are expected to be stable for patients and show little differences between time points. Thus, comparing the QOL data between the LAPPG and LADG groups might be clinically acceptable despite of the variation in time.
Another limitation of this study is that we could not collect all patients’ QOL data, which can result in selection bias. This study was retrospectively performed, and complete QOL data collection was impossible due to the limited number of interviewers and interview time. Therefore, future studies with complete QOL datasets are required to reach more reliable conclusions.
In conclusion, LAPPG was beneficial to the recovery of postoperative anemia and bile reflux, however, it might be unfavorable in terms of long-term pain and reflux symptoms compared to LADG with Billroth II anastomosis. When a physician informs all possible advantages and disadvantages to a patient, and the patient wants to undergo LAPPG, LAPPG could be a treatment option for middle third early gastric cancer.
Laparoscopy-assisted pylorus-preserving gastrectomy (LAPPG) was known to have benefits of function-preserving surgery compared to laparoscopy-assisted distal gastrectomy (LADG). However, in clinical settings, delayed gastric emptying and esophageal reflux following LAPPG can be serious issues, making surgeons reluctant to perform LAPPG.
It is unclear that LAPPG had better long-term functional outcomes and quality of life compared to LADG.
To evaluate the long-term functional outcomes and patient-reported quality of life of LAPPG compared to those of LADG with Billroth II anastomosis.
We reviewed the clinicopathological data of 195 patients who underwent LADG with Billroth II anastomosis and 101 patients who underwent LAPPG for cT1N0 gastric cancer in the middle third of the stomach between 2012 and 2015. Postoperative complications, nutritional parameters, and survey results of the EORTC QLQ C30 and STO22 questionnaire were compared between the two groups.
The serum hemoglobin level was significantly higher in the LAPPG group than in the LADG group (P < 0.001). In the endoscopic findings, incidence of bile reflux was lower (P < 0.001); however, the incidence of residual food was higher in the LAPPG group than in the LADG group (P < 0.001). Regarding the quality of life score, the LAPPG group had a better physical functioning score (86.7 vs 90.0, P = 0.032) but also greater pain and reflux when compared to the LADG group (8.3 vs 16.7 in pain, 11.1 (interquartile range, 0, 22.2) vs 11.1 (interquartile range, 11.1, 33.3) in reflux, P = 0.034 and 0.001, respectively).
LAPPG is beneficial to recovery of anemia and to bile reflux, however, it might be unfavorable in terms of pain and reflux symptoms compared to LADG with Billroth II anastomosis.
LAPPG has both advantages and disadvantages in reference to long-term functional outcomes. When a physician informs all possible advantages and disadvantages to a patient, and the patient wants to undergo LAPPG, LAPPG could be a treatment option for middle third early gastric cancer. Additional large-scale study is needed to determine the functional superiority of LAPPG and patient-reported QOL.
We appreciate contributions from Hyunju Cho, BS, Deok Hee Kim, BS, and Yang Seung Geun, BS Center for Gastric Cancer, National Cancer Center for QOL Data Collection. No individual received direct compensation for involvement in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tsunoda S, Shiryajev YN S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Maki T, Shiratori T, Hatafuku T, Sugawara K. Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery. 1967;61:838-845. [PubMed] |
| 2. | Kodama M, Koyama K. Indications for pylorus preserving gastrectomy for early gastric cancer located in the middle third of the stomach. World J Surg. 1991;15:628-33; discussion 633-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 3. | Xiao XM, Gaol C, Yin W, Yu WH, Qi F, Liu T. Pylorus-Preserving versus Distal Subtotal Gastrectomy for Surgical Treatment of Early Gastric Cancer: A Meta-Analysis. Hepatogastroenterology. 2014;61:870-879. [PubMed] |
| 4. | Song P, Lu M, Pu F, Zhang D, Wang B, Zhao Q. Meta-analysis of pylorus-preserving gastrectomy for middle-third early gastric cancer. J Laparoendosc Adv Surg Tech A. 2014;24:718-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 5. | Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 505] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 6. | Kim YW, Yoon HM, Eom BW, Park JY. History of minimally invasive surgery for gastric cancer in Korea. J Gastric Cancer. 2012;12:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Urushihara T, Sumimoto K, Shimokado K, Kuroda Y. Gastric motility after laparoscopically assisted distal gastrectomy, with or without preservation of the pylorus, for early gastric cancer, as assessed by digital dynamic x-ray imaging. Surg Endosc. 2004;18:964-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Hiki N, Kaminishi M. Pylorus-preserving gastrectomy in gastric cancer surgery--open and laparoscopic approaches. Langenbecks Arch Surg. 2005;390:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Hiki N, Shimoyama S, Yamaguchi H, Kubota K, Kaminishi M. Laparoscopy-assisted pylorus-preserving gastrectomy with quality controlled lymph node dissection in gastric cancer operation. J Am Coll Surg. 2006;203:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (2)] |
| 10. | Nunobe S, Hiki N, Fukunaga T, Tokunaga M, Ohyama S, Seto Y, Yamaguchi T. Laparoscopy-assisted pylorus-preserving gastrectomy: preservation of vagus nerve and infrapyloric blood flow induces less stasis. World J Surg. 2007;31:2335-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Tanaka N, Katai H, Saka M, Morita S, Fukagawa T. Laparoscopy-assisted pylorus-preserving gastrectomy: a matched case-control study. Surg Endosc. 2011;25:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Suh YS, Han DS, Kong SH, Kwon S, Shin CI, Kim WH, Kim HH, Lee HJ, Yang HK. Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg. 2014;259:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (2)] |
| 13. | Tomikawa M, Korenaga D, Akahoshi T, Kohshi K, Sugimachi K, Nagao Y, Tsutsumi N, Takenaka K, Kakeji Y, Hashizume M, Maehara Y. Quality of life after laparoscopy-assisted pylorus-preserving gastrectomy: an evaluation using a questionnaire mailed to the patients. Surg Today. 2012;42:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hosoda K, Yamashita K, Sakuramoto S, Katada N, Moriya H, Mieno H, Watanabe M. Postoperative quality of life after laparoscopy-assisted pylorus-preserving gastrectomy compared With laparoscopy-assisted distal gastrectomy: A cross-sectional postal questionnaire survey. Am J Surg. 2017;213:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1896] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 16. | Owens WD, Felts JA, Spitznagel EL. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1437] [Cited by in RCA: 1474] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 17. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. WHO, 2010. |
| 18. | Ajani JA, In H, Sano T, Gaspar LE, Erasmus JJ, Tang LT, Washington MK, Gerdes H, Wittekind CW, Mansfield PF, Rimmer C, Hofstetter WL, Kelsen D, Amin MB. Stomach. Amin MB. In: Amin MB, editor. AJCC Cancer Staging Manual, 8th ed. New York: Springer-Verlag, 2016: 203-220. |
| 19. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8608] [Article Influence: 538.0] [Reference Citation Analysis (0)] |
| 20. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11461] [Article Influence: 358.2] [Reference Citation Analysis (0)] |
| 21. | Fayers PM, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomley A. Brussels: European Organization for Research and Treatment of Cancer 2001; 1-12 EORTC QLQ-C30 Scoring Manual. |
| 22. | Lee JH, Kim CG, Kim YW, Choi IJ, Lee JY, Cho SJ, Kim YI, Eom BW, Yoon HM, Ryu KW. Botulinum Toxin Injection for the Treatment of Delayed Gastric Emptying Following Pylorus-Preserving Gastrectomy: an Initial Experience. J Gastric Cancer. 2017;17:173-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 23. | Jiang X, Hiki N, Nunobe S, Fukunaga T, Kumagai K, Nohara K, Sano T, Yamaguchi T. Postoperative outcomes and complications after laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Ann Surg. 2011;253:928-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 24. | Annibale B, Capurso G, Lahner E, Passi S, Ricci R, Maggio F, Delle Fave G. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut. 2003;52:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Chen YQ, Guo WH, Chen ZM, Shi L, Chen YX. Effect of gastrectomy on G-cell density and functional activity in dogs. World J Gastroenterol. 2000;6:419-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 26. | Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106:1559S-1566S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 438] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 27. | Furukawa H, Ohashi M, Honda M, Kumagai K, Nunobe S, Sano T, Hiki N. Preservation of the celiac branch of the vagal nerve for pylorus-preserving gastrectomy: is it meaningful? Gastric Cancer. 2018;21:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (1)] |