Published online Sep 21, 2019. doi: 10.3748/wjg.v25.i35.5310
Peer-review started: May 27, 2019
First decision: July 21, 2019
Revised: July 30, 2019
Accepted: August 7, 2019
Article in press: August 7, 2019
Published online: September 21, 2019
Processing time: 122 Days and 2.5 Hours
Long non-coding RNAs (lncRNAs) play important roles in many diseases, including hepatocellular carcinoma (HCC). Autophagy is a metabolic pathway that facilitates cancer cell survival in response to stress. The relationship between autophagy and the lncRNA-activated by transforming growth factor beta (lncRNA-ATB) in HCC remains unknown.
To explore the influence of lncRNA-ATB in regulating autophagy in HCC cells and the underlying mechanism.
In the present study, we evaluated lncRNA-ATB expression in tumor and adjacent non-tumor tissues from 72 HCC cases by real-time PCR. We evaluated the role of lncRNA-ATB in the proliferation and clonogenicity of HCC cells in vitro. The effect of lncRNA-ATB on autophagy was determined using a LC3-GFP reporter and transmission electron microscopy. Furthermore, the mechanism by which lncRNA-ATB regulates autophagy was explored by immunofluorescence staining, RNA immunoprecipitation (RIP), and Western blot.
The expression of lncRNA-ATB was higher in HCC tissues than in normal liver tissues, and lncRNA-ATB expression was positively correlated with tumor size, TNM stage, and poorer survival of patients with HCC. Moreover, ectopic overexpression of lncRNA-ATB promoted cell proliferation and clonogenicnity of HCC cells in vitro. LncRNA-ATB promoted autophagy by activating Yes-associated protein (YAP). Moreover, lncRNA-ATB interacted with autophagy-related protein 5 (ATG5) mRNA and increased ATG5 expression.
LncRNA-ATB regulates autophagy by activating YAP and increasing ATG5 expression. Our data demonstrate a novel function for lncRNA-ATB in autophagy and suggest that lncRNA-ATB plays an important role in HCC.
Core tip: In the present study, we identified the relationship between lncRNA-activated by transforming growth factor beta (lncRNA-ATB) and autophagy in hepatocellular carcinoma (HCC). We demonstrated that lncRNA-ATB promoted autophagic flux in HCC cells. We found that lncRNA-ATB regulated autophagy by activating Yes-associated protein and increasing autophagy-related protein 5 expression. Our findings provide a novel link between lncRNA-ATB and autophagy, and suggest that lncRNA-ATB may be a potential therapeutic target in the treatment of HCC.
- Citation: Wang CZ, Yan GX, Dong DS, Xin H, Liu ZY. LncRNA-ATB promotes autophagy by activating Yes-associated protein and inducing autophagy-related protein 5 expression in hepatocellular carcinoma. World J Gastroenterol 2019; 25(35): 5310-5322
- URL: https://www.wjgnet.com/1007-9327/full/v25/i35/5310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i35.5310
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related mortality among males, and is the fifth most common cancer worldwide[1,2]. Although therapy for HCC has seen significant improvement in recent years, clinical outcome prognosis remains poor for patients with HCC. A large number of aberrantly expressed genes influence the progression of HCC, but the molecular mechanisms governing HCC malignancy are still not entirely clear, and the potential connection between long non-coding RNAs (lncRNAs) and autophagy remains to be fully elucidated. There is an important and unmet need to elucidate molecular mechanisms of autophagy, and to capitalize on that knowledge to develop autophagy-related methods as therapeutic strategies for treatment of HCC.
LncRNAs are a class of RNA transcripts that are longer than 200 nucleotides and exhibit limited protein-coding capacity[3]. LncRNAs regulate many aspects of cancer progression and can influence different malignant behaviors, including cancer cell proliferation, apoptosis, metastasis, glycolysis, and angiogenesis[4,5]. LncRNA-activated by transforming growth factor beta (lncRNA-ATB) is a lncRNA transcript regulated by transforming growth factor beta signaling; it mediates induction of epithelial-mesenchymal transition (EMT) downstream of transforming growth factor beta signaling by competitively binding to members of the miR-200 family[6]. Recently, up-regulation of lncRNA-ATB was reported in a variety of human cancers, and was found to influence a multitude of cellular functions in cancer cells[7]. Emerging reports have identified the role of lncRNAs in regulating autophagy[8], but the involvement of lncRNA-ATB in autophagy in HCC is not entirely clear.
Autophagy is an evolutionarily conserved catabolic process that regulates the coordinated lysosomal degradation of cellular components and damaged organelles. Autophagy can support cell survival and maintenance of homeostasis in response to different forms of stress, such as hypoxia, or deprivation of nutrients and energy[9]. Additionally, autophagy can promote the invasion and migration of HCC cells[10,11]. Nevertheless, whether and how autophagy facilitates cancer progression remains controversial[12]. Based on the cytoprotective properties of autophagy in cancer cells, most research into autophagy has focused on exploring the value of autophagy-targeted therapy[13]. There are currently more than 50 randomized controlled trials evaluating the effects of autophagy as relates to cancer therapy. However, the molecular mechanism of autophagy and the exploitation of autophagy as a therapeutic strategy in HCC remain understudied.
In the present study, we evaluated the relationship between lncRNA-ATB and autophagy in HCC. We demonstrated that lncRNA-ATB promotes autophagic flux in HCC cells. We found that lncRNA-ATB regulates autophagy by activating Yes-associated protein (YAP) and increasing autophagy-related protein 5 (ATG5) expression. Our findings provide a novel link between lncRNA-ATB and autophagy, and suggest that lncRNA-ATB may be a potential therapeutic target in the treatment of HCC.
Seventy-two HCC tissue samples and adjacent non-tumor tissue samples were obtained from the Cancer Hospital of China Medical University (Shenyang, China). All patients on this study provided informed consent. The human subject research performed in this study was approved by the Clinical Research Ethics Committee of the Hospital of China Medical University. Fresh patient tissue samples were frozen in liquid nitrogen and were immediately stored at -80 °C. The clinical characteristics of the 72 patients with HCC are provided in Table 1.
| Clinical feature | n | LncRNA-ATB | P-value | |
| High expression (n = 36) | Low expression (n = 36) | |||
| Gender | 0.096 | |||
| Male | 41 | 17 | 24 | |
| Female | 31 | 19 | 12 | |
| Age (yr) | 0.617 | |||
| ≤ 60 | 48 | 23 | 25 | |
| > 60 | 24 | 13 | 11 | |
| HBsAg | 0.306 | |||
| Negative | 50 | 27 | 23 | |
| Postive | 22 | 9 | 13 | |
| AFP (ng/mL) | 0.216 | |||
| ≤ 400 | 25 | 15 | 10 | |
| > 400 | 47 | 21 | 26 | |
| Tumor size | 0.032a | |||
| ≤ 5cm | 41 | 16 | 25 | |
| > 5cm | 31 | 20 | 11 | |
| TNM stage | 0.017a | |||
| I + II | 30 | 10 | 20 | |
| III + IV | 42 | 26 | 16 | |
| Differentiation | 0.465 | |||
| Well/moderate | 45 | 21 | 24 | |
| Poor | 27 | 15 | 12 | |
The human HCC cell lines SMMC-7721 and HepG2 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). HCC cells were cultured in RPMI 1640 medium (BioWhittaker, Walkersville, MD, United States) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, United States) and 1% penicillin/streptomycin in a humidified atmosphere containing 5% CO2 at 37 °C.
DNA vectors were transfected into cells using the Lipofectamine 3000 reagent (Invitrogen, CA, United States), according to the manufacturer’s instructions. Briefly, pcDNA3.1 or pcDNA3.1-lncRNA-ATB (Genechem, Shanghai, China) was introduced into cells when cell growth reached approximately 80% confluence. Cells were collected 48 h after transfection. Small interfering RNAs (siRNA) targeting YAP were synthesized by Sigma (Shanghai, China) and the sequences used are as follows: YAP siRNA#1: 5′-GACAUCUUCUGGUCAGAGATT-3′ and YAP siRNA#2: 5′-GGUGAUACUAUCAACCAAATT-3′.
Proliferation of HCC cells was measured using a Cell Counting Kit-8 (CCK-8, Dojindo, Japan). First, cells transfected with pcDNA3.1-lncRNA-ATB or pcDNA3.1 were seeded at a density of 3000 cells/well into 96-well plates. Cells were cultured at 37 °C for 0, 24, 48, and 72 h. At these indicated time points, 20 μL of CCK8 solution was added to each well. Plates were then incubated at 37 °C for 2 h. Absorbance was measured at 490 nm using a microplate reader. Data are presented as the mean of three independent experiments.
For colony formation assays, cells transfected with pcDNA3.1-lncRNA-ATB or pcDNA3.1 were plated in 6-well plates and cultured at 37 °C in growth medium containing 10% FBS. After 14 d, colonies were fixed with 4% polyoxymethylene for 10 min and then stained with 0.1% crystal violet solution for 10 min. Colonies were observed using an Olympus microscope (Tokyo, Japan), and the number of colonies was recorded.
Protein lysates were prepared from cells using RIPA buffer supplemented with a protease inhibitor cocktail (Roche, China). Protein lysates were separated by SDS-PAGE and then transferred to PVDF membranes (Sigma, United States). Membranes were then incubated with specific antibodies. Protein expression was assessed using ECL chemiluminescent reagents. The primary antibodies used are as follows: antibodies against ATG5, LC3, and β-actin were purchased from Cell Signaling Technology (United States) and those against phospho-PI3K (Tyr458), PI3K, phospho-AKT (Ser473), AKT, phospho-mTOR (Ser2248), mTOR, phospho-YAP (S127), and YAP were purchased from Abcam (United Kingdom).
For immunofluorescence, cells were fixed with 4% paraformaldehyde (Sigma-Aldrich, United States) for 30 min at room temperature. Cells were then permeabilized with 0.4% Triton X-100 for 5 min at room temperature, and then blocked in 5% bovine serum albumin at 37 ºC for 25 min. Next, cells were incubated with primary antibodies at 4 °C overnight. Following overnight incubation, cells were incubated with specific secondary antibodies for 1 h at 37 °C, and were then washed with PBS three times. After a final wash, nuclei were stained with DAPI for 3 min at room temperature. Immunofluorescence was observed and evaluated using a confocal microscope (Olympus, United States).
HCC cell samples were processed and autophagosomes were visualized by TEM as described previously[14]. In brief, samples were fixed with 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer for 4 h and underwent post-fixation with 1% OsO4 in 0.1 mol/L cacodylate buffer for 2 h. Next, samples were dehydrated, embedded in resin-propylene oxide, and sectioned using a Leica UFC6 ultra-thin microtome at 80 nm thickness. Finally, images were captured using a Hitachi Model H-7650 transmission electron microscope.
Isolation of total RNA, cDNA reverse transcription, and quantitative real-time PCR were performed as previously described[10]. The PCR amplification primers used are as follows: LncRNA-ATB forward, 5’-CTTCACCAGCACCCAGAGA-3’ and reverse, 5’-AAGACAGAAAAACAGTTCCGAGTC-3’; GAPDH forward, 5’-AAAGATGTG CTTCGAGATGTGT-3’ and reverse, 5’-CACTTTGTCAGTTACCAACGTCA-3’; ATG3 forward 5’-GACCCCGGTCCTCAAGGAA-3’ and reverse, 5’-TGTAGCCCATTGCCATGTTGG-3’; ATG5 forward, AAAGATGTGCTTCGAGATGTGT-3’ and reverse, 5’-CACTTTGTCAGTTACCAACGTCA-3’; ATG7 forward 5’-CAGTTTGCCCCTTTTAGTAGTGC-3’ and reverse, 5’-CCAGCCGATACTCGTTCAGC-3’; ATG10 forward 5’-AGACCATCAAAGGACTGTTCTGA-3’ and reverse, 5’-GGGTAGATGC TCCTAGATGTGAC-3’; ATG12 forward 5’-CTGCTGGCGACACCAAGAAA-3’ and reverse, 5’-CGTGTTCGCTCTACTGCCC-3’; ATG16L forward 5’-AAGAAACGTGGGGAGTT AGC-3’ and reverse, 5’-AGAGACAGAGCGTCTCCCAA-3’.
HepG2 and SMMC-7721 cells were co-transfected with pcDNA3.1-MS2, pcDNA3.1-ATB-MS2, pcDNA3.1-ATB-MS2-mut (ATG5), and pMS2-GFP. The plasmids were synthesized by Genechem (Shanghai, China). After transfection for 48 h, cells were cultured to use in experiments for RIP. RIP was performed using a GFP antibody and the Magna RIPTM RNA-Binding protein, according to the manufacturer’s instructions.
All of data analyses were performed using SPSS software (version 17.0, SPSS). Data are presented as the mean ± SD. Significant differences between groups were analyzed using the Student’s t-test. Chi-square tests were performed to determine the relationship between lncRNA-ATB and clinicopathological characteristics. Pearson’s correlation analysis was used to evaluate correlations in expression between two genes. Kaplan-Meier survival analysis was used to evaluate overall survival, and the log-rank test was used to determine differences in survival between groups. Differences were considered to be statistically significant at aP < 0.05 and bP < 0.01.
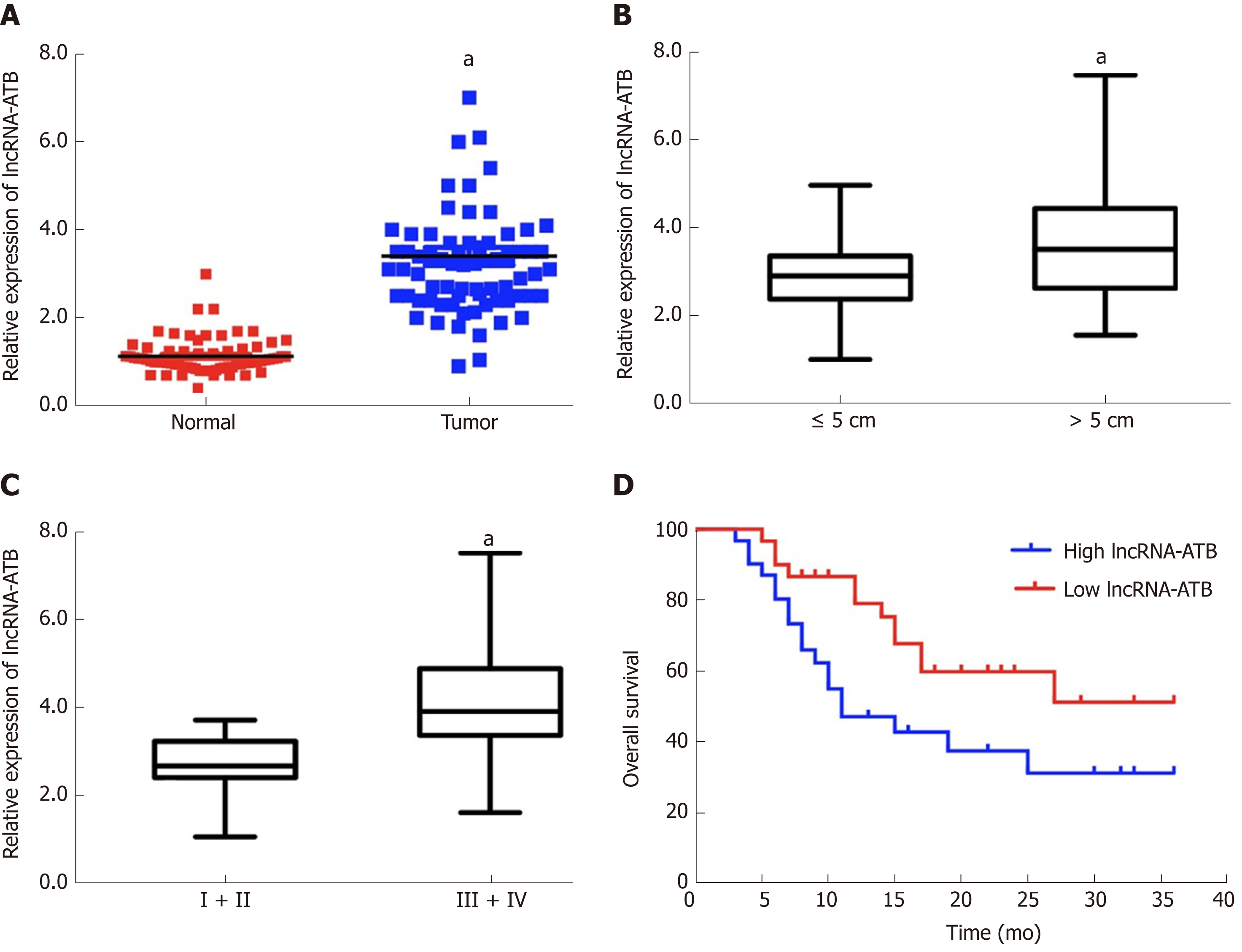
We evaluated expression profiles of lncRNA-ATB in HCC samples by measuring lncRNA-ATB levels in 72 pairs of HCC and adjacent non-tumor tissues by qRT-PCR. LncRNA-ATB was expressed at higher levels in HCC tissues than in non-tumor tissue (Figure 1A). High expression of lncRNA-ATB was associated with larger tumor size and advanced TNM stage. The expression of lncRNA-ATB in patients with HCC tumors larger than 5 cm was significantly higher than in patients with tumor size smaller than 5 cm (Figure 1B). LncRNA-ATB was significantly higher in HCC patients with advanced TNM stage (III/IV) than in patients with local TNM stage (I/II) (Figure 1C). We stratified the 72 patients into a high lncRNA-ATB group and a low lncRNA-ATB group, based on the median expression of lncRNA-ATB. We then examined the relationship between lncRNA-ATB expression and prognosis in patients with HCC. Kaplan-Meier survival analysis revealed that patients with higher lncRNA-ATB expression had a poorer prognosis (Figure 1D). These results indicated that lncRNA-ATB is overexpressed in HCC compared to normal tissues, and that higher lncRNA-ATB expression predicts poor survival for patients with HCC.
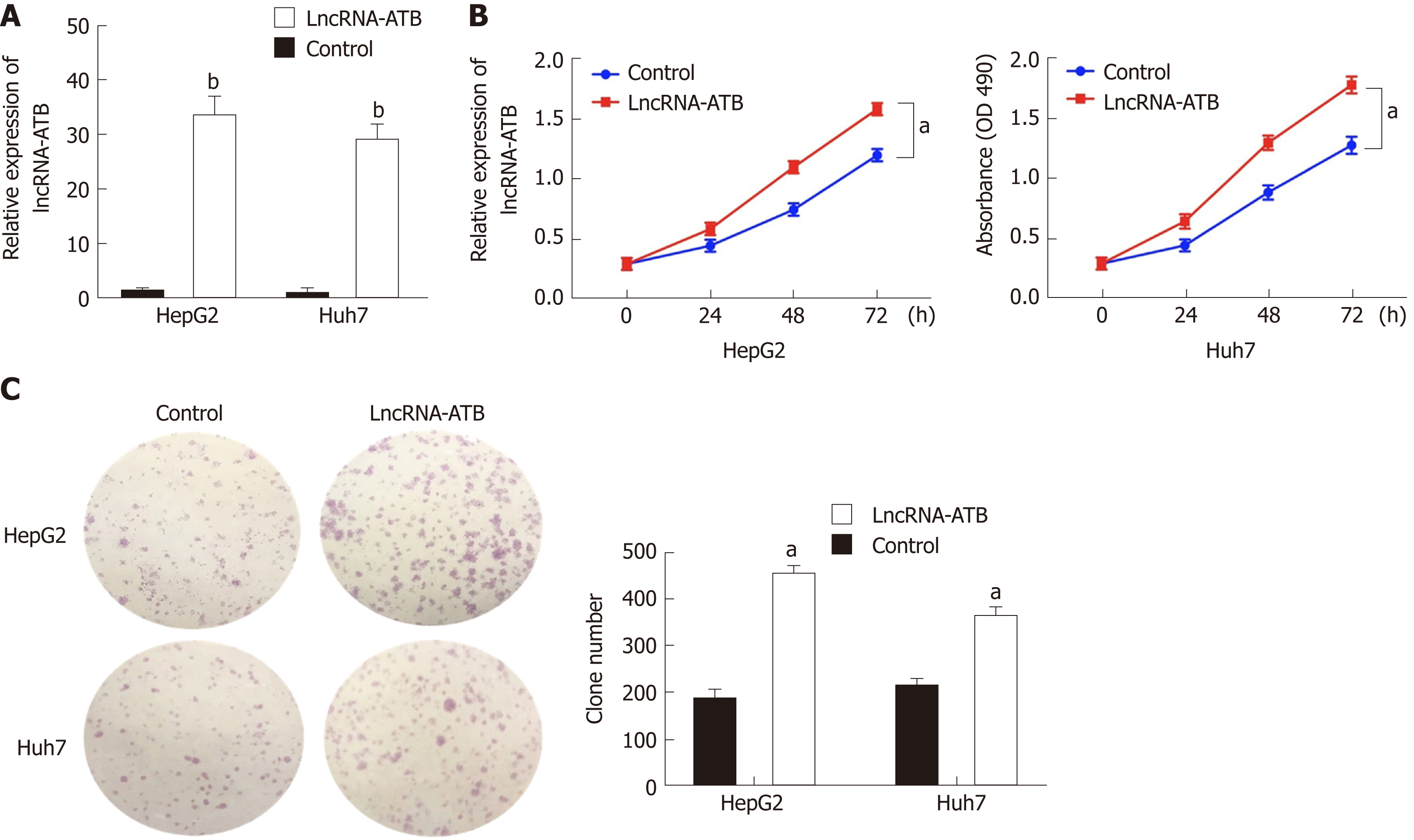
To explore the function of lncRNA-ATB in HCC cells, we established lncRNA-ATB overexpressing cell lines by transfecting SMMC-7721 and HepG2 cells with pcDNA3.1-ATB. At 48 h post-transfection, lncRNA-ATB expression was about 29-fold and 34-fold higher than that in cells transfected with negative control (pcDNA3.1), respectively (Figure 2A). Overexpression of lncRNA-ATB significantly promoted HCC cell proliferation, as indicated by the CCK-8 proliferation assay (Figure 2B). In vitro colony formation assays demonstrated that overexpression of lncRNA-ATB increased the number of colonies formed by both SMMC-7721 and HepG2 cells (Figure 2C). These results show that lncRNA-ATB has an oncogenic capacity to facilitate the proliferation and clonogenicity of HCC cells.
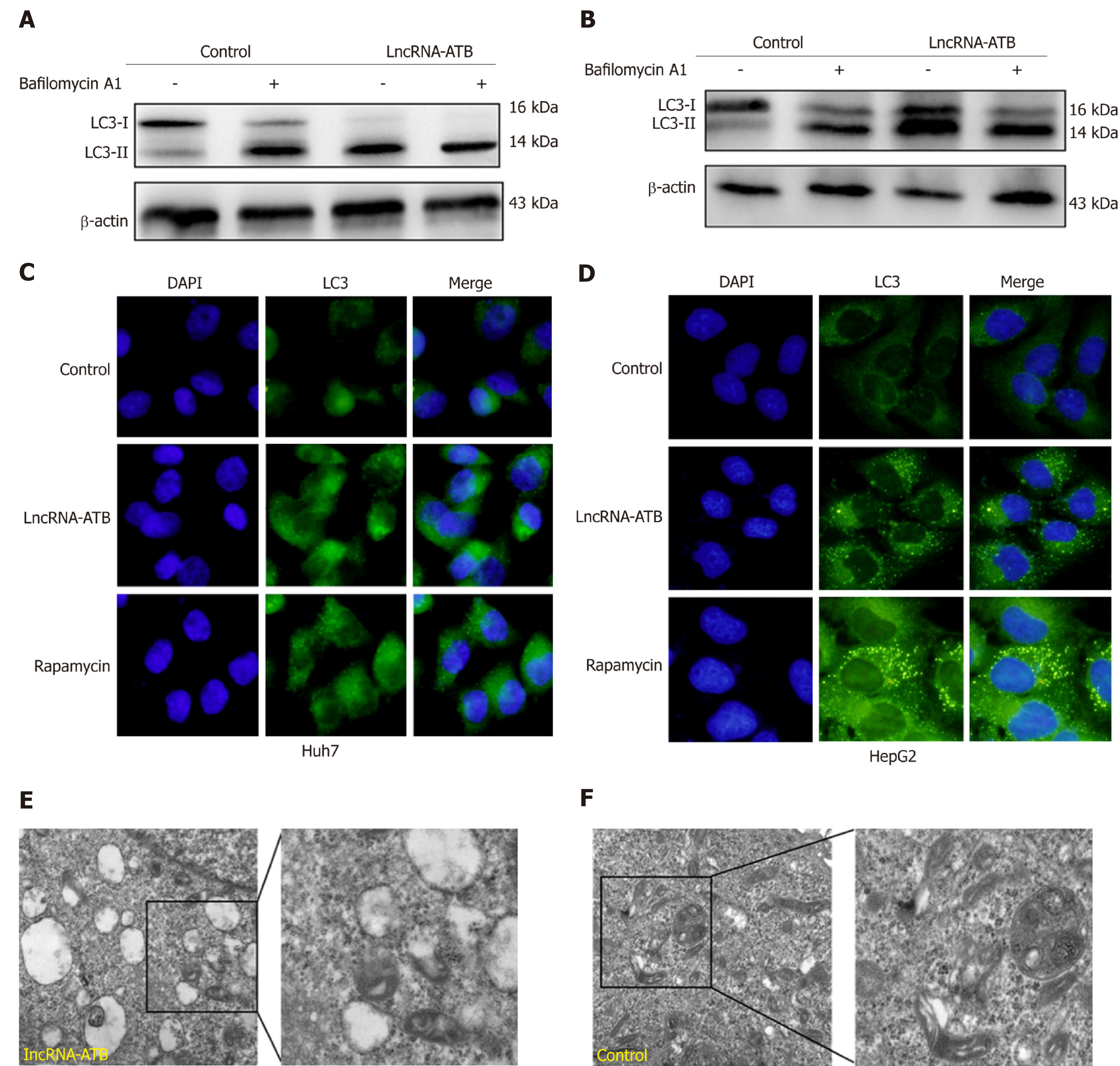
To determine the biological effect of lncRNA-ATB on autophagy, we evaluated the effects of lncRNA-ATB overexpression on autophagy in HCC cells. Overexpression of lncRNA-ATB increased the accumulation of LC3-II, which is generally considered to be a marker of autophagosome formation. Furthermore, we used bafilomycin A1, an inhibitor of the fusion of autophagosomes and lysosomes, to examine autophagic flux by evaluating changes in the degradation of LC3-II. Accumulation of LC3-II was significantly increased in HCC cells treated with bafilomycin A1, after transfection with pcDNA3.1-lncRNA-ATB (Figure 3A and B), indicating that lncRNA-ATB induces autophagic flux. By using a LC3-GFP reporter, the abundance of LC3 puncta was measured, and HCC cells transfected with pcDNA3.1-lncRNA-ATB exhibited similar LC3 puncta accumulation to cells treated with rapamycin (Figure 3C and D). TEM analysis detected autophagosomes and autolysosomes in HCC cells overexpressing lncRNA-ATB, and fewer were observed in negative control cells (Figure 3E and F). These results indicate that lncRNA-ATB promotes autophagic flux and autolysosome formation.
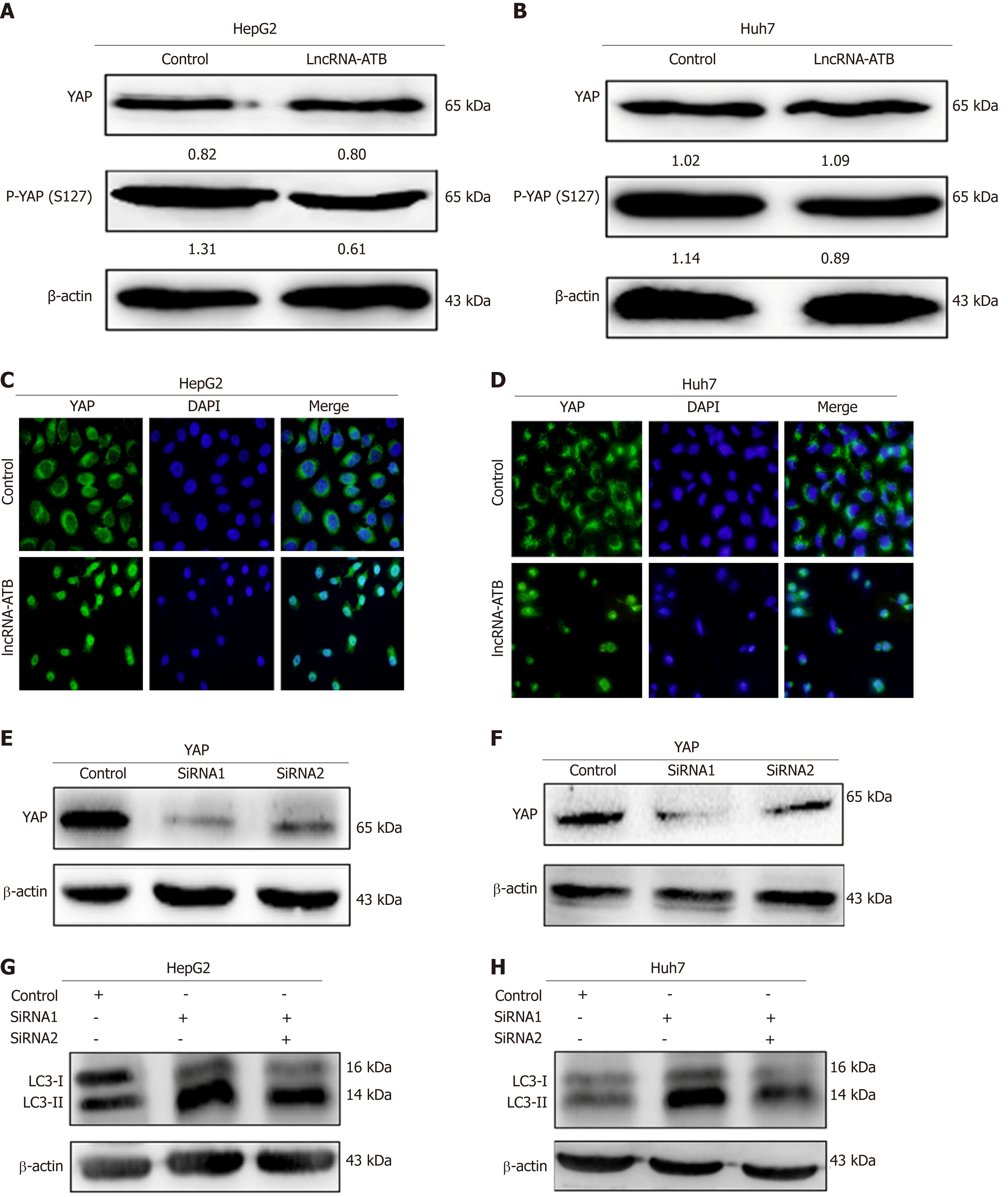
YAP is a key coactivator of the Hippo pathway, and has been demonstrated to promote cancer cell survival by enhancing autophagic flux[15]. Moreover, a growing number of studies have found that lncRNAs are able to regulate Hippo-YAP signaling in cancer cells. We therefore hypothesized that lncRNA-ATB promotes autophagy by activating YAP. To explore this, we measured YAP expression in HCC cells overexpressing lncRNA-ATB, and found that overexpression of lncRNA-ATB did not change the expression of total YAP protein, but decreased p-YAP expression in SMMC-7721 and HepG2 cells (Figure 4A and B). In addition, consistent with the decrease in p-YAP, overexpression of lncRNA-ATB induced YAP translocation from the cytoplasm to the nucleus, as indicated by immunofluorescence (Figure 4C and D). These data demonstrate that lncRNA-ATB induces nuclear translocation of YAP. To determine whether lncRNA-ATB promotes autophagy by activating YAP, we inhibited YAP expression with siRNA. YAP expression was significantly decreased by si-YAP1, as demonstrated by Western blot analysis (Figure 4E and F). Consistently, we found that YAP knockdown partially attenuated lncRNA-ATB-induced activation of autophagy in HCC cells (Figure 4G and H). These results reveal that lncRNA-ATB promotes autophagy in HCC cells by modulating YAP activation.
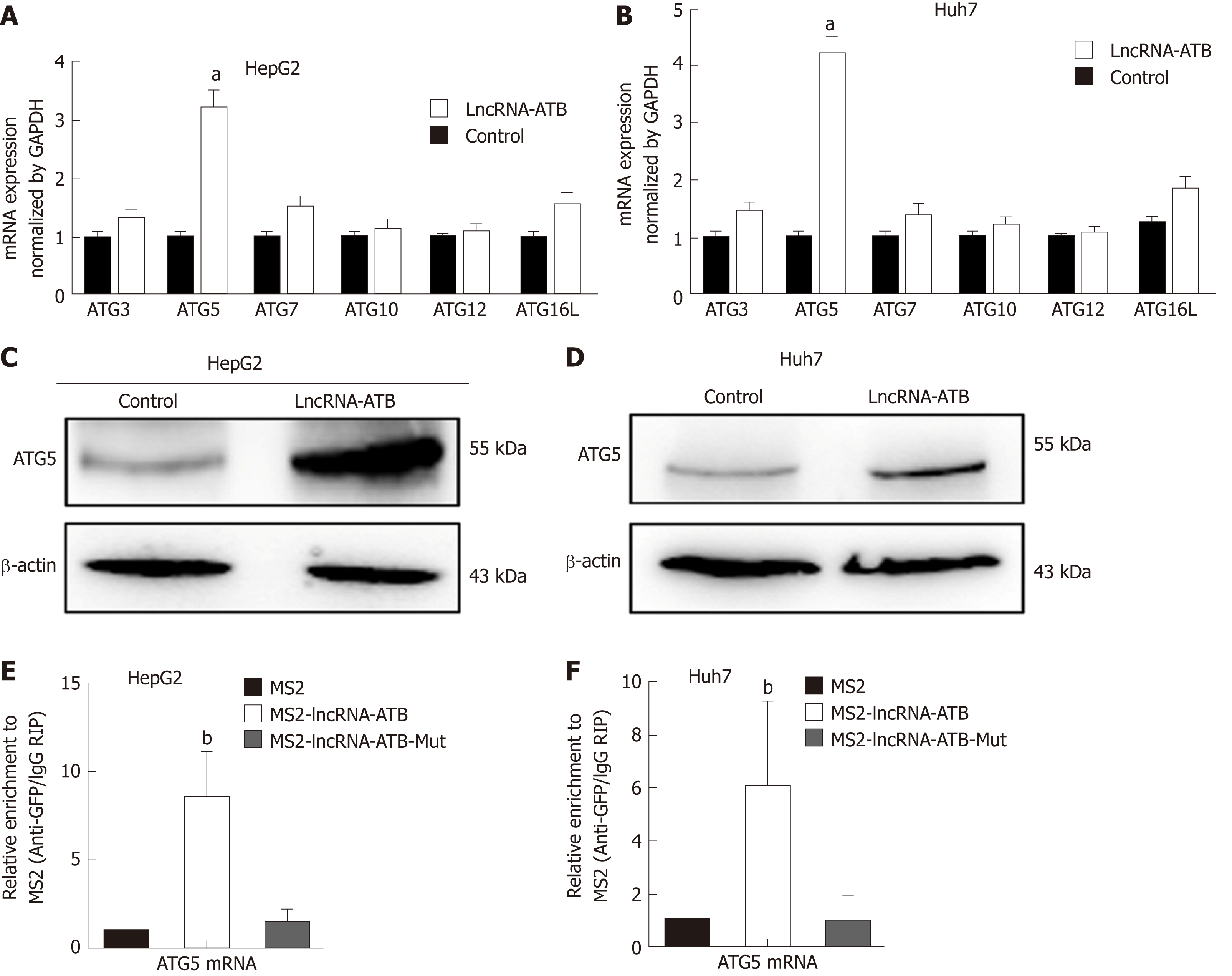
Autophagy is a dynamic, continuous, and tightly coordinated metabolic process that is regulated by a series of autophagy-related genes (ATGs). ATG proteins are essential for the formation of the autophagosome, and are crucial for delivery of autophagic cargo to fuse with the lysosome[16]. To determine which ATG genes are involved in regulation of autophagy by lncRNA-ATB, we used real-time PCR to evaluate the expression of ATG genes in HCC cells following overexpression of lncRNA-ATB. ATG5 mRNA expression was significantly up-regulated by lncRNA-ATB overexpression (Figure 5A and B). In addition, Western blot analysis confirmed that ATG5 protein expression was increased by lncRNA-ATB overexpression (Figure 5C and D). Next, we explored the mechanism by which lncRNA-ATB regulates ATG5. LncRNAs can interact with mRNAs to increase mRNA stability, which promotes gene expression[6,17]. We used BLAST to find potential binding sites between lncRNA-ATB and the ATG5 mRNA, and identified several regions of high complementarity. We used RIP to investigate direct interactions of lncRNA-ATB and ATG5 mRNA, and found that LncRNA-ATB was significantly enriched for ATG5 mRNA compared to the empty vector or to lncRNA-ATB with mutated binding sites (Figure 5E and F). We then determined the expression correlation between ATG5 and lncRNA-ATB in HCC tissue. These demonstrate that lncRNA-ATB interacts with ATG5 mRNA and regulates autophagy by increasing ATG5 expression.
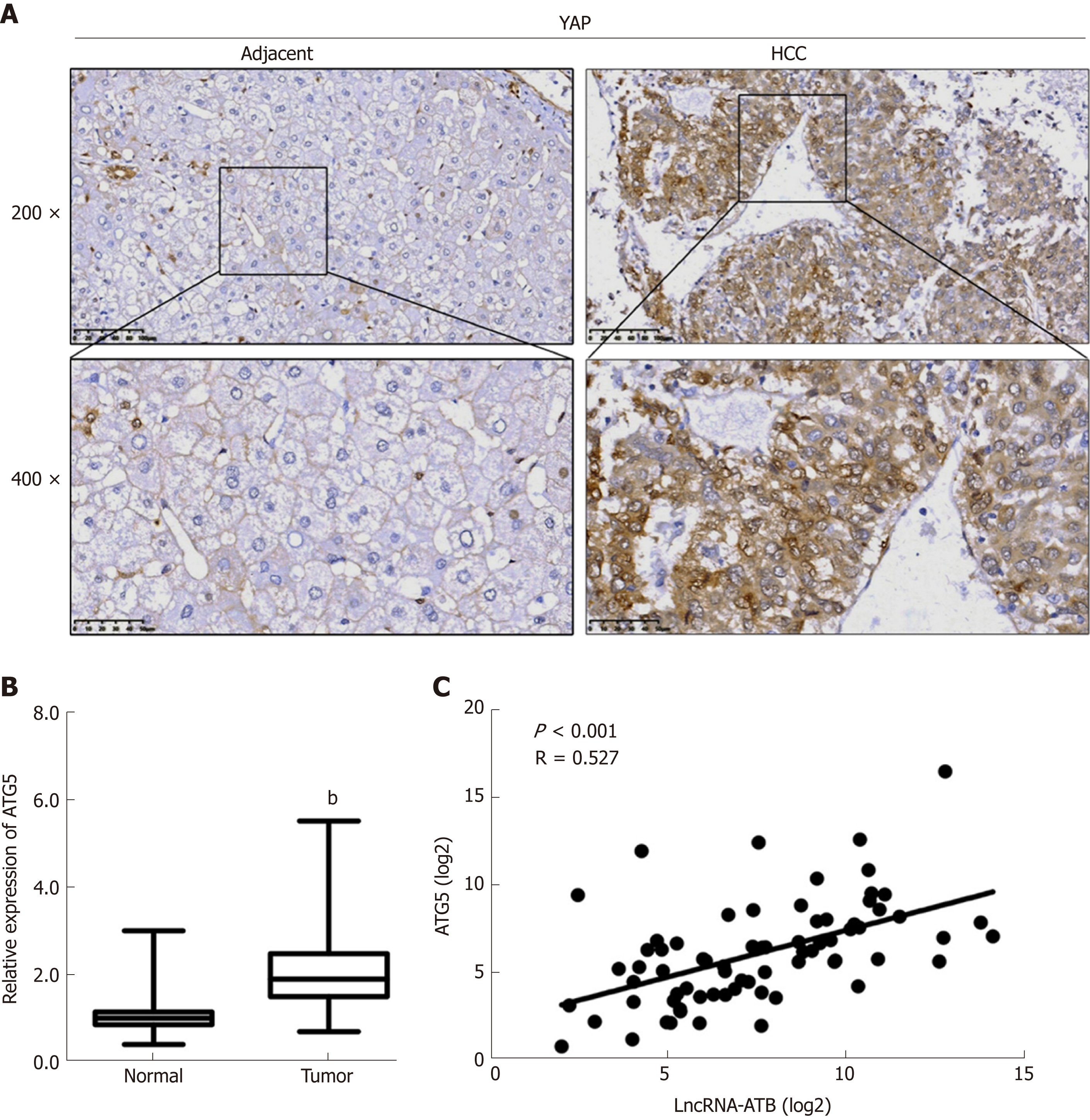
We examined YAP expression in 72 HCC tissues by immunohistochemistry. We found that 62.5% (45/72) of HCC samples showed stronger YAP staining than adjacent non-tumor tissues (Figure 6A). YAP localization showed stronger nuclear staining in HCC tissues than in normal tissues. We then evaluated ATG5 expression by real-time PCR and found that ATG5 expression was significantly higher in HCC tissues compared with adjacent normal tissues (Figure 6B). Additionally, we determined the correlation between the expression of lncRNA-ATB and ATG5, and found that ATG5 mRNA expression was positively correlated with lncRNA-ATB expression (Figure 6C).
LncRNAs play vital roles in tumor progression[18], and more and more lncRNAs are being shown to influence tumor cells biology[19]. In the present study, we found that lncRNA-ATB is expressed at high levels in HCC tissues. We report that elevated expression of lncRNA-ATB associates with increased tumor size and higher TNM stage, suggesting that lncRNA-ATB may serve as a prognostic biomarker to identify those HCC patients who are at higher risk of disease progression. In previous studies, lncRNA-ATB was found to be overexpressed in various tumors of the digestive system, such as gastric cancer, colorectal cancer, and pancreatic cancer[20-22]. In gastric cancer, Iguchi et al[21] found that expression of lncRNA-ATB significantly correlates with postoperative overall survival of patients with GC, and induced EMT in GC cells. They reported that lncRNA-ATB expression is correlated to tumor size, depth of tumor invasion, and TNM stage in colorectal tumors. In functional experiments, lncRNA-ATB was found to influence multiple biological processes of cancer cells. LncRNA-ATB promotes cell viability, migration, and invasion in T24 cells by regulating miR-126 as a molecular sponge[23]. Here we report that lncRNA-ATB overexpression promotes proliferation of HCC cells. These data suggest that lncRNA-ATB plays an oncogenic role in HCC progression.
Autophagy can be considered a kind of metabolic reprogramming pathway, and is a topic of increasing relevance to cancer research. The role of autophagy as an anti-tumor or tumor-promoting pathway is context dependent[24], and emerging studies have indicated that autophagy can facilitate survival of tumor cells in response to stress[9,25]. Reports have also demonstrated that non-coding RNAs can act as regulators in the autophagy pathway[8]. In our current study, we analyzed the potential link between lncRNA-ATB and autophagy. We identified enrichment of autophagy signatures in the gene expression data from SMMC-7721 cells engineered to overexpress lncRNA-ATB. We investigated the influence of lncRNA-ATB on autophagy in HCC cells, and found that overexpression of lncRNA-ATB significantly promoted autophagy flux in HCC cells. The Hippo-YAP pathway regulates autophagy and promotes cancer cell survival during nutrient deprivation[15]. Moreover, lncRNAs play a vital role in activating YAP signaling[26,27]. Therefore, we explored whether lncRNA-ATB induces autophagy by regulating YAP. We found that lncRNA-ATB overexpression decreased p-YAP expression and induced the nuclear translocation of YAP. Rescue experiments showed that lncRNA-ATB promotes autophagy by modulating YAP activation. Our previous study found Hippo-YAP signaling plays an essential role in liver cancer development. Our present data suggest that YAP activation acts as a mediator between lncRNA-ATB expression and autophagy.
Autophagy is a dynamic and continuous process that is regulated by a series of ATG proteins. Yoshinori Ohsumi’s laboratory identified the core ATGs that are essential for formation of the autophagosome and delivery of autophagic cargo to the lysosome[28]. In addition, lncRNAs have been shown to coordinate autophagy by influencing the expression and post-transcriptional regulation of ATG genes[8]. For example, lncRNA PVT1 activates autophagy in glioma vascular endothelial cells by upregulating the expression of ATG7 and beclin1[29]. In our study, we found that lncRNA-ATB increases ATG5 expression by interacting with ATG5 mRNA. ATG5 is part of the ATG12-ATG5–ATG16L1 complex, and it enhances ATG3-mediated conjugation of ATG8 family proteins, resulting in phagophore elongation[30]. Previous studies have also found that lncRNA HNF1A-AS1 functions as an oncogene and promotes autophagy by regulating ATG5. Our results indicate that lncRNA-ATB promotes autophagy by regulating ATG5 expression.
In summary, our study elucidates the role of lncRNA-ATB in regulating autophagy in HCC. We report that lncRNA-ATB is often overexpressed in HCC tissues and acts as an oncogene to facilitate the progression of HCC. Our data show that lncRNA-ATB promotes autophagy by modulating YAP activation. Additionally, we report that lncRNA-ATB interacts with ATG5 mRNA and influences autophagy by increasing the expression of ATG5. Our study provides novel insights into the molecular mechanisms by which lncRNA-ATB regulates autophagy in HCC cells.
Long non-coding RNAs (lncRNAs) play a vital role in the progression of hepatocellular carcinoma (HCC). Autophagy is a dynamic metabolic process that supports cancer cell survival in response to stress. The relationship between autophagy and the lncRNA-activated by transforming growth factor beta (lncRNA-ATB) in HCC remains unknown.
A large number of aberrantly expressed genes influence the progression of HCC, but the molecular mechanisms governing HCC malignancy are still not entirely clear, and the potential connection between lncRNAs and autophagy remains to be fully elucidated. There is an important and unmet need to elucidate the molecular mechanisms of autophagy, and to capitalize on that knowledge to develop autophagy-related methods as therapeutic strategies for treatment of HCC.
To explore the influence of lncRNA-ATB in regulating autophagy in HCC cells and the underlying mechanism.
We compared the expression of lncRNA-ATB in 72 HCC tissues and adjacent non-tumor tissues by real-time PCR. The role of lncRNA-ATB in cell proliferation and colony formation was evaluated in vitro. The effect of lncRNA-ATB on autophagy was determined using a LC3-GFP reporter and transmission electron microscopy. Furthermore, the mechanism by which lncRNA-ATB regulates autophagy was explored by immunofluorescence staining, RNA immunoprecipitation, and Western blot.
We found that lncRNA-ATB was up-regulated in HCC and lncRNA-ATB expression positively correlated with tumor size, TNM stage, and poor survival of HCC patients. Overexpression of lncRNA-ATB promoted cell proliferation and colony formation in vitro. LncRNA-ATB promoted autophagy by activating Yes-associated protein (YAP). Moreover, lncRNA-ATB interacted with autophagy-related protein 5 (ATG5) mRNA and increased ATG5 expression.
Our study reveals that lncRNA-ATB regulates autophagy by activating YAP and increasing ATG5 expression. These results provide new insights into the role of lncRNA-ATB in autophagy in HCC.
Our findings provide a novel link between lncRNA-ATB and autophagy, and suggest that lncRNA-ATB may be a potential therapeutic target in the treatment of HCC.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kimkong I S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Ingle PV, Samsudin SZ, Chan PQ, Ng MK, Heng LX, Yap SC, Chai AS, Wong AS. Development and novel therapeutics in hepatocellular carcinoma: a review. Ther Clin Risk Manag. 2016;12:445-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2486] [Article Influence: 248.6] [Reference Citation Analysis (0)] |
| 3. | Evans JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Invest. 2016;126:2775-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 345] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 4. | Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev. 2016;96:1297-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 1328] [Article Influence: 147.6] [Reference Citation Analysis (1)] |
| 5. | Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1470] [Article Influence: 113.1] [Reference Citation Analysis (0)] |
| 6. | Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, Wang SB, Wang YZ, Yang Y, Yang N, Zhou WP, Yang GS, Sun SH. A long noncoding RNA activated by transforming growth factor beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1274] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 7. | Xiao H, Zhang F, Zou Y, Li J, Liu Y, Huang W. The Function and Mechanism of Long Non-coding RNA-ATB in Cancers. Front Physiol. 2018;9:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Sun T. Long noncoding RNAs act as regulators of autophagy in cancer. Pharmacol Res. 2018;129:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Rybstein MD, Bravo-San Pedro JM, Kroemer G, Galluzzi L. The autophagic network and cancer. Nat Cell Biol. 2018;20:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 10. | Fan Q, Yang L, Zhang X, Ma Y, Li Y, Dong L, Zong Z, Hua X, Su D, Li H, Liu J. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2018;37:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 11. | Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo Y, Song Z, Zheng Q, Xiong J. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis. 2013;34:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 12. | Morel E, Mehrpour M, Botti J, Dupont N, Hamaï A, Nascimbeni AC, Codogno P. Autophagy: A Druggable Process. Annu Rev Pharmacol Toxicol. 2017;57:375-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 13. | Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1946] [Article Influence: 243.3] [Reference Citation Analysis (0)] |
| 14. | Yang L, Zhang X, Li H, Liu J. The long noncoding RNA HOTAIR activates autophagy by upregulating ATG3 and ATG7 in hepatocellular carcinoma. Mol Biosyst. 2016;12:2605-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Song Q, Mao B, Cheng J, Gao Y, Jiang K, Chen J, Yuan Z, Meng S. YAP enhances autophagic flux to promote breast cancer cell survival in response to nutrient deprivation. PLoS One. 2015;10:e0120790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Klionsky DJ, Baehrecke EH, Brumell JH, Chu CT, Codogno P, Cuervo AM, Debnath J, Deretic V, Elazar Z, Eskelinen EL, Finkbeiner S, Fueyo-Margareto J, Gewirtz D, Jäättelä M, Kroemer G, Levine B, Melia TJ, Mizushima N, Rubinsztein DC, Simonsen A, Thorburn A, Thumm M, Tooze SA. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy. 2011;7:1273-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | Yoon JH, Abdelmohsen K, Srikantan S, Yang X, Martindale JL, De S, Huarte M, Zhan M, Becker KG, Gorospe M. LincRNA-p21 suppresses target mRNA translation. Mol Cell. 2012;47:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 790] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 18. | Schmitt AM, Chang HY. Long Noncoding RNAs in Cancer Pathways. Cancer Cell. 2016;29:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2202] [Cited by in RCA: 2419] [Article Influence: 268.8] [Reference Citation Analysis (0)] |
| 19. | Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao SD, Zhang Y, Yang L, Shan W, He Q, Fan L, Kandalaft LE, Tanyi JL, Li C, Yuan CX, Zhang D, Yuan H, Hua K, Lu Y, Katsaros D, Huang Q, Montone K, Fan Y, Coukos G, Boyd J, Sood AK, Rebbeck T, Mills GB, Dang CV, Zhang L. Comprehensive Genomic Characterization of Long Non-coding RNAs across Human Cancers. Cancer Cell. 2015;28:529-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 531] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 20. | Lei K, Liang X, Gao Y, Xu B, Xu Y, Li Y, Tao Y, Shi W, Liu J. Lnc-ATB contributes to gastric cancer growth through a MiR-141-3p/TGFβ2 feedback loop. Biochem Biophys Res Commun. 2017;484:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Iguchi T, Uchi R, Nambara S, Saito T, Komatsu H, Hirata H, Ueda M, Sakimura S, Takano Y, Kurashige J, Shinden Y, Eguchi H, Sugimachi K, Maehara Y, Mimori K. A long noncoding RNA, lncRNA-ATB, is involved in the progression and prognosis of colorectal cancer. Anticancer Res. 2015;35:1385-1388. [PubMed] |
| 22. | Qu S, Yang X, Song W, Sun W, Li X, Wang J, Zhong Y, Shang R, Ruan B, Zhang Z, Zhang X, Li H. Downregulation of lncRNA-ATB correlates with clinical progression and unfavorable prognosis in pancreatic cancer. Tumour Biol. 2016;37:3933-3938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Zhai X, Xu W. Long Noncoding RNA ATB Promotes Proliferation, Migration, and Invasion in Bladder Cancer by Suppressing MicroRNA-126. Oncol Res. 2018;26:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Kreuzaler P, Watson CJ. Killing a cancer: what are the alternatives? Nat Rev Cancer. 2012;12:411-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Edinger AL, Thompson CB. Defective autophagy leads to cancer. Cancer Cell. 2003;4:422-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Zhuo W, Kang Y. Lnc-ing ROR1-HER3 and Hippo signalling in metastasis. Nat Cell Biol. 2017;19:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Yang C, Wu K, Wang S, Wei G. Long non-coding RNA XIST promotes osteosarcoma progression by targeting YAP via miR-195-5p. J Cell Biochem. 2018;119:5646-5656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1305] [Cited by in RCA: 1387] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 29. | Ma Y, Wang P, Xue Y, Qu C, Zheng J, Liu X, Ma J, Liu Y. PVT1 affects growth of glioma microvascular endothelial cells by negatively regulating miR-186. Tumour Biol. 2017;39:1010428317694326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 2035] [Article Influence: 339.2] [Reference Citation Analysis (0)] |