Published online Sep 14, 2019. doi: 10.3748/wjg.v25.i34.5185
Peer-review started: May 17, 2019
First decision: July 21, 2019
Revised: August 4, 2019
Accepted: August 19, 2019
Article in press: August 19, 2019
Published online: September 14, 2019
Processing time: 118 Days and 19.8 Hours
Compared with traditional open surgery, laparoscopic surgery is preferred due to the advantages of less trauma, less pain, and faster recovery. Nevertheless, many patients still suffer from postoperative pain resulting from the surgical incision and associated tissue injury. Many researchers have reported methods to improve postoperative pain control, but there is not a simple and effective method that can be clinically adopted in a widespread manner. We designed this study to prove the hypothesis that application of ropivacaine in the port site and operative site in patients is an effective and convenient method which can decrease postoperative pain and accelerate recovery.
To evaluate the effects of ropivacaine on pain control after laparoscopic hepatectomy and its contribution to patient recovery.
From May 2017 to November 2018, 146 patients undergoing laparoscopic hepatectomy were randomized to receive infiltration of either 7.5 mg/mL ropivacaine around the trocar insertions, incision, and cutting surface of the liver (with a gelatin sponge soaked with ropivacaine) at the end of surgery (ropivacaine group), or normal saline (5 mL) at the same sites at the end of surgery (control group). The degree of pain, nausea, vomiting, heart rate (HR), and blood pressure were collected. The length of postoperative hospitalization, complications, and the levels of stress hormones were also compared between the two groups.
Compared with the control group, the ropivacaine group showed reduced postoperative pain at rest within 12 h (P < 0.05), and pain on movement was reduced within 48 h. The levels of epinephrine, norepinephrine, and cortisol at 24 and 48 h, HR, blood pressure, and cumulative sufentanil consumption in the ropivacaine group were significantly lower than those in the control group (P < 0.05). In the ropivacaine group, hospitalization after operation was shorter, but the difference was not statistically significant. There were no significant differences in postoperative nausea, vomiting, or other complications, including hydrothorax, ascites, peritonitis, flatulence, and venous thrombus (P > 0.05), although fewer patients in the ropivacaine group experienced these situations.
Infiltration with ropivacaine in the abdominal wound and covering the cutting surface of the liver with a gelatin sponge soaked with ropivacaine significantly reduce postoperative pain and the consumption of sufentanil.
Core tip: This study confirmed the efficacy of ropivacaine in pain control after laparoscopic hepatectomy and its contribution to fast track recovery surgery. Ropivacaine not only infiltrated the subcutaneous and deep muscle fasciae and peritoneum but also covered the liver cutting surface in a soaked gelatin sponge to relieve the pain caused by capsule injury. We examined the efficacy using not only visual analog scale, but also blood biochemistry and other standards.
- Citation: Zhang H, Du G, Liu YF, Yang JH, A-Niu MG, Zhai XY, Jin B. Overlay of a sponge soaked with ropivacaine and multisite infiltration analgesia result in faster recovery after laparoscopic hepatectomy. World J Gastroenterol 2019; 25(34): 5185-5196
- URL: https://www.wjgnet.com/1007-9327/full/v25/i34/5185.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i34.5185
For hepatectomy, laparoscopic surgery is preferred in the clinic with the advantages of less trauma, less pain, faster recovery, and a shorter hospital stay than traditional open surgery[1]. However, many patients still suffer from postoperative pain resulting from the surgical incision and associated tissue injury, which lengthens the bedridden time and increases the risk of complications. Therefore, optimal and reliable analgesia for postoperative pain control is very important. Thus, many researchers have reported methods to improve postoperative pain control, accelerate recovery, and decrease hospital stays, such as local anesthesia at the trocar site[2], decreased pneumoperitoneum[3], and intraperitoneal nebulization of anesthetic[4]. Therefore, many methods have been developed and have proven useful, but there is not a simple and effective method that can be clinically adopted in a widespread manner. The focal point of the controversy regarding local anesthesia after an operation centers around the following questions: What the anesthetic is, what the concentration is, and where it should be used. Most previous trials have estimated the degree of analgesia using a visual analog scale (VAS), which is rather subjective. Therefore, we designed this case-control study to examine the efficacy of multisite infiltrative analgesia in trocar sites and sponges soaked with ropivacaine after laparoscopic hepatectomy using VAS, blood biochemistry, and other standards.
To evaluate the effects of ropivacaine on pain control after laparoscopic hepatectomy, we designed a randomized, double-blind, placebo-controlled clinical trial to find an effective and convenient method to decrease postoperative pain and accelerate recovery.
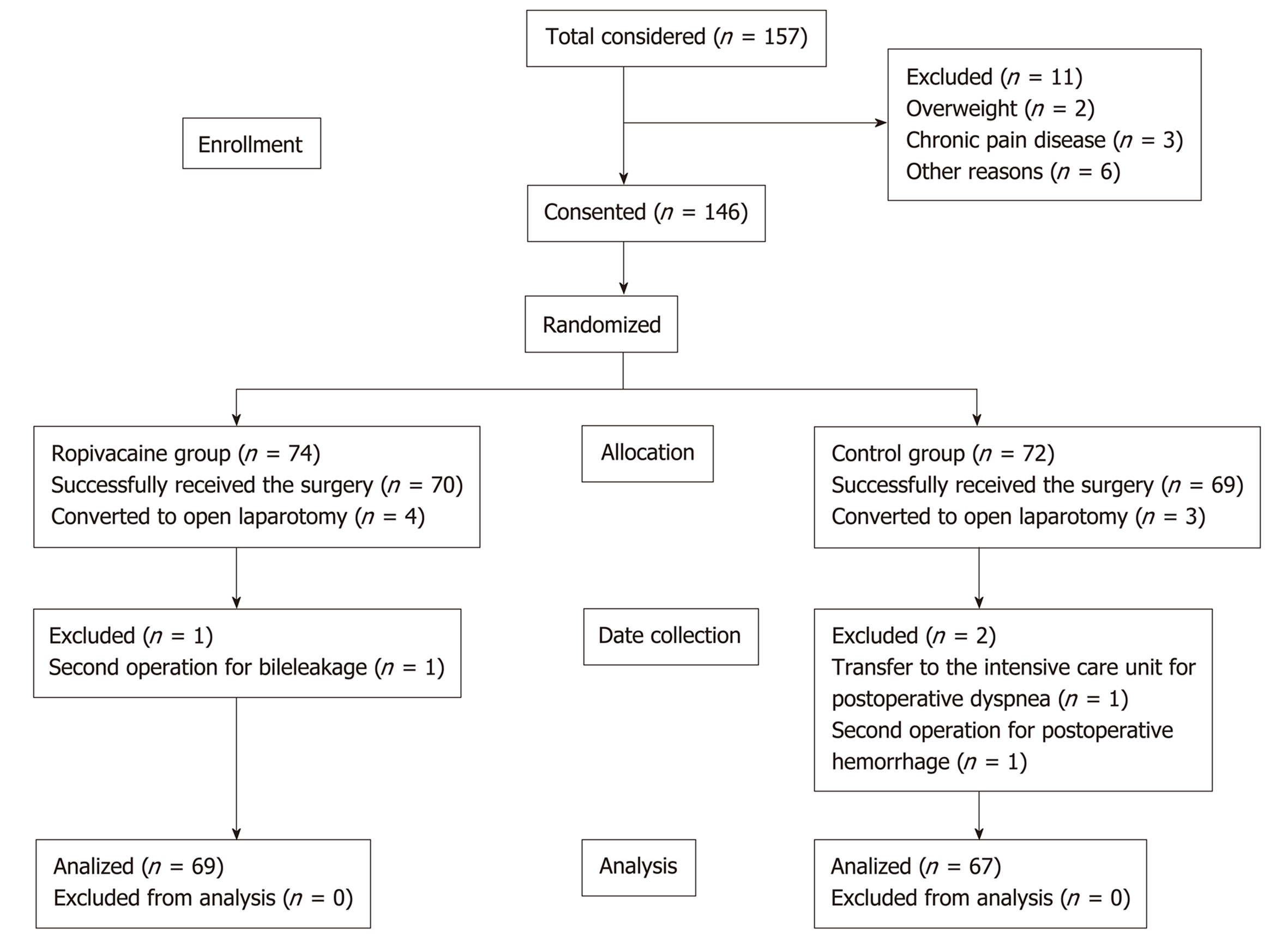
The study was approved by the Ethics Committee of Qilu Hospital of Shandong University (No. 2017052). From May 2017 to November 2018, 146 patients (age range: 20-80 years; weight: 50-90 kg) undergoing laparoscopic hepatectomy performed by our team at the Department of Surgery, Qilu Hospital of Shandong University (Jinan, China) were included in this study. All patients signed an informed consent form before the operation. Patients were excluded if they had acute cholecystitis, serious chronic pain disease, history of alcohol abuse, tranquilizer treatment before surgery, cognitive impairment, or history of allergy to any of the drugs used in the study.
Before the start of the study, random sequence was generated with the Statistical Package for Social Sciences (SPSS) 20.0 (SPSS Inc., Chicago, IL, United States). The patients were randomized into two groups with the table of random numbers on the day of surgery. A group of patients received ropivacaine infiltration around the trocar insertion and abdominal wounds for specimen extraction, and another group of patients received the same volume of normal saline; at the end of the operation, uniform disposal was similar across groups.
All patients received standard general anesthesia administered by the same anesthetist not involved in the study. Anesthesia was induced with propofol, midazolam, rocuronium, and sufentanil. General anesthesia was maintained with 2% sevoflurane.
Twenty milliliter solutions containing either 7.5 mg/mL ropivacaine for the ropivacaine group or normal saline for the control group were prepared and provided by an identical anesthetist, and the surgeons were not informed of the contents of the solution or of the patient allocation. Before trocar withdrawal, a gelatin sponge was soaked with 10 mL of the prepared solution and used to cover the cutting surface of the liver. At the end of the surgery, subcutaneous tissue and deep muscle around the trocar incision and abdominal wound for specimen extraction were infiltrated with 10 mL of the solution with a needle introduced through the skin. Finally, one drainage tube was routinely used for the patient, and the tissue surrounding the tube was also infiltrated with the solution. All patients were given a patient-controlled analgesic (PCA) device containing 100 mL of 1 μg/mL sufentanil that was delivered at a rate of 2 µg/h to the venous catheter. When the patient felt pain, they pushed a button on the device, and 0.5 mL of additional sufentanil was delivered. The gelatin sponge used in the two groups were the same (Xiangen Medical Technology Development Co., Ltd., Jiangxi, China).
During the preoperative communication, all patients were taught how to describe their postoperative pain level using a VAS ranging from 0 (no pain) to 10 (maximal pain). Pain was assessed with the VAS at rest and on movement including coughing and leaning to one side. Pain scores, nausea, vomiting, mean blood pressure, and heart rate (HR) were recorded before the operation and at 0 h, 6 h, 12 h, 24 h, and 48 h after the operation. The cumulative consumption of sufentanil by the PCA device was recorded at 6, 12, 24, and 36 h. If the patients experienced nausea or vomiting, metoclopramide was given. Blood was sampled for detection of levels of epinephrine, norepinephrine, and cortisol at 0 h, 24 h, and 48 h after the operation. Quantitative changes in the levels of catecholamines, including adrenaline, noradrenaline, and dopamine, were analyzed. The surgical stress hormones (epinephrine, norepinephrine, and cortisol) were detected using commercial enzyme-linked immunosorbent assay kits (Cusabio Biotech Co., Ltd., Wuhan, China).
A total of 146 patients were included in the study from May 2017 to November 2018. Finally, 69 patients in the ropivacaine group and 67 patients in the control group were included in the statistical analysis.
Continuous data (age, weight, duration of surgery, trocar number, incision length, VAS, and level of hormone) are presented as the mean ± SD. These data were analyzed by t-tests. The P-value and 95% confidence interval (CI) were calculated. Categorical data [sex, American society of anesthesiologists (ASA) grade, and operation type] are presented as the number of patients and were analyzed by chi-square tests. All data were checked for normal distribution. Data analysis was accomplished with SPSS 20.0 (SPSS Inc., Chicago, IL, United States). P < 0.05 was considered statistically significant.
In the end, 139 patients successfully received the surgery, including laparoscopic hepatectomy and the use of the prearranged solution. Four patients and three patients were converted to open laparotomy due to operational difficulties and uncontrolled bleeding during the operation, respectively. Subsequently, three additional patients were excluded from the study: One in the ropivacaine group (because of a second operation for bile leakage) and two in the control group (due to a transfer to the intensive care unit for postoperative dyspnea and a second operation for postoperative hemorrhage). For the statistical analysis, 69 patients in the ropivacaine group and 67 patients in the control group were included.
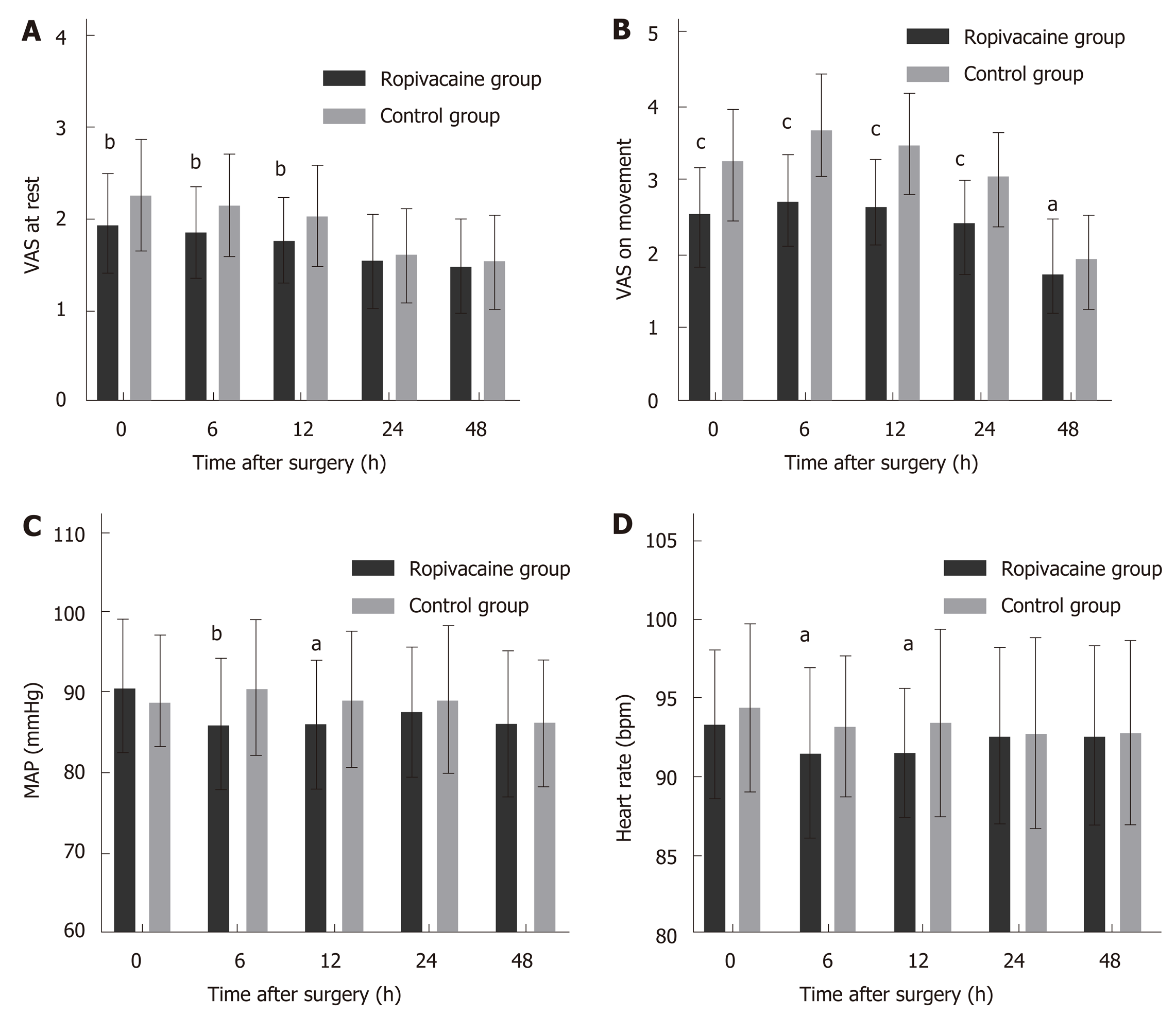
The demographic data, ASA grade, and details of the surgery, including the surgery type, duration, trocar number, and the length of incision for specimen extraction, were similar between the two groups (Table 1). Postoperative pain scores were significantly lower at rest in the ropivacaine group than in the control group, at 0 h, 6 h, and 12 h and significantly lower on movement at 0 h, 6 h, 12 h, and 24 h (P < 0.05). Mean arterial pressure (MAP) was significantly higher in the control group than in the ropivacaine group at 6 h (P = 0.002) and 12 h (P = 0.032) (Figure 1 and Table 2).
| Characteristic | Ropivacaine group | Control group | t/χ2 | P-value |
| Age (yr) | 52.87 ± 12.463 | 50.31 ± 13.984 | 1.126 | 0.2621 |
| Sex | ||||
| Male/female | 38/31 | 38/29 | 38/29 | 0.8469 |
| Weight (kg) | 67.88 ± 11.634 | 65.75 ± 11.992 | 1.051 | 0.2951 |
| ASA grade | ||||
| I/II/III | 8/44/17 | 12/42/13 | 1.351 | 0.5090 |
| Duration of surgery (min) | 156.16 ± 82.748 | 152.75 ± 87.325 | 0.234 | 0.8153 |
| Trocar number | 4.58 ± 0.715 | 4.73 ± 0.770 | -1.190 | -1.190 |
| Incision length (cm) | 5.25 ± 1.035 | 5.45 ± 0.784 | -1.276 | 0.2040 |
| Operation type | ||||
| Lap right hepatectomy | 5 | 6 | 5.230 | 0.1557 |
| Lap left hepatectomy | 21 | 12 | ||
| Lap caudate hepatectomy | 1 | 5 | ||
| Lap irregular hepatectomy | 42 | 44 |
| Characteristic | 0 h | 6 h | 12 h | 24 h | 48 h1 |
| VAS at rest | |||||
| Ropivacaine group | 1.94 ± 0.539 | 1.86 ± 0.493 | 1.77 ± 0.458 | 1.54 ± 0.502 | 1.49 ± 0.504 |
| Control group | 1.49 ± 0.504 | 2.15 ± 0.557 | 2.03 ± 0.550 | 1.63 ± 0.487 | 1.54 ± 0.502 |
| t | -3.155 | -3.362 | -3.019 | -1.068 | -0.516 |
| P-value | 0.0020 | 0.0014 | 0.0030 | 0.2876 | 0.6064 |
| 95%CI | [-0.523, -0.136] | [-0.478, -0.126] | [-0.461, -0.117] | [-0.258, 0.074] | [-0.233, 0.104] |
| VAS on movement | |||||
| Ropivacaine group | 2.55 ± 0.631 | 2.74 ± 0.610 | 2.65 ± 0.614 | 2.42 ± 0.579 | 1.75 ± 0.604 |
| Control group | 3.27 ± 0.687 | 3.70 ± 0.739 | 3.51 ± 0.660 | 3.06 ± 0.574 | 1.97 ± 0.521 |
| t | -6.349 | -8.691 | -7.991 | -6.021 | -1.903 |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0271 |
| 95%CI | [-0.942, -0.494] | [-1.192, -0.733] | [-1.071, -0.639] | [-0.835, -0.444] | [-0.408, -0.025] |
| MAP (mmHg) | |||||
| Ropivacaine group | 90.81 ± 8.432 | 86.00 ± 8.070 | 86.07 ± 7.930 | 87.61 ± 8.037 | 86.17 ± 9.160 |
| Control group | 88.78 ± 8.348 | 90.54 ± 8.412 | 89.15 ± 8.504 | 89.24 ± 9.160 | 86.28 ± 7.792 |
| t | 1.414 | -3.210 | -2.183 | -1.104 | -0.075 |
| P-value | 0.1596 | 0.0017 | 0.0308 | 0.2716 | 0.9402 |
| 95%CI | [-0.811, 4.882] | [-7.333, -1.742] | [-5.864, -0.289] | [-4.550, 1.290] | [-2.998, 2.779] |
| HR (bpm) | |||||
| Ropivacaine group | 93.36 ± 4.706 | 91.57 ± 5.377 | 91.58 ± 4.120 | 92.60 ± 5.592 | 92.63 ± 5.696 |
| Control group | 94.42 ± 5.326 | 93.25 ± 4.427 | 93.46 ± 5.925 | 92.81 ± 6.079 | 92.83 ± 5.828 |
| t | -1.231 | -1.991 | -2.155 | -0.214 | -0.202 |
| P-value | 0.2204 | 0.0486 | 0.0331 | 0.8308 | 0.8406 |
| 95%CI | [-2.768, 0.644] | [-3.348, -0.011] | [-3.617, -0.146] | [-2.197, 1.768] | [-2.154, 1.756] |
| Cumulative sufentanil (µg) | |||||
| Ropivacaine group | 0 | 13.66 ± 2.437 | 27.91 ± 4.176 | 55.60 ± 6.117 | 81.66 ± 7.729 |
| Control group | 0 | 15.43 ± 3.273 | 30.93 ± 6.414 | 61.45 ± 5.405 | 88.89 ± 7.937 |
| t | -3.579 | -3.242 | -5.897 | -5.386 | |
| P-value | 0.0005 | 0.0016 | <0.0001 | <0.0001 | |
| 95%CI | [-2.744, -0.791] | [-4.863, -1.174] | [-7.803, -3.884] | [-9.891, -4.578] |
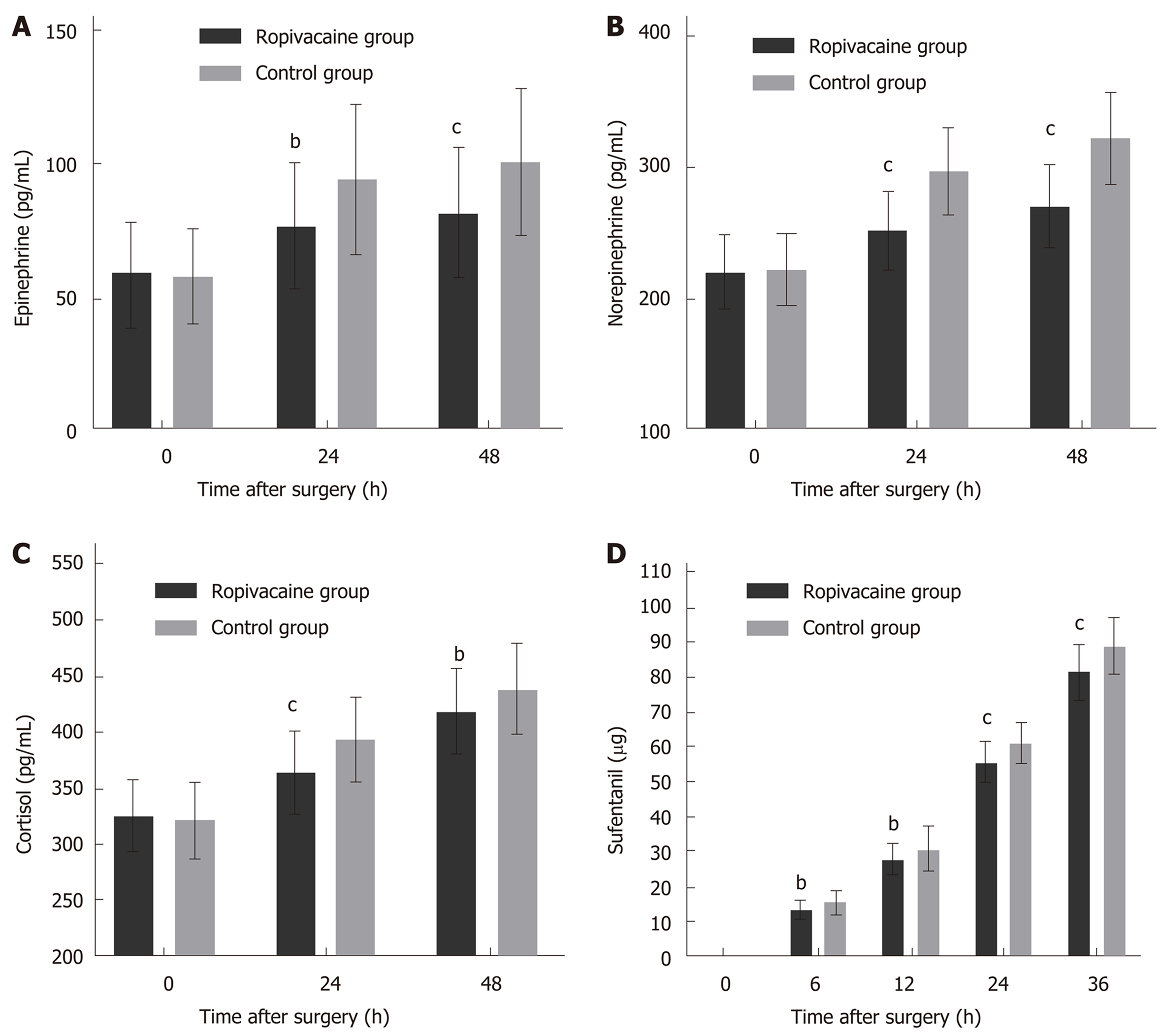
The magnitude of the changes in epinephrine, norepinephrine and cortisol levels above the normal levels was significantly different between the two groups at 24 h and 48 h after surgery (Figure 2A-C, Table 3). Cumulative sufentanil consumption was significantly different between the two groups at 6 h, 12 h, 24 h, and 36 h (P < 0.05 for all comparisons, Figure 2D and Table 2).
| Stress hormone | 0 h | 24 h | 48 h |
| Epinephrine | |||
| Ropivacaine group | 58.05 ± 19.614 | 76.48 ± 23.884 | 81.58 ± 24.529 |
| Control group | 57.60 ± 17.608 | 94.29 ± 28.439 | 100.89 ± 27.413 |
| t | 0.141 | -3.959 | -4.333 |
| P-value | 0.8879 | 0.0001 | <0.0001 |
| 95%CI | [-5.876, 6.780] | [-26.707, -8.913] | [-28.131, -10.498] |
| Norepinephrine | |||
| Ropivacaine group | 220.57 ± 27.623 | 252.01 ± 29.539 | 270.68 ± 30.792 |
| Control group | 222.52 ± 27.907 | 296.90 ± 32.601 | 322.12 ± 34.942 |
| t | -0.409 | -8.421 | -9.116 |
| P-value | 0.6831 | <0.0001 | <0.0001 |
| 95%CI | [-11.367, 7.470] | [-55.441, -34.351] | [-62.602, -40.280] |
| Cortisol | |||
| Ropivacaine group | 325.82 ± 29.790 | 365.06 ± 35.820 | 417.79 ± 36.971 |
| Control group | 320.74 ± 34.618 | 393.82 ± 37.302 | 438.14 ± 40.814 |
| t | 0.918 | -4.586 | -3.048 |
| P-value | 0.3600 | <0.0001 | 0.0028 |
| 95%CI | [-5.862, 16.025] | [-41.158, -16.356] | [-33.544, -7.145] |
Postoperative hospitalization was shorter in the ropivacaine group (7.24 ± 2.50) than in the control group (7.70 ± 2.95), but there was no significant difference (P = 0.324, Table 4). There were similar findings between the two groups regarding vomiting (55.7% and 60.0% for the ropivacaine group and the control group, respectively), and the difference between the two groups for nausea (12.9% and 18.6% for the ropivacaine group and the control group, respectively) was not statistically significant (Table 4). Other complications, including hydrothorax, ascites, peritonitis, flatulence, venous thrombus, and incision infection, in the two groups were not significantly different (P = 0.339) (Table 4).
| Characteristic | Ropivacaine group | Control group | t/χ2 | P-value |
| Hospitalization after operation (d) | 7.28 ± 2.502 | 7.76 ± 2.990 | -1.029 | 0.3055 |
| Postoperative nausea and vomiting | ||||
| Neither nausea nor vomiting | 22 | 15 | 1.819 | 0.4027 |
| Nausea without vomiting | 39 | 41 | ||
| Nausea with vomiting | 8 | 11 | ||
| Complication (+/-) | ||||
| Hydrothorax | 25/44 | 27/40 | 0.238 | 0.6256 |
| Ascites | 10/59 | 8/59 | 0.193 | 0.6606 |
| Peritonitis | 1/68 | 0/67 | 0.978 | 0.3226 |
| Flatulence | 32/37 | 39/28 | 1.907 | 0.1673 |
| Venous thrombus | 3/66 | 5/62 | 0.596 | 0.4402 |
| Incision infection | 2/67 | 1/66 | 0.312 | 0.5768 |
Compared with open surgery, the laparoscopic technique has largely reduced surgical trauma and hospitalization; however, postoperative pain is still an important problem for physicians and patients[5]. Postoperative pain is a phenomenon in which different variables must be taken into account[6]. Surgical incision is the main origin of the pain stimuli, which is influenced by the length of the incision, the number of trocars, and trauma occurring when removing the specimen. Another important factor is pneumoperitoneum created with CO2, which causes peritoneal stretch, diaphragmatic stretch and chemical irritation, and the attendant carbonic acid formation. Additional causes of pain may involve increased inflammatory mediators after tissue injury, increased lactate concentrations and low pH in the skin and muscle wounds, and central neuronal sensitization[7]. To date, not all of these components are known to be involved, and therapeutic approaches still need to be improved.
Ropivacaine is a long-acting amino amide local anesthetic drug. Ropivacaine blocks the generation and conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential[8]. Ropivacaine is a well-tolerated regional anesthetic effective for surgical anesthesia and postoperative relief. The nerve block effect is related to the drug concentration. When the concentration of ropivacaine is 0.2%, the sensory nerve block effect is better, but there is almost no motor nerve block effect; when the concentration is 0.75%, the nerve block effect is better. This "separation block" is the characteristic effect of ropivacaine. Thus, ropivacaine, with its efficacy, low propensity for motor block, and reduced potential for central nervous system (CNS) toxicity and cardiotoxicity, appears to be an important option for regional anesthesia and the management of postoperative pain[9]. In this study, adverse effects of ropivacaine for local anesthesia, such as allergic reactions, local tissue toxicity, cardiovascular toxicity, central nervous system toxicity, and systemic toxicity, were not observed.
Measures that have been reported to assist in the treatment of postoperative pain include inducing local anesthesia, decreasing pneumoperitoneum pressure[3], and administering non-steroidal anti-inflammatory drugs (NSAIDs) and opioids[10]. We found several trials that assessed the effects of local anesthesia in intraperitoneal, subdiaphragmatic, and trocar sites, which significantly decreased postoperative pain scores[11,12]. Postoperative pain mainly comes from wounds of the abdominal muscle and peritoneum. Beyond those causes, injury of Glisson’s capsule can also influence pain[13,14]. Therefore, in our study, ropivacaine not only infiltrated the subcutaneous and deep muscle fasciae and peritoneum but also covered the liver cutting surface in a soaked gelatin sponge to relieve the pain caused by capsule injury.
This study has shown the analgesic efficacy of local infiltrative and soaked sponge cover with ropivacaine after laparoscopic hepatectomy, suggesting the method as a reliable strategy for analgesia after operation. Based on the pain score at rest and the significantly lower movement seen in the ropivacaine group than in the control group, ropivacaine was considered to have a clear analgesic effect that lasted approximately 24 h.
Surgical stress could cause changes in the neuroendocrine, metabolic, immune, and hematological systems[15]. Surgical stress is mainly caused by injury during the operation, including somatic and visceral trauma. Surgical stress causes a series of hormonal changes; in the study, we chose catecholamines (epinephrine and norepinephrine) and cortisol as a reference to estimate the degree of surgery stress[16]. The levels of epinephrine, norepinephrine, and cortisol were significantly reduced in the first 48 h after surgery in the ropivacaine group compared to the control group, which means that the ropivacaine method produced clear effects. The use of ropivacaine significantly reduced surgical stress.
Pain relief plays a leading role in enhanced recovery after surgery (ERAS), which will bring many benefits[17]. Theoretically, exercising the lower limbs will promote blood circulation, which can reduce the formation of venous thrombosis[18]. Early ambulation increases lung ventilation, which promotes the exclusion of tracheal secretions and prevents pulmonary complications[19]. Early ambulation after surgery can also promote gastrointestinal peristalsis, reduce abdominal distention, improve appetite, and prevent constipation. However, in the present study, the complications described above showed no significant difference between the groups. One reason may be that compared with traditional open surgery, the laparoscopic technique clearly reduced pain, so the complications between the two groups were not sufficiently distinct. Another potential reason is that the incidence of thrombus of the lower extremity veins and incision infection is low in laparoscopic hepatectomy, so the differences were not significant with a limited sample size.
Although the results support the notion that multisite local infiltration and the sponge soaked with ropivacaine significantly decreased postoperative pain, there are also deficiencies in this study. First, we have not validated the analgesic effects of ropivacaine at different concentrations. More research is needed to determine the best concentration and dose used for local anesthesia after laparoscopic hepatectomy. Second, this study did not determine whether the analgesic effect of ropivacaine was effective for all patients or whether sex, age, weight, disease conditions, and other factors influence the analgesic effect of ropivacaine. Third, the adverse reactions of ropivacaine, such as wound infection and muscle damage, were not significantly different in this study, which may have been because the sample was not large enough or the ropivacaine concentration was too low. Moreover, the incidence of nausea, vomiting, and abdominal distension caused mainly by general anesthesia and pneumoperitoneum is not obviously reduced by the use of ropivacaine-soaked sponges. To overcome these deficiencies, more research is needed.
Taken together, local anesthesia with ropivacaine is a simple and inexpensive way to achieve analgesic effects after laparoscopic hepatectomy (Figure 3). With sponges soaked with ropivacaine covering the cutting surface of the liver, the concentration of ropivacaine can be maintained for a longer time with a lower incidence of abdominal infection than seen from the intraperitoneal administration of ropivacaine. Ropivacaine infiltration anesthesia reduces postoperative somatic pain, while sponges soaked with ropivacaine can reduce visceral pain and last for a long time, thus avoiding the administration of high doses of opioid analgesic drugs. In addition to analgesia, ropivacaine can also shorten hospitalization and the time spent in bed, potentially leading to a faster recovery. Considering the simplicity, safety, and efficacy of multisite local anesthesia and the sponge covering of the liver with ropivacaine after laparoscopic hepatectomy, this method is very worthy of application and promotion. In the future, the method could be applied to more areas such as gastrectomy and rectal resection with further study.
The postoperative pain caused by laparoscopic hepatectomy delays patients’ recovery, although the pain has reduced a lot compared with laparotomy. Although many methods have been reported for pain relief including intravenous analgesia, epidural analgesia and so on, these methods have their limitations and contraindications. Local wound infiltration is a simple and safe method that can effectively relieve the pain after surgery. The current study was designed to evaluate the effects of ropivacaine on pain control after laparoscopic hepatectomy and to examine whether this local anesthetic technique accelerates patient recovery, thus contributing to the idea of fast track recovery surgery.
Many methods have been reported to improve postoperative pain control, but there is not a simple and effective method that can be clinically adopted in a widespread manner. We designed this study to find an effective and convenient method which can be can be clinically adopted to decrease postoperative pain and accelerate recovery.
The aim of the study was to assess the effectiveness of ropivacaine injections in the port site as well as in the operative site in patients undergoing a hepatectomy.
Before the start of the study, random sequence was generated to make sure that the allocation was completely random. For allocation concealment, envelope method was adopted. Continuous data were analyzed by t-tests. The P-value and 95% confidence interval were calculated. Categorical data were analyzed by chi-square tests. All data were checked for normal distribution. Double blindness was ensured, and the researcher in charge of allocation and anesthetic preparation did not participate in the data collection and analysis.
Infiltration with ropivacaine in the abdominal wound and covering the cutting surface of the liver with a gelatin sponge soaked with ropivacaine could provide effective analgesia after laparoscopic hepatectomy, with a lower visual analog scale (VAS) score and sufentanil consumption, accelerated postoperative recovery, and reduced stress response. These results suggest that this method is a simple, convenient, and effective analgesic method that can provide postoperative analgesia and short-term benefits after surgery. There are still problems need to be solved: The best concentration and dose used for local anesthesia need more research to determine, and more effective anesthetics and better method of application need to be found.
This study provides evidence supporting that infiltration with ropivacaine in the abdominal wound and covering the cutting surface of the liver with a gelatin sponge soaked with ropivacaine after laparoscopic hepatectomy can improve postoperative pain relief, reduce surgical stress response, and accelerate postoperative recovery. This method is very worthy of application and promotion for its simplicity, safety and efficacy.
In this study, the effect of pain relief of ropivacaine remained no more than 24 h to 48 h, so anesthetics with a more lasting effect need to be found. Furthermore, more research is needed to determine the best concentration and dose used for local anesthesia. Methods of anesthetics application are variable, so researchers could try to find more effective and convenient techniques for pain relief.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Biondi A, Mutter D, Kang H S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Lim KH, Chung BS, Kim JY, Kim SS. Laparoscopic surgery in abdominal trauma: A single center review of a 7-year experience. World J Emerg Surg. 2015;10:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Das NT, Deshpande C. Effects of Intraperitoneal Local Anaesthetics Bupivacaine and Ropivacaine versus Placebo on Postoperative Pain after Laparoscopic Cholecystectomy: A Randomised Double Blind Study. J Clin Diagn Res. 2017;11:UC08-UC12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Singla S, Mittal G, Raghav, Mittal RK. Pain management after laparoscopic cholecystectomy-a randomized prospective trial of low pressure and standard pressure pneumoperitoneum. J Clin Diagn Res. 2014;8:92-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Allegri M, Ornaghi M, Ferland CE, Bugada D, Meghani Y, Calcinati S, De Gregori M, Lovisari F, Radhakrishnan K, Cusato M, Scalia Catenacci S, Somaini M, Fanelli G, Ingelmo P. Peritoneal Nebulization of Ropivacaine during Laparoscopic Cholecystectomy: Dose Finding and Pharmacokinetic Study. Pain Res Manag. 2017;2017:4260702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Kasai M, Cipriani F, Gayet B, Aldrighetti L, Ratti F, Sarmiento JM, Scatton O, Kim KH, Dagher I, Topal B, Primrose J, Nomi T, Fuks D, Abu Hilal M. Laparoscopic versus open major hepatectomy: A systematic review and meta-analysis of individual patient data. Surgery. 2018;163:985-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 6. | Brennan TJ. Pathophysiology of postoperative pain. Pain. 2011;152:S33-S40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Carvalho B, Clark DJ, Angst MS. Local and systemic release of cytokines, nerve growth factor, prostaglandin E2, and substance P in incisional wounds and serum following cesarean delivery. J Pain. 2008;9:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Toda S, Sakai A, Ikeda Y, Sakamoto A, Suzuki H. A local anesthetic, ropivacaine, suppresses activated microglia via a nerve growth factor-dependent mechanism and astrocytes via a nerve growth factor-independent mechanism in neuropathic pain. Mol Pain. 2011;7:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Kuthiala G, Chaudhary G. Ropivacaine: A review of its pharmacology and clinical use. Indian J Anaesth. 2011;55:104-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Shibasaki S, Kawamura H, Homma S, Yosida T, Takahashi S, Takahashi M, Takahashi N, Taketomi A. A comparison between fentanyl plus celecoxib therapy and epidural anesthesia for postoperative pain management following laparoscopic gastrectomy. Surg Today. 2016;46:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Sun JX, Bai KY, Liu YF, Du G, Fu ZH, Zhang H, Yang JH, Wang B, Wang XY, Jin B. Effect of local wound infiltration with ropivacaine on postoperative pain relief and stress response reduction after open hepatectomy. World J Gastroenterol. 2017;23:6733-6740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 12. | Bisgaard T, Klarskov B, Kristiansen VB, Callesen T, Schulze S, Kehlet H, Rosenberg J. Multi-regional local anesthetic infiltration during laparoscopic cholecystectomy in patients receiving prophylactic multi-modal analgesia: A randomized, double-blinded, placebo-controlled study. Anesth Analg. 1999;89:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Hall H, Leach A. Paravertebral block in the management of liver capsule pain after blunt trauma. Br J Anaesth. 1999;83:819-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Basak F, Hasbahceci M, Sisik A, Acar A, Ozel Y, Canbak T, Yucel M, Ezberci F, Bas G. Glisson's capsule cauterisation is associated with increased postoperative pain after laparoscopic cholecystectomy: A prospective case-control study. Ann R Coll Surg Engl. 2017;99:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Iwasaki M, Edmondson M, Sakamoto A, Ma D. Anesthesia, surgical stress, and "long-term" outcomes. Acta Anaesthesiol Taiwan. 2015;53:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Krikri A, Alexopoulos V, Zoumakis E, Katsaronis P, Balafas E, Kouraklis G, Karayannacos PE, Chrousos GP, Skalkeas G. Laparoscopic vs. open abdominal surgery in male pigs: Marked differences in cortisol and catecholamine response depending on the size of surgical incision. Hormones (Athens). 2013;12:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Kapritsou M, Papathanassoglou ED, Bozas E, Korkolis DP, Konstantinou EA, Kaklamanos I, Giannakopoulou M. Comparative Evaluation of Pain, Stress, Neuropeptide Y, ACTH, and Cortisol Levels Between a Conventional Postoperative Care Protocol and a Fast-Track Recovery Program in Patients Undergoing Major Abdominal Surgery. Biol Res Nurs. 2017;19:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Iverson RE, Gomez JL. Deep venous thrombosis: prevention and management. Clin Plast Surg. 2013;40:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Lewis CE. Preventing postoperative pneumonia. JAMA Surg. 2014;149:919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |