Published online Sep 14, 2019. doi: 10.3748/wjg.v25.i34.5162
Peer-review started: June 26, 2019
First decision: July 20, 2019
Revised: August 7, 2019
Accepted: August 19, 2019
Article in press: August 19, 2019
Published online: September 14, 2019
Processing time: 78 Days and 17.6 Hours
Acute pancreatitis (AP) is a common acute abdominal disease worldwide, and its incidence rate has increased annually. Approximately 20% of AP patients develop into necrotizing pancreatitis (NP), and 40% to 70% of NP patients have infectious complications, which usually indicate a worse prognosis. Infection is an important sign of complications in NP patients.
To investigate the difference in infection time, infection site, and infectious strain in NP patients with infectious complications.
The clinical data of AP patients visiting the Department of General Surgery of Xuanwu Hospital of Capital Medical University from January 1, 2014 to December 31, 2018 were collected retrospectively. Enhanced computerized tomography or magnetic resonance imaging findings in patients with NP were included in the study. Statistical analysis of infectious bacteria, infection site, and infection time in NP patients with infectious complications was performed, because knowledge about pathogens and their antibiotic susceptibility patterns is essential for selecting an appropriate antibiotic. In addition, the factors that might influence the prognosis of patients were analyzed.
In this study, 539 strains of pathogenic bacteria were isolated from 162 patients with NP infection, including 212 strains from pancreatic infections and 327 strains from extrapancreatic infections. Gram-negative bacteria were the main infectious species, the most common of which were Escherichia coli and Pseudomonas aeruginosa. The extrapancreatic infection time (9.1 ± 8.8 d) was earlier than the pancreatic infection time (13.9 ± 12.3 d). Among NP patients with early extrapancreatic infection (< 14 d), bacteremia (25.12%) and respiratory tract infection (21.26%) were predominant. Among NP patients with late extrapancreatic infection (> 14 d), bacteremia (15.94%), respiratory tract infection (7.74%), and urinary tract infection (7.71%) were predominant. Drug sensitivity analysis showed that P. aeruginosa was sensitive to enzymatic penicillins, third- and fourth-generation cephalosporins, and carbapenems. Acinetobacter baumannii and Klebsiella pneumoniae were sensitive only to tigecycline; Staphylococcus epidermidis and Enterococcus faecium were highly sensitive to linezolid, tigecycline, and vancomycin.
In this study, we identified the timing, the common species, and site of infection in patients with NP.
Core tip: In our study, Gram-negative bacteria were the main pathogens in necrotizing pancreatitis patients with infectious complications in our hospital. The most common Gram-negative bacteria were Escherichia coli and Pseudomonas aeruginosa. Additionally, the proportion of multidrug-resistant bacteria was relatively large, and caution should be used in the application of antibiotics. The extrapancreatic infection time was usually earlier than that of pancreatic infection. For patients with suspected infection, blood and respiratory pathogens should be cultured first. Third- or fourth-generation cephalosporins or carbapenems can be used as empirical drugs. Persistent organ failure, multidrug resistance, and multiple operations were risk factors for death.
- Citation: Lu JD, Cao F, Ding YX, Wu YD, Guo YL, Li F. Timing, distribution, and microbiology of infectious complications after necrotizing pancreatitis. World J Gastroenterol 2019; 25(34): 5162-5173
- URL: https://www.wjgnet.com/1007-9327/full/v25/i34/5162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i34.5162
Acute pancreatitis (AP) is a common acute abdominal disease worldwide, and its incidence rate has increased annually[1]. With the progression of the disease, 20% of AP patients develop into necrotizing pancreatitis (NP). Approximately 40% to 70% of NP patients have infectious complications, which usually indicate a worse prognosis. Infection is an important sign of complications in NP patients[1-5]. To date, studies on pancreatic infection have mainly focused on pancreatic and peripancreatic infection, but few have focused on extrapancreatic infection and coinfection.
Therefore, this study collected clinical data of NP patients who visited our hospital from January 1, 2014 to December 31, 2018. The aim was to analyze the difference in infection time, infection site, and bacterial strains in NP patients with infectious complications and the influence of these factors on mortality, in order to help determine treatment strategies for NP patients with coinfection.
The clinical data of NP patients who visited our hospital from January 1, 2014 to December 31, 2018 were retrospectively collected.
Patients diagnosed with NP by enhanced computerized tomography (CT) or magnetic resonance imaging (MRI) were included in the study.
The exclusion criteria were: (1) Acute attack of chronic pancreatitis; (2) Abdominal exploratory surgery due to pancreatitis; (3) Pancreatitis due to abdominal surgery; and (4) Acute abdominal events such as visceral organ perforation, bleeding, and abdominal compartment syndrome
AP: AP can be diagnosed by any two of the following three items: (1) Acute epigastric pain, radiating to the waist and back; (2) Blood amylase and/or lipase levels at least three times higher than the normal limit; and (3) The presence of typical pancreatic lesions upon imaging examination (such as abdominal ultrasound, enhanced CT, or MRI).
NP: NP presents inflammation associated with pancreatic parenchymal necrosis and/or peripancreatic necrosis. On contrast-enhanced CT, there is lack of pancreatic parenchymal enhancement by intravenous contrast agent and/or the presence of findings of peripancreatic necrosis[3]. According to Balthazar et al[6], the degree of pancreatic necrosis in NP patients is classified as < 30%, 30%-50%, and > 50%.
Infectious NP (INP): In clinical treatment, pancreatic infection is suspected in NP patients when one of the following symptoms is present: (1) Sudden high fever (> 38.5 °C) or persistent fever (> 38.5 °C) that does not return; (2) A significant increase in leukocyte count, or the percentage of neutrophils and inflammatory markers (CRP, IL-6, and PCT); and (3) Continuous deterioration of clinical symptoms such as new-onset organ failure or multiple organ failure. Diagnostic methods include the following: (1) Enhanced CT showing gas configurations in the necrotic collection; and (2) Positive culture from pancreatic necrosis obtained by puncture or operation.
Extrapancreatic infection: Extrapancreatic infection is defined as infection occurring in other organs of AP patients. Common sites of extrapancreatic infection include the blood, respiratory tract, urinary tract, abdominal cavity, biliary tract, and surgical incision site[7-9]. When infection symptoms are suspected in AP patients, but no definite signs of infection (bubbles) are found in the necrotic pancreatic tissue on imaging, and the culture of necrotic tissue and pus from puncture and drainage is negative, suspected patients with extrapancreatic infection can be diagnosed or excluded by multiple, multisite pathogen culture[4,5,7-9].
Multidrug-resistant (MDR) bacteria: MDR bacteria, which are bacteria that are usually sensitive to three or more commonly used antibiotics but show simultaneous resistance to these drugs, were evaluated according to the Chinese expert consensus on the prevention and control of nosocomial infection by MDR bacteria.
After admission, patients with confirmed NP were treated conservatively with analgesics, fluid resuscitation, and supportive treatment. Patients with clinically severe AP (suspected or confirmed symptoms of organ failure) were admitted to the intensive care unit (ICU) with all possible organ support systems, including ventilatory support, vasopressors, and dialysis as and when required. Patients with sterile necrosis are usually treated conservatively. For NP patients with suspected or confirmed infectious complications, broad-spectrum antibiotics (such as third- and fourth-generation cephalosporins and carbapenems) were used to control infection, and sensitive antibiotics were selected according to the drug sensitivity results of the patients. The indications for surgical intervention in NP patients were as follows: (1) After conservative treatment, the clinical symptoms did not improve significantly or worsened continuously; (2) Pancreatic infection was confirmed in the patient; and (3) The range of sterile necrosis in the patient was enlarged, and the surrounding organs were compressed. Minimally invasive approaches to remove necrotic tissue are usually selected according to the necrotic site in the patient.
Data were input into Excel 2018, the baseline data are recorded as the mean ± SD or medians (ranges) as appropriate. Independent samples t-tests were performed to analyze the differences between the groups. Qualitative data were scored, and chi-square or Fisher’s exact tests were performed to analyze the differences between the groups. Logistic regression was used to determine the risk of death in the patients via SPSS 23.0 statistical software, with the level of statistical significance set as P < 0.05.
We collected the clinical data of patients with AP who visited our department from January 1, 2014 to December 31, 2018. A total of 205 NP patients were enrolled according to the results of enhanced CT in our hospital or another hospital. There were 140 males and 65 females, with an average age of 49.5 ± 15.7 years. The causes of NP were gallstones in 109 patients, hyperlipidemia in 67 patients, unknown etiology in 12 patients, alcoholism in 10 patients, endoscopic retrograde cholangio-pancreatography in 6 patients, and posttraumatic pancreatitis in 1 patient. There was no significant difference in age, sex, or etiology between the infection group and the non-infection group (Table 1).
| Variable | Total (n = 205) | Infection (n = 163) | Non-infection (n = 42) | P-value |
| Age (yr) | 49.5 ± 15.7 | 50.3 ± 15.5 | 46.6 ± 16.7 | 0.173 |
| Male | 140 | 112 | 28 | 0.801 |
| Etiology | 0.898 | |||
| Biliary | 109 | 88 | 21 | |
| Alcohol | 10 | 7 | 3 | |
| Hyperlipemia | 67 | 52 | 15 | |
| ERCP | 6 | 5 | 1 | |
| Others | 13 | 11 | 2 | |
| Referral time | 24 (0-300) | 30 (0-300) | 2.5 (0-180) | 0.013a |
| CTSI | 4 (2-10) | 6 (2-10) | 4 (2-8) | 0.127 |
| Persistent OF | 50 | 49 | 1 | 0.005b |
| Need for any intervention | 120 | 113 | 7 | < 0.001c |
| Death | 20 | 18 | 2 | 0.191 |
| TPN | 17 (0-163) | 18 (0-163) | 10 (0-47) | < 0.001c |
| EN | 1 (0-112) | 2 (0-112) | 0 (0-34) | 0.004b |
| Length of hospital stay(d) | 29 (0-172) | 32 (0-172) | 19 (5-48) | < 0.001c |
| Length of stay ICU stay | 11 (0-117) | 13 (0-28) | 7 (0-117) | 0.004b |
| Necrosis degree | 0.018a | |||
| < 30% | 40 | 26 | 14 | |
| 30%-50% | 80 | 65 | 15 | |
| > 50% | 85 | 72 | 13 |
Among the 205 NP patients, 163 had infectious complications, and 42 had sterile necrosis. The different degrees of pancreatic necrosis in the patients with NP were as follows: 40 (26/14) patients with less than 30% necrosis, 80 (65/15) with 30%-50% necrosis, and 85 (72/13) with more than 50% necrosis. The median modified computer tomography severity scan (CTSI) score was 4 (range, 0-10). There was a significant difference in the degree of necrosis between the infection group and the non-infection group (Table 1).
Among the 205 NP patients, 179 were referred, with a median referral time of 24 d; 150 had infectious complications and 29 had aseptic NP. There was no significant difference in the referral time between the infection group and the non-infection group (Table 1).
Among the 162 NP patients with confirmed infectious complications, 539 strains of pathogenic bacteria were isolated, of which 21 were cultured from 112 patients with pancreatic infection. The most common Gram-negative bacteria were P. aeruginosa and E. coli (n = 34). The most common Gram-positive bacterial species was Enterococcus faecium (n = 18), and the most common fungus was Candida albicans (n = 3). A total of 327 strains were cultured from 132 patients with extrapancreatic infection, including 165 strains from blood, 77 from the respiratory tract, 35 from the urinary tract, 25 from the abdominal cavity, 20 from the biliary tract, and 5 from wound infections. The most common Gram-negative bacterial species was P. aeruginosa (n = 32), the most common Gram-positive bacterial species was Staphylococcus epidermidis (n = 32), and the most common fungus was C. albicans (n = 19). Fungal infection was found in 39 patients; 4 strains of pathogenic fungi were cultured from three patients with pancreatic infections, and 52 strains of pathogenic fungi were cultured from 36 patients with extrapancreatic infections (24 from blood, 5 from the respiratory tract, and 17 from the urinary system) (Table 2).
| Organism | INP (n = 212) | Extrapancreatic infection (n = 327) |
| Gram-negative | ||
| P. aeruginosa | 34 | 32 |
| A. baumanii | 22 | 22 |
| E. coli | 34 | 20 |
| K. pneumoniae | 30 | 16 |
| Gram-positive | ||
| S. epidermidis | 5 | 32 |
| E. faecium | 18 | 30 |
| S. haemolyticus | 5 | 19 |
| S. aureus | 5 | 8 |
| Fungal | ||
| C. albicans | 3 | 19 |
| C. parapsilosis | 0 | 12 |
| C. tropicalis | 0 | 12 |
| C. glabrata | 1 | 9 |
Gram-negative bacteria: Almost all gram-negative bacteria cultured from cases of INP were resistant to first- and second-generation cephalosporins and nonenzymatic penicillin. The most common E. coli was sensitive to carbapenems and to third- and fourth-generation cephalosporins. Klebsiella pneumoniae was generally resistant to commonly used antibiotics, and the resistance rate to carbapenems was more than 50%. P. aeruginosa was more sensitive to third- and fourth-generation cephalosporins than to other antibiotic classes. The percentages of E. coli and K. pneumoniae strains producing extended-spectrum beta-lactamase (ESBL) were 41.18% (14/34) and 20% (6/30), respectively. The drug sensitivity of Gram-negative pathogenic bacteria in NP cases with extrapancreatic infection was low. A. baumannii and K. pneumoniae were the most sensitive of the identified species to tigecycline. E. coli and P. aeruginosa were still susceptible to cephalosporins and carbapenems (approximately 50%). The percentages of ESBL-positive E. coli and K. pneumoniae strains were both 50% (10/20) (Table 3).
| Pancreatic | Extrapancreatic | |||||||
| Antibiotic | P. aeruginosa | E. coli | A. baumannii | K. pneumoniae | P. aeruginosa | E. coli | A. baumannii | K. pneumoniae |
| MDR bacteria | 9/34 | 9/34 | 16/22 | 15/30 | 8/32 | 4/20 | 17/22 | 11/16 |
| ESBL +: | — | 14/34 | — | 6/30 | — | 10/20 | — | 2/16 |
| Penicillins | ||||||||
| Ampicillin | 0/34 | 8/34 | 0/22 | 1/30 | 0/32 | 4/20 | 1/22 | 0/16 |
| Piperacillin | 12/34 | 4/34 | 0/22 | 3/30 | 14/32 | 4/20 | 1/22 | 0/16 |
| Enzymatic penicillins | ||||||||
| Piperacillin/tazobactam | 13/34 | 10/34 | 4/22 | 8/30 | 19/32 | 11/20 | 1/22 | 4/16 |
| Cefoperazone/sulbactam | 8/34 | 0/34 | 4/22 | 0/30 | 9/32 | 0/20 | 4/22 | 0/16 |
| Ampicillin/sulbactam | 0/34 | 4/34 | 0/22 | 4/30 | 0/32 | 6/20 | 4/22 | 0/16 |
| quinolones | ||||||||
| Levofloxacin | 21/34 | 11/34 | 3/22 | 11/30 | 17/32 | 6/20 | 3/22 | 3/16 |
| First- and second-generation cephalosporins | ||||||||
| Cefoxitin | 0/34 | 0/34 | 0/22 | 0/30 | 0/32 | 0/20 | 0/22 | 0/16 |
| Cefuroxime | 0/34 | 0/34 | 0/22 | 0/30 | 0/32 | 0/20 | 0/22 | 0/16 |
| Third- and fourth-generation cephalosporins | ||||||||
| Ceftazidime | 14/34 | 13/34 | 3/22 | 7/30 | 16/32 | 11/20 | 3/22 | 2/16 |
| Cefepime | 17/34 | 12/34 | 3/22 | 10/30 | 15/32 | 11/20 | 3/22 | 3/16 |
| Carbapenems | ||||||||
| Imipenem | 10/34 | 21/34 | 2/22 | 11/30 | 10/32 | 12/20 | 3/22 | 2/16 |
| Meropenem | 10/34 | 11/34 | 3/22 | 6/30 | 9/32 | 6/20 | 3/22 | 2/16 |
| Sulfonamides | ||||||||
| Compound trimethoprim | 1/34 | 14/34 | 9/22 | 14/30 | 0/32 | 10/20 | 8/22 | 9/16 |
| Tetracycline | ||||||||
| Tigecycline | 1/34 | 1/34 | 17/22 | 13/30 | 0/32 | 2/20 | 18/22 | 9/16 |
| Minocycline | — | — | 9/22 | — | 0/32 | — | 10/22 | — |
Gram-positive bacteria: Gram-positive bacteria in INP patients were sensitive to vancomycin and tigecycline, but resistant to other antibiotics. The most common E. faecium was highly susceptible to macrolides (77.78%, 14/18). Staphylococcus aureus was almost 100% sensitive to quinolones, tetracyclines, penicillins, aminoglycosides, and sulfonamide compounds. The percentages of ESBL-positive S. epidermidis and S. aureus strains were 60% (3/5) and 60% (3/5), respectively. The drug sensitivities of Gram-positive pathogens in NP with extrapancreatic infection were slightly different. Most of these organisms were highly sensitive to macrolides, tetracyclines, and polypeptide antibiotics (> 80%). ESBL producing strains of S. epidermidis and S. aureus accounted for 37% (12/32) and 37.5% (3/8) of the strains, respectively (Table 4).
| Pancreatic | Extrapancreatic | |||||||
| Antibiotic | E. faecium | S. epidermidis | S. aureus | E. faecalis | E. faecium | S. epidermidis | S. aureus | E. faecalis |
| MDR bacteria | 5/18 | 0/5 | 2/5 | 3/6 | 4/30 | 1/32 | 3/8 | 0/5 |
| ESBL +: | — | 3/5 | 3/5 | — | — | 12/32 | 3/8 | — |
| Penicillins | ||||||||
| Ampicillin | 2/18 | 0/5 | 0/5 | 3/6 | 1/30 | 0/32 | 0/8 | 3/5 |
| Penicillin G | 0/18 | 0/5 | 0/5 | 1/6 | 0/30 | 0/32 | 0/8 | 3/5 |
| quinolones | ||||||||
| Ciprofloxacin | 1/18 | 0/5 | 0/5 | 1/6 | 0/30 | 8/32 | 3/8 | 3/5 |
| Levofloxacin | 2/18 | 0/5 | 0/5 | 1/6 | 1/30 | 8/32 | 3/8 | 3/5 |
| Moxifloxacin | 1/18 | 2/5 | 0/5 | 1/6 | 1/30 | 21/32 | 3/8 | 3/5 |
| Macrolactones | ||||||||
| Linezolid | 14/18 | 5/5 | 4/5 | 5/6 | 25/30 | 31/32 | 8/8 | 4/5 |
| Tetracycline | ||||||||
| Tigecycline | 17/18 | 5/5 | 5/5 | 5/6 | 27/30 | 32/32 | 8/8 | 5/5 |
| Sulfonamides | ||||||||
| Compound trimethoprim | 1/18 | 1/5 | 4/5 | 0/6 | 2/30 | 9/32 | 6/8 | 2/5 |
| Polypeptides | ||||||||
| Vancomycin | 11/18 | 5/5 | 5/5 | 6/6 | 25/30 | 31/32 | 8/8 | 5/5 |
Fungal infection: According to the drug sensitivity of NP patients, C. albicans was the main pathogenic fungus cultured in patients with either pancreatic or extrapancreatic infection. C. albicans was sensitive to the antifungal drugs currently used in the clinic, such as 5-fluorocytosine, amphotericin B, and fluconazole.
MDR bacteria: Among the common Gram-negative bacilli identified in INP cases, the rates of multidrug resistance were as follows: P. aeruginosa, 26.47% (9/34); K. pneumoniae, 50% (15/30); E. coli, 26.47% (9/34); and A. baumannii, 72.73% (16/22). The multidrug resistance rate of common Gram-positive cocci was as follows: E. faecium, 27.78% (5/18). The multidrug resistance rates of common gram-negative bacteria in extrapancreatic infections were as follows: P. aeruginosa, 25% (8/32); K. pneumoniae, 68.75% (11/16); E. coli, 20% (4/20); and A. baumannii, 77.27% (17/22). The multidrug resistance rates of common gram-positive bacteria were as follows: S. epidermidis, 3.13% (1/32) and E. faecium, 13.33% (4/30).
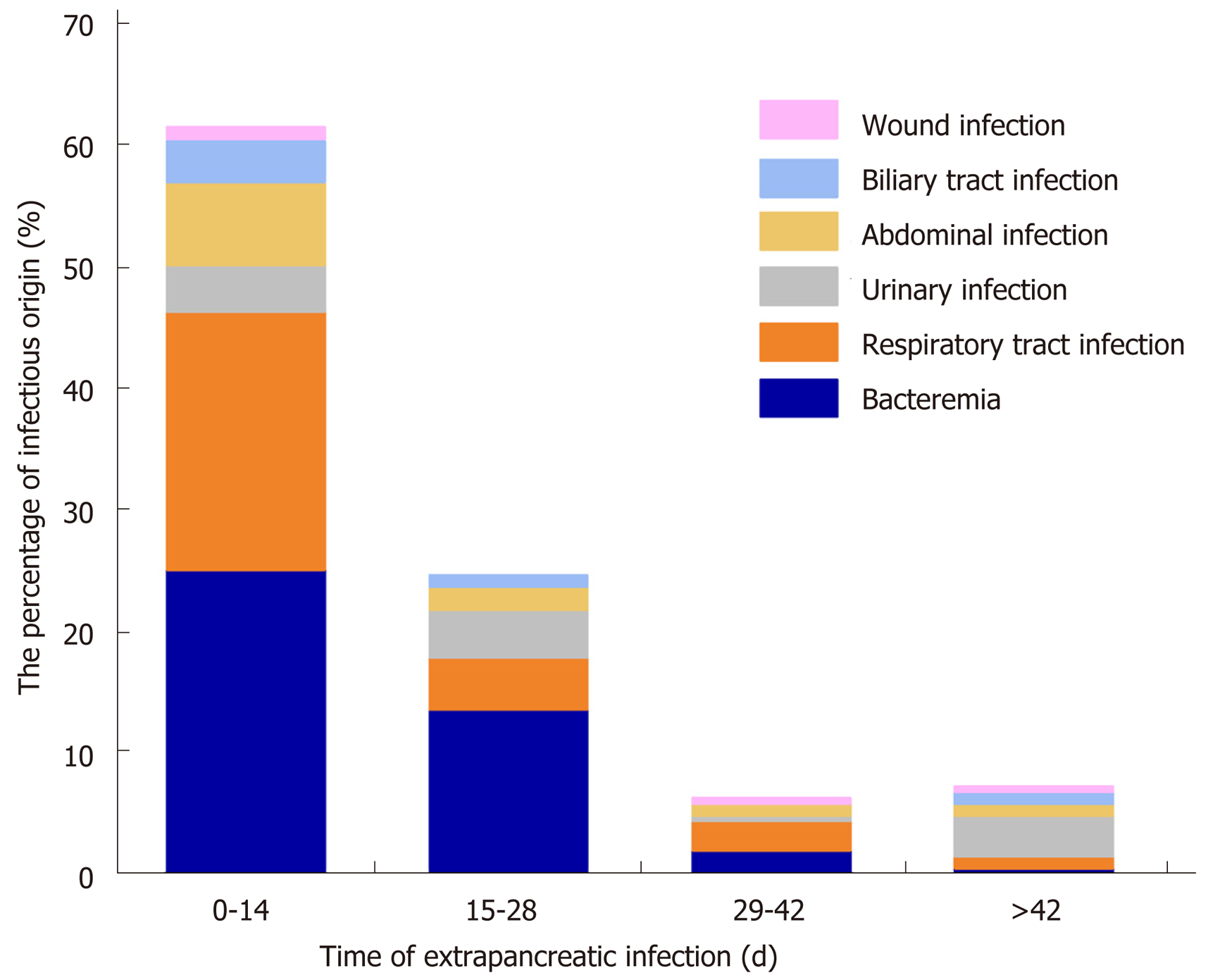
In this study, the extrapancreatic infection time was 9.1 ± 8.8 d, the pancreatic infection time was 13.9 ± 12.3 d, and the fungal infection time was (31.6 ± 26.4 d, P < 0.05). Among the 132 NP patients with extrapancreatic infection, 85 had bacteremia, 60 had respiratory tract infection, 25 had urinary tract infection, 22 had abdominal infection, 11 had biliary tract infection, and 4 had wound infection. The common sites of extrapancreatic infection in patients with early NP (< 14 d) were the blood and respiratory tract. The common sites of extrapancreatic infection in patients with advanced NP (> 14 d) were the blood, respiratory tract, and urinary tract (Figure 1).
In this study, among the 42 cases of sterile NP, 35 were treated conservatively, and 7 were treated surgically. Six patients underwent intracystic drainage or video-assisted retroperitoneal debridement (VARD) to remove necrotic tissue. One patient was treated by interventional embolization for arterial hemorrhage. Among the 163 patients with infection, 50 received only symptomatic supportive treatment such as anti-inflammatory therapy, and 113 patients with NP underwent surgical treatment. Seven patients received only percutaneous catheter drainage (PCD) and anti-inflammatory treatment, while the remaining 106 patients received VARD and anti-inflammatory treatment. Postoperative complications occurred in 21 patients: bleeding in 10, interventional therapy in 3 (hepatic artery in 1 and splenic artery in 2), digestive tract fistula in 8, and pancreatic fistula in 3.
In this study, the overall mortality rate was approximately 9.76% (n = 20). Of the 42 patients with sterile necrosis, 7 treated surgically were discharged from the hospital, and 2 treated conservatively died. Of the 163 NP patients with infectious complications, 7 died of infectious necrosis treated conservatively, and 2 died of PCD only. Nine patients died after VARD treatment. A total of 50 patients suffered from organ failure, including 1 in the noninfection group and 49 in the infection group. A total of 18 patients died, with a mortality rate of 36%. Regression analysis showed that the mortality rate was related to persistent organ failure, infection with multiple drug-resistant bacteria, and the number of operations (Table 5).
| Variable | Univariable analysis | P-value | Multivariable analysis | P-value |
| OR (95%CI) | OR (95%CI) | |||
| Persistent OF | 5.37 (3.785-7.618) | < 0.001 | 0.012 (0.001-0.101) | < 0.001c |
| MDR organism (s) | 2.445 (1.554-3.848) | 0.001 | 0.134 (0.028-0.633) | 0.011b |
| Fungal infection | 2.719 (1.498-4.936) | 0.002 | 0.657 (0.156-2.770) | 0.567 |
| Prophylactic antibiotic | 0.974 (0.783-1.210) | 0.8 | 0.534 (0.095-2.989) | 0.475 |
| INP | 1.151 (0.802-1.651) | 0.48 | 0.277 (0.024-3.234) | 0.306 |
| Extrapancreatic infection | 1.383 (1.124-1.704) | 0.031 | 0.627 (0.042-9.273) | 0.734 |
| Referred patients | 1.102 (0.986-1.232) | 0.25 | 0.524 (0.041-6.655) | 0.619 |
| Operation | 0.789 (0.497-1.255) | 0.262 | 10.533 (1.052-105.491) | 0.045a |
| Complication | 2.921 (1.304-6.540) | 0.011 | 0.892 (0.191-4.162) | 0.885 |
Many guidelines and studies suggest that infection is a risk factor for late mortality in NP patients and an indication for surgery[1-3,7-10]. Currently, the concept of surgery for INP patients has changed from "early, multiple, open debridement" to "delayed, drainage, minimally invasive". The superiority of the “step-up” approach to “open necrosectomy” for NP has been widely recognized[11-15]. Our center delays the progression of NP patients with suspected or confirmed infection by timely conservative treatment such as analgesia, fluid resuscitation, anti-inflammatory agents, and enteral (parenteral) nutrition. In a large number of clinical practices, we believe that the choice of antibiotic treatment for NP patients with infection, especially before the results of the first bacterial culture are obtained, is still relatively blind.
In our study, cultures of pathogenic bacteria from infected NP patients were analyzed. Gram-negative bacteria were the main pathogens in pancreatic and extrapancreatic infections. E. coli and K. pneumoniae were the most common pathogens in pancreatic infections, consistent with the findings in most studies on infectious pathogens[7,8,10,16-21]. Immune suppression in early NP patients may lead to excessive systemic inflammatory response syndrome, which increases intestinal mucosal permeability and damages intestinal commensal microorganisms, resulting in translocation of the intestinal flora and pancreatic infection. The main pathogenic bacteria in extrapancreatic infections were different from those in pancreatic infections. P. aeruginosa and A. baumannii were the most common Gram-negative bacteria in extrapancreatic infection. E. coli and K. pneum oniae were the main Gram-negative bacteria in pancreatic infections. The bacteria in pancreatic infections may originate primarily from the intestinal tract; thus, intestinal bacteria are common, while the bacteria in extrapancreatic infections differ by infection site.
According to the analysis of extrapancreatic infection time in NP patients, the extrapancreatic infection time was earlier than the pancreatic infection time, consistent with the conclusions of other studies[4,5,7,8]. In both early (< 14 d) and late (> 42 d) infections, bacteremia is a common source of infection. In the early stage, bacteremia may occur due to the suppression of immune function by a systemic inflammatory reaction, which may affect the prognosis of NP patients[5]. Brown et al[4] noted that the most common manifestations of extrapancreatic infections in severe AP patients were respiratory tract infections (9.2%) and bacteremia (8.4%). Moka et al[8] noted that INP patients with extrapancreatic infection (pneumonia or bacteremia) had significantly higher total hospitalization lengths, ICU hospitalization lengths, and mortality rates than those without extrapancreatic infection. In a retrospective study by Besselink et al[10], bacteremia was considered an independent risk factor for predicting death in AP patients, and increased the risk of pancreatic parenchymal necrosis and infection. The predominance of bacteremia in late extrapancreatic infections may be related to long hospitalization times, multiple operations, secondary iatrogenic infections, or prolonged indwelling times of deep venous catheters and catheters for long-term parenteral nutrition[22].
In this study, the multidrug resistance rate of common Gram-negative bacteria (K. pneumoniae, P. aeruginosa, E. coli, and A. baumannii) found in INP in our center ranged from 23.68% to 72.73%. The multidrug resistance rate of common Gram-positive bacteria (E. faecium) was 27.78%. The multidrug resistance rates of common Gram-negative bacteria (K. pneumoniae, P. aeruginosa, E. coli, and A. baumannii) in extrapancreatic infections ranged from 25% to 81.82%. The multidrug resistance rate of common G ram-positive bacteria (E. faecium) was 20%. Mourad et al[21] have also reported that prolonged antibiotic use leads to MDR bacterial and fungal infections, which are also associated with prolonged hospitalization and poor prognosis. Therefore, it is necessary to replace antibiotics to which pathogens are sensitive or discontinue drugs in a timely manner, according to the patient's condition, to reduce the risk of the bacteria developing multidrug resistance. In this study, 39 patients developed fungal infections and 56 strains of pathogenic fungi were cultured. The most common pathogenic fungus was C. albicans. Notably, the 39 cases of fungal infection were all mixed infections, and the occurrence time of fungal infections was later than that of bacterial infections. This difference may be related to the long-term use of broad-spectrum antibiotics and the decline of autoimmunity in NP patients. Most studies consider fungal infection a risk factor for mortality in infected patients[7,8,10,19,23-25]. However, whether antifungal drugs should be used prophylactically in patients with suspected or confirmed extrapancreatic (and) or pancreatic infections requiring antibiotics remains controversial. The preventive use of broad-spectrum antibiotics or antifungal therapy may increase the incidence of bacterial or fungal superinfection[8,19]. The preventive use of antibiotics has not increased the incidence of fungal infections and may reduce the incidence of fungal infections in patients, but further large-scale studies are needed to confirm this hypothesis[23,24]. In this study, the degree of pancreatic necrosis, CTSI score, rate of referral, rate of organ failure, need for surgical intervention, need for enteral nutrition, need for parenteral nutrition, and length of hospitalization were higher in NP patients in the infection group than in NP patients in the non-infection group. In the infection group, most patients (n = 150) were referred from an outside hospital. Their basic vital signs and immune function were poor at admission, and the incidence of adverse events such as organ failure and aggravation of pancreatic necrosis increased with prolonged disease course. Long-term nutritional support and even surgery were needed, which prolonged the hospitalization time of the patients. In addition, many studies support the view that the risk of pancreatic infection increases with the degree of pancreatic necrosis[1-3,11-16]. However, in this study, the infection rate of NP patients with simple extrapancreatic infection was also higher than that of the non-infection group, which may be related to the poor autoimmunity and severe inflammation in patients with extrapancreatic infection. In addition, false negative results for the culture of pathogenic bacteria from the pancreas in some patients with simple extrapancreatic infection were not excluded.
In addition, some issues need to be addressed. First, most patients were treated with antibiotics before surgery, and the related effects need to be further analyzed. Second, a few patients were diagnosed with INP during outpatient treatment, but there was no detailed record of the infectious strains, and there was a certain deviation in the number of pathogenic bacteria. Third, the changes in pathogenic bacteria before and after surgery were not analyzed. Finally, the difference between pancreatic necrosis and peri-pancreatic necrosis was not mentioned.
In conclusion, when NP patients present clinical symptoms of suspected infection, such as fever, elevated inflammatory indexes, and sepsis, but no clear indication of pancreatic infection revealed by enhanced CT examination, the culture from site of extrapancreatic infection can be given priority (different extrapancreatic culture sites can be selected according to the onset time). If no extrapancreatic infection was diagnosed, surgical procedures should be considered to determine whether the patient has pancreatic infection. In addition, our study has shown that although Gram-negative bacteria are still dominant in NP patients, MDR bacterial infection tends to increase. Therefore, anti-infection treatment should be carried out after it is clear that the patient has symptoms of infection. For NP patients with confirmed infection, priority can be given to antibiotics to which Gram-negative bacteria are sensitive, and then sensitive antibiotics can be selected according to the results of drug sensitivity. When NP patients are clearly complicated with infection symptoms, they should be paid close attention to the risk of organ failure, MDR bacterial infection, or multiple operations. Hence, understanding the pathogen spectrum and drug sensitivity testing of NP patients with infectious symptoms will help clinicians to use antibiotics effectively and reasonably, improve the therapeutic effect, and reduce the related mortality of NP patients.
As acute pancreatitis patients progress to necrotizing pancreatitis (NP), their hospitalization costs and mortality rates become much higher than those of patients with edematous pancreatitis. Approximately 40%-70% of NP cases present with symptoms of infection, and infection has become the most important risk factor for death from NP. Therefore, it is critical to identify patients with NP who have symptoms of infection.
Studies on pancreatic infectious have mainly focused on pancreatic and peripancreatic infections, and little is known about extrapancreatic infections and coinfections. We considered the difference in infection time, infection site, and infectious strains in NP patients with infectious complications, and found that early intervention may decrease the mortality rate.
Infectious NP, extrapancreatic infection, and coinfection are the common complications of NP. The research is critical to improve the treatment strategy for NP patients with infectious complications.
This study included 205 patients with NP. The baseline data were recorded as the mean ± SD or medians (ranges) as appropriate. Independent samples t-tests were performed to analyze the differences between the groups. Qualitative data were scored, and chi-square or Fisher’s exact tests were performed to analyze the differences between the groups. Logistic regression was used to determine the risk of death in the patients using via SPSS 23.0 statistical software.
A total of 539 strains of pathogenic bacteria were cultured from the 162 NP patients with confirmed infectious complications. The most common Gram-negative bacteria were P. aeruginosa and E. coli (n = 34). The most common Gram-positive bacteria were E. faecium (n = 18), and the most common fungus was C. albicans (n = 3). The extrapancreatic infection time was 9.1 ± 8.8 d, the pancreatic infection time was 13.9 ± 12.3 d, and the fungal infection time was 31.6 ± 26.4 d (P < 0.05). The common sites of extrapancreatic infection in patients with advanced NP (> 14 d) were the blood, respiratory tract, and urinary tract.
This retrospective analysis of NP patients with infectious com-plications in our hospital shows that the main pathogenic bacteria in NP patients were Gram-negative bacteria, the most common of which were E. coli and P. aeruginosa. Additionally, it was found that the proportion of multidrug-resistant bacteria was relatively large, and caution should be used in the application of antibiotics. The extrapancreatic infection time was usually earlier than the pancreatic infection time. For patients with suspected extrapancreatic infection, blood and respiratory pathogen culture should be prioritized. Third- or fourth-generation cephalosporins or carbapenems can be used as empirical antibiotics. Persistent organ failure, multidrug resistance, and surgery were risk factors for death.
We confirmed the common infectious strains, the site of infection, and the time of infection in NP patients with infectious complications. Through these results, we found that it is helpful for clinicians to evaluate the patient's condition in a timely manner to improve the patient's prognosis.
In the process of revise our article, we received the support from the Construction Project of Clinical Advanced subjects of Capital Medical University and the valuable advice and help of Professor Zhang Taiping, from basic surgery of Beijing Union Medical College. We would like to say thank you for them.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barreto SG, Bradley III EL S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, Besselink MG; Dutch Pancreatitis Study Group. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66:2024-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 296] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 2. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4323] [Article Influence: 360.3] [Reference Citation Analysis (45)] |
| 3. | Werge M, Novovic S, Schmidt PN, Gluud LL. Infection increases mortality in necrotizing pancreatitis: A systematic review and meta-analysis. Pancreatology. 2016;16:698-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Brown LA, Hore TA, Phillips AR, Windsor JA, Petrov MS. A systematic review of the extra-pancreatic infectious complications in acute pancreatitis. Pancreatology. 2014;14:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Pando E, Alberti P, Hidalgo J, Vidal L, Dopazo C, Caralt M, Blanco L, Gómez-Gavara C, Bilbao I, Balsells J, Charco R. The role of extra-pancreatic infections in the prediction of severity and local complications in acute pancreatitis. Pancreatology. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 341] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Jain S, Mahapatra SJ, Gupta S, Shalimar, Garg PK. Infected Pancreatic Necrosis due to Multidrug-Resistant Organisms and Persistent Organ failure Predict Mortality in Acute Pancreatitis. Clin Transl Gastroenterol. 2018;9:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Moka P, Goswami P, Kapil A, Xess I, Sreenivas V, Saraya A. Impact of Antibiotic-Resistant Bacterial and Fungal Infections in Outcome of Acute Pancreatitis. Pancreas. 2018;47:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Bohidar NP, Garg PK, Khanna S, Tandon RK. Incidence, etiology, and impact of Fever in patients with acute pancreatitis. Pancreatology. 2003;3:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Besselink MG, van Santvoort HC, Boermeester MA, Nieuwenhuijs VB, van Goor H, Dejong CH, Schaapherder AF, Gooszen HG; Dutch Acute Pancreatitis Study Group. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Rao C, Bhasin DK, Rana SS, Gupta R, Gautam V, Singh K. Implications of culture positivity in acute pancreatitis: does the source matter? J Gastroenterol Hepatol. 2013;28:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Li A, Cao F, Li J, Fang Y, Wang X, Liu DG, Li F. Step-up mini-invasive surgery for infected pancreatic necrosis: Results from prospective cohort study. Pancreatology. 2016;16:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1034] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 14. | Horvath K, Freeny P, Escallon J, Heagerty P, Comstock B, Glickerman DJ, Bulger E, Sinanan M, Langdale L, Kolokythas O, Andrews RT. Safety and efficacy of video-assisted retroperitoneal debridement for infected pancreatic collections: a multicenter, prospective, single-arm phase 2 study. Arch Surg. 2010;145:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Hollemans RA, Bakker OJ, Boermeester MA, Bollen TL, Bosscha K, Bruno MJ, Buskens E, Dejong CH, van Duijvendijk P, van Eijck CH, Fockens P, van Goor H, van Grevenstein WM, van der Harst E, Heisterkamp J, Hesselink EJ, Hofker S, Houdijk AP, Karsten T, Kruyt PM, van Laarhoven CJ, Laméris JS, van Leeuwen MS, Manusama ER, Molenaar IQ, Nieuwenhuijs VB, van Ramshorst B, Roos D, Rosman C, Schaapherder AF, van der Schelling GP, Timmer R, Verdonk RC, de Wit RJ, Gooszen HG, Besselink MG, van Santvoort HC; Dutch Pancreatitis Study Group. Superiority of Step-up Approach vs Open Necrosectomy in Long-term Follow-up of Patients With Necrotizing Pancreatitis. Gastroenterology. 2019;156:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 16. | Kokosis G, Perez A, Pappas TN. Surgical management of necrotizing pancreatitis: an overview. World J Gastroenterol. 2014;20:16106-16112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Mowbray NG, Ben-Ismaeil B, Hammoda M, Shingler G, Al-Sarireh B. The microbiology of infected pancreatic necrosis. Hepatobiliary Pancreat Dis Int. 2018;17:456-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Behrman SW, Bahr MH, Dickson PV, Zarzaur BL. The microbiology of secondary and postoperative pancreatic infections: implications for antimicrobial management. Arch Surg. 2011;146:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Schmidt PN, Roug S, Hansen EF, Knudsen JD, Novovic S. Spectrum of microorganisms in infected walled-off pancreatic necrosis - impact on organ failure and mortality. Pancreatology. 2014;14:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Shen Y, Cui NQ. Clinical observation of immunity in patients with secondary infection from severe acute pancreatitis. Inflamm Res. 2012;61:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Mourad MM, Evans R, Kalidindi V, Navaratnam R, Dvorkin L, Bramhall SR. Prophylactic antibiotics in acute pancreatitis: endless debate. Ann R Coll Surg Engl. 2017;99:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Wu BU, Johannes RS, Kurtz S, Banks PA. The impact of hospital-acquired infection on outcome in acute pancreatitis. Gastroenterology. 2008;135:816-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Kochhar R, Noor MT, Wig J. Fungal infections in severe acute pancreatitis. J Gastroenterol Hepatol. 2011;26:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Trikudanathan G, Navaneethan U, Vege SS. Intra-abdominal fungal infections complicating acute pancreatitis: a review. Am J Gastroenterol. 2011;106:1188-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Shen D, Wang D, Ning C, Lin C, Cao X, Liu Z, Ji L, Huang G. Prognostic factors of critical acute pancreatitis: A prospective cohort study. Dig Liver Dis. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |