Published online Sep 7, 2019. doi: 10.3748/wjg.v25.i33.4999
Peer-review started: April 28, 2019
First decision: May 30, 2019
Revised: July 4, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: September 7, 2019
Processing time: 132 Days and 15.4 Hours
Diarrhea is a major infectious cause of childhood morbidity and mortality worldwide. In clinical trials, Lactobacillus rhamnosus GG ATCC 53013 (LGG) has been used to treat diarrhea. However, recent randomized controlled trials (RCTs) found no evidence of a beneficial effect of LGG treatment.
To evaluate the efficacy of LGG in treating acute diarrhea in children.
The EMBASE, MEDLINE, PubMed, Web of Science databases, and the Cochrane Central Register of Controlled Trials were searched up to April 2019 for meta-analyses and RCTs. The Cochrane Review Manager was used to analyze the relevant data.
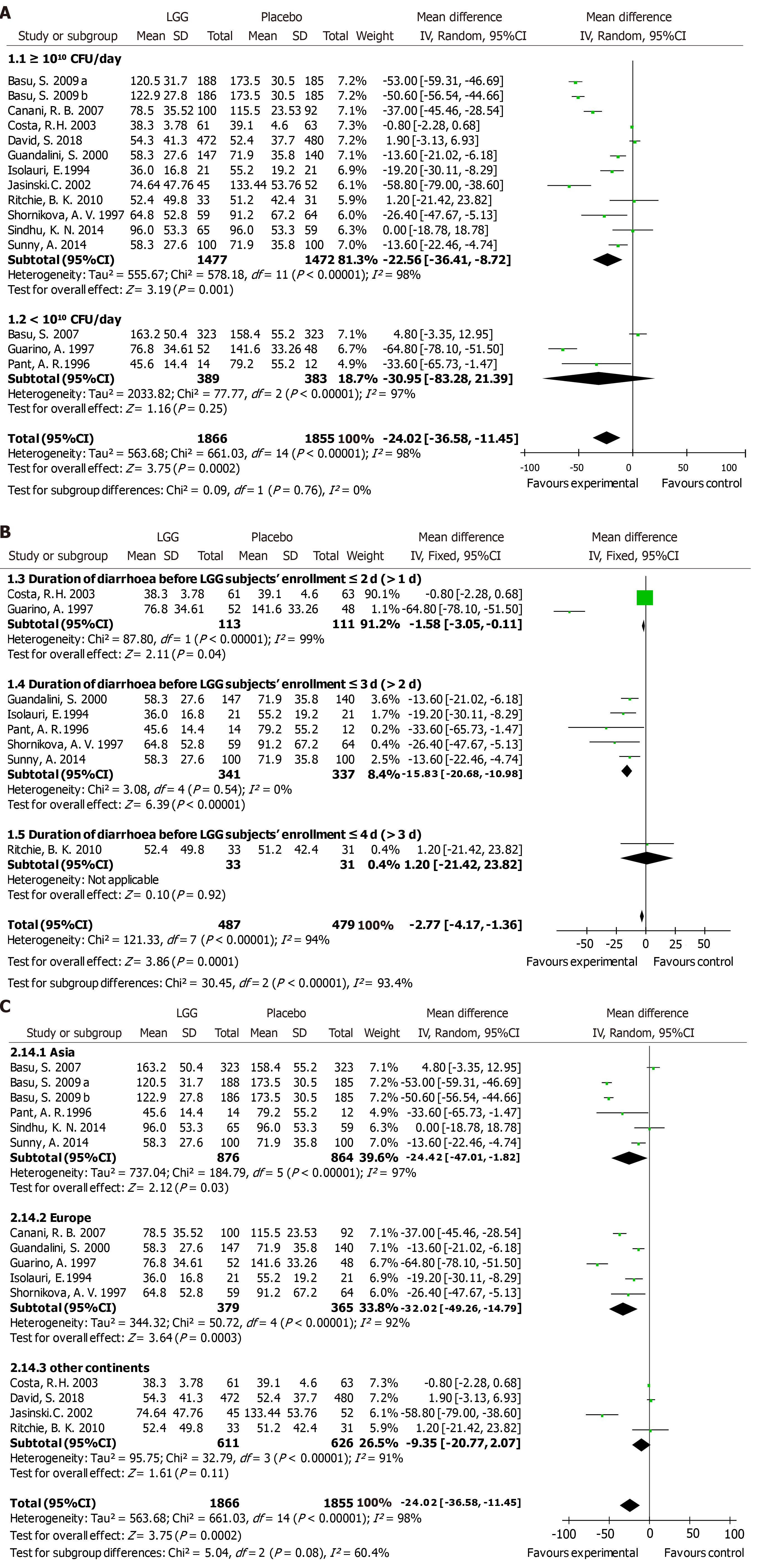
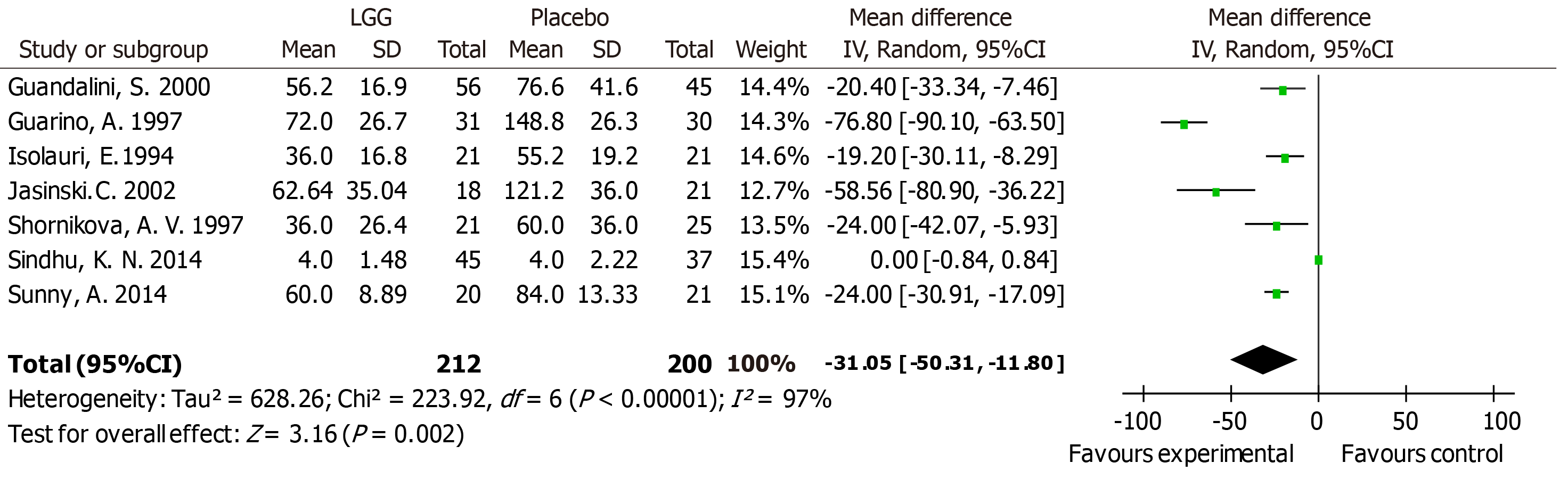
Nineteen RCTs met the inclusion criteria and showed that compared with the control group, LGG administration notably reduced the diarrhea duration [mean difference (MD) -24.02 h, 95% confidence interval (CI) (-36.58, -11.45)]. More effective results were detected at a high dose ≥ 1010 CFU per day [MD -22.56 h, 95%CI (-36.41, -8.72)] vs a lower dose. A similar reduction was found in Asian and European patients [MD -24.42 h, 95%CI (-47.01, -1.82); MD -32.02 h, 95%CI (-49.26, -14.79), respectively]. A reduced duration of diarrhea was confirmed in LGG participants with diarrhea for less than 3 d at enrollment [MD -15.83 h, 95%CI (-20.68, -10.98)]. High-dose LGG effectively reduced the duration of rotavirus-induced diarrhea [MD -31.05 h, 95%CI (-50.31, -11.80)] and the stool number per day [MD -1.08, 95%CI (-1.87, -0.28)].
High-dose LGG therapy reduces the duration of diarrhea and the stool number per day. Intervention at the early stage is recommended. Future trials are expected to verify the effectiveness of LGG treatment.
Core tip: The treatment effectiveness of Lactobacillus rhamnosus GG (LGG) for acute diarrhea in children was assessed in our study. LGG was confirmed to effectively reduce the duration of diarrhea and the stool number per day. LGG was particularly efficacious in patients treated at a dose > 1010 CFU/day, those treated at an early stage of illness, and those diagnosed with rotavirus-positive diarrhea.
- Citation: Li YT, Xu H, Ye JZ, Wu WR, Shi D, Fang DQ, Liu Y, Li LJ. Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: A systematic review with meta-analysis. World J Gastroenterol 2019; 25(33): 4999-5016
- URL: https://www.wjgnet.com/1007-9327/full/v25/i33/4999.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i33.4999
The World Health Organization and United Nations International Children's Emergency Fund define diarrhea as more than three loose or watery stools during a 24-h period. A duration of 14 days is the proposed criterion for acute diarrhea or persistent diarrhea. Diarrhea is a major infectious cause of childhood morbidity and mortality worldwide, especially in developing countries[1]. As the second most common cause of death among children under 5 years of age[2], the frequency of acute diarrhea in one year is approximately two to three episodes per child[1]. Previous data showed that the incidence of diarrhea was 6 to 12 episodes in 12 months per child in developing countries[3].
The goals of treatment are prevention or resolution of dehydration and reduction of the diarrhea duration and infectious period[4]. Oral rehydration, gut motility inhibitors, and antibiotics are used to treat acute gastroenteritis[4]. Oral rehydration contributes to a reduced likelihood of dehydration but has no appreciable effects on bowel movements or the duration of diarrhea and is not utilized to its full extent[5]. Antibiotics should be considered if pathogenic bacteria are detected. Smectite and zinc remain under-utilized as adjuvant therapies[6,7].
Probiotic supplements have gained considerable popularity in the global market and are predicted to generate 64 billion United States dollars in revenue by 2023[8]. Probiotics have health benefits for hosts[9] and have been evaluated in the treatment of diarrhea, and multiple mechanisms of diarrhea improvement have been identified. Probiotics modulate the host immune response[10]. Furthermore, colonic bacterial metabolites such as short-chain fatty acids increase colonic Na and fluid absorption through a cyclic adenosine monophosphate-independent mechanism[5]. In clinical trials, the well-known probiotics Saccharomyces boulardii, Lactobacillus reuteri DSM 17938, and Lactobacillus rhamnosus GG ATCC 53013 (LGG) have been used to treat diarrhea[2,4]. Previously, rotavirus-induced diarrhea was considered an adaptation disease associated with LGG treatment[11]. Wolvers D revealed that the probiotic dose mediated the effectiveness of treatment, and 1010-1011 CFU per day was recommended[12]. In addition, a greater effect was observed in the early stage of illness, and a poorer effect on invasive bacterial diarrhea versus watery diarrhea was observed. LGG treatment has been endorsed by leading experts[13-15]. However, most recent randomized controlled trials (RCTs) conducted by Schnadower et al[8] yielded no evidence of a beneficial effect of LGG treatment. Therefore, we conducted a meta-analysis to evaluate the available validated data and update existing knowledge and thus provide guidance to patients.
Relevant studies published before April 2019 were retrieved from the EMBASE, MEDLINE, PubMed, Web of Science databases, and the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library). The search strategy was conducted with medical subject headings and the search terms “diarrhoea, diarrhea, diarrh*, gastroenteritis, probiotic*, Lactobacillus rhamnosus GG, Lactobacillus GG, and LGG”. No language restrictions were applied. Additional studies were identified by manually searching review articles.
Nineteen RCTs describing LGG interventions for acute diarrhea were included. The PRISMA statement and the guidelines from the Cochrane Collaboration were followed for this evidence-based medicine study[16,17]. The participants were children aged less than 18 years. The dose of LGG was provided in various forms at different times. Antibiotic-associated diarrhea and persistent diarrhea were excluded. Other applications of LGG, such as preventive strategies, were not included. Some particular article types without complete data were excluded, such as abstracts and letters. We also excluded studies using mixtures of more than one probiotic strain. The primary outcomes were directly related to the development of persistent diarrhea, including the duration of diarrhea and diarrhea lasting ≥ 3 and ≥ 4 d. Secondary outcomes included the hospital stay duration, stool frequency, and improvement in stool consistency and vomiting.
Two investigators (Li YT and Xu H) independently identified eligible articles and extracted applicable data following the inclusion criteria. Quality control was assessed by another reviewer (Wu WR). The data set included the baseline characteristics of the participants, the duration of diarrhea, the hospital stay duration, the time to improvement in stool consistency, the mean number of stools per day during diarrhea episodes, the proportion of patients with vomiting, the duration of vomiting, stool frequency on days 2 and 3 after treatment, and the number of patients with diarrhea lasting ≥ 3 or 4 d. Cochrane Review Manager (Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) and STATA version 12.0 (StataCorp LP, College Station, TX, United States) were used for data analyses. Any discrepancies were resolved by discussion.
All included trials were evaluated following the Cochrane Collaboration’s risk of bias tool. Seven domains were examined to identify the bias risk: selection bias, including random sequence generation and allocation concealment, performance bias, including blinding of participants and personnel, detection bias, including blinding of outcome assessments, attrition bias, including incomplete outcome data, reporting bias, including selective reporting, and other bias. Adequate allocation concealment was implemented to ensure blinding of the participants and investigators to avoid influences on the measures. Randomization was performed based on confirmed allocation concealment. Unclear allocation concealment was noted when no method was mentioned. The integrity of the data was evaluated, including the proportion of excluded participants (http://http://www.cochrane-handbook.org).
The Cochrane Review Manager was used to analyze the relevant data. The mean differences (MDs) in continuous data under LGG or placebo treatment were measured. Dichotomous results are pooled and presented as risk ratios. Additionally, 95% confidence intervals (CIs) are reported for all types of outcomes. I2 and χ2 values were calculated to quantify and reflect heterogeneity. A P-value < 0.05 indicates that heterogeneity should not be ignored; thus, a random-effects model was used. A fixed-effects model was employed when no statistically significant inconsistency was detected. Publication bias was assessed by funnel plot asymmetry[18]. Sensitivity analyses were conducted to detect the robustness of results by assessing randomization, missing data, blinding, and allocation concealment. Each individual study was systematically removed from the meta-analysis, and the effect was recalculated and estimated from the remaining studies (Supporting information Figure S1). Regression analysis was conducted, and the relationships between the duration of diarrhea and other covariates, including publication year, participant age, the duration of diarrhea before study enrollment, and the LGG dosage, were examined. Subgroup analyses were performed to diminish significant inconsistency. Preplanned subgroup analyses were performed according to the following clinical characteristics and results from sensitivity or regression analysis: (1) The dosage of LGG per day. A dosage of 1010 CFU/day was observed to be a critical element of effective treatment in the study by Szajewska et al[13]. In addition, a larger dose was suggested in other studies[19,20]; (2) The etiology of diarrhea. Diarrhea mortality and severe diarrhea were most frequently caused by rotavirus in children[21]. Compared to control children, several rotavirus-positive children with watery stools in a probiotic group were reported to exhibit a marked reduction in diarrhea symptoms after 24 h[22]. A meta-analysis performed by Szajewska et al[23] in 2007 concluded that the duration of rotavirus-induced diarrhea was significantly attenuated by LGG supplementation; (3) The site of treatment (inpatient vs outpatient); (4) Vaccination status; (5) Geography of the clinical trials. The location of the study affected the sanitary habits, exposure to various pathogens, and nutrient status of the participants. All studied environmental factors contribute to various outcomes; (6) Early probiotic administration. A beneficial effect of probiotics was reported in the course of disease when initiated early[12]; and (7) Publication date.
A total of 349 potentially relevant studies were identified. The process of screening was carried out according to the flow diagram shown in Figure S2 (Supporting information). The characteristics of each included study are summarized in Table 1. With 988 participants in a 2007 meta-analysis and 2683 participants in a 2013 meta-analysis, a total of 4073 participants in 19 RCTs were identified in the literature. Two experimental arms in the study of Basu et al[24] were listed separately to exhibit different doses of probiotics, which were marked as Basu 2009a and Basu 2009b. Therefore, the figures, tables, and full texts of 18 articles were reviewed[8,24-40]. A large number of trials were conducted in Europe and Asia. Patients were recruited from outpatient, inpatient, and emergency departments. Inconsistency existed in the daily doses and routes of LGG supplementation during the treatment period. Different criteria were used to define diarrhea in the included studies. Diarrhea resolution was commonly defined as passage of the first normal stool or the last watery stool.
| Article | Type of article | Age group | Country | Patient source | n (exp/control) | Inclusion criteria | Exclusion criteria | LGG (dosage) | Control group | Duration of intervention | Etiology |
| Basu et al[25], 2007 | RCT; 1 center; Duration: 1 yr | Children | India | Inpatients | 323/323 | ≥ 3 watery stools/day without visible blood or mucus; <10 white blood cells/high-power field and no red cells, mucus flakes, or bacteria on stool microscopy; negative hanging drop preparation; negative bacterial stool culture | Systemic illness other than diarrhea on admission; systemic complications of diarrhea during hospitalization; failure to provide informed consent | 120 × 106 ; CFU/day | ORF | 7 d | Bacterial diarrhea excluded; Rotavirus-induced diarrhea 75.8% |
| Basu et al[24], 2009a | RCT; 1 center Duration: 1 yr | Children | India | Inpatients | 188/185 | ≥ 3 watery stools/day without macroscopic blood or mucus, <10 white blood cells/high-power field, and no red blood cells, mucus flakes, or bacteria on stool microscopy; negative hanging drop preparation; negative bacterial stool culture | Symptoms of illness other than diarrhea; development of any systemic complication of diarrhea during hospitalization; failure to provide informed consent | 2 × 1010 CFU/day | ORF | 7 d or until diarrhea stopped | Bacterial diarrhea excluded; Rotavirus diarrhea 57.1% |
| Basu et al[24] 2009b | RCT; 1 center Duration: 1 yr | Children | India | Inpatients | 186/185 | ≥ 3 watery stools/day without macroscopic blood or mucus, <10 white blood cells/high-power field, and no red blood cells, mucus flakes, or bacteria on stool microscopy; negative hanging drop preparation; negative bacterial stool culture | Symptoms of illness other than diarrhea; development of any systemic complication of diarrhea during hospitalization; failure to provide informed consent | 2 × 1012 CFU/day | ORF | 7 d or until diarrhea stopped | Bacterial diarrhea excluded; Rotavirus-induced diarrhea 56.06% |
| Canani et al[26], 2007 | RCT; 6 centers Duration: 12 mo | 3-36 mo | Italy | Outpatients | 100/92 | > 2 loose or liquid stools/day for <48 h | Malnutrition; severe dehydration; coexisting acute systemic illness; immunodeficiency; underlying severe chronic disease; cystic fibrosis; food allergy or other chronic GI diseases; use of probiotics in the previous 3 wk; antibiotics or any other antidiarrheal medication in the previous 3 wk; poor compliance | 12 × 109 CFU/day | No details given | 5 d | Stool culture in only a few participants and no data presented |
| Costa et al[27] 2003 | RCT; 1 center | Boys, 1-24 mo | Brazil | Inpatients | 61/63 | Acute diarrhea (3 or more watery or loose stools per 24 h during at least one 24-h period in the 72 h before admission) with moderate or severe dehydration after correction with rapid IV fluids | Systemic infections requiring antibiotics; severe malnutrition (weight for age < 65% of NCHS standards; bloody diarrhea | 1010 CFU/day | Inulin 320 mg/day | Unclear | Rotavirus-induced diarrhea 50%; Bloody diarrhea excluded |
| Czerwionka-Szaflarska et al[28], 2009 | RCT; 1 center | Unclear | Poland | Inpatients | 50/50 | Infants and children with acute infectious diarrhea and failed oral rehydration | Bloody stools; coexisting disease that may influence the course of diarrhea | 50 ml/kg/day | Unclear | Unclear | Bloody diarrhea excluded; Rotavirus-induced diarrhea 58% |
| Schnadower et al[8] 2018 | RCT | 3-48 mo | United States | University-affiliated PED | 483/488 | ≥ 3 watery stools per day, with or without vomiting, for fewer than 7 d | Pancreatitis, bilious emesis, or hematochezia; a known allergy to L. rhamnosus GG or to microcrystalline cellulose or a known allergy to erythromycin, clindamycin, and beta-lactam antibiotic agents; caregiver did not speak English or Spanish; children receiving antibiotics | 1 × 1010 CFU twice daily | Matching placebo | 5 d | Norovirus GI or GII 19.6%; Rotavirus 17.7%; Adenovirus 9.1%; Clostridium difficile 7.4%; Shigella 5.0% |
| Guandalini et al[29], 2000 | RCT; multicenter Duration: 1 yr | 1-36 mo | Listed as follows | Inpatients and outpatients | 147/140 | Infants and children with > 4 liquid or semiliquid stools/day for 1 to 5 d | Previous probiotic usage; untreated underlying chronic small bowel disease; inflammatory bowel disease; any underlying chronic disease or immunosuppressive disease or treatment | ≥10 × 109 CFU/250 mL/day with ORF | ORF | As tolerated for 4-6 h, then ad libitum | Rotavirus 35%; Bacteria 24%; Parasites 4.5%; No pathogens 34.5%; Bloody diarrhea 8.7% |
| Guarino et al[30], 1997 | RCT; 1 center Duration: 3 mo | 3-36 mo | Italy | Outpatients | 52/48 | Infants and children with ≥ 3 watery stools/day for < 48 h | Antibiotic treatment in the last 3 wk, breastfeeding; a weight: height ratio < the 5th percentile | 6 × 109 CFU/day with ORF | ORF | ≤5 d | Rotavirus-induced diarrhea 61% |
| Isolauri et al[31], 1994 | RCT; 1 center Duration: not stated | ≤ 36 mo | Finland | Inpatients | 21/21 | Infants and children with > 3 watery stools/day for < 7 d and stools positive for rotavirus; average dehydration of approximately 5% in both groups | Not stated | 2 × 1010 CFU/day | No probiotic | 5 d | Rotavirus-induced diarrhea 100% |
| Jasinski et al[32], 2002 | RCT; 12 centers Duration: not stated | 1-36 mo | Africa Egypt Europe America | Inpatients and outpatients | 45⁄52 | > 3 watery stools in 12 h or 1 liquid or semiliquid stool with mucus, pus, or blood; < 5 d | Antibiotic or probiotic use in the last 5 d; chronic diseases of the small or large intestine; immunosuppression; phenylketonuria | ORS + LGG 1010 CFU⁄ day | ORS with no LGG | Unclear | Bacterial pathogens 68%; Rotavirus 40.0%; parasites 6%; No pathogens identified: probiotic group 25% |
| Misra et al[33], 2009 | RCT; 1 center Duration: not stated | ≤ 36 mo | India | Inpatients | 105/ 105 | > 3 stools per day (watery or assuming the shape of the container) | Parents refused consent; children living outside the municipal area; bloody diarrhea; severe dehydration; shock, inability to take and retain oral foods; suspected systemic infection | 1 × 106-9 CFU/day | Crystalline micro cellulose | Unclear | Rotavirus 25.6%; Bloody diarrhea excluded; White blood cells in stools 14.3%; Bacterial diarrhea 4.7% |
| Nixon et al[34], 2012 | RCT | 6-72 mo | United States | PED | 77/78 | More than 2 loose stools in the last 24 h | Risk factors for non-viral diarrhea (prolonged diarrhea lasting more than 7 d, gross blood, antibiotic exposure, or inflammatory bowel disease); immune compromise; risk factors for probiotic-associated systemic illness or an allergy to milk products | LGG powder twice daily | Inulin | 5 d | Unclear |
| Pant et al[35], 1996 | RCT; 1 center Duration: 6 wk | 1-24 mo | Thailand | Inpatients | 20/19 | Infants and children with > 3 watery stools in last 24 h and diarrhea for < 14 d | Exclusive breastfeeding; septicemia | 109-10 CFU twice daily | Placebo | 2 d | Bloody stools 33.3%; Rotavirus 17.9%; Astrovirus 2.5% |
| Raza et al[36], 1995 | RCT; 1 center Duration: 2 mo | 1-24 mo | Pakistan | Inpatients | 21/19 | Undernourished infants and children with > 3 watery stools in the last 24 h for < 14 d and at least moderate dehydration | Severe malnutrition; septicemia | 2 × 1011-12 CFU/day | Placebo | 2 d | Bloody diarrhea |
| Ritchie et al[37], 2010 | RCT; 1 center Duration: 21 mo | 4-24 mo | Australia | Unclear | 33/31 | Aboriginal children with acute diarrhea defined as ≥ 3 loose stools during 24 h before presentation for < 7 d and able to tolerate ORF | Oxygen required during the study period; chronic cardiac, renal, or respiratory disease; previous gastrointestinal surgery; proven sucrose intolerance; suspected on known immunodeficiency; probiotic use before enrollment; younger than 4 mo of age | > 15 × 109 CFU/day | Identical placebo | 3 d | Bacterial pathogens 12.5%; Rotavirus 8.5%; Parasites 6% |
| Shornikova et al[38], 1997 | RCT; 1 center Duration: 1 yr | 1-36 mo | Russia | Inpatients | 59/64 | ≥ 1 watery stool in the last 24 h and diarrhea for < 5 d | Not stated | 1010 CFU/day | Placebo | 5 d | Rotavirus 27.4%; Bacterial diarrhea 21% |
| Sindhu et al[39], 2014 | RCT | 6-60 mo | India | Unclear | 65/59 | Diarrhea was defined as ≥ 3 loose watery stools within a 24-h period | Coinfections (the presence of both rotavirus and Cryptosporidium); severe malnutrition; probiotic consumption in the preceding month; allergy to probiotics; acute abdomen or colitis | 1010 CFU and 170 mg of microcrystalline /day cellulose | 170 mg of cellulose | 4 wk | Rotavirus 52.4%; Cryptosporidium species 47.6% |
| Sunny et al[40] 2014 | Open-label RCT | 6-60 mo | India | OPD or PED | 100/100 | Passage of three or more loose stools in the last 24 h | Severe malnutrition; dysentery; clinical evidence of coexisting acute systemic illnesses; clinical evidence of chronic disease; probiotic use in the preceding three weeks; antibiotic use | 1 × 1010 CFU per day | ORS and zinc 20 mg/d | 5 d | Rotavirus 24.1% |
Antibiotic treatment before recruitment was assessed, and different studies varied regarding the use of antibiotics. Similarly, the duration of treatment varied. Studies of moderate to high quality were adequately assessed and are summarized in Figure S3 (Supporting information).
Before enrollment, age was assessed in 16 studies, and the duration of diarrhea was reported in nine studies (Supporting information Figures S4 and S5). No obvious difference in age was found. The statistical differences and high heterogeneity resulting from the duration of diarrhea [MD -6.21 h, 95%CI (-9.04, -3.38)] could be reduced by subgrouping according to the outcomes of the sensitivity analysis (Supporting information Figure S1). The subgroup excluding the study of Ritchie et al[37] performed in 2010 showed acceptable heterogeneity, and no statistical significance was observed for the duration of diarrhea before study enrollment [MD -0.9 h, 95%CI (-4.02, 2.22)] (I2 = 10%). Sensitivity analysis revealed differences in the duration of diarrhea before study enrollment between the two groups in the study of Ritchie et al[37], which recruited aboriginal children in the Northern Territory of Australia. Social disadvantages and poverty contributed to malnutrition in these children[4]. However, no significant differences in the primary and secondary outcomes were found by sensitivity analysis, which is inconsistent with the findings reported in previous meta-analyses[4,13] (Supporting information Figure S1).
A reduced duration of diarrhea was found in the LGG group compared to that in the matched group according to 15 RCTs submitted to meta-analysis, which included 3721 participants [MD -24.02 h, 95%CI (-36.58, -11.45)] (Figure 1A). Significantly heterogeneous results were detected among the included trials (I2 = 98%). Our data support the results of the prior meta-analyses[4] indicating that LGG treatment reduced participants’ duration of diarrhea.
Subgroup analyses were conducted based on clinical features such as age, geographical location, treatment time, outpatient or inpatient settings, the time of enrollment, and literature quality scores. Differences in methodological quality could not explain the statistically significant heterogeneity (Supporting information Figure S6). Regression analysis between the duration of diarrhea and LGG dose revealed that different doses of LGG contributed to the heterogeneity (P = 0.009, adjusted R-squared = 40.21%), suggesting that subgroups according to a high or low dose of LGG should be assessed. A reduced duration of diarrhea was noted in the studies applying > 1010 CFU/day of LGG [MD -22.56 h, 95%CI (-36.41, -8.72)] (Figure 1A). In contrast, although only three studies used lower dosages, no statistically significant differences were detected in the groups receiving lower dosages [MD -30.95 h, 95%CI (-83.28, -21.39)] (Figure 1A). A reduced duration of diarrhea was supported in the studies with participants who received LGG treatment before the second day of diarrhea symptoms [MD -1.58 h, 95%CI (-3.05, -0.11)] and during the second to third days of diarrhea symptoms [MD -15.83 h, 95%CI (-20.06, -10.98)] (Figure 1B). However, Ritchie et al[37] enrolled participants with diarrhea for more than 3 d, and no statistically significant differences were found in the duration of diarrhea [MD 1.2 h, 95%CI (-21.42, 23.82)] (Figure 1B). A reduced diarrhea duration was reported in studies performed in both Asia and Europe [MD -24.42 h, 95%CI (-47.10, -1.82); MD -32.02 h, 95%CI (-49.26, -14.79), respectively]. Paradoxically, the reduction in the diarrhea duration in other regions was not statistically significant [MD -9.35 h, 95%CI (-20.77, 2.07)] (Figure 1C). In the etiological analysis, the effectiveness of LGG was clearly demonstrated in rotavirus-induced diarrhea cases [seven RCTs; MD -31.05 h, 95%CI (-50.31, -11.80)] (Figure 2). Analysis with the studies carried out in the 1990s and 2000s revealed a clear reduction in the diarrhea duration [MD -36.32 h, 95%CI (-62.20, -10.45); MD -29.40 h, 95%CI (-50.56, -8.25), respectively] (Supporting information Figure S7). In contrast, no reduction in the diarrhea duration was observed in the analysis with studies carried out in the 2010s [MD -3.43 h, 95%CI (-13.25, 6.39)] (Supporting information Figure S7). No studies evaluated the effectiveness of LGG in children vaccinated against rotavirus.
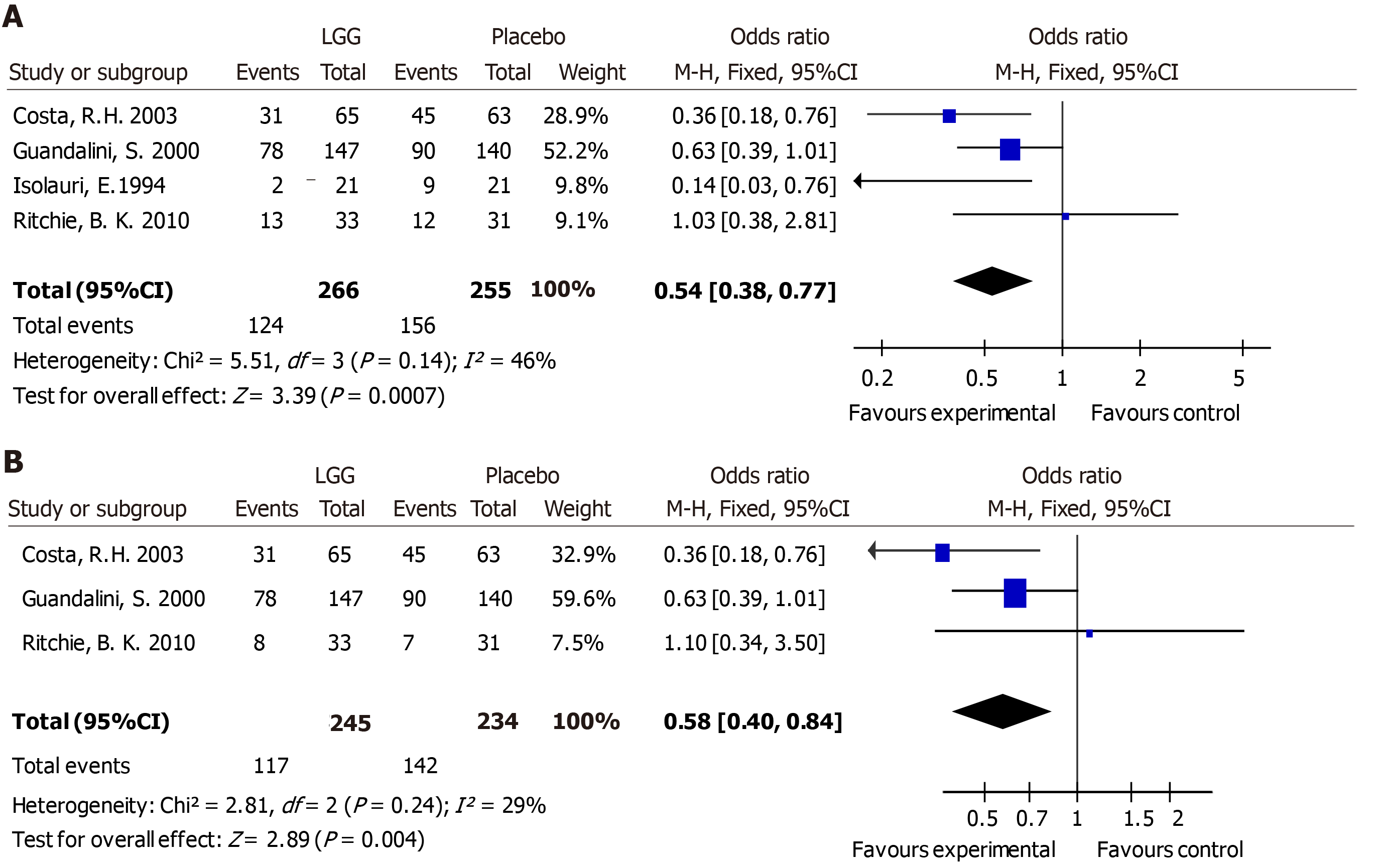
A meta-analysis of four RCTs was performed using a fixed-effects model. The risk of experiencing diarrhea for 3 or more days was reduced when patients received LGG [odds ratio (OR) 0.54, 95%CI (0.38, 0.77)] (Figure 3A).
Three studies were pooled (n = 479) and showed a reduction in the risk of diarrhea lasting for 4 or more days for participants treated with LGG [OR 0.58, 95%CI (0.4, 0.84)] (Figure 3B).
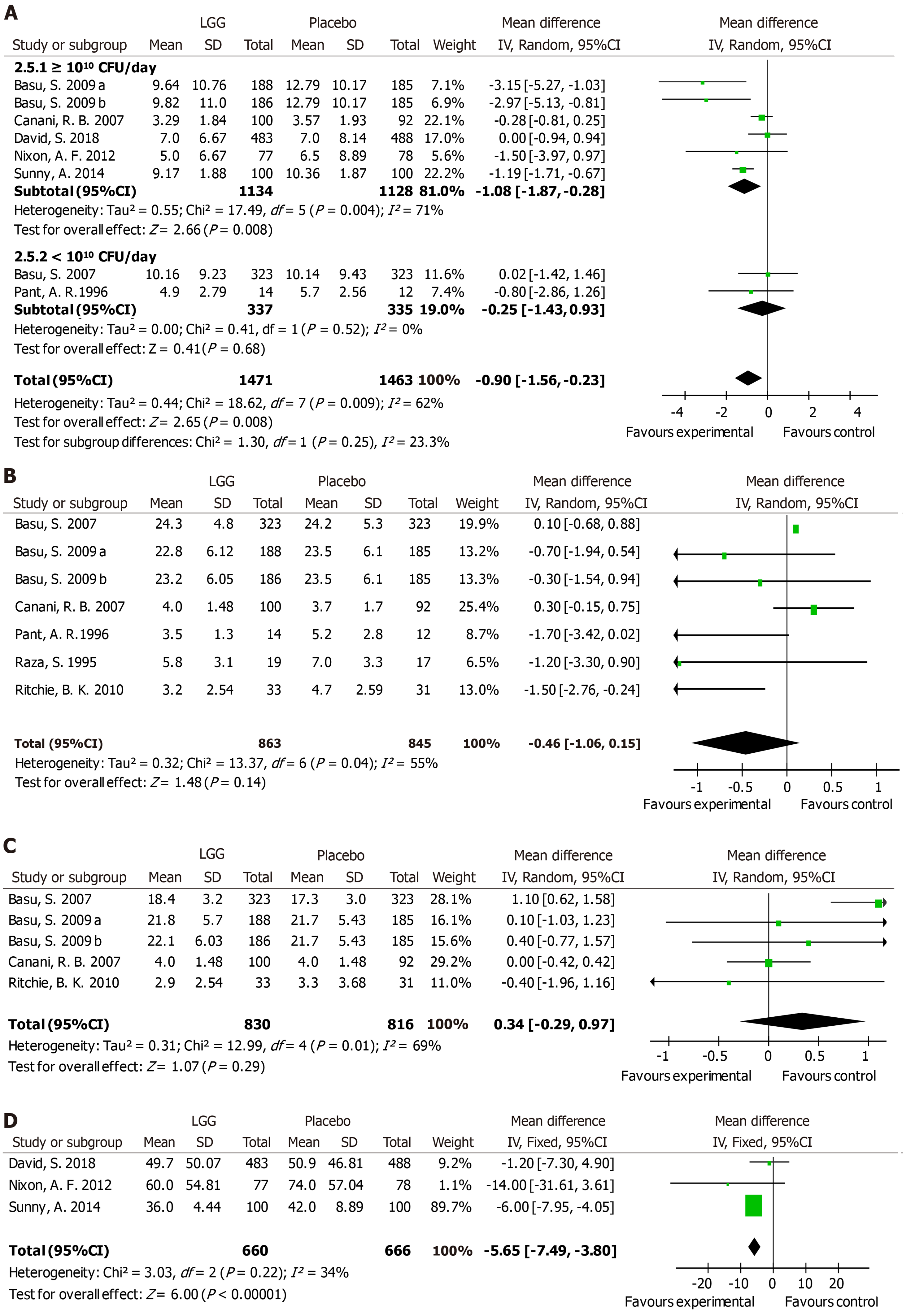
Stool number and consistency were evaluated in most trials. Eight trials reported the mean number of stools in one day during diarrhea episodes. A notable decrease in the stool number per day was noted in the LGG group [MD -0.9, 95%CI (-1.56, -0.23)] (Figure 4A). However, a significantly reduced stool number was observed in the high-dose LGG groups receiving no less than 1010 CFU/day [MD -1.08, 95%CI (-1.87, -0.28)], while the lower-dose groups showed no significant reduction [MD -0.25 d, 95%CI (-1.43, 0.93)] (Figure 4A). After the intervention, stool frequency was evaluated on days 2 and 3. Seven trials provided data on day 2, and the overall effect did not differ between the two groups [MD -0.46, 95%CI (-1.06, 0.15)] (Figure 4B). In addition, similar frequencies were observed in the two groups on day 3, with no differences between them [MD 0.34, 95%CI (-0.29, 0.97)] (Figure 4C). Three trials calculated the mean time to improvement in stool consistency, and an obvious reduction was reported [MD -5.65, 95%CI (-7.49, -3.80)] (Figure 4D).
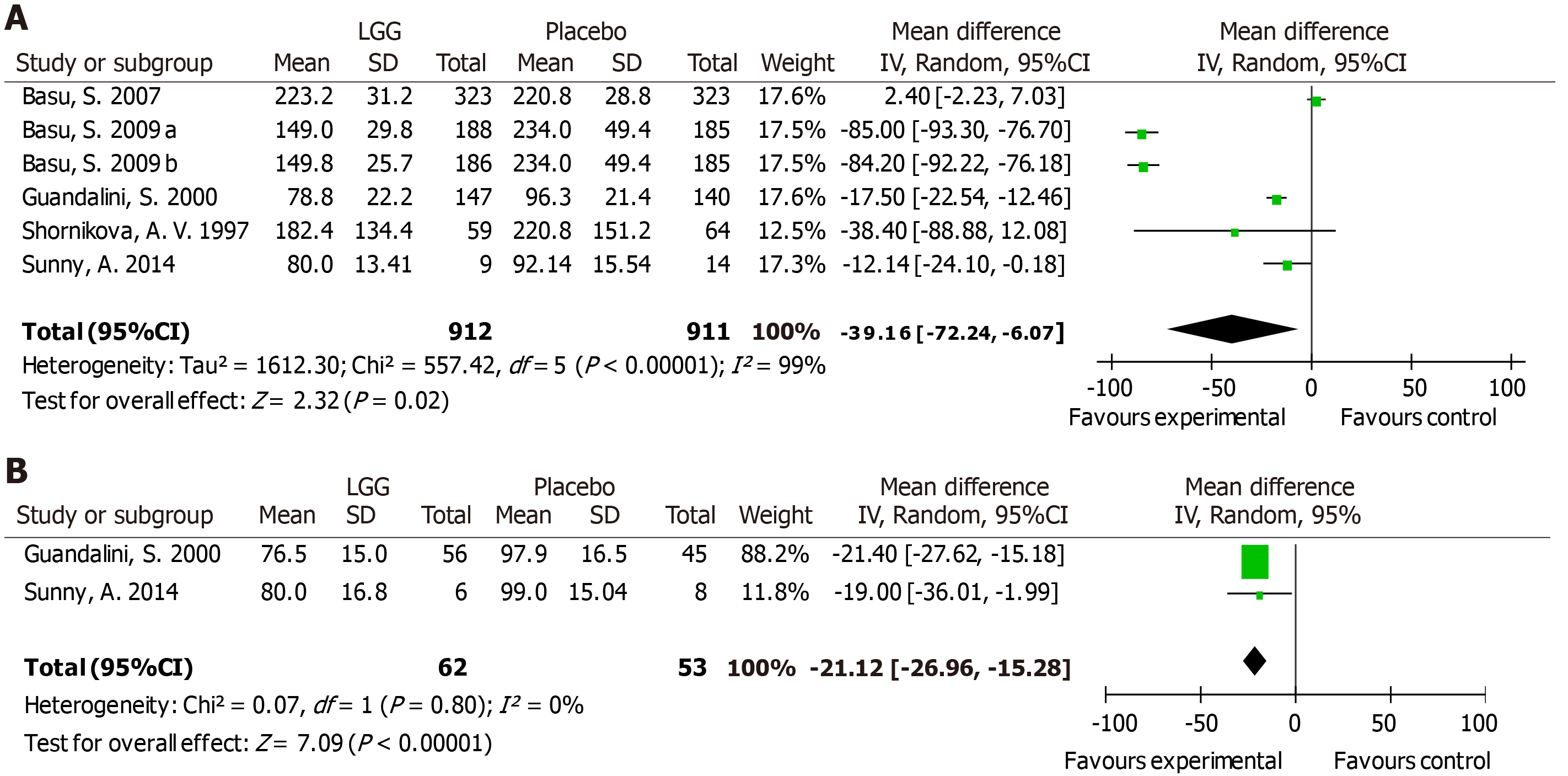
A total of 1823 participants from six RCTs were analyzed. Due to statistically significant heterogeneity, a random-effects model was used, which revealed a significant reduction in the hospital stay duration in the two groups [MD -39.16 h, 95%CI (-72.24, -6.07)] (Figure 5A). A reduction in the hospital stay duration was found in rotavirus-positive children [MD -21.12 h, 95%CI (-26.96, -15.28)] (Figure 5B).
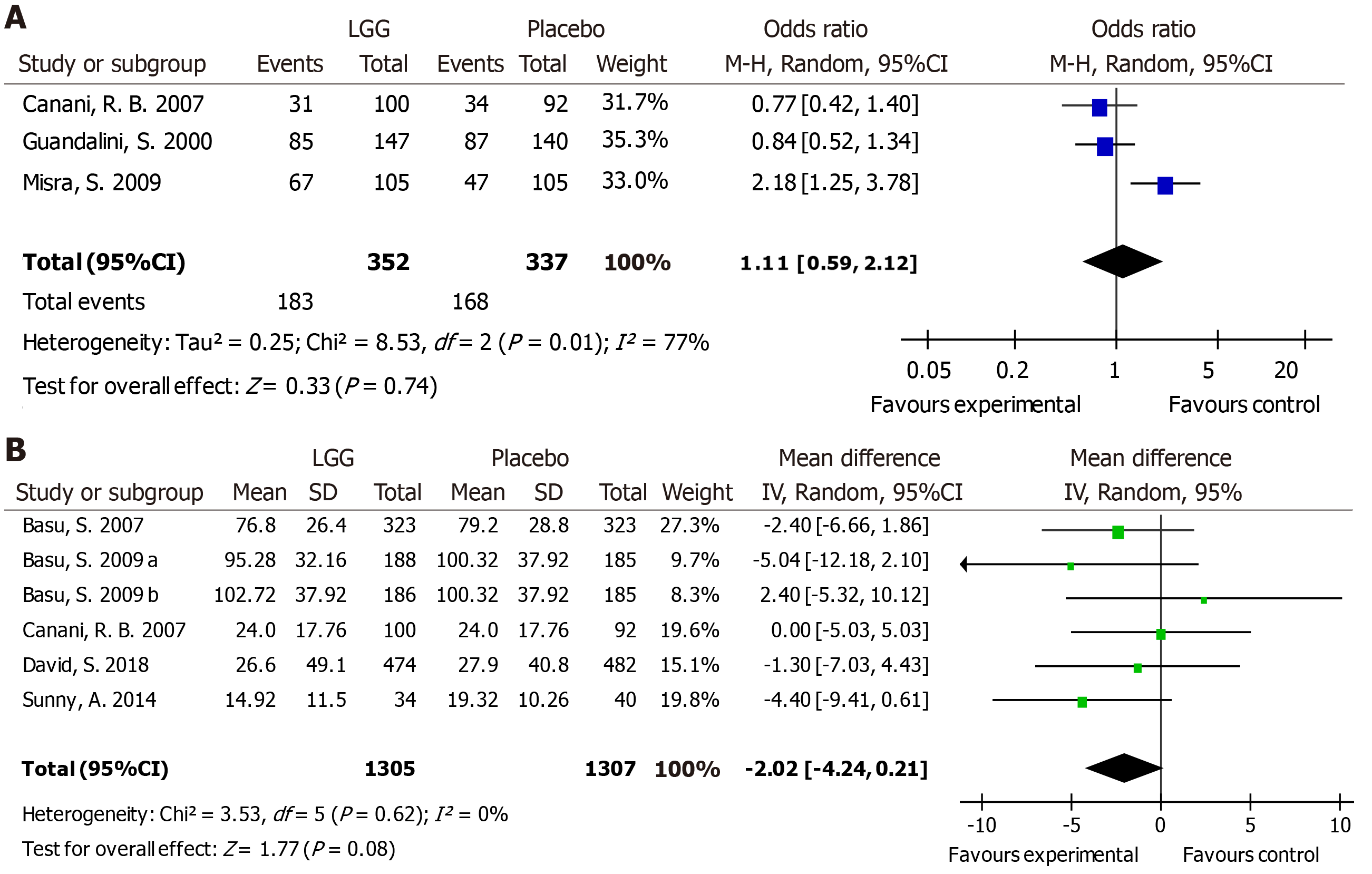
Vomiting in different trials was reported as the number of participants with vomiting [number (%)] or as the duration of vomiting (hours). Compared with the placebo group, no difference in the risk of vomiting was reported in the experimental group [OR 1.11, 95%CI (0.59, 2.12)] (Figure 6A). Furthermore, no reduction in the duration of vomiting was noted with LGG treatment [MD -2.02 h, 95%CI (-4.24, 0.21)] (Figure 6B).
Probiotics have been proposed to be well-tolerated and safe therapeutic agents. Most authors did not report adverse effects. Raza et al[36] reported one case of myoclonic jerks in their trial. Lower rates of respiratory infection, wheezing, and even vulvar abscess were noted in Schnadower’s trial[8,39], but these effects were not thought to be correlated with LGG use[40]. Aggarwal et al[40] reported no adverse effects, and a meta-analysis performed in 2013 showed comparable rates of adverse effects among study groups[13]. In our study, eight studies effectively evaluated the safety of LGG. Adverse effects were reported on a coded reporting form or during daily telephone calls[26,34]. In Schnadower’s study, the caregivers completed a daily diary that was collected by telephone or through email[8]. However, the reporting methods were unclear in five articles[24,37,39-41]. In general, no adverse effects or similar rates of side effects were documented in the LGG and placebo groups.
The risk of bias in 18 articles was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions. One trial employed alternating group allocation, and the random sequence generation method was not reported in five trials. Other RCTs provided detailed randomization methods, which mainly included computer-generated strategies, resulting in a low risk of selection bias. Allocation concealment was not applied in two trials and was not mentioned in seven. Nine trials used the sealed envelope technique for allocation concealment. Double blinding was strictly executed in 12 trials, while four trials allowed openness to patients or doctors, and two trials did not report a detailed blinding method. For detection bias, investigators were blinded to the group assignments in ten trials, while blinding assessments were not performed in three trials. Most trials provided complete data with a loss to follow-up rate less than 10%, although one trial had an unknown risk of incomplete outcome data, reflecting a low risk of attrition bias (Supporting information Figure S3).
According to Egger’s[18] regression asymmetry test, no small sample or publication bias was found in a funnel plot [P = 0.10, 95%CI (-11.33, -1.14)] (Supporting information Figure S8).
Nineteen trials comparing a control group with an experimental group treated with LGG were identified in this meta-analysis. In summary, the analysis revealed that treatment with LGG reduced both the duration of diarrhea and the hospital stay duration, especially in specific patient subsets. A striking finding was the time to improvement in stool consistency, which more investigators have confirmed since 2010[8,34,40]. In the whole range of diarrhea cases, the management of stools with this probiotic strain showed a modest beneficial effect on the number of stools per day and the time to improvement in stool consistency. However, no reduction in stool frequency was observed on days 2 and 3. Compared with the placebo group, the risk of diarrhea lasting more than 3 and 4 d was reduced by LGG administration. In both groups, similar rates of vomiting and adverse effects were observed.
Evidence from RCTs confirmed the beneficial effect of LGG on rotavirus-induced diarrhea[42]. In addition to interference with viral replication, most recent studies have shown that LGG prevented injuries to the epithelium and ameliorated rotavirus-induced diarrhea by modulating immune cells, such as dendritic cells and inflammatory cytokines[43,44]. The marked statistical difference in the diarrhea duration with a higher dosage of probiotics reflected greater effectiveness, which confirmed the dose dependence of dendritic cell activation. Treatment efficacy was related to the dose of LGG[45]. As confirmed in the study of Szajewska et al[13] in 2013, the importance of a daily LGG dose is high, and a dosage of 1010 CFU/day is needed for a positive effect. The statistical heterogeneity between studies can be explained by the timing of the LGG intervention, which was directly correlated with indicators such as the duration of diarrhea before study enrollment. Although the heterogeneity persisted in the subgroup with the shortest duration of diarrhea before study enrollment, probiotics should be applied early in the course of disease. Moreover, symptoms are usually mild at the early stage. Differences in prominent pathogens, sanitation conditions, and common comorbidities lead to dissimilarities between various study locations. Due to differences in the treatment effect among regions, the implications for clinical practice should be evaluated. The nutrient intake and dietary structure of humans have continuously changed in recent decades, which may have caused the reduced effectiveness of LGG reflected in the results of the trials conducted in the 2010s.
Probiotics manipulate and restore the gut microbiota, thus benefitting the prevention of diarrhea. Various therapeutic interventions designed to alter the microbiota range from probiotic administration to fecal microbiota trans-plantation[46,47]. However, due to the limited number of included studies and the self-limiting nature of disease, strategies should also be discussed in detail. Vomiting was reported as an adverse event in numerous studies[48,49], and it is one of the most common symptoms associated with diarrhea[50,51]. Additionally, less frequent clinical symptoms were observed in the probiotic groups[4], although our meta-analysis showed no improvement in the risk or duration of vomiting.
The safety of probiotic supplementation is generally certain. Nevertheless, pathologies correlated with the use of probiotic products to treat gastrointestinal disorders have been identified, such as endocarditis, sepsis, and bacteremia[52-54]. Unfortunately, the most prevalent strain implicated in the adverse effects was Lactobacillus rhamnosus. Conversely, most authors in our analysis did not report adverse effects or the adverse effects were not thought to be correlated with LGG treatment. In addition to the interventions, the primary illness contributed the most to the participant drop-out rate. A higher frequency of negative effects attributed to probiotics was found in catheterized (82.5%) and immunosuppressed (66%) participants[55]. Further safety evaluations of probiotics are necessary in the clinical setting, especially for susceptible individuals, such as those with immunodeficiency, immunosuppression, or malnourishment.
Preventing or correcting dehydration through treatment with zinc or 0.9% saline solution is the main approach used for diarrhea management[56]. However, during diarrhea episodes, infectious symptoms are not fully alleviated and the gut microbiota is not restored by rehydration measures[57]. Probiotics were investigated as therapeutic agents for diarrhea. The mechanisms by which probiotics alleviate diarrhea are described below. Host defenses are reinforced by enhanced antimicrobial peptide secretion. Probiotics prevent disruption of gut barrier integrity and stimulate the expression of junctional adhesion and tight junction molecules[58-61]. They produce short-chain fatty acids and induce the production of IgA to resist infections[62-64]. In epithelial cells and mucin, probiotics compete for binding sites to arrest pathogen colonization[65]. Probiotics can specifically and nonspecifically interfere with the viral cycle, thus impeding the progression of rotavirus-induced diarrhea[66-68]. The prevalence of diarrhea is seasonal, and almost all cases of rotavirus-induced diarrhea occur from January to May in Russia[38]. By contrast, in regions where rotavirus is not prevalent, bacterial diarrhea commonly occurs from June to October[38]. Influenza seasons, dietary habits, and antibiotic use must be considered when evaluating heterogeneity in further studies. The efficacy of probiotic treatment was altered based on host and environmental factors[12]. Overall, our study supported the previous systematic reviews which concluded that LGG is an effective treatment for children with acute diarrhea.
Although most studies have suggested that LGG is efficacious, limited identification of pathogens, small sample sizes, varying therapeutic strategies, and methodological limitations such as articles without a strictly blinded design, including a lack of a standard clinical parameter format, weakened the conclusions and precluded further analyses across studies[69]. For example, Czerwionka-Szaflarska et al[28] did not specifically define the treatment applied, although a significantly reduced duration of diarrhea was detected. Salazar-Lindo et al[41] partially depicted the duration of diarrhea in children with or without LGG treatment. Although factors varied in the trials, according to the same criterion for both groups, no evidence suggests that a poor study design leads to overestimation of probiotic efficacy[4]. Appropriate subgroups, such as those stratified by etiology and nutritional status, are indispensable. In 2016, approximately 8.4% of children (480000) presenting with diarrhea ultimately died due to the condition worldwide (https://data.unicef. org/topic/child-health/diarrhoeal-disease/). Assessments of the availability of vaccines, the applicability of probiotics, and the effectiveness of current treatments under severe conditions and cost-effect analyses must be performed to optimize therapeutic strategies for acute diarrhea management in children.
In summary, the following conclusions were cautiously established: LGG reduces the duration of diarrhea, particularly in patients with rotavirus-positive diarrhea receiving a dosage no less than 1010 CFU per day and in patients treated at the early stage. In addition, studies conducted in Asia and Europe showed greater treatment efficacy. The therapeutic effect of LGG supplementation on the stool number per day and hospital stay duration associated with rotavirus-induced diarrhea is high.
Diarrhea is a major infectious cause of childhood morbidity and mortality worldwide. Preventing or correcting dehydration through treatment with zinc or 0.9% saline solution is the main approach for diarrhea management; however, during diarrhea episodes, infectious symptoms are not fully alleviated by rehydration measures. Probiotics restore the gut microbiota and have been reported to reduce the duration of diarrhea.
Although previous studies have reported that Lactobacillus rhamnosus GG (LGG) is an effective therapeutic agent for acute diarrhea in children, a recent large, high-quality RCT found no adequate evidence of a beneficial effect of LGG treatment.
To evaluate the efficacy of LGG in treating acute diarrhea in children and provide some reference for future trials of treatments for diarrhea.
The EMBASE, MEDLINE, PubMed, Web of Science databases, and the Cochrane Central Register of Controlled Trials were searched up to April 2019 for meta-analyses and randomized controlled trials (RCTs). Cochrane Review Manager was used to analyze the relevant data and primary outcomes, including the duration of diarrhea and diarrhea lasting ≥ 3 and ≥ 4 d. Secondary outcomes included the hospital stay duration, stool frequency, and improvement in stool consistency and vomiting.
The systematic review identified 19 RCTs that met the inclusion criteria and indicated that compared with the control group, LGG administration notably reduced the diarrhea duration [mean difference (MD) -24.02 h, 95% confidence interval (CI) (-36.58, -11.45)]. Greater reductions were detected at a high dose of ≥ 1010 CFU per day [MD -22.56 h, 95%CI (-36.41, -8.72)] and in LGG participants with diarrhea for less than 3 days at study enrollment [MD -15.83 h, 95%CI (-20.68, -10.98)]. The study locations contributed to differences in the reduction in the diarrhea duration in Asia and Europe [MD -24.42 h, 95%CI (-47.01, -1.82); MD -32.02 h, 95%CI (-49.26, -14.79), respectively]. High-dose LGG treatment was confirmed to effectively reduce the duration of rotavirus-induced diarrhea [MD -31.05 h, 95%CI (-50.31, -11.80)] and stool number [MD -1.08, 95%CI (-1.87, -0.28)].
The following conclusions were cautiously established: compared to control children, children who received a course of LGG had better outcomes, including a markedly reduced duration of diarrhea, especially those with rotavirus-positive diarrhea, those who received no less than 1010 CFU per day, and those treated at the early stage. Furthermore, studies conducted in Asia and Europe reported greater treatment efficacy. The therapeutic effect of LGG supplementation on the stool number per day and hospital stay duration associated with rotavirus-induced diarrhea was high.
Our study found better outcomes among children with acute diarrhea who were treated by LGG supplementation. Limited identification of pathogens, small sample sizes, and a lack of a standard clinical parameter format precluded further analyses across studies, thus weakening the evidence required to guide clinical practice. Investigations are required to assess the cost-effectiveness of treating diarrhea with probiotics.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tuna Kirsaclioglu CT, Reyes VEE, Rhoads JM S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1417] [Cited by in RCA: 1500] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 2. | do Carmo MS, Santos CID, Araújo MC, Girón JA, Fernandes ES, Monteiro-Neto V. Probiotics, mechanisms of action, and clinical perspectives for diarrhea management in children. Food Funct. 2018;9:5074-5095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Savarino SJ, Bourgeois AL. Diarrhoeal disease: Current concepts and future challenges. Epidemiology of diarrhoeal diseases in developed countries. Trans R Soc Trop Med Hyg. 1993;87 Suppl 3:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;CD003048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (2)] |
| 5. | Binder HJ, Brown I, Ramakrishna BS, Young GP. Oral rehydration therapy in the second decade of the twenty-first century. Curr Gastroenterol Rep. 2014;16:376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Bryce J, Terreri N, Victora CG, Mason E, Daelmans B, Bhutta ZA, Bustreo F, Songane F, Salama P, Wardlaw T. Countdown to 2015: Tracking intervention coverage for child survival. Lancet. 2006;368:1067-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Pérez-Gaxiola G, Cuello-García CA, Florez ID, Pérez-Pico VM. Smectite for acute infectious diarrhoea in children. Cochrane Database Syst Rev. 2018;4:CD011526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Schnadower D, Tarr PI, Casper TC, Gorelick MH, Dean JM, O'Connell KJ, Mahajan P, Levine AC, Bhatt SR, Roskind CG, Powell EC, Rogers AJ, Vance C, Sapien RE, Olsen CS, Metheney M, Dickey VP, Hall-Moore C, Freedman SB. Lactobacillus rhamnosus GG versus Placebo for Acute Gastroenteritis in Children. N Engl J Med. 2018;379:2002-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39:237-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 475] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: A review of the evidence from studies conducted in humans. Curr Pharm Des. 2009;15:1428-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: A systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33 Suppl 2:S17-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 269] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Wolvers D, Antoine JM, Myllyluoma E, Schrezenmeir J, Szajewska H, Rijkers GT. Guidance for substantiating the evidence for beneficial effects of probiotics: Prevention and management of infections by probiotics. J Nutr. 2010;140:698S-712S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 13. | Szajewska H, Skórka A, Ruszczyński M, Gieruszczak-Białek D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children--updated analysis of randomised controlled trials. Aliment Pharmacol Ther. 2013;38:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Szajewska H, Guarino A, Hojsak I, Indrio F, Kolacek S, Shamir R, Vandenplas Y, Weizman Z; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Use of probiotics for management of acute gastroenteritis: A position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Guarino A, Guandalini S, Lo Vecchio A. Probiotics for Prevention and Treatment of Diarrhea. J Clin Gastroenterol. 2015;49 Suppl 1:S37-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 16. | Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet. 2018;391:1357-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2285] [Cited by in RCA: 1955] [Article Influence: 279.3] [Reference Citation Analysis (0)] |
| 17. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13343] [Article Influence: 833.9] [Reference Citation Analysis (0)] |
| 18. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40493] [Article Influence: 1446.2] [Reference Citation Analysis (2)] |
| 19. | Johnston BC, Goldenberg JZ, Vandvik PO, Sun X, Guyatt GH. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;CD004827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 20. | Johnston BC, Ma SS, Goldenberg JZ, Thorlund K, Vandvik PO, Loeb M, Guyatt GH. Probiotics for the prevention of Clostridium difficile-associated diarrhea: A systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 21. | Cunliffe NA, Kilgore PE, Bresee JS, Steele AD, Luo N, Hart CA, Glass RI. Epidemiology of rotavirus diarrhoea in Africa: A review to assess the need for rotavirus immunization. Bull World Health Organ. 1998;76:525-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Simakachorn N, Pichaipat V, Rithipornpaisarn P, Kongkaew C, Tongpradit P, Varavithya W. Clinical evaluation of the addition of lyophilized, heat-killed Lactobacillus acidophilus LB to oral rehydration therapy in the treatment of acute diarrhea in children. J Pediatr Gastroenterol Nutr. 2000;30:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Szajewska H, Skórka A, Ruszczyński M, Gieruszczak-Białek D. Meta-analysis: Lactobacillus GG for treating acute diarrhoea in children. Aliment Pharmacol Ther. 2007;25:871-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Basu S, Paul DK, Ganguly S, Chatterjee M, Chandra PK. Efficacy of high-dose Lactobacillus rhamnosus GG in controlling acute watery diarrhea in Indian children: A randomized controlled trial. J Clin Gastroenterol. 2009;43:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 25. | Basu S, Chatterjee M, Ganguly S, Chandra PK. Efficacy of Lactobacillus rhamnosus GG in acute watery diarrhoea of Indian children: A randomised controlled trial. J Paediatr Child Health. 2007;43:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 26. | Canani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo MI, De Vincenzo A, Albano F, Passariello A, De Marco G, Manguso F, Guarino A. Probiotics for treatment of acute diarrhoea in children: Randomised clinical trial of five different preparations. BMJ. 2007;335:340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Costa-Ribeiro H, Ribeiro TC, Mattos AP, Valois SS, Neri DA, Almeida P, Cerqueira CM, Ramos E, Young RJ, Vanderhoof JA. Limitations of probiotic therapy in acute, severe dehydrating diarrhea. J Pediatr Gastroenterol Nutr. 2003;36:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Czerwionka-Szaflarska M, Murawska S, Swincow G. Evaluation of influence of oral treatment with probiotic and/or oral rehydration solution on course of acute diarrhoea in children. Przegl Gastroenterol. 2009;4:166-172. |
| 29. | Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A, de Sousa JS, Sandhu B, Szajewska H, Weizman Z. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: A multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 339] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 30. | Guarino A, Canani RB, Spagnuolo MI, Albano F, Di Benedetto L. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr. 1997;25:516-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 178] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Isolauri E, Kaila M, Mykkänen H, Ling WH, Salminen S. Oral bacteriotherapy for viral gastroenteritis. Dig Dis Sci. 1994;39:2595-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 152] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Jasinski C TM, Tanzi MN, Schelotto F, Varela G, Zanetta E, Acuña A, and Arenas C, del Pilar Gadea M, Sirok A, Betancor L, Grotiuz G, Sandín D, Combol A, Xavier B, Vignoli R, Nairac A. Efficacy of Lactobacillus GG in oral rehydration solution. Pediatrica. 2002;22:231-243. |
| 33. | Misra S, Sabui TK, Pal NK. A randomized controlled trial to evaluate the efficacy of lactobacillus GG in infantile diarrhea. J Pediatr. 2009;155:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Nixon AF, Cunningham SJ, Cohen HW, Crain EF. The effect of Lactobacillus GG on acute diarrheal illness in the pediatric emergency department. Pediatr Emerg Care. 2012;28:1048-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Pant AR, Graham SM, Allen SJ, Harikul S, Sabchareon A, Cuevas L, Hart CA. Lactobacillus GG and acute diarrhoea in young children in the tropics. J Trop Pediatr. 1996;42:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Raza S, Graham SM, Allen SJ, Sultana S, Cuevas L, Hart CA. Lactobacillus GG promotes recovery from acute nonbloody diarrhea in Pakistan. Pediatr Infect Dis J. 1995;14:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 119] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Ritchie BK, Brewster DR, Tran CD, Davidson GP, McNeil Y, Butler RN. Efficacy of Lactobacillus GG in aboriginal children with acute diarrhoeal disease: A randomised clinical trial. J Pediatr Gastroenterol Nutr. 2010;50:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Shornikova AV, Isolauri E, Burkanova L, Lukovnikova S, Vesikari T. A trial in the Karelian Republic of oral rehydration and Lactobacillus GG for treatment of acute diarrhoea. Acta Paediatr. 1997;86:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Sindhu KN, Sowmyanarayanan TV, Paul A, Babji S, Ajjampur SS, Priyadarshini S, Sarkar R, Balasubramanian KA, Wanke CA, Ward HD, Kang G. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: A randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2014;58:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 40. | Aggarwal S, Upadhyay A, Shah D, Teotia N, Agarwal A, Jaiswal V. Lactobacillus GG for treatment of acute childhood diarrhoea: An open labelled, randomized controlled trial. Indian J Med Res. 2014;139:379-385. [PubMed] |
| 41. | Salazar-Lindo E, Miranda-Langschwager P, Campos-Sanchez M, Chea-Woo E, Sack RB. Lactobacillus casei strain GG in the treatment of infants with acute watery diarrhea: A randomized, double-blind, placebo controlled clinical trial [ISRCTN67363048]. BMC Pediatr. 2004;4:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Ahmadi E, Alizadeh-Navaei R, Rezai MS. Efficacy of probiotic use in acute rotavirus diarrhea in children: A systematic review and meta-analysis. Caspian J Intern Med. 2015;6:187-195. [PubMed] |
| 43. | Liu F, Li G, Wen K, Wu S, Zhang Y, Bui T, Yang X, Kocher J, Sun J, Jortner B, Yuan L. Lactobacillus rhamnosus GG on rotavirus-induced injury of ileal epithelium in gnotobiotic pigs. J Pediatr Gastroenterol Nutr. 2013;57:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Jiang Y, Ye L, Cui Y, Yang G, Yang W, Wang J, Hu J, Gu W, Shi C, Huang H, Wang C. Effects of Lactobacillus rhamnosus GG on the maturation and differentiation of dendritic cells in rotavirus-infected mice. Benef Microbes. 2017;8:645-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Cai S, Kandasamy M, Rahmat JN, Tham SM, Bay BH, Lee YK, Mahendran R. Lactobacillus rhamnosus GG Activation of Dendritic Cells and Neutrophils Depends on the Dose and Time of Exposure. J Immunol Res. 2016;2016:7402760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Khoruts A. Targeting the microbiome: From probiotics to fecal microbiota transplantation. Genome Med. 2018;10:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Vemuri RC, Gundamaraju R, Shinde T, Eri R. Therapeutic interventions for gut dysbiosis and related disorders in the elderly: Antibiotics, probiotics or faecal microbiota transplantation? Benef Microbes. 2017;8:179-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Boudraa G, Benbouabdellah M, Hachelaf W, Boisset M, Desjeux JF, Touhami M. Effect of feeding yogurt versus milk in children with acute diarrhea and carbohydrate malabsorption. J Pediatr Gastroenterol Nutr. 2001;33:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Kurugöl Z, Koturoğlu G. Effects of Saccharomyces boulardii in children with acute diarrhoea. Acta Paediatr. 2005;94:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Henriksson R, Bergström P, Franzén L, Lewin F, Wagenius G. Aspects on reducing gastrointestinal adverse effects associated with radiotherapy. Acta Oncol. 1999;38:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Uhnoo I, Svensson L, Wadell G. Enteric adenoviruses. Baillieres Clin Gastroenterol. 1990;4:627-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Cannon JP, Lee TA, Bolanos JT, Danziger LH. Pathogenic relevance of Lactobacillus: A retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis. 2005;24:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 53. | De Groote MA, Frank DN, Dowell E, Glode MP, Pace NR. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr Infect Dis J. 2005;24:278-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 54. | Molinaro M, Aiazzi M, La Torre A, Cini E, Banfi R. [Lactobacillus Rhamnosus sepsis in a preterm infant associated with probiotic integrator use: A case report.]. Recenti Prog Med. 2016;107:485-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 55. | Gouriet F, Million M, Henri M, Fournier PE, Raoult D. Lactobacillus rhamnosus bacteremia: An emerging clinical entity. Eur J Clin Microbiol Infect Dis. 2012;31:2469-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | World Health Organization. The Treatment of Diarrhea: A Manual for Physicians and other Senior Health Workers. Geneva: World Health Organization 2005; . |
| 57. | Das RR. Zinc in acute childhood diarrhea: Is it universally effective? Indian J Pharmacol. 2012;44:140; author reply 140-140; author reply 141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298:G851-G859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 472] [Article Influence: 31.5] [Reference Citation Analysis (1)] |
| 59. | Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140-G1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (1)] |
| 60. | Yang F, Wang A, Zeng X, Hou C, Liu H, Qiao S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015;15:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 61. | Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 62. | Fukushima Y, Kawata Y, Hara H, Terada A, Mitsuoka T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol. 1998;42:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 196] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Rautava S, Arvilommi H, Isolauri E. Specific probiotics in enhancing maturation of IgA responses in formula-fed infants. Pediatr Res. 2006;60:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 64. | Naidu AS, Bidlack WR, Clemens RA. Probiotic spectra of lactic acid bacteria (LAB). Crit Rev Food Sci Nutr. 1999;39:13-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 351] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 65. | Bujňáková D, Kmeť V. Inhibitory potential of lactobacilli against Escherichia coli internalization by HT 29 cells. Folia Microbiol (Praha). 2012;57:269-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Gonzalez-Ochoa G, Flores-Mendoza LK, Icedo-Garcia R, Gomez-Flores R, Tamez-Guerra P. Modulation of rotavirus severe gastroenteritis by the combination of probiotics and prebiotics. Arch Microbiol. 2017;199:953-961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Kang JY, Lee DK, Ha NJ, Shin HS. Antiviral effects of Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 on rotavirus-infected Caco-2 cells and a neonatal mouse model. J Microbiol. 2015;53:796-803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 68. | Paim FC, Langel SN, Fischer DD, Kandasamy S, Shao L, Alhamo MA, Huang HC, Kumar A, Rajashekara G, Saif LJ, Vlasova AN. Effects of Escherichia coli Nissle 1917 and Ciprofloxacin on small intestinal epithelial cell mRNA expression in the neonatal piglet model of human rotavirus infection. Gut Pathog. 2016;8:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Kolaček S, Hojsak I, Berni Canani R, Guarino A, Indrio F, Orel R, Pot B, Shamir R, Szajewska H, Vandenplas Y, van Goudoever J, Weizman Z; ESPGHAN Working Group for Probiotics and Prebiotics. Commercial Probiotic Products: A Call for Improved Quality Control. A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2017;65:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |