Published online Sep 7, 2019. doi: 10.3748/wjg.v25.i33.4959
Peer-review started: April 24, 2019
First decision: July 22, 2019
Revised: July 29, 2019
Accepted: August 7, 2019
Article in press: August 7, 2019
Published online: September 7, 2019
Processing time: 136 Days and 19.9 Hours
Liver stiffness measurement (LSM) tends to overestimate fibrosis stage in nonalcoholic fatty liver disease (NAFLD). Controlled attenuation parameter (CAP), provided by LSM device, has been introduced for noninvasive quantification of hepatic steatosis.
To determine the role of CAP values in predicting liver fibrosis stage by LSM in nonalcoholic steatohepatitis (NASH).
One hundred eighty-four patients with biopsy proven NASH had LSM and CAP evaluated at baseline. Among them, 130 patients had 1-year follow up LSM and analyzed for the changes of LSM after pioglitazone or ursodeoxycholic acid (UDCA) treatment.
In Kleiner fibrosis stage F0-1, LSM values increased at higher CAP tertile (P = 0.001), and in F2, at middle and higher tertiles (P = 0.027). No difference across CAP tertiles was noticed in F3-4 (P = 0.752). Receiver operating characteristic curve for LSM cutoff in diagnosis of F ≥ 2 identified 8.05 kPa for lower CAP tertile, 9.35 kPa for middle, and 10.55 kPa for high tertile. When changes in proportion of significant fibrosis (F ≥ 2) were assessed among pioglitazone and UDCA treated patients considering CAP values, pioglitazone treated patients demonstrated decrease in proportion of high LSM.
In patient with NAFLD, interpretation of LSM in association with CAP scores may provide helpful information sparing unnecessary liver biopsy.
Core tip: Liver stiffness measurement (LSM) is said to be exaggerated in nonalcoholic fatty liver disease (NAFLD). We investigated the role of controlled attenuation parameter (CAP), a means of measuring steatosis noninvasively, in predicting liver fibrosis by LSM in 184 biopsy proven nonalcoholic steatohepatitis patients. The optimum LSM cutoff for Kleiner fibrosis stage (F) ≥ 2 reflecting CAP values showed higher cutoff with increased CAP tertile (LSM, 8.05 kPa for lower CAP tertile, 9.35 kPa for middle, 10.45 kPa for high CAP tertile). Therefore, we suggest that interpretation of LSM in patients with NAFLD should take CAP scores into account in order to avoid unnecessary liver biopsy.
- Citation: Lee JI, Lee HW, Lee KS. Value of controlled attenuation parameter in fibrosis prediction in nonalcoholic steatohepatitis. World J Gastroenterol 2019; 25(33): 4959-4969
- URL: https://www.wjgnet.com/1007-9327/full/v25/i33/4959.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i33.4959
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease around worldwide. The spectrum of NAFLD ranges from simple steatosis without evidence of liver injury to nonalcoholic steatohepatitis (NASH) with or without liver fibrosis[1]. Although the natural history of NAFLD requires further investigation, studies demonstrate that the severity of liver fibrosis is the most important determinant of mortality and morbidity in patients with NAFLD[2-4]. Patients with significant liver fibrosis [Kleiner classification fibrosis stage (F) ≥ 2] showed decreased survival compared to those with no or minimal fibrosis (F0-1)[4]. NAFLD may progress from simple steatosis to NASH with fibrosis, and estimation of severity of liver fibrosis is critical not only for the initial workup but also for follow- up[5].
Although liver biopsy is considered the gold standard for assessing the severity of fibrosis[6], it is an invasive procedure that might not be practical to perform sequentially. Instead, the liver stiffness measurement (LSM), obtained by transient elastograpy (TE) is a useful noninvasive means of assessing liver fibrosis. LSM values are well correlated with the biopsy determined severity of fibrosis[7-9]. However, the diagnostic performance of LSM is known to be affected by obesity and the severity of steatosis, which are closely associated with NAFLD, resulting in overestimation of the LSM in patients with NAFLD[10-12]. Recently, FibroScan, a type of TE, has been equipped with controlled attenuation parameter (CAP), software to enable noninvasive quantification of hepatic steatosis. The CAP value was strongly correlated with the histologically assessed percentage of liver fat in patients with NAFLD, but is susceptible to interference by liver fibrosis[13]. However, CAP may enhance the accuracy of TE measured LSM in patients with NAFLD[14]. We evaluated the role of the CAP value in predicting the liver fibrosis stage based on LSM in patients with biopsy proven NASH.
This retrospective study involved patients with biopsy proven NASH evaluated at Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea. Liver biopsy was performed to confirm the diagnosis of NASH in patients with ultrasound findings of fatty liver and persistent (> 6 mo) elevation of the alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level without excessive alcohol consumption (30 g/day in men and 20 g/day in women). Patients in whom liver stiffness was evaluated within 1 mo before the liver biopsy were included in the analysis. The exclusion criteria were as follows: (1) Liver disease of other or mixed etiology (such as hepatitis B infection, hepatitis C infection, alcohol abuse, autoimmune liver disease, Wilson’s disease, or drug-induced liver disease); (2) LSM evaluated while the AST or ALT level was more than fivefold the upper limit of normal (ULN); (3) Hepatocellular carcinoma; (4) Advanced liver cirrhosis (Child-Turcotte-Pugh B and C); (5) Previous treatment with steatosis-inducing drugs such as tamoxifen, aromatase inhibitor, valproic acid, amiodarone or corticosteroid; (6) Human immunodeficiency virus infection; (7) Active intravenous drug addiction or use of cannabis; and (8) Insufficient clinical data.
This study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital (permit no: 3-2019-0010). The requirement for written informed consent was exempted by the IRB since the database was accessed only for analysis purposes and the patients’ personal information was anonymized by coding.
Demographic, clinical and anthropometric data were collected at the time of liver biopsy. Hypertension was defined as use of antihypertensive medication and type 2 diabetes mellitus was considered present if the fasting glucose level was ≥ 126 mg/dL or antidiabetic agents were being used. Body mass index (BMI) was calculated as body weight in kilogram divided by the square of height in meters, and a BMI ≥ 25 kg/m2 was considered to indicate obesity based on the criteria used in the Asian-Pacific region[15].
TE was performed using a FibroScan (Echosens, Paris, France), medical device, with a standard probe. Only LS values with at least 10 valid measurements, a success rate of at least 60%, and an interquartile range-to-median ratio of < 30% were considered reliable, as suggested by previous studies[16,17]. In addition, patients in whom LS was measured while the AST or ALT > 5 × ULN was present, were excluded from the analysis due to possible exaggerated LSM values as previous studies demonstrated[18,19]. The baseline LSM was obtained within 1 month before liver biopsy. The follow-up LSM was performed after 12 mo of NASH treatment with daily dose of 15 mg pioglitazone, peroxisome proliferator-activated receptor (PPAR)-γ agonist, or 300 mg/day ursodeoxycholic acid (UDCA) following liver biopsy.
A single liver-dedicated expert pathologist, blinded to the patients’ identity, performed the histologic analysis. A ≥ 15-mm-long biopsy specimen or the presence of at least 10 complete portal tracts was considered adequate for the analysis[20]. NASH was diagnosed according to the NASH Clinical Research Network System, and was defined as the presence of ≥ 5% hepatic steatosis and inflammation with hepatocyte injury such as ballooning with or without fibrosis[21].
All statistical tests were performed using IBM SPSS Statistics 22.0 (IBM, Armonk, NY, United States). Continuous variables are expressed as means ± standard deviation (SD) or medians (range). The area under the receiver-operating characteristic (ROC) curve was calculated to reflect the overall accuracy of LSM for diagnosing significant fibrosis (F2-4). Categorical variables were compared by using two-sided χ2-test (or Fisher’s exact test, or McNemar test, as appropriate) and continuous variables by independent or Mann-Whitney test as appropriate. A paired t test was performed to evaluate changes in LSM. A two-sided P value of < 0.05 was considered indicative of statistical significance.
From January 2010 to December 2017, 325 patients underwent liver biopsy and LSM assessed due to suspicion of NASH. Among them, 184 patients met the inclusion and exclusion criteria and were thus included in the analysis. The baseline characteristics of the 184 patients with NASH are listed in Table 1.
| Variable | NAFLD (n = 184) |
| Age (yr) | 44.6 ± 14.5 |
| Male gender | 127 (69.0%) |
| Body mass index, median (range, kg/m2) | 29.3 (19.8-44.5) |
| Body mass index ≥ 25 kg/m2 | 156 (84.8%) |
| Diabetes | 69 (37.5%) |
| Hypertension | 54 (29.3%) |
| Hyperlipidemia | 75 (40.8%) |
| Fasting glucose (mg/dL) | 117.9 ± 32.9 |
| HDL cholesterol (md/dL) | 47.1 ± 11.8 |
| Triglycerides (mg/dL) | 193.8 ± 127.5 |
| LDL cholesterol (mg/dL) | 131.7 ± 31.3 |
| Alanine aminotransferase (U/L) | 92.4 ± 68.1 |
| Aspartate aminotransferase (U/L) | 67.3 ± 39.2 |
| Gamma glutamyltransferase (U/L) | 74.1 ± 86.7 |
| Platelet (× 1000/mm3) | 244.8 ± 58.6 |
| Albumin (g/dL) | 4.5 ± 0.3 |
| Liver stiffness (kPa) | 10.9 ± 4.9 |
| Stiffness IQR (kPa) | 1.8 ± 3.3 |
| CAP (dB/m) | 320.9 ± 37.1 |
| Lower tertile | 223-310 |
| Middle tertile | 311-339 |
| Higher tertile | 340-400 |
| CAP IQR (dB/m) | 24.7 ± 9.5 |
| Histology at biopsy | |
| Steatosis grade | |
| 1 (5%-33%) | 44 (23.9%) |
| 2 (34%-66%) | 81 (44.0%) |
| 3 (> 66%) | 59 (32.1%) |
| Stage of fibrosis (Kleiner) | |
| 0 | 20 (10.9%) |
| 1 | 84 (45.7%) |
| 2 | 53 (28.8%) |
| 3 | 21 (11.4%) |
| 4 | 6 (3.3%) |
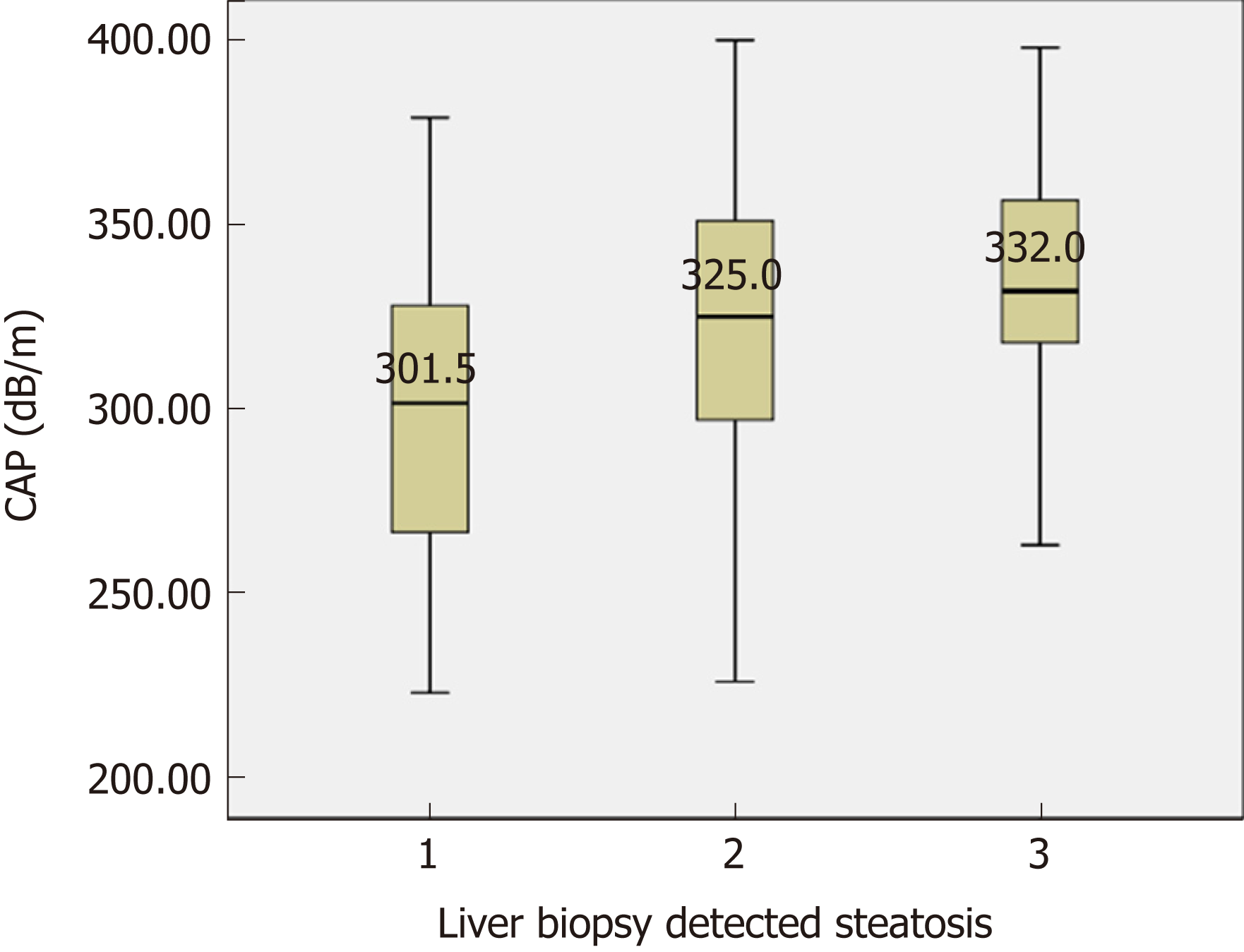
Among the 184 patients with biopsy proven NASH, the distribution of the histologically assessed steatosis grade (S) was as follows: S1, n = 44 (2.9%); S2, n = 81 (44.0%); S3, n = 59 (32.1%) (Figure 1). The CAP scores were significantly different between S1 and S2-S3 (P < 0.001). However, no significant difference was detected between S2 and S3 (P = 0.075). The ROC curve showed the optimum CAP cutoff for ≥ S2 was 313.5 dB/m [area under the curve (AUC), 0.736; sensitivity, 72.9%; specificity, 63.6%]. On the other hands, the accuracy dropped to an AUC of 0.656 for S3, with a cutoff of 323.5 dB/m (sensitivity 64.4%; specificity 55.2%).
LSM significantly increased with the histologically detected fibrosis stage (F0, 7.5 ± 2.1; F1, 9.8 ± 2.7; F2, 11.8 ± 4.9; F3, 15.4 ± 6.9; and F4, 20.3 ± 8.8 kPa) (P < 0.001). The ROC curve showed that the optimum LSM cutoff for ≥ F2 was 8.95 kPa (AUC, 0.730; sensitivity, 72.5%; specificity, 65.4%). According to univariate and multivariate analyses, the CAP values and pathologically detected fibrosis stage was significantly associated with LSM (Table 2).
| Univariate analysis | Multivariate analysis | |||||
| Variable | β | SE | P | β | SE | P |
| Age (yr) | 0.053 | 0.025 | 0.037a | 0.017 | 0.022 | 0.451 |
| Male gender | 0.957 | 0.792 | 0.228 | |||
| BMI (kg/m2) | -0.032 | 0.083 | 0.700 | |||
| CAP (dB/m) | 0.022 | 0.010 | 0.026a | 0.041 | 0.009 | < 0.001b |
| LS IQR (kPa) | 0.329 | 0.109 | 0.003b | 0.294 | 0.022 | 0.451 |
| Histology at biopsy | ||||||
| Steatosis | -0.895 | 0.490 | 0.069 | -0.532 | 0.445 | 0.233 |
| Lobular inflammation | 1.134 | 0.082 | 0.152 | 0.776 | ||
| 1.366 | 0.650 | 0.008b | 0.638 | 0.532 | 0.131 | |
| Ballooning | 2.769 | 0.509 | < 0.001b | 2.665 | 0.421 | < 0.001b |
| Fibrosis | 0.331 | 0.344 | ||||
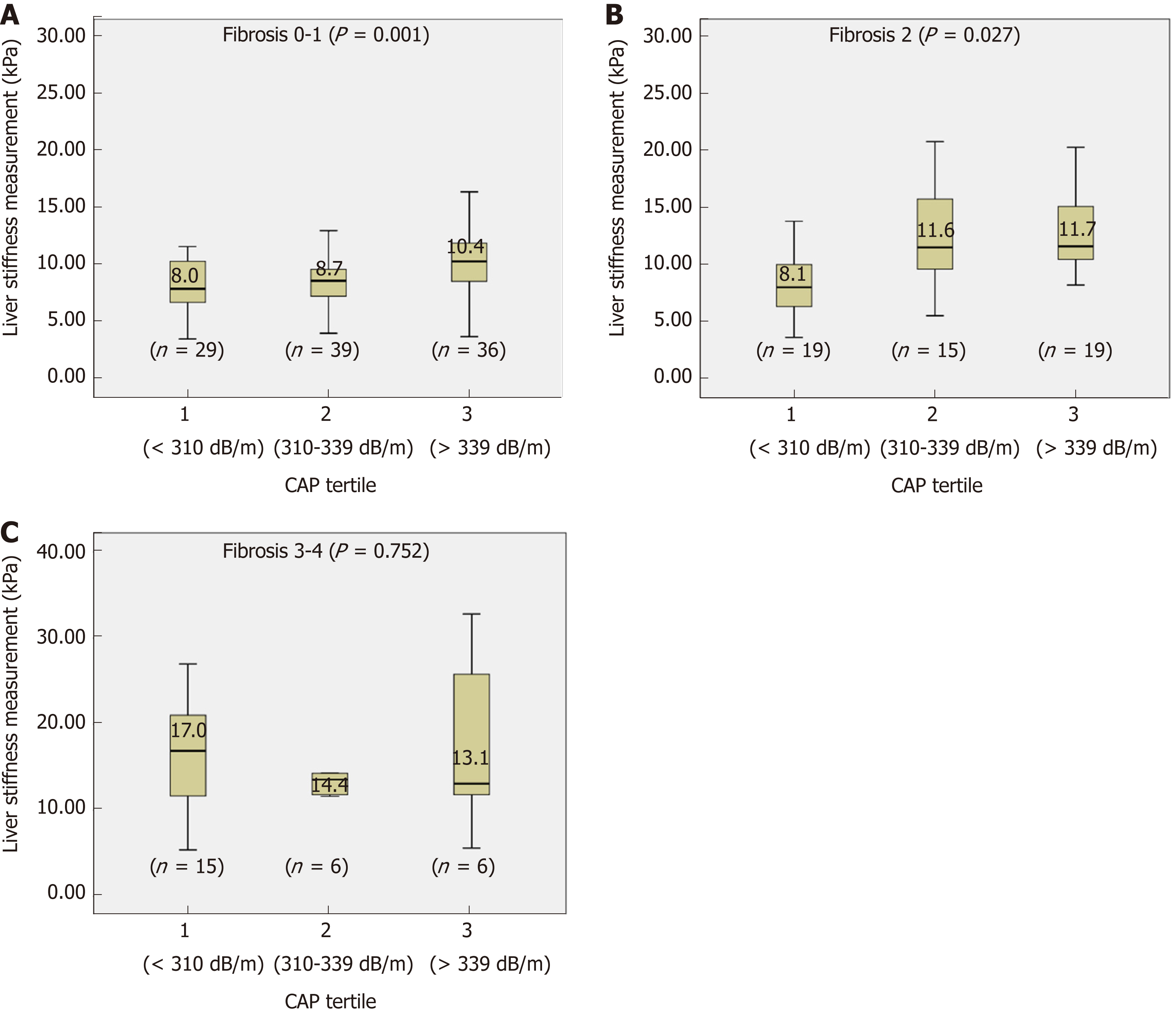
Although there were significant differences in CAP scores between S1 and S2-S3, no cutoff could differentiate S3 from S2. S2 and S3 accounted for the majority of the patients (76.1%, 140/184). Furthermore, in a multivariate analysis, the CAP value, but not the pathologically detected steatosis grade, was associated with LSM. Therefore, the variations in the LSM value for each stage of liver fibrosis was evaluated according to arbitrary CAP tertiles (lower, 223-310; middle, 311-339; high 340-400 dB/m) (Figure 2). For F0-1, LSM values significantly increased at high CAP tertile (P = 0.001) (Figure 2A). For F2, LSM values were higher for the middle and high CAP tertiles (P = 0.027) (Figure 2B). However, the LSM values did not differ significantly according to CAP tertile in patients with NASH and advanced fibrosis (F3-4) (P = 0.752) (Figure 2C).
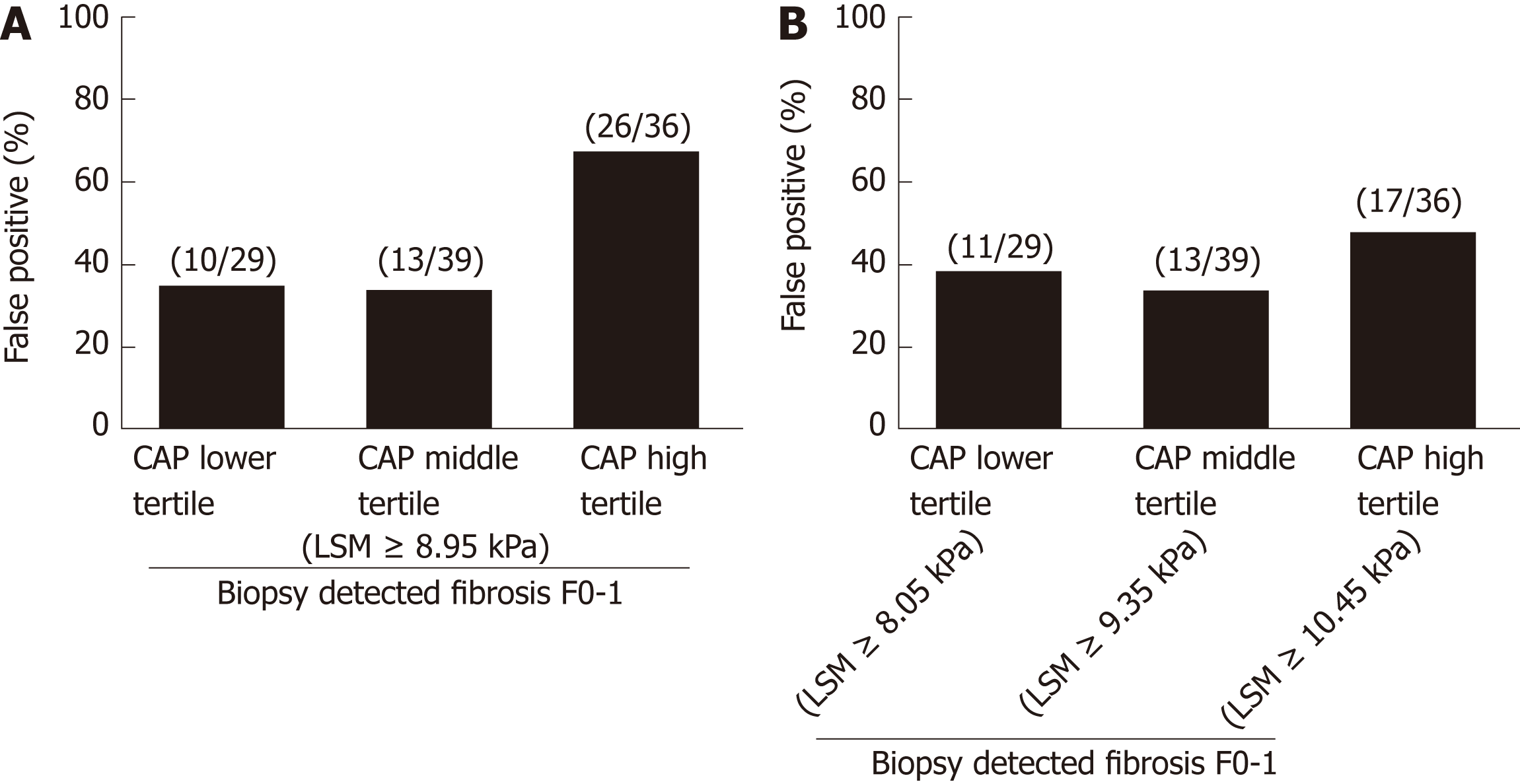
When cutoff of 8.95 kPa was used to diagnose significant fibrosis (F ≥ 2), positive predictive values in lower, middle and high CAP tertiles was 21/31 (67.7%), 19/32 (59.4%) and 23/49 (46.9%), respectively. False positive rates increased with increasing CAP tertile (Figure 3A). However, when different cutoffs were used for each CAP tertile, differences in false positive rates among different CAP tertile were reduced and the false positive rate in high CAP tertile decreased (Figure 3B). ROC curves showed that the optimum LSM cutoff for diagnosis of F ≥ 2 was 8.05 kPa (AUC, 0.682; sensitivity, 73.5%; specificity 51.7%) for the lower CAP tertile, LSM of 9.35 kPa (AUC, 0.843; sensitivity, 90.5%; specificity 71.8%) for the middle CAP tertile, and LSM of 10.55 kPa (AUC 0.682; sensitivity, 76.0%; specificity 52.8%) for high CAP tertile.
Among the 184 patients with biopsy proven NASH, 130 patients had LS measured 1 year after liver biopsy and were treated with PPAR-γ agonist (pioglitazone) 15 mg/day (n = 80) or UDCA 300 mg/day (n = 50). Regarding the baseline characteristics, there was no significant difference in BMI, LS value or CAP score between the two groups (Table 3). However, the pioglitazone group had higher rates of DM and hypertension (P = 0.048, P = 0.049, respectively).
| Variable | Pioglitazone group (n = 80) | UDCA group (n = 50) | P value |
| Age (yr) | 47.6 ± 14.5 | 44.9 ± 14.5 | 0.303 |
| Male gender | 60 (75.0%) | 30 (60.0) | 0.071 |
| Body mass index ≥ 25 kg/m2 | 66 (82.5%) | 41 (82.0%) | 0.942 |
| Diabetes | 38 (47.5%) | 15 (30.0) | 0.048a |
| Hypertension | 29 (36.2%) | 10 (20.0%) | 0.049a |
| Hyperlipidemia | 36 (45.0%) | 26 (52.0%) | 0.437 |
| Fasting glucose (mg/dL) | 121.3 ± 34.5 | 112.5 ± 24.1 | 0.090 |
| HDL cholesterol (md/dL) | 47.9 ± 10.0 | 47.7 ± 14.1 | 0.923 |
| Triglycerides (mg/dL) | 199.5 ± 147.7 | 185.9 ± 105.2 | 0.590 |
| LDL cholesterol (mg/dL) | 130.5 ± 29.4 | 136.4 ± 34.8 | 0.435 |
| Alanine aminotransferase (U/L) | 103.0 ± 76.1 | 74.6 ± 48.7 | 0.011a |
| Aspartate aminotransferase (U/L) | 70.2 ± 40.5 | 65.7 ± 42.8 | 0.554 |
| Gamma glutamyltransferase (U/L) | 63.9 ± 61.3 | 62.3 ± 41.8 | 0.856 |
| Platelet (× 1000/mm3) | 229.8 ± 54.8 | 253.2 ± 54.4 | 0.019a |
| Albumin (g/dL) | 4.5 ± 0.3 | 4.5 ± 0.3 | 0.866 |
| Liver stiffness (kPa) | 11.7 ± 4.8 | 10.5 ± 5.4 | 0.183 |
| Stiffness IQR (kPa) | 2.2 ± 4.7 | 1.4 ± 0.9 | 0.387 |
| CAP (dB/m) | 318.2 ± 37.9 | 319.3 ± 37.1 | 0.873 |
| CAP IQR (dB/m) | 25.2 ± 9.0 | 23.0 ± 10.1 | 0.216 |
| Fibrosis score (Kleiner) | 0.017a | ||
| F0 | 2 (2.5%) | 9 (18.0%) | |
| F1 | 41 (51.2%) | 21 (42.0%) | |
| F2 | 24 (30.0%) | 15 (30.0%) | |
| F3-4 | 13 (16.3%) | 5 (10.0%) |
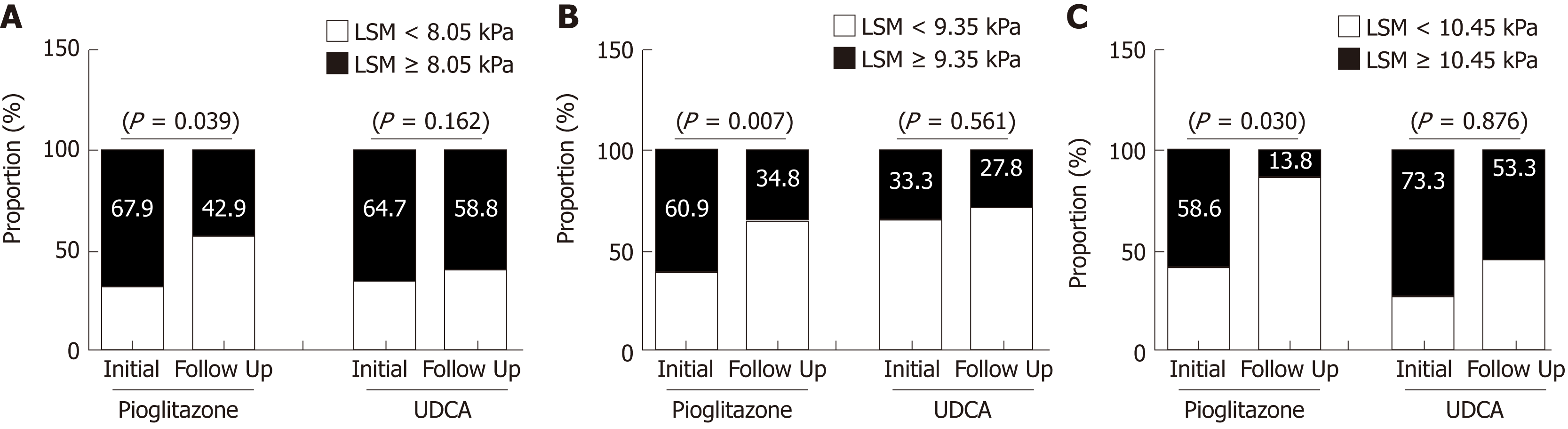
The patients treated with pioglitazone demonstrated a decreased LSM value after 1- year of treatment (P < 0.001), when that in UDCA-treated patients did not change significantly (P = 0.068) (Figure 4). The CAP values did not show significant changes after treatment with pioglitazone (318.2 ± 37.9 vs 313.2 ± 41.5 dB/m, P = 0.197) or UDCA (319.3 ± 37.1 vs 309.2 ± 38.2 dB/m, P = 0.057). Changes in the proportion of patients with LSM suggesting significant fibrosis (F ≥ 2) were assessed separately in the pioglitazone and UDCA groups according to the LSM cutoffs for each CAP tertile, that were obtained from the analysis with 184 patients. Among the patient with lower CAP tertile (223-309 dB/m), those treated with pioglitazone showed a decreased proportion of high LSM values (F ≥ 2) but the proportion of high LSM values did not change significantly among the patients treated with UDCA (Figure 4A). Similar results were noted in patients with middle (310-332 dB/m) and high CAP tertile (333-400 dB/m) (Figure 4B and C).
This study of 184 patients with biopsy proven NASH demonstrated that high CAP scores are associated with increased LSM values at the same fibrosis stage, resulting in overestimation of liver fibrosis. Lower positive predictive values were noted in patients in the high CAP tertile, particularly those with F0-2. Therefore, higher LSM cutoffs might be useful for identifying significant fibrosis in patients with NAFLD and high CAP values. Although a “high CAP cutoff value” had yet to be defined, the cutoff for the high CAP tertile in this study was 330-340 dB/m.
Two prospective cohort studies on the natural history of NAFLD proposed that the severity of liver fibrosis is the most important predictor of liver-related complications as well as survival in patients with NAFLD[2,3]. In addition, recent studies suggested that only the severity of fibrosis is an important prognostic factor for NAFLD, and is independent of NASH and the severity of inflammation[4,22]. These studies investigated the prognostic value of the baseline liver fibrosis stage, and one also assessed the progression of liver fibrosis in patients with NAFLD[5]. Therefore, when sequential liver biopsy is not practical, accurate prediction of fibrosis stage using noninvasive methods is important. To reduce the effect of hepatic steatosis, as indicated by CAP scores, on the prediction of fibrosis based on LSM, we calculated the cutoff values for significant fibrosis in according to CAP tertile. We applied arbitrary CAP tertiles since the CAP score did not accurately differentiate S2 from S3 which accounted for the majority of the patients. Also, CAP reportedly cannot differentiate adjacent grades of steatosis with high precision[23]. As a noninvasive means of steatosis measurement, magnetic resonance imaging (MRI)-proton density fat traction (PDFF) is reported to be more accurate for predicting hepatic steatosis compared to CAP[24]. Magnetic resonance elastography (MRE) has the highest diagnostic accuracy for staging fibrosis in patients with NAFLD. However, both MRI-PDFF and MRE are MRI-based tools that require more space and more costly than FibroScan with CAP[25].
Cutoff values according to CAP tertile were applied to estimate the effect of pioglitazone and UDCA on the LSM values at the 1-year follow up. Treatment with pioglitazone reduced LSM-estimated proportion of significant fibrosis. However, the CAP score did not change significantly after pioglitazone or UDCA administration. In recent NAFLD practice guidance, pioglitazone and 800 IU/day vitamin E are recommended to improve liver histology in patients with NASH[26]. The 184 patients from which the LSM cutoff values for significant fibrosis were estimated included 130 patients treated with pioglitazone or UDCA. Although pioglitazone improved the LSM value, the utility of this result is limited by several factors. Ironically, the accuracy of LSM cutoff determined based on the CAP score cannot be validated without follow-up biopsy after pioglitazone or UDCA treatment in patients with NASH. Therefore, studies involving paired liver biopsies are needed, until more reliable LSM standards for NAFLD are established. Secondly, being a retrospective, observational study, the baseline demographic parameters of the pioglitazone and UDCA-treated groups were not matched. Patients treated with pioglitazone were more likely to be diabetic and hypertensive which are important elements of metabolic syndrome. Among NAFLD patients with fibrosis progression, 80% were diabetic, suggesting that diabetes promotes the progression of NASH[5]. Although it involved a larger number of patients with diabetes, our study showed that pioglitazone resulted in a reduced proportion of patients with high LSM values compared to UDCA. However, the sample size was too small to reach a definite conclusion. Finally, unlike previous investigations of the effect of pioglitazone on NASH, the patients in this study received low dose of pioglitazone (15 mg/day). Two randomized studies of the effect of pioglitazone on NASH prescribed pioglitazone 30 mg or 45 mg daily to patients with NASH[27,28]. The mean BMIs of the patients in these previous studies were about 33-35 kg/m2, compare to 29.2 ± 4.5 kg/m2 in this study, and the lower dose of pioglitazone may have been effective due to the lower BMI of our patients. Further studies investigating smaller doses of pioglitazone on patients with NASH are needed to verify our results.
In conclusion, LSM in patients with NASH may overestimate the liver fibrosis stage, particularly in those with high CAP values. Interpretation of LSM results taking into consideration the simultaneously measured CAP scores may prevent the performance of unnecessary liver biopsy in patients with NAFLD.
In nonalcoholic fatty liver disease (NAFLD), studies demonstrate that the severity of liver fibrosis is the most important determinant of the disease prognosis. Although liver biopsy is considered the gold standard for identifying fibrosis stage, it is an invasive procedure, and liver stiffness measurement (LSM) is widely preformed as a noninvasive means. However, LSM tends to overestimate fibrosis stage in NAFLD.
Controlled attenuation parameter (CAP), provided by LSM device, has been introduced for noninvasive quantification of hepatic steatosis. It also has been suggested that CAP may contribute in enhancing the accuracy of transient elastography measured LSM in patients with NAFLD.
Our aim was to determine the role of CAP values in predicting liver fibrosis stages by LSM.
This retrospective study involves 184 patients with biopsy proven nonalcoholic steatohepatitis (NASH), seen at a tertiary hospital in Seoul, Republic of Korea between 2010 and 2017. These patients had LSM and CAP evaluated within one month before the liver biopsy. Liver stiffness and CAP scores were measured by the FibroScan (Echosens, Paris, France), a medical device, using a standard probe. The patients in whom liver stiffness was measured when aspartate aminotransferase or alanine aminotransferase level was more than fivefold the upper limit of normal were excluded from the analysis due to the possibility of exacerbated LSM values. From 184 patients, 130 patients had 1-year follow-up LSM and analyzed for the changes of LSM after pioglitazone or ursodeoxycholic acid (UDCA) treatment.
Among 184 NASH patients with liver biopsy, histologically assessed steatosis grade (S) was distributed as follows: S1, n = 44 (2.9%); S2, n = 81 (44.0%); S3, n = 59 (32.1%). CAP scores were significantly different between S1 and S2-S3 (P < 0.001). However, no significant difference was found between S2 and S3 (P = 0.075). LSM significantly increased in accordance with the liver biopsy detected fibrosis stage (P < 0.001). After multivariate analysis, CAP value along with pathologically detected fibrosis stages was identified as a significant factor associated with LSM. Since our assessment showed that no reliable cutoff was demonstrated to differentiate S3 from S2 and 76.1% (140/184) of our study patients were either S2 or S3, variations of LSM within the same stage of liver fibrosis was evaluated according to the arbitrary CAP tertiles (lower 223-310, middle 311-339, high 340-400 dB/m). In Kleiner fibrosis stage F0 - 1, LSM values increased at high CAP tertile (P = 0.001), and in F2, at middle and high tertile (P = 0.027). No difference was noticed in F3-4 (P = 0.752) according to CAP tertile. Receiver operating characteristic curves for LSM cutoff in diagnosis of F ≥ 2 identified 8.05 kPa for lower CAP tertile, 9.35 kPa for middle, and 10.55 kPa for high tertile. The patients treated with pioglitozone demonstrated decreased LSM values after 1 year of the treatment (P < 0.001), when that in UDCA treated patients did not show significant changes (P = 0.068). CAP values did not show significant changes after pioglitazone (P = 0.197) or UDCA treatment (P = 0.057). When changes in proportion of significant fibrosis (F ≥ 2) were assessed among pioglitazone or UDCA treated patients reflecting CAP values, pioglitazone treated patients demonstrated decrease in proportion of high LSM.
In conclusion, LSM in NASH may overestimate the liver fibrosis stage particularly in patients with high CAP values. Interpretation of LSM considering simultaneously measured CAP scores may provide more helpful information preventing unnecessary liver biopsy in patients with NAFLD.
In patients with NAFLD with high CAP scores, LSM cutoff that leads to liver biopsy may need to be set higher than in those with other chronic liver diseases. Validation studies for more precise LSM cutoffs should be performed incorporating larger number of patients with biopsy proven NAFLD. With more reliable LSM cutoffs for noninvasive diagnosis of liver fibrosis in NAFLD, clinical studies evaluating efficacies of treatment would be more widely preformed in NAFLD.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo JS, Serrano-Luna J, Sherif Z S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
| 1. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1756] [Cited by in RCA: 1822] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 2. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1703] [Article Influence: 170.3] [Reference Citation Analysis (1)] |
| 3. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-97.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2229] [Article Influence: 222.9] [Reference Citation Analysis (0)] |
| 4. | Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 775] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 5. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 6. | Spinzi G, Terruzzi V, Minoli G. Liver biopsy. N Engl J Med. 2001;344:2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1736] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 7. | Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 393] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 8. | Verveer C, Zondervan PE, ten Kate FJ, Hansen BE, Janssen HL, de Knegt RJ. Evaluation of transient elastography for fibrosis assessment compared with large biopsies in chronic hepatitis B and C. Liver Int. 2012;32:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Li Y, Huang YS, Wang ZZ, Yang ZR, Sun F, Zhan SY, Liu XE, Zhuang H. Systematic review with meta-analysis: The diagnostic accuracy of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2016;43:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Petta S, Di Marco V, Cammà C, Butera G, Cabibi D, Craxì A. Reliability of liver stiffness measurement in non-alcoholic fatty liver disease: The effects of body mass index. Aliment Pharmacol Ther. 2011;33:1350-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Wong GL, Chan HL, Choi PC, Chan AW, Lo AO, Chim AM, Wong VW. Association between anthropometric parameters and measurements of liver stiffness by transient elastography. Clin Gastroenterol Hepatol. 2013;11:295-302.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Petta S, Maida M, Macaluso FS, Di Marco V, Cammà C, Cabibi D, Craxì A. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology. 2015;62:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 13. | Fujimori N, Tanaka N, Shibata S, Sano K, Yamazaki T, Sekiguchi T, Kitabatake H, Ichikawa Y, Kimura T, Komatsu M, Umemura T, Matsumoto A, Tanaka E. Controlled attenuation parameter is correlated with actual hepatic fat content in patients with non-alcoholic fatty liver disease with none-to-mild obesity and liver fibrosis. Hepatol Res. 2016;46:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Petta S, Wong VW, Cammà C, Hiriart JB, Wong GL, Marra F, Vergniol J, Chan AW, Di Marco V, Merrouche W, Chan HL, Barbara M, Le-Bail B, Arena U, Craxì A, de Ledinghen V. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017;65:1145-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 15. | Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35:561-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 16. | Jung KS, Kim SU, Ahn SH, Park YN, Kim DY, Park JY, Chon CY, Choi EH, Han KH. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 17. | Kim MN, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, Song KJ, Park YN, Han KH. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. 2015;61:1851-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Fung J, Lai CL, Cheng C, Wu R, Wong DK, Yuen MF. Mild-to-moderate elevation of alanine aminotransferase increases liver stiffness measurement by transient elastography in patients with chronic hepatitis B. Am J Gastroenterol. 2011;106:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Kim SU, Kim JK, Park YN, Han KH. Discordance between liver biopsy and Fibroscan® in assessing liver fibrosis in chronic hepatitis B: Risk factors and influence of necroinflammation. PLoS One. 2012;7:e32233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: The smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 596] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 21. | Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: Pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 22. | Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, Gonzalez-Fabian L, Alvarez-Quiñones Sanz M, Conde-Martin AF, De Boer B, McLeod D, Hung Chan AW, Chalasani N, George J, Adams LA, Romero-Gomez M. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155:443-457.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 599] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 23. | Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): A novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 636] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 24. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264-1281.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1035] [Article Influence: 172.5] [Reference Citation Analysis (0)] |
| 25. | Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 641] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 26. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4946] [Article Influence: 706.6] [Reference Citation Analysis (9)] |
| 27. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 28. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 737] [Article Influence: 81.9] [Reference Citation Analysis (0)] |