Published online Sep 7, 2019. doi: 10.3748/wjg.v25.i33.4945
Peer-review started: March 12, 2019
First decision: March 27, 2019
Revised: April 4, 2019
Accepted: May 18, 2019
Article in press: May 18, 2019
Published online: September 7, 2019
Processing time: 180 Days and 22.9 Hours
Carcinoembryonic antigen (CEA) is a commonly used biomarker in colorectal cancer. However, controversy exists regarding the insufficient prognostic value of preoperative serum CEA alone in rectal cancer. Here, we combined preoperative serum CEA and the maximum tumor diameter to correct the CEA level, which may better reflect the malignancy of rectal cancer.
To assess the prognostic impact of preoperative CEA/tumor size in rectal cancer.
We retrospectively reviewed 696 stage I to III rectal cancer patients who underwent curative tumor resection from 2007 to 2012. These patients were randomly divided into two cohorts for cross-validation: training cohort and validation cohort. The training cohort was used to generate an optimal cutoff point and the validation cohort was used to further validate the model. Maximally selected rank statistics were used to identify the optimum cutoff for CEA/tumor size. The Kaplan-Meier method and log-rank test were used to plot the survival curve and to compare the survival data. Univariate and multivariate Cox regression analyses were used to determine the prognostic value of CEA/tumor size. The primary and secondary outcomes were overall survival (OS) and disease-free survival (DFS), respectively.
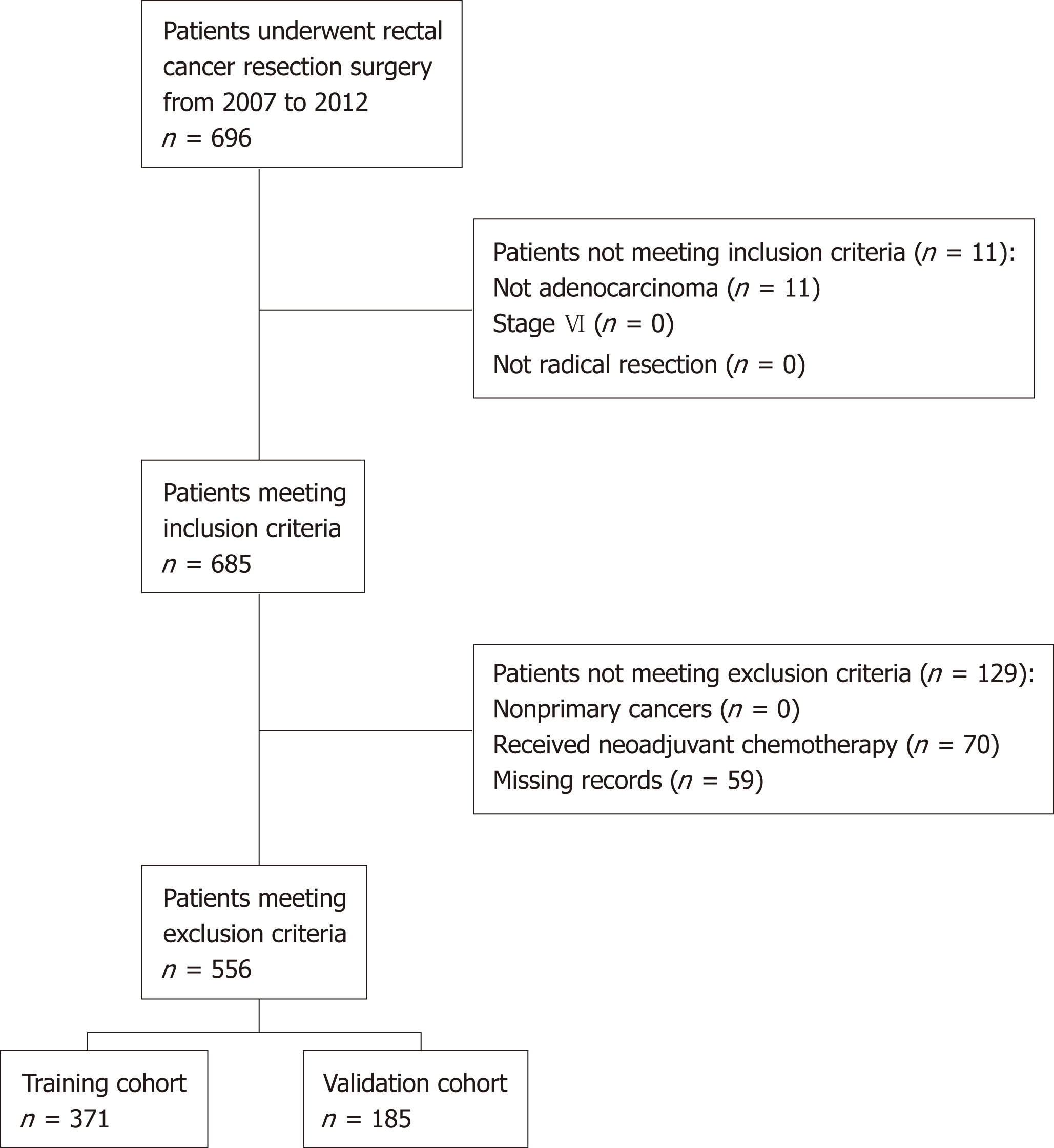
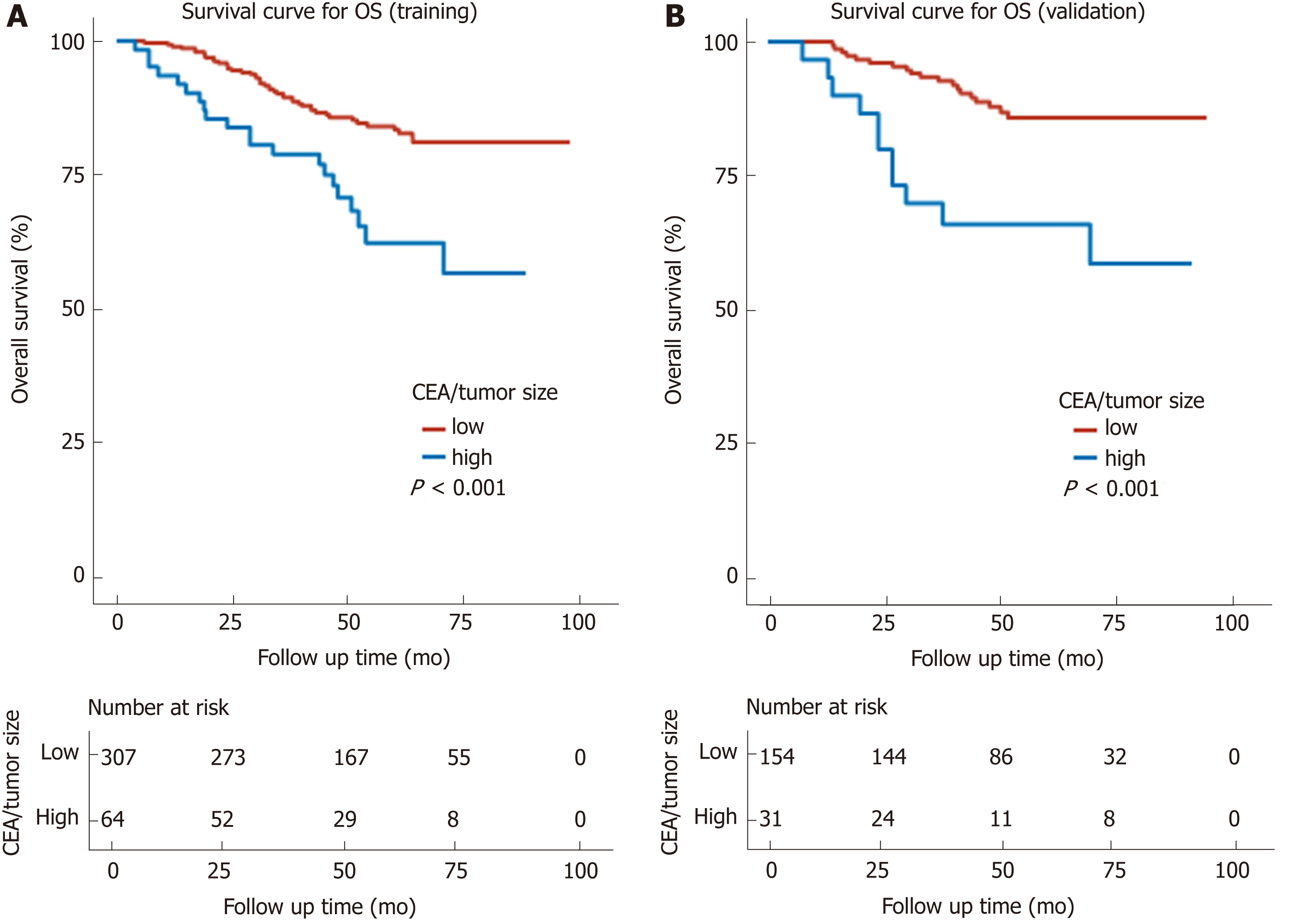
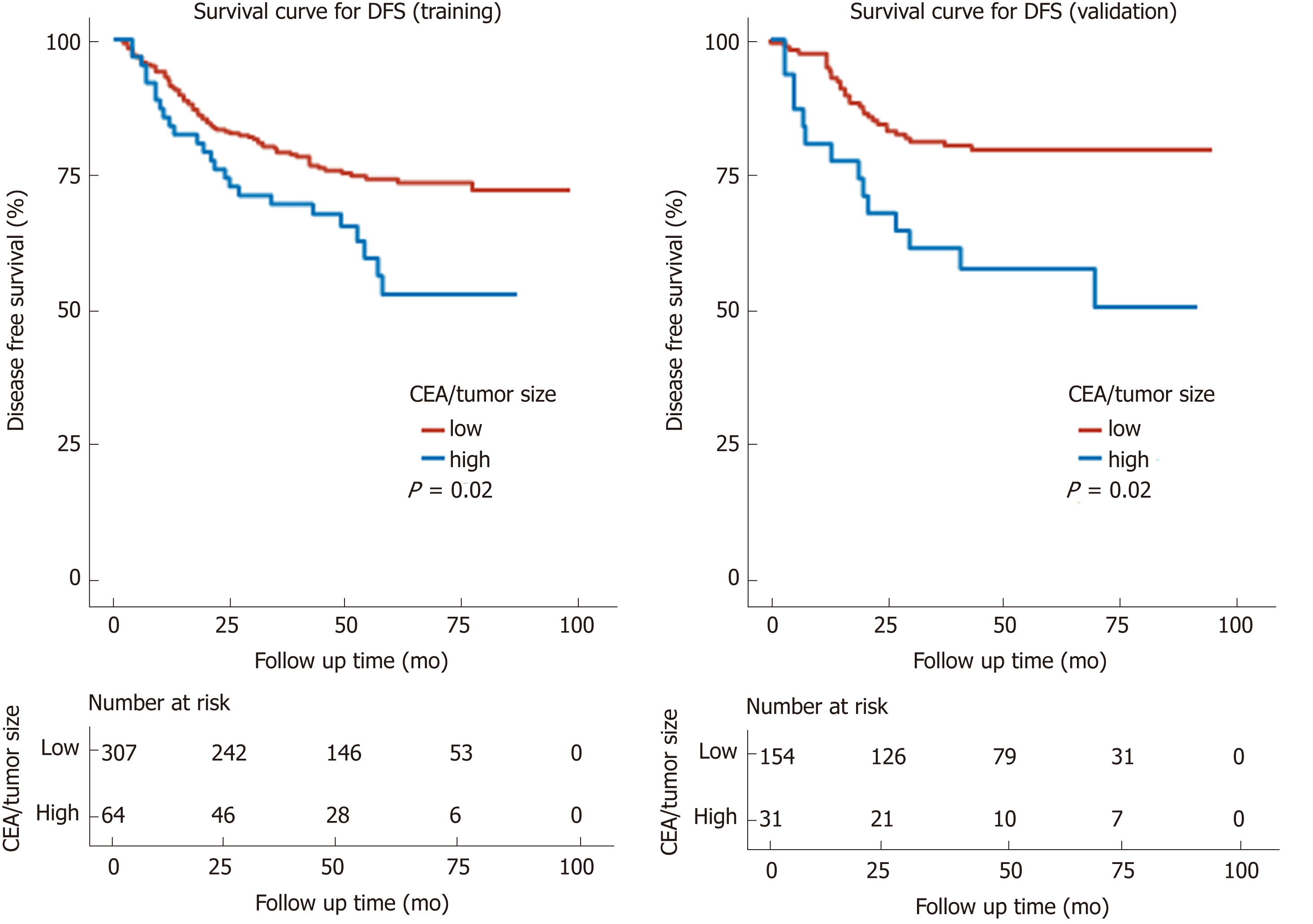
In all, 556 patients who satisfied both the inclusion and exclusion criteria were included and randomly divided into the training cohort (2/3 of 556, n = 371) and the validation cohort (1/3 of 556, n = 185). The cutoff was 2.429 ng/mL per cm. Comparison of the baseline data showed that high CEA/tumor size was correlated with older age, high TNM stage, the presence of perineural invasion, high CEA, and high carbohydrate antigen 19-9 (CA 19-9). Kaplan-Meier curves showed a manifest reduction in 5-year OS (training cohort: 56.7% vs 81.1%, P < 0.001; validation cohort: 58.8% vs 85.6%, P < 0.001) and DFS (training cohort: 52.5% vs 71.9%, P = 0.02; validation cohort: 50.3% vs 79.3%, P = 0.002) in the high CEA/tumor size group compared with the low CEA/tumor size group. Univariate and multivariate analyses identified CEA/tumor size as an independent prognostic factor for OS (training cohort: hazard ratio (HR) = 2.18, 95% confidence interval (CI): 1.28-3.73, P = 0.004; validation cohort: HR = 4.83, 95%CI: 2.21-10.52, P < 0.001) as well as DFS (training cohort: HR = 1.47, 95%CI: 0.93-2.33, P = 0.096; validation cohort: HR = 2.61, 95%CI: 1.38-4.95, P = 0.003).
Preoperative CEA/tumor size is an independent prognostic factor for patients with stage I-III rectal cancer. Higher CEA/tumor size is associated with worse OS and DFS.
Core tip: This is a retrospective study that sought to evaluate the prognostic value of carcinoembryonic antigen (CEA)/tumor size in rectal cancer, which may better reflect the tumor malignancy. Maximally selected rank statistics identified an optimal cutoff point of 2.429 ng/mL per cm for CEA/tumor size. Kaplan-Meier curves showed a significant reduction in the 5-year overall survival and disease-free survival in the high CEA/tumor size group. Univariate and multivariate analyses identified CEA/tumor size as an independent prognostic factor for stage I to III rectal cancer.
- Citation: Cai D, Huang ZH, Yu HC, Wang XL, Bai LL, Tang GN, Peng SY, Li YJ, Huang MJ, Cao GW, Wang JP, Luo YX. Prognostic value of preoperative carcinoembryonic antigen/tumor size in rectal cancer. World J Gastroenterol 2019; 25(33): 4945-4958
- URL: https://www.wjgnet.com/1007-9327/full/v25/i33/4945.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i33.4945
Colorectal cancer (CRC) is the third most frequently diagnosed malignancy and one of the leading causes of cancer-related mortality worldwide[1]. Although Western developed countries show a steady or slightly declining trend, the morbidity and mortality of CRC in developing countries like China are still on the rise[2]. Unlike Western countries, the incidence of rectal cancer is higher than that of colon cancer in China and the prognosis of rectal cancer still needs to be improved[3]. Therapy options for CRC have been developed rapidly in the past decade, but selecting optimal treatments for individuals remains a great challenge for clinicians due to the lack of effective markers[4]. In recent years, biomarkers have played an increasingly vital role in the detection and management of CRC[5]. Among the biomarkers, carcinoembryonic antigen (CEA) is one of the most common and most convenient preoperative detecting indexes in patients with colorectal cancer[6].
CEA, a large glycoprotein, has been recommended by the American Society of Clinical Oncology (ASCO) and the European Group on Tumor Markers (EGTM) as a prognostic biomarker that can be used to determine the prognosis and stage of CRC[5,7]. However, controversy still exists regarding the prognostic value of the absolute preoperative serum CEA level in colorectal cancer. Recent studies have noted that CEA is insufficiently sensitive to be used alone, and some researchers have sought new ways to improve its prognostic value by the addition of another factor, such as CD44v6, carbohydrate antigen (CA) 19-9, neutrophil-to-lymphocyte ratio (NLR), or peritoneal carcinomatosis index ratio (PCI)[6,8-11]. Intriguingly, a recent study indicated that postoperative tissue CEA (t-CEA) rather than serum CEA (s-CEA) is an independent prognostic factor in stage I to III CRC[12]. This indicated that we should pay more attention to the local CEA produced by tumor cells rather than the overall serum CEA level. Considering that detecting the CEA produced and secreted by all tumor cells is not realistic, using the ratio of CEA to tumor size may somehow reflect the ability of tumor cells to secrete CEA. Another research group demonstrated that CEA density is a prognostic factor for percutaneous ablation of pulmonary colorectal metastases[13]. Using tumor size to adjust and improve the prognostic value of tumor marker is not uncommon, such as prostate specific antigen density and tumor-infiltrating CD8+ T-cell density[14,15]. Maximum tumor diameter is also a prognostic indicator for some solid tumors including prostate cancer and colorectal liver metastases[16,17]. While the volume-adjusted prostate-specific antigen has been widely studied as a useful marker in prostate cancer[18,19], whether the combination of CEA level and tumor size serves as a novel prognostic factor for rectal cancer remains unresolved.
In this study, we considered both the preoperative serum CEA level and the rectal tumor size and devised the CEA/tumor size, which represents the CEA level adjusted by tumor size, to better reflect the malignancy of rectal cancer. We also refined the insufficient prognostic value of serum CEA. We aimed to apply this new approach to investigate the prognostic impact of the preoperative CEA/tumor size in patients with rectal cancer.
Patients who were diagnosed with stage I to III rectal cancer and underwent a radical excision at the Sixth Affiliated Hospital of Sun Yat-Sen University from 2007 to 2012 were studied. This study was approved by the Medical Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University and did not cause any harm to the patients. All retrospective data were obtained from a database maintained by the Sixth Affiliated Hospital of Sun Yat-sen University. The inclusion criteria were as follows: (1) Histologically confirmed adenocarcinoma; (2) Stage I to III according to the 8th edition of the American Joint Committee on Cancer (AJCC); and (3) Radical resection. The following patients were excluded: (1) Those with nonprimary cancers; (2) Patients who received neoadjuvant chemotherapy and/or radiotherapy; and (3) Patients with missing data on preoperative CEA or tumor size. Patients who satisfied both the inclusion and exclusion criteria were randomly divided into two cohorts for cross-validation: Training cohort and validation cohort. The training cohort was used to generate an optimal cutoff point and the validation cohort was used to test the applicability of this cutoff point and the model.
The following data were collected using the Electronic Medical Record System: Age, sex, histological features, TNM stage (AJCC), differentiation degree, presence of lymphovascular invasion, presence of perineural invasion, preoperative serum CA 19-9 and CEA levels, maximum tumor diameter, recurrence, and survival time. Follow-up was conducted every three months during the first year after resection, every six months during the next two years, and once a year thereafter. Routine physical examination, serum CEA test, and radiographic examinations including chest radiography, abdominopelvic computed tomographic scanning, or ultrasonography, whole-body bone scanning, double-contrast barium enema, and colonoscopy were performed and recorded six months after resection and yearly thereafter. The follow-up time ended in June 2016, and the follow-up interval varied from three to ten years.
In our study, we used the maximum diameter in the maximum cross section to represent the tumor size, which was measured by radiologists and pathologists (pathological data are preferred). We defined the CEA/tumor size as the ratio of preoperative CEA level to the maximum tumor diameter. The primary outcome was overall survival (OS), which was defined as the time in months from surgery to death. The secondary endpoint was disease-free survival (DFS), which was defined as the time in months from surgery to disease recurrence, whether radiological or histological. Maximally selected rank statistics were used to identify the optimal discriminator value for the CEA/tumor size, which was conducted in the training cohort. For every potential cutoff point, the absolute value of the standardized log-rank statistic was computed. The cutoff that provided the best separation of the survival outcome into two groups, where the standardized statistics reached their maximum, was selected as the cutoff point. Based on this cutoff, we divided the validation cohort into two groups: High CEA/tumor size group and low CEA/tumor size group. The intergroup comparisons of the clinicopathological variables were performed using the two independent samples t-test or Mann-Whitney U test for continuous variables, and the chi-square test or two-tailed Fisher’s exact test for discrete variables. The Kaplan-Meier method and log-rank test were used to plot the survival curve and to compare the survival data. Univariate analysis of potential risk factors for each variable was performed using the Cox proportional hazards regression model. Variables with a P-value < 0.10 in the univariate analysis were selected to fit the multivariate Cox model. Multivariate analysis using the Cox proportional hazards regression model was used to identify independent risk factors. Variable selection methods, including forward, backward, and stepwise algorithms, as determined by the Akaike information criterion (AIC), were used to construct the appropriate model. The proportional hazards assumption of the Cox regression models was tested by Schoenfeld residuals. All tests were bilateral, and P-values < 0.05 were considered statistically significant. All analyses were performed using the R Language for Statistical Computing (version 3.5.1).
Of the 696 patients diagnosed with rectal cancer who underwent surgical resection from 2007 to 2012, 11 were not histologically confirmed to have adenocarcinoma, 70 received neoadjuvant chemotherapy and/or radiotherapy, and 59 had missing data. Excluding these patients left 566 patients who satisfied both the inclusion and exclusion criteria (Figure 1). These patients were randomly divided into two cohorts: The training cohort (n = 371, 2/3 of 566) and the validation cohort (n = 185, 1/3 of 566).
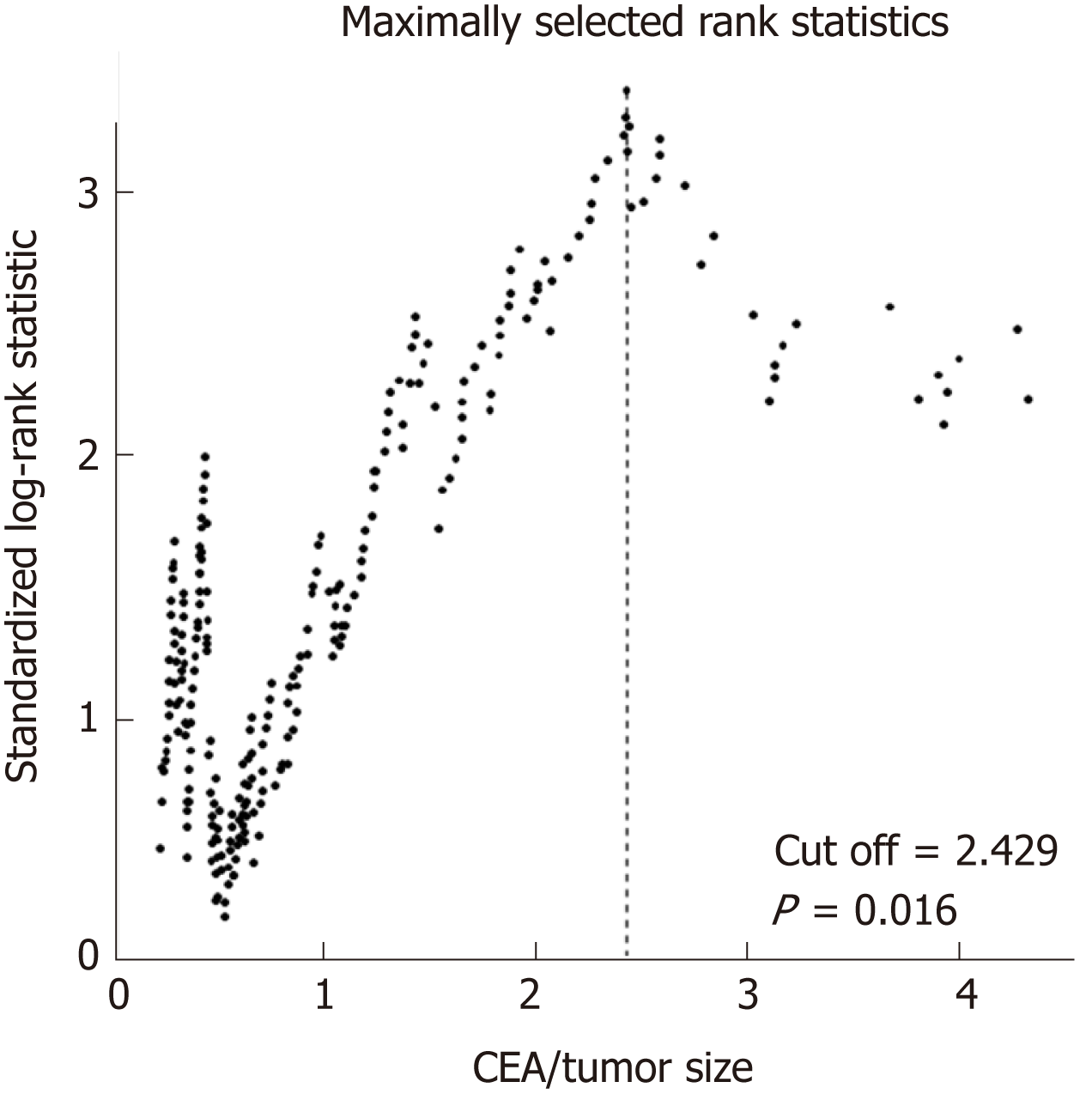
Maximally selected rank statistics were performed to determine the optimal value with maximal standardized log-rank statistics. For all 371 rectal cancer patients in the training cohort, the CEA/tumor size of 2.429 ng/mL per cm (P = 0.016) provided the best separation of the survival outcomes of the two groups (Figure 2). Based on this cutoff value, 371 patients from the training cohort and 185 patients from the validation cohort were divided into the high CEA/tumor size group and the low CEA/ tumor size group, respectively. As shown in Table 1, high CEA/tumor size was correlated with older age, high TNM stage, the presence of perineural invasion, and high CEA and CA 19-9 levels in the training cohort. Somewhat differently, in the validation cohort, patients with a higher CEA/tumor size only tended to have higher preoperative CEA and CA 19-9 levels. Tumor size, sex, differentiation, and lymphovascular invasion did not differ significantly between the two groups in both cohorts.
| Training cohort (n = 371) | Validation cohort (n = 185) | |||||||
| Cases | Low | High | P-value | Cases | Low | High | P-value | |
| Age | 371 | 58 (21-89) | 65 (32-86) | < 0.001a | 185 | 61 (25-87) | 57 (35-79) | 0.149 |
| Tumor size | 371 | 4.3 (0.8-13) | 4.3 (0.8-13.5) | 0.773 | 185 | 4.5 (1-13) | 4.3 (0.8-10) | 0.472 |
| Sex | 0.419 | 0.199 | ||||||
| Male | 218 | 177 (58) | 41 (64) | 103 | 82 (53) | 21 (68) | ||
| Female | 153 | 130 (42) | 23 (36) | 82 | 72 (47) | 10 (32) | ||
| TNM stage | 0.008a | 0.350 | ||||||
| I | 104 | 96 (31) | 8 (12) | 48 | 43 (28) | 5 (16) | ||
| II | 127 | 99 (32) | 28 (44) | 74 | 61 (40) | 13 (42) | ||
| III | 140 | 112 (36) | 28 (44) | 63 | 50 (32) | 13 (42) | ||
| Differentiation | 0.395 | 0.826 | ||||||
| Poor | 60 | 51 (17) | 9 (14) | 24 | 19 (12) | 5 (16) | ||
| Moderate | 209 | 176 (57) | 33 (52) | 102 | 85 (55) | 17 (55) | ||
| High | 102 | 80 (26) | 22 (34) | 59 | 50 (32) | 9 (29) | ||
| Lymphovascular invasion | 0.697 | 0.683 | ||||||
| Negative | 338 | 281 (92) | 57 (89) | 173 | 143 (93) | 30 (97) | ||
| Positive | 33 | 26 (8) | 7 (11) | 12 | 11 (7) | 1 (3) | ||
| Perineural invasion | 0.039a | 0.073 | ||||||
| Negative | 340 | 286 (93) | 54 (84) | 172 | 146 (95) | 26 (84) | ||
| Positive | 31 | 21 (7) | 10 (16) | 13 | 8 (5) | 5 (16) | ||
| CEA | < 0.001a | < 0.001a | ||||||
| 0-5 ng/mL | 263 | 262 (85) | 1 (2) | 127 | 126 (82) | 1 (3) | ||
| > 5 ng/mL | 108 | 45 (15) | 63 (98) | 58 | 28 (18) | 30 (97) | ||
| CA 19-9 | 0.006a | 0.027a | ||||||
| 0-37 ng/mL | 325 | 276 (90) | 49 (77) | 158 | 136 (88) | 22 (71) | ||
| > 37 ng/mL | 46 | 31 (10) | 15 (23) | 27 | 18 (12) | 9 (29) | ||
Kaplan-Meier curves showed a manifest reduction in the 5-year OS (56.7% vs 81.1%, P < 0.001) and DFS (52.5% vs 71.9%, P = 0.02) in the high CEA/tumor size group compared with the low CEA/tumor size group in the training cohort (Figures 3A and 4A). The worse outcome of those with high CEA/tumor size was confirmed in the validation cohort, as those patients exhibited a lower 5-year OS (58.8% vs 85.6%, P < 0.001) and DFS (50.3% vs 79.3%, P = 0.002) (Figures 3B and 4B).
According to the univariate analysis, age, TNM stage, differentiation, lymphovascular invasion, preoperative CEA and CA 19-9 levels, and CEA/tumor size were selected for the multivariate analysis for OS in both cohorts. As for DFS, the univariate analysis indicated that advanced TNM stage, the presence of lymphovascular invasion, high CEA level, and high CEA/tumor size might be associated with a poor outcome in both cohorts. However, the presence of perineural invasion only showed a significant association with DFS in the training cohort, while poor differentiation and high CA 19-9 level were associated with poor DFS only in the validation cohort (Tables 2 and 3).
| Variable | Training cohort (n = 371) | Validation cohort (n = 185) | ||||
| Hazard ratio | 95%CI | P-value | Hazard ratio | 95%CI | P-value | |
| Age | 1.02 | 1.00-1.04 | 0.024a | 1.03 | 1.00-1.06 | 0.070 |
| Tumor size | 1.04 | 0.92-1.18 | 0.506 | 1.14 | 0.95-1.37 | 0.164 |
| Sex (ref = male) | 1.43 | 0.88-2.31 | 0.145 | 0.85 | 0.41-1.77 | 0.665 |
| TNM1 (ref = stage I) | 1.74 | 1.26-2.41 | 0.001a | 1.93 | 1.15-3.22 | 0.012a |
| Differentiation1 (ref = poor) | 0.58 | 0.40-0.84 | 0.004a | 0.53 | 0.30-0.94 | 0.030a |
| Lymphovascular invasion (ref = negative) | 1.88 | 0.96-3.68 | 0.066 | 3.11 | 1.19-8.13 | 0.021a |
| Perineural invasion (ref = negative) | 1.03 | 0.41-2.56 | 0.954 | 1.29 | 0.31-5.46 | 0.729 |
| CEA (ref = CEA < 5) | 1.81 | 1.11-2.94 | 0.017a | 2.72 | 1.33-5.59 | 0.006a |
| CA 19-9 (ref = CA 19-9 < 37) | 1.88 | 1.04-3.39 | 0.036a | 2.14 | 0.92-4.99 | 0.078 |
| CEA/tumor size (ref = low) | 2.45 | 1.46-4.11 | 0.001a | 3.57 | 1.70-7.52 | 0.001a |
| Variable | Training cohort (n = 371) | Validation cohort (n = 185) | ||||
| Hazard ratio | 95%CI | P-value | Hazard ratio | 95%CI | P-value | |
| Age | 1 | 0.99-1.02 | 0.572 | 1.02 | 0.99-1.04 | 0.173 |
| Tumor size | 1.01 | 0.91-1.12 | 0.828 | 1.12 | 0.97-1.30 | 0.128 |
| Sex (ref = male) | 1.26 | 0.85-1.87 | 0.247 | 0.75 | 0.41-1.37 | 0.353 |
| TNM1 (ref = stage I) | 1.9 | 1.45-2.50 | <0.001a | 1.6 | 1.07-2.39 | 0.023a |
| Differentiation1 (ref = poor) | 0.78 | 0.58-1.06 | 0.113 | 0.6 | 0.38-0.95 | 0.031a |
| Lymphovascular invasion (ref = negative) | 2.44 | 1.45-4.12 | 0.001a | 2.63 | 1.11-6.22 | 0.028a |
| Perineural invasion (ref = negative) | 2.17 | 1.23-3.82 | 0.008a | 1.98 | 0.78-5.03 | 0.151 |
| CEA (ref = CEA < 5) | 1.55 | 1.03-2.32 | 0.034a | 1.9 | 1.05-3.41 | 0.033a |
| CA 19-9 (ref = CA 19-9 < 37) | 1.43 | 0.85-2.42 | 0.177 | 1.96 | 0.97-3.96 | 0.061 |
| CEA/tumor size (ref = low) | 1.72 | 1.10-2.71 | 0.018a | 2.58 | 1.37-4.85 | 0.003a |
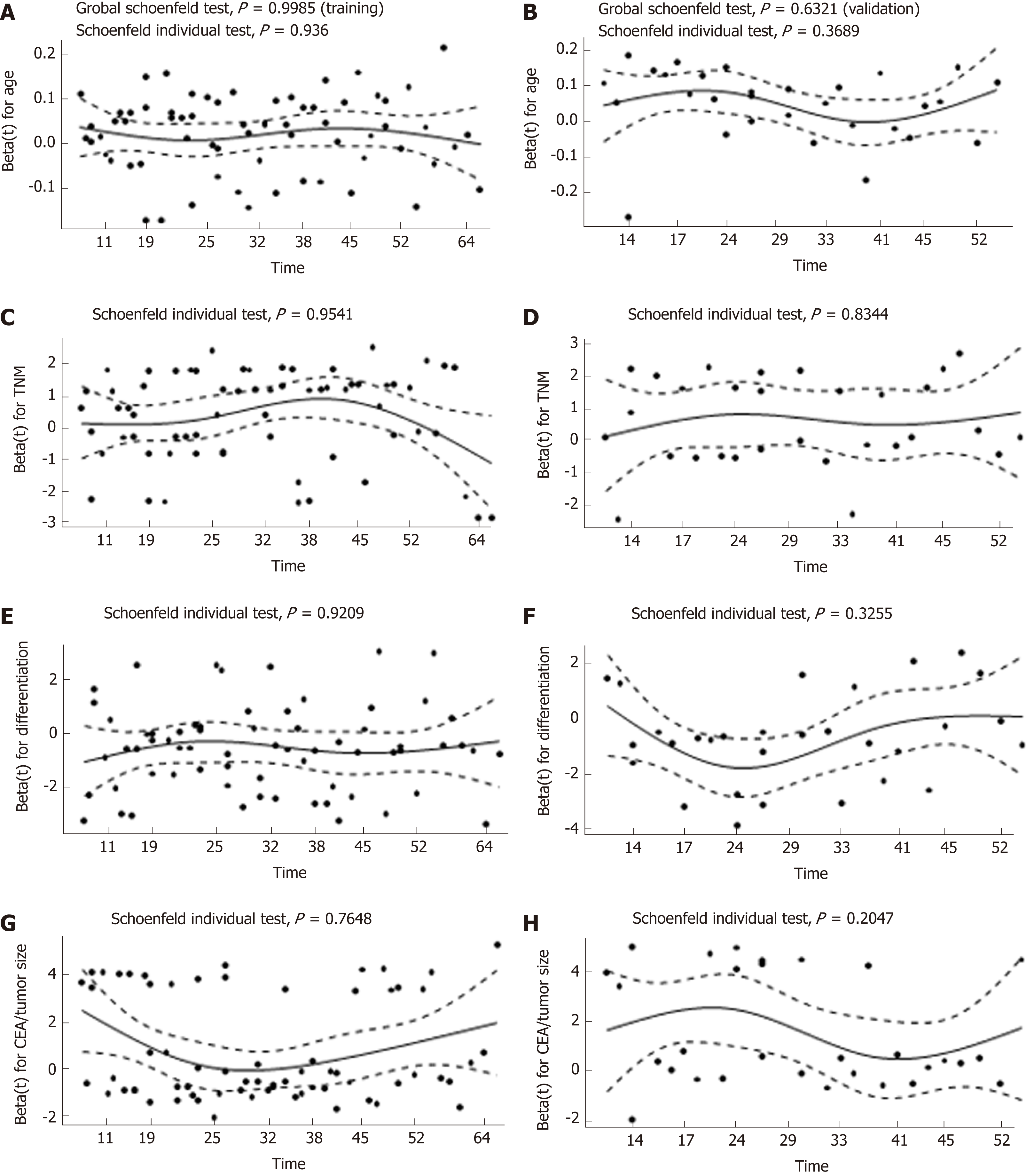
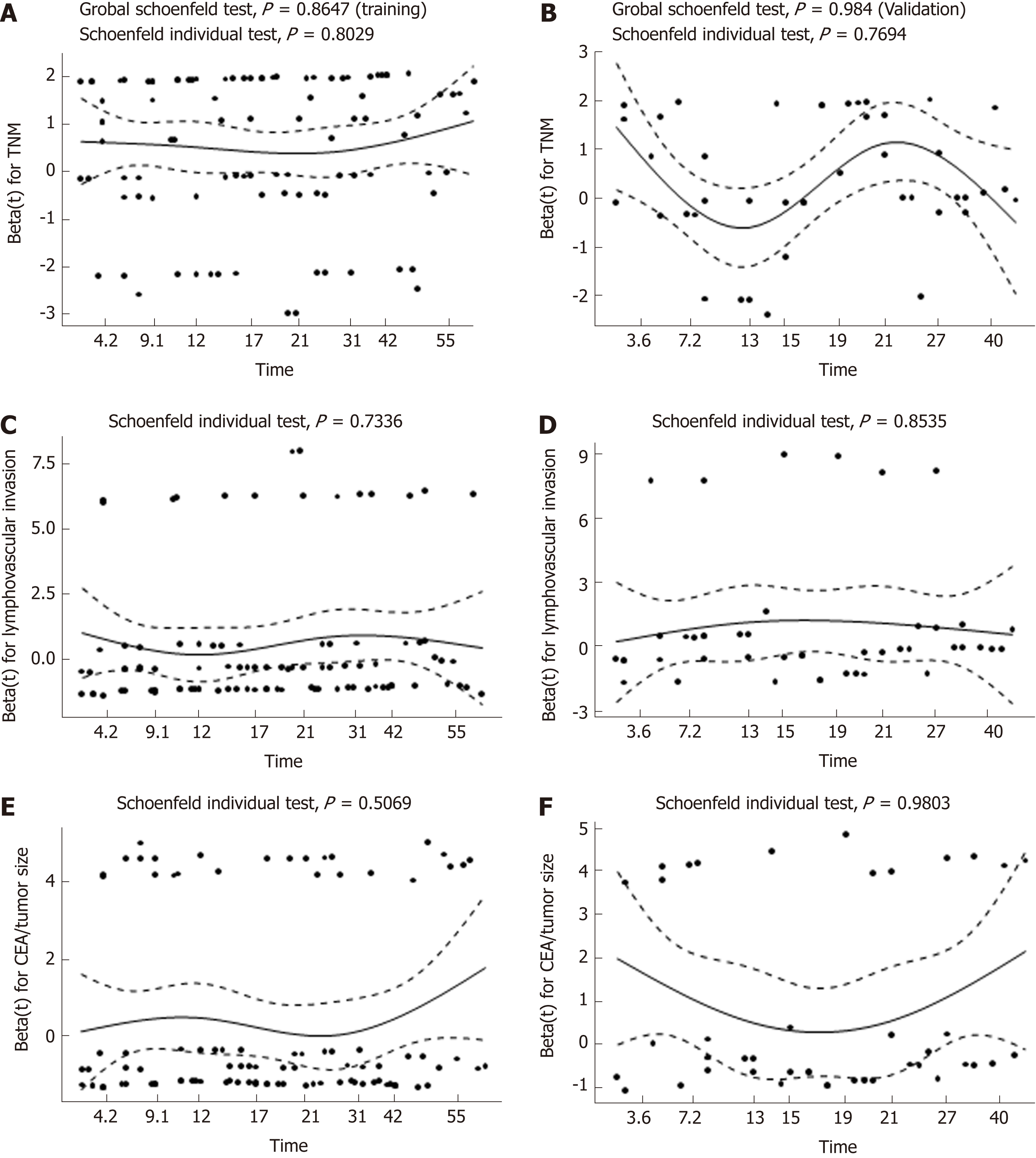
To adjust for the influence of potential confounders, the prognostic impact of CEA/tumor size on OS and DFS was further explored by constructing a multivariate Cox proportional hazards model. Forward, backward, and stepwise algorithms determined by the AIC were used to construct the optimum model. All of the above methods generated identical models, and the results were similar in both cohorts. According to the multivariate analysis, older age, poor differentiation, advanced TNM stage, and higher CEA/tumor size were all significantly correlated with a worse OS. With respect to DFS, the significance of TNM stage, lymphovascular invasion, and CEA/tumor size was retained in the final model in both cohorts (Table 4). As a result, CEA/tumor size was significantly associated with OS in both the training cohort [hazard ratio (HR) = 2.18, 95%CI: 1.28-3.73] and in the validation cohort (HR = 4.83, 95%CI: 2.21-10.51). However, CEA/tumor size showed a critical association with DFS in the training cohort (HR = 1.47, 95%CI: 0.93-2.33) and a significant association in the validation cohort (HR = 2.61, 95%CI: 1.38-4.95). Plotting the Schoenfeld residuals against time showed that all the covariates in the Cox proportional hazards model for OS and DFS met the proportional hazard assumption (P > 0.05, Figures 5 and 6).
| Training cohort (n = 371) | Validation cohort (n = 185) | |||||
| OS | Hazard radio | 95%CI | P-value | Hazard radio | 95%CI | P-value |
| Age | 1.02 | 1.00-1.04 | 0.023a | 1.05 | 1.02-1.09 | 0.003a |
| TNM1 (ref = stage I) | 1.47 | 1.04-2.07 | 0.031a | 1.84 | 1.04-3.24 | 0.035a |
| Differentiation1 (ref = poor) | 0.57 | 0.39-0.85 | 0.006a | 0.50 | 0.28-0.90 | 0.021a |
| CEA/tumor size (ref = low) | 2.18 | 1.28-3.73 | 0.004a | 4.83 | 2.21-10.52 | <0.001a |
| DFS | ||||||
| TNM1 (ref = stage I) | 1.75 | 1.32-2.32 | <0.001a | 1.43 | 0.94-2.17 | 0.091 |
| Lymphovascular invasion (ref = negative) | 1.85 | 1.08-3.16 | 0.024a | 2.45 | 1.00-6.03 | 0.05 |
| CEA/tumor size (ref = low) | 1.47 | 0.93-2.33 | 0.096 | 2.61 | 1.38-4.95 | 0.003a |
CEA is reliable for the detection of rectal cancer recurrence and is recommended by the ASCO and EGTM as a prognostic biomarker during routine follow-up for CRC after surgical resection[5,7]. Despite many published studies that have demonstrated the prognostic impact of CEA among CRC patients, no agreement concerning the cutoff values has been established[20-24]. Moreover, Tong et al[12] found that postoperative tissue CEA is significantly associated with the prognosis of CRC, and Huo et al[13] illustrated that serum CEA density was an independent prognostic factor in patients with colorectal pulmonary metastasis. CEA, as a classic tumor marker, is used to evaluate the biological activity of malignancies, but biological activity will also be affected by tumor quantity. When tumors grow, no matter how clumsily or aggressively, serum CEA level will increase as the expression of CEA increases in proliferating adenocarcinoma cells. Therefore, tumor size is a confounding factor that should be minimized. A new prognostic factor that better reflects the intra-tumor CEA concentration without omission of the tumor volume will be much more accurate than a classic serum CEA test. A comprehensive study stated that tumor size, especially the maximum horizontal tumor diameter, represented a valuable prognosticator in gastric cancer[25]. Another study found a direct relationship between tumor volume in rectal cancer and overall survival[26]. Therefore, we decided to use CEA/tumor size, which is a simple parameter that could reduce the confounding effect of tumor size. Taken together, these results indicate that the ratio of serum CEA to the maximum tumor diameter might be a better marker to assess the tumor’s biological activity and to refine the insufficient prognostic value of serum CEA for rectal cancer.
This is the first study to evaluate the prognostic value of CEA/tumor size for stage I to III rectal cancer. We found that patients with a high CEA/tumor size (over 2.429 ng/mL per cm) had a significantly worse 5-year OS and DFS. Therefore, a correlation exists between the preoperative CEA/tumor size and the prognosis of rectal cancer patients after resection. Patients with high CEA/tumor size tended to have a worse outcome. In our study, no correlation was found between tumor size and survival outcome. Univariate and multivariate analyses showed that CEA/tumor size was independently associated with OS and DFS, while absolute serum CEA was not. This implied that adjusting the confounding effect of tumor size may improve the prognostic value of CEA. Thus, preoperative CEA/tumor size can be used as an independent prognostic factor for patients with stage I-III rectal cancer.
Notably, this study highlights the important relationship between serum CEA and tumor volume, which is in agreement with previous studies. With respect to the prevalence of serum CEA in clinical applications, additional improvement in the accuracy of estimating 5-year outcomes will benefit more patients. In addition, a growing tumor with little change in biological activity will exhibit an increased CEA level and a relatively unchangeable CEA/tumor size. Therefore, CEA/tumor size is not only more accurate but more stable than serum CEA. In patients with identical serum CEA levels, it is necessary to make a decision regarding clinical intervention for patients with smaller maximum tumor diameter. In contrast, a low CEA/tumor size may indicate less aggressive and malignant tumors.
However, we admit that our study has some inherent limitations. First, maximum tumor diameter as an indication of tumor volume is not so precise. Huo et al[13] used the spherical formula (4 × π × radius3)/3 to represent the tumor volume since they assumed that pulmonary tumors were spherical. Nevertheless, unlike pulmonary metastases, rectal tumors are not a fixed geometric shape, which means this method is unreliable[26]. Alternatively, the careful delineation of the tumor boundary combined with specific software may provide a more accurate estimation of tumor size. However, maximum tumor diameter represents a quick and convenient method that can be used to roughly estimate tumor volume, and as a result, has more prospects for clinical application. Second, CEA/tumor size cannot be used as part of a routine follow-up index to dynamically monitor the recurrence and metastasis of rectal cancer after surgery. Surgical resection will remove the local tumor, and therefore CEA/tumor size will be unable to be continually calculated. For patients with new-found relapse and metastasis, the value of CEA/tumor size requires further investigation. Beyond that, we also noticed a newly published research study suggesting that postoperative CEA is a better prognostic marker for survival than preoperative CEA in colon cancer[27]. However, postoperative CEA indicates complete resection of the tumor, while CEA/tumor size is focused on tumor malignancy. Third, we did not include patients with neoadjuvant chemotherapy and/or radiotherapy because both of them can influence preoperative CEA and tumor size and may bias our result. Finally, in both cohorts, CEA/tumor size was included in the final Cox model for DFS, which means that CEA/tumor size is an essential factor for DFS. But the P-value was 0.003 in the validation cohort and 0.096 in the training cohort, which may result from the insufficient sample size or discrepancy between the two cohorts. Whether CEA/tumor size is really associated with DFS still needs further study.
Preoperative CEA/tumor size is a new method that can be used to predict the outcomes of patients with stage I-III rectal cancer, which may influence the decision-making process for a specific treatment regimen and patient counselling. Since both CEA level and tumor size are routinely measured before surgery, the data of CEA/tumor size can be obtained by simple calculation. This will facilitate the application of CEA/tumor size in clinical practice. Compared with CEA, a great advantage of CEA/tumor size is the ability to figure out those patients with higher CEA but relatively small tumor size. The result of our study suggests that these easily neglected tumors may represent higher malignancy and worse outcome. With the optimization of risk stratification, clinicians can choose individualized treatment options and the outcome of rectal cancer patients can be improved accordingly.
Of course, some limitations of our study design still need to be discussed. As a retrospective study, we were not able to obtain high-level clinical evidence. We also found that some patients did not reach an enough follow-up time, which may influence the accuracy of our result. Since the estimated cutoff point was relatively high, the high-risk group and low-risk group accounted for 20% and 80%, respectively. Although the number of events per variable > 10 in our Cox model, a larger sample size would be better to obtain more reliable results[28]. Therefore, a large-scale prospective study and longer follow-up time are needed and we will try our best to validate our conclusion in future studies. It is also worthwhile for other researchers to further validate our study with new evidence, as we are looking forward to a more accurate prognostic factor for rectal cancer.
In summary, patients with a high preoperative CEA/tumor size have a worse outcome than those with a low CEA/tumor size. Preoperative CEA/tumor size may play an important role in prognosis and treatment decisions of rectal cancer patients after surgery.
Colorectal cancer (CRC) is the third most frequently diagnosed malignancy and one of the leading causes of cancer-related mortality worldwide. Therapy options for CRC have been developed rapidly in the past decade, but selecting optimal treatments for individuals remains a great challenge for clinicians due to the lack of effective markers.
Controversy exists regarding the insufficient prognostic value of preoperative serum CEA alone, which is a widely used biomarker in rectal cancer. Recent studies have found that local CEA may play a more important role in the prognosis of CRC than overall serum CEA. Some studies have tried to add another factor like tumor size to improve the prognostic value of biomarker, such as prostate specific antigen density and tumor-infiltrating CD8+ T-cell density. Here, we combined preoperative serum CEA and the maximum tumor diameter to correct the CEA level, which may better reflect the malignancy of rectal cancer and improve the risk stratification system.
We aimed to investigate the prognostic impact of the preoperative CEA/tumor size in patients with rectal cancer, which may influence the decision-making process for a specific treatment regimen and patient counselling.
We retrospectively reviewed 696 stage I to III rectal cancer patients who underwent curative tumor resection from 2007 to 2012. These patients were randomly divided into two cohorts for cross-validation: Training cohort and validation cohort. The training cohort was used to generate an optimal cutoff point and the validation cohort was used to further validate the model. Maximally selected rank statistics were used to identify the optimum cutoff for CEA/tumor size. The Kaplan-Meier method and log-rank test were used to plot the survival curve and to compare the survival data. Univariate and multivariate Cox regression analyses were used to determine the prognostic value of CEA/tumor size. The primary and secondary outcomes were overall survival (OS) and disease-free survival (DFS), respectively.
In all, 556 patients who satisfied both the inclusion and exclusion criteria were included and randomly divided into a training cohort (2/3 of 556, n = 371) and a validation cohort (1/3 of 556, n = 185). The cutoff was 2.429 ng/mL per cm. Comparison of the baseline data showed that high CEA/tumor size was correlated with older age, high TNM stage, presence of perineural invasion, high CEA, and high carbohydrate antigen 19-9 (CA 19-9). Kaplan-Meier curves showed a manifest reduction in 5-year OS (training cohort: 56.7% vs 81.1%, P < 0.001; validation cohort: 58.8% vs 85.6%, P <0.001) and DFS (training cohort: 52.5% vs 71.9%, P = 0.02; validation cohort: 50.3% vs 79.3%, P = 0.002) in the high CEA/tumor size group compared with the low CEA/tumor size group. Univariate and multivariate analyses identified CEA/tumor size as an independent prognostic factor for OS (training cohort: hazard ratio (HR) = 2.18 95% confidence interval (CI): 1.28-3.73, P = 0.004; validation cohort: HR = 4.83, 95%CI: 2.21-10.52, P < 0.001) as well as DFS (training cohort: HR = 1.47, 95% CI: 0.93-2.33, P = 0.096; validation cohort: HR: 2.61, 95%CI = 1.38-4.95, P = 0.003).
This is the first study to evaluate the prognostic value of CEA/tumor size for stage I to III rectal cancer. We found that patients with high CEA/tumor size tended to have a worse outcome. Adjusting the confounding effect of tumor size can improve the prognostic value of CEA. Compared with CEA, another great advantage of CEA/tumor size is the ability to figure out those patients with higher CEA but relatively small tumor size. The results of our study suggest that these easily neglected tumors may represent higher malignancy and worse outcome, which may challenge the conventional risk stratification system. Since both CEA level and tumor size are routinely measured before surgery, the data of CEA/tumor size can be obtained by simple calculation. Therefore, CEA/tumor size can be easily applied in clinical practice.
As a retrospective study, we were not able to obtain high-level clinical evidence, but the current retrospective study will provide an important basis for us to carry out a prospective study. A large-scale prospective study and longer follow-up time are needed in future study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkan A, Ziogas DE S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55782] [Article Influence: 7968.9] [Reference Citation Analysis (132)] |
| 2. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3299] [Article Influence: 412.4] [Reference Citation Analysis (3)] |
| 3. | Deng Y. Rectal Cancer in Asian vs. Western Countries: Why the Variation in Incidence? Curr Treat Options Oncol. 2017;18:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. J Clin Oncol. 2015;33:1787-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 271] [Article Influence: 27.1] [Reference Citation Analysis (1)] |
| 5. | Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousová M, Holubec L, Sturgeon C. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134:2513-2522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 6. | Stiksma J, Grootendorst DC, van der Linden PW. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer. 2014;13:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC; ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1111] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 8. | Nicholson BD, Shinkins B, Pathiraja I, Roberts NW, James TJ, Mallett S, Perera R, Primrose JN, Mant D. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev. 2015;CD011134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Chen L, Jiang B, Wang Z, Liu M, Yang H, Xing J, Zhang C, Yao Z, Zhang N, Cui M, Su X. Combined preoperative CEA and CD44v6 improves prognostic value in patients with stage I and stage II colorectal cancer. Clin Transl Oncol. 2014;16:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Zhan X, Sun X, Hong Y, Wang Y, Ding K. Combined Detection of Preoperative Neutrophil-to-Lymphocyte Ratio and CEA as an Independent Prognostic Factor in Nonmetastatic Patients Undergoing Colorectal Cancer Resection Is Superior to NLR or CEA Alone. Biomed Res Int. 2017;2017:3809464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Kozman MA, Fisher OM, Rebolledo BJ, Parikh R, Valle SJ, Arrowaili A, Alzahrani N, Liauw W, Morris DL. CEA to peritoneal carcinomatosis index (PCI) ratio is prognostic in patients with colorectal cancer peritoneal carcinomatosis undergoing cytoreduction surgery and intraperitoneal chemotherapy: A retrospective cohort study. J Surg Oncol. 2018;117:725-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Tong G, Xu W, Zhang G, Liu J, Zheng Z, Chen Y, Niu P, Xu X. The role of tissue and serum carcinoembryonic antigen in stages I to III of colorectal cancer-A retrospective cohort study. Cancer Med. 2018;7:5327-5338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Huo YR, Glenn D, Liauw W, Power M, Zhao J, Morris DL. Evaluation of carcinoembryonic antigen (CEA) density as a prognostic factor for percutaneous ablation of pulmonary colorectal metastases. Eur Radiol. 2017;27:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Aminsharifi A, Howard L, Wu Y, De Hoedt A, Bailey C, Freedland SJ, Polascik TJ. Prostate Specific Antigen Density as a Predictor of Clinically Significant Prostate Cancer When the Prostate Specific Antigen is in the Diagnostic Gray Zone: Defining the Optimum Cutoff Point Stratified by Race and Body Mass Index. J Urol. 2018;200:758-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Shimizu S, Hiratsuka H, Koike K, Tsuchihashi K, Sonoda T, Ogi K, Miyakawa A, Kobayashi J, Kaneko T, Igarashi T, Hasegawa T, Miyazaki A. Tumor-infiltrating CD8<sup>+</sup> T-cell density is an independent prognostic marker for oral squamous cell carcinoma. Cancer Med. 2019;8:80-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Eichelberger LE, Koch MO, Eble JN, Ulbright TM, Juliar BE, Cheng L. Maximum tumor diameter is an independent predictor of prostate-specific antigen recurrence in prostate cancer. Mod Pathol. 2005;18:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Yoshimoto T, Morine Y, Imura S, Ikemoto T, Iwahashi S, Saito YU, Yamada S, Ishikawa D, Teraoku H, Yoshikawa M, Higashijima J, Takasu C, Shimada M. Maximum Diameter and Number of Tumors as a New Prognostic Indicator of Colorectal Liver Metastases. In Vivo. 2017;31:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Tanaka N, Fujimoto K, Chihara Y, Torimoto M, Hirao Y, Konishi N, Saito I. Prostatic volume and volume-adjusted prostate-specific antigen as predictive parameters for prostate cancer patients with intermediate PSA levels. Prostate Cancer Prostatic Dis. 2007;10:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Peng Y, Shen D, Liao S, Turkbey B, Rais-Bahrami S, Wood B, Karademir I, Antic T, Yousef A, Jiang Y, Pinto PA, Choyke PL, Oto A. MRI-based prostate volume-adjusted prostate-specific antigen in the diagnosis of prostate cancer. J Magn Reson Imaging. 2015;42:1733-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Becerra AZ, Probst CP, Tejani MA, Aquina CT, González MG, Hensley BJ, Noyes K, Monson JR, Fleming FJ. Evaluating the Prognostic Role of Elevated Preoperative Carcinoembryonic Antigen Levels in Colon Cancer Patients: Results from the National Cancer Database. Ann Surg Oncol. 2016;23:1554-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol. 2010;101:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Ann Surg Oncol. 2009;16:3087-3093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Peng Y, Wang L, Gu J. Elevated preoperative carcinoembryonic antigen (CEA) and Ki67 is predictor of decreased survival in IIA stage colon cancer. World J Surg. 2013;37:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Thirunavukarasu P, Sukumar S, Sathaiah M, Mahan M, Pragatheeshwar KD, Pingpank JF, Zeh H, Bartels CJ, Lee KK, Bartlett DL. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011;103:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 25. | Jun KH, Jung H, Baek JM, Chin HM, Park WB. Does tumor size have an impact on gastric cancer? A single institute experience. Langenbecks Arch Surg. 2009;394:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Tayyab M, Razack A, Sharma A, Gunn J, Hartley JE. Correlation of rectal tumor volumes with oncological outcomes for low rectal cancers: does tumor size matter? Surg Today. 2015;45:826-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Konishi T, Shimada Y, Hsu M, Tufts L, Jimenez-Rodriguez R, Cercek A, Yaeger R, Saltz L, Smith JJ, Nash GM, Guillem JG, Paty PB, Garcia-Aguilar J, Gonen M, Weiser MR. Association of Preoperative and Postoperative Serum Carcinoembryonic Antigen and Colon Cancer Outcome. JAMA Oncol. 2018;4:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 28. | Austin PC, Allignol A, Fine JP. The number of primary events per variable affects estimation of the subdistribution hazard competing risks model. J Clin Epidemiol. 2017;83:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |