Published online Aug 28, 2019. doi: 10.3748/wjg.v25.i32.4779
Peer-review started: April 22, 2019
First decision: May 9, 2019
Revised: June 10, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 28, 2019
Processing time: 130 Days and 4.4 Hours
Liver cirrhosis is a chronic hepatic disease which is associated with cardiovascular abnormalities. Hyperdynamic circulation in liver cirrhosis causes functional and structural cardiac alterations. The prevalence of left ventricle diastolic dysfunction (LVDD) in cirrhotic patients ranges from 25.7% to as high as 81.4% as reported in different studies. In several studies the severity of diastolic dysfunction (DD) correlated with a degree of liver failure and the rate of dysfunction was higher in patients with decompensated cirrhosis compared with compensated. Future directions of comprehensive assessment of cardiac function in cirrhotic patients might provide a better prognosis for these patients.
To clarify the correlation between the severity of liver cirrhosis and left ventricle diastolic dysfunction in the existing literature.
Through January and February of 2019 at Vilnius University we conducted a systematic review of the global existing literature on the prevalence of left ventricle diastolic dysfunction in patients with liver cirrhosis. We searched for articles in PubMed, Medline and Web of science databases. Articles were selected by using adequate inclusion and exclusion criteria. Our interest was the outcome of likely correlation between the severity of cirrhosis [evaluated by Child-Pugh classes, Model For End-Stage Liver Disease (MELD) scores] and left ventricle diastolic dysfunction [classified according to American Society of Echocardiography (ASE) guidelines (2009, 2016)], as well as relative risk of dysfunction in cirrhotic patients. Subgroup analyses were performed to evaluate the ratio and grades of left ventricle diastolic dysfunction with respect to cirrhosis severity.
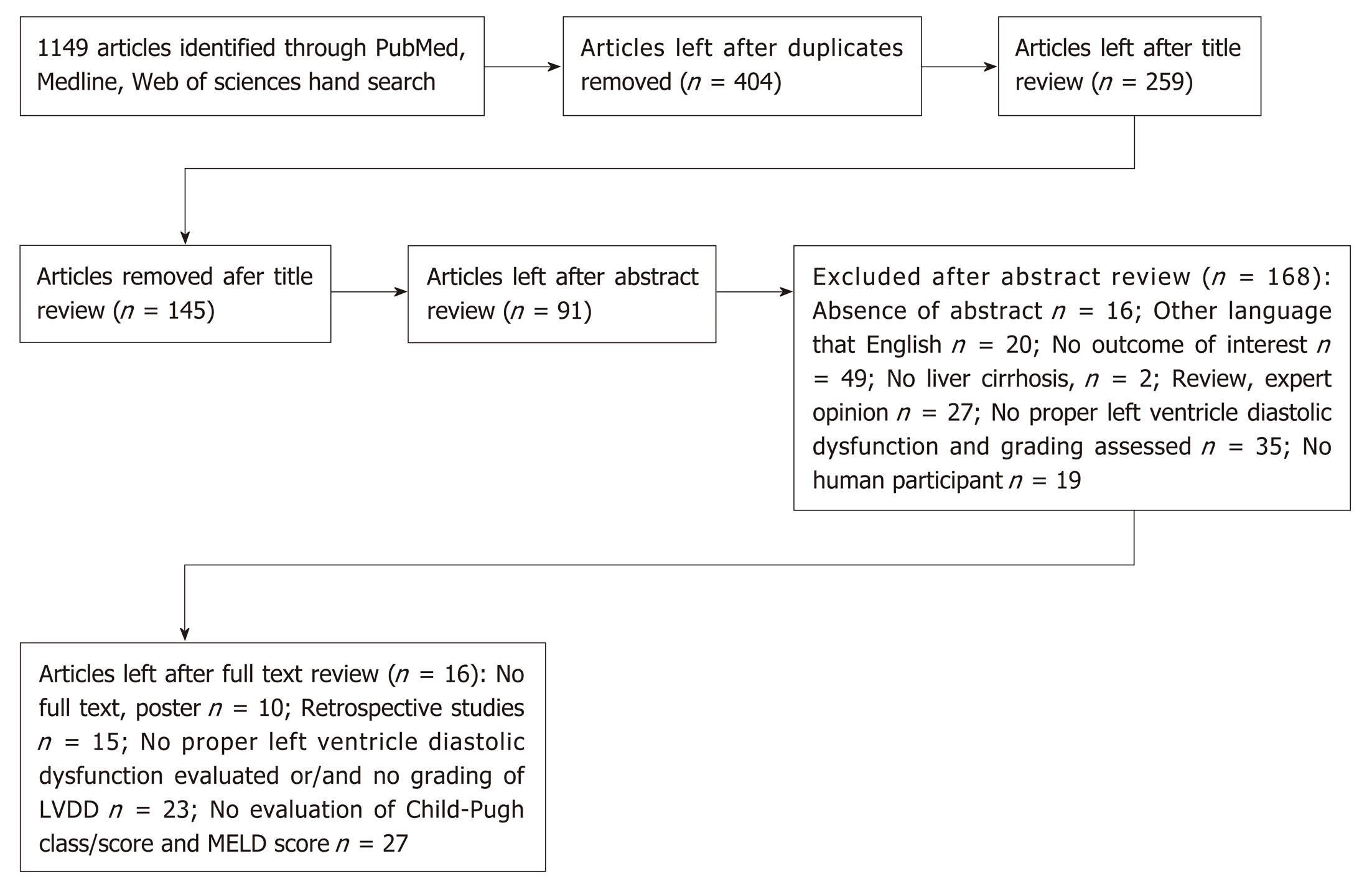
A total of 1149 articles and abstracts met the initial search criteria. Sixteen articles which met the predefined eligibility criteria were included in the final analysis. Overall, 1067 patients (out of them 723 men) with liver cirrhosis were evaluated for left ventricle diastolic dysfunction. In our systemic analysis we have found that 51.2% of cirrhotic patients had left ventricle diastolic dysfunction diagnosed and the grade 1 was the most prevalent (59.2%, P < 0.001) among them, the grade 3 had been rarely diagnosed - only 5.1%. The data about the prevalence of diastolic dysfunction in cirrhotic patients depending on Child-Pugh Classes was available from 5 studies (365 patients overall) and only in 1 research diastolic dysfunction was found being associated with severity of liver cirrhosis (P < 0.005). We established that diastolic dysfunction was diagnosed in 44.6% of Child-Pugh A class patients, in 62% of Child B class and in 63.3% of Child C patients (P = 0.028). The proportion of patients with higher diastolic dysfunction grades increases in more severe cirrhosis presentation (P < 0.001). There was no difference between mean MELD scores in patients with and without diastolic dysfunction and in different diastolic dysfunction groups. In all studies diastolic dysfunction was more frequent in patients with ascites.
This systemic analysis suggests that left ventricle diastolic dysfunction is an attribute of liver cirrhosis which has not received sufficient attention from clinicians so far. Future suggestions of a comprehensive assessment of cardiac function in cirrhotic patients might provide a better prognosis for these patients and give hint for better understanding of the left ventricle diastolic dysfunction pathogenesis in liver cirrhosis.
Core tip: In this systematic review we aimed to assess the association between left ventricle diastolic dysfunction and the severity of liver cirrhosis, evaluated by Child-Pugh classes. The proportion of patients with higher diastolic dysfunction grades increases in more severe cirrhosis presentation (P < 0.001). These results suggest that left ventricle diastolic dysfunction and its severity is an attribute of liver cirrhosis. Future directions of a comprehensive assessment of cardiac function in cirrhotic patients might provide a better prognosis to these patients and give hint for better understanding of the left ventricle diastolic dysfunction pathogenesis in liver cirrhosis.
- Citation: Stundiene I, Sarnelyte J, Norkute A, Aidietiene S, Liakina V, Masalaite L, Valantinas J. Liver cirrhosis and left ventricle diastolic dysfunction: Systematic review. World J Gastroenterol 2019; 25(32): 4779-4795
- URL: https://www.wjgnet.com/1007-9327/full/v25/i32/4779.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i32.4779
Cirrhosis is a chronic hepatic disease that typically presents in individuals aged 50-60 years[1,2]. This major health problem is one of the most common causes of death worldwide and is associated with a wide range of cardiovascular abnormalities[3,4]. Patients with cirrhosis typically demonstrate disturbance of circulatory system. Hyperdynamic circulation presented in patients with liver cirrhosis causes functional and structural cardiac alterations[5-7]. These changes induce myocardial remodelling and left ventricle hypertrophy resulting in systolic and diastolic dysfunctions and cardiomyopathy[8-10].
Cirrhotic cardiomyopathy is characterized by the following factors: impaired contractile responsiveness to stress, diastolic dysfunction and electrophysiological abnormalities without overt cardiac disease[11,12]. Although this type of liver cirrhosis complication has been described since the 1960s, just recently it has been recognized not only as a feature of alcoholic cardiotoxicity but as an ailment occurring in cirrhosis of any aetiology[13].
The most common cardiac abnormality that occurs among cirrhotic patients is left ventricular diastolic dysfunction (LVDD) related to development of myocardial fibrosis, hypertrophy and subendothelial edema[5-7]. Diastolic dysfunction occurs when the passive elastic traits of the myocardium are reduced due to the increased myocardial mass and changes in the extracellular collagen[14].
According to different studies, the prevalence of LVDD in cirrhotic patients is ranging from 25.7% to as high as 81.4%[15,16]. Evidence suggests that patients with cirrhosis display primarily LVDD with normal systolic function at rest [1]. Diastolic dysfunction may progress to systolic dysfunction, although this has not been directly shown in cirrhotic patients[17-19]. In several studies severity of LVDD correlated with the degree of liver failure[20-22]. Furthermore, the rate of LVDD was higher in decompensated cirrhosis compared with compensated cirrhosis[23]. On the contrary, several studies have not identified any association between severity of liver disease and LVDD[24-26]. Therefore an attentive analysis of already performed studies on LVDD causes and prevalence in cirrhotic patients as well as LVDD complication influence on patients’ quality of life and their survival is needed to develop appropriate treatment strategy. It is important to assess cardiac changes in especially those patients, who are waiting for the liver transplantation, paracentesis or transjugular intrahepatic portosystemic shunt (TIPSS) implantation, because cardiovascular decompensation can be the main cause of operative failure.
The aim of our study was to conduct a systematic review of the existing literature to collate all data about the cause, diagnosis, prognosis of LVDD in cirrhotic patients and try to elucidate the association between severity of liver disease and LVDD with the intention to propose measures for the therapy of cirrhotic patients with LVDD.
An electronic search of the global literature on the prevalence of left ventricle diastolic dysfunction in liver cirrhosis was performed. Articles available in PubMed, Medline and Web of Science databases were reviewed in January and February 2019. The period of publications collected was 1969-2019.
The Medical Subject Heading (MeSH) database was used as a terminological search filter. The last search date was 7/3/2019. The main key phrases for searching the articles were: “left ventricle diastolic dysfunction” and “liver cirrhosis“. These terms were connected together to narrow our search by using a Boolean operator AND. Our MeSH Major Topic was “Liver cirrhosis/*complications“ and the main MeSH Subheading was “left ventricle diastolic dysfunction/*etiology, diagnosis, treatment”. To describe diastolic dysfunction we used these terms: Left ventricle diastolic dysfunction, cirrhotic cardiomyopathy, diastolic function of left ventricle, ventricular dysfunction, left. The full terms used in PubMed search can be found in electronic supplementary material.
Studies were considered eligible if they met the following criteria: (1) Articles published in English only; (2) Only patients with liver cirrhosis diagnosed by clinical view, laboratory and imaging tests were included; (3) Severity of liver cirrhosis was evaluated by using Model for End-Stage Liver Disease score, (MELD score) or/and Child-Pugh classification A/B/C and scores.; (4) Left ventricle diastolic function was evaluated by tissue Doppler imaging method; and (5) Left ventricle diastolic dysfunction was defined and its grading (1, 2, 3) classified according to ASE 2009 or 2016 guidelines[27,28].
The exclusion criteria were: (1) Studies with children (< 18 years old); (2) Retrospective studies; (3) No proper definition of left ventricle diastolic dysfunction according to ASE guidelines (2009, 2016); (4) No grading of left ventricle diastolic dysfunction according to ASE guidelines (2009, 2016); (5) Only systolic function evaluated; (6) Other causes of liver insufficiency, for example, toxic liver disease, septicaemia; (7) No severity of cirrhosis evaluated by using MELD score and Child-Pugh classification; (8) Patients who had chronic heart disease, for example, valvular pathology, coronary heart disease, arrhythmias, as atrial fibrillation (Patients with chronic heart disease other than cirrhotic cardiomyopathy); (9) No human participants in the study; (10) No full article available; and (11) Article not available in English.
Our interest was the correlation between the severity of cirrhosis and left ventricle diastolic dysfunction, relative risk of LVDD in liver cirrhosis group.
Three investigators separately reviewed all the titles, abstracts and full articles. Reviewers excluded irrelevant articles. The disagreement on whether to include or not the article was solved by other two investigators. Data extraction was performed by three investigators and reviewed for accuracy by another investigator.
Total number of patients, number of healthy controls, age, etiology of cirrhosis, Child-Pugh scores and classification A/B/C, MELD score, the presence of ascites, LVDD, the grading of diastolic dysfunction, the year of the LVDD guidelines used, influence of LVDD on survival of the patients with cirrhosis were recorded. Some studies had missing data for Child-Pugh classification, MELD scores or did not have a healthy controls group.
The risk of bias for each study was evaluated by using ROBINS-I tool from the Cochrane Method group at the study level[29]. All of the major domains were selected: confounding, selection of the participants, evaluation of LVDD and grading, missing data, selection of the reported results and measurement of outcomes[29].
This systematic review was prepared and revised according to the PRISMA 2009 Checklist[30]. Statistical evaluation was performed with R version 3.5.3 (GNU Project).
Studies meeting the inclusion criteria were tabulated. Statistically and clinically appropriate studies were combined to estimate the LVDD prevalence and LVDD grades’ distribution in patients with respect to cirrhosis severity.
To evaluate the overall distribution of LVDD grades in cirrhotic patients χ2 goodness-of-fit test was applied. Pearson’s χ2 was used for comparing the distribution of patients with and without LVDD in three Child-Pugh groups.
For analysing of LVDD grades ratio in three Child-Pugh groups and LVDD prevalence in cirrhotics with and without ascites Pearson’s χ2 and Fisher exact tests were used. P value < 0.05 was considered as statistically significant.
A total of 1149 articles and abstracts met the initial search criteria.
Out of 1149 articles 91 were selected for full text review. As many as 16 articles that met the predefined eligibility criteria were included in the final analysis[15,16,21,22,24-26,31-39]. The process of identification, screening and eligibility is described in Figure 1.
Included studies and patients’ characteristics are shown in Table 1. Overall 1067 patients (of which 723 were men) with liver cirrhosis were evaluated for LVDD. Out of 16 studies 9 had control group, altogether 318 control subjects included.
| Author | Study type | Number of cases, n | Gender (male), n | Control, n | Etiology of cirrhosis, n | Means1 of Child-Pugh scores' | Child-Pugh classification, n | Means1 of MELD scores' | ||||
| V | A | O | A | B | C | |||||||
| Dadhich et al[31] | Cross sectional case control study | 40 | ND | 20 | 26 | 10 | 4 | 7.5 ± 1.08 pre-ascitic group, 9.4 ± 2.11 ascitic group | 4 | 22 | 14 | ND |
| Lee et al[32] | Cohort study | 70 | 55 | 0 | 16 | 47 | 7 | ND | 18 | 35 | 14.1 ± 5.9 | |
| Karagiannakis et al[33] | Cohort study | 45 | E33 | 0 | 19 | 22 | 4 | 6.43 ± 1.9 | 26 | 15 | 3 | 11.5 ± 4.2 |
| Alexopoulou et al[34] | Cross-sectional observa-tional study | 76 | 57 | 0 | 41 | 20 | 15 | 9.2 ± 2.7 | 11 | 28 | 37 | 17 ± 7 |
| Farouk et al[15] | Cross-sectional study | 35 | 22 | 16 | 35 | ND | 6 | 14 | 15 | ND | ||
| Bhuin et al[16] | Descrip-tive study | 70 | 32 | 0 | 70 | ND | 4 | 38 | 28 | ND | ||
| Cesari et al[35] | Case series | 117 | 106 | 46 | 57 | 60 | 8 ± 2 | ND | ND | ND | 12 ± 5 | |
| Ru ız-del- Arbol, et al[21] | Cross-sectional | 80 | 67 | 0 | 32 | 35 | 13 | 8 ± 2 grade 0; 9 ± 2 grade 1; 10 ± 2 grade 2; | 12 | 30 | 38 | 15 ± 6 grade 0; 16 ± 5 grade 1; 21 ± 6 grade 2; |
| Rimbas et al[36] | Cross-sectional observa-tional study | 46 | 30 | 46 | 19 | 24 | 3 | 7 ± 2 | 23 | 16 | 7 | 13 ± 5 |
| Hammami et al[25] | Cross-sectional study | 80 | 42 | 80 | 42 | 38 | ND | 24 | 36 | 20 | 14.2 ± 4.98 | |
| Merli et al[37] | Cross-sectional observa-tional study | 90 | 59 | 31 | 49 | 28 | 13 | ND | 48 | 26 | 16 | 11.9 ± 4.7 |
| Merli et al[26] | Case series | 74 | 44 | 26 | 41 | 21 | 12 | ND | 29 | 26 | 19 | 13 ± 5 |
| Kazankov et al[38] | Cross-sectional observa-tional study | 44 | 27 | 23 | 5 | 32 | 7 | 7.1 ± 2.2 | 20 | 12 | 8 | 12.3 ± 4.9 |
| Nazar et al[22] | Case series | 100 | 71 | 0 | 41 | 46 | 15 | with LVDD 9 ± 2; no LVDD 8 ± 2.2 | 26 | 39 | 37 | 15 ± 7 |
| Devauchelle et al[39] | Descrip-tive study | 40 | 30 | 0 | 9 | 24 | 7 | ND | 13 | 9 | 18 | 16 |
| Somani et al[24] | Cross-sectional observa-tional study | 60 | 48 | 30 | 22 | 30 | 8 | ND | 8 | 26 | 26 | 15.2 ± 4.6 without LVDD;14.6 ± 4.3 with LVDD |
| Total | 1067 | 723 | 318 | |||||||||
The risk of bias for each study is demonstrated in Table 2.
| Author | Bias due to confounding | Bias in selection of participants into the study | Bias in evaluation of LVDD and grading | Bias due to missing data | Bias in selection of the reported result | Bias in measurement of outcomes |
| Dadhich et al[31] | Moderate | Low | Low | No information | Low | Low |
| Lee et al[32] | Moderate | Moderate | Low | No information | Low | Low |
| Karagiannakis et al[33] | Moderate | Moderate | Low | Serious | Low | Low |
| Alexopoulou et al[34] | Moderate | Moderate | Low | No information | Low | Low |
| Farouk et al[15] | Moderate | Low | Low | No information | Low | Low |
| Bhuin et al[16] | Moderate | Moderate | Low | No information | Low | Low |
| Cesari et al[35] | Moderate | Serious | Low | Serious | Low | Low |
| Ru ız-del- Arbol, et al[21] | Low | Moderate | Low | No information | Low | Low |
| Rimbas et al[36] | Moderate | Moderate | Low | Seriuos | Moderate | Low |
| Hammami et al[25] | Moderate | Serious | Low | Low | Low | Moderate |
| Merli et al[37] | Moderate | Low | Low | Serious | Moderate | Moderate |
| Merli et al[26] | Moderate | Low | Low | Serious | Low | Low |
| Kazankov et al[38] | Serious | Moderate | Low | Serious | No information | Serious |
| Nazar et al[22] | Low | Moderate | Low | Serious | No information | Moderate |
| Devauchelle et al[39] | Critical | No information | Low | No information | Low | No information |
| Somani et al[24] | Low | Low | Low | No information | Low | Low |
All patients and controls had echocardiography with tissue Doppler imaging performed. Tested parameters of LVDD are shown in Table 3.
| Author | DT | IVRT | E | A | E/A | E/e' = E/E' | e'(E') | e'(E') medial | e'(E') lateral |
| Dadhich et al[31] | + | + | + | + | + | + | + | ||
| Lee et al[32] | + | + | + | + | + | + | + | ||
| Karagiannakis et al[33] | + | + | + | + | + | + | + | ||
| Alexopoulou et al[34] | + | + | + | + | + | + | |||
| Farouk et al[15] | + | + | + | + | + | + | |||
| Bhuin et al[16] | + | + | + | + | + | + | + | ||
| Cesari et al[35] | + | + | + | + | + | + | |||
| Ruız-del-Arbol et al[21] | + | + | + | + | + | + | + | ||
| Rimbas et al[36] | + | + | + | + | + | + | + | + | |
| Hammami et al[25] | + | + | + | + | + | ||||
| Merli et al[37] | + | + | + | + | + | ||||
| Merli et al[26] | + | + | + | + | |||||
| Kazankov et al[38] | + | + | + | + | |||||
| Nazar et al[22] | + | + | + | + | |||||
| Devauchelle et al[39] | + | + | + | + | + | ||||
| Somani et al[24] | + | + | + | + | + | + | + |
The data about LVDD, its grades and guidelines used in every study is depicted in Table 4.
| Author | Diagnosis of LVDD in patients, n (%) | Grade 1, n | Grade 2, n | Grade 3, n | Diagnosis of LVDD in controls, n (%) | Year of LVDD guidelines used |
| Dadhich et al[31] | 28 (70) | 11 | 17 | 2009 | ||
| Lee et al[32] | 44 (62.8) | 34 | 10 | 2009 | ||
| Karagiannakis et al[33] | 17 (37.7) | 9 | 8 | 2009 | ||
| Alexopoulou et al[34] | 51 (67.1) | 37 | 11 | 3 | 2009 | |
| Farouk et al[15] | 9 (25.7) | 9 | 2009 | |||
| Bhuin et al[16] | 57 (81.4) | 29 | 28 | 2009 | ||
| Cesari et al[35] | 43 (37) | 4 | 28 | 11 | 7 (16) | 2009 |
| Ru ız-del- Arbol, et al[21] | 37 (46.2) | 19 | 18 | 2009 | ||
| Rimbas et al[36] | 22 (47.8) | 12 | 8 | 2 | 2016 | |
| Hammami et al[25] | 41 (51.2) | 19 | 11 | 11 | 8 (10) | 2016 |
| Merli et al[37] | 36 (40) | 24 | 12 | 2009 | ||
| Merli et al[26] | 47 (63.5) | 37 | 10 | 2009 | ||
| Kazankov et al[38] | 24 (54.5) | 11 | 12 | 1 | 2009 | |
| Nazar et al[22] | 58 (58) | 42 | 16 | 2009 | ||
| Devauchelle et al[39] | 14 (35) | 11 | 3 | 2009 | ||
| Somani et al[24] | 18 (30) | 15 | 3 | 2009 | ||
| Total | 546 (51.2) | 323 | 195 | 28 | 15 (4.7) |
In our systemic analysis we found 51.2% of cirrhotic patients had LVDD diagnosed and the grade 1 was the most prevalent (59.2%, P < 0.001) among them. The grade 3 had been rarely diagnosed - only in 5.1%.
In most studies controls had no cardiac dysfunction but in two studies[35,25] 4.7% (overall 15 out of 318) controls were diagnosed with LVDD.
In our systemic analysis the data about LVDD prevalence in cirrhotic patients depending on Child-Pugh classes was available from 5 studies (365 patients overall)[16,24,25,32,34] and only Bhuin et al[16] found LVDD being associated with severity of liver cirrhosis (P < 0.005). Other four studies did not find any difference in the severity of LVDD among Child classes[24,25,32,34].
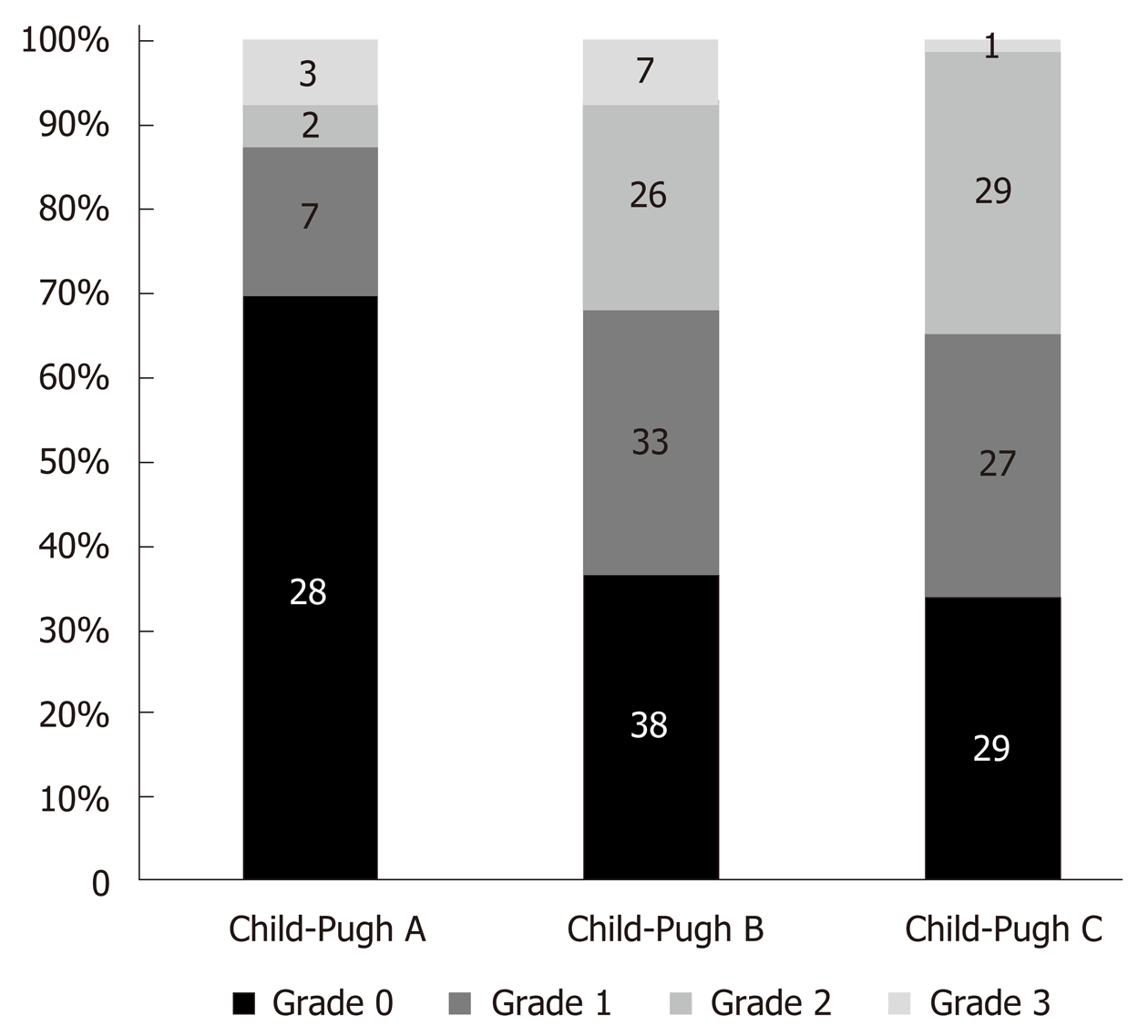
After analysing this data we observed that LVDD was diagnosed in 44.6% (29) of Child-Pugh A class patients, in 62% (101) of Child B class and in 63.3% (81) of Child C patients (P = 0.028).
Only three studies reported how many patients with different LVDD grades were in different Child-Pugh classes[16,21,25]. Figure 2 shows that the proportion of patients with higher LVDD grades increases in the presence of more severe cirrhosis.
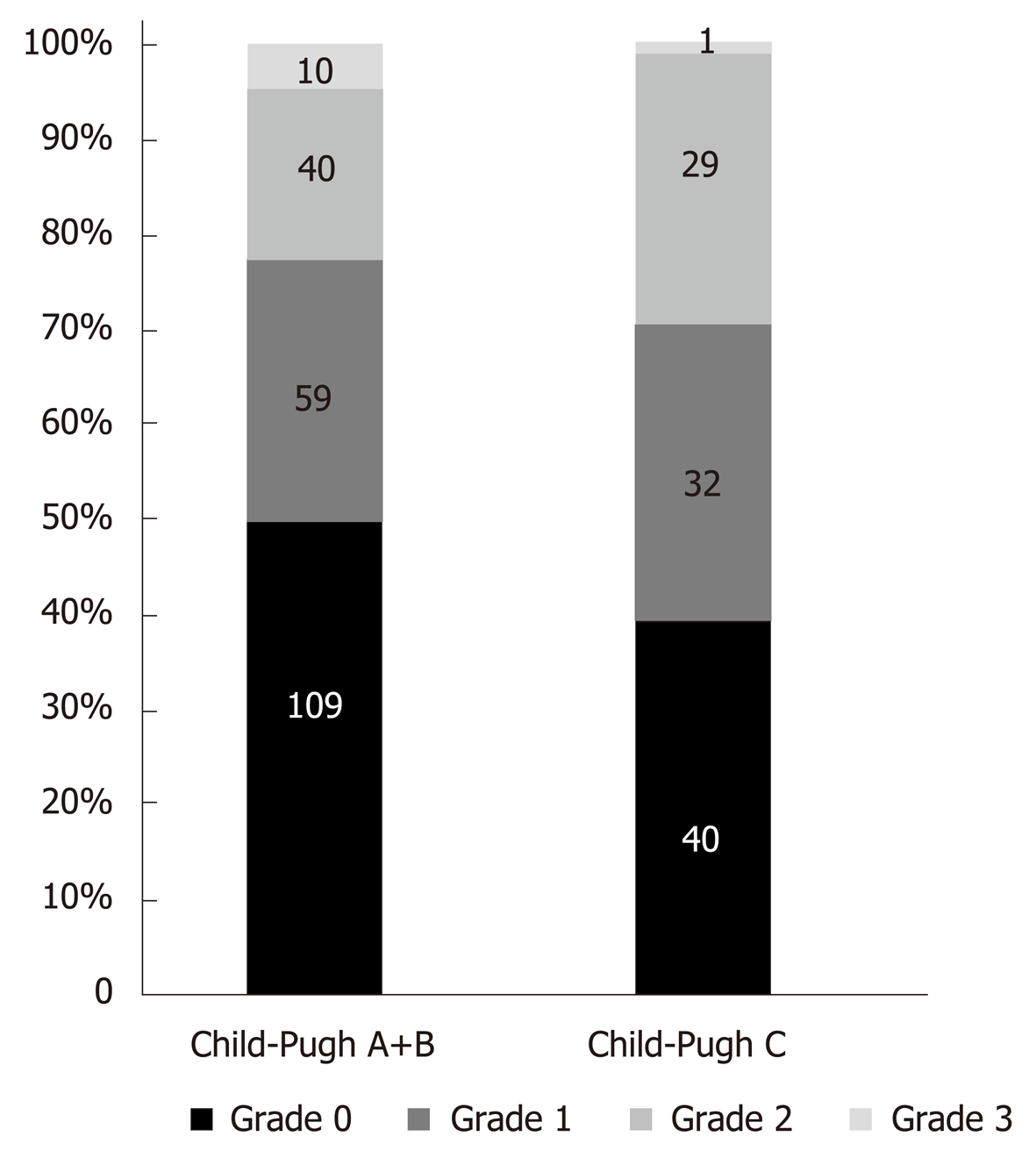
In Merli et al[37] study patients were divided in two groups: Child-Pugh A-B classes and Child-Pugh C class patients. In order not to lose the data reported in this study we also divided all patients from other 5 studies into the same two groups and analysed all patient data from 6 studies[16,24,25,32,34,37]. LVDD was found in 49.3% (149) of patients of Child - Pugh A+B classes and in 59.7% (86) of Child - Pugh C class patients (P = 0.051).
The prevalence of different LVDD grades in grouped Child-Pugh classes is shown in Figure 3.
The difference in means of Child-Pugh scores between LVDD grades was analysed in 5 studies[21,24,25,33,34,36] (Table 5). In two studies the considerable difference was observed. Ruız-del-Arbol identified the substantial difference (P < 0.01) between mean Child-Pugh scores in LVDD grade 2 and LVDD grade 0 groups[21]. In Rimbas et al[36] study Child-Pugh scores were markedly higher in patients with liver cirrhosis of LVDD grade 2 than in those with grades 1 and no LVDD.
| Author | Number of cases, n | Difference in means of Child-Pugh scores between LVDD grades Yes/No/Not assessed | Mean Child-Pugh scores in patients without LVDD | Mean Child-Pugh scores in patients with LVDD | P value |
| Dadhich et al[31] | 40 | NA | NA | NA | - |
| Lee et al[32] | 70 | NA | NA | NA | - |
| Karagiannakis et al[33] | 45 | No | 6.5 ± 2.1 | 6.4 ± 1.6 | NS |
| Alexopoulou et al[34] | 76 | No | 9 ± 2.8 | 9.2 ± 2.6 | NS |
| Farouk et al[15] | 35 | NA | NA | NA | - |
| Bhuin et al[16] | 70 | NA | NA | NA | - |
| Cesari et al[35] | 117 | NA | NA | NA | - |
| Ru ız-del- Arbol, et al[21] | 80 | Yes | 8 ± 2 (7-9) | 10 ± 2 (9-11) | aP < 0.01 |
| Rimbas et al[36] | 46 | Yes | 7.1 ± 2 | 7.3 ± 2.1 | aP < 0.01 |
| Hammami et al[25] | 80 | NA | NA | - | |
| Merli et al[37] | 90 | NA | NA | NA | - |
| Merli et al[26] | 74 | NA | NA | NA | - |
| Kazankov et al[38] | 44 | No | ND | ND | NS |
| Nazar et al[22] | 100 | NA | NA | NA | - |
| Devauchelle et al[39] | 40 | NA | NA | NA | - |
| Somani et al[24] | 60 | NA | NA | NA | - |
The difference in means of MELD scores between LVDD groups and in patients without LVDD was estimated in 8 studies[21,24,32,33,34,36,38,39] (Table 6). Two studies found that patients from the grade 2 LVDD subgroup had a higher MELD score, compared to patients with grade 1 LVDD and without LVDD (P < 0.01 and P < 0.005, respectively)[21,36]. The means of MELD scores did not markedly differ in patients with and without LVDD in remaining 6 studies[24,32,33,34,38,39].
| Author | MELD1 score in patients with LVDD | MELD1 score in patients without LVDD | P value |
| Lee et al[32] | 13.9 ± 5.7 | 14.5 ± 6.4 | NS |
| Karagiannakis et al[33] | 11 ± 3.5 | 11.7 ± 4.6 | NS |
| Alexopoulou et al[34] | 15.5 ± 6.5 | 14.3 ± 5.7 | NS |
| Nazar et al[22] | 16 ± 8 | 14 ± 6 | P = 0.07 |
| Somani et al[24] | 14.6 ± 4.3 | 15.2 ± 4.6 | NS |
| Rimbas et al[36] | 13 ± 6 | 13 ± 5 | NS |
| Ru ız-del- Arbol, et al[21] | 16 ± 53 and 21 ± 64 | 15 ± 6 | aP < 0.005 |
| Kazankov et al[38] | ND | ND | ND |
| Devauchelle et al[39] | 14 (4)2 | 16 (11)2 | NS |
In the study of Lee et al[32] the survival rate was substantially lower in patients with LVDD than in those without LVDD (31.1 mo vs 42.6 mo, P = 0.01). Patients with a ratio of early filling velocity to early diastolic mitral annular velocity (E/e’) ≥ 10 (LVDD grade 2) had lower survival than the patients with E/e’ ratio < 10.
In Ruiz-Del-Albor study patients without LVDD had the longest and those with grade 2 LVDD the shortest probability of survival [95% vs 39% (P < 0.01), res-pectively][21]. The value of the E/e’ ratio with higher sensitivity and specificity to predict 12-mo survival was 10. Survival was significantly greater in E/e’ < 10, compared to the E/e’ 10 group [91% and 29% (P < 0.0001), respectively][21].
On Kaplan-Meier analysis in Karagiannakis et al[33] study patients with LVDD had markedly worse prognosis compared to those without (P = 0.013, log rank: 5.495).
A multivariate analysis in the study of Alexopoulou et al[34] showed that age, MELD and plasma sodium but no LVDD were predictive of death. In three other studies no substantial difference in survival was found between patients with or without LVDD[24,36,37].
The association of ascites and LVDD was assessed in 8 studies (Table 7)[21,22,26,31,35]. Overall 604 patients were studied and in 56.9% of those with ascites LVDD was diagnosed (in 211 patients out of 371) while in 48.5% (113 out of 233) of patients without ascites LVDD presented, P = 0.04451.
| Author | Overall patients with LVDD, n (%) | LVDD in patients with ascites, n (%) | LVDD in patients without ascites, n (%) | P value |
| Dadhich et al[31] | 28/70 | 16/80 | 12/60 | P = 0.09 |
| Lee et al[32] | NA | |||
| Karagiannakis et al[33] | 17/37.8 | 9/40.9 | 8/34.8 | NA |
| Alexopoulou et al[34] | 51/67.1 | 14/93.3 | 37/60.7 | P = 0.016 |
| Farouk et al[15] | NA | |||
| Bhuin et al[16] | 47/67.1 | 47/67.1 | 0 | |
| Cesari et al[35] | 37/32.7 | 22/28.6 | 15/41.7 | P < 0.005 |
| Ru ız-del- Arbol et al[21] | 37/46.3 | 31/57.4 | 6/23.1 | aP < 0.01 and bP < 0.025 |
| Rimbas et al[36] | NA | |||
| Hammami et al[25] | 49/61.0 | 25/64.1 | 24/58.5 | NA |
| Merli et al[37] | NA | |||
| Merli et al[26] | P = 0.04 | |||
| Kazankov et al[38] | NA | |||
| Nazar et al[22] | 58/58.0 | 47/63.5 | 11/42.3 | P = 0.03 |
| Devauchelle et al[39] | NA | |||
| Somani et al[24] | NA |
Dadlich et al[31] showed that the majority of the cirrhotic patients with ascites (80%) had LVDD compared to those without ascites but the difference was not proved statistically (P = 0.09). Ruiz-del-Arbor et al[21] in their research showed that ascites was more frequent in LVDD grade 2 comparing with LVDD grade 1 (P < 0.025) and normal diastolic function (P < 0.01). Alexopoulou et al[34] investigated that severe ascites was more frequent in the group with LVDD (P = 0.016). In 2013 Merli et al[26] noticed that LVDD was more prevalent in patients with ascites compared to those without it (77% vs 56%; P = 0.04).
The older age and the presence of LVDD was assessed in 10 studies (Table 8). LVDD was associated with an older age in 4 studies[32-35]. On the contrary, 6 studies found that LVDD was not associated with an older age but there was no evidence of age impact on LVDD in all these studies[21,22,24,31,38,39].
| Author | Number of cases, n | Older age association with the presence of LVDD, Yes/No/Not assessed | Age (patients without LVDD)1 | Age (patients with LVDD)1 | P value |
| Dadhich et al[31] | 40 | No | ND | ND | NS |
| Lee et al[32] | 70 | Yes | 47.8 ± 8.0 | 58.2 ± 9.9 | P < 0.001 |
| Karagiannakis et al[33] | 45 | Yes | 53.8 ± 13 | 62.8 ± 9 | P = 0.016 |
| Alexopoulou et al[34] | 76 | Yes | 53.4 ± 16.5 | 62.4 ± 12.7 | P = 0.04 |
| Farouk et al[15] | 35 | NA | NA | NA | - |
| Bhuin et al[16] | 70 | NA | NA | NA | - |
| Cesari et al[35] | 117 | Yes | ND | ND | P = 0.005 |
| Ru ız-del- Arbol et al[21] | 80 | No | ND | ND | NS |
| Rimbas et al[36] | 46 | NA | NA | NA | - |
| Hammami et al[25] | 80 | NA | NA | NA | - |
| Merli et al[37] | 90 | NA | NA | NA | - |
| Merli et al[26] | 74 | NA | NA | NA | - |
| Kazankov et al[38] | 44 | No | ND | ND | NS |
| Nazar et al[22] | 100 | No | 55 ± 10 | 57 ± 10 | NS |
| Devauchelle et al[39] | 40 | No | 57 (10) | 59 (13) | NS |
| Somani et al[24] | 60 | No | 50.5 ± 9.9 | 49.5 ± 8.5 | NS |
To our knowledge this is the first systematic review which summarizes the articles demonstrating the association between the severity of liver cirrhosis and LVDD.
Numerous studies have shown changes in the diastolic function in patients with liver cirrhosis. The prevalence of diastolic dysfunction in the articles analysed by our systematic review ranged from 25.7% to 81.4%[15,16]. More than half of all patients included in our review had some degree of diastolic dysfunction (51.2 %). As many as 59.2% of all patients with LVDD was grade 1 dysfunction. Similar results were presented by Hammami et al[25] (23.8% of those who had grade 1 LVDD), by Lee et al[32] (34 out of 44 patients with LVDD had grade 1 disorder), and by Merli et al[37] - 36 patients had LVDD and 24 out of 36 had grade 1 LVDD.
Older studies used only E/A ratio from the echocardiographic parameters to define LVDD but this ratio is very reliable on preload, and is age-related[29,32]. With increasing age E/A ratio normally decreases[29]. So, we did not include those publications in our review. For subjects without history of cardiovascular disease the risk of having diastolic dysfunction increases with age > 60 years[28,29]. Not all the studies in our review excluded the elderly patients. Ruiz-del-arbor et al[21] excluded the patients who were > 60 years old, Somani et al[34] also did not include in their study the patients who were > 75 years old, Merli only applied the patients < 70 years old in order to prevent from false positive detection of LVDD[37]. The newest studies included in our review used TDI (tissue Doppler imaging) method to detect the mitral annulus velocities, as E/e’ (the ratio between early mitral inflow velocity and mitral annular early diastolic velocity)[21,28,29,32]. For example, Lee et al[32] on their research noted that E/e’ parameter is a very relevant echocardiographic parameter for diagnosing and grading LVDD. We think that E/A ratio alone could not be used to detect the disorder of diastolic function.
This systematic review was performed based on our study of 47 patients with diagnosed liver cirrhosis waiting for the liver transplantation in Vilnius University Hospital Santaros Clinics, Lithuania[40]. It was a retrospective study about the association between the LVDD and the severity of cirrhosis, evaluated by MELD score and Child-Pugh classification. The data obtained was consistent with the studies analysed in this review. Normal diastolic function was present in 20 (42.6%), first grade dysfunction in 7 (14.9%), second grade in 18 (38.3 %) and the third grade in 2 (4.3%) cirrhotic patients. These results are similar to Cesari et al[35] research, where 28 out of 43 patients had grade 2 diastolic dysfunction, but in other studies the most common LVDD grade was 1. For example, in Alexopoulou et al[34] study 37 (72.6%) out of 51 patients with LVDD had grade 1 dysfunction. In our research we did not observe considerable difference in the severity of diastolic function among different groups of Child A, B, and C (P = 0.536; r = -0.093) and in distribution of diastolic dysfunction between Child-Pugh classes (P = 0.098). Hammami et al[25] did not capture the difference in the grades of LVDD between Child-Pugh classes as well.
However, the results of our research are discordant with other studies. Bhuin et al[16] revealed a noteworthy prevalence of LVDD between Child-Pugh classes (P = 0.0204). Ruız-Del- Arbol et al[21] observed notable differences in means of Child-Pugh scores and LVDD grades (P < 0.01). Also, Ruız-Del- Arbol et al[21] showed that changes in the cardiac function were related to the severity of the liver failure defined by a higher prevalence of hepatic encephalopathy, ascites and higher Child-Pugh and MELD scores in patients with grade 2 LVDD than in those with normal diastolic function. Merli et al[26] investigated that LVDD occurred more frequently in patients with the ascites group than in the group without it (77% vs 56%; P = 0.04). Rimbas et al[36] found that LVDD is present in all classes of Child-Pugh, not only in patients with advanced liver cirrhosis.
We also detected a correlation between the diastolic function and MELD scores (P = 0.007; r = 0.4) in our study and there was a substantial difference in means of the MELD scores (P = 0.004) between grades of LVDD. The difference was significant between grades: 0 and 3 (P = 0.008), 1 and 3 (P = 0.034).
Ruız-Del- Arbol et al[21] established a notable difference in means of MELD scores between the patients with and without LVDD (P < 0.005). On the contrary, Kazankov et al[38] did not observe any significance of MELD scores between the groups with and without LVDD.
Having summarized the data from the research of Lee et al[17], Alexopoulou et al[34], Bhuin et al[16], Somani et al[24], Hammami et al[25] we observed that there is a substantial correlation between the prevalence of LVDD in Child-Pugh classes (P = 0.028). More cases of LVDD were detected in classes B and C comparing to class A (62% and 63.3 % vs 44.6%). Summarizing the data from the Bhuin et al[16], Ruız-Del- Arbol et al[21] and Hammami et al[25], we observed considerable difference (P < 0.001) between the grades of LVDD and Child-Pugh classes. Child-Pugh classes B and C had a certain degree of LVDD more often than Child-Pugh class A. For example, grade 1 LVDD occurred in 31.7% of Child B class, 31.4% of Child C and only in 17.5% of Child A class.
Control groups of healthy subjects were formed in 9 studies[15,24-26,31,35-38]. It is interesting to note that in Cesari et al[25] and in Hammami et al[35] research some controls had LVDD. Hammami et al[25] documented 8 healthy participants out of 80 to have LVDD (6 had grade 1, and 2 had grade 3). As many as 16% of 46 controls of Cesari et al[25,35] research had low or moderate grade of LVDD. These findings might show the risk of bias in confounding domain.
Some of the patients from studies we reviewed were treated with β-blockers in order to lower portal pressure[26,31,36-38]. Dadhich et al[31] investigated that 23 patients involved in their study consumed propranolol as a treatment of portal hypertension, and most of those patients had ascites (15 vs 8, P = 0.03). β-blockers reduce the risk of variceal bleeding, bacterial translocation and developing of spontaneous bacterial peritonitis[41]. In 2010 Wong et al[41] in 2010 stated that the negative effect of β-blockers might be possible in patients with cardiac disorder due to cirrhosis and refractory ascites. According to the window theory, using β-blockers is effective for those patients between early cirrhosis and end-stage cirrhosis[42]. In severe end-stage cirrhosis β-blockers have negative impact on cardiac output. The decrease in cardiac output causes renal hypoperfusion, and hepatorenal syndrome type 2 might develop[41]. Those patients with liver cirrhosis and refractory ascites are reliant on cardiac output in order to maintain a sufficient arterial blood pressure[43]. Mandorfer et al[44] in their research suspected that use of non-selective β-blockers at the time of diagnosis of spontaneous bacterial peritonitis was related to a higher rate of developing hepatorenal syndrome and mortality. Also, Sersté et al[45] noticed that using propranolol was related to a higher mortality compared to those not having β-blocker therapy. Garcia-Tsao et al[46] in European Association for the Study of the Liver (EASL) Journal of Hepatology stated that non-selective β-blockers should be discontinued in patients with liver cirrhosis who develop hypotension and any signs of impaired organ perfusion. Kazankov et al[38] investigated that there was no difference in cardiac and liver function among the patients treated or not treated with β-blockers. Devauchelle et al[39] did not notice a statistically significant difference (P = 0.7) in using β-blockers between LVDD and no LVDD groups. Even if the influence of prophylactic treatment on LVDD was not assessed in all of our chosen studies, we think it may have had an impact on the LVDD grade.
In our review we noticed that patients with ascites had a higher tendency to develop LVDD (P = 0.04451). The diagnosis of diastolic dysfunction in patients with liver cirrhosis is strongly influenced by circulatory dysfunction, predominantly by splanchnic vasodilatation, which triggers fluid retention[26,47-49,22]. Ascites affects ventricular filling probably by the increase in intra-thoracic pressure[50]. Removal of ascitic fluid by paracentesis changes echocardiography parameters: Reduces the A wave velocity and increases the E/A ratio, but compared with healthy controls it still remains abnormal[50]. Ascites is an important factor included in Child-Pugh score calculation and not in MELD score formula. This fact might have affected the results - why we detected a relation between LVDD and Child-Pugh class but not with MELD score. We believe that fluid retention in the abdomen have an influence on the left ventricle diastolic function.
The risk of bias for each study was evaluated by using ROBINS-I tool (Table 2)[29]. Most of the studies had bias linked with confounding and selection of the participants. There were doubts about the inclusion and exclusion criteria (there were studies with no decent inclusion criteria), selecting the healthy controls, which may have had an influence on the results (no precise definition of healthy individuals, some cases of LVDD in controls group were observed in a couple of studies); not all studies excluded patients, who were taking β-blockers, or other medication, as antihypertensive treatment, diuretics, which could have affected the results of echocardiography. On the contrary, all studies perfectly fulfilled the evaluation of LVDD and its grades. We considered a research as being at low risk of bias if all domains were properly assured and no serious/critical bias was detected. More than half of publications fulfilled the low risk of bias.
The limitation of our systematic review is relatively small number of patients and the bias of confounding and selection domains. Not all the studies reviewed all prognostic factors, which could have affected the presence of LVDD. There was insufficient data, which also could have affected the measurement of outcomes. Nevertheless, several studies in our review did not observe MELD scores, so we could not assess its relation with LVDD.
Cardiac involvement in patients with liver cirrhosis seems to be clear, and there is correlation between the severity of cirrhosis and LVDD.
Although the data of our study and published results of other studies included in present review had limited number of participants, their systemic analysis suggests that LVDD and its severity depends on the stage of liver cirrhosis. To our knowledge this is the first systematic review which summarizes the investigations demonstrating the association between the severity of liver cirrhosis and LVDD. The cardiac assessment in patients with Child-Pugh class B and C would be useful for the prevention of mortality risk because of heart function impairment. Echocardiography should be done at least once in patients‘ case history. However, larger studies are needed for final and detailed conclusions.
Cardiovascular abnormalities occur in patients with liver cirrhosis. The prevalence of left ventricle diastolic dysfunction (LVDD) in cirrhotic patients ranges from 25.7% to as high as 81.4% as reported in different studies. In several studies the severity of diastolic dysfunction (DD) correlated with a degree of liver failure and the rate of dysfunction was higher in patients with decompensated cirrhosis compared with compensated. It is important to assess cardiac changes in especially those patients who are waiting for the liver transplantation, paracentesis or transjugular intrahepatic portosystemic shunt (TIPSS) implantation, because cardiovascular decompensation can be the main cause of operative failure.
Due to the lack of studies, well designed studies are necessary to determine the role of assesment of cardiac function in cirrhotic patients. Therefore, an attentive analysis of already performed studies on LVDD prevalence in cirrhotic patients is very important because LVDD has an influence on patients’ quality of life and their survival, especially in patients with advanced liver cirrhosis. Developing an appropriate treatment strategy for such patients also is the key to future research. Future directions of comprehensive assessment of cardiac function in cirrhotic patients might provide a better prognosis to these patients so it is an attractive field of research.
The aim of our study was to clarify the association between the severity of liver cirrhosis and left ventricle diastolic dysfunction in the published studies.
In January and February, 2019 at Vilnius University we conducted a systematic review of the global existing literature on the prevalence of left ventricle diastolic dysfunction in patients with liver cirrhosis. We searched for articles in PubMed, Medline and Web of science databases. Eligibility criteria were: (1) Articles published in English only; (2) Only patients with liver cirrhosis diagnosed by clinical view, laboratory and imaging tests were included; (3) Severity of liver cirrhosis was evaluated by using Model for End-Stage Liver Disease score, (MELD score) or/and Child-Pugh classification A/B/C and scores; (4) Left ventricle diastolic function was evaluated by tissue Doppler imaging method; and (5) Left ventricle diastolic dysfunction was defined and its grading (I, II, III) classified according to ASE guidelines issued in 2009 or 2016. Analyses were performed to evaluate the ratio and grades of left ventricle diastolic dysfunction with respect to cirrhosis severity.
A total of 1149 articles and abstracts met the initial search criteria. 16 articles which met the predefined eligibility criteria were included in the final analysis. Overall, 1067 patients (out of them 723 men) with liver cirrhosis were evaluated for left ventricle diastolic dysfunction. In our systemic analysis we have found that 51.2% of cirrhotic patients had left ventricle diastolic dysfunction diagnosed and the grade 1 was the most prevalent (59.2 %, P < 0.001) among them, the grade 3 had been rarely diagnosed - only 5.1%. The data about the prevalence of diastolic dysfunction‘s prevalence in cirrhotic patients depending on Child-Pugh Classes was available from 5 studies (365 patients overall) and only 1 study found diastolic dysfunction being associated with severity of liver cirrhosis (P < 0.005). We established that diastolic dysfunction was diagnosed in 44.6% of Child-Pugh A class patients, in 62% of Child B class and in 63.3% of Child C patients (P = 0.028). The proportion of patients with higher diastolic dysfunction grades increases in more severe cirrhosis presentation (P < 0.001). There was no difference between mean MELD scores in patients with and without diastolic dysfunction. In all studies diastolic dysfunction was more frequent in patients with ascites (P = 0.04451). The influence of prophylactic treatment with of β-blockers on LVDD grade was not assessed in all of our chosen studies, but we think it may have had an impact on the cardiac function.
To our knowledge this is the first systematic review which summarizes the articles demonstrating the association between the severity of liver cirrhosis and LVDD. Our review of the existing literature allowed us to interpret the data supporting the correlation between the severity of liver cirrhosis evaluated by Child-Pugh classification and LVDD. Several studies in our review did not observe MELD scores, so we could not assess its‘ potential relation with LVDD. We highly recommend cardiac assessment of patients with Child-Pugh class B and C. Echocardiography should be done at least once in patients‘ case history, because these patients are at high risk of mortality. However, more extensive studies are needed for final and detailed conclusions.
Future directions of comprehensive assessment of cardiac function in cirrhotic patients might provide a better prognosis for such patients. We highly suggest that future clinical trials should evaluate how using of β-blockers in cirrhotics affects cardiac function, especially LVDD and its grade. In order to clarify the correlation between the severity of liver cirrhosis and LVDD and to elucidate the pathogenetic association between liver damages and cardiac impairment, the more large-scale prospective research is needed.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Lithuania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Manenti A, Peng B, Tripathi D, Voiosu AM, Zapater P S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
| 1. | Valeriano V, Funaro S, Lionetti R, Riggio O, Pulcinelli G, Fiore P, Masini A, De Castro S, Merli M. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95:3200-3205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Karasu Z, Mindikoğlu AL, Van Thiel DH. Cardiovascular problems in cirrhotic patients. Turk J Gastroenterol. 2004;15:126-132. [PubMed] |
| 3. | Al-Hamoudi WK. Cardiovascular changes in cirrhosis: pathogenesis and clinical implications. Saudi J Gastroenterol. 2010;16:145-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733-746, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Ma Z, Lee SS. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology. 1996;24:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 205] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1022] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 7. | Fouad YM, Yehia R. Hepato-cardiac disorders. World J Hepatol. 2014;6:41-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Mircoli L, Rivera R, Bonforte G, Fedele L, Genovesi S, Surian M, Ferrari AU. Influence of left ventricular mass, uremia and hypertension on vagal tachycardic reserve. J Hypertens. 2003;21:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Braverman AC, Steiner MA, Picus D, White H. High-output congestive heart failure following transjugular intrahepatic portal-systemic shunting. Chest. 1995;107:1467-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Carvalheiro F, Rodrigues C, Adrego T, Viana J, Vieira H, Seco C, Pereira L, Pinto F, Eufrásio A, Bento C, Furtado E. Diastolic Dysfunction in Liver Cirrhosis: Prognostic Predictor in Liver Transplantation? Transplant Proc. 2016;48:128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Ruiz-del-Árbol L, Serradilla R. Cirrhotic cardiomyopathy. World J Gastroenterol. 2015;21:11502-11521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Fede G, Privitera G, Tomaselli T, Spadaro L, Purrello F. Cardiovascular dysfunction in patients with liver cirrhosis. Ann Gastroenterol. 2015;28:31-40. [PubMed] |
| 14. | Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004;351:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 316] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Farouk H, Al-Maimoony T, Nasr A, El-Serafy M, Abdel-Ghany M. Echocardiographic assessment of the left ventricular diastolic function in patients with non-alcoholic liver cirrhosis. Cor et Vasa. 2017;59:540-545. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Patel RH, Pandya S, Nanjappa S, Greene JN. A Case of Refractory Pulmonary Coccidioidomycosis Successfully Treated with Posaconazole Therapy. J Fam Med. 2017;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Lee RF, Glenn TK, Lee SS. Cardiac dysfunction in cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:125-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Møller S, Henriksen JH. Cardiovascular dysfunction in cirrhosis. Pathophysiological evidence of a cirrhotic cardiomyopathy. Scand J Gastroenterol. 2001;36:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Pozzi M, Redaelli E, Ratti L, Poli G, Guidi C, Milanese M, Calchera I, Mancia G. Time-course of diastolic dysfunction in different stages of chronic HCV related liver diseases. Minerva Gastroenterol Dietol. 2005;51:179-186. [PubMed] |
| 20. | Deliu R, Donoiu I, Militaru C, Istrătoaie O, Ciurea T. Correlates of diastolic function in patients with alcoholic liver cirrhosis. CorSalud. 2018;10:106-112. |
| 21. | Ruíz-del-Árbol L, Achécar L, Serradilla R, Rodríguez-Gandía MÁ, Rivero M, Garrido E, Natcher JJ. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology. 2013;58:1732-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Nazar A, Guevara M, Sitges M, Terra C, Solà E, Guigou C, Arroyo V, Ginès P. LEFT ventricular function assessed by echocardiography in cirrhosis: relationship to systemic hemodynamics and renal dysfunction. J Hepatol. 2013;58:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Falletta C, Filì D, Nugara C, Di Gesaro G, Minà C, Baravoglia CM, Romano G, Scardulla C, Tuzzolino F, Vizzini G, Clemenza F. Diastolic dysfunction diagnosed by tissue Doppler imaging in cirrhotic patients: Prevalence and its possible relationship with clinical outcome. Eur J Intern Med. 2015;26:830-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Somani PO, Contractor Q, Chaurasia AS, Rathi PM. Diastolic dysfunction characterizes cirrhotic cardiomyopathy. Indian Heart J. 2014;66:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Hammami R, Boudabbous M, Jdidi J, Trabelsi F, Mroua F, Kallel R, Amouri A, Abid D, Tahri N, Abid L, Kammoun S. Cirrhotic cardiomyopathy: is there any correlation between the stage of cardiac impairment and the severity of liver disease? Libyan J Med. 2017;12:1283162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Merli M, Calicchia A, Ruffa A, Pellicori P, Riggio O, Giusto M, Gaudio C, Torromeo C. Cardiac dysfunction in cirrhosis is not associated with the severity of liver disease. Eur J Intern Med. 2013;24:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2270] [Cited by in RCA: 2334] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 28. | Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2879] [Cited by in RCA: 3843] [Article Influence: 427.0] [Reference Citation Analysis (0)] |
| 29. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10855] [Article Influence: 1206.1] [Reference Citation Analysis (2)] |
| 30. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47173] [Article Influence: 2948.3] [Reference Citation Analysis (0)] |
| 31. | Dadhich S, Goswami A, Jain VK, Gahlot A, Kulamarva G, Bhargava N. Cardiac dysfunction in cirrhotic portal hypertension with or without ascites. Ann Gastroenterol. 2014;27:244-249. [PubMed] |
| 32. | Lee SK, Song MJ, Kim SH, Ahn HJ. Cardiac diastolic dysfunction predicts poor prognosis in patients with decompensated liver cirrhosis. Clin Mol Hepatol. 2018;24:409-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Karagiannakis DS, Vlachogiannakos J, Anastasiadis G, Vafiadis-Zouboulis I, Ladas SD. Diastolic cardiac dysfunction is a predictor of dismal prognosis in patients with liver cirrhosis. Hepatol Int. 2014;8:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 34. | Alexopoulou A, Papatheodoridis G, Pouriki S, Chrysohoou C, Raftopoulos L, Stefanadis C, Pectasides D. Diastolic myocardial dysfunction does not affect survival in patients with cirrhosis. Transpl Int. 2012;25:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Cesari M, Fasolato S, Rosi S, Angeli P. Cardiac dysfunction in patients with cirrhosis: is the systolic component its main feature? Eur J Gastroenterol Hepatol. 2015;27:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Rimbaş RC, Baldea SM, Guerra RDGA, Visoiu SI, Rimbaş M, Pop CS, Vinereanu D. New Definition Criteria of Myocardial Dysfunction in Patients with Liver Cirrhosis: A Speckle Tracking and Tissue Doppler Imaging Study. Ultrasound Med Biol. 2018;44:562-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Merli M, Torromeo C, Giusto M, Iacovone G, Riggio O, Puddu PE. Survival at 2 years among liver cirrhotic patients is influenced by left atrial volume and left ventricular mass. Liver Int. 2017;37:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Kazankov K, Holland-Fischer P, Andersen NH, Torp P, Sloth E, Aagaard NK, Vilstrup H. Resting myocardial dysfunction in cirrhosis quantified by tissue Doppler imaging. Liver Int. 2011;31:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Devauchelle P, Schmitt Z, Bonnet A, Duperret S, Viale JP, Mabrut JY, Aubrun F, Gazon M. The evolution of diastolic function during liver transplantation. Anaesth Crit Care Pain Med. 2018;37:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Sarnelyte J, Stundiene I, Valantinas J. Liver cirrhosis and left ventricle diastolic dysfunction: retrospective study. United Eur Gastroenterol J. 2018;6:A135-A747. |
| 41. | Wong F, Salerno F. Beta-blockers in cirrhosis: friend and foe? Hepatology. 2010;52:811-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Krag A, Wiest R, Albillos A, Gluud LL. The window hypothesis: haemodynamic and non-haemodynamic effects of β-blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61:967-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 43. | Garcia-Tsao G. The Use of Nonselective Beta Blockers for Treatment of Portal Hypertension. Gastroenterol Hepatol (NY). 2017;13:617-619. [PubMed] |
| 44. | Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, Hagmann M, Blacky A, Ferlitsch A, Sieghart W, Trauner M, Peck-Radosavljevic M, Reiberger T. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 45. | Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, Moreau R, Lebrec D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 46. | Garcia-Tsao G. Beta blockers in cirrhosis: The window re-opens. J Hepatol. 2016;64:532-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Møller S, Bendtsen F, Henriksen JH. Determinants of the renin-angiotensin-aldosterone system in cirrhosis with special emphasis on the central blood volume. Scand J Gastroenterol. 2006;41:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Arroyo V, Fernández J. Management of hepatorenal syndrome in patients with cirrhosis. Nat Rev Nephrol. 2011;7:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Schrier RW, Masoumi A, Elhassan E. Role of vasopressin and vasopressin receptor antagonists in type I cardiorenal syndrome. Blood Purif. 2009;27:28-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Pozzi M, Carugo S, Boari G, Pecci V, de Ceglia S, Maggiolini S, Bolla GB, Roffi L, Failla M, Grassi G, Giannattasio C, Mancia G. Evidence of functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatology. 1997;26:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |