Published online Aug 28, 2019. doi: 10.3748/wjg.v25.i32.4764
Peer-review started: March 6, 2019
First decision: April 11, 2019
Revised: May 14, 2019
Accepted: May 31, 2019
Article in press: June 1, 2019
Published online: August 28, 2019
Processing time: 175 Days and 20.5 Hours
Non-invasive evaluation for liver fibrosis is clinically important, especially in patients with undetectable hepatitis B virus (HBV) DNA treated with nucleoside analogs.
To clarify the monitoring power of hepatitis B core-related antigen (HBcrAg) for hepatic histologic changes in patients with chronic hepatitis B (CHB) treated with entecavir.
This prospective multicenter study used multiple ordinal and multivariate logistics regression analysis to assess variables associated with Ishak fibrosis score and regression for fibrosis regression, respectively, in 403 CHB patients, including 374 with entecavir for 72 weeks (291 underwent paired liver biopsy) and 29 as controls.
Level of HBcrAg correlated negatively with liver fibrosis staging (γ = -0.357, P < 0.001) in hepatitis B e antigen (HBeAg)-positive patients, and positively with liver fibrosis staging in HBeAg-negative patients. Higher HBcrAg concentration was associated with younger age, HBeAg positive status, high HBV DNA loads, high level of hepatitis B surface antigen (HBsAg) and higher necroinflammation, but not with HBV genotype. Serum concentration of HBcrAg, basal core promoter/precore (BCP/PC) mutant, quantitation of HBsAg (qHBsAg) and platelet counts were independently associated with Ishak fibrosis score on multiple ordinal regression. HBV DNA was undetectable in 88.37% of patients treated with entecavir at week 72, while their level of HBcrAg was still detectable. A greater reduction in post-treatment HBcrAg concentration was associated with the regression of hepatic fibrosis and histological improvement. HBcrAg concentration > 6.33 log IU/mL at baseline and logarithmic reduction > 1.03 log IU/mL at week 72 were associated with a higher chance of regression of liver fibrosis and histological improvement, respectively.
HBcrAg level is associated with liver fibrosis progression. HBcrAg is an excellent monitor of hepatic histological changes, especially in CHB patients treated with nucleoside analogs.
Core tip: Hepatitis B core-related antigen (HBcrAg) is an excellent monitor of hepatic histological changes, especially in patients with chronic hepatitis B treated with nucleoside analogs. Baseline HBcrAg level and logarithmic reduction of HBcrAg at week 72 of treatment can predict and monitor the chance of achieving regression of liver fibrosis and histological improvement.
- Citation: Chang XJ, Sun C, Chen Y, Li XD, Yu ZJ, Dong Z, Bai WL, Wang XD, Li ZQ, Chen D, Du WJ, Liao H, Jiang QY, Sun LJ, Li YY, Zhang CH, Xu DP, Chen YP, Li Q, Yang YP. On-treatment monitoring of liver fibrosis with serum hepatitis B core-related antigen in chronic hepatitis B. World J Gastroenterol 2019; 25(32): 4764-4778
- URL: https://www.wjgnet.com/1007-9327/full/v25/i32/4764.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i32.4764
Chronic hepatitis B (CHB) causes liver injury that can progress to cirrhosis, result in hepatocellular carcinoma (HCC), liver failure, and death[1,2]. Early and accurate diagnosis of hepatic fibrosis/cirrhosis is particularly important for appropriate management and monitoring of CHB, and preventing cirrhosis-related morbidity and mortality[3].
Liver biopsy remains the gold standard for fibrosis stage clinically. But, invasiveness, poor acceptance, sampling variability, and complications limit its wide application , and it is unsuitable to dynamically observe liver fibrosis progression[4]. Several non-invasive models have been developed to stage liver fibrosis, including FibroTest[5], aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio[6], AST to platelet index[7], fibrosis index based on 4 factors (FIB-4)[8] and Hepascore[9]. Liver stiffness measurement with FibroScan® (LSM-FS) is a better predictor than serum biomarkers for advanced liver fibrosis and cirrhosis in patients with CHB[10]. However, among patients with intermediate LSM-FS measurements, the accuracy of hepatic fibrosis staging is lower[11,12]. Although these non-invasive methods are simple and available for those with chronic HCV infection[10,13,14], inconsistent results have been reported[15], and some predictive models designed especially for CHB patients have some limitations[15-17], and few of them have been widely validated.
Hepatitis B core-related antigen (HBcrAg)[18-20], a new biomarker of HBV infection, consist of hepatitis B core antigen (HBcAg) and hepatitis B e antigen (HBeAg); they are products of the precore/core gene and have the first 149 amino acids of HBcAg in common.
Serum concentration of HBcrAg is correlated with intrahepatic levels of covalently closed circular DNA[21,22]. HBcrAg is a valuable marker to guide cessation of antivirus and assess CHB related hepatic disease[23]. HBcrAg is a useful predictor of necro-inflammation and fibrosis in HBeAg-positive or -negative patients[23,24]. However, those studies were not ideal. For example, no patient underwent paired liver biopsy; the adequacy criteria for liver biopsy were sub-optimal and developed by retrospective data, assessing patients who met the eligibility criteria; and there was a lack of multicenter and external validation. Therefore, non-invasive markers are needed to assess liver fibrosis and antiviral effectiveness in CHB patients.
The aim of the present study was to develop serum HBcrAg as a simple novel biomarker referencing to central histologic stage of fibrosis by liver biopsy, to monitor liver fibrosis in patients with CHB, and evaluate treatment-related changes in hepatic histology.
This was a multicenter prospective sub-study of our ongoing clinical trial of regression of HBV-related liver fibrosis in China (NCT01965418)[25]. The study meets the requirements of the Declaration of Helsinki, the protocol was approved by the ethics committee of each participating institution, and written informed consent was obtained from all patients. Patients were prospectively recruited from Beijing 302nd Hospital, the First Affiliated Hospital of Wenzhou Medical University, the First Affiliated Hospital of Zhengzhou University, and Fuzhou Infectious Diseases Hospital. Patients who met the following inclusion criteria were enrolled: (1) Men or women aged 18-65 years, positive HBsAg for at least 6 mo; (2) As for HBeAg-positive CHB, HBV DNA ≥ 20000 IU/mL and ALT ≥ 2 × upper normal limit, as for HBeAg-negative CHB, HBV DNA ≥ 2000 IU/mL and ALT ≥ 2 × upper normal limit, or clinically compensated cirrhosis with detectable serum HBV DNA regardless of HBeAg status and the ALT level at screening before liver biopsy[12,26]; (3) A baseline liver-biopsy specimen obtained showing an Ishak fibrosis score stage ≥ 3 within 4 wk before enrollment; and (4) Nucleos(t)ide analogues treatment naïve or anti-fibrotic therapy within 6 mo before enrollment. The exclusion criteria included: (1) Co-infection with other virus hepatitis or chronic liver diseases; (2) Liver biopsy was inadequate for grading and/or staging; (3) One more variables were missing; and (4) Decompensated cirrhosis or history of any concurrent malignancy.
All patients were required for follow-up liver biopsy at treatment week 72. Ultrasound-guided liver biopsy was performed according to a standard protocol[27]. A Quick-cut needle or Menghini needle, 16 G (Allegiance Corporation, McGaw Park, IL, United States), was used for biopsy, and a minimal 2.0-cm length of liver tissue with at least 11 portal tracts was obtained for pathological evaluation[27]. All liver biopsies were reviewed in a central pathology laboratory by two liver pathologists blinded to treatment assignment and time of biopsy. Biopsies with inconsistent reports were re-reviewed by both pathologists together to reach a consensus. Histological assessment included: (1) Fibrosis stage evaluated by the Ishak modified histological activity index grading scale (0 indicates no fibrosis and ≥ 5 indicates cirrhosis)[28]; and (2) Inflammatory activity assessed by the Knodell scoring system (≤ 3 indicates mild or no necro-inflammation, ≥ 10 indicates severe necro-inflammation)[29].
Fasting blood samples were collected on the day of biopsy and and served for immediate use (10 mL fresh blood), except for serum hyaluronic acid (HA) etc. that was measured on frozen samples (5 mL serum stored at -80 °C). Blood analysis was performed using standard methodologies. Serum biochemical parameters included total bilirubin, indirect bilirubin, ALT, AST, gamma glutamyl transpeptidase, alkaline phosphatase, albumin, blood urea nitrogen, creatinine, α-fetoprotein, prothrombin time, triglycerides, total cholesterol, low-density lipoprotein and high-density lipoprotein, apolipoprotein A1, haptoglobin, HA, propeptide of type III procollagen (PIIINP) and α2-macroglobulin. All parameters were measured using standard methodologies.
Markers of HBV included HBsAg, HB surface antibody (HBsAb), HBeAg, HB e antibody (HBeAb), HB core antibody (HBcAb) (Abbott, Shanghai, China), and serum HBcrAg was measured using a CLEIA Lumipulse G1200 automated analyzer (Fujirebio Inc., Tokyo, Japan). The reagents were provided by Fujirebio Inc., lot number: SAX5031 (Japan). The lower and upper detection limits were 1 kU/mL and 10000 kU/mL, respectively. If the serum exceeded the upper detection limit, it was diluted 10 times and retested. α2-Macroglobulin, haptoglobin and apolipoprotein A1 were measured by immunonephelometry using a BN ProsPec analyser (Siemens diagnostics, Deerfield, IL, United States). Serum HA concentration (Lumino Analyzer and Maglumi Reagent; STRATEC Biomedical Systems AG, Berlin, Germany). PIIINP was analyzed using kits produced by Elabscience (Beijing, China). Markers of HBV included HBsAg, HBsAb, HBeAg, HBeAb and HBcAb (Abbott, Shanghai, China), and quantitation of HBsAg (qHBsAg) was detected at the central laboratory using the Abbott Architect Assay (Abbott Laboratories, Abbott Park, IL, United States). HBV DNA levels were determined using the COBAS Taq Man assay (Roche Molecular Systems, Branchburg, NJ, United States) at Beijing 302 Hospital with the detection limit of 20 IU/mL.
Viral DNA was extracted from samples of 100 μL serum. Complete HBV genomes were amplified and sequenced as previously described[30]. The direct sequencing was performed using an ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, United States). HBV genomic sequences were deposited in GenBank under accession numbers MK171258 - MK171652 for those from CHB patient.
Data analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, United States). Categorical data were expressed as numbers (percentages) and continuous variables as either mean or median. Comparisons between groups were conducted by Student’s t-test (variance homogeneity), Satterthwaite test (variance not homogeneity) or Wilcoxon rank sum test (variance not symmetric) for continuous variables and the χ2 test for categorical variables. For efficacy evaluations based on categorical variables, the Cochran-Mantel-Haenszel test was applied to test the central effect, when the Breslow-Day test showed there was no significant central effect (P > 0.05). The χ2 test was directly applied to the classification data. To evaluate which variables were associated with Ishak fibrosis score and histological improvement in our cohort, we performed multiple ordinal logistic regression, and the variables are presented as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was assessed at the 0.05 level, and defined as two-sided P < 0.05.
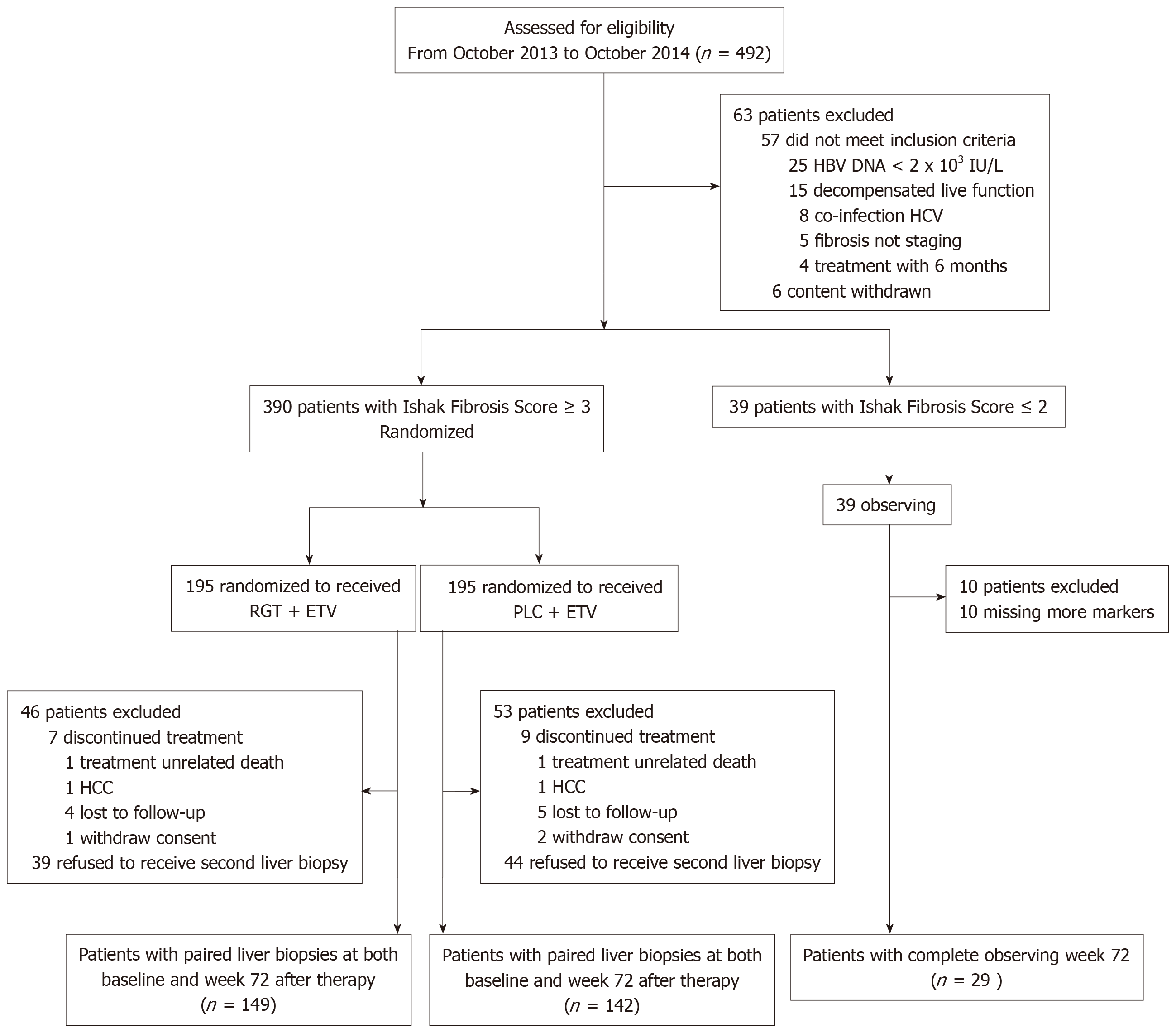
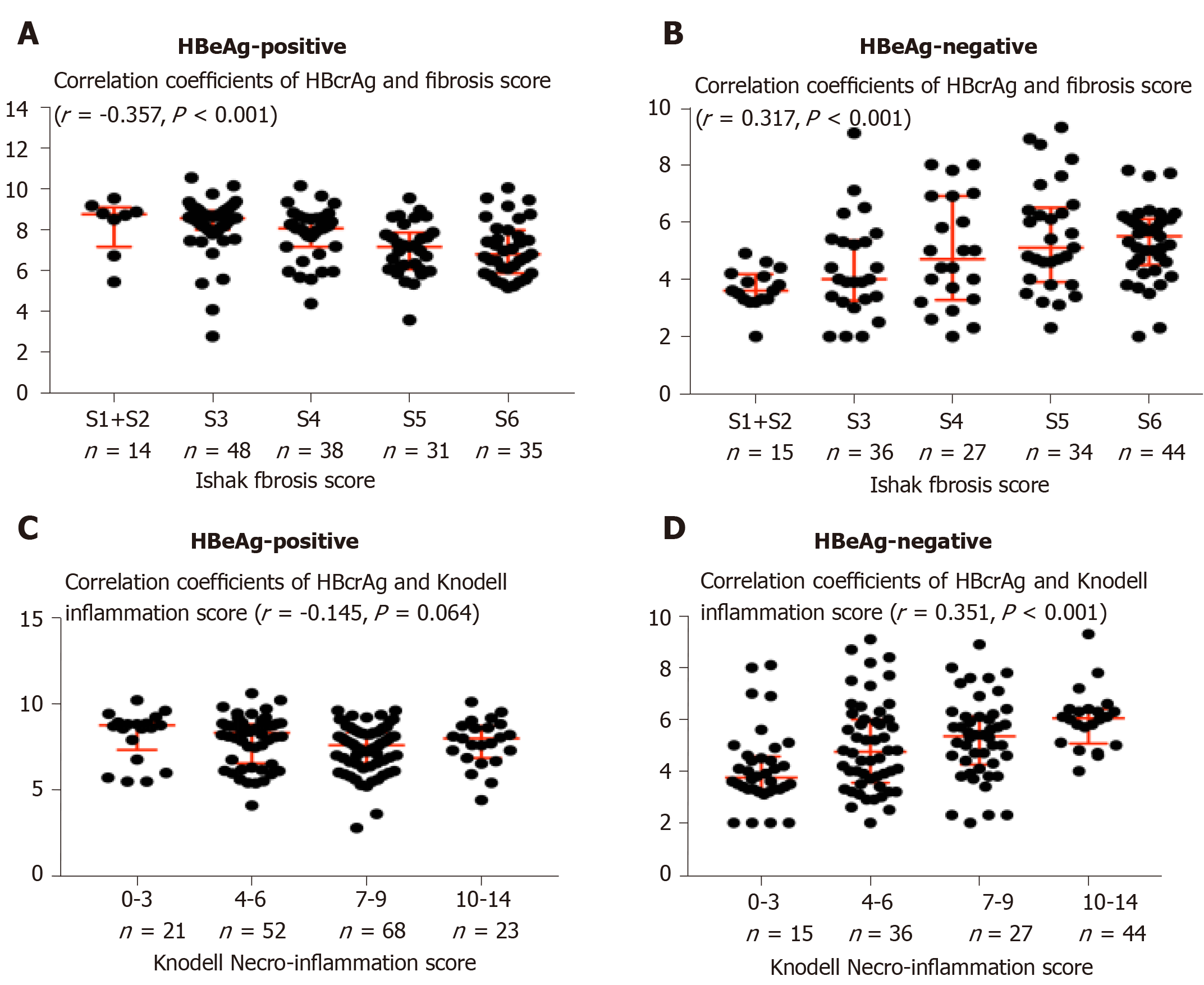
Four hundred and ninety-two CHB patients were screened for eligibility, of whom 390 patients with Ishak fibrosis score ≥ 3 underwent randomization and 39 with Ishak fibrosis score ≤ 2 served as observed controls. A total of 403 (374 randomized and 29 observed controls) patients finished a 72-wk follow-up and 291/403 (72.2%) underwent a second liver biopsy. Finally, 320 patients (291 with paired liver biopsies and 29 observed controls) were included in the statistical analysis (Figure 1). The baseline characteristics of the study population are summarized in Table 1. The two groups showed comparable baseline characteristics, while the HBeAg-positive patients had a higher median HBcrAg concentration (8.0 log10 IU/mL vs 4.9 log10 IU/mL; P < 0.001), HBV DNA (7.5 log10 IU/mL vs 4.9 log10 IU/mL; P < 0.001), qHBsAg (3.9 log10 IU/mL vs 3.3 log10 IU/mL; P < 0.001) than the HBeAg-negative patients had. HBeAg-positive patients were younger, with low α2-Macroglobulin concentration compared to HBeAg-negative patients (both P < 0.001). The baseline proportion of patients with genotype C in HBeAg-poitive group was higher (75% vs 60%, P = 0.038). In HBeAg-positive patients, 14 had S1/2 (8.5%), 48 S3 (29.3%), 36 S4 (22%), 31 S5 (19%), and 35 S6 (21%) fibrosis. HBcrAg was significantly negatively correlated with liver fibrosis staging (γ = -0.357, P < 0.001, Table 1 and Figure 2), with median concentrations of 8.8, 8.6, 8.1, 7.2 and 6.8 log10 IU/mL for S1/2, S3, S4, S5 and S6, respectively. However, In HBeAg-negative patients, 15 patients had S1/2 (9.6%), 36 S3 (23%), 27 S4 (17%), 34 S5 (22%) and 44 S6 (28%) fibrosis. Level of HBcrAg was significantly positively correlated with liver fibrosis staging (γ = 0.317, P < 0.001, Table 1 and Figure 2), with median concentrations of 3.6, 4.4, 5.0, 5.3 and 5.7 log10 IU/mL for S1/2, S3, S4, S5 and S6, respectively.
| Variables | HBeAg-positive(n = 164) | HBeAg-negative(n = 156) | P value |
| Age (median, IQR) (yr) | 39 (14) | 45 (11) | < 0.001 |
| Male | 117 (71) | 100 (64) | 0.166 |
| Platelet counts (Median, IQR) (109/L) | 165.5 (84) | 160 (83) | 0.074 |
| Prothrombin time (Median, IQR) (s) | 12.1 (2.3) | 12.1 (2.5) | 0.574 |
| Alanine transaminase (Median, IQR) (IU/L) | 60.5 (87) | 43 (54) | 0.041 |
| Aspartate aminotransferase (Median, IQR) (IU/L) | 46.5 (49) | 35 (39) | 0.144 |
| Alkaline phosphatase (Median, IQR) (U/L) | 81 (33) | 77.5 (39) | 0.687 |
| γ-glutamyltransferase (Median, IQR) (U/L) | 37 (59) | 31 (36) | 0.134 |
| Albumin (Median, IQR) (g/L) | 42.0 (6.4) | 43.0 (5.9) | 0.014 |
| Globulin (Median, IQR) (g/L) | 30.5 (6.2) | 30.1 (6.1) | 0,239 |
| Cholinesterase (Median, IQR) (U/L) | 6547 (2148) | 7113.5 (2621) | 0.029 |
| Adenosine deaminase (Median, IQR) (U/L) | 16.0(9.9) | 15.8 (10.6) | 0.787 |
| Haptoglobin (Median, IQR) (g/L) | 384 (518) | 401.5 (495) | 0.339 |
| α2-Macroglobulin (Median, IQR) (μg/mL) | 2080 (900) | 2555 (905) | <0.001 |
| Hyaluronic acid (Median, IQR) (μg/L) | 74 (66) | 60 (71) | 0.391 |
| Propeptide of type III procollagen (Median, IQR) (μg/L) | 8 (5) | 7 (3) | 0.375 |
| α-Fetoprotein (Median, IQR) (ng/mL) | 5.1 (9.2) | 4.3 (5.8) | 0.581 |
| Spleen thickness (Median, IQR) (mm) | 35 (7) | 35 (6) | 0.562 |
| Spleen length (Median, IQR) (mm) | 107 (20) | 110.5 (19) | 0.373 |
| HBVDNA (Median, IQR) (log IU/mL) | 7.5 (2.1) | 4.9 (1.8) | < 0.001 |
| quantitative of HBsAg (Median, IQR) (log IU/mL) | 3.9 (1.1) | 3.3 (0.7) | < 0.001 |
| HBcrAg (Median, IQR) (log IU/mL) | 8.0 (2.0) | 4.9 (2.3) | < 0.001 |
| Genotype | |||
| B | 28 (17) | 38 (24) | 0.038 |
| C | 123 (75) | 93 (60) | |
| BCP/PC/C mutation | |||
| T1753C/A/G | 24 (14.6) | 48 (31) | 0.002 |
| T1754G | 2 (1.2) | 12 (7.7) | 0.061 |
| T1758C | 15 (9.1) | 5 (3.2) | 0.135 |
| A1762T | 102 (62.1) | 113 (72.4) | 0.083 |
| G1764A | 102 (62.1) | 119 (76.3) | 0.019 |
| C1766T | 16 (9.8) | 14 (8.9) | 0.789 |
| A1846T/C | 15 (9.1) | 62 (39.7) | < 0.001 |
| G1862T | 5 (3.0) | 10 (6.4) | 0.377 |
| G1896A | 32 (19.7) | 106 (67.9) | < 0.001 |
| G1899A | 6 (3.7) | 36 (23.1) | < 0.001 |
| C1913A/G | 21 (12.90 | 19 (12.2) | 0.909 |
| Ishak Fibrosis Score (mean ± SD) | 4.3 ± 1.2 | 4.7 ± 1.2 | 0.006 |
| S1+ S2 | 14 (8.5) | 15 (9.6) | 0.406 |
| S3 | 48 (29.3) | 36 (23.1) | |
| S4 | 36 (22.0) | 27 (17.3) | |
| S5 | 31 (18.9) | 34 (21.8) | |
| S6 | 35 (21.3) | 44 (28.2) | |
| Knodell necroinflammation (mean ± SD) | 6.8 ± 2.7 | 6.0 ± 3.0 | 0.013 |
| 0-3 | 21 (13) | 36 (23) | 0.046 |
| 4-6 | 52 (32%) | 52 (33) | |
| 7-9 | 68 (41) | 46 (30) | |
| 10-14 | 23 (14) | 22 (14) |
According to the median serum concentration of HBcrAg [6.33 log10 IU/mL, interquartile range (IQR): 4.80-8.18], these 320 cases were divided into two groups: those with low concentration of HBcrAg (≤ 6.33 log10 IU/mL) and those with high concentration (> 6.33 log10 IU/mL). A higher serum concentration of HBcrAg was significantly associated with younger age, HBeAg positivity, high HBVDNA load, high level of qHBsAg, higher necroinflammation, elevated level of ALT and AST, but not with HBV genotype (Table 2).
| Variables | HBcrAg-high (n = 160) | HBcrAg-low (n = 160) | P value |
| Age (Median, IQR) (yr) | 39 (15) | 44 (12) | < 0.001 |
| Male | 110 (69) | 107 (67) | 0.720 |
| Platelet counts (Median, IQR) (109/L) | 169 (84) | 159.5 (78) | 0.066 |
| Prothrombin time (Median, IQR) (s) | 12.1 (2.6) | 12.0 (2.2) | 0.966 |
| Alanine transaminase (Median, IQR) (IU/L) | 67 (84) | 41 (48) | 0.020 |
| Aspartate aminotransferase (Median, IQR) (IU/L) | 49 (48) | 34 (32) | 0.013 |
| Alkaline phosphatase (Median, IQR) (U/L) | 81 (33) | 77 (37) | 0.454 |
| γ-glutamyltransferase (Median, IQR) (U/L) | 35 (60) | 33 (36) | 0.135 |
| Albumin (Median, IQR) (g/L) | 42.0 (6.4) | 43.0 (5.3) | 0.032 |
| Globulin (Median, IQR) (g/L) | 30.6 (7.0) | 29.0 (5.8) | 0.003 |
| Cholinesterase (Median, IQR) (U/L) | 6438 (2265) | 7133.5 (2327) | 0.065 |
| Adenosine deaminase (Median, IQR) (U/L) | 16.4 (10.0) | 15.2 (9.2) | 0.582 |
| Haptoglobin (Median, IQR) (g/L) | 384 (515) | 391.5 (486.5) | 0.664 |
| α2-Macroglobulin (Median, IQR) (μg/mL) | 2200 (960) | 2480 (990) | 0.003 |
| Hyaluronic acid (Median, IQR) (μg/L) | 74 (66.0) | 63.5 (72.0) | 0.787 |
| Propeptide of type III procollagen (Median, IQR) (μg/L) | 6438 (5.0) | 7 (3.0) | 0.434 |
| α-Fetoprotein (Median, IQR) (ng/mL) | 5.0 (9.1) | 4.7 (6.5) | 0.597 |
| Spleen thickness (Median, IQR) (mm) | 35 (7.0) | 35 (5.0) | 0.239 |
| Spleen length (Median, IQR) (mm) | 109 (21.0) | 109 (18.0) | 0.410 |
| HBVDNA (Median, IQR) (log IU/mL) | 7.5 (2.0) | 4.9 (1.8) | < 0.001 |
| quantitative of HBsAg (Median, IQR) (log IU/mL) | 3.9 (1.2) | 3.3 (0.7) | < 0.001 |
| Genotype | |||
| B | 35 (22) | 31 (19) | 0.971 |
| C | 114 (71) | 102 (64) | |
| BCP/PC/C mutation | |||
| T1753C/A/G | 18 (11.3) | 54 (33.8) | < 0.001 |
| T1754G | 5 (3.1%) | 8 (5.0) | 0.602 |
| T1758C | 11 (6.9) | 10 (6.3) | 0.792 |
| A1762T | 92 (57.5) | 124 (77.5) | 0.001 |
| G1764A | 96 (60.0) | 125 (78.1) | 0.002 |
| C1766T | 7 (4.4) | 24 (15.0) | 0.006 |
| A1846T/C | 16 (10.0) | 61 (38.1) | < 0.001 |
| G1862T | 5 (3.1) | 10 (6.3%) | 0.256 |
| G1896A | 42 (26.3) | 96 (60.0) | < 0.001 |
| G1899A | 7 (4.4) | 35 (21.9) | < 0.001 |
| C1913A/G | 18 (11.3) | 22 (13.8) | 0.577 |
| HBeAg-positive | 131 (79.9) | 33 (21.2) | < 0.001 |
| Ishak Fibrosis Score (mean ± SD) | 4.2 ± 1.1 | 4.8 ± 1.1 | < 0.001 |
| Knodell Necroinflammation Score (mean ± SD) | 6.9 ± 2.7 | 5.8 ± 2.9 | 0.001 |
A hierarchical clustering analysis was performed on complete genome sequencing for HBV according to the median of serum HBcrAg concentration (6.33 log IU/mL, IQR: 4.80-8.18). The 320 cases were divided into two distinct subclasses, cluster1 (> 6.33 log IU/mL as high serum concentration of HBcrAg) and cluster 2 (≤ 6.33 log IU/mL as low serum concentration of HBcrAg) (Figure 3A). Compared to cluster 1, cluster 2 had a higher proportion of HBV BCP/PC/C mutations, such as T1753G/C/A (33.8% vs 11.3%, P < 0.001), G1762T (77.5% vs 57.5%, P = 0.001), G1764A (78.1% vs 60.0%, P = 0.002), C1766T (15.0% vs 4.4%, P = 0.006), A1846T/C (38.1% vs 10.0%, P < 0.001), G1896A (60.0% vs 26.3%, P < 0.001) and G1899A (21.9% vs 4.4%, P < 0.001) (Figure 3B). At these loci, patients with non-mutations had significantly higher concentration of HBcrAg than patients with mutations had (Figure 3C). The patients with BCP non-mutation had significant higher serum concentration of HBcrAg than that of those with BCP mutation at baseline (8.33 log IU/mL, IQR: 6.07-8.73 vs 6.14 log IU/mL, IQR: 5.0-7.63, P < 0.001, Figure 3D). At week 72, no significant differences in the serum concentration of HBcrAg (BCP non-mutation, 5.63 log IU/mL, IQR: 4.30-7.63 vs BCP mutation, 5.27 log IU/mL, IQR: 4.20-6.25, P = 0.061, Figure 3D), but the median decline of HBcrAg level was higher in BCP non-mutation patients compared with BCP mutation patients (-1.19 vs -0.87 log IU/mL, P = 0.041, Figure 3D).
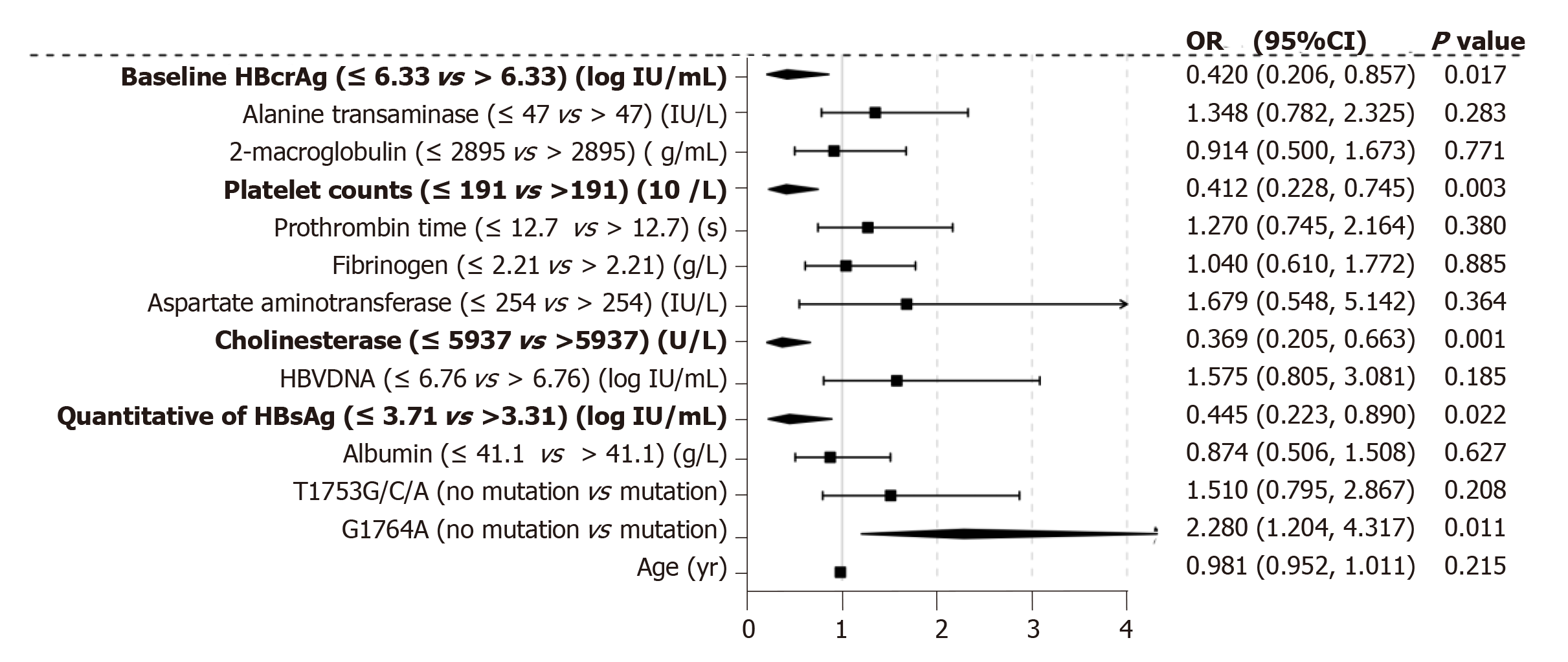
We performed multiple ordinal regression analysis to determine which factors were associated with significant fibrosis to cirrhosis (S3-S6; Figure 4). The presence of G1764A mutation showed the strongest independent association with S3-6 fibrosis (OR = 2.28, 95%CI: 1.204-4.317, P = 0.011). Significant fibrosis was also associated with qHBsAg (OR = 0.445, 95%CI: 0.223-0.890, P = 0.022), baseline serum concentration of HBcrAg (OR = 0.420, 95%CI: 0.206-0.875, P = 0.017), platelet counts (OR = 0.412, 95%CI: 0.228-0.745, P = 0.003) and cholinesterase (OR = 0.369, 95%CI: 0.205-0.663, P = 0.001).
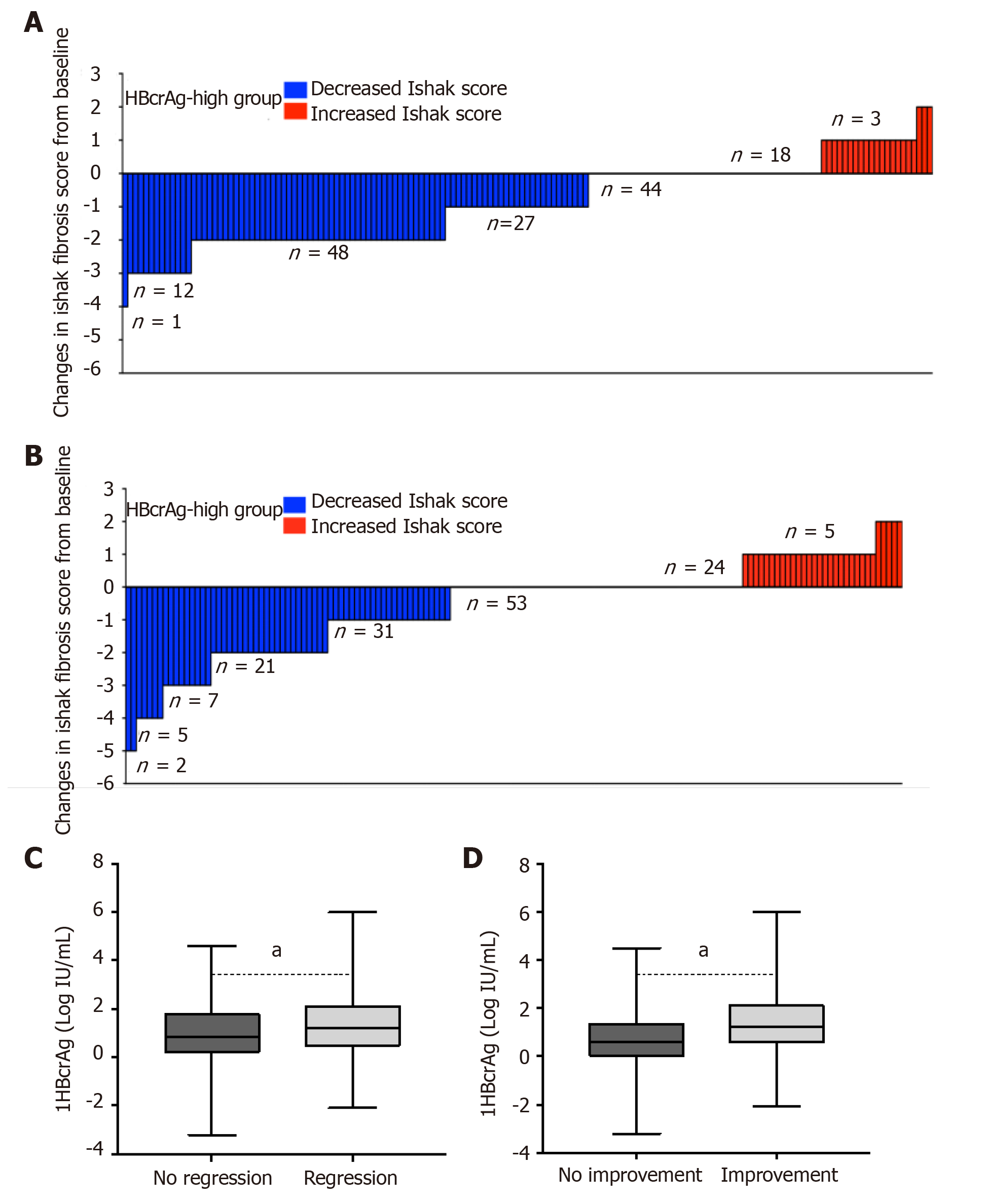
Of the 425 patients, 403 patients completed week 72 of follow-up. Two hundred and ninety-one patients had the second liver biopsy. HBV DNA was undetectable by PCR in 88.37% of patients treated with entecavir for 72 weeks, while HBcrAg was still detectable. The incidence of regression of fibrosis was 57.5% in patients with high baseline HBcrAg (> 6.33 log IU/mL) and low concentration of HBcrAg (40.6%, P = 0.004; Figure 5A and B). There were 50 (17.2%, 50/291) patients with liver fibrosis progression, included 27 HBeAg negative and 23 HBeAg positive after 72 weeks of entecavir therapy. Compared with baseline, the proportion of increased serum concentration of HBcrAg in HBeAg-negative patients was significantly higher than that in HBeAg-positive patients (77%, 19/27 vs 30%, 7/23; P = 0.005). The median logarithmic reduction of HBcrAg concentration was higher in those with regression of liver fibrosis (n = 134), compared with those with no regression of fibrosis (n = 147; -1.2 vs --0.8 log IU/mL, P = 0.042; Figure 5C). Compared to those with no histological improvement, the patients with histological improvement had significantly greater median logarithmic reduction of HBcrAg concentration (-1.2 log IU/mL vs -0.6 log IU/mL, P = 0.001; Figure 5D).
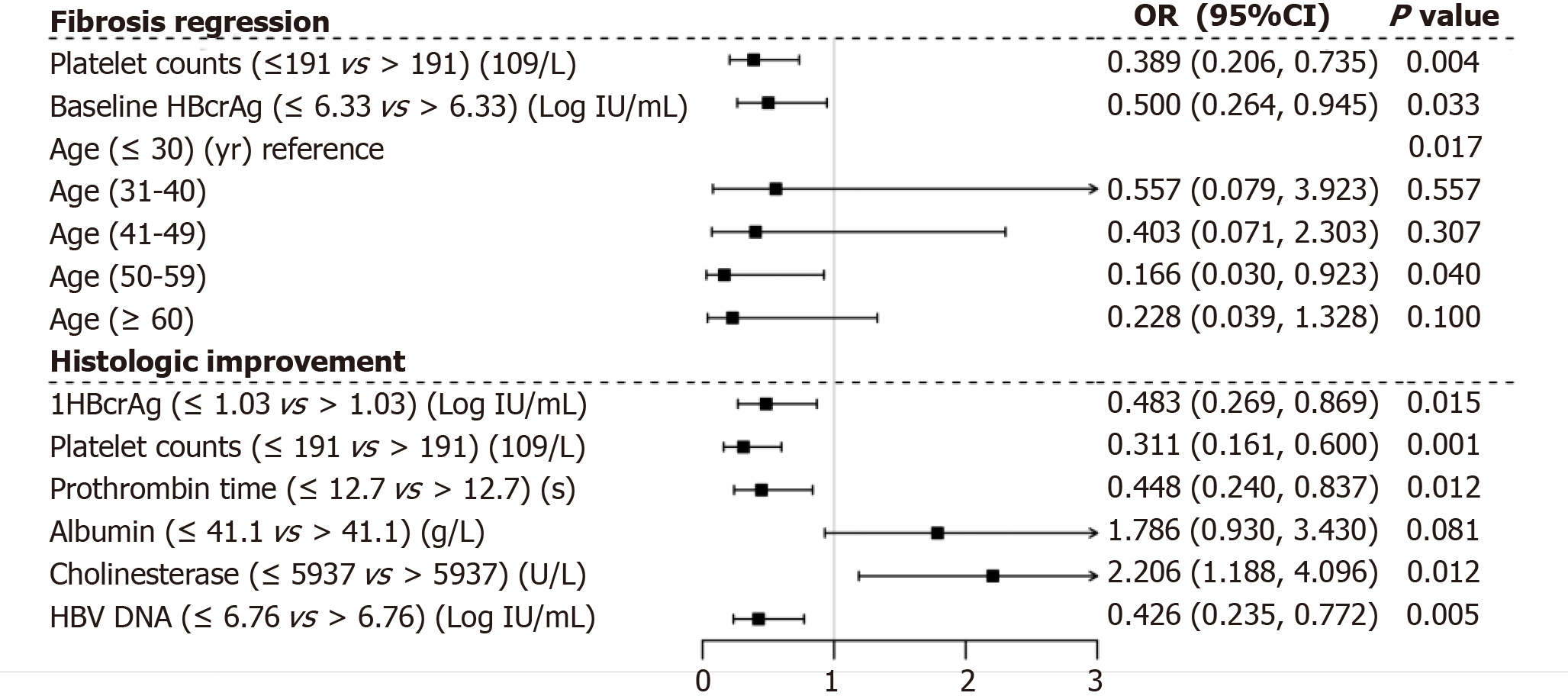
We performed multiple ordinal regression analysis to determine which variables were associated with regression of liver fibrosis (Figure 6). Baseline serum concentration HBcrAg > 6.33 log IU/mL showed the strongest independent association with regression of liver fibrosis (OR = 0.50, 95%CI: 0.264-0.945, P = 0.033). Regression of liver fibrosis was also associated with platelet counts > 191 × 109/L (OR = 0.389, 95%CI: 0.206-0.735, P = 0.004) and age < 50 years (OR = 0.166, 95%CI: 0.030-0.923, P = 0.04). However, cholinesterase > 5937 U/L had the strongest independent association with histological improvement (OR = 2.206, 95%CI: 1.188-4.096, P = 0.012). Histological improvement was also found to be associated with 1HBcrAg level (median logarithmic reduction of HBcrAg from baseline) > 1.03 log IU/mL (OR = 0.483, 95%CI: 0.269-0.869, P = 0.015), prothrombin time > 12.7 s (OR = 0.448, 95%CI: 0.240-0.837, P = 0.012), HBV DNA > 6.76 log IU/mL (OR = 0.426, 95%CI: 0.235-0.772, P = 0.005) and platelet count > 191 × 109/L (OR = 0.311, 95%CI: 0.161-0.600, P = 0.001).
Few studies have investigated the association between serum concentration of HBcrAg and the pathological status of liver tissues until recently[23,31,32], especially the kinetics of serum HBcrAg and treatment-related changes in hepatic histology. In the present study, we intend to analyze the kinetics of serum HBcrAg in treatment-naïve CHB patients receiving nucleos(t)ide analogues and to identify on-treatment predictors of regression of liver fibrosis as a treatment end-point. This has not been investigated before and our results have generated clinically useful thresholds, which further demonstrate the value of serum HBcrAg in management of patients with CHB, especially with long-term NAs. The main strengths of this study were: (1) The size, prospective, multicenter design and 291 patients underwent paired liver biopsy; (2) The nature of study population was relatively homogeneous (i.e., covering a full spectrum of fibrosis stage); (3) Central pathological analysis was performed to unify the stage of liver fibrosis; and (4) Except for cirrhosis as an observed endpoint, significant and advanced fibrosis was evaluated to prevent spectrum bias.
The distribution of serum HBcrAg level was higher in HBeAg-positive than HBeAg-negative patients. Our data showed that serum HBcrAg level was significantly associated with younger age, HBeAg positivity, higher HBV-DNA titers, and high qHBsAg level, but not HBV genotypes. As noted earlier, the HBV genotype might depend on the biogeography, and not be affected by any host or viral proteins, but can influence the progression of liver disease[33-35]. Our results confirmed that there was no independent association between HBV genotype and serum HBcrAg level, indicating that HBV-triggered liver injury is mediated by host immune response to viral proteins[33]. Interestingly, we found that patients with BCP/PC mutation had a significantly lower serum concentration of HBcrAg when compared with their wild-type counterparts. Furthermore, after treated with entecavir for 72 wk, the decline of HBcrAg level in patients with BCP mutation was significantly less than that of patients with BCP non-mutation. BCP mutation, which down-regulate HBeAg production, have been reported to be associated with advanced liver disease [36]. Therefore, selection of BCP/PC mutants can affect the HBcrAg level and explain changes of the HBcrAg level. Indeed, patients with BCP/PC mutants whose the decline of serum HBcrAg level less after treated by entecavir had a lower proportion of the regression of liver fibrosis. More importantly, the present study showed that the risk of having significant fibrosis (stage ≥ 3) at a specific point in time was independently associated with the presence of BCP mutation (2.28-fold increase), qHBsAg (0.445-fold increase), baseline serum concentration of HBcrAg (0.420-fold increase), platelet counts (0.412-fold increase) and cholinesterase level (0.369-fold increase). Notably, the finding that BCP mutation was independently associated with liver fibrosis progression suggests that serum HBcrAg level reflects CHB disease activity in the liver and can monitor treatment-related changes in hepatic histology.
Whether the on-treatment monitoring of liver fibrosis with serum HBcrAg levels is unknown[19,23,37]. In our study, the serum of HBcrAg level was significantly higher in treatment-naïve HBeAg-positive patients compared to treatment-naïve HBeAg-negative patients, due to the fact that HBeAg production (one of the components of HBcrAg) was declined after HBeAg seroconversion[38]. More importantly, the serum of HBcrAg level showed a strong negative correlation with liver fibrosis staging in treatment-naïve HBeAg-positive individuals, and positive correlation with that in treatment-naïve HBeAg-negative individuals[23], extended our current knowledge and suggested that the serum of HBcrAg level may be able to differentiate between chronic infection and chronic hepatitis[19]. We also found that HBV DNA was undetectable by PCR in 88.37% of patients treated with entecavir for 72 wk, while HBcrAg was still detectable. Since nucleos(t)ide analogues have no direct inhibitory action on the transcription and translation of viral mRNA[31,39], HBcrAg continues to be produced in spite of adequate suppression of viral DNA synthesis. The present study demonstrated that patients with high baseline serum concentration of HBcrAg resulted in a significantly higher regression rate of fibrosis/cirrhosis. Moreover, patients with significantly declined in HBcrAg level were more likely to have a significantly higher regression rate of liver fibrosis and better histologic improvement after treatment week 72. Hence, in nucleos(t)ide analogues treated patients whose HBV DNA has become undetectable by PCR, the use of HBcrAg measurement would be particularly useful for monitoring hepatic histological changes, especially in HBeAg-negative CHB.
It should be noted that our study had some limitations. Although the serum concentration of HBcrAg for noninvasive evaluation of treatment-related changes in hepatic histology was demonstrated after 72 wk treatment with entecavir, longer observation would yield additional value in confirming its prediction of HCC development. As the current study is ongoing, we will be able to provide further update in the near future.
In conclusion, in CHB patients treated by entecavir with paired liver biopsy, serum HBcrAg levels are associated with liver fibrosis progression. In NA-treated patients whose HBV DNA has become undetectable by PCR, HBcrAg measurement would be particularly useful for monitoring hepatic histological changes, especially in HBeAg-negative CHB.
Cirrhosis can lead to hepatocellular carcinoma, liver failure, even death. Early and accurate diagnosis of hepatic fibrosis/cirrhosis is pretty important for managing chronic hepatitis B (CHB) patients and preventing cirrhosis-related morbidity and mortality. Liver biopsy as the gold standard for fibrosis stage is invasive, poor acceptive, those limit its wide application. Many models for fibrosis stage have some limitations and few of them have been validated.
Some studies have reported that hepatitis B core-related antigen (HBcrAg) is closely related with liver fibrosis stage in CHB patients. However, whether HBcrAg can be a potential biomarker for assessing liver fibrosis stage and monitoring the change of liver histology after antivirus is unknown.
In this study, we aimed to evaluate the value of serum HBcrAg level in liver fibrosis stage and changes of liver histology after nucleos(t)ide analogues treated . If clarified, the liver fibrosis stage and fibrosis regression could be assessed and monitored by a non-invasive biomarker, serum HBcrAg level, without liver biopsy.
Base on a prospective multicenter study, multiple ordinal regression analysis was used to screen variables associated with Ishak fibrosis score in 403 CHB patients, including 374 with entecavir for 72 wk (291 underwent paired liver biopsy) and 29 as controls. Multivariate regression analysis was used to explore key factors associated with fibrosis regression and histological improvement.
Serum HBcrAg level, basal core promoter/precore (BCP/PC) mutant, qHBsAg and platelet counts were independently associated with fibrosis staging on multiple ordinal regression. HBcrAg concentration > 6.33 log IU/mL at baseline and its logarithmic reduction > 1.03 log IU/mL at week 72 were associated with a higher chance of regression of liver fibrosis and histological improvement, respectively.
Serum HBcrAg level can assess liver fibrosis staging and monitor changes of liver histology, especially in CHB patients treated with nucleoside analogs.
This study strongly suggests HBcrAg is an excellent non-invasive biomarker for assessing liver fibrosis staging and monitoring changes of liver histology. In order to more accurately assess liver fibrosis staging and predict fibrosis regression, the model including HBcrAg should be established in further research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Consort 2010 statement: The manuscript was prepared according to the CONSORT 2010 Checklist.
P-Reviewer: Broering R, Osna NA S-Editor: Ma RY L-Editor: Filipodia E-Editor: Qi LL
| 1. | Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, Forouzanfour MH, Groeger J, Hanafiah KM, Jacobsen KH, James SL, MacLachlan J, Malekzadeh R, Martin NK, Mokdad AA, Mokdad AH, Murray CJL, Plass D, Rana S, Rein DB, Richardus JH, Sanabria J, Saylan M, Shahraz S, So S, Vlassov VV, Weiderpass E, Wiersma ST, Younis M, Yu C, El Sayed Zaki M, Cooke GS. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 985] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 2. | Tang LSY, Covert E, Wilson E, Kottilil S. Chronic Hepatitis B Infection: A Review. JAMA. 2018;319:1802-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 484] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 3. | Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, Hu J, Kramvis A, Lampertico P, Janssen HLA, Levrero M, Li W, Liang TJ, Lim SG, Lu F, Penicaud MC, Tavis JE, Thimme R; Members of the ICE-HBV Working Groups; ICE-HBV Stakeholders Group Chairs; ICE-HBV Senior Advisors, Zoulim F. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 374] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 4. | McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396-1400. [PubMed] |
| 5. | Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302-1304. [PubMed] |
| 6. | Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734-739. [PubMed] |
| 7. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3232] [Article Influence: 146.9] [Reference Citation Analysis (0)] |
| 8. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1601] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 9. | Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 391] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 10. | Nakagomi R, Tateishi R, Masuzaki R, Soroida Y, Iwai T, Kondo M, Fujiwara N, Sato M, Minami T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Kondo Y, Tanaka Y, Otsuka M, Kato N, Moriya K, Ikeda H, Koike K. Liver stiffness measurements in chronic hepatitis C: Treatment evaluation and risk assessment. J Gastroenterol Hepatol. 2019;34:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Dong XQ, Wu Z, Li J, Wang GQ, Zhao H; China HepB-Related Fibrosis Assessment Research Group. Declining in liver stiffness cannot indicate fibrosis regression in patients with chronic hepatitis B: A 78-week prospective study. J Gastroenterol Hepatol. 2019;34:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1328] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 13. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 786] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 14. | Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 266] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 15. | Dong XQ, Wu Z, Zhao H, Wang GQ; China HepB-Related Fibrosis Assessment Research Group. Evaluation and comparison of thirty noninvasive models for diagnosing liver fibrosis in chinese hepatitis B patients. J Viral Hepat. 2019;26:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Zeng MD, Lu LG, Mao YM, Qiu DK, Li JQ, Wan MB, Chen CW, Wang JY, Cai X, Gao CF, Zhou XQ. Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology. 2005;42:1437-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Mohamadnejad M, Montazeri G, Fazlollahi A, Zamani F, Nasiri J, Nobakht H, Forouzanfar MH, Abedian S, Tavangar SM, Mohamadkhani A, Ghoujeghi F, Estakhri A, Nouri N, Farzadi Z, Najjari A, Malekzadeh R. Noninvasive markers of liver fibrosis and inflammation in chronic hepatitis B-virus related liver disease. Am J Gastroenterol. 2006;101:2537-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Tada T, Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S, Yama T, Tanaka J. HBcrAg predicts hepatocellular carcinoma development: An analysis using time-dependent receiver operating characteristics. J Hepatol. 2016;65:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Mak LY, Seto WK, Fung J, Yuen MF. New Biomarkers of Chronic Hepatitis B. Gut Liver. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Honda M, Shirasaki T, Terashima T, Kawaguchi K, Nakamura M, Oishi N, Wang X, Shimakami T, Okada H, Arai K, Yamashita T, Sakai Y, Yamashita T, Mizukoshi E, Kaneko S. Hepatitis B Virus (HBV) Core-Related Antigen During Nucleos(t)ide Analog Therapy Is Related to Intra-hepatic HBV Replication and Development of Hepatocellular Carcinoma. J Infect Dis. 2016;213:1096-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Suzuki F, Miyakoshi H, Kobayashi M, Kumada H. Correlation between serum hepatitis B virus core-related antigen and intrahepatic covalently closed circular DNA in chronic hepatitis B patients. J Med Virol. 2009;81:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, Loglio A, Facchetti F, Lampertico P, Levrero M, Zoulim F. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 23. | Zhang ZQ, Lu W, Wang YB, Weng QC, Zhang ZY, Yang ZQ, Feng YL. Measurement of the hepatitis B core-related antigen is valuable for predicting the pathological status of liver tissues in chronic hepatitis B patients. J Virol Methods. 2016;235:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Tada T, Kumada T, Toyoda H, Kobayashi N, Akita T, Tanaka J. Hepatitis B virus core-related antigen levels predict progression to liver cirrhosis in hepatitis B carriers. J Gastroenterol Hepatol. 2018;33:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Qu J, Yu Z, Li Q, Chen Y, Xiang D, Tan L, Lei C, Bai W, Li H, Shang Q, Chen L, Hu X, Lu W, Li Z, Chen D, Wang X, Zhang C, Xiao G, Qi X, Chen J, Zhou L, Chen G, Li Y, Zeng Z, Rong G, Dong Z, Chen Y, Lou M, Wang C, Lu Y, Zhang C, Yang Y. Blocking and reversing hepatic fibrosis in patients with chronic hepatitis B treated by traditional Chinese medicine (tablets of biejia ruangan or RGT): study protocol for a randomized controlled trial. Trials. 2014;15:438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Kim MN, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, Song KJ, Park YN, Han KH. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. 2015;61:1851-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 27. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1573] [Article Influence: 98.3] [Reference Citation Analysis (1)] |
| 28. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] |
| 29. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [PubMed] |
| 30. | Xu Z, Liu Y, Xu T, Chen L, Si L, Wang Y, Ren X, Zhong Y, Zhao J, Xu D. Acute hepatitis B infection associated with drug-resistant hepatitis B virus. J Clin Virol. 2010;48:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Wong DK, Tanaka Y, Lai CL, Mizokami M, Fung J, Yuen MF. Hepatitis B virus core-related antigens as markers for monitoring chronic hepatitis B infection. J Clin Microbiol. 2007;45:3942-3947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Lam YF, Seto WK, Wong D, Cheung KS, Fung J, Mak LY, Yuen J, Chong CK, Lai CL, Yuen MF. Seven-Year Treatment Outcome of Entecavir in a Real-World Cohort: Effects on Clinical Parameters, HBsAg and HBcrAg Levels. Clin Transl Gastroenterol. 2017;8:e125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 33. | Khatun M, Mondal RK, Pal S, Baidya A, Bishnu D, Banerjee P, Santra AK, Dhali GK, Banerjee S, Chowdhury A, Datta S. Distinctiveness in virological features and pathogenic potentials of subgenotypes D1, D2, D3 and D5 of Hepatitis B virus. Sci Rep. 2018;8:8055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Halfon P, Bourlière M, Pol S, Benhamou Y, Ouzan D, Rotily M, Khiri H, Renou C, Pénaranda G, Saadoun D, Thibault V, Serpaggi J, Varastet M, Tainturier MH, Poynard T, Cacoub P. Multicentre study of hepatitis B virus genotypes in France: correlation with liver fibrosis and hepatitis B e antigen status. J Viral Hepat. 2006;13:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Lapalus M, Laouenan C, Cardoso AC, Estrabaud E, Carvalho-Filho RJ, Zhang Q, Lada O, Appourchaux K, Mouri F, Boyer N, Bedossa P, Asselah T, Martinot-Peignoux M, Marcellin P. Precore/Core promoter variants to predict significant fibrosis in both HBeAg positive and negative chronic hepatitis B. Liver Int. 2015;35:2082-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Tseng TC, Liu CJ, Yang HC, Chen CL, Yang WT, Tsai CS, Kuo SF, Verbree FC, Su TH, Wang CC, Liu CH, Chen PJ, Chen DS, Kao JH. Higher proportion of viral basal core promoter mutant increases the risk of liver cirrhosis in hepatitis B carriers. Gut. 2015;64:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 37. | Mak LY, Wong DK, Cheung KS, Seto WK, Lai CL, Yuen MF. Review article: hepatitis B core-related antigen (HBcrAg): an emerging marker for chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2018;47:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 38. | Seto WK, Wong DK, Fung J, Huang FY, Liu KS, Lai CL, Yuen MF. Linearized hepatitis B surface antigen and hepatitis B core-related antigen in the natural history of chronic hepatitis B. Clin Microbiol Infect. 2014;20:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 39. | Hsu YC, Nguyen MH, Mo LR, Wu MS, Yang TH, Chen CC, Tseng CH, Tai CM, Wu CY, Lin JT, Tanaka Y, Chang CY. Combining hepatitis B core-related and surface antigens at end of nucleos(t)ide analogue treatment to predict off-therapy relapse risk. Aliment Pharmacol Ther. 2019;49:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |