Published online Aug 28, 2019. doi: 10.3748/wjg.v25.i32.4739
Peer-review started: April 28, 2019
First decision: May 30, 2019
Revised: July 2, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 28, 2019
Processing time: 124 Days and 5.5 Hours
Severe acute pancreatitis (SAP) is a common condition in the intensive care unit (ICU) and has a high mortality. Early evaluation of the severity and prognosis is very important for SAP therapy. Recently, red blood cell distribution (RDW) was associated with mortality of sepsis patients and could be used as a predictor of prognosis. Similarly, RDW may be associated with the prognosis of SAP patients and be used as a prognostic indicator for SAP patients.
To investigate the prognostic value of RDW for SAP patients.
We retrospectively enrolled SAP patients admitted to the ICU of the First Affiliated Hospital of China Medical University from June 2015 to June 2017. According to the prognosis at 90 d, SAP patients were divided into a survival group and a non-survival group. RDW was extracted from a routine blood test. Demographic parameters and RDW were recorded and compared between the two groups. The receiver operator characteristic (ROC) curve was constructed and Cox regression analysis was performed to investigate the prognostic value of RDW for SAP patients.
In this retrospective cohort study, 42 SAP patients were enrolled, of whom 22 survived (survival group) and 20 died (non-survival group). The baseline parameters were comparable between the two groups. The coefficient of variation of RDW (RDW-CV), standard deviation of RDW (RDW-SD), Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and Sequential Organ Failure Assessment (SOFA) score were significantly higher in the non-survival group than in the survival group (P < 0.05). The RDW-CV and RDW-SD were significantly correlated with the APACHE II score and SOFA score, respectively. The areas under the ROC curves (AUCs) of RDW-CV and RDW-SD were all greater than those of the APACHE II score and SOFA score, among which, the AUC of RDW-SD was the greatest. The results demonstrated that RDW had better prognostic value for predicting the mortality of SAP patients. When the RDW-SD was greater than 45.5, the sensitivity for predicting prognosis was 77.8% and the specificity was 70.8%. Both RDW-CV and RDW-SD could be used as independent risk factors to predict the mortality of SAP patients in multivariate logistic regression analysis and univariate Cox proportional hazards regression analysis, similar to the APACHE II and SOFA scores.
The RDW is greater in the non-surviving SAP patients than in the surviving patients. RDW is significantly correlated with the APACHE II and SOFA scores. RDW has better prognostic value for SAP patients than the APACHE II and SOFA scores and could easily be used by clinicians for the treatment of SAP patients.
Core tip: Our study aimed to investigate the prognostic value of red blood cell distribution (RDW) for severe acute pancreatitis (SAP) patients. We retrospectively enrolled 42 SAP patients admitted to the intensive care unit in two years. The results suggested that RDW is greater in the non-surviving SAP patients than in the surviving patients. RDW is significantly correlated with the Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores. RDW has better prognostic value for SAP patients than the APACHE II and SOFA scores and could easily be used by clinicians for the treatment of SAP patients.
- Citation: Zhang FX, Li ZL, Zhang ZD, Ma XC. Prognostic value of red blood cell distribution width for severe acute pancreatitis. World J Gastroenterol 2019; 25(32): 4739-4748
- URL: https://www.wjgnet.com/1007-9327/full/v25/i32/4739.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i32.4739
Acute pancreatitis (AP) is an inflammatory process of the pancreas that often leads to local and systemic complications[1]. It is also the most common pancreatic disease worldwide[2]. According to the 2012 revised Atlanta classification for AP, severe AP (SAP) has been redefined as AP with persistent organ failure (organ failure lasting for more than 48 hours), whose mortality rate is between 20%-50%[3,4]. There are two phases during AP; systemic inflammatory response syndrome and the resultant organ failure dominate the early phase. There are currently no effective drugs available to treat AP, and thus most care is supportive[1]. Thus, rapid assessment of disease severity and the evaluation of prognosis are pivotal to determine therapeutic strategies as effective treatment could significantly decrease mortality in patients with SAP[5].
The red blood cell distribution width (RDW) is a part of the routine complete blood count and can easily be obtained by clinicians. RDW is a means of evaluating the variability in the size of erythrocytes and has been used widely in the differential diagnosis of anemia[6]. Recently, RDW was shown to be associated with inflammatory reactions and has been used as a prognostic biomarker in hypertension[7], coronary disease[8,9], stroke[10], pulmonary hypertension[11], and acute kidney injury[12]. RDW was further demonstrated to be an independent predictor of in-hospital mortality in elderly patients with sepsis[13]. For patients with AP, RDW was shown to be positively associated with AP severity, and is likely a useful predictive parameter for AP severity[14]. However, it is not yet clear whether RDW is associated with the prognosis of SAP patients or whether it can be used as a prognostic indicator for SAP patients. The main aim of our study was to examine the difference in RDW between the surviving and non-surviving SAP patients and to evaluate the prognostic value of RDW for SAP patients.
This retrospective cohort study enrolled 42 patients diagnosed with SAP who were admitted to the intensive care unit (ICU) of the First Affiliated Hospital of China Medical University from June 2015 to June 2017. Patients were diagnosed as having AP by meeting two out of the following three criteria: (1) Typical clinical symptoms with consistent abdominal pain; (2) Serum amylase and/or lipase greater than 3 times the upper limit of normal; (3) Characteristic findings from abdominal ultrasonography and/or computed tomography. Based on the 2012 revised Atlanta classification criteria, SAP was defined as AP with persistent single or multiple organ failure which lasted at least 48 hours, or a Marshall score greater than 2[3].
The exclusion criteria included any of the following: (1) The time from abdominal pain onset to hospital admission ≥ 72 h; (2) Age younger than 18 years; (3) Pancreatitis induced by trauma; (4) Chronic pancreatitis; (5) Unavailable laboratory measurements or medical records; (6) Patients with anemia; (7) Advanced malignant tumors or malignant tumors with chemotherapy and radiotherapy; (8) Pregnancy; and (9) Expected stay in ICU shorter than 24 h. The study was conducted according to the principles of the Declaration of Helsinki. Informed consent from individual patients was not obtained since all data were retrieved retrospectively from the laboratory test information system without additional blood samples or laboratory analysis.
Laboratory data were obtained from the blood screening test at ICU admission, including RDW, C reactive protein (CRP), white blood cells, serum albumin, serum calcium, platelet distribution width (PDW), and neutrophil to lymphocyte ratio (NLR). RDW was implied as RDW-CV (coefficient of variation of RDW) and RDW-SD (standard deviation of RDW), respectively, both of which are indicators of inhomogeneity of red blood cells. The electronic medical records and paper charts of all enrolled SAP patients were reviewed for information on demographics, physiologic variables, and disease severity, including the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, and length of stay in the ICU (LOS-ICU). The prognosis at 90 d of all enrolled SAP patients was recorded. According to the prognosis at 90 d, the patients were divided into a survival group and a non-survival group. The RDW value was compared between the two groups. We used receiver operator characteristic (ROC) curves and Cox regression analysis to verify the prognostic value of RDW for SAP patients.
Continuous variables are presented as the mean and standard derivation. Categorical data are reported as number (frequency). Student’s t-test and Mann-Whitney U test were used to evaluate the difference in baseline characteristics between the two groups. Multiple group comparisons were performed using the Chi-square test for categorical variables and the Kruskal-Wallis test for continuous data. ROC curves were constructed to evaluate the prognostic value of different parameters in predicting prognosis. Multivariate logistic regression analysis and univariate Cox proportional hazards regression analysis were used to evaluate the risk factors for predicting mortality in SAP patients. Hazard ratios and 95% confidence intervals are presented. A P-value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 24.0 software package (SPSS Inc, Chicago IL, United States).
Forty-two SAP patients were enrolled in this retrospective cohort study. The clinical characteristics of these patients are summarized in Table 1. According to the prognosis at 90 d, the patients were divided into a survival group (n = 22) and a non-survival group (n = 20). There was no difference in gender, age, or BMI between the two groups, suggesting baseline comparability (Table 2). There was also no difference in PDW, CRP, NLR, or LOS-ICU between the two groups. The RDW-CV, RDW-SD, APACHE II score, and SOFA score were significantly greater in the non-survival group than in the survival group (P < 0.05).
| Characteristic | Number |
| Total number of enrolled patients | 42 |
| Age (mean ± SD, yr) | 47.15 ± 15.91 |
| Gender, n (%) | |
| Male | 27 (64.29) |
| Female | 15 (35.71) |
| BMI | 26.67 ± 4.30 |
| Etiology of SAP | |
| Biliary tract disease | 20 |
| Alcoholism | 8 |
| Hypertriglyceridemia | 7 |
| Drug-induced | 0 |
| Others | 7 |
| Number of failed organs | |
| 1 | 12 |
| 2 | 9 |
| ≥ 3 | 7 |
| Mortality at 90 d, n (%) | 18 (42.86) |
| Demographic parameter | Survival group | Non-survival group | P-value |
| Number | 22 | 20 | |
| Age (yr) | 50.50 ± 17.78 | 44.33 ± 13.28 | 0.206 |
| Gender | Male (12): female (10) | Male (15): female (5) | 0.720 |
| BMI (kg/m2) | 26.72 ± 4.61 | 26.59 ± 3.96 | 0.924 |
| LOS-ICU (d) | 7.71 ± 6.64 | 11.22 ± 14.40 | 0.347 |
| RDW-CV (%) | 13.59 ± 0.88 | 15.09 ± 1.63 | 0.002 |
| RDW-SD (fL) | 43.96 ± 3.11 | 49.94 ± 5.51 | 0.000 |
| PDW (fL) | 14.72 ± 3.35 | 14.95 ± 3.76 | 0.842 |
| CRP (mg/L) | 212.10 ± 107.04 | 212.19 ± 144.97 | 0.998 |
| NLR | 14.05 ± 10.13 | 9.92 ± 4.53 | 0.085 |
| Serum calcium | 1.64 ± 0.27 | 1.77 ± 0.45 | 0.253 |
| APACHE II | 12.42 ± 4.27 | 15.67 ± 5.70 | 0.041 |
| SOFA | 4.83 ± 2.65 | 7.94 ± 2.99 | 0.001 |
We performed an analysis to examine the correlation of RDW-CV and RDW-SD with the APACHE II and SOFA scores. The results showed that RDW-CV and RDW-SD were significantly correlated with the APACHE II and SOFA scores (Table 3).
| Variable | RDW-CV | RDW-SD | ||
| r | P-value | r | P-value | |
| APACHE II | 0.504 | 0.001 | 0.434 | 0.004 |
| SOFA | 0.414 | 0.006 | 0.380 | 0.012 |
| Mortality | 0.504 | 0.001 | 0.490 | 0.001 |
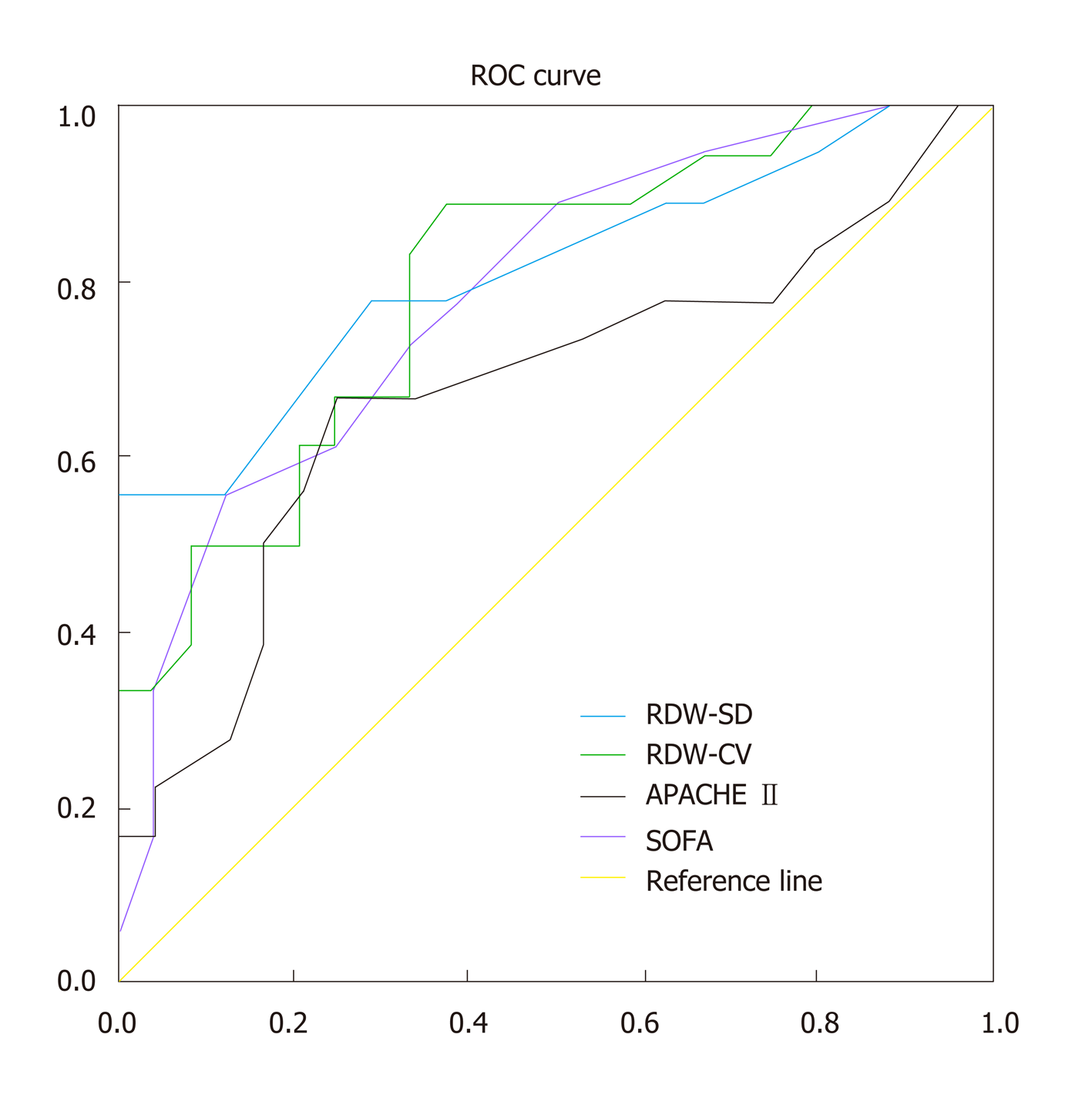
According to the prognosis of SAP patients at 90 d, we constructed different ROC curves for RDW-CV, RDW-SD, APACHE II score, and SOFA score to examine their clinical value for predicting prognosis of SAP patients. We found that the areas under the curves (AUCs) of RDW-CV and RDW-SD were all significantly larger than those of the APACHE II and SOFA scores (Table 4, Figure 1), with the RDW-SD being the greatest. Our results suggested that RDW was superior to both the APACHE II score and SOFA score in predicting the prognosis of SAP patients. The cutoff value for RDW-CV was 13.55, with an 83.3% sensitivity and 66.7% specificity (Table 5). When the RDW-SD was greater than 45.5, the sensitivity of predicting mortality for SAP patients was 77.8%, while the specificity was 70.8% (Table 5).
| Parameter | Sensitivity | Specificity | Youden index | |
| RDW-CV | 13.45 | 0.889 | 0.625 | 0.514 |
| 13.55 | 0.833 | 0.667 | 0.500 | |
| 13.35 | 0.889 | 0.583 | 0.472 | |
| 13.65 | 0.778 | 0.667 | 0.445 | |
| RDW-SD | 50.5 | 0.556 | 1 | 0.556 |
| 51.5 | 0.5 | 1 | 0.500 | |
| 45.5 | 0.778 | 0.708 | 0.486 | |
| 49.5 | 0.556 | 0.917 | 0.473 |
To further investigate the prognostic value of RDW for SAP patients, we performed multivariate logistic regression analysis and univariate Cox proportional hazards regression analysis to determine whether RDW could be used as an independent risk factor to predict mortality. The results from both regression analyses showed that RDW-CV and RDW-SD could be used as independent risk factors to predict the prognosis of SAP patients, similar to the APACHE II score and SOFA score (Table 6 and 7).
| Variable | Multivariate OR (95%CI) | P-value |
| RDW-CV | 3.283 (1.503-7.174) | 0.003 |
| RDW-SD | 1.368 (1.120-1.670) | 0.002 |
| APACHE II | 1.172 (1.017-1.351) | 0.028 |
| SOFA | 1.682 (1.212-2.332) | 0.002 |
| Variable | Multivariate OR (95%CI) | P-value |
| RDW-CV | 1.615 (1.258-2.072) | 0.000 |
| RDW-SD | 1.166 (1.077-1.261) | 0.000 |
| RDW-CV and RDW-SD | 1.005 (1.003-1.008) | 0.000 |
| APACHE II | 1.151 (1.048-1.264) | 0.003 |
| SOFA | 1.363 (1.175-1.582) | 0.000 |
In this retrospective cohort study, we found that RDW, which was assessed as RDW-CV and RDW-SD, was significantly elevated in non-surviving SAP patients. A similar elevation was seen in both the APACHE II score and SOFA score. RDW-CV and RDW-SD were significantly correlated with the APACHE II score and SOFA score. The AUCs of RDW-CV and RDW-SD were greater than those of the APACHE II score and SOFA score, with RDW-SD being the greatest. We also found that RDW-CV and RDW-SD could be used as independent risk factors to predict the prognosis of SAP patients via both the multivariate logistic regression analysis and univariate Cox proportional hazards regression analysis, similar to the APACHE II score and SOFA score. These results suggested that RDW has superior predictive value for the prognosis of SAP patients.
AP is the most common pancreatic disease worldwide, and SAP has a very high mortality[2-4]. Although early diagnosis and management of SAP patients remain very important, early evaluation of the severity and prognosis has equal significance for SAP patients. Thus, clinicians could rapidly recognize SAP patients with high mortality risk and provide more active therapy in order to save their lives. In our study, we found that the RDW had a greater ROC AUC vs either the APACHE II or SOFA score in predicting the prognosis of SAP patients, suggesting that RDW may have better prognostic value for SAP patients.
RDW is a parameter that reflects the heterogeneity of red blood cells measured with an automated blood cell analyzer and is routinely performed as part of a complete blood count[14]. The RDW level can be easily obtained and is an inexpensive measure. It is often expressed as RDW-CV and RDW-SD, but the latter has better sensitivity and is less affected by other factors[15]. Higher RDW levels indicate greater variation in the size of red blood cells, which could be used for the differential diagnosis of nutritional deficiency-related anemia due to iron, folic acid, and vitamin B12 deficiency[16]. Recently, studies have showed that RDW is associated with inflammatory reactions and can predict the severity and prognosis of many diseases including cardiovascular diseases[17], chronic obstructive pulmonary disease[18], pulmonary hypertension[11], rheumatoid arthritis[19], and malignancy[20]. Higher RDW was also associated with poorer outcome in severe sepsis and septic shock[21], and all-cause mortality in critically ill patients[22]. Previous studies demonstrated that RDW was associated with pro-inflammatory cytokines[23], tumor necrosis factor alpha[24], and oxidative stress reactions[25]. The inflammatory response alters the half-life of red blood cells, erythropoiesis, disorders of iron metabolism, and increases hemolysis, which results in impaired hematopoiesis and increases RBC size heterogeneity. Thus, RDW could be used as a nonspecific inflammatory indicator[26]. During sepsis, the inflammatory cytokines induced by pathogen associated molecular patterns could directly induce red blood cell injury, disturb the iron steady state, induce bone marrow suppression, and downregulate the expression of the erythropoietin receptor, all of which could lead to the elevation of RDW during sepsis[27].
AP is an inflammatory event of the pancreas. Excessive and uncontrolled systemic inflammatory reactions are key to the pathogenesis of SAP and related multiple organ dysfunction during the course of SAP. Therefore, as an inflammatory indicator, the level of RDW should theoretically increase in SAP and should have predictive value for both disease severity and mortality in SAP patients. Zhang et al[14] confirmed that RDW was positively associated with AP severity and was a useful predictive parameter for AP severity at the early admission stage. Wang et al[13] demonstrated that for each 1% increase in the RDW level, the mortality rate of elderly septic patients increased by 18%. Our results further confirmed that RDW was greater in the non-surviving SAP patients than in the surviving patients, and had better prognostic value for SAP patients than either the APACHE II score or the SOFA score. Therefore, clinicians could rapidly recognize those SAP patients with a higher risk for mortality when their RDW level was increased. More active therapy could then be promptly given in order to save their lives.
The APACHE II score and SOFA score are both commonly used for mortality prediction in critically ill patients[28]. They are comprised of many routine variables and their calculation usually requires at least 24 hours. To some extent, they are more complicated and cannot be obtained as easily as RDW. Studies have shown that the prognostic value of RDW for patients with septic shock was better than the APACHE II score and the SOFA score[29]. RDW was also confirmed to be an independent risk factor for mortality in septic neonates, and the effectiveness of mortality prediction was superior than that of the SOFA score[30]. In our study, the AUC of RDW was greater than those of the APACHE II score and SOFA score, and both RDW-CV and RDW-SD were independent risk factors for predicting the prognosis of SAP patients in the regression analysis. The prognostic value of RDW was superior to that of either the APACHE II score or the SOFA score. Our results are similar to previous studies and further verified that RDW could predict the prognosis of critically ill patients. Therefore, RDW is an easily obtained, inexpensive, and useful indicator that should be widely used in ICU practice.
There are some limitations to our study. First, this is a retrospective study executed in a single center. Second, the study sample is small. Third, we did not perform a prospective validation study to further confirm the prognostic value of RDW for SAP patients. Fourth, we only examined the prognostic value of RDW in SAP patients. Whether RDW has a similar prognostic value in mild AP and moderate-severe AP patients remains to be examined. Therefore, we expect that large prospective randomized controlled trials will further verify our results.
In conclusion, we have found that the RDW is significantly greater in the non-surviving SAP patients than in the surviving patients. RDW is significantly associated with both the APACHE II score and the SOFA score. The ROC AUC for mortality prediction by RDW is greater than those of the APACHE II score and the SOFA score. Both RDW-CV and RDW-SD are independent risk factors that are predictive of mortality in SAP patients. RDW has better prognostic value for SAP patients than either the APACHE II score or the SOFA score. Clinicians could use RDW as a valuable indicator for early recognition of SAP patients with higher mortality risk and promptly provide more active therapies to save more lives.
Severe acute pancreatitis (SAP) is a common acute and severe clinical disease. There are a large number of inflammatory mediators and cytokines released, which cause systemic inflammatory response, accompanied by continuous multiple organ dysfunction, such as intestinal dysfunction and metabolic dysfunction. Therefore, it is important to evaluate the prognostic factors for SAP. Red cell distribution width (RDW) is an indicator of erythrocyte variability in the blood circulation, and can reflect the difference in red blood cell size and the dispersion degree of red blood cell volume. At present, it is not yet clear whether RDW is associated with the prognosis of SAP patients or whether it can be used as a prognostic indicator for SAP patients.
Timely and effective judgment of the condition of SAP patients is of great value for the treatment of those patients. RDW may be related to the severity of SAP. Our study aimed to investigate the prognostic value of RDW for SAP patients.
The main aim of our study was to examine the difference of RDW between the surviving SAP patients and non-surviving SAP patients and evaluate the prognostic value of RDW for SAP patients.
We retrospectively enrolled SAP patients admitted to intensive care unit for two years. According to the prognosis at 90 d, the SAP patients were divided into a survival group and a non-survival group. The RDW was extracted from a routine blood test. The demographic parameters and RDW were recorded and compared between the two groups. The receiver operator characteristic (ROC) was constructed and the Cox regression analysis was done to investigate the prognostic value of RDW for SAP patients.
Of 42 SAP patients enrolled in this retrospective cohort study, 22 survived (survival group) and 20 died (non-survival group). The baseline parameters were comparable between the two groups. The coefficient of variation of RDW (RDW-CV), standard deviation of RDW (RDW-SD), Physiology and Chronic Health Evaluation II (APACHE II) score, and Sequential Organ Failure Assessment (SOFA) scores were significantly higher in the non-survival group than in the survival group (P < 0.05). The RDW-CV and RDW-SD were significantly correlated with the APACHE II score and SOFA score, respectively. The areas of the ROC curves (AUCs) of RDW-CV and RDW-SD were all larger than those of APACHE II score and SOFA score, among which the AUC of RDW-SD was the largest one. The results demonstrated that RDW had the better prognostic value for predicting the mortality of SAP patients. When the RDW-SD was higher than 45.5, the sensitivity of predicting prognosis was 77.8% and the specificity was 70.8%. Both RDW-CV and RDW-SD could be used as independent risk factors for predicting mortality of SAP patients in multivariate logistic regression analysis and univariate Cox proportional hazards regression analysis, similar to the APACHE II score and SOFA score.
The RDW is higher in the non-surviving SAP patients than that in the surviving patients. RDW has better prognostic value for SAP patients than APACHE II and SOFA scores, and it could be easily used for clinicians in treating SAP patients. RDW has superior value in predicting the prognosis of SAP patients. RDW is associated with inflammatory reactions and can predict severity and prognosis of many diseases including cardiovascular diseases, chronic obstructive pulmonary disease, pulmonary hypertension, rheumatoid arthritis, and malignancy. Higher RDW is also associated with poorer outcome in severe sepsis and septic shock. RDW may be associated with mortality of SAP patients and could be used as a predictor of prognosis. Clinicians could rapidly recognize those SAP patients with higher risk for mortality when the RDW level is increased. More active therapy could be given promptly to those patients in order to save their lives.
This is a retrospective study executed in a single center and the sample of our study is small. We expect large prospective randomized controlled trials to further verify our results. And we would perform a prospective validation study to further confirm the prognostic value of RDW for SAP patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen YW, Goldaracena N, Snowdon VK S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial Medical Treatment of Acute Pancreatitis: American Gastroenterological Association Institute Technical Review. Gastroenterology. 2018;154:1103-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 2. | Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 3. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4328] [Article Influence: 360.7] [Reference Citation Analysis (45)] |
| 4. | Lytras D, Manes K, Triantopoulou C, Paraskeva C, Delis S, Avgerinos C, Dervenis C. Persistent early organ failure: Defining the high-risk group of patients with severe acute pancreatitis? Pancreas. 2008;36:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Peng T, Peng X, Huang M, Cui J, Zhang Y, Wu H, Wang C. Serum calcium as an indicator of persistent organ failure in acute pancreatitis. Am J Emerg Med. 2017;35:978-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Miyamoto K, Inai K, Takeuchi D, Shinohara T, Nakanishi T. Relationships among red cell distribution width, anemia, and interleukin-6 in adult congenital heart disease. Circ J. 2015;79:1100-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 7. | Bilal A, Farooq JH, Kiani I, Assad S, Ghazanfar H, Ahmed I. Importance of Mean Red Cell Distribution Width in Hypertensive Patients. Cureus. 2016;8:e902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 8. | Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M; for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation. 2008;117:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 603] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 9. | Sangoi MB, Da Silva SH, da Silva JE, Moresco RN. Relation between red blood cell distribution width and mortality after acute myocardial infarction. Int J Cardiol. 2011;146:278-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol. 2009;104:868-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Oh HJ, Park JT, Kim JK, Yoo DE, Kim SJ, Han SH, Kang SW, Choi KH, Yoo TH. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol Dial Transplant. 2012;27:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Wang AY, Ma HP, Kao WF, Tsai SH, Chang CK. Red blood cell distribution width is associated with mortality in elderly patients with sepsis. Am J Emerg Med. 2018;36:949-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Zhang T, Liu H, Wang D, Zong P, Guo C, Wang F, Wu D, Tang M, Zhou J, Zhao Y. Predicting the Severity of Acute Pancreatitis With Red Cell Distribution Width at Early Admission Stage. Shock. 2018;49:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med. 1991;9 Suppl 1:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 248] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Viswanath D, Hegde R, Murthy V, Nagashree S, Shah R. Red cell distribution width in the diagnosis of iron deficiency anemia. Indian J Pediatr. 2001;68:1117-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Sangoi MB, Guarda Ndos S, Rödel AP, Zorzo P, Borges PO, Cargnin LP, De Carvalho JA, Premaor MO, Moresco RN. Prognostic value of red blood cell distribution width in prediction of in-hospital mortality in patients with acute myocardial infarction. Clin Lab. 2014;60:1351-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Seyhan EC, Özgül MA, Tutar N, Ömür I, Uysal A, Altin S. Red blood cell distribution and survival in patients with chronic obstructive pulmonary disease. COPD. 2013;10:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Yunchun L, Yue W, Jun FZ, Qizhu S, Liumei D. Clinical Significance of Red Blood Cell Distribution Width and Inflammatory Factors for the Disease Activity in Rheumatoid Arthritis. Clin Lab. 2016;62:2327-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Montagnana M, Danese E. Red cell distribution width and cancer. Ann Transl Med. 2016;4:399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Jo YH, Kim K, Lee JH, Kang C, Kim T, Park HM, Kang KW, Kim J, Rhee JE. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am J Emerg Med. 2013;31:545-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 23. | Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 24. | Lorente L, Martín MM, Abreu-González P, Solé-Violán J, Ferreres J, Labarta L, Díaz C, González O, García D, Jiménez A, Borreguero-León JM. Red blood cell distribution width during the first week is associated with severity and mortality in septic patients. PLoS One. 2014;9:e105436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10:1923-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 26. | Orfanu AE, Popescu C, Leuștean A, Negru AR, Tilişcan C, Aramă V, Aramă ȘS. The Importance of Haemogram Parameters in the Diagnosis and Prognosis of Septic Patients. J Crit Care Med (Targu Mures). 2017;3:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25:609-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 348] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 28. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Sadaka F, O'Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med. 2013;28:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Ellahony DM, El-Mekkawy MS, Farag MM. A Study of Red Cell Distribution Width in Neonatal Sepsis. Pediatr Emerg Care. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |