Published online Aug 28, 2019. doi: 10.3748/wjg.v25.i32.4614
Peer-review started: April 30, 2019
First decision: June 5, 2019
Revised: July 12, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 28, 2019
Processing time: 120 Days and 20.8 Hours
Liver cancers are the second most frequent cause of global cancer-related mortality of which 90% are attributable to hepatocellular carcinoma (HCC). Despite the advent of screening programmes for patients with known risk factors, a substantial number of patients are ineligible for curative surgery at presentation with limited outcomes achievable with systemic chemotherapy/external radiotherapy. This has led to the advent of numerous minimally invasive options including but not limited to trans-arterial chemoembolization, radiofrequency/microwave ablation and more recently selective internal radiation therapy many of which are often the first-line treatment for select stages of HCC or serve as a conduit to liver transplant. The authors aim to provide a comprehensive overview of these various image guided minimally invasive therapies with a brief focus on the technical aspects accompanied by a critical analysis of the literature to assess the most up-to-date evidence from comparative systematic reviews and meta-analyses finishing with an assessment of novel combination regimens and future directions of travel.
Core tip: Hepatocellular carcinoma (HCC) is the most frequently observed primary malignant liver tumors and is a major cause of worldwide mortality. Despite the advances in minimally invasive surgery, such as laparoscopic and robotic, they are reserved only in early stage patients. Thus, percutaneous locoregional treatments have now a pivotal role in HCC management; in this review, we discuss state of the art of currently available locoregional treatment for HCC and their future perspectives.
- Citation: Inchingolo R, Posa A, Mariappan M, Spiliopoulos S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J Gastroenterol 2019; 25(32): 4614-4628
- URL: https://www.wjgnet.com/1007-9327/full/v25/i32/4614.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i32.4614
Hepatocellular carcinoma (HCC) represents 75%-90% of primary liver malignancies and is a major cause of worldwide mortality[1]. According to the National Cancer Institute, estimated HCC deaths for the year 2016 in the United States were 27170, while the incidence of the disease will continue to increase until 2030. Acknowledged risk factors are closely related with life-style choices and include chronic hepatitis B and C virus infection, fatty liver disease, cirrhosis, diabetes, obesity, and smoking[2-4]. The prognosis of HCC remains poor, especially if diagnosed at an advanced stage, while mainstream curative options for very early and early stage HCC in good surgical candidates are liver transplantation and surgical resection. Nevertheless, at the time of diagnosis, a substantial number of patients are ineligible for surgical treatment, due to intermediate or advanced disease stage or severe comorbidities which increase the surgical risk[5]. Unfortunately, the prognosis of HCC following systemic pharmacotherapy, external radiotherapy or plain supportive treatments is also poor. As a result, various percutaneous, image-guided, locoregional therapies, have emerged in order to improve outcomes, initially among inoperable patients[6,7] (Table 1). After decades of thorough investigation and clinical experience in the field of interventional oncology, numerous minimal invasive treatment options have been developed and include: (1) Curative modalities such as percutaneous radiofrequency ablation (RFA), microwave ablation (MWA), percutaneous ethanol injection (PEI), cryoablation (CA), irreversible electroporation (IRE); and (2) Palliative therapies such as bland trans-arterial embolization (TAE), conventional trans-arterial che-moembolization (TACE) or chemoembolization with drug-eluting beads (DEB-TACE) and more recently local endovascular radiotherapy via the trans-arterial delivery of beta-emitting microparticles (selective internal radiation therapy; SIRT). Moreover, the effectiveness of various combinations of locoregional treatments with or without systemic chemotherapy has been also investigated, aiming in down staging inoperable disease or increasing overall survival rates and improving quality of life[7-10]. This review analyses currently available locoregional treatment options for HCC and highlights their importance in the development of more efficient treatment algorithms.
| Indications | Advantages | Disadvantages | |
| Ablation (RFA) | BCLC-A patients | Curative | Low complication rates rates in HCC > 3 cm |
| New devices (MWA, Cryoablation, HIFU, Laser, IRE) | Relatively unfeasible in “complex” sites/lesions | ||
| TACE | BCLC-B patients without PVT | Super-selective delivery | Palliative |
| Great variety of materials | Heterogeneous population | ||
| No standardization | |||
| Combined therapy (RFA + TACE) | Selected BCLC-A/B patients | Complimentary and synergistic effect | No standardization |
| Better than RFA and TACE alone | |||
| SIRT | Selected BCLC-B/C patients not amenable for TACE or Sorafenib | Super-selective delivery | High costs |
Transcatheter arterial chemoembolization (TACE) represents the therapeutic gold-standard in patients unsuitable for surgery and for percutaneous ablation techniques, with multinodular HCC and preserved liver function, without vascular invasion or extra-hepatic spread (intermediate stage, BCLC-B)[11,12].
The TACE treatment is based on the occlusion of the arterial blood supply of the target neoplastic lesion by embolizing microparticles, combined with the injection of chemotherapeutic drugs in a super-selective manner, sparing the adjacent healthy liver[13-16].
There is great variety, in the literature, among therapeutic protocols, and middle-/long-term results are poor, mostly due to the tumour burden, incomplete embolization, and presence of undetectable satellite lesions[17,18].
Nonetheless, BCLC stage B includes a heterogeneous population, with different tumour burden, as well as greatly different liver function (from Child-Pugh class A5 to B9), that cannot all be treated with the same weapons; there is, therefore, the need to perform individualized and personalized treatments. The ideal TACE target is represented by BCLC stage B asymptomatic patients with preserved liver function and without portal vein thrombosis, which are suitable for a more complete and effective treatment[19,20].
Less than 2% of patients obtain a complete response after the first TACE treatment, due to the presence of viable tissue and neo-angiogenesis which allows the continuous growth of the neoplasm; therefore, TACE should be performed more than once, at regular intervals[21], however, there is no consensus nor guidelines on the correct number on TACE treatments and on the time interval between TACE sessions, leaving the choice in the hands of the operators, with the expert’s suggestion of “on-demand” treatments with 1- or 2-mo interval between sessions and of ceasing TACE after 2-3 unsuccessful sessions[22].
Meta-analysis and randomized controlled trials have demonstrated that treatment response is associated with a good 2-years patient’s survival (about 60%), even though there is great heterogeneity among these trials in terms of patient’s characteristics, treatment modalities and materials[23,24].
The principal contraindication to TACE treatment is the presence of a poor venous blood supply from the portal vein (mostly due to chemical or neoplastic thrombosis of the main portal vein or of its lobar and segmental branches, as well as porto-systemic anastomosis and hepatofugal portal flow), due to the increased risk of ischaemic necrosis of the liver and thus liver failure. In a similar manner, patients with advanced hepatic disease (Child-Pugh class B and C) should not be considered for TACE due to their increased risk of liver failure and death[25].
Adverse effects of selective transarterial administration of the chemotherapeutic drugs may be similar those seen with systemic administration: Nausea, vomiting, myelotoxicity, alopecia, and kidney failure.
Hepatic artery occlusion, causing acute ischaemia of the HCC lesion is associated, in more than 50% of the patients, with post-embolization syndrome, which usually lasts less than 48 hours and is characterized by fever, abdominal pain, and slowed peristalsis: Fever is determined by the tumoral necrosis with cytokines release. A small percentage of patients can present with infectious complications such as hepatic abscess or cholecystitis[20].
Various chemotherapic agents have been utilized for TACE, the most common choices being doxorubicin and cisplatin[20].
Liu et al[26] showed in a randomized trial that TACE with the use of more than one chemotherapic drug has a better efficacy on overall survival and overall response rates when compared to doxorubicin monotherapy.
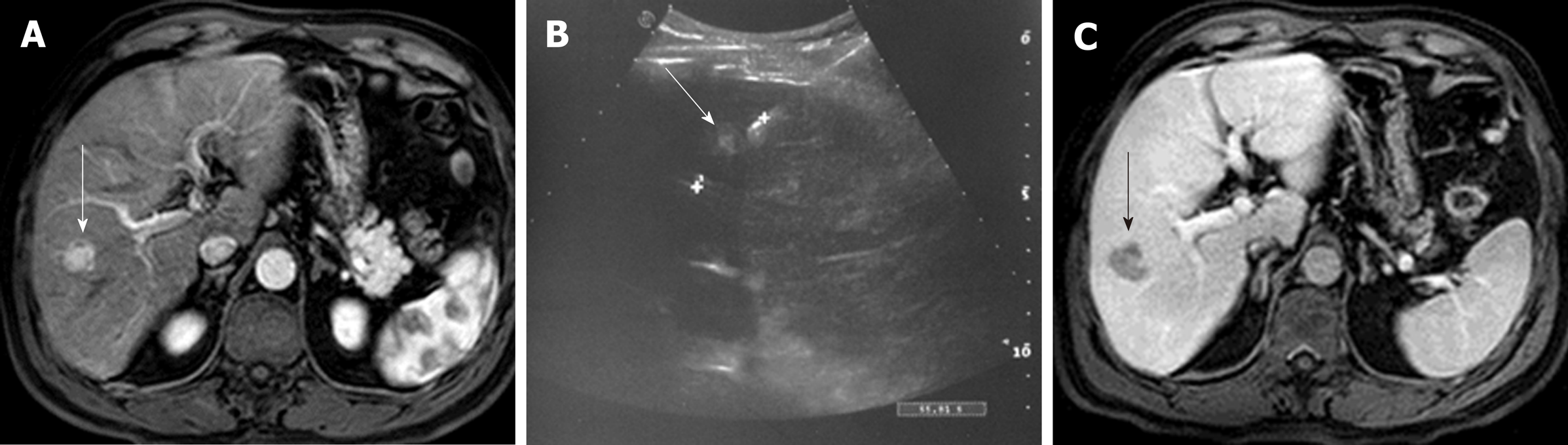
One of the greatest matters of debate when dealing with TACE, is the choice between conventional TACE (c-TACE) and drug-eluting beads TACE (DEB-TACE). c-TACE is performed with the infusion of a suspension of iodized oil mixed with the chemotherapic drug; the iodized oil acts as a carrier for the drug, and undergoes selective uptake by the neoplastic cells, increasing HCC exposureto the drug, followed by administration of the embolic particles[25,27-29]. On the other hand, DEB-TACE uses embolizing polyvinylchloride microspheres of different sizes, pre-loaded with the chemotherapic drug, which are injected in the tumour feeding artery releasing the drug in the neoplastic bloodstream, in a sustained, prolonged, and predictable manner; this determines a reduction of collateral effects due to the passage of the chemotherapeutic agent into the systemic circulation. Moreover, due to the predetermined microparticles’ calibre, the arterial occlusion is a predictable and homogeneous process, increasing the anti-neoplastic activity and safety profile[30-35] (Figure 1).
Burrel et al[34] observed that 1-, 3- and 5-years survival in a group of patients treated with DEB-TACE was 89.9%, 66.3% and 38.3% respectively, with a median survival time of 48.6 mo.
Various studies evaluated and compared the efficacy of c-TACE versus DEB-TACE, as the PRECISION-V trial, which showed higher - even though not significant - complete response, objective response and disease control rates in DEB-TACE, and a significant reduction in liver toxicity and doxorubicin-related side effects; other studies did not confirm that superiority. Therefore, the comparison between c-TACE and DEB-TACE is still a matter of debate[36-38].
A recent improvement to TACE was made with the introduction of embolizing microparticles containing iodine atoms in their structure, thus visible during fluoroscopy, granting a precise and controlled delivery of the chemotherapeutic drug during the treatment[39].
Furthermore, patients with intermediate-stage HCC (BCLC-B) and impaired liver function, or with portal vein thrombosis/invasion, can nowadays benefit from a particular form of chemoembolization, based on degradable starch microspheres (DSM-TACE), which carry the chemotherapic drug but are rapidly digested once delivered in the hepatic blood stream, reducing the ischaemic effect on the liver parenchyma[40,41]. This treatment has also been performed as a second-line treatment in BCLC-C stage patients ineligible for the anti-angiogenic drug Sorafenib, with similar results in terms of progression-free survival (6.4 months) and overall survival (11.3 months), and of 1- and 16.5-months overall disease control of 80% and 52.5% respectively[42].
Ablative techniques using chemical or thermal energy have been developed and established in the loco-regional therapy of hepatocellular carcinomas over the last three decades[43,44]. The 2017 European association for the study of the liver (EASL) clinical practice guidelines on the management of HCCs recommend the use of ablative therapy in very early stage (single lesion < 2 cm) and early stage (single or 2-3 nodules < 3 cm) cancers amongst patients who are not candidates for surgical resection or transplant[44,45]. Prototypical amongst ablation therapies was PEI[46], used to cause coagulative necrosis of the lesion via cellular dehydration. By the early 90s however, the advent of RFA offered better survival and local disease control versus PEI[47,48] with the latter demonstrating local recurrence rates exceeding 43% in lesions > 3 cm[49], but retaining a role in the management of tumours < 2 cm where thermal ablation is not feasible. RFA is now established as the first-line ablative therapy while concomitant advances have been made with MWA and CA. Newer technologies such as laser ablation (LA) and irreversible electroporation (IRE) remain under investigation for their efficacy[44-46].
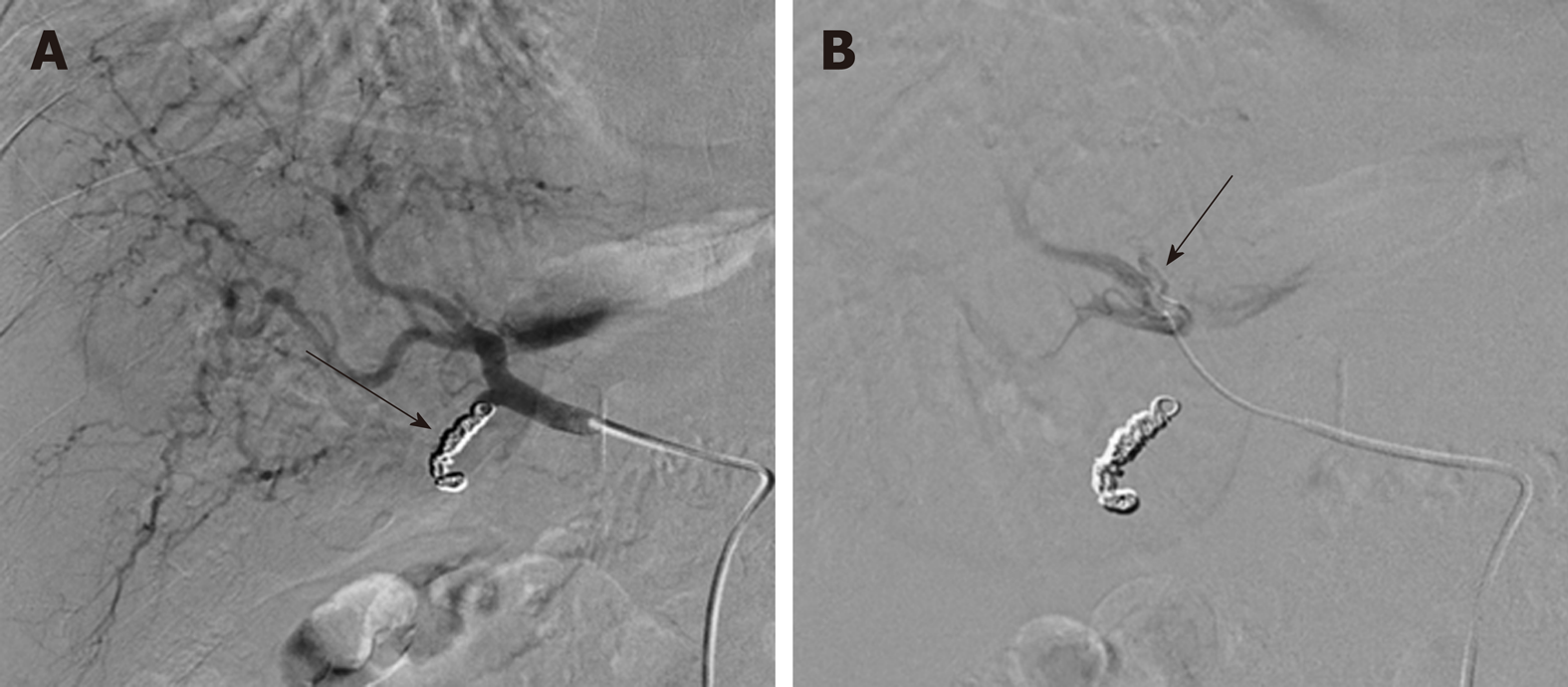
In RFA, an alternating electric current between (460-500 kHz) is applied to the target lesion via a radio-frequency (RF) electrode placed under imaging/laparoscopic guidance and returning through grounding pads on the skin surface. An induced electromagnetic field causes oscillation of tissue ions and frictional heating leading to coagulative necrosis and cell death at temperatures of 60-100 °C[50,51]. Overall efficacy of RFA is limited by local tissue charring, which increases impedance, limiting the zone of ablation, and the well described ‘heat-sink’ effect whereby, slow in vivo heat transfer from the electrode is counteracted by local high flow vascular perfusion[50]. Strategies to mitigate these limitations include the use of internal electrode cooling and the use of bipolar mode with multiple electrodes to create overlapping ablation zones though these carry an increased risk of bleeding and adjacent organ damage[50,51]. Imaging guidance is generally achieved with the use of B-mode ultrasound (Figure 2), however one study by Kim et al[52] found up to a third of lesions were not visible on this modality alone with increased use of fusion imaging with volumetric CT/MRI data to circumvent this challenge[53]. An inadequate acoustic window may be improved by infusion of fluid into the pleural or peritoneal cavity with the added benefit of minimizing adjacent organ injury. Consideration must be given to the location of the tumour and RFA should be avoided in lesions with close proximity to other abdominal viscera or in a peripheral subcapsular location[54]. For patients with very early/early stage HCC (overall size < 3 cm) RFA is the principle loco-regional therapeutic option in contrast to transplantation or surgical resection (SR). Comparison of the outcomes between RFA and SR has yielded several inconclusive studies in the literature; however results from a 2014 Cochrane review by Weis et al and three other contemporaneous systematic reviews and meta-analyses[55-58] demonstrate similar overall survival at 1 and 3 years between RFA and SR groups for tumours < 2 cm in subgroup analysis. Conclusions about recurrence rates in this cohort are contradictory amongst the various studies with recurrence generally lower following resection which is associated with longer in hospital stay and more overall complications. Cucchetti et al[56] also demonstrate a favourable cost analysis of RFA over SR in this subgroup of patients. Amongst early stage tumours (2-3 cm, up to 3 nodules), Pompili et al[59] demonstrated no significant difference in survival (RFA 66.2% vs SR 74.4%, P = 0.353) or cumulative recurrence (RFA 57.1% vs SR 56.0%, P = 0.765) at 4 years, though a trend was noted towards lower recurrence in the resection group. These findings were further confirmed with propensity score matching to minimise confounding factors for overall survival and recurrence (P = 0.450 and P = 0.152 respectively) with similar results demonstrated in a more recent RCT of 218 patients by Ng et al[60]. RFA therefore remains the mainstay of ablative treatments very early and early stage HCC despite the dearth of large scale multi-centric RCTs in this field.
Microwave ablation was initially developed to work around the heat-sink and tissue impedance limitations of RFA within the liver[50]. While the underlying mechanism of cell death in MWA is similar to RFA, tissue temperature is raised by causing the continuous realignment of polar (principally water) molecules within an oscillating microwave field at frequencies of 915/2450 MHz[50,61]. Microwaves radiate equally through all biological tissue without impedance allowing a much larger volume of tissue to be heated with each application. The latest third generation systems incorporate antenna cooling and high-power generators in combination with different antenna designs, which contribute to variable size and shape of the ablation zones necessitating careful planning on the part of the operator. With these attributes, MWA shares a similar application profile as RFA but with advantages over the latter with regards to larger lesions or those closer to blood vessels and other visceral structures[62]. Given its relative novelty there is a lack of high powered studies comparing its efficacy to RFA and only 2 meta-analyses assessing outcomes of HCC treatment between the modalities[63,64] which largely included the same studies with similar cumulative numbers of approximately 400 patients in the MWA and RFA arms apiece. Chinnaratha et al[63] demonstrated no difference in local re-currence/progression between RFA and MWA with pooled OR (95%CI) 1.01 (0.67-1.50, P = 0.98) or overall survival at 3 years with pooled OR (95%CI) 0.76 (0.44-1.32, P = 0.33). Complete ablation was achieved in MWA at rates of between 91%-100% between studies. Of note, a subgroup analysis of the use of MWA in HCC beyond the Milan criteria (single tumor > 5 cm or > 3 nodules) in 450 patients demonstrated lower local tumor progression over RFA with pooled OR (95%CI) 1.88 (1.10-3.23, P = 0.02) supporting the use of MWA in larger lesions. Facciorusso et al excluded lower powered studies and congress abstracts finding no significant difference in local recurrence with OR (95%CI) 1.01 (0.53-1.87, P = 0.98), higher (though insignificant) overall survival at 3 years, OR (95%CI) 0.95 (0.58-1.57, P = 0.85) and a non-significant higher rate of complete ablation in MWA (P = 0.67). A systematic review by Lahat et al[65] analysing major complications (defined as symptoms persisting for > 1 wk, delaying discharge, causing significant morbidity/disability and death) following percutaneous ablation reported mortality of 0.15% and 0.23% for RFA and MWA respectively with major complications occurring in 4.1% and 4.6% of cases respectively the most common being haemorrhage but also including portal vein thrombosis, bile leak/duct injury, intestinal/diaphragmatic injury and liver abscess/dysfunction. Ding et al[66] found no statistically significant difference in types or number of complications between MWA and RFA in their large retrospective analysis of 879 patients (P > 0.05). Ultimately while MWA has shown promising results for local disease its proven benefits over RFA are limited and further study is required.
In addition to RFA and MFA, several novel modalities of ablation are entering clinical practice including CA, LA, IRE and high-intensity focused ultrasound (HIFU). Cryoablation causes tumour necrosis by freezing at temperatures between −35 °C to −20 °C using the Joule-Thomson theory of expanding gases within a needle-like cryoprobe causing cooling at the probe tip[50]. Heat transfer to probe is by passive diffusion and so probe surface area limits cooling capacity. Procedures are therefore usually carried out with several probes with ablation times of up to 25-30 min with the advantage of precise intra-procedural monitoring of ice ball formation on image guidance (CT/MRI) and the ability to include larger ablative zones[67,68]. Cryotherapy is susceptible to the “cool-sink” effect whereby thermal energy exchange is disrupted near the cryoprobe owing to adjacent vascular structures[51] and the possibility of the serious and possibly life threating complication of Cryoshock whereby ablation of large tumours with large ablation volume eliciting an aggressive inflammatory response with pleural effusions, thrombocytopenia, haemorrhage, myoglobinemia and renal/respiratory failure[67]. A recent meta-analysis by Luo et al[69] including several cohort studies and one RCT demonstrated high rates of complete ablation (73.3%-100%) and no statistically significant difference in recurrence rates or overall survival between CA and RFA. In their RCT included within the meta-analysis, Wang et al[70], demonstrated improved 3 year survival (11% vs 7 %, P = 0.043), and for lesions > 3 cm, significantly lower local progression (7.7% vs 18.2%, P = 0.041) in patients treated with CA rather than RFA indicating some possible benefit with CA in larger lesions which needs to be weighed against the potentially serious associated complications. LA remains poorly studied in comparison to most other methods of ablations. Light is delivered via multiple flexible quartz fibers within water-cooled laser application sheaths and LA has been demonstrated to be safe and feasible[62]. Luo et al[69] found higher tumor recurrence, lower overall survival and complete ablation in LA vs RFA however none of these were statistically significant.
IRE is a recently developed non-thermal ablation technology that uses low-voltage, high-energy, electrical pulses to induce cell death by pore creation within the cell lipid membrane. The procedure is performed using multiple monopolar 19-gauge probes and, due to its non-thermal nature, represents a valid alternative to thermal for peri-vascular lesions and those in proximity to large bile ducts. However, despite the fact that initial results following IRE for HCC are optimistic for selected < 3 cm lesions, and the safety profile of the method has been established, data remain limited. As a result, application of liver IRE for the treatment of HCC requires further investigation and its clinical application for the moment remains limited[71].
The paucity of literature assessing HIFU limits conclusions that can be drawn about its effectiveness.
RFA remains the mainstay of ablative therapy at present with further high-quality randomised studies needed to evaluate the performance of the newer modalities in comparison.
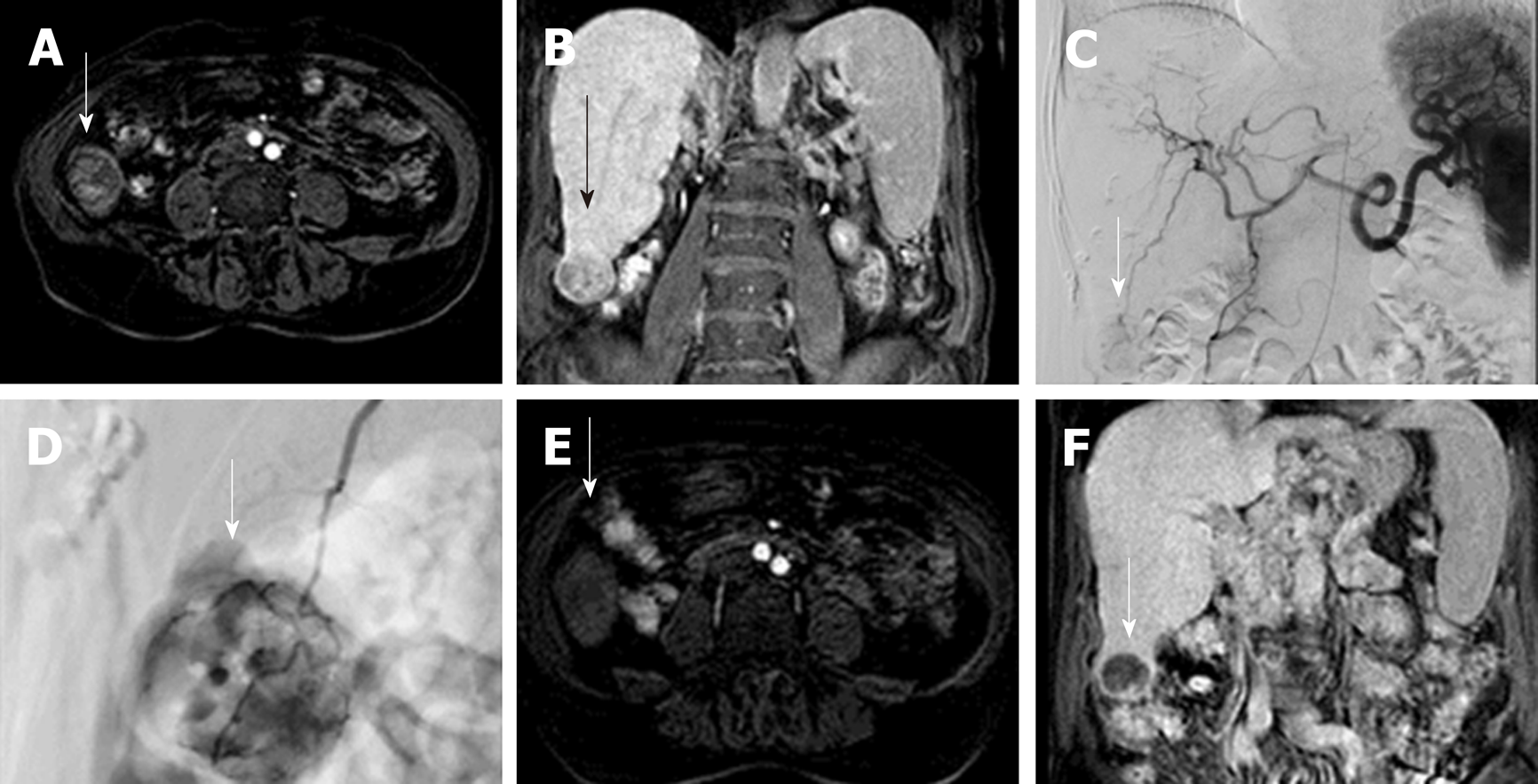
SIRT or also known as trans arterial radio-embolization (TARE) is a well-recognised loco-regional treatment modality used in patients with intermediate-stage or advanced stages of HCC (BLCL – B/C) in patients who are not eligible or cannot tolerate TACE/Sorafenib respectively[72,73]. Similar to TACE, delivery of treatment relies upon the hepatic arterial predominant blood supply of HCCs (80%) thereby reducing its effect on normal hepatic parenchyma which derives most (75%) of its supply from the portal vein[74]. In contradistinction to TACE which uses a combination of chemotherapy and ischaemia, SIRT has a minor effect from microembolisation and principally acts by irradiation from Yttrium-90(Y-90) bearing microspheres though other radioactive substances such as 131-iodine labelled lipiodol[44] may be considered. Emission of a beta particle (maximum energy 2.27 MeV; mean energy: 0.94 MeV) with decay of 90Y to 90Zr (Zirconium) is able to deliver targeted radiation to the lesion limiting radiation exposure to normal parenchyma while reducing the risk of radiation induced liver disease (up to 50% of patients with 40Gy delivered)[74]. High energy beta radiation triggers DNA double strand breaks resulting in tumour cell damage. Pre-procedure planning requires a separate angiographic procedure delivering 99mTc macroaggregated albumin (MAA) at the most ideal arterial position to target the tumour (Figure 3), followed by single photon emission computer tomography (SPECT) to detect extra-hepatic uptake and assess lung shunt fraction (proportion deposited in lung potentially causing radiation pneumonitis). Dosage of Y-90 to be delivered is calculated based on factors including type of microspheres being used, percentage involvement of tumour within the liver, lung shunt fraction and overall liver mass estimate from cross-sectional imaging with multiple formulas available but not completely evaluated at present[75]. Intra-arterial CT angiography (hybrid angiography-MDCT or cone-beam CT) allows volumetric assessment of tumour coverage by the chosen vessel and non-target vessels close to this should be selectively coil embolised to prevent extra-hepatic passage of Y-90. Careful administration of the Y-90 is carried out in conjunction with a physicist to reduce exposure to personnel.
Of both available prospective RCT results comparing SIRT and TACE amongst intermediate stage patients, PREMIERE (n = 43) and SIRTACE (n = 28), the former demonstrated significantly longer time to progression for SIRT (14.5 mo vs 6.4 mo, P = 0.0019) and no significant difference in overall survival (23.8 mo vs 17.7 mo, P = 0.9772) and the latter (pilot) study, by Kollig et al, demonstrated similar efficacy and health-related quality of life (HRQoL)[76,77]. All other studies being retrospective, a meta-analysis by Lobo et al[78] demonstrated comparable overall survival and complication rates with one study by Soydal et al[79] demonstrating a survival advantage for SIRT (39 mo vs 31 mo, P = 0.014). Kollig et al[77] also described a similar profile of safety between TACE and SIRT. Y-90 has also been demonstrated to have a role in early stage cancers as a bridge to liver transplantation and in order to downstage tumours from United network for organ sharing stage T3 to T2 (58% vs 31%, P = 0.023)[80,81].
Sorafenib remains the mainstay of treatment in advanced stage HCC (BCLC – C). Results from the SIRveNIB comparing SIRT with Sorafenib demonstrated no statistically significant differences in overall survival however progression free survival and time to progression in patients treated with SIRT vs Sorafenib with a similar trend demonstrated in the SARAH trial[82,83]. Complication rates of up to 4.9% and a mortality rate of 1.5% were reported in one multi-centre Australian study[84]. The most common complications were post-embolisation syndrome (0-70%) and Radiation-induced liver disease (0-31%). Other complications include biliary system damage and pneumonitis. Given both the SIRveNIB and SARAH trials were not able to statistically demonstrate superiority and the studies were not aimed at simply demonstrating non-inferiority, the status quo of Sorafenib as first line treatment for advanced stage HCC remains and further study is suggested in specific patient cohorts where SIRT may prove useful such as those with tumoral portal vein thrombosis. Further large-scale trials are required prior to conclusive guidelines regarding the use of SIRT in advanced HCC.
The combined treatment of HCC lesions implies the utilization of both RFA and TACE; this approach is used in early and intermediate stage (BCLC-A or -B) patients with large (> 3 cm) unresectable HCC nodules. Combined treatments were mostly investigated in retrospective studies, showing, however, better results when compared to RFA or TACE alone, both in terms of complete response and disease-free survival rates, as well as being less time and cost-consuming than performing the two treatments alone[85].
In fact, even if RFA provides excellent results in terms of local disease control and represents a curative treatment for HCC lesions up to 3 cm, it is ineffective in HCCs larger than 3 cm in size, with low rates of complete response, and high rates of local recurrence even after an initial complete response, as showed by Peng et al. in a prospective randomized trial, which compared combined treatment versus RFA alone in HCC up to 7 cm: The combined treatment group had significantly better overall survival and recurrence-free survival rates than the RFA-alone group, with a 1-, 3- and 4-year overall survival of 92.6%, 66.6% and 61.8% vs 85.3%, 59% and 45% respectively, and a recurrence-free survival of 79.4%, 60.6% and 54.8% versus 66.7%, 44.2% and 38.9% respectively[86].
On the other hand, TACE is considered a palliative treatment, showing decreased effectiveness with increasing size of the target HCC lesion, with only a few treated HCCs obtaining a stable complete response. High rates of local recurrence, generally being due to an incomplete embolization of the target lesion or due to tumoral neoangiogenesis[33].
Many authors have demonstrated the efficacy of the combined treatment in achieving complete tumour necrosis and increasing patient’s survival rates, especially in HCC lesions larger than 3 cm[85].
RFA and TACE can be combined in different but complimentary and synergistic ways; however, it is not clear which is the best combination and the optimal time interval between TACE and RFA to enhance the synergic effect and balance local therapeutic efficacy, with preservation of safety and liver function.
In particular, performing RFA first allows use of the sublethal heating area surrounding the central post-ablative necrosis, where the residual neoplastic cells have increased vascular permeability and blood flow. This in turn grants better delivery of the chemotherapeutic drug and improves efficacy due to a lower cellular resistance, as well as a better treatment of satellite nodules[87,88].
On the flipside, performing TACE first reduces the heat-sink effect secondary to the blood flow in the adjacent vessels, amplifying the RFA treatment, even though there could be a greater degradation of the chemotherapic drug when exposed to the high temperatures of the RFA. In addition, performing TACE first could lead to reduced ultrasound visibility of the target lesion, impairing the correct RFA electrode positioning; this issue has been partly overcome with the introduction of cone-beam CT (CBCT), which allows an accurate RFA electrode positioning using multiplanar and three-dimensional reconstructions[89,90].
Nonetheless, the individual steps of RFA and TACE, of combined treatment can be performed in a single session or with a wide time interval (ranging from 1 to 30 d)[86,91-93].
Various meta-analysis have shown that the combined treatment determines a significant increase of patient’s 1-, 3-, and 5-years overall survival rates when compared to RFA alone (P = 0.0004, 0.0002 and 0.0001 respectively), in particular when dealing with HCC lesions larger than 3 cm, as shown by the meta-analysis of randomized controlled trials performed by Lu et al., whereas there was no difference in terms of overall survival rates in HCCs smaller than 3 cm[94]. Moreover, even if there are ambiguous results, when compared to surgical resection, combined treatment seems to grant the same overall survival, even though surgical resection has better disease-free survival rates, in particular in lesions larger than 3 cm[95,96].
In recent years, the introduction of a new ablative technique represented by MWA, which overcomes the RFA limitations and can produce greater necrosis volumes, has also expanded the possibilities combined treatments; MWA plus TACE has been demonstrated to have good complete response rates in HCC lesions up to 5 cm, with a better efficacy when compared to RFA plus TACE, even if there are only a few studies comparing these two kinds of combined treatment. For example, Sheta et al., showed 1-month recurrence rates of 0% and 5% for MWA+TACE and RFA+TACE respectively, with similar complication rates[97-103].
One other great advantage of the combined treatment is the possibility to overcome the classical contraindications of the ablative treatment; in particular, when dealing with complex lesions, such as nodules located in unfavourable positions and so with a greater risk of complications, as well as in “complex” patients (those with a high risk of bleeding due to their cirrhosis which leads to a low platelet count), performing TACE after the ablative treatment allows prompt treatment of eventual post-ablative bleedings[88,102,103]. Additionally, performing TACE after RFA allows, treatment of those not-so-rare hypovascular HCC lesions, thanks to the vasodilator and hyperaemic effects of thermal ablation.
When considering combined therapies, in HCC lesions larger than 3 cm, the recently-introduced treatment with RFA plus intravenous systemic lyso-thermosensitive liposomal doxorubicin (LTLD) is worth a mention: The liposomes contains doxorubicin, and the RFA-induced target heating, when performed for more than 45 min, determines a high target drug concentration, almost 25 times greater than that of free doxorubicin[104,105].
A final, special mention is deserved for the combination of TACE and systemic chemotherapy with Sorafenib: The TACE-induced ischaemia determines the production of neoplastic angiogenic growth-factors that can be promptly blocked by the anti-angiogenic action of Sorafenib, with a good tolerability for the patients[106]. Most trials, however, failed to show a significant advantage of combined therapy versus TACE alone in terms of overall survival and time to progression[107,108]. On the other hand, the TACTICS trial showed a very favorable result of its primary end-point (progression-free survival rates) in the TACE+Sorafenib group versus TACE alone[109]. The reason of this different trend in comparison to the other trials can be identified both in the different primary end-point (Time-To-Progression (TTP) for SPACE, PFS for TACTICS), and in the different definition of Time-To-UnTACEable-Progression (TTUP, the time until TACE is no more effective or feasible): Even if both the SPACE and TACTICS trial considered vascular invasion and extra-hepatic lesions as a sign of unTACEable progression, TACTICS trial did not considered the development of a new liver lesion as tumour progression, whereas the SPACE trial added Child-Pugh B, persistent ascites and low platelet count as other criteria, limiting the treatment possibilities and leading to a precocious stop in sorafenib administration; moreover, in TACTICS trial, TACE was administered “on-demand” at the growth of the viable lesions, whereas, in SPACE trial, TACE sessions were scheduled, leading to less treatments[106,109].
Alternatively to Sorafenib, Kudo et al. investigated the efficacy of combination therapy between doxorubicin-TACE and brivanib, an inhibitor of vascular-endothelial and fibroblast growth factor given as an adjuvant for TACE; the trial did not show improvements in terms of OS between TACE plus brivanib and TACE plus placebo[110].
The optimal treatment algorithm for the management of HCC is still under meticulous investigation, as survival and recurrence rates are still far from satisfactory, and many unresolved issues remain to be determined. Surgery and liver transplantation have for years provided the best results. However, recent advances in minimal invasive locoregional treatments are continuously gaining ground not only in the management of non-operable HCC but also in the curative treatment of small T1 lesions. This is attributed to the fact that surgical options are often not indicated due to the advanced stage of underlying cirrhosis, or other severe comorbidities. Notably, in patients with HCC the prevalence of cirrhosis ranges between 85%-95%. However, despite the AASLD suggestion for surgical resection over RFA for T1 or T2 lesions in patients with Child- Pugh class A cirrhosis, the effectiveness of other ablative techniques, such as MWV or stereotactic body radiation as well as their combination with TACE and SIRT should also be evaluated compared to surgery[2]. Recurrence of HCC, especially in cases in which the ablated treatment margin was deemed sufficient (1 cm), could be attributed to non-visible microscopic satellite nodules. Histopathology studies following curative hepatectomy for lesions measuring from 2.5 to 5 cm, have demonstrated the presence of non-detectable microsatellite disease (portal vein invasion or intrahepatic metastasis) in 46% of the patients with a mean distance from the primary tumor of 9 mm (range 8 to 30 mm). The authors believe that more potent ablative technologies achieving treatment margins beyond 1cm as well as the combination of ablative/embolization therapies, in lesions measuring over 2.5 cm, would improve overall survival and recurrence rates due to the possibility to expand the necrotic zone to also include non-identified satellite lesions. Interestingly, according to a recent network metanalysis of RCTs, comparing available percutaneous locoregional treatments combined with local ablative or adjuvant systemic treatments for non-operable HCC, TACE combined with external radiotherapy or local ablation significantly improve patient survival and tumor response compared with embolization therapies alone, indicating the utility of the synergic effect provided by different locoregional treatments. However, the quality of evidence remains low to moderate and future RCTs to provide further level A evidence are required. The combination of locoregional modalities in the same patient in order to improve both quality of life and overall survival should also be assessed. Future studies should focus on the effect of the sequential alternation of treatments such as ablation, TACE and SIRT, based on the existing staging of the disease and the realistic therapeutic target.
Another major issue remains the lack of high-quality evidence to verify a significant survival improvement of chemo-embolization over bland embolization, or the superiority of other TACE techniques versus conventional TACE for intermediate stage HCC. Therefore, carefully designed multicenter RCTs comparing various embolization options are still awaited.
The authors believe that a major breakthrough in the management of HCC treatment would be the genetic characterization of HCC in every-day clinical practice in order to enable a personalized treatment plan. Today, non-invasive, liquid biopsy by sequencing cell-free DNA in plasma is currently under investigation and aims in the identification of driver mutations and tumor heterogeneity as to enable targeted HCC therapy. Published studies demonstrated variability in the efficacy of targeted agents in different populations and high inter-patient heterogeneity attributed to genetic mutations[111]. Personalized medicine could contribute in patient selection and individualized decision-making could optimize the outcomes of locoregional treatments.
To summarize, future research direction should focus on the combination of loco regional therapies. High quality data from multi-center RCTs are required to investigate the possibility of improving the overall survival in unresectable HCC by applying the available ablative and embolization techniques. Crucial issues regarding the combination of minimal invasive therapies that remain to be determined by large-scale trials, include the choice between bland TAE, particle-mediated TACE, or conventional TACE, the choice to embolize prior or after ablation, as well as the optimal timing of embolization (same session? after 2 wk, etc.). Moreover, the authors strongly believe that the investigation of aggressive sequential alternation of various locoregional therapies in the ambit of personalized patient selection will provide evidence that could modify the existing therapeutic protocols and improve both quality of life and survival outcomes. Finally, radioembolization is a very promising therapy and the initial failure to improve survival in patients with intermediate or advanced HCC should not discourage investigators. Well- designed trials with better patient selection would certainly define its role in HCC treatment.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding SZ, Iliescu L, Nah YW, Niu ZS, Piñero F, Sandhu DS S-Editor: Gong ZM L-Editor: A E-Editor: Zhang YL
| 1. | Wong MC, Jiang JY, Goggins WB, Liang M, Fang Y, Fung FD, Leung C, Wang HH, Wong GL, Wong VW, Chan HL. International incidence and mortality trends of liver cancer: a global profile. Sci Rep. 2017;7:45846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 2. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3017] [Article Influence: 431.0] [Reference Citation Analysis (3)] |
| 3. | Blonski W, Kotlyar DS, Forde KA. Non-viral causes of hepatocellular carcinoma. World J Gastroenterol. 2010;16:3603-3615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1791] [Article Influence: 85.3] [Reference Citation Analysis (2)] |
| 5. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1275] [Article Influence: 141.7] [Reference Citation Analysis (2)] |
| 6. | Delicque J, Boulin M, Guiu B, Pelage JP, Escal L, Schembri V, Assenat E, Fohlen A. Interventional oncology for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2016;40:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Katsanos K, Kitrou P, Spiliopoulos S, Maroulis I, Petsas T, Karnabatidis D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0184597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Becker G, Soezgen T, Olschewski M, Laubenberger J, Blum HE, Allgaier HP. Combined TACE and PEI for palliative treatment of unresectable hepatocellular carcinoma. World J Gastroenterol. 2005;11:6104-6109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Wang YB, Chen MH, Yan K, Yang W, Dai Y, Yin SS. Quality of life after radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: comparison with transcatheter arterial chemoembolization alone. Qual Life Res. 2007;16:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Pitton MB, Kloeckner R, Ruckes C, Wirth GM, Eichhorn W, Wörns MA, Weinmann A, Schreckenberger M, Galle PR, Otto G, Dueber C. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2015;38:352-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2871] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 12. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4517] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 13. | Chuang VP, Wallace S, Soo CS, Charnsangavej C, Bowers T. Therapeutic Ivalon embolization of hepatic tumors. AJR Am J Roentgenol. 1982;138:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Carr BI, Zajko A, Bron K, Orons P, Sammon J, Baron R. Phase II study of Spherex (degradable starch microspheres) injected into the hepatic artery in conjunction with doxorubicin and cisplatin in the treatment of advanced-stage hepatocellular carcinoma: interim analysis. Semin Oncol. 1997;24:S6-97-S6-99. [PubMed] |
| 15. | Bruix J, Castells A, Montanyà X, Calvet X, Brú C, Ayuso C, Jover L, García L, Vilana R, Boix L. Phase II study of transarterial embolization in European patients with hepatocellular carcinoma: need for controlled trials. Hepatology. 1994;20:643-650. [PubMed] |
| 16. | Gunji T, Kawauchi N, Ohnishi S, Ishikawa T, Nakagama H, Kaneko T, Moriyama T, Matsuhashi N, Yazaki Y, Imawari M. Treatment of hepatocellular carcinoma associated with advanced cirrhosis by transcatheter arterial chemoembolization using autologous blood clot: a preliminary report. Hepatology. 1992;15:252-257. [PubMed] |
| 17. | Dufour JF, Bargellini I, De Maria N, De Simone P, Goulis I, Marinho RT. Intermediate hepatocellular carcinoma: current treatments and future perspectives. Ann Oncol. 2013;24 Suppl 2:ii24-ii29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Schmidt S, Follmann M, Malek N, Manns MP, Greten TF. Critical appraisal of clinical practice guidelines for diagnosis and treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1779-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Cabibbo G, Latteri F, Antonucci M, Craxì A. Multimodal approaches to the treatment of hepatocellular carcinoma. Nat Clin Pract Gastroenterol Hepatol. 2009;6:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179-S188. [PubMed] |
| 21. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2270] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 22. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 23. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 24. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 25. | Brown DB, Cardella JF, Sacks D, Goldberg SN, Gervais DA, Rajan DK, Vedantham S, Miller DL, Brountzos EN, Grassi CJ, Towbin RB; SIR Standards of Practice Committee, Angle JF, Balter S, Clark TW, Cole PE, Drescher P, Freeman NJ, Georgia JD, Haskal Z, Hovsepian DM, Kilnani NM, Kundu S, Malloy PC, Martin LG, McGraw JK, Meranze SG, Meyers PM, Millward SF, Murphy K, Neithamer CD Jr, Omary RA, Patel NH, Roberts AC, Schwartzberg MS, Siskin GP, Smouse HR, Swan TL, Thorpe PE, Vesely TM, Wagner LK, Wiechmann BN, Bakal CW, Lewis CA, Nemcek AA Jr, Rholl KS. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2009;20:S219-S226, S226.e1-S226.10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Liu B, Huang JW, Li Y, Hu BS, He X, Zhao W, Zheng YB, Lu LG. Single-Agent versus Combination Doxorubicin-Based Transarterial Chemoembolization in the Treatment of Hepatocellular Carcinoma: A Single-Blind, Randomized, Phase II Trial. Oncology. 2015;89:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 518] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 28. | Duran R, Chapiro J, Schernthaner RE, Geschwind JF. Systematic review of catheter-based intra-arterial therapies in hepatocellular carcinoma: state of the art and future directions. Br J Radiol. 2015;88:20140564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Lewis AL, Holden RR. DC Bead embolic drug-eluting bead: clinical application in the locoregional treatment of tumours. Expert Opin Drug Deliv. 2011;8:153-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, Delis S, Gouliamos A, Kelekis D. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 32. | Woo HY, Heo J. Transarterial chemoembolization using drug eluting beads for the treatment of hepatocellular carcinoma: Now and future. Clin Mol Hepatol. 2015;21:344-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, Stefaniotou A, Marinis A, Kelekis A, Alexopoulou E, Chatziioannou A, Chatzimichael K, Dourakis S, Kelekis N, Rizos S, Kelekis D. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012;35:1119-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 35. | Spreafico C, Cascella T, Facciorusso A, Sposito C, Rodolfo L, Morosi C, Civelli EM, Vaiani M, Bhoori S, Pellegrinelli A, Marchianò A, Mazzaferro V. Transarterial chemoembolization for hepatocellular carcinoma with a new generation of beads: clinical-radiological outcomes and safety profile. Cardiovasc Intervent Radiol. 2015;38:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y, Avajon Y, Gruenberger T, Pomoni M, Langenberger H, Schuchmann M, Dumortier J, Mueller C, Chevallier P, Lencioni R; PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 37. | Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM, Lee HG, Yoon SK. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 38. | Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D, Cucchetti A, Bolondi L, Trevisani F, PRECISION ITALIA STUDY GROUP. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 483] [Article Influence: 43.9] [Reference Citation Analysis (1)] |
| 39. | Levy EB, Krishnasamy VP, Lewis AL, Willis S, Macfarlane C, Anderson V, van der Bom IM, Radaelli A, Dreher MR, Sharma KV, Negussie A, Mikhail AS, Geschwind JF, Wood BJ. First Human Experience with Directly Image-able Iodinated Embolization Microbeads. Cardiovasc Intervent Radiol. 2016;39:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Iezzi R, Pompili M, Rinninella E, Annicchiarico E, Garcovich M, Cerrito L, Ponziani F, De Gaetano A, Siciliano M, Basso M, Zocco MA, Rapaccini G, Posa A, Carchesio F, Biolato M, Giuliante F, Gasbarrini A, Manfredi R; HepatoCatt Study Group. TACE with degradable starch microspheres (DSM-TACE) as second-line treatment in HCC patients dismissing or ineligible for sorafenib. Eur Radiol. 2019;29:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Schicho A, Pereira PL, Haimerl M, Niessen C, Michalik K, Beyer LP, Stroszczynski C, Wiggermann P. Transarterial chemoembolization (TACE) with degradable starch microspheres (DSM) in hepatocellular carcinoma (HCC): multi-center results on safety and efficacy. Oncotarget. 2017;8:72613-72620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10258] [Article Influence: 603.4] [Reference Citation Analysis (2)] |
| 43. | Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: From diagnosis to treatment. Surg Oncol. 2016;25:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 333] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 44. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6038] [Article Influence: 862.6] [Reference Citation Analysis (3)] |
| 45. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4092] [Article Influence: 584.6] [Reference Citation Analysis (6)] |
| 46. | Lee DH, Lee JM. Recent Advances in the Image-Guided Tumor Ablation of Liver Malignancies: Radiofrequency Ablation with Multiple Electrodes, Real-Time Multimodality Fusion Imaging, and New Energy Sources. Korean J Radiol. 2018;19:545-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgrò G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol. 2010;52:380-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 48. | Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 49. | Ishii H, Okada S, Nose H, Okusaka T, Yoshimori M, Takayama T, Kosuge T, Yamasaki S, Sakamoto M, Hirohashi S. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1996;77:1792-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 50. | Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics. 2014;34:1344-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 51. | Krokidis ME, Kitrou P, Spiliopoulos S, Karnabatidis D, Katsanos K. Image-guided minimally invasive treatment for small renal cell carcinoma. Insights Imaging. 2018;9:385-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Kim JE, Kim YS, Rhim H, Lim HK, Lee MW, Choi D, Shin SW, Cho SK. Outcomes of patients with hepatocellular carcinoma referred for percutaneous radiofrequency ablation at a tertiary center: analysis focused on the feasibility with the use of ultrasonography guidance. Eur J Radiol. 2011;79:e80-e84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Kim YS, Lim HK, Rhim H, Lee MW. Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28:897-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Crocetti L, Bargellini I, Cioni R. Loco-regional treatment of HCC: current status. Clin Radiol. 2017;72:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 55. | Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2013;CD003046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 56. | Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, Pinna AD. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 57. | Xu Q, Kobayashi S, Ye X, Meng X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. 2014;4:7252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 58. | Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency Ablation versus Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology. 2018;287:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 59. | Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, Brunello F, Pinna AD, Giorgio A, Giulini SM, De Sio I, Torzilli G, Fornari F, Capussotti L, Guglielmi A, Piscaglia F, Aldrighetti L, Caturelli E, Calise F, Nuzzo G, Rapaccini GL, Giuliante F. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 60. | Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, Yuen J, Poon RTP, Fan ST, Lo CM. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 61. | Dou JP, Liang P, Yu J. Microwave ablation for liver tumors. Abdom Radiol (NY). 2016;41:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: An updated review. World J Gastrointest Pharmacol Ther. 2016;7:477-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 63. | Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 64. | Chinnaratha MA, Chuang MY, Fraser RJ, Woodman RJ, Wigg AJ. Percutaneous thermal ablation for primary hepatocellular carcinoma: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 65. | Lahat E, Eshkenazy R, Zendel A, Zakai BB, Maor M, Dreznik Y, Ariche A. Complications after percutaneous ablation of liver tumors: a systematic review. Hepatobiliary Surg Nutr. 2014;3:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 66. | Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, Du Z. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol. 2013;68:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 67. | Dunne RM, Shyn PB, Sung JC, Tatli S, Morrison PR, Catalano PJ, Silverman SG. Percutaneous treatment of hepatocellular carcinoma in patients with cirrhosis: a comparison of the safety of cryoablation and radiofrequency ablation. Eur J Radiol. 2014;83:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Rong G, Bai W, Dong Z, Wang C, Lu Y, Zeng Z, Qu J, Lou M, Wang H, Gao X, Chang X, An L, Li H, Chen Y, Hu KQ, Yang Y. Long-term outcomes of percutaneous cryoablation for patients with hepatocellular carcinoma within Milan criteria. PLoS One. 2015;10:e0123065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Luo W, Zhang Y, He G, Yu M, Zheng M, Liu L, Zhou X. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017;15:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 70. | Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, Bai W, Dong Z, Lu Y, Zeng Z, Lou M, Wang H, Gao X, Chang X, An L, Qu J, Li J, Yang Y. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 71. | Zimmerman A, Grand D, Charpentier KP. Irreversible electroporation of hepatocellular carcinoma: patient selection and perspectives. J Hepatocell Carcinoma. 2017;4:49-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Wang EA, Stein JP, Bellavia RJ, Broadwell SR. Treatment options for unresectable HCC with a focus on SIRT with Yttrium-90 resin microspheres. Int J Clin Pract. 2017;71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Venkatanarasimha N, Gogna A, Tong KTA, Damodharan K, Chow PKH, Lo RHG, Chandramohan S. Radioembolisation of hepatocellular carcinoma: a primer. Clin Radiol. 2017;72:1002-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Hsieh TC, Wu YC, Sun SS, Yen KY, Kao CH. Treating hepatocellular carcinoma with 90Y-bearing microspheres: a review. Biomedicine (Taipei). 2016;6:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Mosconi C, Cappelli A, Pettinato C, Golfieri R. Radioembolization with Yttrium-90 microspheres in hepatocellular carcinoma: Role and perspectives. World J Hepatol. 2015;7:738-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Gordon A, Lewandowski R, Hickey R, Kallini J, Gabr A, Sato K, Desai K, Thornburg B, Gates V, Ganger D, Kulik L, Salem R. Prospective randomized phase 2 study of chemoembolization versus radioembolization in hepatocellular carcinoma: results from the PREMIERE trial. JVIR. 2016;27:S61-S62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Kolligs FT, Bilbao JI, Jakobs T, Iñarrairaegui M, Nagel JM, Rodriguez M, Haug A, D'Avola D, op den Winkel M, Martinez-Cuesta A, Trumm C, Benito A, Tatsch K, Zech CJ, Hoffmann RT, Sangro B. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 78. | Lobo L, Yakoub D, Picado O, Ripat C, Pendola F, Sharma R, ElTawil R, Kwon D, Venkat S, Portelance L, Yechieli R. Unresectable Hepatocellular Carcinoma: Radioembolization Versus Chemoembolization: A Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol. 2016;39:1580-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 79. | Soydal C, Arslan MF, Kucuk ON, Idilman R, Bilgic S. Comparison of survival, safety, and efficacy after transarterial chemoembolization and radioembolization of Barcelona Clinic Liver Cancer stage B-C hepatocellular cancer patients. Nucl Med Commun. 2016;37:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Riaz A, Lewandowski RJ, Kulik LM, Mulcahy MF, Sato KT, Ryu RK, Omary RA, Salem R. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20:1121-30; quiz 1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 81. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 82. | Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, Choo SP, Cheow PC, Chotipanich C, Lim K, Lesmana LA, Manuaba TW, Yoong BK, Raj A, Law CS, Cua IHY, Lobo RR, Teh CSC, Kim YH, Jong YW, Han HS, Bae SH, Yoon HK, Lee RC, Hung CF, Peng CY, Liang PC, Bartlett A, Kok KYY, Thng CH, Low AS, Goh ASW, Tay KH, Lo RHG, Goh BKP, Ng DCE, Lekurwale G, Liew WM, Gebski V, Mak KSW, Soo KC; Asia-Pacific Hepatocellular Carcinoma Trials Group. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol. 2018;36:1913-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 473] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 83. | Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, Sibert A, Bouattour M, Lebtahi R, Allaham W, Barraud H, Laurent V, Mathias E, Bronowicki JP, Tasu JP, Perdrisot R, Silvain C, Gerolami R, Mundler O, Seitz JF, Vidal V, Aubé C, Oberti F, Couturier O, Brenot-Rossi I, Raoul JL, Sarran A, Costentin C, Itti E, Luciani A, Adam R, Lewin M, Samuel D, Ronot M, Dinut A, Castera L, Chatellier G; SARAH Trial Group. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 594] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 84. | Kuo JC, Tazbirkova A, Allen R, Kosmider S, Gibbs P, Yip D. Serious hepatic complications of selective internal radiation therapy with yttrium-90 microsphere radioembolization for unresectable liver tumors. Asia Pac J Clin Oncol. 2014;10:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Kim JW, Kim JH, Won HJ, Shin YM, Yoon HK, Sung KB, Kim PN. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol. 2012;81:e189-e193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 86. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 87. | Kamal A, Barakat E, ElMoez A, Mawad M, El-Fouly N, Shaker M. Combined radiofrequency and chemoembolization vs. chemoembolization in management of hepatocellular carcinoma. Hepatoma Res. 2015;1:19-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Iezzi R, Pompili M, Posa A, Coppola G, Gasbarrini A, Bonomo L. Combined locoregional treatment of patients with hepatocellular carcinoma: State of the art. World J Gastroenterol. 2016;22:1935-1942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 89. | Solbiati L, Tonolini M, Cova L. Monitoring RF ablation. Eur Radiol. 2004;14 Suppl 8:P34-P42. [PubMed] |
| 90. | Wang ZJ, Wang MQ, Duan F, Song P, Liu FY, Wang Y, Yan JY, Li K, Yuan K. Clinical application of transcatheter arterial chemoembolization combined with synchronous C-arm cone-beam CT guided radiofrequency ablation in treatment of large hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:1649-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Kang SG, Yoon CJ, Jeong SH, Kim JW, Lee SH, Lee KH, Kim YH. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol. 2009;20:1570-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 92. | Iezzi R, Pompili M, La Torre MF, Campanale MC, Montagna M, Saviano A, Cesario V, Siciliano M, Annicchiarico E, Agnes S, Giuliante F, Grieco A, Rapaccini GL, De Gaetano AM, Gasbarrini A, Bonomo L, HepatoCATT Study Group for the Multidisciplinary Management of HCC. Radiofrequency ablation plus drug-eluting beads transcatheter arterial chemoembolization for the treatment of single large hepatocellular carcinoma. Dig Liver Dis. 2015;47:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Yuan H, Liu F, Li X, Guan Y, Wang M. Transcatheter arterial chemoembolization combined with simultaneous DynaCT-guided radiofrequency ablation in the treatment of solitary large hepatocellular carcinoma. Radiol Med. 2019;124:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 95. | Saviano A, Iezzi R, Giuliante F, Salvatore L, Mele C, Posa A, Ardito F, De Gaetano AM, Pompili M; HepatoCATT Study Group. Liver Resection versus Radiofrequency Ablation plus Transcatheter Arterial Chemoembolization in Cirrhotic Patients with Solitary Large Hepatocellular Carcinoma. J Vasc Interv Radiol. 2017;28:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Takuma Y, Takabatake H, Morimoto Y, Toshikuni N, Kayahara T, Makino Y, Yamamoto H. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology. 2013;269:927-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 97. | Sheta E, El-Kalla F, El-Gharib M, Kobtan A, Elhendawy M, Abd-Elsalam S, Mansour L, Amer I. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study. Eur J Gastroenterol Hepatol. 2016;28:1198-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 98. | Liu C, Liang P, Liu F, Wang Y, Li X, Han Z, Liu C. MWA combined with TACE as a combined therapy for unresectable large-sized hepotocellular carcinoma. Int J Hyperthermia. 2011;27:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 99. | Xu LF, Sun HL, Chen YT, Ni JY, Chen D, Luo JH, Zhou JX, Hu RM, Tan QY. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol. 2013;28:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 100. | Ginsburg M, Zivin SP, Wroblewski K, Doshi T, Vasnani RJ, Van Ha TG. Comparison of combination therapies in the management of hepatocellular carcinoma: transarterial chemoembolization with radiofrequency ablation versus microwave ablation. J Vasc Interv Radiol. 2015;26:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 101. | Ni JY, Sun HL, Chen YT, Luo JH, Chen D, Jiang XY, Xu LF. Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol. 2014;20:17483-17490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 628] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 103. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J, EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 104. | Zamboni WC. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist. 2008;13:248-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 105. | Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 2000;60:6950-6957. [PubMed] |
| 106. | Chao Y, Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 107. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del Arbol LR, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 543] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 108. | Kudo M. Proposal of Primary Endpoints for TACE Combination Trials with Systemic Therapy: Lessons Learned from 5 Negative Trials and the Positive TACTICS Trial. Liver Cancer. 2018;7:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 109. | Kudo M, Ueshima K, Torimura T, Tanabe N, Ikeda M, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Isoda N, Yasui K, Kuzuya T, Okusaka T, Furuse J, Kokudo N, Okita K, Yoshimura K, Arai Y. Randomized, open label, multicenter, phase II trial of transcatheter arterial chemoembolization (TACE) therapy in combination with sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. J Clin Oncol. 2018;36:4017-4017. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 110. | Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, Yang J, Lu L, Tak WY, Yu X, Lee JH, Lin SM, Wu C, Tanwandee T, Shao G, Walters IB, Dela Cruz C, Poulart V, Wang JH. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology. 2014;60:1697-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 111. | Chan SL, Wong AM, Lee K, Wong N, Chan AK. Personalized therapy for hepatocellular carcinoma: Where are we now? Cancer Treat Rev. 2016;45:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |