Published online Aug 28, 2019. doi: 10.3748/wjg.v25.i32.4567
Peer-review started: March 29, 2019
First decision: June 10, 2019
Revised: July 30, 2019
Accepted: August 7, 2019
Article in press: August 7, 2019
Published online: August 28, 2019
Processing time: 152 Days and 17.1 Hours
The prevalence of obesity continues to rise, and along with it comes a multitude of health-related consequences. The healthcare community has consistently struggled with providing treatment options to obese patients, in part due to the reluctance of patients in pursuing the more effective (yet invasive) surgical approaches such as sleeve gastrectomy and Rou-en-Y gastric bypass. On the other hand, the less invasive approach such as lifestyle/behavioral interventions and pharmacotherapy (Orlistat, Phenteramine, Phentermine/Topiramate, Locaserin, Naltrexon/Buproprion, and Liraglutide) have very limited efficacy, especially in the morbidly obese patients. Despite our best efforts, the epidemic of obesity continues to rise and pose enormous costs on our healthcare system and society. Bariatric endoscopy is an evolving field generated to combat this epidemic through minimally invasive techniques. These procedures can be performed in an ambulatory setting, are potentially reversible, repeatable, and pose less complications than their invasive surgical counterparts. These modalities are designed to alter gut metabolism by means of space occupation, malabsorption, or restriction. In this review we will discuss different bariatric endoscopic options (such as intragastric balloons, endoscopic sleeve gastroplasty, endoscopic aspiration therapies and gastrointestinal bypass sleeves), their advantages and disadvantages, and suggest a new paradigm where providers may start incorporating this modality in their treatment approach for obese patients.
Core tip: The prevalence of obesity has risen to an alarming level. The associated morbidity and mortality of this epidemic affects the community and health related economics directly. Current options include lifestyle modifications, pharmacotherapy, and surgery; the latter being the most effective, however most invasive and prone to complications. Bariatric endoscopy, with methods including intragastric balloons, sleeve gastroplasty, and aspiration techniques, provides an effective and less invasive option for weight loss in obese patients.
- Citation: Glass J, Chaudhry A, Zeeshan MS, Ramzan Z. New Era: Endoscopic treatment options in obesity–a paradigm shift. World J Gastroenterol 2019; 25(32): 4567-4579
- URL: https://www.wjgnet.com/1007-9327/full/v25/i32/4567.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i32.4567
The prevalence of obesity has seen a drastic increase over the last thirty years, now approaching 35% in men and 40% in women[1]. Obesity has been associated with a multitude of adverse health outcomes including all cause cardiovascular mortality, diabetes, hyperlipidemia, and all-cause mortality[2]. Lifestyle modifications, pharmacological agents, and surgical options are all amongst the myriad treatments in obesity.
Lifestyle modifications, despite being the least expensive and less invasive, have been shown to be the least successful of the options. Intensive behavioral in-terventions (such as identifying barriers, self-monitoring of weight, peer support, etc.) with a combination of dietary changes and increased physical activity over multiple sessions can result in > 5% weight loss in adults with obesity. Hence, US Preventive Services Task Force recommends that clinicians offer or refer adults with a body mass index (BMI) of 30 or higher to intensive, multicomponent behavioral interventions (grade B recommendation)[3].
Pharmacological agents currently Food and Drug Administration (FDA) approved for weight loss include Orlistat, Phenteramine, Phentermine/Topiramate, Locaserin, Naltrexon/Buproprion, and Liraglutide[4]. These agents have been approved for patients with a BMI of ≥ 27 kg/m2 with one obesity related complication or patients with a BMI of ≥ 30 kg/m2. They have shown modest benefits for weight loss with an estimate weight loss of 3%-7% efficacy compared with placebo. At 12 to 18 mo, participants in pharmacotherapy-based weight loss trials (32 trials) had more weight loss compared with placebo groups [mean or least squares mean difference in weight change, -1.0 kg (-2.2 lb) to -5.8 kg (−12.8 lb)][5]. Participants also experienced a greater decrease in waist circumference and a greater likelihood of losing 5% of their initial weight compared with placebo groups. Three pharmacotherapy-based weight loss maintenance trials showed that participants receiving the intervention had better weight loss maintenance compared with placebo groups over 12 to 36 mo (mean difference, -0.6 to -3.5 kg)[6]. Although these options have shown promise, along with some intolerable side effects, their longstanding efficacy has not been particularly encouraging[7].
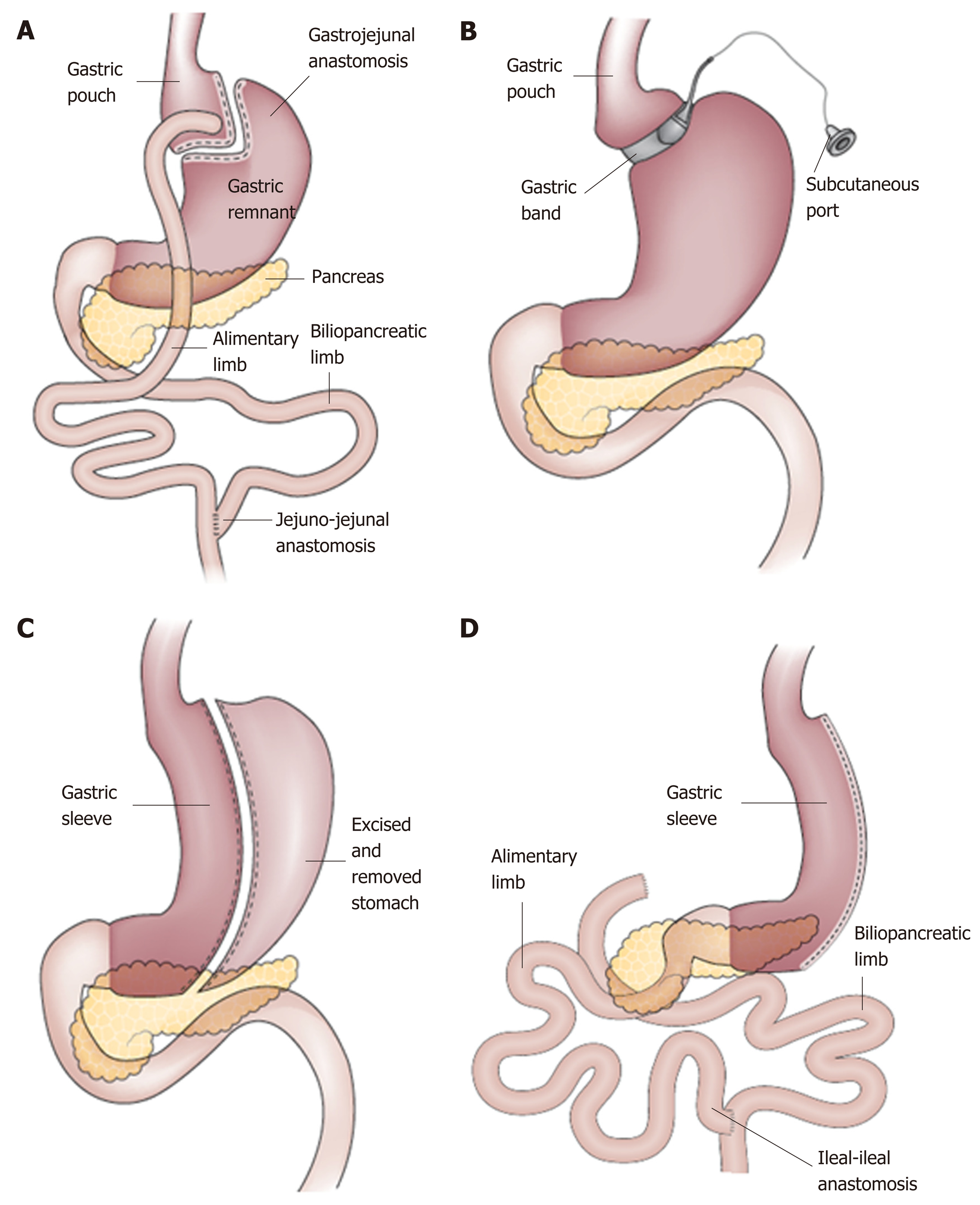
For patients with BMI ≥ 40 or BMI ≥ 35 with comorbidity, referral to an experienced bariatric surgeon should be considered. To date, bariatric surgery has shown the most dramatic results in treatment of obesity. Current literature has shown this treatment to be lasting and effective, with improvement of obesity related co-morbidity. Despite these advantages, bariatric surgery poses several disadvantages. As with all procedures, bariatric surgery comes with possible complications, the most common including anastomotic ulceration, anastomotic stenosis, gastro-gastric fistulas, surgical leaks, intestinal obstruction, and choledocholithiasis[8]. In addition, the process of undergoing bariatric surgery is complex, requiring multiple physician visits over a prolonged period of time. As a result, only < 1% of all eligible patients actually undergo bariatric surgery[9]. A detailed discussion of various surgical options, advantages and disadvantages, adverse effect profile, etc. is beyond the scope of this article. A brief overview has been summarized in Figure 1.
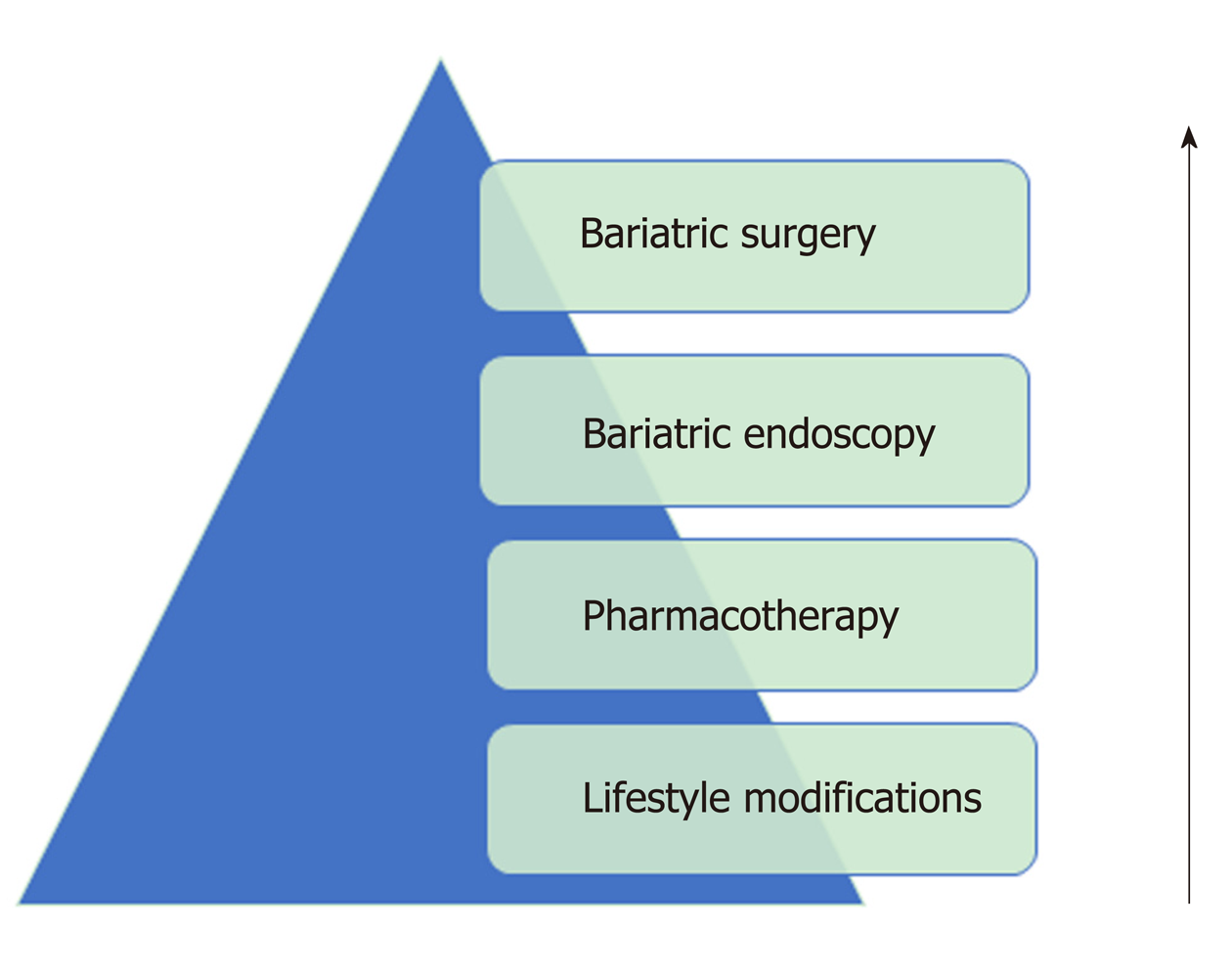
Given the limited efficacy of lifestyle/behavioral interventions and pharmacotherapy, as well as the barriers associated with bariatric surgery, new therapies must be discovered to handle the growing population of obese patients. In this review, we introduce bariatric endoscopy as a methodology and tool that physicians may start incorporating in their treatment algorithms for management of obesity (Figure 2).
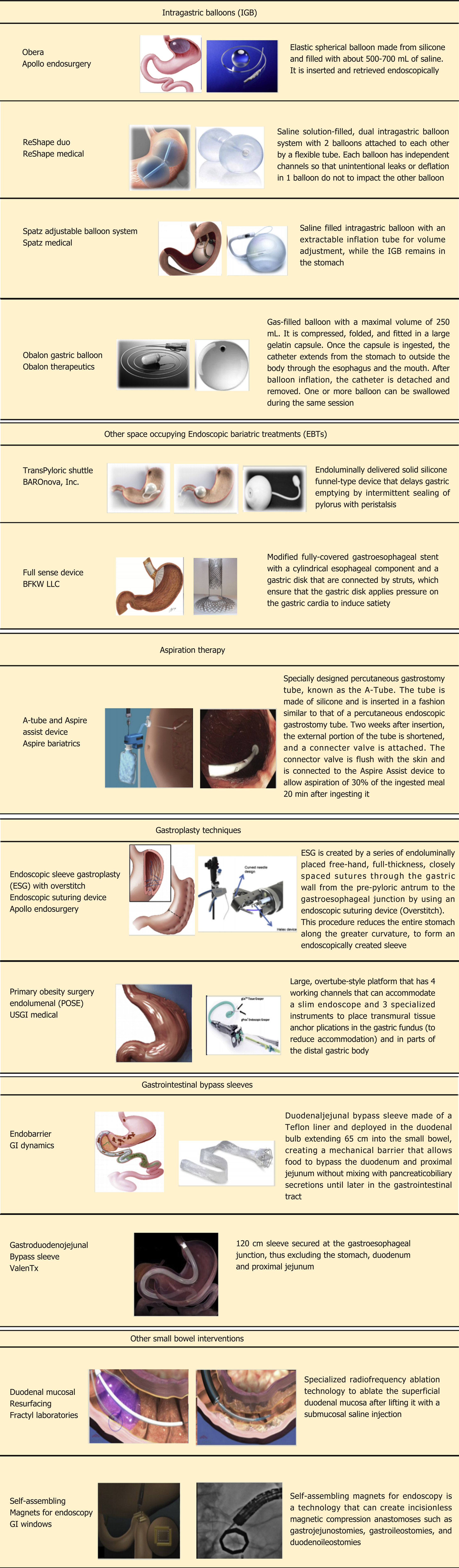
Bariatric endoscopy is an innovative technology designed to mimic weight loss surgery without the associated co-morbidities. There are several modalities used in endoscopic bariatrics, each with its own physiologic method behind weight loss. These include malabsorption techniques, use of space occupying devices, restrictive methods, and aspiration therapies. In this section we will discuss three forms of bariatric endoscopy including intragastric balloon (IGB) therapy, endoscopic sleeve gastroplasty (ESG), and aspiration techniques (AspireAssist). Further we will discuss other small bowel therapies currently in clinical trial. Figure 3 illustrates a summary of all FDA approved and non-FDA approved bariatric endoscopic modalities, whereas Table 1 summarizes an overview of the commonly used modalities in the world of bariatrics.
| Treatment options | Indication | Duration | Efficacy | Advantages | Disadvantages |
| Pharmacological methods | BMI of ≥ 27 kg/m2 with one obesity related complication or patients with a BMI of ≥ 30 kg/m2 | Varies depending on the agent | 2%-10% weight loss depending on the agent[33] | Mild to moderately effective | Adverse side effect profile dependent on the medication |
| Non-invasive | |||||
| Reusable | No evidence for long term effectiveness | ||||
| Intragastric Balloons | BMI between 30 and 40 kg/m2 | 6 mo | %TBWL 7%-15% | Reversible | High rates of nausea and vomiting |
| %EWL 30%-47% | Repeatable | ||||
| At 6 mo duration[34] | Can be used in combination of other modalities | Some reports of gastric perforation in rare cases | |||
| Aspiration therapy | BMI of 35-55 kg/m2 | Long term duration | %TBWL 14%-18% | Effective | Must see doctors at scheduled intervals |
| %EWL 37-54% | Minimal side effects | ||||
| From 6-24 mo[35] | Long duration of use | Abdominal pain, peristomal complications | |||
| Endoscopic Gastroplasty | BMI between 30 and 40 kg/m2 | Long term follow-up studies in progress | %TBWL 12%-19% | Adaptive new technology with a lot of promise | Requires expertise and technical skills |
| At 6-24 mo duration[35] | Minimally invasive | Perigastric fluid collections, extragastric bleeding | |||
| Lap Band | BMI of 35 kg/m2 with obesity related complications or BMI of 40 kg/m2 | Reversible | Mean percentage weight loss at 5 yr was 15.9 ± 12.4%[35] | Sustained weight loss at 5 yr | Need for explant up to 12% Vomiting, nausea, dysphagia, GERD |
| Improvements in hgb a1c, cholesterol | |||||
| Sleeve gastrectomy | BMI of 35 kg/m2 with obesity related complications or BMI of 40 kg/m2 | Permanent, but can be bridged to RYGB | 49%EWL at 5 yr[36] | Long term sustained weight loss | Significant adverse events |
| Cardiometabolic risk factor modifying; hyperlipidemia, diabetes | Overall morbidity rate of 19% at 5 yr | ||||
| RYGB | BMI of 35 kg/m2 with obesity related complications or BMI of 40 kg/m2 | Permanent | 57%EWL at 5 yr[36] | Long term sustained weight loss | Significant adverse events related to surgery |
| Cardiometabolic risk factor modifying; hyperlipidemia, diabetes | Overall morbidity rate of 26% at 5 yr |
IGBs were first introduced in the United States in 1985 as the Garren-Edwards gastric bubble which was made of polyurethane and filled with 200-220cc of air[10]. This balloon gave way for the three IGBs which are currently FDA approved for use. These include the Orbera IGB (Apollo Endosurgery, Austin, TX, United States), the ReShape Duo (ReShape Medical, San Clemente, CA, United States), and the most recent Obalon IGB (Obalon Therapeutics, Inc, Carlsbad, CA, United States). The balloons act as space-occupying device thereby inducing early satiety leading to weight reduction and altering gut neuroendocrinology[11]. Each balloon has been approved for a 6 mo duration for those with a BMI between 30 to 40 mg/kg2 who have failed lifestyle modifications[12]. The most common complication of the IGBs include nausea (29%) and pain (33.7%), which are usually self-limited. Other rare side effects include small bowel obstruction, perforation, and death at rates of 0.3%, 0.1%, and 0.08%, respectively[13].
The Orbera balloon is an elastic spherical balloon made of silicone. It is inserted in the stomach blindly via transoral advancement on a preloaded catheter. An endoscope is advanced to confirm correct placement, after which the balloon is injected with 450-700 mL of normal saline mixed with methylene blue. The methylene blue provides a marker for balloon malfunction. If the balloon ruptures, the methylene blue will be systemically absorbed and change the color of urine to blue, prompting the patient to seek medical attention. Pain and nausea are frequent side effects of Orbera IGB implantation, occurring in about 33.7% of subjects, with serious side effects being balloon migration and gastric perforation of 1.4% and 0.1%, respectively.
Orbera has shown efficacy in several trials. It has been used in other countries for many years before being approved by FDA for use in the United States in 2015. Based on a large meta-analysis of 17 studies, the percentage of excess weight loss (%EWL) with the Orbera IGB at 12 mo was 25.44% and total body weight loss (%TBWL) was 12.3% 13.16%, and 11.27% at 3, 6, and 12 mo after implantation, respectively[13,14]. In a recent multicenter, randomized, open-label clinical trial, 255 adults with a BMI of 30-40 kg/m2 were treated and outcomes were assessed up to 12 mo. Patients were randomized to IGB (Obera) plus lifestyle modifications (n = 125) vs lifestyle modification alone (control; n = 130). Balloons were removed at 6 mo and lifestyle intervention continued for both groups through 12 mo. At 6 mo, weight loss was -3.3% (-3.2 kg) in the lifestyle modification arm vs -10.2 % (-9.9 kg) in the balloon plus lifestyle intervention arm (P < 0.001); at 9 mo (3 mo post balloon removal), weight loss was -3.4% (-3.2 kg) vs -9.1% (-8.8 kg, P < 0.001); and at 12 mo, -3.1% (-2.9 kg) vs -7.6% (-7.4 kg, P < 0.001). The authors concluded that IGB was more successful than life style modifications alone in achieving short term weight loss at 9 mo (3 mo post balloon removal) and 12 mo (6 mo post balloon removal.) The study lacked the long term follow up needed to determine effectiveness on diabetes, hyperlipidemia, and cardiovascular outcomes[15]. IGBs have now been studied in conjunction with bariatric surgery, with a more recent study showing the Orbera balloon plus bariatric surgery [Roux-en-Y gastric bypass (RYGB)/Sleeve gastrectomy] to be more effective in the super obese (BMI > 50 kg/m2) than either alone[16].
The ReShape Duo (ReShape Medical, San Clemente, CA, United States) is another type of IGB with two balloons attached to each other by flexible tube. Each balloon requires about 450 mL of saline solution (mixed with methylene blue). This unique shape enables one balloon to continue working as a space occupying device even if the other balloon gets deflated spontaneously. Initial data on the efficacy of ReShape Duo balloon has shown promise. The REDUCE trial was the first large multicenter prospective study looking at efficacy and safety of the ReShape dual balloon. In this study, 326 participants with a BMI between 30-40 kg/m2 were randomized to endoscopic ReShape Duo balloon placement plus diet and exercise vs. sham endoscopy plus diet and exercise alone. Duo patients had significantly greater %EWL at 24 wk (25.1% intent-to-treat (ITT), 27.9% completed cases (CC, n = 167) compared with DIET patients (11.3%ITT, P = 0.004, 12.3% CC, n = 126). This significant weight loss noted with ReShape Duo balloon (i.e., %EWL noted with Duo balloon was double that of lifestyle modification) was coupled with very low adverse event (AE) profile (such as accommodative symptoms, balloon deflation, gastric ulceration)[17]. High incidence of gastric ulcers and erosions (39%) was initially observed at or near the gastric incisura during the study period but the frequency reduced to 10.3% with design modification.
The most recent data was published in 2018 through John’s Hopkins where they analyzed 202 adult patients who underwent ReShape Duo IGB insertion and they determined %TBWL and %EWL over a 12 mo period. Mean %TBWL at 1, 3, 6, 9 and 12 mo was 4.8%, 8.8%, 11.4%, 13.3% and 14.7%, respectively. Of the data available, 60.4% of patients achieved more than 10% TBWL and 55.4% had more than 25% EWL. Common AE included nausea, vomiting, and abdominal pain, with more severe AE being one small bowel obstruction. Secondary endpoints such as systolic and diastolic blood pressure, hemoglobin a1c, fasting blood sugar, and total cholesterol were found to be statistically significantly lower at balloon removal than at baseline[18].
Obalon (Obalon Therapeutics Inc, Carlsbad, CA, United States) is the first and only swallowable, FDA approved balloon system for weight loss. This is approved to facilitate weight loss in patients with a BMI between 30 and 40 kg/m2 in conjunction with diet and exercise. The Obalon balloon is packaged with a gelatin capsule. The patient swallows the capsule which is attached to a small catheter. Fluoroscopy is used to confirm intragastric location. The gelatin capsule dissolves allowing the balloon to enter the stomach. The catheter is used to inflate the balloon by using a gas filled canister, after which the catheter is detached and removed. The balloons should be removed after 6 mo.
The advantage of this mechanism is that the placement of the IGB endoscopically (with all procedural and anesthesia related risks) is not required; however, it still must be retrieved after a 6-mo time period. Up to three balloons may be placed at one time equivalent to 750 mL of gastric space which allows modifiability[19]. The most recent data showing efficacy of this system was published in 2018 where the authors conducted a double-blind, randomized sham controlled trial of Obalon balloon plus lifestyle therapy compared to lifestyle therapy alone for weight loss at 6 mo. The authors found that compared with lifestyle alone, the Obalon IGB resulted in twice the weight loss compared to controls with minimal AEs[20].
The IGB offers several advantages. One, it is a relatively simple procedure that could be adopted within the realm of general gastroenterology with minimal additional training needed. The procedure can be performed on an outpatient basis, thereby saving costs. It is minimally invasive with little risk as compared to the most common surgical bariatric procedures (RYGB and Sleeve gastrectomy). Further, studies are now revealing superior weight loss effect with combination of IGB and bariatric surgical procedures than either alone, suggesting that it can be used in combination with standard surgical approaches. Lastly, it can be easily reversed and repeated multiple times over. Although there is promise for IGB’s, there still remains a need for further evidence of safety, efficacy, and established guidelines that can be followed by the medical community.
Other IGBs are available worldwide but not approved in United States at the current time. Spatz adjustable balloon system (Spatz Medical, Great Neck, NY, United States) is an endoscopically placed saline filled IGB with a unique design that allows for volume adjustment. Hence, increasing or decreasing balloon volume may result in better patient tolerance, making it adjustable per patient’s preference. Another IGB, Elipse Balloon (Allurion Technologies, Wellesley, MA, United States) remains in the stomach for approximately 4 mo, at which time a valve opens spontaneously, deflating the balloon and allowing it to be spontaneously excreted through the GI tract.
Other gastric non-balloon space-occupying devices have been introduced. TransPyloric Shuttle (BAROnova, Inc, Goleta, CA, United States) is composed of a large spherical silicone bulb connected to a smaller cylindrical silicone bulb by a flexible catheter. This unique design allows the device to assume transpyloric positioning creating an intermittent seal resulting in delayed gastric emptying and early satiety. The Full Sense Device (Barker, Foote, Kemmeter, Walburn, LLC, Grand Rapids, MI, United States) is a modified, fully covered, gastroesophageal stent. After deployment, the exclusive design allows the gastric disk component to apply pressure on the gastric cardia resulting in satiety.
ESG is an incisionless method whereby full thickness sutures (OverStitch; Apollo Endosurgery) are placed within the greater curvature of the stomach during endoscopy. This, in essence, creates a gastric restriction by decreasing gastric volumes by approximately 70%, thereby leading to early satiety and weight loss. Additionally, this method has been shown to alter insulin sensitivity, gastric emptying, satiation, and appetite regulatory hormones; which becomes important in the altered neurohormonal physiology in obese individuals[21].
This method for endoscopic weight loss has shown promise in clinical trials and retrospective reviews. In a recent study published in 2017, Lopez-Nava et al[22] evaluated 248 patients to examine the short term and long-term outcomes after ESG. There they were able to show that %TBWL was 15.2 and 18.6 at 6 and 24 mo respectively. At 24 mo the proportion of patients achieving > 10% TBWL was 84.2% and 53% with per protocol and intention-to-treat analysis, respectively[22]. In a more recent multi-centered trial, 112 patients (baseline BMI 37.9 kg/m2) who underwent ESG were followed prospectively. TBWL at 1, 3, and 6 mo was 8.4%, 11.9%, and 14.9% respectively. The proportion of patients who attained greater than 10% TBWL and 25% EWL was 62.2 and 78.0% at 3 mo post-ESG and 81.0 and 86.5% at 6 mo post-ESG. Multivariable analysis revealed that male gender, greater baseline body weight, and no prior endoscopic bariatric treatment were predictors of weight loss at 6 mo follow up. The safety of this procedure was also evaluated. Mild AEs such as self-limited nausea and vomiting occurring in a large proportion of patients were noted. Three major AEs were noted (two patients had gastrointestinal (GI) bleeding and one patient had perigastric fluid collection; no patient required conversion to surgery for management of these complications)[23]. In summary, the current evidence suggests that ESG results in greater weight loss compared to gastric balloon but not as much as with bariatric surgery (although high quality head to head studies to answer this question are in progress).
In another study performed by Sharaiha et al[24], ESG has been able to reduce obesity associated medical co-morbidities. In this study, 91 consecutive patients who underwent ESG between August 2013 and March 2016 were analyzed. At 24 mo follow up, statistically significant reductions in levels of hemoglobin A1c, systolic blood pressure, waist circumference, low density lipoprotein cholesterol, alanine aminotransferase, and serum triglycerides were noted[24]. A multi-center ESG Randomized Interventional Trial (MERIT-Trial) with estimated 200 participants is underway and is expected to complete at the end of 2020.
ESG provides a unique intermediary between gastric bariatric surgery and the gastric balloon. Gastric balloons have only been studied and approved for a short-term period, with long lasting weight loss a limitation of this modality thus far. On the other hand, ESG may provide a feasible means of weight loss with a more durable effect than the IGB. Moreover, compared to bariatric surgery, ESG is less invasive, efficient, safe and less costly making it an attractive possibility for patients who want to lose weight without undergoing surgery.
Another similarly designed treatment modality, primary obesity surgery endoluminal (POSE), uses a peroral incisionless operating platform (USGI Medical, San Clemente, CA, United States) to place transmural tissue anchor plications that reduce accommodation of the gastric fundus. Additional plications are placed in the distal gastric body to delay gastric emptying. In a pivotal United States multicenter randomized blinded clinical trial, the %TBWL at 12 mo for the POSE group was 4.94% ± 7% compared to 1.38% ± 5.6% in the control group. The rate of serious AEs was 4.7%[25].
The AspireAssist (Aspire Bariatrics, King of Prussia, PA, United States) is an FDA approved device for patients with BMI of 35-55 kg/m2. It is a large caliber tube that is inserted percutaneously through the stomach (like a polyethylene glycol tube)[26]. Two weeks after insertion, the external portion of the tube is shortened and a skin port with a valve is attached to the skin. An Aspire Assist device is connected to the skin port to provide aspiration. A water reservoir is used to flush water into the stomach to help with aspiration. Usually, aspiration is performed over a 5-10 min period about 20 to 30 min following each meal, effectively removing up to one third of the ingested meal. Regular visits to a physician very one to two months is required in the first year (and less frequently thereafter) to help monitor the progress.
The PATHWAY trial was the largest multicentered randomized controlled trial looking at 207 patients, randomized in a 2:1 fashion, where 137 patients underwent AspireAssist and 70 patients received Lifestyle Counseling. 111 patients underwent final analysis (26 withdrew). At 52 wk, participants in AspireAssist group had lost a mean (± SD) of 31.5 ± 26.7% of their excess body weight (12.1 ± 9.6% total body weight), whereas those in the Lifestyle Counseling group had lost a mean of 9.8 ± 15.5% of their excess body weight (3.5 ± 6.0% total body weight, P < 0.001). A total of 58.6% of participants in the AspirteAssist group and 15.3% in the Lifestyle Counseling group lost at least 25% of their excess body weight (P < 0.001). Each of these outcomes achieved statistical significance. The AspireAssist group was also able to show statistically significant changes in glycated hemoglobin levels as well[27]. The advantages of aspiration therapies include reversibility, outpatient procedure, no alteration to the internal anatomy, limited risks, and long-term duration of use.
The proximal small bowel plays an important role in glucose homeostasis and pathogenesis of diet-induced diabetes. Enteroendocrine cells within the small intestinal mucosa release gut peptides that mediate satiety and increase insulin secretion. Secretion of biliary and pancreatic enzymes within the proximal small bowel helps in digestion of food. To mitigate these beneficial physiological effects of the proximal small bowel mucosa, several barrier devices have been developed, which will be explained in the next section.
The small intestine is primarily used for nutrient absorption of carbohydrates, fats, and proteins. GI bypass sleeves are intended to bypass a part of small intestine to cause malabsorption of nutrients. This has shown promise in both weight reduction as well as control of diabetes.
Endobarrier (GI dynamics, Lexington, MA, United States) is a long duodenojejunal bypass (DJBS) sleeve made up of an impermeable polymer liner and a nitinol crown anchoring system which attaches to the duodenal bulb. The sleeve is designed to cause malabsorption by allowing food passage from the stomach into the small bowel while bypassing the first 65 cm of the small bowel. Due to the barrier function of the sleeve, contact of the food with pancreatic enzymes and biliary secretions in the duodenum is eliminated and hence, results in malabsorption of nutrients and resultant weight loss. This has shown excitement as it is designed to mimic the RYGB without the associated morbidity (1 year 14.9%) and 30 d mortality (0.5%)[28]. The sleeve is removed endoscopically in 12 mo.
Although this technology has yet to be approved by FDA within the United States, there have been multiple clinical trials which have shown promise with this device. In a large metanalysis by Force et al[29], published in 2015, EndoBarrier was associated with 35.3% EWL at 12 mo (95% confidence interval, 24.6-46.1). An interesting finding associated with this bypass sleeves was that hemoglobin a1c levels were markedly improved with the use of this device, even in as little as 24 wk[30]. This finding has been seen in RYGB, and is thought to be secondary to increased glucagon synthesis after exclusion of the proximal small bowel, as well as enhanced secretion of incretins, like GLP-1, in response to nutrients being delivered to the distal small bowel[31].
However, a pivotal multicenter double-blinded sham control United States trial was terminated early because of a 3.5% incidence of hepatic abscess formation. Interestingly, subjects who received the DJBS had more weight loss compared to the sham group at 12 mo (%TBWL, 7.7% ± 9.6% vs 2.1% ± 5.4%, P < 0.0001) and had more significant improvement in HgA1c level (-1.1% ± 1.5% vs -0.3% ± 1.6%). AEs occurred in 10.9% of patients requiring early device retrieval]. Unfortunately, long term follow up study revealed that the weight reduction of initial DJBL treatment seems to be diminished after 4 years of follow up[32]. Due to the results discussed above, the current system fell out of favor but paved the way for second generation DJBSs with atraumatic anchoring and retrieval systems, which are being currently studied in clinical human trials.
Other small bowel endoscopic bariatric therapies are in the initial phase of development and are being studied in clinical trials. Gastroduodenojejunal bypass sleeve (ValenTx) is technology similar to EndoBarrier; however, it is a much longer sleeve and is anchored to the GE junction. The ValenTx endoluminal bypass therapy mimics the permanent anatomical changes made by the RYGB procedure, but does this with an adjustable, removable, and replaceable device. This technology is still undergoing early clinical trials.
A duodenal mucosal resurfacing procedure (Fractyl Laboratories, Cambridge, MA, United States) has been developed. In this procedure, a special catheter is used to deliver heat energy resulting in thermal ablation of the superficial duodenal mucosa. This results in mucosal remodeling and subsequent resetting of the signaling pathway of the duodenal neuroendocrine cells, translating into better control of hyperglycemia and diabetes. Self-assembling magnets for endoscopy (GI windows, Boston, MA, United States) have been developed with the idea of creating a communication between proximal jejunum and ileum. This would allow the nutrients to bypass the absorptive surface of a major part of small bowel leading to malabsorption and weight loss.
The medical community is exploring options to feed the unmet need of the United States obesity epidemic. These options include lifestyle/behavioral modifications, pharmacotherapy, bariatric surgery, or combination therapy. Studies have shown that lifestyle modifications and pharmacotherapy have only been able to achieve modest weight loss effects. Bariatric surgery has been shown to be a landmark intervention in obesity, but many patients find this invasive approach unacceptable due to the side effect profile and potential late complications. Endoscopic bariatric treatment options, although relatively novel, has shown efficacy in the treatment of obesity. It has potential to become more popular in near future considering being less invasive and having a more favorable side effect profile compared to surgery. Long term efficacy is not well known at this time but should be available in a few years as more studies are being reported. Additional studies must also include comparison of different modalities and outcomes on obesity related illnesses (cardiovascular disease, diabetes, hyperlipidemia, etc.). The future of this developing arena will also depend upon training future gastroenterologists in the technical and medical aspects of this field. To date, there is no formalized bariatric endoscopic training amongst gastroenterology fellowship programs. Primary care, bariatric medicine physicians, and bariatric surgeons must incorporate and determine appropriateness of bariatric endoscopy when evaluating patients. Given the promise of this new modality, the authors of this review believe the future of bariatric medicine will include endoscopic intervention as a major tool in its armamentarium.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang YP S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284-2291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2161] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 2. | Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1495] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 3. | US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Grossman DC, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB. Behavioral Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 388] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 4. | Narayanaswami V, Dwoskin LP. Obesity: Current and potential pharmacotherapeutics and targets. Pharmacol Ther. 2017;170:116-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 5. | LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O'Connor EA. Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018;320:1172-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 320] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 6. | LeBlanc EL, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018; 18-05239-EF-1. [PubMed] |
| 7. | Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14:12-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 264] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 8. | Schulman AR, Thompson CC. Complications of Bariatric Surgery: What You Can Expect to See in Your GI Practice. Am J Gastroenterol. 2017;112:1640-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: A review using Andersen’s Model of Health Services Use. Surg Obes Relat Dis. 2018;14:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Gleysteen JJ. A history of intragastric balloons. Surg Obes Relat Dis. 2016;12:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Mathus-Vliegen EM, de Groot GH. Fasting and meal-induced CCK and PP secretion following intragastric balloon treatment for obesity. Obes Surg. 2013;23:622-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Laing P, Pham T, Taylor LJ, Fang J. Filling the Void: A Review of Intragastric Balloons for Obesity. Dig Dis Sci. 2017;62:1399-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | ASGE Bariatric Endoscopy Task Force. ASGE Technology Committee, Abu Dayyeh BK, Edmundowicz SA, Jonnalagadda S, Kumar N, Larsen M, Sullivan S, Thompson CC, Banerjee S. Endoscopic bariatric therapies. Gastrointest Endosc. 2015;81:1073-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Genco A, Maselli R, Frangella F, Cipriano M, Forestieri P, Delle Piane D, Furbetta F, Micheletto G, Ciampaglia F, Granelli P, Zilli M, Lorenzo M, Di Rocco G, Giannotti D, Redler A. Intragastric balloon for obesity treatment: Results of a multicentric evaluation for balloons left in place for more than 6 months. Surg Endosc. 2015;29:2339-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Courcoulas A, Abu Dayyeh BK, Eaton L, Robinson J, Woodman G, Fusco M, Shayani V, Billy H, Pambianco D, Gostout C. Intragastric balloon as an adjunct to lifestyle intervention: A randomized controlled trial. Int J Obes (Lond). 2017;41:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 16. | Ashrafian H, Monnich M, Braby TS, Smellie J, Bonanomi G, Efthimiou E. Intragastric balloon outcomes in super-obesity: A 16-year city center hospital series. Surg Obes Relat Dis. 2018;14:1691-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Ponce J, Woodman G, Swain J, Wilson E, English W, Ikramuddin S, Bour E, Edmundowicz S, Snyder B, Soto F, Sullivan S, Holcomb R, Lehmann J; REDUCE Pivotal Trial Investigators. The REDUCE pivotal trial: A prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015;11:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 18. | Agnihotri A, Xie A, Bartalos C, Kushnir V, Islam S, Islam E, Lamet M, Lamet A, Farboudmanesch R, Overholt BF, Altawil J, Early DS, Bennett M, Lowe A, Mullady DK, Adeyeri CS, El Zein M, Mishra P, Fayad L, Dunlap M, Oberbach A, Cheskin LJ, Kalloo AN, Khashab MA, Kumbhari V. Real-World Safety and Efficacy of Fluid-Filled Dual Intragastric Balloon for Weight Loss. Clin Gastroenterol Hepatol. 2018;16:1081-1088.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Mion F, Ibrahim M, Marjoux S, Ponchon T, Dugardeyn S, Roman S, Deviere J. Swallowable Obalon® gastric balloons as an aid for weight loss: A pilot feasibility study. Obes Surg. 2013;23:730-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Sullivan S, Swain J, Woodman G, Edmundowicz S, Hassanein T, Shayani V, Fang JC, Noar M, Eid G, English WJ, Tariq N, Larsen M, Jonnalagadda SS, Riff DS, Ponce J, Early D, Volckmann E, Ibele AR, Spann MD, Krishnan K, Bucobo JC, Pryor A. Randomized sham-controlled trial of the 6-month swallowable gas-filled intragastric balloon system for weight loss. Surg Obes Relat Dis. 2018;14:1876-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Abu Dayyeh BK, Acosta A, Camilleri M, Mundi MS, Rajan E, Topazian MD, Gostout CJ. Endoscopic Sleeve Gastroplasty Alters Gastric Physiology and Induces Loss of Body Weight in Obese Individuals. Clin Gastroenterol Hepatol. 2017;15:37-43.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 22. | Lopez-Nava G, Sharaiha RZ, Vargas EJ, Bazerbachi F, Manoel GN, Bautista-Castaño I, Acosta A, Topazian MD, Mundi MS, Kumta N, Kahaleh M, Herr AM, Shukla A, Aronne L, Gostout CJ, Abu Dayyeh BK. Endoscopic Sleeve Gastroplasty for Obesity: A Multicenter Study of 248 Patients with 24 Months Follow-Up. Obes Surg. 2017;27:2649-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 23. | Sartoretto A, Sui Z, Hill C, Dunlap M, Rivera AR, Khashab MA, Kalloo AN, Fayad L, Cheskin LJ, Marinos G, Wilson E, Kumbhari V. Endoscopic Sleeve Gastroplasty (ESG) Is a Reproducible and Effective Endoscopic Bariatric Therapy Suitable for Widespread Clinical Adoption: A Large, International Multicenter Study. Obes Surg. 2018;28:1812-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 24. | Sharaiha RZ, Kumta NA, Saumoy M, Desai AP, Sarkisian AM, Benevenuto A, Tyberg A, Kumar R, Igel L, Verna EC, Schwartz R, Frissora C, Shukla A, Aronne LJ, Kahaleh M. Endoscopic Sleeve Gastroplasty Significantly Reduces Body Mass Index and Metabolic Complications in Obese Patients. Clin Gastroenterol Hepatol. 2017;15:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 25. | Sullivan S, Swain JM, Woodman G, Antonetti M, De La Cruz-Muñoz N, Jonnalagadda SS, Ujiki M, Ikramuddin S, Ponce J, Ryou M, Reynoso J, Chhabra R, Sorenson GB, Clarkston WK, Edmundowicz SA, Eagon JC, Mullady DK, Leslie D, Lavin TE, Thompson CC. Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: The ESSENTIAL trial. Obesity (Silver Spring). 2017;25:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Kumar N, Sullivan S, Thompson CC. The role of endoscopic therapy in obesity management: Intragastric balloons and aspiration therapy. Diabetes Metab Syndr Obes. 2017;10:311-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Thompson CC, Abu Dayyeh BK, Kushner R, Sullivan S, Schorr AB, Amaro A, Apovian CM, Fullum T, Zarrinpar A, Jensen MD, Stein AC, Edmundowicz S, Kahaleh M, Ryou M, Bohning JM, Ginsberg G, Huang C, Tran DD, Glaser JP, Martin JA, Jaffe DL, Farraye FA, Ho SB, Kumar N, Harakal D, Young M, Thomas CE, Shukla AP, Ryan MB, Haas M, Goldsmith H, McCrea J, Aronne LJ. Percutaneous Gastrostomy Device for the Treatment of Class II and Class III Obesity: Results of a Randomized Controlled Trial. Am J Gastroenterol. 2017;112:447-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Dixon JB, le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet. 2012;379:2300-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 29. | ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee; Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, Sullivan S, Thompson CC, Banerjee S. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82:425-38.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 288] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 30. | de Jonge C, Rensen SS, Verdam FJ, Vincent RP, Bloom SR, Buurman WA, le Roux CW, Schaper NC, Bouvy ND, Greve JW. Endoscopic duodenal-jejunal bypass liner rapidly improves type 2 diabetes. Obes Surg. 2013;23:1354-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Knop FK. Resolution of type 2 diabetes following gastric bypass surgery: Involvement of gut-derived glucagon and glucagonotropic signalling? Diabetologia. 2009;52:2270-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | van Rijn S, Roebroek YGM, de Jonge C, Greve JWM, Bouvy ND. Effect of the EndoBarrier Device: A 4-Year Follow-up of a Multicenter Randomized Clinical Trial. Obes Surg. 2019;29:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: New drugs and emerging targets. Clin Pharmacol Ther. 2014;95:53-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 34. | Abu Dayyeh BK, Edmundowicz S, Thompson CC. Clinical Practice Update: Expert Review on Endoscopic Bariatric Therapies. Gastroenterology. 2017;152:716-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Dixon JB, Eaton LL, Vincent V, Michaelson R. LAP-BAND for BMI 30-40: 5-year health outcomes from the multicenter pivotal study. Int J Obes (Lond). 2016;40:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Salminen P, Helmiö M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, Hurme S, Soinio M, Nuutila P, Victorzon M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA. 2018;319:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 713] [Article Influence: 101.9] [Reference Citation Analysis (0)] |