Published online Aug 21, 2019. doi: 10.3748/wjg.v25.i31.4555
Peer-review started: March 28, 2019
First decision: June 6, 2019
Revised: June 25, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 21, 2019
Processing time: 142 Days and 11.4 Hours
Documentation of disease activity in patients affected by Crohn’s disease (CD) is mandatory in order to manage patients properly. Magnetic resonance imaging (MRI) is considered the reference cross-sectional technique for the assessment of CD activity. Among MRI findings, layered pattern (LP) of contrast enhancement seems to be one of the most significant signs of severe disease activity; however, it has also been associated with chronic disease and mural fibrosis.
To systematically evaluate the accuracy of LP of contrast enhancement in the diagnosis of active inflammation in patients with CD.
In February 2019, we searched the MEDLINE and Cochrane Central Register of Controlled Trials databases for studies evaluating the diagnostic accuracy of LP of contrast enhancement on MRI for the detection of active inflammation in patients with CD. To be included, studies had to use histopathologic analysis (endoscopy or surgery) as the reference standard. Risk of bias and applicability concerns of the included studies were evaluated by using items from the Quality Assessment for Diagnostic Accuracy Studies 2 (QUADAS-2) tool. Pooled sensitivity and specificity were determined using a bivariate random-effect model. Heterogeneity was quantified by using the I2 statistic. Our meta-analysis received no funding, and the review protocol was not published or registered in advance.
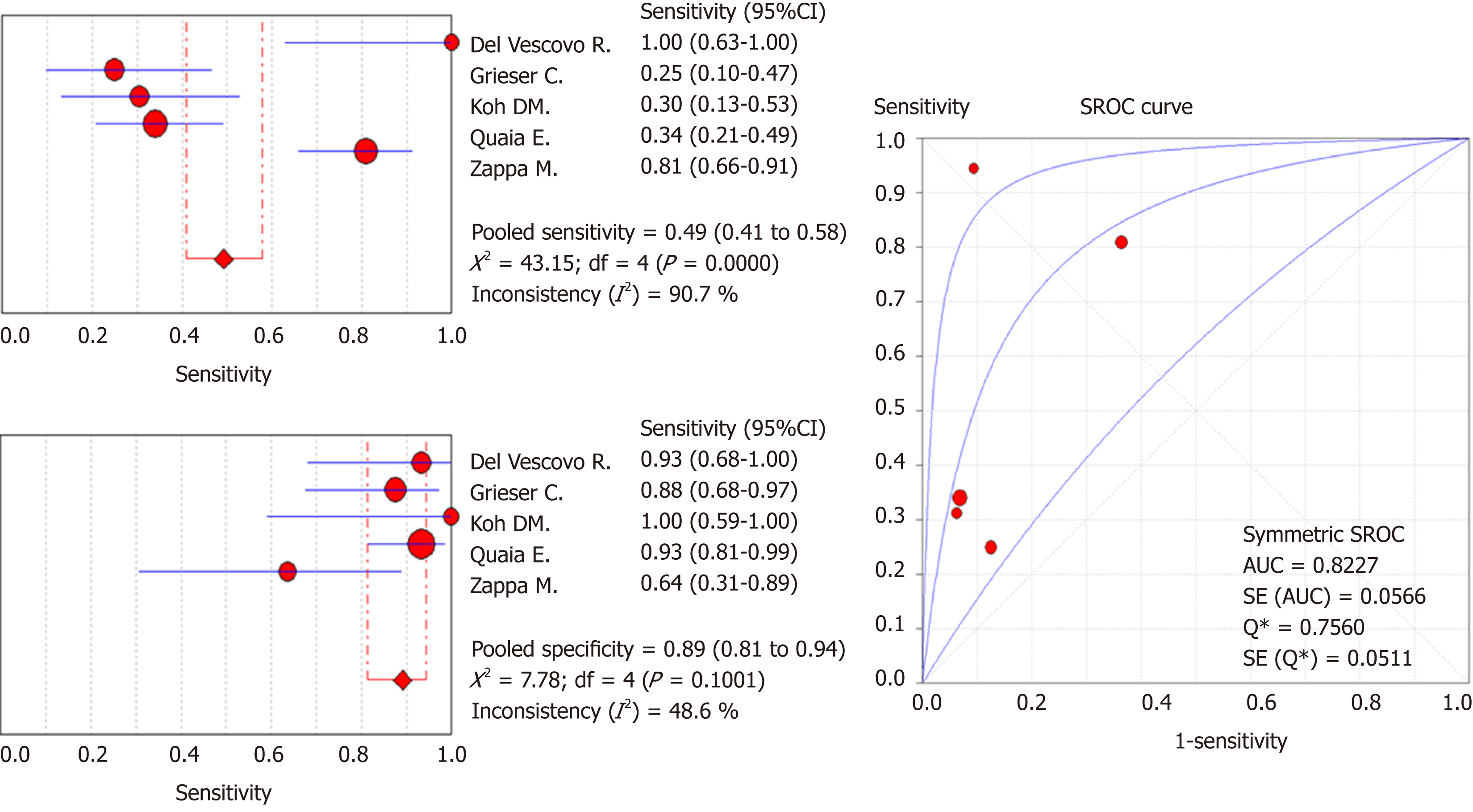
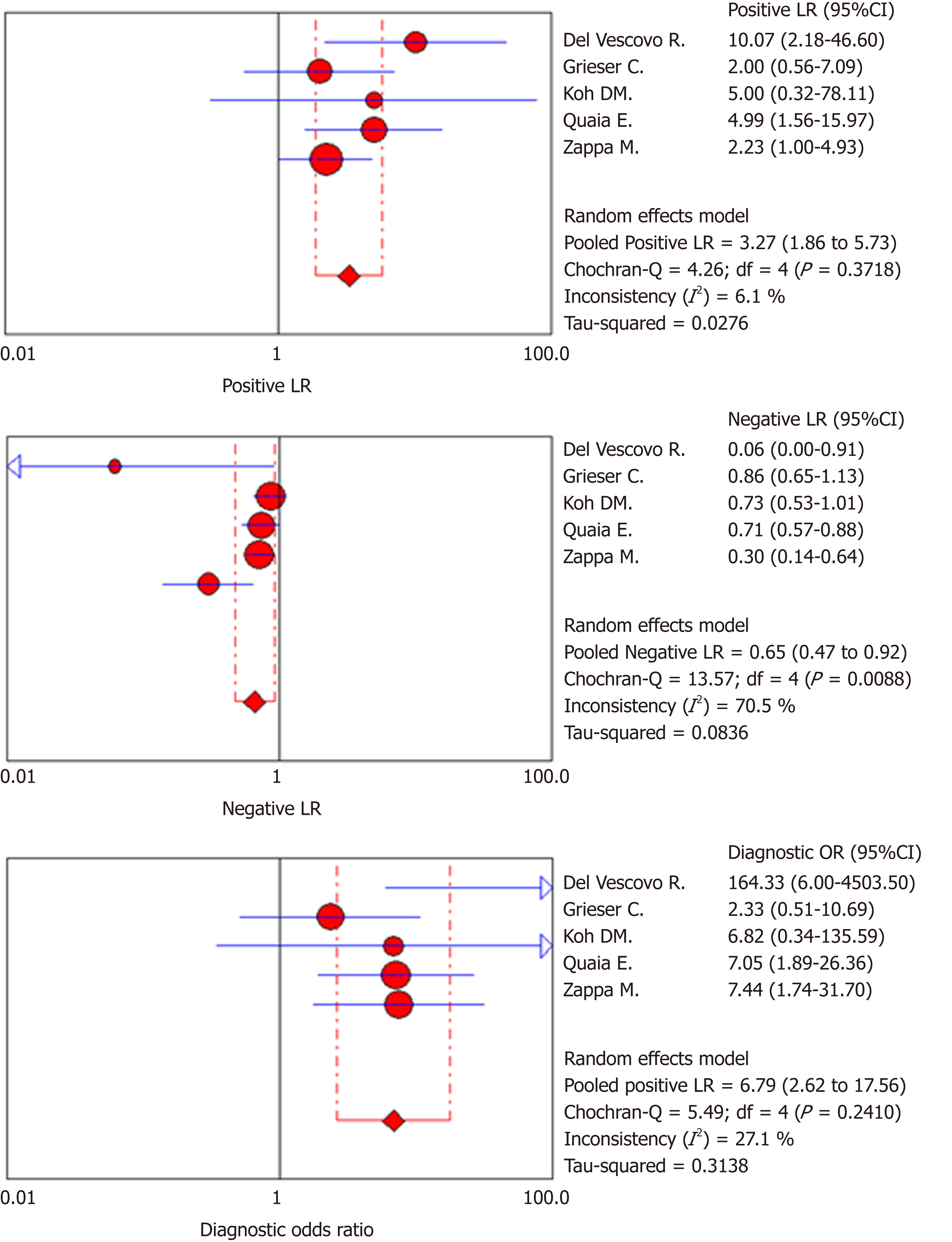
Of the 1383 studies identified, five articles were finally selected for quantitative and qualitative synthesis (245 patients, 238 of whom had histopathologically confirmed CD, 144 with active inflammation and 94 with inactive disease). The meta-analysis showed a pooled sensitivity of 49.3% (95%CI: 41%-57.8%; I2: 90.7%) and specificity of 89.1% (95%CI: 81.3%- 94.4%; I2: 48.6%). Pooled PLR and NLR were 3.3 (95%CI: 1.9-5.7; I2: 6.1%) and 0.6 (95%CI: 0.5-0.9; I2 70.5%), respectively. SDOR was 6.8 (95%CI: 2.6-17.6; I2: 27.1%). The summary ROC curve showed an area under the curve (AUC) of 0.82 (SE 0.06; Q* 0.76). High risk of bias and applicability concerns were observed in the domains of patient selection for one included study.
LP on contrast-enhanced MRI is a specific finding to rule out active inflammation in patients with CD. Further studies using a prespecified definition of LP on contrast-enhanced MRI are needed to support our findings.
Core tip: Magnetic resonance imaging (MRI) plays a critical role in the assessment of Crohn’s disease (CD) activity and severity. Layered pattern (LP) of contrast enhancement is frequently observed in patients with active disease; however, its relevance remains controversial, since it has also been correlated with the presence of mural fibrosis and chronic disease. Our systematic review and meta-analysis showed that LP on contrast-enhanced MRI yields high specificity for active inflammation and can reliably rule out the presence of active disease in patients with CD.
- Citation: Bellini D, Rivosecchi F, Panvini N, Rengo M, Caruso D, Carbone I, Ferrari R, Paolantonio P, Laghi A. Layered enhancement at magnetic resonance enterography in inflammatory bowel disease: A meta-analysis. World J Gastroenterol 2019; 25(31): 4555-4566
- URL: https://www.wjgnet.com/1007-9327/full/v25/i31/4555.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i31.4555
Assessment of inflammatory activity in patients affected by Crohn’s disease (CD) is mandatory in order to manage patients properly. Currently, a single reference standard to diagnose CD is not available. The diagnosis is composed of clinical and laboratory findings, evaluation by endoscopy and cross-sectional imaging, and histological examinations[1].
In case of suspected CD, the small bowel has to be assessed with an imaging method[1]. Among several cross-sectional imaging methods able to evaluate the entire bowel wall, magnetic resonance enterography/enteroclysis (MRE) is widely considered the best imaging modality to determine disease activity and the grade of severity, because of better contrast resolution, safety profile and reproducibility[1-3]. Numerous relevant papers have reported that the use of MRE in daily clinical practice significantly alters the management of CD patients[4,5]. Indeed, owing to its ability to provide both luminal and extra-luminal disease evaluation, MRE is critical in guiding timely and tailored management, determining disease phenotype, extent, and activity and defining the presence of stricturing or penetrating complications, and has been shown to positively influence clinician’s diagnostic confidence and therapeutic strategies[6,7].
There are several MRI findings that contribute to define the stage and the activity of CD: Mural changes (bowel wall thickening, wall edema, fibrosis, fat infiltration), different patterns of contrast enhancement (transmural, layered), mesentery involvement (fibrofatty proliferation, fat stranding, enhancing lymphadenopathies), and the presence of complications (luminal stenosis, fistulas, abscesses). However, among all these features, transmural enhancement seems to be one of the most significant signs of severe disease activity, being unequivocally associated with an active disease with almost no fibrosis, and it is usually observed at the time of first diagnosis, especially in children or young patients[8]. On the other hand, a layered pattern (LP) of enhancement, defined as strong enhancement of the inner layer (mucosa) and the outer layer (serosa) with no enhancement of the middle layer (representing submucosa and muscularis)[9], has been associated not only with severe disease activity[9-11] but also with chronic disease[8]; there is other relevant evidence that correlate LP with mural fibrosis or fat deposition[8,12].
Seeking evidence-based information on diagnostic accuracy of imaging features for detecting active or inactive CD, we conducted a systematic review and meta-analysis to define the diagnostic yield of LP on contrast-enhanced MRI in the evaluation of active inflammation in patients affected by CD.
This systematic review was written by using the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy Studies (PRISMA-DTA)[13] and the Cochrane Handbook of Diagnostic Test Accuracy Reviews[14]. The review protocol was not published or registered in advance. The authors received no financial support for this meta-analysis.
In February 2019, two indipendent observers searched the MEDLINE (United States National Library of Medicine) and Cochrane Central Register of Controlled Trials (Cochrane Library) databases for studies that reported the diagnostic accuracy of LP on contrast-enhanced MRI in detecting active inflammation in patients with CD. The last search was performed on February 8, 2019. We proposed the following PICO(S) question: Patients affected by CD; Index test consisting of LP; Comparison with histopathological findings; Outcome consisting in diagnostic accuracy in detecting active inflammation; any type of Study that is not a case report or case series was eligible for initial screening.
The search used the following keywords: inflammatory bowel diseases, CD, MRI, LP, specificity, sensitivity, diagnostic accuracy, active inflammation, and fibrosis combined using “OR” and “AND”. Additional articles were searched for using the "Related Articles" function in PubMed (United States National Library of Medicine). Authors crosschecked the references of the selected papers to identify any additional pertinent manuscript. Full search strategies for all databases are described in detail in the online-only supplementary material(Appendix S1).
Potentially eligible studies were at first examined by two authors (blinded to the review process, with 4 and 8 years of subspecialty abdominal imaging experience). Studies performed on human patients whose title or abstracts reported the search terms were selected. Review and commentary articles as well as case report or case series were excluded. The full-text review of eligible papers was carried out independently by two other reviewers (blinded to the review process, with 2 and 6 years of experience); discrepancies regarding potential eligibility and inclusion were resolved by consensus. Reviewers were aware of authors’ names and journal of publication of the screened papers. Studies were included in the meta-analysis if they met the following criteria: (1) Diagnostic accuracy of LP on contrast-enhanced MRI in detecting active inflammation in patients with CD was investigated; (2) Data to determine 2 × 2 contingency tables were available; (3) Per-patient analysis was performed; (4) A proper reference standard to confirm imaging-based diagnoses was used for all patients (i.e., endoscopy with biopsy and/or surgical pathologic examination); and (5) Patients were not a subgroup from any other included study population
Two reviewers independently extracted relevant data from the included studies using a data extraction form. Disagreements were resolved by discussion with a third reviewer (blinded to the review process, an MRI radiologist with more than 25 years of experience). The extracted data were as follows.
Study characteristics: These characteristics included year of publication, study design and country, sample size, main outcome, reference standards, interval between index test and reference standard, and total number of true-positive (TP), false-positive (FP), true-negative (TN) and false-negative (FN) findings.
Patient characteristics: These characteristics included patient’s age and gender, patient spectrum (i.e., known or suspected CD), clinical setting and information about CD activity.
Imaging characteristics: These characteristics included detailed information about the imaging equipment and basic specifications (vendor, model, and magnetic field strength), assessment techniques used (MRI pulse sequences, postcontrast sequences timing), bowel preparation (diet, cathartic or spasmolytic drugs), and enteral and intravenous contrast agent used.
Reviewers tried repeatedly to get in contact with authors whose papers reported incomplete or apparently conflicting or inconsistent data to be clarified.
Risk of bias and applicability concerns of the included studies were assessed independently by two authors (blinded to the review process, with 2 and 6 years of experience) using items from a customized Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool[15]. Disagreement was resolved by consensus discussion with a third senior author (blinded to the review process, with 25 years of experience in abdominal radiology). For each domain (patient selection, index test, reference standard, and patient flow and timing), risks of bias and applicability concerns were rated as low (1), high (0), or unclear (0.5). The risk for bias across studies (i.e., “publication bias’’) was not assessed, since there is no generally accepted method for this task and the number of included studies was low[16].
The primary end point in this systematic review and meta-analysis was to evaluate sensitivity, specificity, positive likelihood ratio (PLR) value, negative likelihood ratio (NLR), summary diagnostic odds ratio (SDOR), and summary receiver-operating-characteristic curve (SROC) of LP on contrast-enhanced MRI in detecting active inflammation in patients with CD.
Data on numbers of TP, FN, TN, and FP findings were used to calculate a pooled sensitivity and specificity along with 95% confidence intervals (CI), using random-effect models according to heterogeneity in a per-patient analysis. For studies that provided for additional reference standard tests other than endoscopy or surgery (e.g., CD Activity Index and serum or fecal markers), only results from histopathologic analysis were considered for data synthesis.
For each analysis, between-study heterogeneity was quantified computing I2 values with the following equation: I2 = [(χ2-df)/ χ2] x 100%, where χ2 was the chi-squared statistic and df the degrees of freedom. I2 values were rated as follows: I2 ≥ 25%, low heterogeneity; I2 ≥ 50% moderate heterogeneity; and I2 ≥ 75%, high heterogeneity.
A P value of <0.05 was used as the threshold for statistical significance for all analyses. Data were analyzed by using Comprehensive Meta Analyses (version 2.2.064, July 27, 2011, Biostat), Excel 365 (Microsoft), and MetaDiSc (version 1.4, Hospital Ramon y Cajal and Universidad Complutense de Madrid) statistical software interfaces.
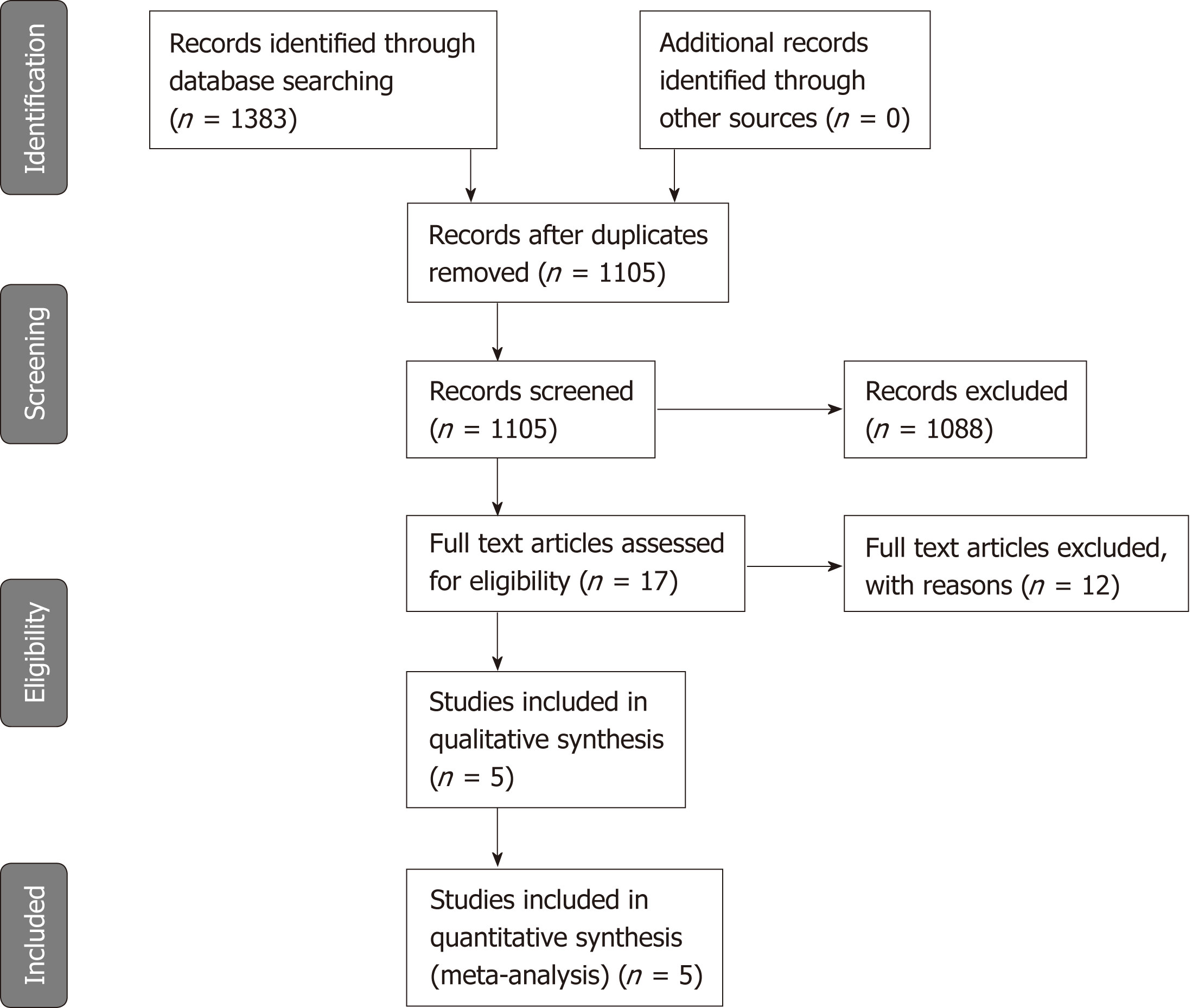
Electronical search returned 1383 papers, of which 1105 were evaluated for inclusion, after duplicates removal. After title and abstract review, 1088 articles not meeting the inclusion criteria were excluded, and 17 papers underwent full-text review (Figure 1). No additional studies were identified after checking the references. Twelve studies were excluded due to impossibility of reconstructing a 2 x 2 contingency table or because data were analyzed on a per-bowel segments analysis (Appendix S2). Five articles were ultimately selected for quantitative and qualitative synthesis[10,11,17-19].
The detailed study and patient characteristics are reported in Table 1. The 5 studies eligible for review[10,11,17-19] involved 245 patients, 238 of whom had histopathologically confirmed CD, 144 with active inflammation and 94 with inactive disease. The median sample size was 48 patients (ranging between 23 and 91 patients), and the median patient age was 37 years (ranging between 15 and 78 years). The median year of publication of the included studies was 2011 (range 2001-2014). All five studies were single-center studies conducted in Europe. Further detailed characteristics on the index test and reference standard of the included studies are summarized in Table 2.
| Ref. | Year of publication | Country | Study design | Founding sources | Patient spectrum | Inclusion criteria | Disease activity | No. of patients | Age, yr, mean (range)or mean ± SD | Gender, male: female |
| Del Vescovo et al[19] | 2008 | Italy | Prospective | NR | Known CD/healthy controls | Proven terminal ileum CD/no history of bowel disease | 8 active CD, 8 non-active CD, 7 healthy controls | 23 | 37.5 ± 17.41 | 15:8 |
| Grieser et al[18] | 2012 | Germany | Retrospective | NR | Known CD | Referred to MRI for evaluation of disease activity | 24 active CD, 24 patients non-active CD | 48 | 37 ± 11 | 16:32 |
| Koh et al[10] | 2001 | England | Prospective | NR | Known CD | Clinically symptoma-tic patients referred to MRI by gastroenterologists | 23 active CD, 7 patients non-active CD | 30 | 37.6 (18-58) | 14:16 |
| Quaia et al[17] | 2014 | Italy | Retrospective | NR | Known CD | Clinically symptoma-tic patients referred to MRI for evaluation of disease activity | 47 active CD, 44 patients non-active CD | 91 | 39.6 ± 17.1 | 47:44 |
| Zappa et al[11] | 2011 | France | Retrospective | NR | Known CD | Patients who were to undergo bowel resection for small bowel CD | 42 active CD, 11 patients non-active CD | 53 | 35 (15-74) | 28:25 |
| Ref. | Vendor (Model) | Magnetstrength, T | Technique | Bowelpreparation | Luminal contrast | Spasmolytic agent | Intravenous contrast | MRI sequences | Postcontrast sequence timing | Index test readers (Years of experience) | Reference standard | Interval1 |
| Del Vescovo et al[19] | Siemens Corp. (Magnetom Symphony) | 1.5 | EG | NR | 1.2-2 L PEG solution orally 45 min prior | Buscopan 20 mg iv | 0.1 mmol/kg Gadodiamide (Omniscan) | T2-HASTE, True-FISP, T1-FLASH fs (pre/postcontrast) | 15 s, 60 s, 100 s, 160 s, 220 s, 280 s, 340 s, 400 s, 460 s | 2 blinded radiologists in consensus (NR) | Endoscopy with biopsy | NR |
| Grieser et al[18] | GE Healthcare (Twin Speed) | 1.5 | EC | 8 hrs fasting | 1-2 L water, 80 mL/min, 20 min prior; 30 mL/min during MRI | Buscopan 10 mg iv | 0.2 mL/kg Gd-DTPA (Magnevist) | SSFP, FIESTA, FRFSE, T1-SPGR fs (pre/postcontrast) | 60-70 s | 2 blinded radiologists in consensus (6,12) | Endoscopy with biopsy and/or surgery | Endoscopy ≤ 3 d; surgery ≤ 10 d |
| Koh et al[10] | Siemens Corp. (Magnetom Impact) | 1 | EG | NR | 600 mL water orally 30 min prior | Buscopan 1 mg im | 0.1 mmol/kg Gadodiamide (Omniscan) | T2-TSE, T1-FLASH, T1-FLASH fs (pre/postcontrast) | 20 s | 2 blinded radiologists in consensus (NR) | Endoscopy with biopsy and/or surgery | Median 21 d |
| Quaia et al[17] | Philips (Achieva) | 1.5 | EG | 8 hrs fasting | 2 L PEG solution orally 60 min prior and diatrizoate meglumine and diatrizoate sodium solution rectally | Buscopan 40 mg iv | 0.2 mL/kg Gadobenate dimeglumine (Multihance) | T2-HASTE, T2-SPAIR, T1-FISP, T1-THRIVE 3D fs (pre/postcontrast) | 30 s, 70 s, 3 min, 5 min | 2 blinded radiologists in consensus (10,12) | Endoscopy with biopsy or surgery | ≤ 30 d |
| Zappa et al[11] | Philips (Intera) | 1.5 | EG | NR | 1 L mannitol solution orally | Glucagon 1 mg iv | 0.2 mL/kg Gadoterate meglumine (Dotarem) | T2-SSH-TSE fs, True-FISP, T1-FLASH 3D (pre/postcontrast) | 90 s, 8 min | 2 blinded radiologists in consensus (NR) | Surgery | ≤ 90 d (mean 24 d, range 1–90 d, median 14 d) |
Accuracy of LP on MRI exams in the diagnosis of active inflammation: Two-by-two data for diagnostic accuracy were summarized into a forest plot (Figures 2 and 3 and Table 3). Cumulative data for diagnostic accuracy were sensitivity 49.3% (95%CI: 41.0%-57.8%; I2: 90.7%), specificity 89.1% (95%CI: 81.3%-94.4%; I2: 48.6%), pooled PLR 3.3 (95%CI: 1.9-5.7; I2: 6.1%), and pooled NLR 0.6 (95%CI: 0.5-0.9; I2: 70.5%). The SDOR was 6.8 (95%CI: 2.6-17.6; I2: 27.1%). The SROC curve showed an area under the curve (AUC) of 0.82 (SE: 0.06; Q*: 0.76).
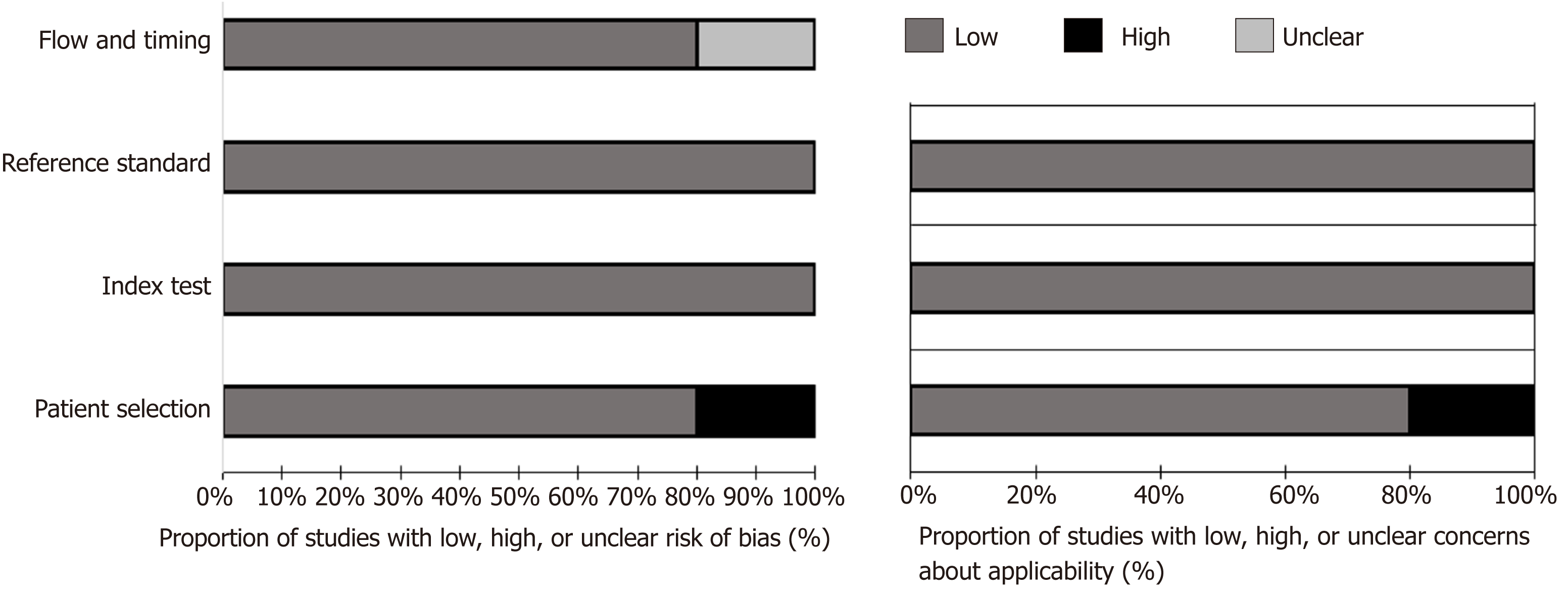
The results of quality assessment are shown in Figure 4. Reference standard, patient selection, and flow and timing domains were the major sources of concerns regarding potential bias. Four included studies did not report whether the readers who interpreted the reference standard were aware of MRE findings[10,17-19]. In two studies, the treatment was not withheld or interrupted before the completion of both the index test and the reference standard[11,17], while this was unclear in the remaining three included studies[10,18,19]. One study had high risk of bias and applicability concerns in the patient selection domain because it was a case-control study and patients with involvement of small bowel segments apart from terminal ileum were excluded[19]. The comprehensive results from the quality assessment of each article using a customized QUADAS-2 tool are reported in online-only Supplemental Material (Appendix S3).
Our systematic review and meta-analysis, which included 245 patients (144 of them with active inflammation), demonstrates that layered pattern has high diagnostic accuracy for the identification of active CD.
By pooling and comparing data on different diagnostic accuracy measures, our most significant result is the high value of specificity (close to 90%), underlying the importance of this pattern of enhancement in excluding active inflammation. The clinical impact of this finding is of utmost importance, and assessing whether or not active inflammation is present is crucial to guide the therapeutic management of patients with CD, allowing physicians to distinguish patients who would benefit from medical therapy[20]. Biological therapies aimed to control active inflammation have notable side effect profiles. Clinical evaluation is always the first step to assess disease activity. However, it has been shown that clinical evaluation of disease activity is often unreliable and blood or fecal markers lack specificity to accurately identify active inflammation[21,22]. Therefore, specific imaging signs, such as LP, which are able to accurately identify active inflammation, are crucial to avoid overtreatment and to reduce healthcare costs.
Despite the relatively low value of cumulative sensitivity (approximately 50%), the overall effectiveness of LP should be considered high. Indeed, our meta-analysis showed a SDOR of 6.8 (95%CI: 2.6-17.5) and a cumulative AUC of 0.82; in other words, it is almost 7 times more probable to identify LP in active CD than in non-active CD.
Notwithstanding the importance of LP, the multiparametric approach of MRI demands, in routine clinical practice, a more comprehensive assessment of all other radiological signs. Rimola and coworkers [12] recently published an interesting paper aimed to identify MRE features able to discriminate between active inflammatory process and fibrosis. According to their results, the following MRE findings proved to be significantly correlated with the presence of active inflammation at pathological examination: mural edema (high signal on T2-weighted images; P = 0.02), increased enhancement of the inner layer of the bowel wall (P = 0.03), ulcerations (P < 0.01), and indistinct margins (P = 0.05). The same group proposed the use of an MR index of activity (MaRIa), pooling and powering different findings in a quantitative and objective manner, to assess the overall disease activity at MRI. Other quantitative measurements of fibrosis are proposed by the same group for the identification of severe disease with both active inflammation and fibrosis, assessing the gain of percentage enhancement between the 70 s and 7 min phase[12].
However, to the best of our knowledge, neither the abovementioned paper nor other articles already published assessed the diagnostic value of MRE-specific findings in a systematic way. The high evidence level that comes from the present meta-analysis makes LP of enhancement a practical and reliable sign to be used routinely for the evaluation of active inflammation.
Our study had notable limitations. First, the heterogeneity in number of patients among different studies, with a large proportion of subjects from a few centers, may have affected our work and may have been further compounded by the relatively small number of studies enrolled. On the other hand, the number of included studies is comparable to that commonly reported in the literature for meta-analyses of diagnostic test accuracy studies[23]. Second, the data extracted from the included studies were collected retrospectively from a relatively large patient cohort. Third, three included studies used both endoscopy with biopsy and surgery as reference standard[10,17,18], however we could not perform a sub-analysis to assess the potential influence of the reference standard used on diagnostic accuracy, since necessary data were not provided by the Authors. Fourth, a significant bias in the domain of flow and timing could have negatively affected results. It is not clear in the three studies included in our analysis, if the treatment of patients changed between the index test and the reference standard. However, the interval between the MRE and histopathological evaluation was fewer than 30 d in most of the studies, which is reasonably acceptable considering the course of CD. Fifth, none of the studies had set a threshold to define the “LP of enhancement” for which evaluation was performed qualitatively and in a dichotomic way. This represents a source of bias in the index text domain and is mainly derived from retrospective analyses only (rather than a priori), which is known to overestimate diagnostic accuracy.
To conclude, our meta-analysis’ results demonstrate that layered pattern of enhancement is a specific radiological sign of active inflammation in CD. However, because of concerns about risk of bias and limited sample size, additional prospective studies using a prespecified definition of layered pattern may be appropriate.
Cross-sectional imaging evaluation of the small bowel is recommended in Crohn’s Disease (CD) to determine the grade of disease activity and the extent of bowel involvement. Magnetic resonance enterography/enteroclysis (MRE) is often preferred over other cross-sectional imaging modalities due to its ability to demonstrate transmural involvement or extraenteric complications and the lack of radiation exposure.
Layered pattern (LP) of bowel walls’ contrast enhancement is commonly observed at MRE in patients with CD. Nevertheless, it remains uncertain whether LP has to be considered as a sign of active inflammation or rather correlates with chronic changes and the presence of coexisting fibrosis. A better characterization of the clinical significance of LP may further expand the role of MRE, helping clinicians to choose the best treatment option and to monitor response to therapies over the course of the disease.
We performed a systematic review and meta-analysis aiming to estimate the diagnostic performance of LP of bowel walls’ enhancement at MRE in detecting inflammatory activity in CD.
Electronic search was performed to identify studies that investigated the diagnostic accuracy of LP for the recognition of active inflammation in patients with known or suspected CD using ileocolonoscopy with biopsy or surgical specimens’ histopathological analysis as reference standard. Quality Assessment for Diagnostic Accuracy Studies 2 (QUADAS-2) was employed to assess methodological quality of the included studies. Pooled data on diagnostic accuracy were estimated by means of bivariate random-effect model analysis.
After full-text review, five studies met the inclusion criteria for quantitative analysis. Cumulative data on LP diagnostic accuracy demonstrated by meta-analysis were as follows: pooled sensitivity, 49.3% (95%CI: 41.0%-57.8%); pooled specificity, 89.1% (95%CI: 81.3%-94.4%); pooled PLR, 3.3 (95%CI: 1.9-5.7); pooled NLR, 0.6 (95%CI: 0.5-0.9); and SDOR, 6.8 (95%CI: 2.6-17.6). Summary ROC curve returned an area under the curve (AUC) of 0.82 (SE 0.06). High risk of bias and applicability concerns were raised up in relation to patient selection in one of the included studies.
LP of bowel walls’ enhancement at MRE yields high specificity for active inflammation in patients with CD.
Our findings may further refine the role of MRE in characterizing inflammatory activity in CD, providing relevant information to ensure proper therapeutic management. Future prospective studies adopting a prespecified definition of LP are advisable to further support our findings.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Can G, Sivandzadeh G, Sultan K S-Editor: Ma YJ L-Editor: A E-Editor: Ma YJ
| 1. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J. European Crohn's and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]; ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1149] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 2. | Gourtsoyiannis NC, Papanikolaou N, Karantanas A. Magnetic resonance imaging evaluation of small intestinal Crohn's disease. Best Pract Res Clin Gastroenterol. 2006;20:137-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Bruining DH, Loftus EV, Ehman EC, Siddiki HA, Nguyen DL, Fidler JL, Huprich JE, Mandrekar JN, Harmsen WS, Sandborn WJ, Fletcher JG. Computed tomography enterography detects intestinal wall changes and effects of treatment in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2011;9:679-683.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Mendoza JL, González-Lama Y, Taxonera C, Suárez-Ferrer C, Matute F, Vera MI, López-Palacios N, Rodríguez P, Calvo M, Méndez R, Pastrana M, González C, Lana R, Rodríguez R, Abreu L. Using of magnetic resonance enterography in the management of Crohn's disease of the small intestine: first year of experience. Rev Esp Enferm Dig. 2012;104:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Messaris E, Chandolias N, Grand D, Pricolo V. Role of magnetic resonance enterography in the management of Crohn disease. Arch Surg. 2010;145:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | García-Bosch O, Ordás I, Aceituno M, Rodríguez S, Ramírez AM, Gallego M, Ricart E, Rimola J, Panes J. Comparison of Diagnostic Accuracy and Impact of Magnetic Resonance Imaging and Colonoscopy for the Management of Crohn's Disease. J Crohns Colitis. 2016;10:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Hafeez R, Punwani S, Boulos P, Bloom S, McCartney S, Halligan S, Taylor SA. Diagnostic and therapeutic impact of MR enterography in Crohn's disease. Clin Radiol. 2011;66:1148-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Tielbeek JA, Ziech ML, Li Z, Lavini C, Bipat S, Bemelman WA, Roelofs JJ, Ponsioen CY, Vos FM, Stoker J. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn's disease assessment with histopathology of surgical specimens. Eur Radiol. 2014;24:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Bloom S, Cohen R, Windsor A, Obichere A, Hansmann A, Novelli M, Halligan S, Taylor SA. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology. 2009;252:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 10. | Koh DM, Miao Y, Chinn RJ, Amin Z, Zeegen R, Westaby D, Healy JC. MR imaging evaluation of the activity of Crohn's disease. AJR Am J Roentgenol. 2001;177:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 249] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Zappa M, Stefanescu C, Cazals-Hatem D, Bretagnol F, Deschamps L, Attar A, Larroque B, Tréton X, Panis Y, Vilgrain V, Bouhnik Y. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn's disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis. 2011;17:984-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Rimola J, Planell N, Rodríguez S, Delgado S, Ordás I, Ramírez-Morros A, Ayuso C, Aceituno M, Ricart E, Jauregui-Amezaga A, Panés J, Cuatrecasas M. Characterization of inflammation and fibrosis in Crohn's disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 13. | McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM; the PRISMA-DTA Group, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 2057] [Article Influence: 293.9] [Reference Citation Analysis (0)] |
| 14. | Deeks JJ, Bossuyt PM, Gatsonis C, editors. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration. 2013; Available from: http://srdta. cochrane.org/. Edited September 13, 2013. |
| 15. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9512] [Article Influence: 679.4] [Reference Citation Analysis (0)] |
| 16. | McInnes MD, Bossuyt PM. Pitfalls of Systematic Reviews and Meta-Analyses in Imaging Research. Radiology. 2015;277:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Quaia E, Cabibbo B, Sozzi M, Gennari AG, Pontello M, Degrassi F, Cova MA. Biochemical markers and MR imaging findings as predictors of crohn disease activity in patients scanned by contrast-enhanced MR enterography. Acad Radiol. 2014;21:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Grieser C, Denecke T, Steffen IG, Werner S, Kröncke T, Guckelberger O, Pape UF, Meier J, Thiel R, Kivelitz D, Sturm A, Hamm B, Röttgen R. Magnetic resonance enteroclysis in patients with Crohn's disease: fat saturated T2-weighted sequences for evaluation of inflammatory activity. J Crohns Colitis. 2012;6:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Del Vescovo R, Sansoni I, Caviglia R, Ribolsi M, Perrone G, Leoncini E, Grasso RF, Cicala M, Zobel BB. Dynamic contrast enhanced magnetic resonance imaging of the terminal ileum: differentiation of activity of Crohn's disease. Abdom Imaging. 2008;33:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1443] [Article Influence: 180.4] [Reference Citation Analysis (0)] |
| 21. | Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 633] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 22. | Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Löfberg R, Modigliani R, Present DH, Rutgeerts P, Schölmerich J, Stange EF, Sutherland LR. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122:512-530. [PubMed] |
| 23. | Davey J, Turner RM, Clarke MJ, Higgins JP. Characteristics of meta-analyses and their component studies in the Cochrane Database of Systematic Reviews: a cross-sectional, descriptive analysis. BMC Med Res Methodol. 2011;11:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 263] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 24. | Wagner M, Ko HM, Chatterji M, Besa C, Torres J, Zhang X, Panchal H, Hectors S, Cho J, Colombel JF, Harpaz N, Taouli B. Magnetic Resonance Imaging Predicts Histopathological Composition of Ileal Crohn's Disease. J Crohns Colitis. 2018;12:718-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Iannicelli E, Martini I, Fantini C, Papi C, Gigante P, Carbonetti F, Di Pietropaolo M, David V. Magnetic resonance enterography in Crohn's disease: new simple proposal to assess disease activity. Clin Imaging. 2016;40:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Spieler B, Hindman N, Levy J, Zabrieski K, Sahlein D, Seuss C, Kim S. Contrast-enhanced MR enterography as a stand-alone tool to evaluate Crohn's disease in a paediatric population. Clin Radiol. 2013;68:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Macarini L, Stoppino LP, Centola A, Muscarella S, Fortunato F, Coppolino F, Della Valle N, Ierardi V, Milillo P, Vinci R. Assessment of activity of Crohn's disease of the ileum and large bowel: proposal for a new multiparameter MR enterography score. Radiol Med. 2013;118:181-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Malagò R, D'Onofrio M, Mantovani W, D'Alpaos G, Foti G, Pezzato A, Caliari G, Cusumano D, Benini L, Pozzi Mucelli R. Contrast-enhanced ultrasonography (CEUS) vs. MRI of the small bowel in the evaluation of Crohn's disease activity. Radiol Med. 2012;117:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Lasocki A, Pitman A, Williams R, Lui B, Kalade AV, Farish S. Relative efficacy of different MRI signs in diagnosing active Crohn's disease, compared against a histological gold standard. J Med Imaging Radiat Oncol. 2011;55:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Oto A, Kayhan A, Williams JT, Fan X, Yun L, Arkani S, Rubin DT. Active Crohn's disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging. 2011;33:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 31. | Parisinos CA, McIntyre VE, Heron T, Subedi D, Arnott ID, Mowat C, Wilson DC, McGurk S, Glancy S, Zealley IA, Satsangi J, Lees CW. Magnetic resonance follow-through imaging for evaluation of disease activity in ileal Crohn's disease: an observational, retrospective cohort study. Inflamm Bowel Dis. 2010;16:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Malagò R, Manfredi R, Benini L, D'Alpaos G, Mucelli RP. Assessment of Crohn's disease activity in the small bowel with MR-enteroclysis: clinico-radiological correlations. Abdom Imaging. 2008;33:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |