Published online Aug 21, 2019. doi: 10.3748/wjg.v25.i31.4502
Peer-review started: May 27, 2019
First decision: June 16, 2019
Revised: July 8, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 21, 2019
Processing time: 87 Days and 2.5 Hours
As one effective treatment for lateral pelvic lymph node (LPLN) metastasis (LPNM), laparoscopic LPLN dissection (LPND) is limited due to the complicated anatomy of the pelvic sidewall and various complications after surgery. With regard to improving the accuracy and completeness of LPND as well as safety, we tried an innovative method using indocyanine green (ICG) visualized with a near-infrared (NIR) camera system to guide the detection of LPLNs in patients with middle-low rectal cancer.
To investigate whether ICG-enhanced NIR fluorescence-guided imaging is a better technique for LPND in patients with rectal cancer.
A total of 42 middle-low rectal cancer patients with clinical LPNM who underwent total mesorectal excision (TME) and LPND between October 2017 and March 2019 at our institution were assessed and divided into an ICG group and a non-ICG group. Clinical characteristics, operative outcomes, pathological outcomes, and postoperative complication information were compared and analysed between the two groups.
Compared to the non-ICG group, the ICG group had significantly lower intraoperative blood loss (55.8 ± 37.5 mL vs 108.0 ± 52.7 mL, P = 0.003) and a significantly larger number of LPLNs harvested (11.5 ± 5.9 vs 7.1 ± 4.8, P = 0.017). The LPLNs of two patients in the non-IVG group were residual during LPND. In addition, no significant difference was found in terms of LPND, LPNM, operative time, conversion to laparotomy, preoperative complication, or hospital stay (P > 0.05).
ICG-enhanced NIR fluorescence-guided imaging could be a feasible and convenient technique to guide LPND because it could bring specific advantages regarding the accuracy and completeness of surgery as well as safety.
Core tip: Lateral pelvic lymph node (LPLN) metastasis is an important factor for local recurrence after surgery in patients with advanced low rectal cancer. As one of the effective treatment methods, laparoscopic LPLN dissection is limited due to complicated anatomy of the pelvic sidewall and various complications after surgery. In this paper, we introduce an innovative method using indocyanine green visualized with a near-infrared camera system to guide the detection of LPLNs in patients with middle-low rectal cancer and to evaluate the safety and availability of this method.
- Citation: Zhou SC, Tian YT, Wang XW, Zhao CD, Ma S, Jiang J, Li EN, Zhou HT, Liu Q, Liang JW, Zhou ZX, Wang XS. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J Gastroenterol 2019; 25(31): 4502-4511
- URL: https://www.wjgnet.com/1007-9327/full/v25/i31/4502.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i31.4502
As a common malignant tumour of the digestive system, rectal cancer is associated with high morbidity and mortality[1]. Local recurrence greatly affects the treatment efficiency and the survival outcomes for patients with rectal cancer. Lateral pelvic lymph node (LPLN) metastasis (LPNM) is an important factor for local recurrence after surgery in patients with middle-low rectal cancer, and approximately 8.6% to 21.0% of patients with middle-low rectal cancer have associated LPNM[2-4]. As one of the effective treatment methods, laparoscopic LPLN dissection (LPND) can significantly reduce the local recurrence rate compared with simple total mesorectal excision (TME) surgery[3,5-7], and its safety and feasibility have also been confirmed by previous studies[8-10]. In clinical applications, laparoscopic LPND is limited by various complications because the ureters and hypogastric nerves might be damaged without efficient guidance.
Indocyanine green (ICG) is an inexpensive and safe non-specific fluorescent probe that has been approved by the FDA for clinical use since 1959 for cardiac and liver function tests. Recently, ICG fluorescence-guided imaging, as a new technique, has been applied to guide sentinel lymph node detection in breast cancer[11,12], gastric cancer[3,5,13-16], colorectal cancer[17,18], and other malignant tumours[19-21]. On this basis, using a near-infrared (NIR) camera system, the current study took advantage of ICG to guide the detection of LPLNs in patients with middle-low rectal cancer, aiming to investigate whether this technology could be safely and efficiently used for LPND in patients with rectal cancer.
After approval by the ethics committee of Cancer Hospital, Chinese Academy of Medical Sciences (NCC 2017-YZ-026, Oct 17, 2017), a total of 42 consecutive middle-low rectal cancer patients with clinical LPNM who underwent TME and LPND at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College between October 2017 and March 2019 were enrolled. The inclusion criteria of this study were as follows: (1) Patients with rectal adenocarcinoma confirmed by endoscopic biopsy; (2) Patients with suspected LPNM based on magnetic resonance imaging (MRI) evaluation; and (3) The tumour was located under the retroperitoneum (within 8 cm of the anal margin). The exclusion criteria were (1) recurrent patients and (2) patients with distant metastasis.
Patients who underwent TME and LPND with ICG-enhanced NIR fluorescence-guided imaging were assigned to an ICG group (n = 12), and patients who received conventional TME and LPND without ICG-enhanced NIR fluorescence-guided imaging were assigned to a non-ICG group (n = 30). All patients underwent the same preoperative examinations: routine blood test, hepatorenal function test, serum carcinoembryonic antigen (CEA), chest radiography, abdominal computed tomography (CT), endorectal ultrasonography, and pelvic MRI. Clinical LPNM was diagnosed based on MRI by two imaging specialists who specialized and had more than 10 years of experience in colorectal cancer. The assessment criteria were as follows: (1) ≥ 0.7 cm in short diameter; (2) ≥ 0.5 cm but ≤ 0.7 cm in short diameter with inhomogeneous or intense enhancement; and (3) Irregular shape and rough edges. Meeting one or more of the above criteria resulted in a diagnosis of LPNM. The pathological specimens were also examined by two pathologists who specialized in colorectal cancer. All patients in this study underwent surgery performed by four surgeons with more than 20 years of clinical experience. The American Joint Committee on Cancer (AJCC, the eighth edition) staging system was used for tumour staging. Additionally, written informed consent was obtained from each patient included in the study.
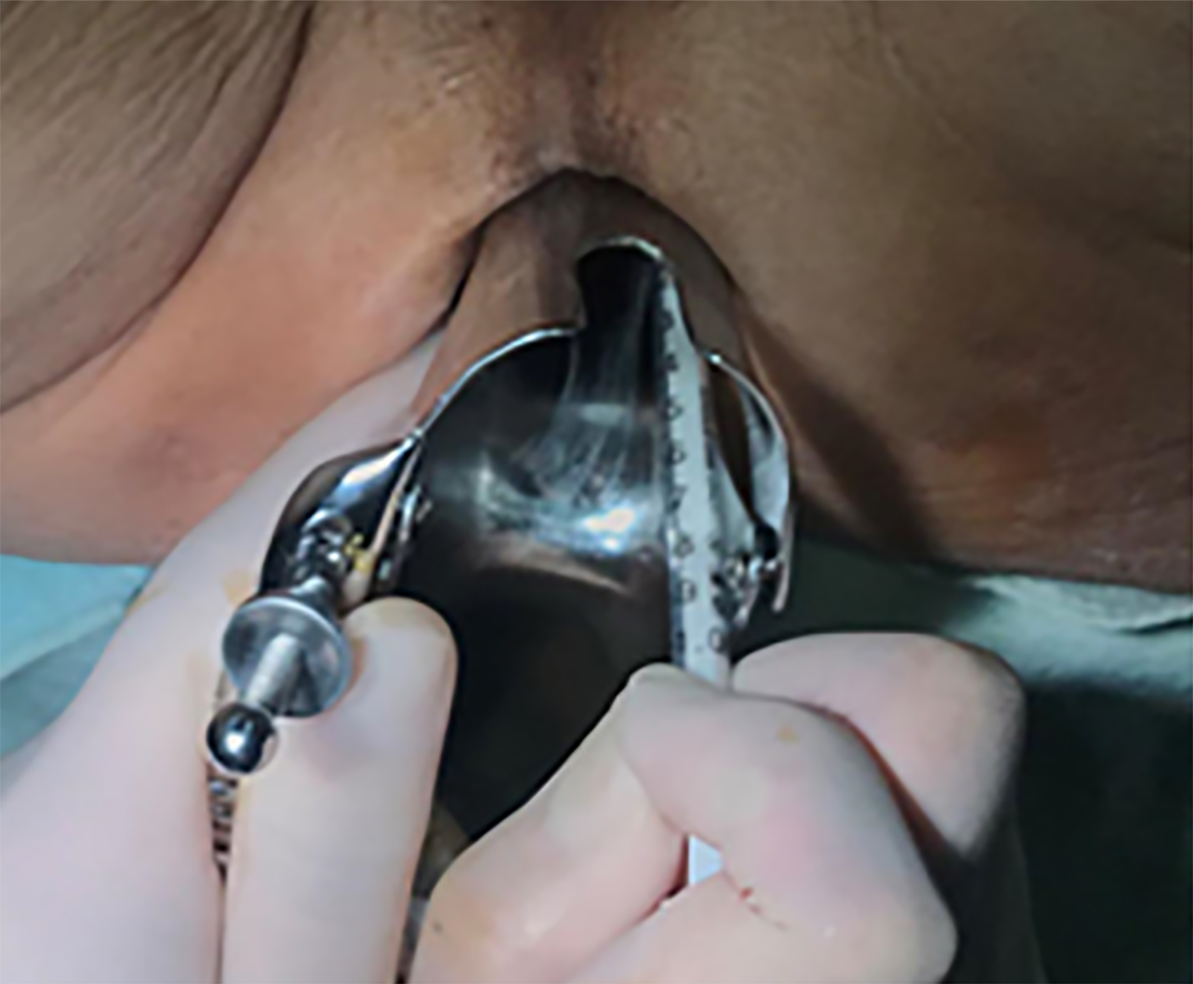
Perineal phase: All the patients were placed in the modified lithotomy position after anaesthesia. The anus was gently dilated until it was large enough to accommodate four fingers. To dilate the anus further and clearly observe the lower margin of the tumour, an anal dilator was placed into the proximal lip of the exposed mucosal edge with a vertical orientation. A fine needle (4.5 gauge) was inserted into the submucosal layer of the rectum through the anus, and ICG dye (Product Model: H20055881) was slowly injected into four sites on the anal side of the tumour. The total amount of injection for each patient was 4 mL (0.1 mg/mL) (Figure 1).
Laparoscopic phase: The five abdominal ports placed for laparoscopic LPND were the same as those used for TME. The laparoscopic skill was applied according to the radical principle. High ligation of the inferior mesenteric vessel, mobilization of the bowel, and dissection of the lymph nodes were performed laparoscopically, and the total mesorectal excision procedure with nerve-sparing techniques for rectal cancer was followed.
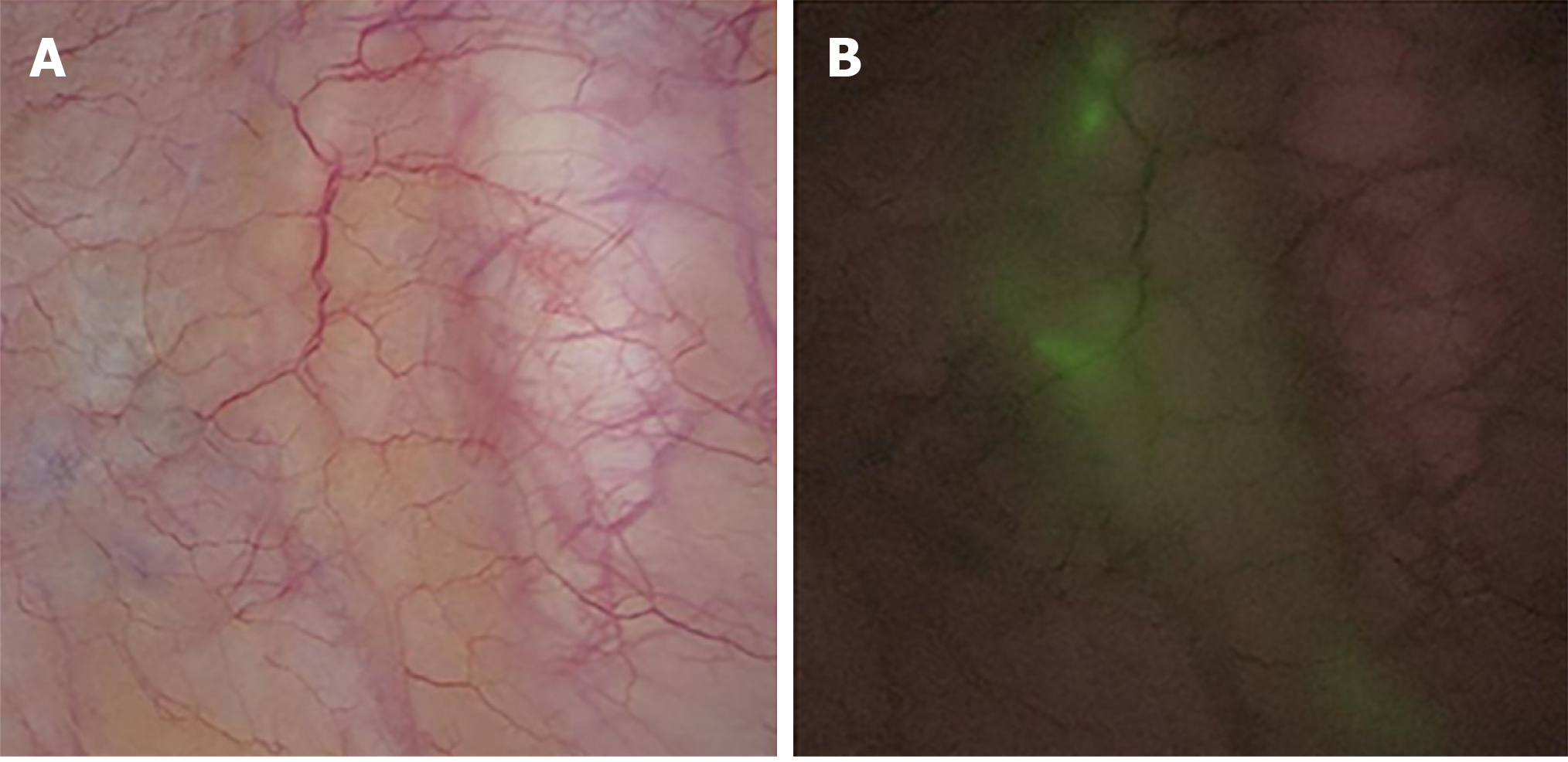
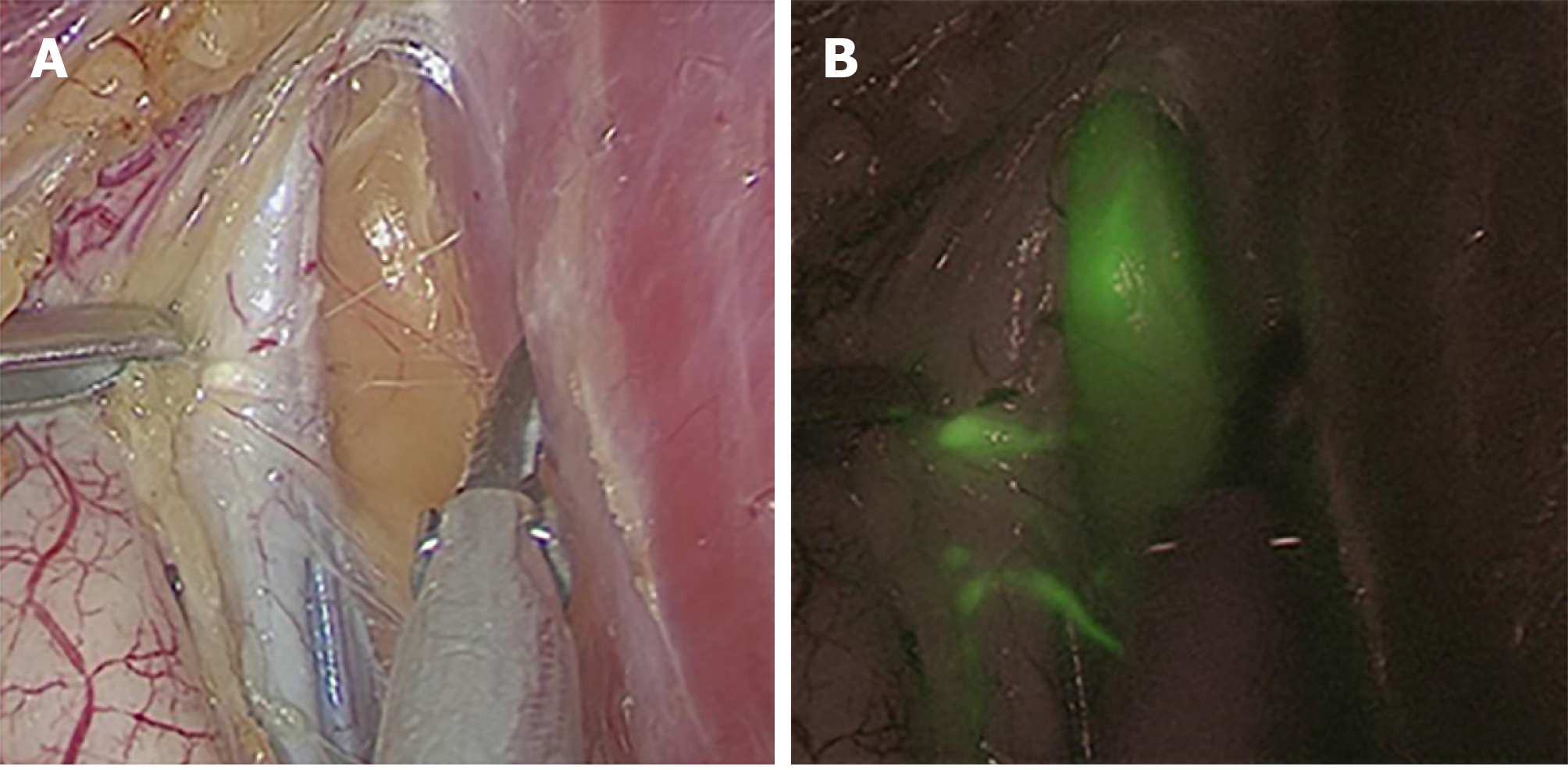
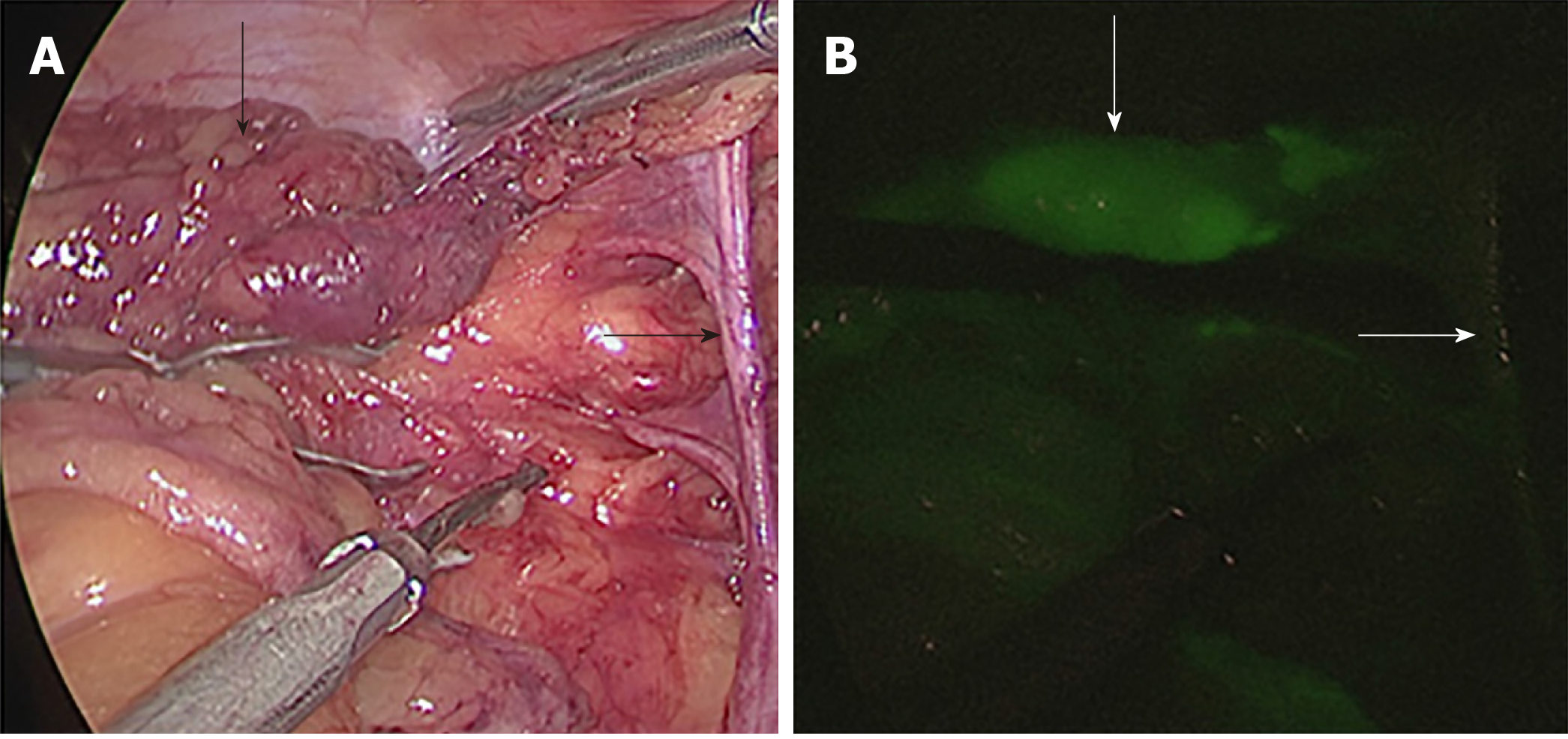
After the rectal tumour specimen was resected, ICG fluorescence-guided imaging was used via the near-infrared camera system (Karl Storz Endoskope spies TM GmbH & Co. KG, Tuttlingen, Germany) to guide lateral lymph node dissection. LPLNs were defined as lymph nodes distributed outside the pelvic plexus, including lymph nodes surrounding the internal iliac vessels, obturator fossa, and external iliac vessels. First, the direction of lateral lymph node drainage was determined by overall scanning under the guidance of ICG-enhanced NIR fluorescence-guided imaging (Figure 2). When performing LPND, the ureters and hypogastric nerve were first separated and elevated to prevent damage to these structures. Then, the lymph nodes were dissected in order along the external iliac vessels and the common iliac vessels as well as in the obturator fossa and along the internal iliac vessels, separately and carefully, and the ureters and hypogastric nerve were protected carefully. The light sources were switched via a footswitch to help the surgeon quickly and accurately distinguish between lymph nodes and non-lymphatic soft tissue (Figure 3). The blood vessels, nerves, and residual soft tissues were examined by ICG-enhanced NIR fluorescence-guided imaging to prevent the omission of lymph nodes during dissection (Figure 4).
Patients in the non-ICG group underwent the same surgical approach as those in the ICG group, except that no ICG-enhanced NIR fluorescence-guided imaging was used for LPND.
The Statistical Package for the Social Sciences (SPSS) version 24.0 for Windows (IBM Corp, Armonk, NY, United States) was used for data analyses. Quantitative data are shown as the mean ± standard deviation and were analysed by Student’s t-test. Categorical data are shown as frequencies and percentages and were analysed by the Chi-squared test or Fisher’s exact test. All tests were two-sided, and a P-value less than 0.05 was considered to indicate statistical significance.
The baseline data for the patients in the ICG group and the non-ICG group are shown in Table 1. There was no significant difference between the ICG group and the non-ICG group in terms of age, sex, BMI, ASA category, concomitant disease, type of operation, preoperative chemoradiotherapy, tumour differentiation, or tumour size (P > 0.05).
| Parameter | ICG group(n = 12) | Non-ICG group(n = 30) | P-value |
| Age (yr, mean ± SD) | 60.3 ± 9.6 | 58.5 ± 9.5 | 0.587 |
| Gender | 0.200 | ||
| Male | 5 (41.7) | 19 (63.3) | |
| Female | 7 (58.3) | 11 (36.7) | |
| BMI (kg/m2, mean ± SD) | 24.8 ± 2.8 | 25.3 ± 3.1 | 0.602 |
| ASA category | 0.785 | ||
| I | 2 (16.7) | 3 (10) | |
| II | 8 (66.7) | 23 (76.7) | |
| III | 2 (16.7) | 4 (13.3) | |
| Concomitant disease | 1.000 | ||
| Yes | 4 (33.3) | 9 (30) | |
| No | 8 (66.7) | 21 (70) | |
| Type of operation | |||
| Low anterior resection | 8 (66.7) | 16 (53.3) | 0.430 |
| Abdominoperineal resection | 4 (33.3) | 14 (46.7) | |
| Preoperative chemoradiotherapy | 2 (16.7) | 10 (33.3) | 0.483 |
| Tumor differentiation | 0.879 | ||
| Moderate | 7 (58.3) | 20 (66.7) | |
| Poor | 5 (41.7) | 10 (33.3) | |
| Tumor size (cm, mean ± SD) | 3.5 ± 1.4 | 3.4 ± 1.9 | 0.996 |
The surgical outcomes are shown in Table 2. There was a significant between-group difference in terms of intraoperative blood loss and the number of lateral lymph nodes harvested. Compared to the non-ICG group, the ICG group had significantly lower intraoperative blood loss (55.8 ± 37.5 mL vs 108.0 ± 52.7 mL, P = 0.003) and a greater number of LPLNs harvested (11.5 ± 5.9 vs 7.1 ± 4.8, P = 0.017). Except for these two parameters, there was no significant difference in other factors, including LPND, LPNM, operative time, conversion to laparotomy, pT stage, pN stage, pTNM stage, postoperative complication, and hospital stay (P > 0.05). There were two (16.7%) patients in the ICG group and seven (23.3%) patients non-ICG group who underwent bilateral dissection (P = 0.953), and LPNM was diagnosed in three (25%) patients in the ICG group and three (10%) patients in the non-ICG group. The mean operative time was 255.7 ± 65.2 min in the ICG group and 273.1 ± 73.3 min in the non-ICG group (P = 0.108). No patient in the ICG group was converted to open resection, and two patients in the non-ICG group were converted to open resection due to intraoperative injury of the internal iliac vein and obturator artery. There was no positive circumferential resection margin or distal margin in the two groups. Postoperative anastomotic leakage occurred in one patient in the ICG group and was cured by washout and drainage. Four patients in the non-ICG group had postoperative complications: one patient had an intraperitoneal haemorrhage and was cured by administering haemostatics and transfusion; two patients had wound infections and were healed after open drainage and using antibiotics; and one patient had lymphatic leakage and was cured by fasting and adequate drainage. There was no statistically significant difference in the postoperative hospital stay between the two groups (P = 0.393).
| Outcome | ICG group(n = 12) | Non-ICG group (n = 30) | P-value |
| LPND | 0.953 | ||
| Unilateral dissection | 10 (83.3) | 23 (76.7) | |
| Bilateral dissection | 2 (16.7) | 7 (23.3) | |
| LPNM | 0.443 | ||
| Yes | 3 (25) | 3 (10) | |
| No | 9 (75) | 27 (90) | |
| Operative time (min, mean ± SD) | 255.7 ± 65.2 | 273.1 ± 73.3 | 0.108 |
| Blood loss (mL, mean ± SD) | 55.8 ± 37.5 | 108.0 ± 52.7 | 0.003 |
| Conversion to laparotomy (case) | 0 (0) | 2 (6.67) | 1.000 |
| Number of LPLNs harvested | 11.5 ± 5.9 | 7.1 ± 4.8 | 0.017 |
| pT stage | 0.694 | ||
| I | 3 (25) | 3 (10) | |
| II | 2 (16.7) | 6 (20) | |
| III | 6 (50) | 18 (60) | |
| IV | 1 (8.3) | 3 (10) | |
| pN stage | 0.516 | ||
| 0 | 6 (50.0) | 13 (43.3) | |
| 1 | 2 (16.7) | 10 (33.3) | |
| 2 | 4 (33.3) | 7 (23.4) | |
| pTNM stage | 0.805 | ||
| I | 4 (33.3) | 7 (23.3) | |
| II | 2 (16.7) | 6 (20) | |
| III | 6 (50) | 17 (56.7) | |
| Postoperative complication (case) | 1 (8.33) | 4 (23.33) | 1.000 |
| Hospital stay (d, mean ± SD) | 9.2 ± 1.6 | 9.7 ± 2.0 | 0.393 |
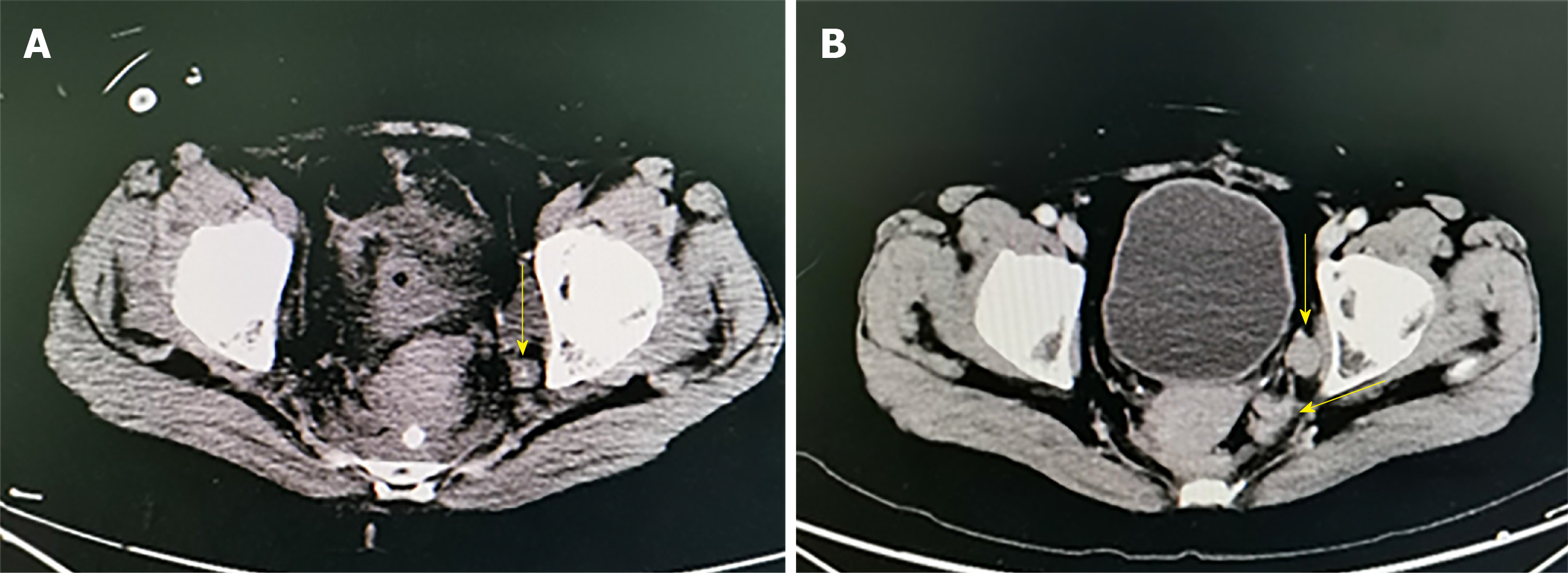
During follow-up, enlarged LPLNs were found in two cases under CT imaging in the non-ICG group. As shown in Figure 5, one patient developed ileus 10 days after surgery, and the CT revealed a residual lymph node in the distal left internal iliac artery. Another patient was found to have residual lymph nodes in the left obturator foramen region on CT re-examination 3 mo after surgery. There were omissions during LPND. Carcinoma infiltration was found based on the postoperative pathology after supplement surgery for further dissection of LPLNs. In addition, no residual tumours or recurrence was found in the ICG group.
The incidence of LPNM, which is one of the important metastatic pathways involved in middle-low rectal cancer, ranges from 8.6% to 21.0%[2-4]. LPNM indicates a worse prognosis and increased local recurrence. Sugihara et al[5] reported that the overall survival of patients with positive LPNM was significantly worse than that of stage III patients with negative LPNM (45.8% vs 71.2%, P < 0.001). In addition, the JCOG0212 clinical study of Japan showed that the recurrence rate of stage II/III patients was 13% (44/350) after TME surgery, while the LPLN recurrence rate was 56.8% (25/44)[22]. Therefore, LPLNs suspected of LPLM should be thoroughly dissected if the technique is feasible. However, LPND is controversial due to its technical difficulties and the complicated anatomy of the pelvic sidewall. Moreover, the tissue oedema and fibrosis induced by chemoradiotherapy further increase the difficulty of the surgery and thus have reduced the number of lymph node dissections.
Medical knowledge is constantly updated and changing. With the accumulation of clinical experience and new research broadening our knowledge, new surgical techniques and methods are being introduced. ICG-enhanced NIR fluorescence-guided imaging can provide a higher tissue penetration depth and better signal-to-background ratio, so it is widely used in gastrointestinal perfusion assessment and lymph node dissection in various malignant tumours[3,5,13-21]. After peritumoural injection of ICG dye, sentinel lymph nodes and lymphatic channels between the tumour and lymph nodes can be identified. Noura et al[23] showed that the ICG fluorescence-guided imaging system was considered to be a practical and feasible technique for the detection of lateral sentinel nodes in middle-low rectal cancer and that it could be used to determine indications for LPND. In the current study, ICG-enhanced NIR fluorescence-guided imaging helped the surgeon to distinguish lymph nodes from non-lymphatic soft tissues to increase the intra-operative protection of blood vessels and nerves and to improve the accuracy of detection in order to enable radical lymph node dissection. The results showed that compared to the non-ICG group, the ICG group had significantly lower intraoperative blood loss (55.8 ± 37.5 mL vs 108.0 ± 52.7 mL, P = 0.003) and a greater number of LPLNs harvested (11.5 ± 5.9 vs 7.1 ± 4.8, P = 0.017). This might be because ICG-enhanced NIR fluorescence-guided imaging can provide better sensitivity and specificity for lymph node detection. We believe that this novel technique could improve intra-operative surgical staging and ultimately lead to a better oncological outcome[24].
In this paper, we preferred to inject ICG into the submucosal layer of the rectum through the anus. Because of the high risk of ICG leakage during or after subserosal injection, this approach can significantly affect the target of fluorescence guidance. In addition, when ICG solution is administered at a higher concentration, the preparation penetrates into the soft tissue surrounding the lymph nodes during the dispersal process, and some lymph nodes are prone to accumulating ICG. This may lead to a local decrease in the initial fluorescence intensity after injection, and it may further interfere with the detection of lymph node fluorescence. Therefore, an ICG solution at a concentration of 0.1 g/mL was used in the present study.
Ogura et al[25] reported that in patients with short axes of equal to or greater than 7 mm after neoadjuvant chemoradiotherapy, the 5-year local recurrence rate of LPLNs was 5.7% after the patients underwent TME + LPND, and 51% of the patients demonstrated pathologically proven lateral lymph node metastases. Postoperative recurrence of LPLNs may be related to missing some lymph node metastases or to omission in difficult areas such as the internal iliac artery during intraoperative dissection of the LPLNs[8,26]. In the present study, two patients in the non-ICG group experienced incomplete LPND. Consequently, ICG-enhanced NIR fluorescence-guided imaging can accurately display the routes of lymph nodes and lymphatic vessels, providing clues for the detection of occult or missing lymph nodes, improving the accurate resection range, and thereby further reducing the risk of recurrence of LPLNs.
This study was limited by the small sample size, which might have caused some differences between the ICG group and non-ICG group that were not found in the current study. For example, the results showed a certain advantage for the conversion to laparotomy, as there was no patient that was converted to laparotomy in the ICG group, while two patients were converted to laparotomy in the non-ICG group, but no significant difference was found. Hence, the sample size should be expanded in further studies to fully analyse the effect of ICG-enhanced NIR fluorescence-guided imaging on laparoscopic LPND in rectal cancer.
In conclusion, this study showed that ICG-enhanced NIR fluorescence-guided imaging could be a feasible and convenient technique to assist LPND, as it could bring specific advantages regarding accuracy and completeness of surgery as well as safety. Moreover, this novel technique might make the surgery much easier and shorten the learning curve for surgeons who are not familiar with LPND.
Recently, ICG fluorescence-guided imaging has been applied to guide sentinel lymph node detection in various malignant tumours. As an effective treatment for lateral pelvic lymph node (LPLN) metastasis (LPNM), laparoscopic LPLN dissection (LPND) is limited due to the complicated anatomy of the pelvic sidewall and various complications after surgery. With regard to improving the accuracy and completeness of LPND as well as in terms of safety, we tried an innovative method using ICG visualized with a near-infrared camera (NIR) system to guide the detection of LPLNs in patients with middle-low rectal cancer.
The purpose of this study was to compare and analyse the clinical and pathological outcomes of LPND via an ICG-enhanced NIR fluorescence-guided imaging procedure vs a traditional procedure. The significance of this study is that it introduces a more effective and safe method for rectal cancer patients who undergo LPND.
The study aimed to evaluate the safety and availability of LPND via ICG-enhanced NIR fluorescence-guided imaging in patients with rectal cancer.
Middle-low rectal cancer patients who underwent total mesorectal excision (TME) and LPND were systemically reviewed between October 2017 and March 2019 at our institution. Clinical characteristics, operative outcomes, pathological outcomes, and postoperative complication information were collected and analysed using SPSS version 24.0 between the two groups.
The results showed that intraoperative blood loss was significantly lower in the ICG group than in the non-ICG group (P = 0.003). Compared to the non-ICG group, the ICG group had a significantly larger number of LPLNs harvested (11.5 ± 5.9 vs 7.1 ± 4.8, P = 0.017). In addition, no significant difference was found in terms of LPND, LPNM, operative time, conversion to laparotomy, preoperative complication, or hospital stay (P > 0.05).
ICG-enhanced NIR fluorescence-guided imaging could be a feasible and convenient technique to guide LPND because it could increase the number of LPLNs harvested and bring specific advantages regarding the accuracy and completeness of surgery as well as safety.
In this study, we emphasized that the location and concentration of ICG injection are critical for surgical outcome. Moreover, this is a retrospective study with a small sample size, and bias may exist. Further randomized prospective controlled trials are needed to confirm our results.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brisinda G, Ciocalteu A, Hori T, Perse M, Seow-Choen F S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Li X
| 1. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3301] [Article Influence: 412.6] [Reference Citation Analysis (3)] |
| 2. | Sato H, Maeda K, Maruta M. Prognostic significance of lateral lymph node dissection in node positive low rectal carcinoma. Int J Colorectal Dis. 2011;26:881-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Ishihara S, Kawai K, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Morikawa T, Watanabe T. Oncological Outcomes of Lateral Pelvic Lymph Node Metastasis in Rectal Cancer Treated With Preoperative Chemoradiotherapy. Dis Colon Rectum. 2017;60:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Nagasaki T, Akiyoshi T, Fujimoto Y, Konishi T, Nagayama S, Fukunaga Y, Ueno M. Preoperative Chemoradiotherapy Might Improve the Prognosis of Patients with Locally Advanced Low Rectal Cancer and Lateral Pelvic Lymph Node Metastases. World J Surg. 2017;41:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Sugihara K, Kobayashi H, Kato T, Mori T, Mochizuki H, Kameoka S, Shirouzu K, Muto T. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum. 2006;49:1663-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 338] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 6. | Suzuki K, Muto T, Sawada T. Prevention of local recurrence by extended lymphadenectomy for rectal cancer. Surg Today. 1995;25:795-801. [PubMed] |
| 7. | Moriya Y, Sugihara K, Akasu T, Fujita S. Patterns of recurrence after nerve-sparing surgery for rectal adenocarcinoma with special reference to loco-regional recurrence. Dis Colon Rectum. 1995;38:1162-1168. [PubMed] |
| 8. | Liang JT. Technical feasibility of laparoscopic lateral pelvic lymph node dissection for patients with low rectal cancer after concurrent chemoradiation therapy. Ann Surg Oncol. 2011;18:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Furuhata T, Okita K, Nishidate T, Ito T, Yamaguchi H, Ueki T, Akizuki E, Meguro M, Ogawa T, Kukita K, Kimura Y, Mizuguchi T, Hirata K. Clinical feasibility of laparoscopic lateral pelvic lymph node dissection following total mesorectal excision for advanced rectal cancer. Surg Today. 2015;45:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 10. | Ogura A, Akiyoshi T, Nagasaki T, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y, Ueno M, Kuroyanagi H. Feasibility of Laparoscopic Total Mesorectal Excision with Extended Lateral Pelvic Lymph Node Dissection for Advanced Lower Rectal Cancer after Preoperative Chemoradiotherapy. World J Surg. 2017;41:868-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast. 2010;19:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 12. | Hirche C, Murawa D, Mohr Z, Kneif S, Hünerbein M. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat. 2010;121:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Kelder W, Nimura H, Takahashi N, Mitsumori N, van Dam GM, Yanaga K. Sentinel node mapping with indocyanine green (ICG) and infrared ray detection in early gastric cancer: an accurate method that enables a limited lymphadenectomy. Eur J Surg Oncol. 2010;36:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Tajima Y, Murakami M, Yamazaki K, Masuda Y, Kato M, Sato A, Goto S, Otsuka K, Kato T, Kusano M. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann Surg Oncol. 2010;17:1787-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Sherwinter DA, Gallagher J, Donkar T. Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Colorectal Dis. 2013;15:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V, Soler L, Barry B, Namer IJ, Demartines N, Charles AL, Geny B, Marescaux J. Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg. 2014;259:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Andersen HS, Bennedsen ALB, Burgdorf SK, Eriksen JR, Eiholm S, Toxværd A, Riis LB, Rosenberg J, Gögenur I. In vivo and ex vivo sentinel node mapping does not identify the same lymph nodes in colon cancer. Int J Colorectal Dis. 2017;32:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Currie AC, Brigic A, Thomas-Gibson S, Suzuki N, Moorghen M, Jenkins JT, Faiz OD, Kennedy RH. A pilot study to assess near infrared laparoscopy with indocyanine green (ICG) for intraoperative sentinel lymph node mapping in early colon cancer. Eur J Surg Oncol. 2017;43:2044-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Gilmore DM, Khullar OV, Gioux S, Stockdale A, Frangioni JV, Colson YL, Russell SE. Effective low-dose escalation of indocyanine green for near-infrared fluorescent sentinel lymph node mapping in melanoma. Ann Surg Oncol. 2013;20:2357-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Tanaka R, Nakashima K, Fujimoto W. Sentinel lymph node detection in skin cancer using fluorescence navigation with indocyanine green. J Dermatol. 2009;36:468-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Holloway RW, Bravo RA, Rakowski JA, James JA, Jeppson CN, Ingersoll SB, Ahmad S. Detection of sentinel lymph nodes in patients with endometrial cancer undergoing robotic-assisted staging: a comparison of colorimetric and fluorescence imaging. Gynecol Oncol. 2012;126:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Fujita S, Akasu T, Mizusawa J, Saito N, Kinugasa Y, Kanemitsu Y, Ohue M, Fujii S, Shiozawa M, Yamaguchi T, Moriya Y; Colorectal Cancer Study Group of Japan Clinical Oncology Group. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol. 2012;13:616-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 23. | Noura S, Ohue M, Seki Y, Tanaka K, Motoori M, Kishi K, Miyashiro I, Ohigashi H, Yano M, Ishikawa O, Miyamoto Y. Feasibility of a lateral region sentinel node biopsy of lower rectal cancer guided by indocyanine green using a near-infrared camera system. Ann Surg Oncol. 2010;17:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, Sugihara K, Watanabe M, Moriya Y, Kitano S; Japan Clinical Oncology Group Colorectal Cancer Study Group. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg. 2014;260:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 25. | Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, van de Velde CJH, Beets GL, Rutten HJT, Kusters M; Lateral Node Study Consortium. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol. 2019;37:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 369] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 26. | Nagayoshi K, Ueki T, Manabe T, Moriyama T, Yanai K, Oda Y, Tanaka M. Laparoscopic lateral pelvic lymph node dissection is achievable and offers advantages as a minimally invasive surgery over the open approach. Surg Endosc. 2016;30:1938-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |