Published online Aug 21, 2019. doi: 10.3748/wjg.v25.i31.4383
Peer-review started: April 25, 2019
First decision: May 24, 2019
Revised: June 7, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 21, 2019
Processing time: 118 Days and 21.4 Hours
Systemic inflammation is a marker of poor prognosis preoperatively present in around 20%-40% of colorectal cancer patients. The hallmarks of systemic inflammation include an increased production of proinflammatory cytokines and acute phase proteins that enter the circulation. While the low-level systemic inflammation is often clinically silent, its consequences are many and may ultimately lead to chronic cancer-associated wasting, cachexia. In this review, we discuss the pathogenesis of cancer-related systemic inflammation, explore the role of systemic inflammation in promoting cancer growth, escaping antitumor defense, and shifting metabolic pathways, and how these changes are related to less favorable outcome.
Core tip: Increasing evidence indicates that systemic inflammation has wide-ranging effects on colorectal cancer (CRC) pathogenesis, spanning from supporting primary tumor growth by promoting tumor cell proliferation to helping angiogenesis by enhancing the availability of pro-angiogenic molecules, to suppressing anti-tumor immunity by recruiting anti-inflammatory cell types, and to shaping pre-metastatic niches to promote subsequent metastasis. Systemic inflammatory biomarkers, such as circulating acute phase proteins, cytokines, exosomes, and leukocytes, may help to classify CRC patients into useful prognostic categories. However, further larger-scale studies are needed to determine optimal marker combinations for selecting patients to receive specific treatments.
- Citation: Tuomisto AE, Mäkinen MJ, Väyrynen JP. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J Gastroenterol 2019; 25(31): 4383-4404
- URL: https://www.wjgnet.com/1007-9327/full/v25/i31/4383.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i31.4383
Interactions between tumor and host are important regulators of tumor progression[1-3]. These interactions are mediated by a complex network of cytokines, chemokines, growth factors, and matrix remodeling enzymes[4], reaching beyond the local tumor microenvironment and evoking systemic responses[1,5] that have an effect on the course of the disease[6]. Therefore, cancer progression is not only determined by factors intrinsic for the tumor, but is largely directed by multifaceted systemic processes[5].
Colorectal cancer (CRC) is the third most common cancer in the Western World, and the second most common cause of cancer deaths[7]. Systemic inflammation is most common in poorly differentiated and advanced CRC[8,9], but despite that it is also an independent indicator of less favorable outcome in CRC [10,11] and associated with shorter survival[6,12,13]. In the case of resectable disease, 21%–41% of CRC patients have increased serum levels of acute phase proteins such as CRP (C-reactive protein), indicating a systemic inflammatory response to the tumor[8,9,14].
Cancer-associated systemic inflammation is characterized by numerous alterations in many organ systems distant from the site or sites of inflammation. Activation of systemic inflammatory response in the liver results in a rapid increase in the production of acute phase proteins, such as CRP[15]. Many disabling symptoms of cancer patients, such as fever, anemia, fatigue and loss of appetite can be attributed to the presence of systemic inflammation, and finally, metabolic changes such as loss of muscle and negative nitrogen balance manifest in cachexia, a cancer-associated wasting syndrome[16].
Many markers of systemic inflammation are based on counts, ratios, or scores of circulating white cells or acute phase proteins, such as neutrophil/lymphocyte ratio (NLR) and Modified Glasgow Prognostic score (mGPS), a measure based on elevated serum CRP level and decreased serum albumin level[17], but more recent studies have also evaluated the significance of alterations in circulating cytokine, chemokine, and growth factor milieu[18-20], platelet transcriptome[21], or the composition of tumor-derived extracellular vesicles[22]. Released by tumor cells or non-neoplastic cells in tumor-elicited host reaction, IL6 (interleukin-6) is one of the most important mediators of systemic effects of inflammation, such as the production of acute phase proteins in the liver[15] and in cancer cachexia[16].
In this review, we aim to provide an overview of the factors contributing to systemic inflammatory responses in CRC, of their downstream processes in the responding tissues, as well as of the prognostic significance of systemic inflammatory markers in CRC.
A literature search using PubMed was conducted to identify articles relevant to the topic, using search terms: (“colorectal cancer” or “colon cancer” or “rectal cancer” or “colorectal neoplasia”) and (“systemic inflammation” or “CRP” or “Glasgow Prognostic Score” or “interleukin” or “chemokine” or “IL6” or “cytokine” or “CXCL*” or “CCL*” or “cachexia” or “inflammation” or “premetastatic niche”). The last search update was performed in March 2019. The titles and abstracts of the studies were screened for studies relevant to the review topic. Additional relevant publications were identified from the bibliographies of the included studies. Finally, this review was based on 196 publications identified during the search.
The hallmarks of systemic inflammation in cancer patients include an increased production of proinflammatory cytokines and acute phase proteins that enter the circulation. Indeed, many proteins regulating the function of immune cells, or produced in large quantities by immune cells or in inflammatory conditions, have been reported to show altered serum levels in CRC patients compared to controls (Table 1). For example, when evaluating serum profiles of 13 cytokines, chemokines, and growth factors in 116 CRC patients and 86 healthy controls, it was found that serum levels of five of these proteins showed statistically significant alterations, including increased serum IL6, IL7, CXCL8 (IL8), and PDGFB levels, and decreased serum CCL2 levels[18]. Increased serum IL6 and CXCL8 levels in CRC have been reported in many studies, also summarized in two recent meta-analyses[23,24]. Further highlighting the presence of systemic inflammatory markers in the sera of CRC patients, in a systematic review and meta-analysis of diagnostic and prognostic serum biomarkers of CRC[25], several of the most frequently reported diagnostic markers, such as CRP, VEGFA, and TIMP1, were related to the systemic inflammatory response.
| Marker | Function | Detection method | Samples | Ref. | |
| Acute phase proteins | |||||
| CRP (C-reactive protein) | ↑ | Acute phase protein | ELISA | serum | Gunter et al[157] |
| HP (haptoglobin) | ↑ | Hemoglobin-binding acute phase protein | ELISA | serum | Sun et al[158] |
| Ferritin | ↓ | Protein that stores iron | meta-analysis | serum | Feng et al[159] |
| Cytokines and chemokines | |||||
| IL6 | ↑ | Proinflammatory cytokine | meta-analysis | serum | Xu et al[23] |
| IL7 | ↑ | Cytokine involved in lymphocyte maturation | Multiplex magnetic bead assay | serum | Kantola et al[18] |
| IL17A | ↑ | Proinflammatory cytokine | meta-analysis | serum | Yan et al[160] |
| IL22 | ↑ | Cytokine contributing to tissue homeostasis | meta-analysis | serum | Yan et al[160] |
| IL23 | ↑ | Proinflammatory cytokine | meta-analysis | serum | Yan et al[160] |
| CCL2 | ↓ | Recruitment of monocytes and macrophages | Multiplex magnetic bead assay | serum | Kantola et al[18] |
| CXCL5 | ↑ | Recruitment of neutrophils | ELISA | serum | Kawamura et al[161] |
| CXCL8 (IL8) | ↑ | Recruitment of neutrophils | meta-analysis | serum | Jin et al[24] |
| CXCL10 | ↑ | Recruitment of T cells and NK cells | ELISA | serum | Toiyama et el [162] |
| CXCL16 | ↑ | Recruitment of T cells and NK cells | ELISA | serum | Matsushita et al[163] |
| SPP1 (secreted phosphoprotein 1) | ↑ | Leukocyte chemotaxis | streptavidin–biotin sandwich assay | serum | Werner et al[164] |
| Protease enzymes and their inhibitors | |||||
| MMP8 | ↑ | Protease enzyme also cleaving cytokines | immunofluorometric assay | serum | Väyrynen et al[165] |
| MMP9 | Degradation of extracellular matrix and regulation of neutrophil action | ELISA | serum | Wilson et al[166] | |
| TIMP1 | ↑ | Inhibitor of metalloproteinases | meta-analysis | serum | Meng et al[167] |
| Growth factors and their inhibitors | |||||
| ANGPTL2 | ↑ | Growth factor contributing to the regulation of inflammation and angiogenesis | ELISA | serum | Toiyama et al[168] |
| ESM1 | ↑ | Secreted angiogenic factor | ELISA | serum | Jiang et al[169] |
| PDGFB | ↑ | Proliferation of mesenchymal cells | Multiplex magnetic bead assay | serum | Kantola et al[18] |
| VEGFA | ↑ | Vascular growth factor | ELISA | serum | George et el[196] |
| VEGFC | ↑ | Vascular growth factor | ELISA | serum | Wang et al[170] |
| Markers of metabolism | |||||
| glucose (fasting) | ↑ | Energy source | G6PD | serum | Ferroni et al[171] |
| HbA1c | Oxygen carrier | HPLC Analyzer | serum | Ferroni et al[171] | |
| insulin (fasting) | ↑ | Regulator of metabolism | ELISA | serum | Ferroni et al[171] |
| ECM/endothelium-derived signaling proteins | |||||
| Endostatin | ↑ | Angiogenesis inhibitor | ELISA | serum | Kantola et al[173] |
| POSTN (periostin) | ↑ | ECM protein | ELISA | serum | Ben et al[174] |
| VASTATIN | ↑ | Collagen VIII derived matrikine | ELISA | serum | Willumsen et al[172] |
| VCAM-1 (soluble) | ↑ | Multifunctional | ELISA | serum | Toiyama et el[175] |
| Other signaling molecules | |||||
| DAND5 | ↑ | BMP inhibitor | ELISA | serum | Miao et al[176] |
| LRP (leptin) | ↓ | Regulator of metabolism | ELISA | serum | Kumor et al[177] |
| Resistin | ↑ | Regulator of metabolism | ELISA | serum | Kumor et al[177] |
The factors driving the systemic inflammatory response in CRC patients are complex and thereby not clear, but they are related to the interaction between neoplastic cells and the surrounding tumor microenvironment involving in-flammatory cells, fibroblasts, extracellular matrix, and vasculature[5]. While tumor cells can variably express different cytokines and chemokines[26], immune cells and fibroblasts are capable of producing many of these factors at much higher levels[27-29].
Cancer cells express highly variable amounts of different cytokines, chemokines, and growth factors in vitro[26,30]. These include IL6, CCL2, CXCL8, CSF1 (macrophage colony-stimulating factor, M-CSF), and CSF2 (granulocyte-macrophage colony-stimulating factor, GM-CSF) (Table 2). These molecules contribute to a variety of functions related to systemic inflammation and cancer progression. For example, IL6, a seminal proinflammatory cytokine, regulates the acute phase response through the induction of acute phase proteins in hepatocytes and the differentiation of monocytes to macrophages[31], whereas CCL2 is essential for the recruitment of bone marrow derived monocytes into peripheral organs and tumors[32]. CXCL8 is an important proinflammatory chemokine, recruiting granulocytes but also promoting angiogenesis[33]. Both CSF1 and CSF2 stimulate the proliferation, differentiation, and survival of monocytes and macrophages, but while CSF1 is involved in M2-like anti-inflammatory macrophage polarization, CSF2 contributes to M1-like pro-inflammatory macrophage polarization[34].
| Inflammatory mediator | Function | Detection method | Samples | Ref. |
| IL6 | Proinflammatory cytokine | IHC, RT-PCR | FFPE CRC specimens | Zeng et al[178] |
| CSF1 | Proliferation, differentiation, and survival of monocytes, macrophages, and bone marrow progenitor cells; polarization of pro-tumor M2 macrophages | IHC | FFPE CRC specimens | Nebiker et al[179] |
| CSF2 | Proliferation, differentiation, and survival of monocytes, macrophages, granulocytes and bone marrow progenitor cells, polarization of anti-tumor M1 macrophages | IHC | FFPE CRC specimens | Nebiker et al[179] |
| CCL2 | Recruitment of monocytes and macrophages | IHC, WB | CRC cell lines, FFPE CRC specimens | Hu et al[180] |
| CXCL1 | Recruitment of neutrophils | IHC | FFPE CRC specimens | Oladipo et al[181] |
| CXCL8 | Recruitment of neutrophils | IHC, IF, WB | CRC cell lines, FFPE CRC specimens | Xiao et al[30] |
| CXCL8 | Recruitment of neutrophils | IHC | FFPE CRC specimens | Oladipo et al[181] |
| CXCL10 | Recruitment of T cells and NK cells | IHC, RT-PCR | CRC cell lines, FFPE CRC specimens | Jiang et al[182] |
| CXCL12 | Recruitment of lymphocytes and endothelial progenitor cells | IHC | FFPE CRC specimens | Akishima-Fukasawa et al[183] |
| VEGFA | Angiogenesis | IHC | FFPE CRC specimens | Tuomisto et al[184] |
Tumor-derived extracellular vesicles have recently gained more and more interest as potential regulators of tumor cell-immune cell interactions[35,36]. They are a heterogeneous group of lipid bilayer-delimited particles released by tumor cells in the tumor microenvironment and into the circulation[35,36]. They have been implicated in a variety of functions in tumor progression, such as contributing to angiogenesis, vascular leakiness, regulation of immune responses, and reprogramming of stromal recipient cells in subsequent metastatic areas[35,36]. Based on their size and contents, they can be divided into subcategories, such as microvesicles, exosomes, ectosomes, and oncosomes.
The contents of tumor-derived extracellular vesicles can be highly variable. For example, they have been reported to contain immunosuppressive proteins such as TGFB[37,38], protease enzymes such as MMP9[38], and growth factors such as IGF1[38]. Moreover, nucleic acids (micro RNAs, miRNAs; and long non-coding RNAs, lncRNAs) can be found in tumor-derived extracellular vesicles, and these can contribute to tumor cell and stromal cell proliferation and apoptosis, and the regulation of immune responses against the tumor[35,36]. For example, exosome-carried miR-21, miR-29a, and miR-222-3p have been associated with immunoregulatory functions in various tumor types[39]. However, current knowledge of miRNAs in the regulation of immune reactions is limited, and further investigation is required to show more clearly the significance of exosome-carried mRNAs in systemic inflammatory reactions, relative to other factors[35].
CRCs are infiltrated by a heterogeneous population of immune and inflammatory cells, including proinflammatory cells, such as CD8+ cytotoxic T cells, type 1 CD4+ helper T cells (Th1 cells), NK cells, and M1 macrophages, anti-inflammatory cells such as regulatory T cells (Treg), type 2 helper T cells (Th2 cells), M2 macrophages, and myeloid derived suppressor cells (MDSCs). Other cells include B lymphocytes, plasma cells, neutrophils, eosinophils and mast cells that co-operate with both immunoenhancing and immunosuppressing cells[40,41]. In contrast to systemic inflammatory response, which is associated with adverse outcome[12], an intense immune cell infiltrate, evaluated using hematoxylin and eosin stained sections[42-45] or by immunohistochemistry using antibodies to specific immune cell markers[46-50], has frequently been associated with improved survival in CRC, independent of tumor stage or other prognostic parameters. This has been attributed to the ability of immune cells to recognize transformed malignant cells and restrict tumor growth (immunosurveillance hypothesis)[3,51]. However, some types of immune cells such as Th17 cells, characterized by their production of IL17, a proinflammatory cytokine, have been associated with poor survival[52].
Immune cells are considered an important source of cytokines, chemokines, and growth factors in tumor microenvironment (Table 3), but a few recent studies have shown an inverse correlation or lack of correlation between local immune response and systemic inflammation. A recent study evaluated the relationships between serum levels of 13 cytokines and the densities of eight types of tumor-infiltrating immune cells (CD3+, CD8+, and FOXP3+ T cells, CD68+ macrophages, CD1a+ dendritic cells, CD83+ dendritic cells, ELANE+ neutrophils, and tryptase+ mast cells) in a cohort of 147 stage I–IV CRC patients. In that study, serum cytokines and tumor-infiltrating immune cells in CRC represented entities with high intra-group correlations but relatively weak positive inter-group correlations. High macrophage density was associated with increased serum CCL4 levels (which could reflect CCL4 production by macrophages or recruitment of CCR5+ macrophages in tumors as a response to CCL4) and high densities of CD3+ and CD8+ T cells were associated with increased serum IL12 levels (which indicates that systemic IL-12 levels may contribute to or reflect tumor-associated Th1 response). Yet another study reported a trend towards an inverse relationship between local inflammation and systemic inflammation in a cohort of stage II colon cancer patients[53].
| Cell type | Inflammatory mediators | Functions | Ref. |
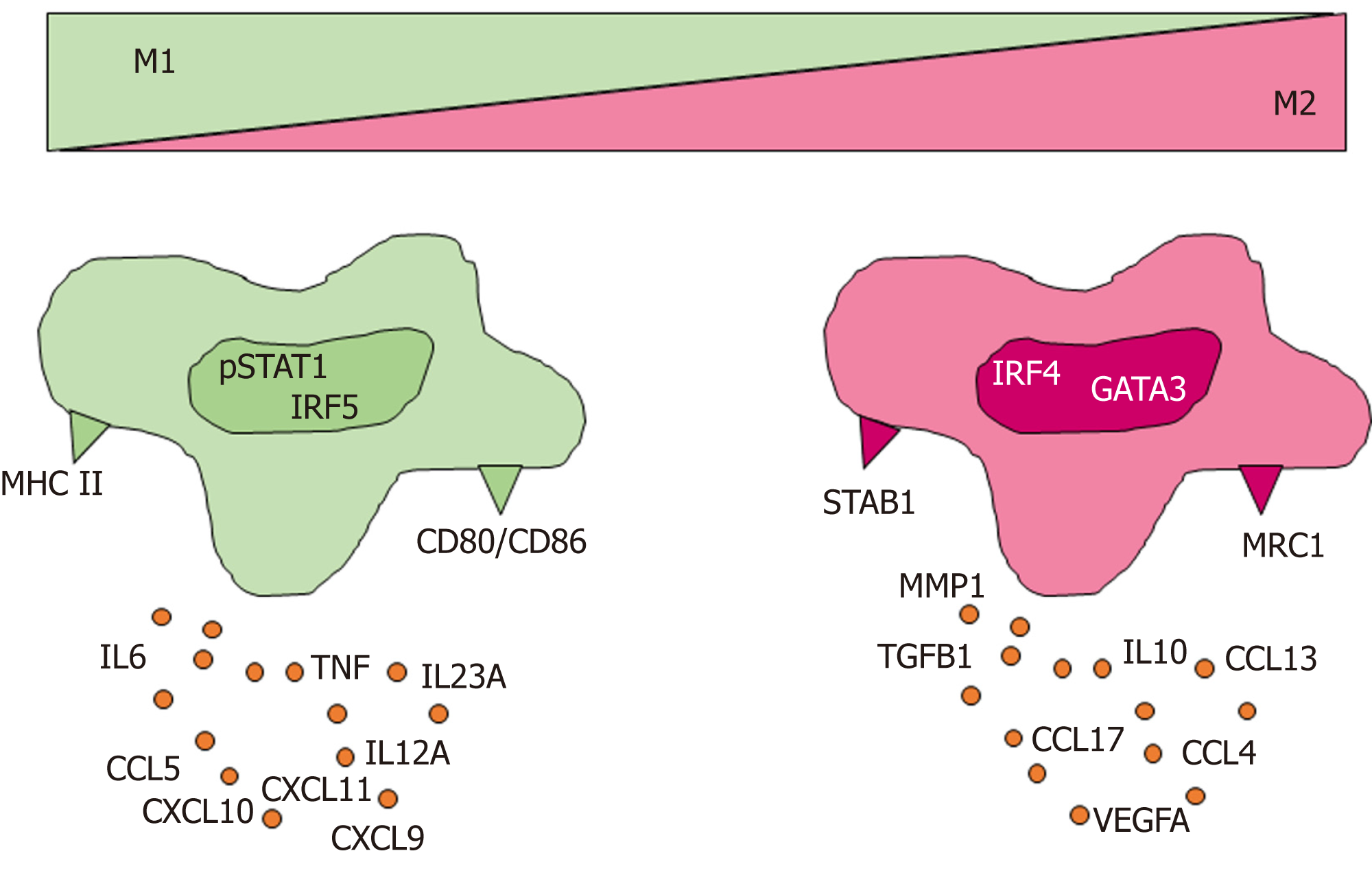
| M1 macrophage | IL6, TNF, IL12A, IL12B, IL23A, CXCL5, CXCL9, CXCL10, CXCL11, | Activation of inflammation | Murray et al[178] |
| M2 macrophage | IL10, CCL4, CCL13, CCL17, CCL18, MMP1, TGFB1 | Resolution of inflammation | Murray et al[47] |
| Th1 lymphocyte | IFNG, IL2 | Activation of cytotoxic immune response | Zhu et al[55] |
| Th2 lymphocyte | IL4, IL5, IL10, IL13 | Activation of humoral immune response | Zhu et al[55] |
| Th17 lymphocyte | IL17A, IL17F, IL21, IL22 | Activation of neutrophils | Zhu et al[55] |
| Treg lymphocyte | TGFB | Immunosuppression | Zhu et al[55] |
| Plasma cell | IL10, IL35. TNF, IL17A, CSF2 | Both pro- and anti-inflammatory mediators | Dang et al[156] |
| Neutrophil | IL1A, IL1B, IL1RA, IL6, IL12 CXCL8, CXCL9, CXCL10, CXCL11, CCL2, CCL3, CCL4, TGFB1, VEGFA | Activation of inflammation; depending on the type of polarization, also anti-inflammatory mediators are secreted | Tecchio et al[58] |
| Eosinophil | IL1A, IL2, IL4, IL6, IL12, CXCL1, CXCL8, CXCL10, CCL3, CCL5, CCL11 | Th2 type immune responses | Davoine et al[185] |
| Myeloid derived suppressor cell | IL10, TGFB | Immunosuppression | Bronte et al[87] |
| Mast cell | IL4, IL5, IL6, TNF, CSF2 | Th2 type immune responses | Amin et al[186] |
The reasons underlying the relative weakness of the observed associations between tumor immune cell densities and serum levels of inflammatory markers are unclear. However, more precise definition of immune cell categories may be needed to show more closely defined associations with circulating inflammatory mediators. For example, general macrophage markers, such as CD68, do not adequately reflect the phenotypic diversity of macrophages, which can produce copious amounts of various cytokines depending on their polarization status (Figure 1)[28,54]. Also other immune cells, including T helper cells[55], B cells[56], neutrophils[57,58], produce different types and quantities of cytokines and chemokines related to the type of their activation. Based on this, for example, neutrophil categorization into proinflammatory N1 and anti-inflammatory N2 subsets has been suggested[59], but it is not as well established as T helper cell classification (Th1, Th2, Th17, Treg) or macrophage classification (M1 and M2)[60].
Cancer-associated fibroblasts (CAFs) contribute to proliferative signaling, invasion and metastasis, angiogenesis, and inflammatory reactions[1,27]. Cancer cells and fibroblasts may form a reciprocal positive feedback loop where tumor cells release growth factors activating fibroblasts and, in return, fibroblasts secrete growth factors, such as IGF1, which stimulate the proliferation of cancer cells. Recently, such an IGF1-dependent feedback loop, promoting disease progression, was demonstrated in radiotherapy-activated CAFs in CRC mouse model and human CRC samples[61].
CAFs also produce several factors contributing to tumor inflammatory reactions (Table 4). For example, two recent studies indicated that stromal fibroblasts are an important source of IL6 in CRC[29,62]. Nagasaki et al[29] found that stromal fibroblasts had higher IL6 production than tumor cells, and that colon cancer cells enhanced IL6 production by isolated stromal fibroblasts. Moreover, in a xenograft mouse model, anti-IL6R antibody targeting stromal tissue showed greater anti-tumor activity than anti-IL6R antibody targeting xenografted cancer cells. Huynh et al[62] also demonstrated that CAFs are a major source of IL6 in human CRC samples, and found that IL6 production was associated with tumor promoting Th17 immune response. De Boeck et al[63] performed secretome profiling of CAFs isolated from human CRC samples, and found that in these experimental conditions, CAFs represent a rich source of cytokines, chemokines, proteases, and growth factors, such as CXCL8 involved in neutrophil recruitment, CCL5 involved in T cell recruitment, VEGFA involved in angiogenesis, and various matrix metalloproteinases (MMPs) (Table 4). MMPs play an important role in extracellular matrix remodeling during tumor invasion, but can also contribute to inflammatory regulation by, for example, cleaving chemokines and cytokines[64,65].
| Inflammatory mediator | Function | Detection method | Samples | Ref. |
| IL6 | Proinflammatory cytokine | IF | FFPE CRC specimens | Nagasaki et al[29] |
| IL6 | Proinflammatory cytokine | LC-MS/MS | Cell culture (human cancer associated fibroblasts) | De Boeck et al[63] |
| IL6 | Proinflammatory cytokine | ELISA | CAFs isolated from human CRC tissue | Zhang et al[187] |
| IL8 | Proinflammatory cytokine | ELISA | CAFs isolated from human CRC tissue | Zhang et al[187] |
| IL11 | Anti-inflammatory cytokine | qRT-PCR | CAFs isolated from human CRC tissue | Calon et al[188] |
| TGFB | Immunosuppression, inhibition of cytotoxic T cells and Th1 cells | IHC, WB | Cell culture (CRC cells, fibroblasts) | Hawingkels et al[68] |
| CXCL5 | Recruitment of neutrophils | IHC, in situ hybridization | FFPE CRC specimens | Li et al[189] |
| CXCL8 | Recruitment of neutrophils | LC-MS/MS | Cell culture (human cancer associated fibroblasts) | De Boeck et al[63] |
| CCL5 | Recruitment of T cells | LC-MS/MS | Cell culture (human cancer associated fibroblasts) | De Boeck et al[63] |
| MMP1 | ECM degradation | LC-MS/MS | Cell culture (human cancer associated fibroblasts) | De Boeck et al[63] |
| MMP2 | ECM degradation | LC-MS/MS | Cell culture (human cancer associated fibroblasts) | De Boeck et al[63] |
| MMP3 | ECM degradation | LC-MS/MS | Cell culture (human cancer associated fibroblasts) | De Boeck et al[63] |
| MMP9 | ECM degradation | LC-MS/MS | Cell culture (human cancer associated fibroblasts) | De Boeck et al[63] |
| TIMP1 | Inhibition of MMPs | LC-MS/MS | Cell culture (human cancer associated fibroblasts) | De Boeck et al[63] |
| TIMP1 | Inhibition of MMPs | IHC, in situ hybridization | FFPE CRC specimens | Joo et al[190] |
| TIMP2 | Inhibition of MMPs | LC-MS/MS | Cell culture (human cancer associated fibroblasts) | De Boeck et al[63] |
| TIMP2 | Inhibition of MMPs | IHC, in situ hybridization | FFPE CRC specimens | Joo et al[190] |
| VEGFA | Angiogenesis | LC-MS/MS | Cell culture (human cancer associated fibroblasts) | De Boeck et al[63] |
TGFB signaling is a central immunosuppressive pathway in CRC progression[66,67]. Recently, Hawinkels et al[68] demonstrated a positive feedback loop, where the interaction of tumor cells with resident fibroblasts results in hyperactivated TGFB signaling in both cell types. In vitro, the treatment of CAFs with TGFB increased their expression of collagen-1, PLAU (urokinase type plasminogen activator), various matrix MMPs, including MMP2, MMP3, and MMP9, tissue inhibitors of matrix metalloproteinases (TIMPs), and TGFB itself[68]. Collectively, these data support the role of CAFs in the regulation of cancer associate inflammatory reactions.
Necrosis, an uncontrolled process of cell death, provokes a rapid systemic inflammatory response that is necessary for the removal of dead tissues from the body by phagocytic cells like neutrophilic granulocytes and macrophages. Necrosis is also prevalent and represents an indicator of less favorable outcome in colorectal, renal, lung, and breast cancer[69-71]. Irreversible cell injury induces the systemic inflammatory response, when dying cells release proinflammatory molecules into the extracellular space, and this is further propagated when intracellular contents of the cells are exposed[72]. In trauma patients, mitochondrional damage-associated components released to the circulation are able to elicit systemic inflammation[73]. Richards et al[74] and Guthrie et al[75] found that increasing amount of tumor necrosis in CRC is associated with higher levels of markers of systemic inflammation, such as modified Glasgow Prognostic Score (mGPS) and serum IL6, supporting the role of tumor necrosis in the induction of systemic inflammatory response. In addition, further supporting the association between hypoxia and systemic inflammation, Bousquet et al[76] showed that hypoxic conditions are related to a reduction of reactive oxygen species (ROS) production and increased damaged mitochondrial DNA (mtDNA) generation in vitro and that in rectal cancer patients with locally advanced disease, a low circulating ROS to damaged mtDNA ratio was associated with systemic inflammation. However, to our knowledge, the activation of systemic inflammation by tumor necrosis has not been demonstrated in more experimental studies.
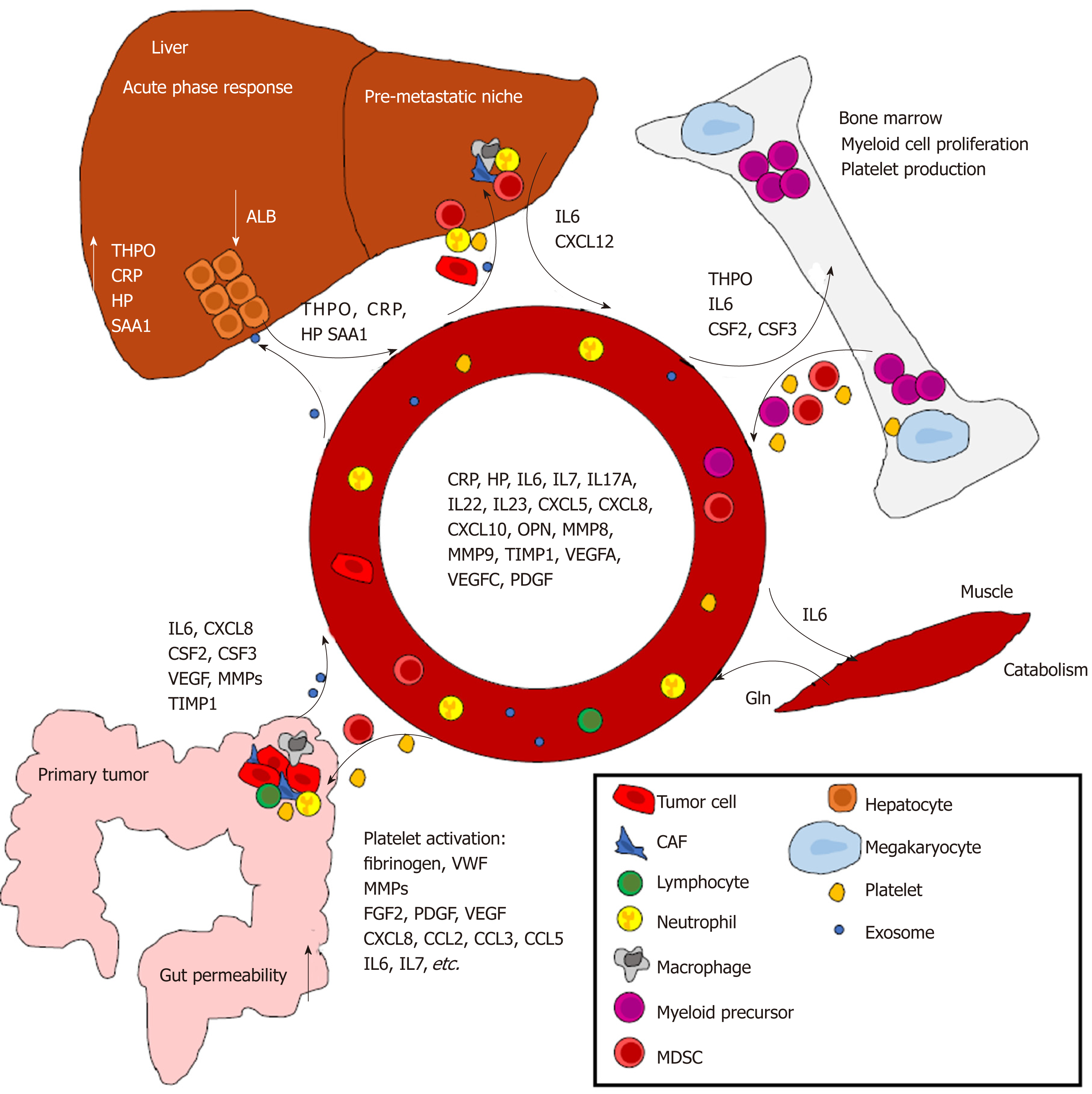
The effects of systemic inflammation span throughout the body; from primary tumors to metastases, liver, bone marrow, gut, skeletal muscle, and other organs (Figure 2). Recent studies have shown that even before metastatic disease, the systemic inflammatory response promotes tumor progression by modifying the interactions between neoplastic and non-neoplastic cells. The concept of pre-metastatic niche describes the process in which instead of being passive receivers of circulating tumor cells, the tissues and organs of a future metastasis are actively modified before the metastatic spread[77].
The liver participates in a large number of tasks, such as macronutrient metabolism, blood volume regulation, detoxification of chemicals and several metabolites, and regulation of immune responses[78]. The liver synthesizes the majority of serum proteins, such as albumin, fibrinogen, clotting factors, transport proteins, complement proteins, and lipoproteins. It maintains whole body homeostasis via metabolism of carbohydrates, lipids, amino acids and vitamins, and it also functions as an immune organ that mediates and regulates systemic and local innate and adaptive immunity. Digestion and nutrient absorption in the gastrointestinal tract provide a constant source of antigens (and a potential route for pathogens) that enter the body, and liver sinusoids are thereby rich in antigen-presenting cells, NK cells and NKT-cells that have a key role both in immunotolerance and in the immune defense against pathogens. Liver mediates immunotolerance via complex interaction of hepatocytes, liver nonparenchymal cells and immune cells[79].
One of the best-known mechanisms of the liver in immunoregulation is the production of acute-phase proteins in response to inflammation[15]. An acute-phase protein has been defined as one whose plasma concentration increases (positive acute-phase proteins) or decreases (negative acute-phase proteins) during inflammation. Examples of positive acute-phase proteins include ceruloplasmin, CRP, haptoglobin, hepcidin, and SAA, whereas negative acute-phase proteins include albumin, transferrin, transthyretin, and alpha-fetoprotein[15]. IL6 has been established as one of the most important contributors to altered protein production in the liver during the acute phase response. During response to infection, circulating IL6 levels quickly increase, propagating inflammatory signaling throughout the body[80]. Notably, IL6 is one of the cytokines showing the greatest increase in CRC patients relative to healthy controls, and a further increase in metastatic disease compared to non-metastatic disease[18].
IL6 also appears to be one of the main contributors to altered hepatic metabolism during systemic inflammation. In a recent study, Flint et al[81] showed that, in a mouse CRC model, tumor-induced IL6 caused systemic metabolic changes, such as suppression of hepatic ketogenesis, which triggered marked glucocorticoid secretion from the liver. In turn, this suppressed intratumoral immunity and caused failure of anti-cancer immunotherapy. The IL6-ketogenesis suppression-glucocorticoid pathway in the liver may represent one of the mechanisms by which immunosuppression in tumor tissue, often observed in CRC patients with advanced cancer[40], is coupled with changes in liver function and systemic metabolic changes.
The immune system is governed by an appropriate balance of the lymphoid and myeloid responses. Hematopoietic stem cells (HSCs) reside in the bone marrow, producing different blood cell lineages in an highly organized manner[82]. HSCs respond rapidly to acute blood cell demand, such as injury or inflammation[83]. In patients with solid cancers, hematopoiesis is abnormal, leading to altered composition of hematopoietic progenitor cells, with myeloid-biased differentiation[84]. Accordingly, the systemic inflammation in cancer patients is widely reflected in hematological parameters, such as neutrophil-to-lymphocyte ratio, with neutrophil predominance over lymphocytes[85]. Together with CSF2, and CSF3, IL6 is among the leading myelopoiesis-driving cytokines[86].
A prolonged demand for myeloid cells – as in the case of a severe prolonged infection – results in sustained myelopoiesis that is characterized by the emergence of immature myeloid cells in the circulation and in peripheral tissues[87]. Many of these cells have been reported to harbor immunosuppressive functions, and this group of myeloid progenitor cells with immunosuppressive activity has been named as myeloid derived suppressor cells (MDSCs)[87,88]. In the peripheral blood, human polymorphonuclear MDSCs are CD11b+CD14-CD15+ and monocytic MDSCs are CD11b+CD14+CD15-HLADR-/low[87]. In addition to these gating criteria, functional suppression assays are required to precisely define MDSCs because of the overlap between their phenotype with that of more mature monocytes and granulocytes[87]. Besides peripheral blood, the presence of cells with MDSC-like phenotype has been reported in human CRC tissue[89].
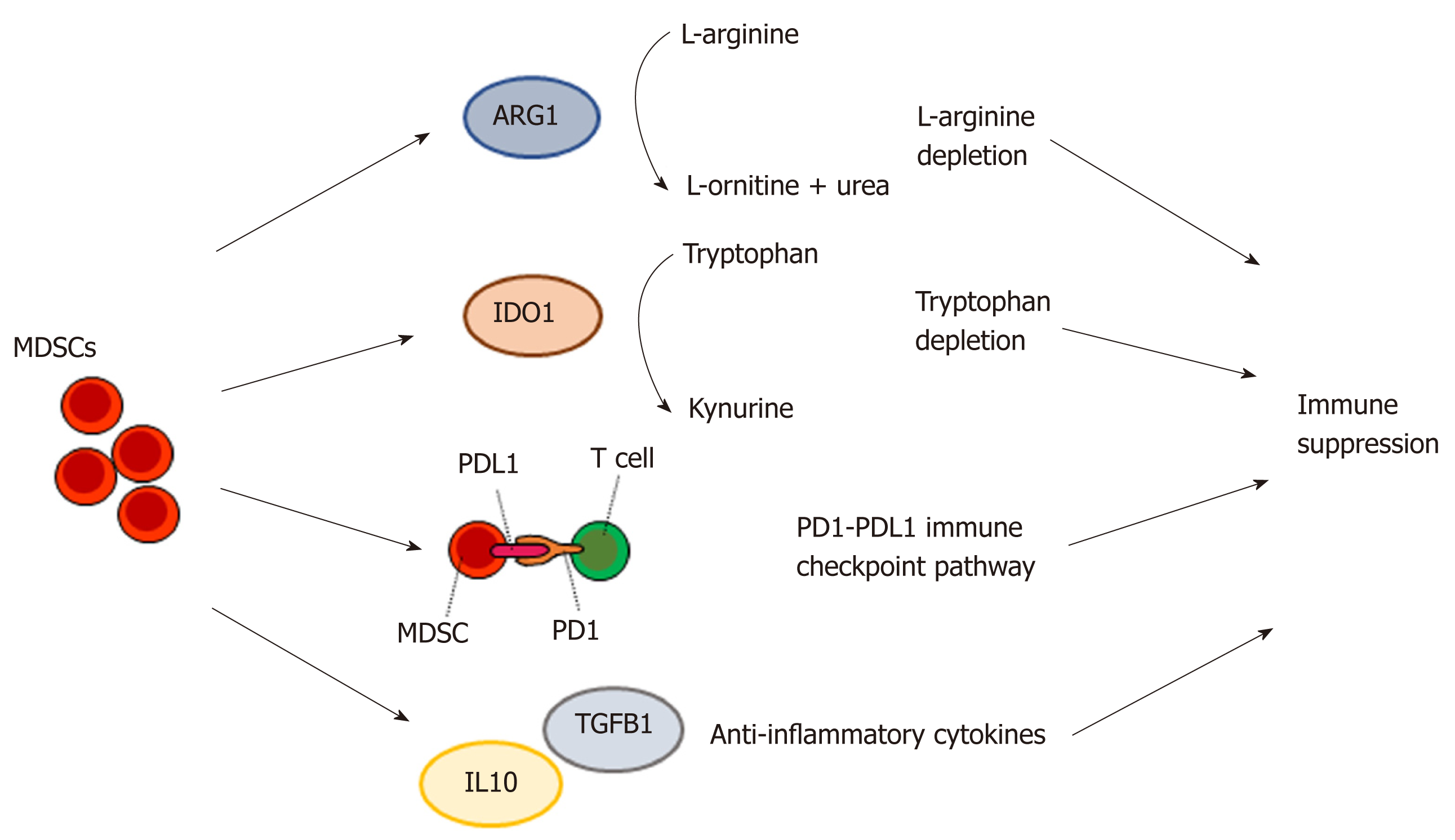
The mechanisms by which MDSCs mediate immunosuppression and support tumor progression are complex and not fully understood[90]. However, several potential key pathways include the ARG1 pathway, IDO1 pathway, PD1/PDL1 pathway, and cytokine pathways (such as IL10 and IL6)[90,91] (Figure 3). L-arginine is an amino acid that is consumed by T cells and many other immune cells. MDSCs are characterized by high production of ARG1, which metabolizes L-arginine to L-ornithine and urea, resulting in L-arginine depletion and thus local immune suppression[92]. IDO1, expressed in a subset of MDSCs[87], converts tryptophan to kynurenine. The depletion of tryptophan suppresses activity in the mTORC1 signaling pathway, leading to autophagy in T cells and immunosuppression[93]. PD1 and PDL1 form an inhibitory immune checkpoint mechanism restricting excessive T cell activation[94]. The binding of PDL1, expressed on a subset of MDSCs[95], to PD1 causes T cell inhibition[96].
Besides myeloid immune cells, such as granulocytes, monocytes, and MDSCs, common myeloid progenitor cells can differentiate into megakaryocytes and red blood cells. However, instead of erythrocytosis (an increase in the number of red blood cells in the blood), CRC patients frequently have anemia (a decrease in the number of red blood cells or hemoglobin concentration), with a prevalence of 33%-43% in resectable disease[97-99]. Colorectal tumors frequently bleed into the lumen[100], explaining the iron deficiency associated with microcytic anemia in a subset of patients. However, systemic inflammation also appears to be one of the main determinants of low blood hemoglobin levels, and, in particular, normocytic anemia, in CRC patients[97-99]. There are several collaborating mechanisms linking systemic inflammation and anemia. First, hepcidin, an acute phase protein produced in the liver, limits iron absorption from small intestine[101,102] and iron availability for erythroid cells[102]. Second, pro-inflammatory cytokines directly inhibit the proliferation of erythroid progenitor cells[103]. Third, proinflammatory cytokines also inhibit erythropoietin synthesis in the kidney, resulting in decreased erythropoiesis[104]. In addition to the main symptoms associated with anemia such as fatigue, weakness, or shortness of breath, decreased availability of oxygen in anemic cancer patients may contribute to systemic metabolic changes, such as alterations in circulating and liver lipid levels, which have been shown to associate with hypoxia in animal models[105].
Platelets are anucleate cells generated in the bone marrow by the megakaryocyte. They contribute to hemostasis but also to cancer pathogenesis by releasing growth factors and cytokines[106]. Thrombocytosis (increased blood platelet count) is common in cancer patients. A recent prospective cohort study in the United Kingdom investigated 1-year cancer incidence in 40000 patients aged ≥ 40 years with thrombocytosis[107]. In that cohort, 11.6% of males and 6.2% of females developed cancer in 1-year follow-up, with CRC and lung cancer as the most common diagnoses[107]. The factors contributing to cancer-associated thrombocytosis include CSF2, CSF3, FGF2 (fibroblast growth factor 2, basic fibroblast growth factor), IL6, and THPO (thrombopoietin)[108]. In cancer patients with systemic inflammation, THPO production in the liver is increased in response to IL6 and other cytokines, resulting in increased platelet production[108,109].
Platelet granules contain a plethora of hemostatic factors (e.g., fibrinogen, VWF), enzymes (e.g., MMP1, MMP2), growth factors (e.g., FGF2, PDGF, VEGF), chemokines (e.g., CXCL8, CCL2, CCL3, CCL5), and cytokines (e.g., IL6, IL7), which are released on platelet activation[110,111]. Reacting to the modified tumor vasculature, platelets can release these factors in the tumor microenvironment, promoting tumor progression. Although platelets lack nucleus, it has been demonstrated that the megakaryocyte packs them with a protein translation machinery that includes ribosomes, initiation and termination factors, miRNAs, and template messenger RNAs (mRNAs)[112]. Moreover, recent studies have indicated that platelets are capable of exchanging nucleic acids and proteins with tumor cells, leading to the concept of tumor-educated platelets, i.e., platelets reflecting the properties of tumors and programmed to support tumor growth[21,106]. Highlighting the alterations in platelet mRNA profile in cancer patients, a recent study performed mRNA sequencing of 283 platelet samples and found that tumor-educated platelets distinguished cancer patients from healthy individuals with 96% accuracy, differentiated between six primary tumor types, including CRC, with 71% accuracy, and identified several genetic alterations found in tumors, such as KRAS mutation[113].
In CRC, metastasis is the major cause of death and the main target organ of metastasis is the liver[114]. The understanding of the biological mechanisms of cancer metastasis is still limited. During the past few decades, it has been established that before metastasis, primary tumors can create a favorable microenvironment, a pre-metastatic niche, at tissue sites for subsequent metastasis. Among the first to describe the phenomenon were Kaplan et al[115], who showed that in Lewis lung carcinoma and melanoma mouse models, VEGFR1+ (vascular endothelial growth factor receptor 1) bone marrow-derived progenitor cells homed to tumor-specific pre-metastatic sites before the arrival of tumor cells, supporting the subsequent metastasis. The pre-metastatic niches are composed of stromal components of the distant organs, bone marrow-derived cells including stromal cells and immunosuppressive immune cells, and tumor-derived secreted factors, such as cytokines, growth factors, and extracellular vesicles[5,116]. Liu and Cao recently proposed that six hallmark characteristics of pre-metastatic niche include inflammation supporting a proliferatory microenvironment; immunosuppression; angiogenesis; lymph-angiogenesis; metabolic, stromal, and epigenetic reprogramming; and organotropism.
Several studies have demonstrated pre-metastatic niches in CRC mouse models. Seubert et al[117] found that high systemic TIMP1 levels led to increased hepatic levels of neutrophil chemokine CXCL12, resulting in recruitment of neutrophils to the liver. Both inhibition of CXCL12-mediated neutrophil recruitment and systemic depletion of neutrophils reduced TIMP1-induced increased liver susceptibility towards metastasis. In another study, Shao et al[118] showed that CRC-derived small extracellular vesicles, also known as exosomes, are targeted to the liver where they promote the formation of premetastatic niche and CRC metastasis. Liver macrophages, Kupffer cells, engulfed these exosomes and their cargo, leading to Kupffer cell polarization toward proinflammatory phenotype and increased CSF3 and IL6 expression. An inflammatory microenvironment was created and expression of apoptosis and matrix remodeling related genes was altered, promoting cancer metastasis to the liver.
In a mouse model of pancreatic cancer, Lee et al[119] showed that IL6, produced by tumor-adjacent non-cancerous fibroblasts, traveled to the liver and mediated STAT3 signaling in hepatocytes, resulting in the secretion of acute phase reactants serum amyloid A1 and A2 (SAA proteins). SAA proteins attracted immunosuppressive myeloid cells to the liver, promoted hepatic stellate cell activation and production of extracellular matrix, creating a metastasis-prone environment in the liver. This study also reported enhanced hepatic SAA expression in CRC patients. All in all, more and more evidence supports the role of systemic inflammation in creating a tumor-favoring environment in distant organs, enabling metastatic tumor cells to survive after colonization.
The large bowel is the dwelling place for a vast set of commensal micro-organisms, mainly bacteria and fungi. Collectively, these are often described as intestinal microbiome or microbiota. Changes in the intestinal microbiota are observed in many situations, including CRC and cachexia[120,121]. The interplay between microbiota and the immune system has gained increasing interest, although our knowledge is still very limited. Still, recent studies have shown that the gut microbiota is able to modulate patients’ responsiveness to PD1 and CTLA4 blocking immu-notherapies[122,123]. Such findings give us a glimpse of the potential significance of microbiota to its host.
In normal circumstances, intestinal bacteria are barred from entering the circulation by several mechanisms, collectively known as the intestinal barrier. Increased permeability of the mucosa allows the entry of bacteria or bacterial components into the portal circulation and subsequently, the liver, where they elicit a succinct response[124]. In mouse cancer models, strong correlation exists between circulating IL6 levels and intestinal permeability[121,125]. Animal models of colon cancer have also indicated that areas adjacent to cancer present a disrupted barrier function[126,127], and that systemic inflammation may induce endotoxemia often associated with cancer. Besides bacteria or bacterial components, also bacterial metabolites such as short-chain fatty acids (SCFAs) can modulate peripheral immune response. SCFAs are capable of shifting the effector T to regulatory T cell balance by facilitating the differentiation of regulatory T cells, and thereby limit the systemic inflammation [128].
The international consensus definition of cachexia is an ongoing loss of skeletal muscle mass – with or without loss of fat mass – that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment[129]. Systemic inflammation is a key driving factor in cancer-associated cachexia[16]. In CRC patients, the presence of systemic inflammation is associated with low skeletal muscle mass[130,131]. Cachexia not only reduces patient’s quality of life and treatment response, but it is also an indirect cause of death in about 20% of patients who eventually die of cancer[16].
The ApcMin/+ mouse is a widely used animal model of CRC and CRC-associated cachexia[132]. In this mouse line, the cachexia progression is associated with increased plasma IL6 levels, and cachexia does not progress in the absence of IL6 despite the presence of intestinal and colon tumors[133]. However, in control mice, the overexpression of IL6 does not induce cachexia[133], suggesting that IL6 is an indispensable but not sufficient factor in cancer cachexia pathogenesis. Systemic inflammation in the absence of malignancy, such as in sepsis, is also able to induce severe muscle wasting[134], verifying that active inflammatory reaction is an important driver in muscle catabolism.
Active immune response is a highly energy-consuming process[135,136]. Thus, activation of systemic inflammation in cancer patients requires the utilization of stored energy and nutrients, especially as anorexia is a common symptom in cancer patients with advanced disease. A recent study investigated the relationships between systemic inflammatory markers and circulating levels of nine amino acids in 336 CRC patients and found that, of the studied factors, systemic inflammation was the main determinant of low serum glutamine level in these patients[137]. Glutamine is the most abundant amino acid in the body, and circulating glutamine is mainly derived from skeletal muscle, functioning as an inter-organ carbon, nitrogen and energy transporter to be utilized by rapidly dividing cells such as enterocytes and lymphocytes[138]. In healthy humans, circulating glutamine is mainly consumed in the gut and kidney[138]. Tumor tissue can either consume or produce glutamine, depending on tissue of origin and oncogene activation[139]. Patients with sepsis have decreased plasma glutamine levels[140] resulting from increased glutamine consumption[141]. Mouse studies have shown that tumor induces a decrease in circulating glutamine levels, stimulates glutamine release and decreases the glutamine content in skeletal muscle[142]. Accordingly, it has been suggested that altered interorgan glutamine homeostasis in cancer patients is an essential driver in cachexia.
The prognostic and predictive classification of CRC has mainly been based on tumor stage[143,144]. However, each patient and tumor is unique[145], and a more exact classification of the disease based on the features of the tumor and host could enable more personalized treatments. Indeed, patient selection for anti-EGFR treatment for metastatic CRC is currently based on RAS and BRAF mutation testing[146], and anti-PD1 antibody treatment has been approved for metastatic CRC with high-level microsatellite instability or mismatch repair deficiency[147]. Considering the impact of systemic inflammation in CRC progression, systemic inflammatory markers represent potential additional prognostic and predictive parameters (Table 5).
| Marker | Ref. | Study design | Study population | Outcome, HR |
| Acute phase proteins | ||||
| CRP | Woo et al[191] | Meta-analysis, 21 studies | 3934 CRC patients, stage I-II | OS, HR 2.04 (1.45–2.86); CSS, HR 4.37 (2.63–7.27); DFS, HR 1.88 (0.97–3.67) |
| Albumin | Gupta et al[192] | Systematic review, 12 studies | 3644 CRC patients, stage I-IV | Low albumin associated with worse survival (no meta-analysis conducted) |
| Albumin | Ghuman et al[193] | Case-case study within a prospective cohort study (AMORIS) | 4764 CRC patients, stage I-IV | OS, HR 0.57 (0.29–1.14); CSS, HR 0.36 (0.16–0.85) |
| mGPS | Lu et al[194] | Meta-analysis, 41 studies | 9839 CRC patients, stage I-IV | OS, HR 2.20 (1.88–2.57); CSS, HR 1.86 (1.59–2.17) |
| HP (haptoglobin) | Ghuman et al[193] | Case-case study within a prospective cohort study (AMORIS) | 4764 CRC patients, stage I-IV | OS, HR 1.28 (1.08–1.51); CSS, HR 1.17 (0.95–1.45) |
| Blood cell count parameters | ||||
| Neutrophil-to-lymphocyte ratio | Li et al[148] | Meta-analysis, 16 studies | 5897 CRC patients, stage I-IV | OS, HR 1.66 (1.36–2.02); CSS, HR 2.27 (1.75–2.96); DFS, HR 1.54, (1.18–2.02) |
| Lymphocyte-to-monocyte ratio | Tan et al[149] | Meta-analysis, 15 studies | 11783 CRC patients, stage I-IV | OS, HR 0.57 (0.52-0.62); CSS, HR 0.55 (0.32-0.95); DFS, HR 0.77 (0.70-0.84) |
| Platelet count | Rao et al[152] | Meta-analysis, 9 studies | 3413 CRC patients, stage I-IV | OS, HR 2.11 (1.68-2.65); DFS, HR 2.51 (1.84-3.43) |
| Platelet-to-lymphocyte ratio | Tan et al[153] | Meta-analysis, 15 studies | 3991 CRC patients, stage I-IV | OS, HR 1.53 (1.24–1.89), DFS, HR 1.68 (1.07–2.62) |
| Anemia | Wilson et al[150] | Meta-analysis, 12 studies | 3588 CRC patients, stage I-IV | OS, HR 1.56 (1.30-1.88), DFS, HR 1.34 (1.11-1.61) |
| Cytokines, chemokines, and their receptors | ||||
| IL6 | Xu et al[23] | Meta-analysis, 10 studies | 860 CRC patients, stage I-IV | OS, HR 1.76 (1.42–2.19); DFS, HR 2.97 (1.76–5.01) |
| TNFRSF11B (Osteoprotegerin) | Birgisson et al[20] | Prospective cohort study | 261 stage II-IV CRC patients | OS, HR 3.33 |
| Protease enzymes and their inhibitors | ||||
| TIMP1 | Lee et al[195] | Meta-analysis, 10 studies | 1477 CRC patients, stage I-IV | OS, HR 2.25 (1.56-3.25) |
Acute phase proteins, including CRP, albumin, and their composite mGPS (mGPS0: serum CRP ≤ 10 mg/L and serum albumin ≥ 35 g/L or < 35 g/L; mGPS1: serum CRP > 10 mg/L and serum albumin ≥ 35 g/L; mGPS2: serum CRP > 10 mg/L and serum albumin < 35 g/L), are among the best-studied systemic inflammation-based prognostic parameters in CRC[10]. Numerous studies have reported that high circulating CRP levels, low albumin levels and high mGPS are associated with adverse patient outcome (Table 5). In addition, blood differential leukocyte count parameters have well-established prognostic value in CRC. Myeloid cell proliferation, associated with systemic response to CRC, leads to an increase in circulating neutrophil and monocyte counts, relative to lymphocytes, which has been associated with adverse outcome[148,149]. Indices based on relative counts of these cell types represent promising prognostic parameters. Preoperative anemia, reflecting systemic inflammation in a subset of patients, has also been associated with adverse outcome[150]. Platelet count and platelet-to-lymphocyte ratio can also provide potentially clinically relevant prognostic information[151-153], with high platelet counts associated with poor survival. In future, more sophisticated analyses of platelet composition and function, such as platelet RNA sequencing, may complement these parameters to provide more nuanced information of the status of platelet activation and education during systemic inflammation[21].
Several studies have indicated that circulating cytokine concentrations provide prognostic information in CRC. Recently, using proximity extension assays, Birgisson et al[20] analyzed plasma levels of 92 oncology-related proteins, including an assemblage of cytokines, chemokines, and growth factors, in a cohort of 261 stage II-IV CRC patients. Many of these molecules, including CSF1, CXCL10, CXCL9, HGF (hepatocyte growth factor), IL6, osteoprotegerin, PGF (placental growth factor), and VEGFA, were significantly associated with survival in univariable analysis, and of these, osteoprotegerin was the best in predicting survival in multivariable survival models[20]. Analyzing multiple markers in one sample may improve the prognostic power relative to measuring the levels of a single marker. However, caution needs to be employed when interpreting the results of such studies because of the well-known risk of multiple hypothesis testing, necessitating confirmation of the findings in independent study populations. As indicated by a recent meta-analysis[154], a few multiple cytokine array studies have been conducted in CRC, but mainly in fairly small populations, with varying markers and methods, making further larger scale studies necessary to draw more convincing conclusion about the prognostic significance of the reported marker combinations.
During the past decade, increasing effort has been directed towards the investigation of circulating tumor-derived extracellular vesicles as potential prognostic and diagnostic biomarkers in CRC and other tumors. Based on this approach, several promising results have been reported. For example, Liu et al[22] recently studied circulating exosomal miRNA content from 369 stage I–IV CRC patients. They found that exosomal miR-27a and miR-130a predicted survival. However, as for multiplex cytokine arrays, the selection of optimal combination of markers as well as independent validation studies would be required to establish circulating exosomal miRNA signatures as clinically relevant prognostic parameters in CRC.
The research conducted during the past few decades indicates that systemic inflammation has wide-ranging effects on CRC progression, including supporting primary tumor invasion, proliferation, angiogenesis, and metastasis, as well as suppressing anti-tumor immunity. Several systemic inflammation-based prognostic parameters, such as neutrophil-lymphocyte ratio, modified Glasgow Prognostic Score, and platelet-lymphocyte ratio, have been found to have impressive prognostic value in a large number of studies and are widely available in clinical laboratories worldwide. However, larger-scale multi-institutional studies of their predictive value for the response to specific adjuvant therapies are needed. Moreover, these markers only begin to scratch the surface of the potential of systemic inflammation-based biomarkers in predicting patient survival. In future, additional tests, such as multiple cytokine-chemokine-growth factor assays, analysis of tumor-derived extracellular vesicles, and profiling of tumor-educated platelet transcriptome may translate into improved prognostic and predictive parameters, ultimately enabling accurate identification of patients who might benefit from specific adjuvant therapies, as well as into improved methods of non-invasive disease monitoring. Factors regulating the formation of pre-metastatic niches in CRC, suppression of anti-tumor immunity, tumor-platelet interactions, and CRC-associated cachexia also represent potential targets for drug development.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sung WW, Temraz S S-Editor: Ma YJ L-Editor:A E-Editor: Ma YJ
| 1. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47087] [Article Influence: 3363.4] [Reference Citation Analysis (5)] |
| 2. | McAllister SS, Weinberg RA. Tumor-host interactions: a far-reaching relationship. J Clin Oncol. 2010;28:4022-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 3. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4538] [Article Influence: 324.1] [Reference Citation Analysis (0)] |
| 4. | Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591-5596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1331] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 5. | McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 726] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 6. | Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 7. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13157] [Article Influence: 1879.6] [Reference Citation Analysis (4)] |
| 8. | Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal Cancer, Systemic Inflammation, and Outcome: Staging the Tumor and Staging the Host. Ann Surg. 2016;263:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Kantola T, Klintrup K, Väyrynen JP, Vornanen J, Bloigu R, Karhu T, Herzig KH, Näpänkangas J, Mäkelä J, Karttunen TJ, Tuomisto A, Mäkinen MJ. Reply: Comment on 'Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma'. Br J Cancer. 2013;108:1917-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 1010] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 11. | Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH, Wan DS, Pan ZZ. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010;25:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: Systematic review and meta-analysis. Sci Rep. 2017;7:16717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 13. | Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J Surg Oncol. 2017;115:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 14. | Park JH, van Wyk H, Roxburgh CSD, Horgan PG, Edwards J, McMillan DC. Tumour invasiveness, the local and systemic environment and the basis of staging systems in colorectal cancer. Br J Cancer. 2017;116:1444-1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4404] [Cited by in RCA: 4612] [Article Influence: 177.4] [Reference Citation Analysis (0)] |
| 16. | Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 986] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 17. | Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer. 2014;110:1409-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 18. | Kantola T, Klintrup K, Väyrynen JP, Vornanen J, Bloigu R, Karhu T, Herzig KH, Näpänkangas J, Mäkelä J, Karttunen TJ, Tuomisto A, Mäkinen MJ. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Chen ZY, Raghav K, Lieu CH, Jiang ZQ, Eng C, Vauthey JN, Chang GJ, Qiao W, Morris J, Hong D, Hoff P, Tran H, Menter DG, Heymach J, Overman M, Kopetz S. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112:1088-1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 20. | Birgisson H, Tsimogiannis K, Freyhult E, Kamali-Moghaddam M. Plasma Protein Profiling Reveal Osteoprotegerin as a Marker of Prognostic Impact for Colorectal Cancer. Transl Oncol. 2018;11:1034-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Best MG, Wesseling P, Wurdinger T. Tumor-Educated Platelets as a Noninvasive Biomarker Source for Cancer Detection and Progression Monitoring. Cancer Res. 2018;78:3407-3412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 22. | Liu X, Pan B, Sun L, Chen X, Zeng K, Hu X, Xu T, Xu M, Wang S. Circulating Exosomal miR-27a and miR-130a Act as Novel Diagnostic and Prognostic Biomarkers of Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2018;27:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 23. | Xu J, Ye Y, Zhang H, Szmitkowski M, Mäkinen MJ, Li P, Xia D, Yang J, Wu Y, Wu H. Diagnostic and Prognostic Value of Serum Interleukin-6 in Colorectal Cancer. Medicine (Baltimore). 2016;95:e2502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Jin WJ, Xu JM, Xu WL, Gu DH, Li PW. Diagnostic value of interleukin-8 in colorectal cancer: a case-control study and meta-analysis. World J Gastroenterol. 2014;20:16334-16342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 25. | Liu Z, Zhang Y, Niu Y, Li K, Liu X, Chen H, Gao C. A systematic review and meta-analysis of diagnostic and prognostic serum biomarkers of colorectal cancer. PLoS One. 2014;9:e103910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Fukuyama T, Ichiki Y, Yamada S, Shigematsu Y, Baba T, Nagata Y, Mizukami M, Sugaya M, Takenoyama M, Hanagiri T, Sugio K, Yasumoto K. Cytokine production of lung cancer cell lines: Correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Sci. 2007;98:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Tao L, Huang G, Song H, Chen Y, Chen L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol Lett. 2017;14:2611-2620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 28. | Guerriero JL. Macrophages: The Road Less Traveled, Changing Anticancer Therapy. Trends Mol Med. 2018;24:472-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 29. | Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 30. | Xiao YC, Yang ZB, Cheng XS, Fang XB, Shen T, Xia CF, Liu P, Qian HH, Sun B, Yin ZF, Li YF. CXCL8, overexpressed in colorectal cancer, enhances the resistance of colorectal cancer cells to anoikis. Cancer Lett. 2015;361:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8:1248-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 32. | Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2327] [Cited by in RCA: 2226] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 33. | Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508-8515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 360] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 34. | Hamilton TA, Zhao C, Pavicic PG, Datta S. Myeloid colony-stimulating factors as regulators of macrophage polarization. Front Immunol. 2014;5:554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 35. | Manning S, Danielson KM. The immunomodulatory role of tumor-derived extracellular vesicles in colorectal cancer. Immunol Cell Biol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30:836-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1487] [Article Influence: 165.2] [Reference Citation Analysis (0)] |
| 37. | Yamada N, Kuranaga Y, Kumazaki M, Shinohara H, Taniguchi K, Akao Y. Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-β1-mediated suppression. Oncotarget. 2016;7:27033-27043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 38. | Chen Y, Xie Y, Xu L, Zhan S, Xiao Y, Gao Y, Wu B, Ge W. Protein content and functional characteristics of serum-purified exosomes from patients with colorectal cancer revealed by quantitative proteomics. Int J Cancer. 2017;140:900-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 39. | Alfonsi R, Grassi L, Signore M, Bonci D. The Double Face of Exosome-Carried MicroRNAs in Cancer Immunomodulation. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Väyrynen JP, Tuomisto A, Klintrup K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer. 2013;109:1839-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pagès F, Speicher MR, Trajanoski Z, Galon J. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1792] [Cited by in RCA: 2952] [Article Influence: 246.0] [Reference Citation Analysis (0)] |
| 42. | Klintrup K, Mäkinen JM, Kauppila S, Väre PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41:2645-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 43. | Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987;1:1303-1306. [PubMed] |
| 44. | Väyrynen JP, Sajanti SA, Klintrup K, Mäkelä J, Herzig KH, Karttunen TJ, Tuomisto A, Mäkinen MJ. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer. 2014;134:2126-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, Glickman JN, Ferrone CR, Mino-Kenudson M, Tanaka N, Dranoff G, Giovannucci EL, Fuchs CS. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412-6420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 347] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 46. | Väyrynen JP, Vornanen JO, Sajanti S, Böhm JP, Tuomisto A, Mäkinen MJ. An improved image analysis method for cell counting lends credibility to the prognostic significance of T cells in colorectal cancer. Virchows Arch. 2012;460:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Wirta EV, Seppälä T, Friman M, Väyrynen J, Ahtiainen M, Kautiainen H, Kuopio T, Kellokumpu I, Mecklin JP, Böhm J. Immunoscore in mismatch repair-proficient and -deficient colon cancer. J Pathol Clin Res. 2017;3:203-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 48. | Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1482] [Article Influence: 211.7] [Reference Citation Analysis (0)] |
| 49. | Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1630] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 50. | Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 51. | Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1859] [Cited by in RCA: 2009] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 52. | Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 896] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 53. | Turner N, Wong HL, Templeton A, Tripathy S, Whiti Rogers T, Croxford M, Jones I, Sinnathamby M, Desai J, Tie J, Bae S, Christie M, Gibbs P, Tran B. Analysis of local chronic inflammatory cell infiltrate combined with systemic inflammation improves prognostication in stage II colon cancer independent of standard clinicopathologic criteria. Int J Cancer. 2016;138:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4289] [Cited by in RCA: 4525] [Article Influence: 411.4] [Reference Citation Analysis (0)] |
| 55. | Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 423] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 56. | Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 435] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 57. | Bird L. Tumour immunology: Neutrophil plasticity. Nat Rev Immunol. 2009;9:672. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 514] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 59. | Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2035] [Cited by in RCA: 2529] [Article Influence: 158.1] [Reference Citation Analysis (0)] |
| 60. | Eruslanov EB, Singhal S, Albelda SM. Mouse versus Human Neutrophils in Cancer: A Major Knowledge Gap. Trends Cancer. 2017;3:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 61. | Tommelein J, De Vlieghere E, Verset L, Melsens E, Leenders J, Descamps B, Debucquoy A, Vanhove C, Pauwels P, Gespach CP, Vral A, De Boeck A, Haustermans K, de Tullio P, Ceelen W, Demetter P, Boterberg T, Bracke M, De Wever O. Radiotherapy-Activated Cancer-Associated Fibroblasts Promote Tumor Progression through Paracrine IGF1R Activation. Cancer Res. 2018;78:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 62. | Huynh PT, Beswick EJ, Coronado YA, Johnson P, O'Connell MR, Watts T, Singh P, Qiu S, Morris K, Powell DW, Pinchuk IV. CD90(+) stromal cells are the major source of IL-6, which supports cancer stem-like cells and inflammation in colorectal cancer. Int J Cancer. 2016;138:1971-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | De Boeck A, Hendrix A, Maynard D, Van Bockstal M, Daniëls A, Pauwels P, Gespach C, Bracke M, De Wever O. Differential secretome analysis of cancer-associated fibroblasts and bone marrow-derived precursors to identify microenvironmental regulators of colon cancer progression. Proteomics. 2013;13:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Van Lint P, Libert C. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev. 2006;17:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 65. | Cathcart J, Pulkoski-Gross A, Cao J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes Dis. 2015;2:26-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 370] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 66. | Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS, Batlle E. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1329] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 67. | Ros XR, Vermeulen L. Turning Cold Tumors Hot by Blocking TGF-β. Trends Cancer. 2018;4:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Hawinkels LJ, Paauwe M, Verspaget HW, Wiercinska E, van der Zon JM, van der Ploeg K, Koelink PJ, Lindeman JH, Mesker W, ten Dijke P, Sier CF. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. 2014;33:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 69. | Richards CH, Roxburgh CS, Anderson JH, McKee RF, Foulis AK, Horgan PG, McMillan DC. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg. 2012;99:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Väyrynen SA, Väyrynen JP, Klintrup K, Mäkelä J, Karttunen TJ, Tuomisto A, Mäkinen MJ. Clinical impact and network of determinants of tumour necrosis in colorectal cancer. Br J Cancer. 2016;114:1334-1342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 71. | Pollheimer MJ, Kornprat P, Lindtner RA, Harbaum L, Schlemmer A, Rehak P, Langner C. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol. 2010;41:1749-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 72. | Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 645] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 73. | Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2990] [Cited by in RCA: 2800] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 74. | Richards CH, Roxburgh CSD, Anderson JH, McKee RF, Foulis AK, Horgan PG, McMillan DC. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg. 2012;99:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Guthrie GJ, Roxburgh CS, Richards CH, Horgan PG, McMillan DC. Circulating IL-6 concentrations link tumour necrosis and systemic and local inflammatory responses in patients undergoing resection for colorectal cancer. Br J Cancer. 2013;109:131-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Bousquet PA, Meltzer S, Sønstevold L, Esbensen Y, Dueland S, Flatmark K, Sitter B, Bathen TF, Seierstad T, Redalen KR, Eide L, Ree AH. Markers of Mitochondrial Metabolism in Tumor Hypoxia, Systemic Inflammation, and Adverse Outcome of Rectal Cancer. Transl Oncol. 2019;12:76-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 1322] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 78. | Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147-R1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 899] [Article Influence: 128.4] [Reference Citation Analysis (0)] |
| 79. | Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 682] [Cited by in RCA: 773] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 80. | Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 572] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 81. | Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, Fearon DT. Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab. 2016;24:672-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 283] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 82. | Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2046] [Cited by in RCA: 1830] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 83. | Mirantes C, Passegué E, Pietras EM. Pro-inflammatory cytokines: emerging players regulating HSC function in normal and diseased hematopoiesis. Exp Cell Res. 2014;329:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 84. | Wu WC, Sun HW, Chen HT, Liang J, Yu XJ, Wu C, Wang Z, Zheng L. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc Natl Acad Sci U S A. 2014;111:4221-4226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 85. | Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman Vujacic I, Lalic D, Milovanovic T, Dumic I, Krivokapic Z. Combined Diagnostic Efficacy of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Mean Platelet Volume (MPV) as Biomarkers of Systemic Inflammation in the Diagnosis of Colorectal Cancer. Dis Markers. 2019;2019:6036979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 86. | Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 473] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 87. | Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1905] [Cited by in RCA: 2131] [Article Influence: 236.8] [Reference Citation Analysis (0)] |
| 88. | Ostrand-Rosenberg S, Sinha P, Chornoguz O, Ecker C. Regulating the suppressors: apoptosis and inflammation govern the survival of tumor-induced myeloid-derived suppressor cells (MDSC). Cancer Immunol Immunother. 2012;61:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 89. | OuYang LY, Wu XJ, Ye SB, Zhang RX, Li ZL, Liao W, Pan ZZ, Zheng LM, Zhang XS, Wang Z, Li Q, Ma G, Li J. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J Transl Med. 2015;13:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 90. | Elliott LA, Doherty GA, Sheahan K, Ryan EJ. Human Tumor-Infiltrating Myeloid Cells: Phenotypic and Functional Diversity. Front Immunol. 2017;8:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 91. | De Veirman K, Van Valckenborgh E, Lahmar Q, Geeraerts X, De Bruyne E, Menu E, Van Riet I, Vanderkerken K, Van Ginderachter JA. Myeloid-derived suppressor cells as therapeutic target in hematological malignancies. Front Oncol. 2014;4:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 92. | Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 521] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 93. | Labadie BW, Bao R, Luke JJ. Reimagining IDO Pathway Inhibition in Cancer Immunotherapy via Downstream Focus on the Tryptophan-Kynurenine-Aryl Hydrocarbon Axis. Clin Cancer Res. 2019;25:1462-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 94. | Väyrynen JP, Tuomisto A, Mäkinen MJ. Regulatory mechanisms of T cell activation-From basic research discoveries to a new principle of cancer therapy and the Nobel Prize. Acta Physiol (Oxf). 2019;225:e13224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 95. | Ballbach M, Dannert A, Singh A, Siegmund DM, Handgretinger R, Piali L, Rieber N, Hartl D. Expression of checkpoint molecules on myeloid-derived suppressor cells. Immunol Lett. 2017;192:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 96. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3572] [Cited by in RCA: 4048] [Article Influence: 161.9] [Reference Citation Analysis (0)] |
| 97. | McSorley ST, Johnstone M, Steele CW, Roxburgh CSD, Horgan PG, McMillan DC, Mansouri D. Normocytic anaemia is associated with systemic inflammation and poorer survival in patients with colorectal cancer treated with curative intent. Int J Colorectal Dis. 2019;34:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Väyrynen JP, Tuomisto A, Väyrynen SA, Klintrup K, Karhu T, Mäkelä J, Herzig KH, Karttunen TJ, Mäkinen MJ. Preoperative anemia in colorectal cancer: relationships with tumor characteristics, systemic inflammation, and survival. Sci Rep. 2018;8:1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 99. | McSorley ST, Tham A, Steele CW, Dolan RD, Roxburgh CS, Horgan PG, McMillan DC. Quantitative data on red cell measures of iron status and their relation to the magnitude of the systemic inflammatory response and survival in patients with colorectal cancer. Eur J Surg Oncol. 2019;45:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Zorzi M, Fedato C, Grazzini G, Stocco FC, Banovich F, Bortoli A, Cazzola L, Montaguti A, Moretto T, Zappa M, Vettorazzi M. High sensitivity of five colorectal screening programmes with faecal immunochemical test in the Veneto Region, Italy. Gut. 2011;60:944-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12:107-111. [PubMed] |
| 102. | Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 632] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 103. | Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2133] [Cited by in RCA: 2165] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 104. | Spivak JL. The anaemia of cancer: death by a thousand cuts. Nat Rev Cancer. 2005;5:543-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 105. | Siques P, Brito J, Naveas N, Pulido R, De la Cruz JJ, Mamani M, León-Velarde F. Plasma and liver lipid profiles in rats exposed to chronic hypobaric hypoxia: changes in metabolic pathways. High Alt Med Biol. 2014;15:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Joosse SA, Pantel K. Tumor-Educated Platelets as Liquid Biopsy in Cancer Patients. Cancer Cell. 2015;28:552-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 107. | Bailey SE, Ukoumunne OC, Shephard EA, Hamilton W. Clinical relevance of thrombocytosis in primary care: a prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br J Gen Pract. 2017;67:e405-e413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 108. | Plantureux L, Mège D, Crescence L, Dignat-George F, Dubois C, Panicot-Dubois L. Impacts of Cancer on Platelet Production, Activation and Education and Mechanisms of Cancer-Associated Thrombosis. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 109. | Schafer AI. Thrombocytosis. N Engl J Med. 2004;350:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 110. | Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 879] [Cited by in RCA: 856] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 111. | Whiteheart SW. Platelet granules: surprise packages. Blood. 2011;118:1190-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 112. | Rowley JW, Schwertz H, Weyrich AS. Platelet mRNA: the meaning behind the message. Curr Opin Hematol. 2012;19:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 113. | Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F, Schellen P, Verschueren H, Post E, Koster J, Ylstra B, Ameziane N, Dorsman J, Smit EF, Verheul HM, Noske DP, Reijneveld JC, Nilsson RJA, Tannous BA, Wesseling P, Wurdinger T. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell. 2015;28:666-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 626] [Cited by in RCA: 634] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 114. | Hugen N, Nagtegaal ID. Distinct metastatic patterns in colorectal cancer patients based on primary tumour location. Eur J Cancer. 2017;75:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 115. | Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2613] [Cited by in RCA: 2396] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 116. | Liu Y, Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell. 2016;30:668-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 826] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 117. | Seubert B, Grünwald B, Kobuch J, Cui H, Schelter F, Schaten S, Siveke JT, Lim NH, Nagase H, Simonavicius N, Heikenwalder M, Reinheckel T, Sleeman JP, Janssen KP, Knolle PA, Krüger A. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology. 2015;61:238-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 118. | Shao Y, Chen T, Zheng X, Yang S, Xu K, Chen X, Xu F, Wang L, Shen Y, Wang T, Zhang M, Hu W, Ye C, Yu X, Shao J, Zheng S. Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis. 2018;39:1368-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 119. | Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, Delman D, Graham K, Gladney WL, Hua X, Black TA, Chien AL, Majmundar KS, Thompson JC, Yee SS, O'Hara MH, Aggarwal C, Xin D, Shaked A, Gao M, Liu D, Borad MJ, Ramanathan RK, Carpenter EL, Ji A, de Beer MC, de Beer FC, Webb NR, Beatty GL. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature. 2019;567:249-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 120. | Tilg H, Adolph TE, Gerner RR, Moschen AR. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018;33:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 548] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 121. | Bindels LB, Neyrinck AM, Loumaye A, Catry E, Walgrave H, Cherbuy C, Leclercq S, Van Hul M, Plovier H, Pachikian B, Bermúdez-Humarán LG, Langella P, Cani PD, Thissen JP, Delzenne NM. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget. 2018;9:18224-18238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 122. | Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2493] [Cited by in RCA: 3791] [Article Influence: 473.9] [Reference Citation Analysis (0)] |
| 123. | Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1834] [Cited by in RCA: 2540] [Article Influence: 254.0] [Reference Citation Analysis (0)] |
| 124. | Invernizzi P. Liver auto-immunology: the paradox of autoimmunity in a tolerogenic organ. J Autoimmun. 2013;46:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 125. | Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochim Biophys Acta. 2011;1812:1601-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 126. | Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 256] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 127. | Bekusova VV, Falchuk EL, Okorokova LS, Kruglova NM, Nozdrachev AD, Markov AG. Increased paracellular permeability of tumor-adjacent areas in 1,2-dimethylhydrazine-induced colon carcinogenesis in rats. Cancer Biol Med. 2018;15:251-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 128. | Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2516] [Cited by in RCA: 3427] [Article Influence: 285.6] [Reference Citation Analysis (0)] |
| 129. | Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 3833] [Article Influence: 273.8] [Reference Citation Analysis (0)] |
| 130. | Richards CH, Roxburgh CS, MacMillan MT, Isswiasi S, Robertson EG, Guthrie GK, Horgan PG, McMillan DC. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One. 2012;7:e41883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 131. | Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, Xiao J, Alexeeff S, Corley D, Weltzien E, Castillo AL, Caan BJ. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. 2017;3:e172319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 314] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 132. | Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668-670. [PubMed] |
| 133. | Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393-R401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 134. | Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch muscles. Am J Physiol. 1992;262:C1513-C1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 135. | Rauw WM. Immune response from a resource allocation perspective. Front Genet. 2012;3:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 136. | Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am J Physiol. 1997;273:R1631-R1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 154] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 137. | Sirniö P, Väyrynen JP, Klintrup K, Mäkelä J, Karhu T, Herzig KH, Minkkinen I, Mäkinen MJ, Karttunen TJ, Tuomisto A. Alterations in serum amino-acid profile in the progression of colorectal cancer: associations with systemic inflammation, tumour stage and patient survival. Br J Cancer. 2019;120:238-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 138. | Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678-3684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 948] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 139. | Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Matés JM, Alonso FJ, Wang C, Seo Y, Chen X, Bishop JM. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 533] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 140. | Kao CC, Bandi V, Guntupalli KK, Wu M, Castillo L, Jahoor F. Arginine, citrulline and nitric oxide metabolism in sepsis. Clin Sci (Lond). 2009;117:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 141. | Kao C, Hsu J, Bandi V, Jahoor F. Alterations in glutamine metabolism and its conversion to citrulline in sepsis. Am J Physiol Endocrinol Metab. 2013;304:E1359-E1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 142. | Chen MK, Espat NJ, Bland KI, Copeland EM, Souba WW. Influence of progressive tumor growth on glutamine metabolism in skeletal muscle and kidney. Ann Surg. 1993;217:655-66; discussion 666-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 143. | Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Köhne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (1)] |
| 144. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1195] [Article Influence: 149.4] [Reference Citation Analysis (0)] |
| 145. | Ogino S, Fuchs CS, Giovannucci E. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert Rev Mol Diagn. 2012;12:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 146. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2427] [Article Influence: 269.7] [Reference Citation Analysis (31)] |
| 147. | Ogino S, Nowak JA, Hamada T, Phipps AI, Peters U, Milner DA, Giovannucci EL, Nishihara R, Giannakis M, Garrett WS, Song M. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut. 2018;67:1168-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 148. | Li H, Zhao Y, Zheng F. Prognostic significance of elevated preoperative neutrophil-to-lymphocyte ratio for patients with colorectal cancer undergoing curative surgery: A meta-analysis. Medicine (Baltimore). 2019;98:e14126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 149. | Tan D, Fu Y, Tong W, Li F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int J Surg. 2018;55:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 150. | Wilson MJ, van Haaren M, Harlaar JJ, Park HC, Bonjer HJ, Jeekel J, Zwaginga JJ, Schipperus M. Long-term prognostic value of preoperative anemia in patients with colorectal cancer: A systematic review and meta-analysis. Surg Oncol. 2017;26:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 151. | Gu D, Szallasi A. Thrombocytosis Portends Adverse Prognosis in Colorectal Cancer: A Meta-Analysis of 5,619 Patients in 16 Individual Studies. Anticancer Res. 2017;37:4717-4726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 152. | Rao XD, Zhang H, Xu ZS, Cheng H, Shen W, Wang XP. Poor prognostic role of the pretreatment platelet counts in colorectal cancer: A meta-analysis. Medicine (Baltimore). 2018;97:e10831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 153. | Tan D, Fu Y, Su Q, Wang H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e3837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 154. | Gunawardene A, Dennett E, Larsen P. Prognostic value of multiple cytokine analysis in colorectal cancer: a systematic review. J Gastrointest Oncol. 2019;10:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 155. | Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1192] [Cited by in RCA: 2072] [Article Influence: 230.2] [Reference Citation Analysis (0)] |
| 156. | Dang VD, Hilgenberg E, Ries S, Shen P, Fillatreau S. From the regulatory functions of B cells to the identification of cytokine-producing plasma cell subsets. Curr Opin Immunol. 2014;28:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 157. | Gunter MJ, Stolzenberg-Solomon R, Cross AJ, Leitzmann MF, Weinstein S, Wood RJ, Virtamo J, Taylor PR, Albanes D, Sinha R. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66:2483-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 158. | Sun L, Hu S, Yu L, Guo C, Sun L, Yang Z, Qi J, Ran Y. Serum haptoglobin as a novel molecular biomarker predicting colorectal cancer hepatic metastasis. Int J Cancer. 2016;138:2724-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 159. | Feng Z, Chen JW, Feng JH, Shen F, Cai WS, Cao J, Xu B. The association between serum ferritin with colorectal cancer. Int J Clin Exp Med. 2015;8:22293-22299. [PubMed] |
| 160. | Yan G, Liu T, Yin L, Kang Z, Wang L. Levels of peripheral Th17 cells and serum Th17-related cytokines in patients with colorectal cancer: a meta-analysis. Cell Mol Biol (Noisy-le-grand). 2018;64:94-102. [PubMed] |
| 161. | Kawamura M, Toiyama Y, Tanaka K, Saigusa S, Okugawa Y, Hiro J, Uchida K, Mohri Y, Inoue Y, Kusunoki M. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer. 2012;48:2244-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 162. | Toiyama Y, Fujikawa H, Kawamura M, Matsushita K, Saigusa S, Tanaka K, Inoue Y, Uchida K, Mohri Y, Kusunoki M. Evaluation of CXCL10 as a novel serum marker for predicting liver metastasis and prognosis in colorectal cancer. Int J Oncol. 2012;40:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 163. | Matsushita K, Toiyama Y, Tanaka K, Saigusa S, Hiro J, Uchida K, Inoue Y, Kusunoki M. Soluble CXCL16 in preoperative serum is a novel prognostic marker and predicts recurrence of liver metastases in colorectal cancer patients. Ann Surg Oncol. 2012;19 Suppl 3:S518-S527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 164. | Werner S, Krause F, Rolny V, Strobl M, Morgenstern D, Datz C, Chen H, Brenner H. Evaluation of a 5-Marker Blood Test for Colorectal Cancer Early Detection in a Colorectal Cancer Screening Setting. Clin Cancer Res. 2016;22:1725-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 165. | Väyrynen JP, Vornanen J, Tervahartiala T, Sorsa T, Bloigu R, Salo T, Tuomisto A, Mäkinen MJ. Serum MMP-8 levels increase in colorectal cancer and correlate with disease course and inflammatory properties of primary tumors. Int J Cancer. 2012;131:E463-E474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 166. | Wilson S, Damery S, Stocken DD, Dowswell G, Holder R, Ward ST, Redman V, Wakelam MJ, James J, Hobbs FD, Ismail T. Serum matrix metalloproteinase 9 and colorectal neoplasia: a community-based evaluation of a potential diagnostic test. Br J Cancer. 2012;106:1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 167. | Meng C, Yin X, Liu J, Tang K, Tang H, Liao J. TIMP-1 is a novel serum biomarker for the diagnosis of colorectal cancer: A meta-analysis. PLoS One. 2018;13:e0207039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 168. | Toiyama Y, Tanaka K, Kitajima T, Shimura T, Kawamura M, Kawamoto A, Okugawa Y, Saigusa S, Hiro J, Inoue Y, Mohri Y, Goel A, Kusunoki M. Elevated serum angiopoietin-like protein 2 correlates with the metastatic properties of colorectal cancer: a serum biomarker for early diagnosis and recurrence. Clin Cancer Res. 2014;20:6175-6186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 169. | Jiang H, Fu XG, Chen YT. Serum level of endothelial cell-specific molecule-1 and prognosis of colorectal cancer. Genet Mol Res. 2015;14:5519-5526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 170. | Wang TB, Chen ZG, Wei XQ, Wei B, Dong WG. Serum vascular endothelial growth factor-C and lymphoangiogenesis are associated with the lymph node metastasis and prognosis of patients with colorectal cancer. ANZ J Surg. 2011;81:694-699. [PubMed] |
| 171. | Ferroni P, Formica V, Della-Morte D, Lucchetti J, Spila A, D'Alessandro R, Riondino S, Guadagni F, Roselli M. Prognostic value of glycated hemoglobin in colorectal cancer. World J Gastroenterol. 2016;22:9984-9993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 172. | Willumsen N, Jorgensen LN, Karsdal MA. Vastatin (the NC1 domain of human type VIII collagen a1 chain) is linked to stromal reactivity and elevated in serum from patients with colorectal cancer. Cancer Biol Ther. 2019;20:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 173. | Kantola T, Väyrynen JP, Klintrup K, Mäkelä J, Karppinen SM, Pihlajaniemi T, Autio-Harmainen H, Karttunen TJ, Mäkinen MJ, Tuomisto A. Serum endostatin levels are elevated in colorectal cancer and correlate with invasion and systemic inflammatory markers. Br J Cancer. 2014;111:1605-1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 174. | Ben QW, Zhao Z, Ge SF, Zhou J, Yuan F, Yuan YZ. Circulating levels of periostin may help identify patients with more aggressive colorectal cancer. Int J Oncol. 2009;34:821-828. [PubMed] |
| 175. | Toiyama Y, Miki C, Inoue Y, Kawamoto A, Kusunoki M. Circulating form of human vascular adhesion protein-1 (VAP-1): decreased serum levels in progression of colorectal cancer and predictive marker of lymphatic and hepatic metastasis. J Surg Oncol. 2009;99:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 176. | Miao X, Zhang Y, Sun J, Cui S, Meng Q, Zhu K, Hu X, Wang T. Elevated serum DAND5 is associated with metastasis and predicts poor prognosis in colorectal cancer. United European Gastroenterol J. 2017;5:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 177. | Kumor A, Daniel P, Pietruczuk M, Małecka-Panas E. Serum leptin, adiponectin, and resistin concentration in colorectal adenoma and carcinoma (CC) patients. Int J Colorectal Dis. 2009;24:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 178. | Zeng J, Tang ZH, Liu S, Guo SS. Clinicopathological significance of overexpression of interleukin-6 in colorectal cancer. World J Gastroenterol. 2017;23:1780-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 179. | Nebiker CA, Han J, Eppenberger-Castori S, Iezzi G, Hirt C, Amicarella F, Cremonesi E, Huber X, Padovan E, Angrisani B, Droeser RA, Rosso R, Bolli M, Oertli D, von Holzen U, Adamina M, Muraro MG, Mengus C, Zajac P, Sconocchia G, Zuber M, Tornillo L, Terracciano L, Spagnoli GC. GM-CSF Production by Tumor Cells Is Associated with Improved Survival in Colorectal Cancer. Clin Cancer Res. 2014;20:3094-3106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 180. | Hu H, Sun L, Guo C, Liu Q, Zhou Z, Peng L, Pan J, Yu L, Lou J, Yang Z, Zhao P, Ran Y. Tumor cell-microenvironment interaction models coupled with clinical validation reveal CCL2 and SNCG as two predictors of colorectal cancer hepatic metastasis. Clin Cancer Res. 2009;15:5485-5493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 181. | Oladipo O, Conlon S, O'Grady A, Purcell C, Wilson C, Maxwell PJ, Johnston PG, Stevenson M, Kay EW, Wilson RH, Waugh DJ. The expression and prognostic impact of CXC-chemokines in stage II and III colorectal cancer epithelial and stromal tissue. Br J Cancer. 2011;104:480-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 182. | Jiang Z, Xu Y, Cai S. CXCL10 expression and prognostic significance in stage II and III colorectal cancer. Mol Biol Rep. 2010;37:3029-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 183. | Akishima-Fukasawa Y, Nakanishi Y, Ino Y, Moriya Y, Kanai Y, Hirohashi S. Prognostic significance of CXCL12 expression in patients with colorectal carcinoma. Am J Clin Pathol. 2009;132:202-10; quiz 307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 184. | Tuomisto A, García-Solano J, Sirniö P, Väyrynen J, Pérez-Guillermo M, Mäkinen MJ, Conesa-Zamora P. HIF-1α expression and high microvessel density are characteristic features in serrated colorectal cancer. Virchows Arch. 2016;469:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 185. | Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014;5:570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 186. | Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 187. | Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, Yuan L, Feng Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019;10:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 188. | Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massagué J, Sancho E, Batlle E. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 899] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 189. | Li Z, Zhou J, Zhang J, Li S, Wang H, Du J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int J Cancer. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 190. | Joo YE, Seo KS, Kim J, Kim HS, Rew JS, Park CS, Kim SJ. Role of tissue inhibitors of metalloproteinases (TIMPs) in colorectal carcinoma. J Korean Med Sci. 1999;14:417-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 191. | Woo HD, Kim K, Kim J. Association between preoperative C-reactive protein level and colorectal cancer survival: a meta-analysis. Cancer Causes Control. 2015;26:1661-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 192. | Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1011] [Cited by in RCA: 1023] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 193. | Ghuman S, Van Hemelrijck M, Garmo H, Holmberg L, Malmström H, Lambe M, Hammar N, Walldius G, Jungner I, Wulaningsih W. Serum inflammatory markers and colorectal cancer risk and survival. Br J Cancer. 2017;116:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 194. | Lu X, Guo W, Xu W, Zhang X, Shi Z, Zheng L, Zhao W. Prognostic value of the Glasgow prognostic score in colorectal cancer: a meta-analysis of 9,839 patients. Cancer Manag Res. 2018;11:229-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 195. | Lee JH, Choi JW, Kim YS. Plasma or serum TIMP-1 is a predictor of survival outcomes in colorectal cancer: a meta-analysis. J Gastrointestin Liver Dis. 2011;20:287-291. [PubMed] |
| 196. | George ML, Eccles SA, Tutton MG, Abulafi AM, Swift RI. Correlation of plasma and serum vascular endothelial growth factor levels with platelet count in colorectal cancer: clinical evidence of platelet scavenging. Clin Cancer Res. 2000;6:3147-3152. [PubMed] |