Published online Jan 21, 2019. doi: 10.3748/wjg.v25.i3.367
Peer-review started: November 14, 2018
First decision: December 5, 2018
Revised: December 12, 2018
Accepted: December 21, 2018
Article in press: December 21, 2018
Published online: January 21, 2019
Processing time: 68 Days and 20.3 Hours
A recent retrospective study confirmed that hepatic stiffness and splenic stiffness measured with magnetic resonance elastography (MRE) are strongly associated with the presence of esophageal varices. In addition, strong correlations have been reported between splenic stiffness values measured with MRE and hepatic venous pressure gradients in animal models. However, most studies have been conducted on adult populations, and previous pediatric MRE studies have only demonstrated the feasibility of MRE in pediatric populations, while the actual clinical application of spleen MRE has been limited.
To assess the utility of splenic stiffness measurements by MRE to predict gastroesophageal varices in children.
We retrospectively reviewed abdominal MRE images taken on a 3T system in pediatric patients. Patients who had undergone Kasai operations for biliary atresia were selected for the Kasai group, and patients with normal livers and spleens were selected for the control group. Two-dimensional spin-echo echo-planar MRE acquisition centered on the liver, with a pneumatic driver at 60 Hz and a low amplitude, was performed to obtain hepatic and splenic stiffness values. Laboratory results for aspartate aminotransferase to platelet ratio index (APRI) were evaluated within six months of MRE, and the normalized spleen size ratio was determined with the upper normal size limit. All Kasai group patients underwent gastroesophageal endoscopy during routine follow-up. The Mann-Whitney U test, Kendall's tau b correlation and diagnostic performance analysis using the area under the curve (AUC) were performed for statistical analysis.
The median spleen MRE value was 5.5 kPa in the control group (n = 9, age 9-18 years, range 4.7-6.4 kPa) and 8.6 kPa in the Kasai group (n = 22, age 4-18 years, range 5.0-17.8 kPa). In the Kasai group, the APRI, spleen size ratio and spleen MRE values were higher in patients with portal hypertension (n = 11) than in patients without (n = 11) (all P < 0.001) and in patients with gastroesophageal varices (n = 6) than in patients without (n = 16) (all P < 0.05), even though their liver MRE values were not different. The APRI (τ = 0.477, P = 0.007), spleen size ratio (τ = 0.401, P = 0.024) and spleen MRE values (τ = 0.426, P = 0.016) also correlated with varices grades. The AUC in predicting gastroesophageal varices was 0.844 at a cut-off of 0.65 (100% sensitivity and 75% specificity) for the APRI, and 0.844 at a cut-off of 9.9 kPa (83.3% sensitivity and 81.3% specificity) for spleen MRE values.
At a cut-off of 9.9 kPa, spleen MRE values predicted gastroesophageal varices as well as the APRI and spleen size ratio in biliary atresia patients after the Kasai operation. However, liver MRE values were not useful for this purpose.
Core tip: Non-invasive monitoring of portal hypertension is important in children with hepatic fibrosis. Spleen magnetic resonance elastography (MRE) values predicted gastroesophageal varices at a cut-off of 9.9 kPa in biliary atresia patients after the Kasai operation, and the diagnostic performance was comparable to that of the aspartate aminotransferase to platelet ratio index and the spleen size ratio. However, liver MRE values did not differ in patients with and without portal hypertension or gastroesophageal varices.
- Citation: Yoon H, Shin HJ, Kim MJ, Han SJ, Koh H, Kim S, Lee MJ. Predicting gastroesophageal varices through spleen magnetic resonance elastography in pediatric liver fibrosis. World J Gastroenterol 2019; 25(3): 367-377
- URL: https://www.wjgnet.com/1007-9327/full/v25/i3/367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i3.367
Biliary atresia is a perinatal disease of unclear etiology characterized by inflammatory obstruction of the biliary tree, leading to biliary cirrhosis and early death if left untreated. The Kasai hepatoportoenterostomy is the first surgical step aimed at restoring bile flow. Performing the Kasai operation earlier increases its chance of success[1]. However, even if the Kasai operation is performed in the first month of life, up to 60% of children who receive the operation will require liver transplantation before the age of 20 years due to liver cirrhosis[2,3]. In 70% of children with successfully established bile drainage, the disease progresses, presenting as fibrosis, portal hypertension and cirrhosis[3]. Therefore, regular long-term monitoring of fibrosis and cholestasis is important for patients with biliary atresia[4].
Liver biopsy is the reference standard for evaluating hepatic fibrosis. However, repeated monitoring with liver biopsy is difficult because of its invasive nature, relatively high cost, small sampling size, interpretational variability and limited patient acceptance, especially in children[5-8]. As a result of these limitations, many investigators have focused on identifying alternative, noninvasive methods to assess hepatic fibrosis. Magnetic resonance elastography (MRE) is an imaging technique that assesses tissue stiffness by measuring the speed of shear wave propagation within the parenchyma. MRE can quantitatively measure the stiffness of the liver parenchyma to facilitate safe, rapid, cost-effective and noninvasive evaluations of a wide range of hepatic diseases. MRE hepatic stiffness values correlate well with hepatic fibrosis, and thus play a crucial role in monitoring disease progression in adults with chronic liver disease[9-11]. Through the use of MRE, it is possible to measure both hepatic stiffness and splenic stiffness and comprehensively assess liver fibrosis and portal hypertension.
Previous studies have revealed that splenic stiffness best correlates with the presence of gastroesophageal varices observed upon endoscopy in chronic liver disease[12,13]. A recent retrospective study confirmed that hepatic stiffness and splenic stiffness measured with MRE are strongly associated with the presence of esophageal varices[14]. In addition, strong correlations have been reported between splenic stiffness values measured with MRE and hepatic venous pressure gradients in animal models[15,16]. However, most studies have been conducted on adult populations, and previous pediatric MRE studies have only demonstrated the feasibility of MRE in pediatric populations, while the actual clinical application of spleen MRE has been limited.
Therefore, the primary aim of this study was to evaluate the utility of MRE for hepatic and splenic stiffness assessments to evaluate portal hypertension in pediatric patients with biliary atresia after the Kasai operation.
Our institutional review board approved this study and waived the requirement for informed consent because of its retrospective nature. From January 2015 to December 2016, we retrospectively reviewed patients who were under the age of 18 and had undergone liver magnetic resonance imaging (MRI), including MRE and T2* mapping, due to various indications. Patients with a history of biliary atresia who had undergone the Kasai operation, with lab results obtained within six months of MRE imaging, were included in the Kasai group. Patients without any previous medical or surgical history who had undergone liver MRI to evaluate the possibility of fatty liver, and who were thereby found to have no liver pathology, were included in the control group. The normal criteria for liver MRI were a hepatic fat fraction < 6%[17], liver MRE values < 2.71 kPa[18,19] and a liver T2* value > 6.7 msec[20].
Clinical charts were reviewed to determine the absence or presence of clinical portal hypertension (PHT). Clinically evident PHT was defined as being present when there was either a history of a complication of PHT (esophageal or gastric variceal bleed, ascites or hepatopulmonary syndrome) or clinical findings consistent with PHT in terms of both splenomegaly and thrombocytopenia (platelet count < 150000 cells/mL)[21]. The modified aspartate aminotransferase to platelet ratio index (APRI) was calculated[22]. In the Kasai group, all patients had previous endoscopy results, which were reviewed by one gastroenterologist to determine the presence of varices. The varices grade was classified as 0, no varices; 1, varices running straight; 2, varices with a beaded appearance; and 3, varices running obliquely and tortuously, with a tumor-like appearance[23].
Liver MRI was performed with a 3T system (Discovery 750w; GE Healthcare, Milwaukee, WI, United States). The routine liver MRI protocol included coronal and axial single-shot fast spin-echo T2-weighted images for morphological liver assessment, a three-dimensional volumetric multi-echo gradient sequence (IDEAL-IQ; GE Healthcare) for fat quantification using the proton density fat fraction and T2* decay assessment, and MRE. Spleen size was measured as the longest diameter of the spleen on either a coronal or axial image. The spleen size ratio was calculated as the spleen length divided by the suggested upper normal limit of spleen length at each age[24], to compensate for growth effects due to age. The total scan time was less than 15 min, and the imaging study was done without sedation. All patients fasted for at least 4 h prior to MRE.
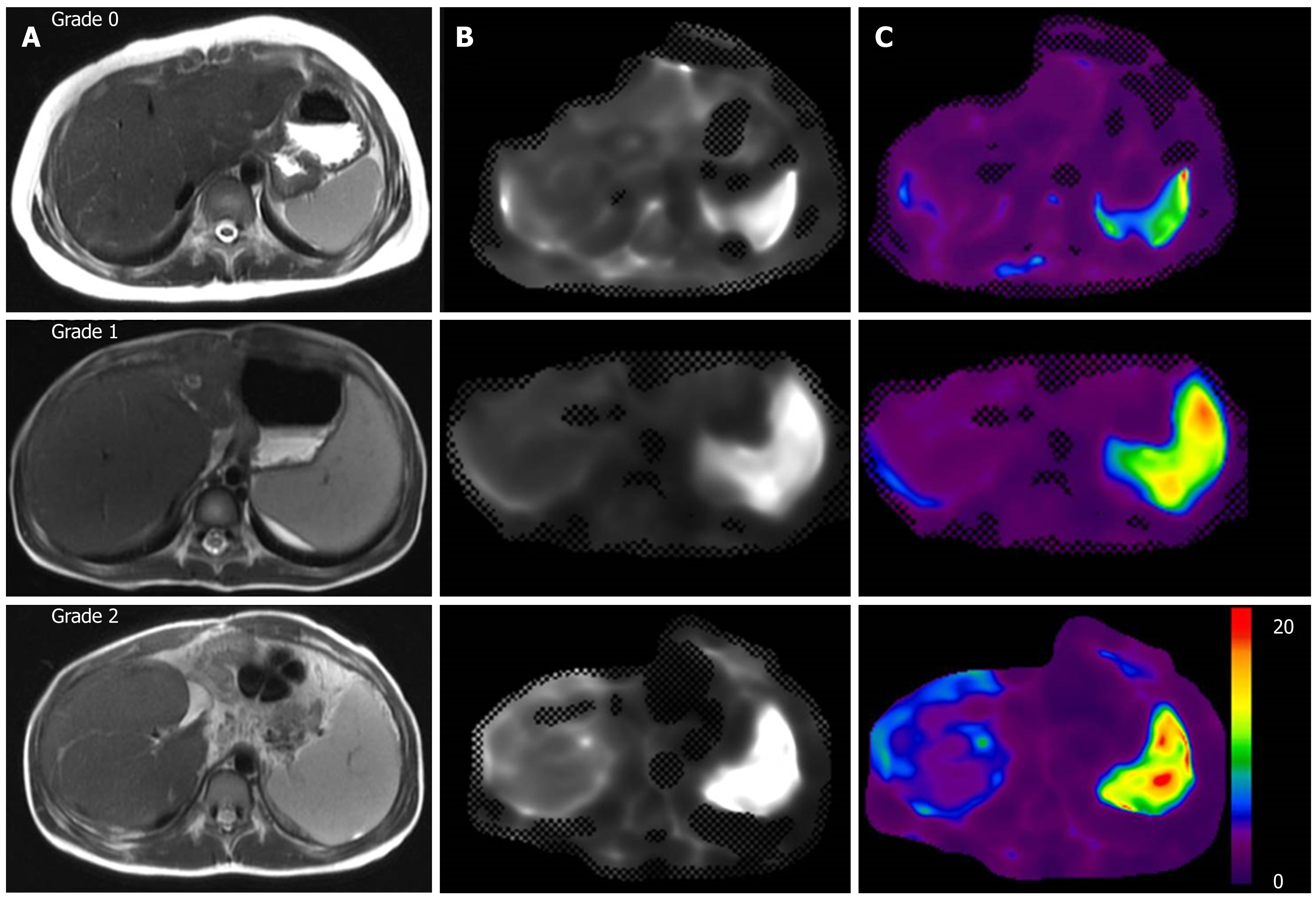
For MRE, a 19-cm diameter passive pneumatic driver was positioned over the right anterior abdominal wall and attached to an acoustic waveform generator. The driver amplitude power was reduced by 20%, and a 60 Hz waveform was applied to the driver to prevent abdominal wall discomfort and pain in pediatric patients[25]. A two-dimensional spin-echo-based echo-planar MRE sequence was acquired with the following parameters: repetitive time/echo time = 1000/62, 60 Hz magnetization encoding gradient, 64 × 64 matrix and 8-mm slice thickness with a 2-mm gap and a 38-cm field of view. Four slices were obtained, including the level of the porta hepatis, during a 24-s breath hold. If the patient could not hold his or her breath, the scan was performed while the patient was breathing freely, with a single average rather than multiple averages. Wave images and MRE images with cross-hatching were automatically generated on the operating console.
The inversion algorithm used for stiffness map calculation involved a multi-scale direct inversion. Hepatic stiffness was measured by one experienced radiologist. A single maximum region of interest (ROI) was placed on each stiffness map, mainly in the right hepatic lobe, avoiding large vessels and areas with inadequate wave propagation or cross-hatching marks. The cross-reference tool in our system was used for guidance. The average of four stiffness maps was used to represent the hepatic stiffness of each patient, and the mean values were recorded in kilopascals (kPa). Splenic stiffness was measured through the placement of a free-hand single maximum ROI at each visible splenic area on the stiffness map. The average of the spleen ROIs was used to represent the splenic stiffness of each patient and was recorded in kPa.
Data management and statistical calculations were performed with MedCalc software (version 18.10.2, Ostend, Belgium). The Mann-Whitney U test was performed for group comparisons. For the varices grade groups, the Kruskal-Wallis test was used for group comparisons, and Kendall's tau b test was used for correlation analysis. Area under the curve (AUC) analyses were also performed to evaluate the diagnostic performance for gastroesophageal varices. P values less than 0.05 were considered significant.
During the study period, 26 biliary atresia patients who had previously undergone the Kasai operation underwent liver MRI including MRE. Four of these patients were excluded: three had a history of splenic artery embolization, and one did not have a spleen MRE value because the spleen was outside the field of view. Thus, a total of 22 patients (10 male and 12 female) were enrolled in the Kasai group, with ages ranging from 4 to 18 years (median age of 10 years). None of the patients had difficulty holding their breath during the liver MRI. All 22 patients had a native liver, and none had a clinical or radiological diagnosis of a hepatic tumor. For the control group, 10 patients met the inclusion criteria. However, one was excluded because the spleen was outside the field of view during MRE. Therefore, nine patients (five male and four female, age 9-18 years with a median of 14 years) were ultimately included in the control group.
Table 1 displays the clinical and radiological results of both groups. The body mass index, hepatic fat fraction and liver T2* values did not differ between the two groups. However, the Kasai group had higher APRI (median 0.61 vs 0.33, P = 0.033), spleen size (median 11.8 cm vs 9.5 cm, P = 0.009), spleen size ratio (median 1.03 vs 0.83, P = 0.004), liver MRE (median 3.4 kPa vs 2.2 kPa, P < 0.001) and spleen MRE values (median 9.0 kPa vs 5.5 kPa, P < 0.001) than the control group. The range of spleen MRE values in the control group was 4.7-6.4 kPa.
| Kasai group (n = 22) | Control group (n = 9) | P value1 | ||
| Clinical and laboratory findings | Age (yr) | 10.0 (4.0-18.0) | 14.0 (9.0-18.0) | 0.012 |
| BMI (kg/m2) | 17.1 (14.8-29.9) | 20.6 (14.7-24.2) | 0.593 | |
| APRI | 0.61 (0.18-5.00) | 0.33 (0.18-0.51) | 0.033 | |
| Varices | n = 6 | n = 0 | ||
| Liver MRI findings | Spleen size (cm) | 11.8 (7.1-19.0) | 9.5 (7.9-10.1) | 0.009 |
| Spleen size ratio | 1.03 (0.63-1.73) | 0.83 (0.68-0.92) | 0.004 | |
| Liver fat fraction (%) | 3.0 (1.0-18.0) | 3.1 (2.5-5.8) | 0.313 | |
| Liver T2* value (msec) | 23.5 (15.0-64.1) | 25.6 (12.0-30.0) | 0.824 | |
| Liver MRE value (kPa) | 3.4 (2.5-6.0) | 2.2 (1.6-2.5) | < 0.001 | |
| Spleen MRE value (kPa) | 9.0 (5.0-17.8) | 5.5 (4.7-6.4) | < 0.001 |
In the Kasai group, 11 patients (11/22, 50%) had clinical PHT. The patients with and without PHT (Table 2) did not differ in age (median 10.0 years vs 10.0 years, P = 0.797). The APRI (median 1.17 vs 0.30, P < 0.001), spleen size (median 14.6 cm vs 9.6 cm, P < 0.001), spleen size ratio (median 1.30 vs 0.91, P < 0.001), and spleen MRE values (median 11.1 kPa vs 7.6 kPa, P < 0.001) were higher in the patients with PHT than in those without PHT. However, the liver MRE values did not differ between these groups (median 3.7 kPa vs 3.1 kPa, P = 0.056).
| With PHT (n = 11) | Without PHT (n = 11) | P value1 | ||
| Clinical and laboratory findings | Age (yr) | 10.0 (7.0-18.0) | 10.0 (4.0-15.0) | 0.797 |
| BMI (kg/m2) | 16.6 (15.1-25.6) | 17.4 (14.8-29.9) | 0.797 | |
| APRI | 1.17 (0.47-5.00) | 0.30 (0.18-1.82) | < 0.001 | |
| Liver MRI findings | Spleen size (cm) | 14.6 (10.9-19.0) | 9.6 (7.1-12.1) | < 0.001 |
| Spleen size ratio | 1.30 (0.97-1.73) | 0.91 (0.63-1.06) | < 0.001 | |
| Liver fat fraction (%) | 3.0 (1.6-7.0) | 2.0 (1.0-18.0) | 0.300 | |
| Liver T2* value (msec) | 22.5 (15.0-38.8) | 24.1 (17.5-64.1) | 0.654 | |
| Liver MRE value (kPa) | 3.7 (2.9-6.0) | 3.1 (2.5-5.7) | 0.056 | |
| Spleen MRE value (kPa) | 11.1 (7.1-17.8) | 7.6 (5.0-9.6) | < 0.001 |
Six patients were found to have esophageal or gastric varices upon endoscopy. The endoscopies were performed within 0-104 mo of the liver MRI (median 52 mo) in all patients, and within 0-98 mo (median 15 mo) in patients with varices. Four patients had grade 1 esophageal varices, one patient had grade 2 esophageal varices and one patient had grade 2 gastric varices.
When patients with and without gastroesophageal varices were compared (Table 3), the APRI (median 1.21 vs 0.40, P = 0.013), spleen size ratio (median 1.33 vs 0.95, P = 0.027) and spleen MRE values (median 12.0 kPa vs 8.4 kPa, P = 0.013) were found to be significantly higher in patients with varices. However, the liver MRE values did not differ between these groups (median 3.7 kPa vs 3.3 kPa, P = 0.541).
| With varices (n = 6) | Without varices (n = 16) | P value1 | ||
| Clinical and laboratory findings | Age (yr) | 9.0 (7.0-11.0) | 10.0 (4.0-18.0) | 0.203 |
| BMI (kg/m2) | 17.1 (15.3-25.6) | 17.1 (14.8-29.9) | 0.914 | |
| APRI | 1.21 (0.83-5.00) | 0.40 (0.18-3.39) | 0.013 | |
| Liver MRI findings | Spleen size (cm) | 14.1 (10.9-17.2) | 11.1 (7.1-19.0) | 0.070 |
| Spleen size ratio | 1.33 (0.97-1.52) | 0.95 (0.63-1.73) | 0.027 | |
| Liver fat fraction (%) | 3.0 (2.2-7.0) | 2.8 (1.0-18.0) | 0.294 | |
| Liver T2* value (msec) | 24.7 (15.0-38.8) | 23.5 (17.2-64.1) | 0.791 | |
| Liver MRE value (kPa) | 3.7 (2.9-4.6) | 3.3 (2.5-6.0) | 0.541 | |
| Spleen MRE value (kPa) | 12.0 (7.1-17.8) | 8.4 (5.0-11.4) | 0.013 |
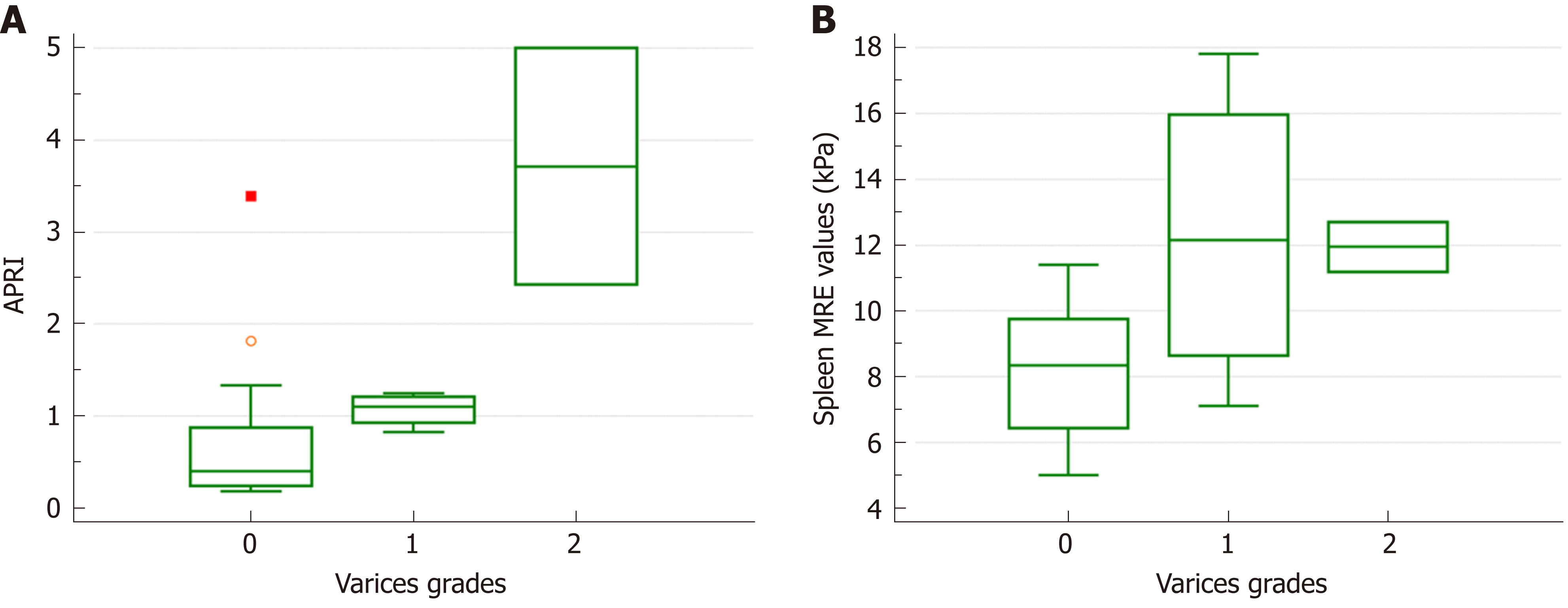
When the groups were compared according to the varices grade, only the APRI (median 0.40, 1.10 and 3.72 for grades 0, 1 and 2; P = 0.029) and spleen MRE values (median 8.4, 12.2 and 12.0 for grades 0, 1 and 2; P = 0.045) differed among the different grade groups (Figure 1). However, the spleen size (P = 0.183) and spleen size ratio (P = 0.084) did not differ in this comparison. In the correlation analysis between the measurement variables and varices grades, the APRI (τ = 0.477, P = 0.007), spleen size ratio (τ = 0.401, P = 0.024) and spleen MRE values (τ = 0.426, P = 0.016) correlated with the varices grade (Figure 2).
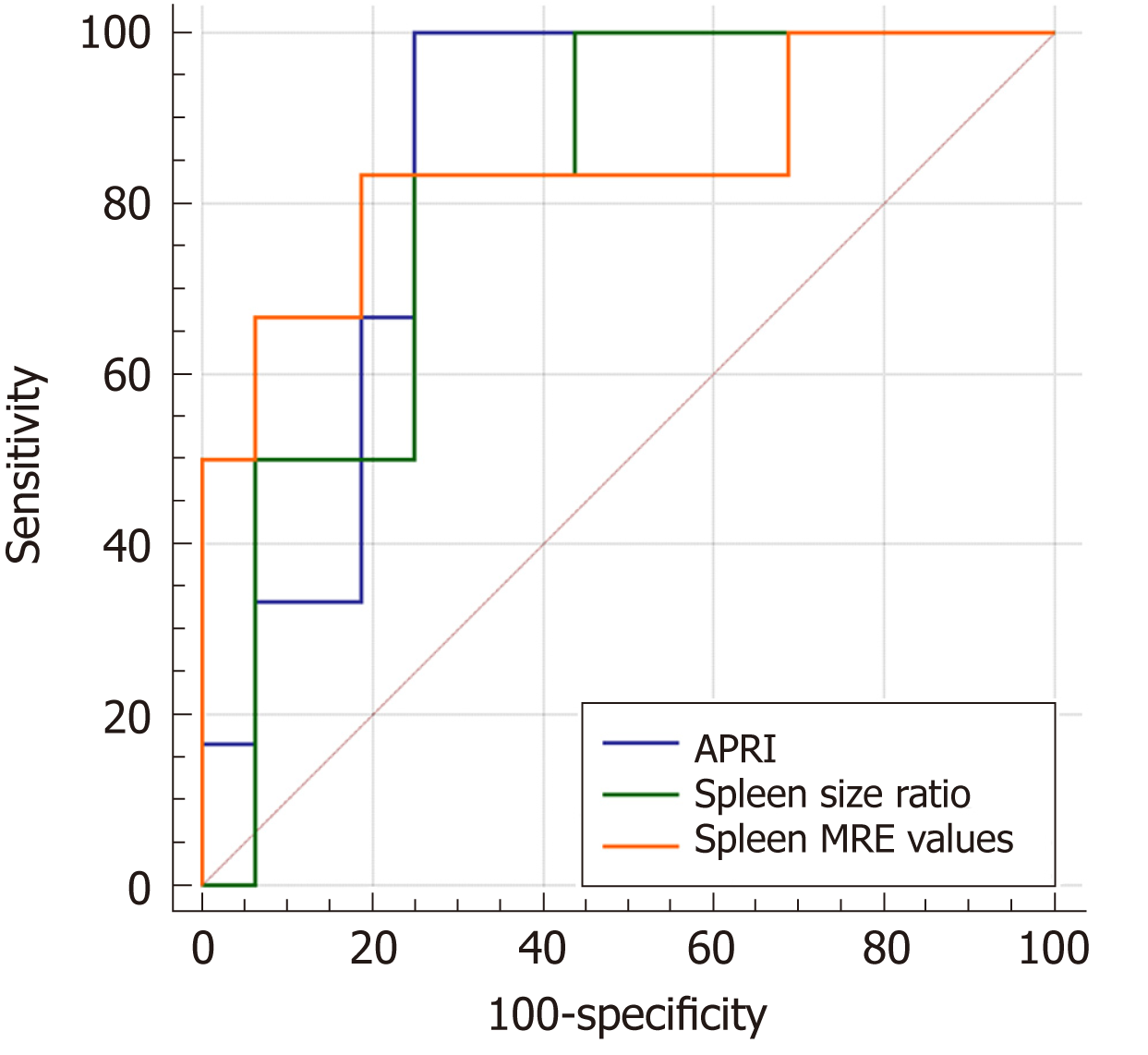
Table 4 summarizes the diagnostic performance of the APRI, spleen size ratio and spleen MRE values. The APRI had 100% sensitivity and 75.0% specificity for predicting varices when a cut-off value of 0.65 was used (AUC = 0.844). The cut-off value for the spleen size ratio was 1.08, with 83.3% sensitivity and 75.0% specificity (AUC = 0.813). The AUC of the spleen MRE values in predicting varices was 0.844 at a cut-off value of 9.9 kPa. When the AUCs of these three methods were compared, the diagnostic performance was not found to differ among them (Figure 3).
| Parameter | AUC | 95%CI | Cut-off value | Sensitivity (%) | Specificity (%) |
| APRI | 0.844 | 0.627-0.961 | > 0.65 | 100.0 | 75.0 |
| Spleen size ratio | 0.813 | 0.591-0.945 | > 1.08 | 83.3 | 75.0 |
| Spleen MRE (kPa) | 0.844 | 0.627-0.961 | > 9.9 | 83.3 | 81.3 |
The results of our study indicate that spleen MRE values can predict gastroesophageal varices as well as the APRI and spleen size ratio in biliary atresia patients after the Kasai operation. However, liver MRE values did not differ between patients with and without portal hypertension and patients with and without varices. These results are in good agreement with a previous study in adults, which also demonstrated that splenic stiffness values were superior to hepatic stiffness values for identifying the presence of varices in patients with chronic liver disease[26].
One of the major complications of biliary atresia is the development of PHT. Often, PHT is defined by the development of complications, or by endoscopic findings. However, surveillance endoscopy of children with cirrhosis is not undertaken by many pediatric gastroenterologists[21]. Therefore, a clinically accessible and reproducible modality for surveillance is required. Monitoring the APRI or splenomegaly can be an easy way for clinicians to predict liver cirrhosis. In 2011, Chongsrisawat et al[27] demonstrated that the APRI (at a cut-off value of 1.92) had 84% sensitivity and 83% specificity for predicting varices in 73 biliary atresia patients after operation. They also found that splenomegaly had high sensitivity and specificity (92% and 85%, respectively). Another study revealed that the APRI (r = 0.5, P < 0.001) and spleen size (r = 0.38, P < 0.001) correlated with portal venous pressure in Kasai patients, but had limitations as prognostic factors for variceal development[28]. The APRI (at a cut-off value of 0.6) also had greater predictive accuracy than liver stiffness measurements for esophageal varices in pediatric patients with various liver diseases[29]. Our study also demonstrated the high diagnostic performance of the APRI and spleen size ratio for predicting gastroesophageal varices in biliary atresia patients. However, the APRI is an indirect method of evaluating portal hypertension, and a wide range of cut-off values were reported in the previous studies. Furthermore, to obtain the spleen size ratio, precise anatomical imaging is essential.
Several quantitative elastography technologies using ultrasound such as transient elastography (TE), acoustic radiation force impulse (ARFI) imaging and supersonic shear wave elastography (SSWE) have been used to evaluate liver fibrosis in pediatric patients and showed good correlation between liver fibrosis and elastographic values[30-32]. However, TE has many pitfalls such as more technical failures in children and inability to avoid other structures such as liver vessels and bile ducts[33]. Unlike TE, real-time elastographic methods such as ARFI and SSWE have been integrated to conventional diagnostic ultrasound. However, ARFI cannot produce a real-time quantitative map of liver tissue stiffness, and only a few studies have been performed in children to predict liver fibrosis using SSWE[31,34]. In our study, we performed short liver MRI, including an anatomical sequence and MRE, which could be completed in less than 15 min and did not require sedation. This allowed us to perform a minimal and noninvasive study to evaluate the status of the liver and combined complications during follow-up in patients with biliary atresia after the Kasai operation. We could not only measure spleen size, evaluate anatomical lesions such as morphological cirrhosis and identify biliary complications such as bile cysts or masses, but also perform a functional evaluation using hepatic and splenic stiffness measurements. The spleen size ratio measured from MRI images and spleen MRE values had a good diagnostic performance for predicting gastroesophageal varices in children. However, liver MRE values were not useful for this purpose.
In previous studies, hepatic fibrosis has been found to correlate with liver MRE values in adult patients[35] and children[36]. However, although liver MRE values appear to be a reliable surrogate for liver biopsies in identifying hepatic fibrosis, the pathophysiological basis for their correlation with PHT remains poorly defined. Hepatic stiffness can reflect increased intrahepatic vascular resistance caused by anatomical changes, but it is not likely to be sensitive to changes in portal pressure due to functional vascular changes[37]. Our study revealed no difference in liver MRE values between patients with and without PHT or gastroesophageal varices.
This is the first report of spleen MRE values in children. We obtained spleen MRE values during routine liver MRI with the driver placed on the right side of the abdomen. The normal range of spleen MRE values in our control group (aged 9-18 years) was 4.7-6.4 kPa, with a median of 5.5 kPa. In a previous study in which a 1.5T MRI system was used, the mean spleen MRE value in healthy adult volunteers was 3.6 kPa (ranging from 2.4 to 4.4 kPa) with the driver placed on the right side of the abdomen, and 4.3 kPa (ranging from 3.2 to 5.6 kPa) with the driver on the left side of the abdomen[38]. This indicates that the location of the driver can affect the stiffness value, and this factor needs to be considered when results are interpreted in each clinic.
Our study also demonstrated the clinical usefulness of spleen MRE values in pediatric patients with biliary atresia. Spleen MRE values were higher in patients with portal hypertension than in patients without (median 11.1 kPa vs 7.6 kPa, P < 0.001) and in patients with gastroesophageal varices than in patients without (median 12.0 kPa vs 8.4 kPa, P = 0.013). The diagnostic performance of spleen MRE values in predicting gastroesophageal varices was comparable to that of the APRI and spleen size ratio. Therefore, clinicians may start gastroesophageal endoscopy in patients with spleen MRE values above the cut-off value of 9.9 kPa. Moreover, spleen MRE values were different at different varices grades, even though the number of patients in each grade was small (four in grade 1 and two in grade 2). Thus, clinicians can use this method to monitor PHT noninvasively.
Although our experience suggests that liver MRI including spleen MRE is a promising tool for assessing biliary atresia patients after the Kasai operation, there are some limitations to this study. First, this was a retrospective study with a limited number of patients in a single center. Also, we lacked portal venous pressure measurements with hepatic venous pressure gradients. At our institution, hepatic venous pressure measurements are not performed in routine practice for patients with biliary atresia after the Kasai operation, and we did not perform an additional invasive procedure for this retrospective analysis. A future study with a large number of patients involving multiple institutions should be considered. Second, there were varying time intervals between gastroesophageal endoscopy and MRE, even though most patients were stable during follow-up. Third, we could not control other factors that can influence hepatic elasticity, since hepatic stiffness reflects not only fibrosis, but also inflammation. Despite these limitations, our study suggests the clinical utility of spleen MRE in pediatric patients with biliary atresia. A further study with a large number of patients with variable varices grades is needed to validate these results.
In conclusion, the median value of normal spleen MRE was 5.5 kPa in children, with a maximum of 6.4 kPa. Spleen MRE values predicted gastroesophageal varices at a cut-off of 9.9 kPa in biliary atresia patients after the Kasai operation, and the diagnostic performance was comparable to that of the APRI and the spleen size ratio. However, liver MRE values did not differ in patients with and without PHT or gastroesophageal varices.
Biliary atresia patients have high chance of disease progression to liver fibrosis even after Kasai operation. Therefore, regular long-term monitoring is required to early diagnose liver cirrhosis and portal hypertension. The primary aim of this study was to evaluate the utility of magnetic resonance elastography (MRE) for hepatic and splenic stiffness assessments to evaluate portal hypertension in pediatric patients with biliary atresia after the Kasai operation.
Hepatic fibrosis has been found to correlate with liver MRE values in adult and children. However, there is little discussion about the relationship between spleen stiffness measurement by MRE and portal hypertension in children.
This study analyzed the role of spleen MRE values in biliary atresia patients after Kasai operation with portal hypertension.
We retrospectively reviewed abdominal MRE images in pediatric patients. Patients who had undergone Kasai operations for biliary atresia were selected for the Kasai group, and patients with normal livers and spleens were selected for the control group. Hepatic and splenic stiffness values were measured by MRE. Aspartate aminotransferase to platelet ratio index (APRI) from laboratory results and the normalized spleen size ratio were calculated. These parameters were compared between the Kasai group and the control group, and also among the Kasai group patients depending on the existence of portal hypertension or gastroesophageal varices.
The median spleen MRE value was 5.5 kPa in the control group and 8.6 kPa in the Kasai group. In the Kasai group, the APRI, spleen size ratio and spleen MRE values were higher in patients with portal hypertension and in patients with gastroesophageal varices, even though their liver MRE values were not different. The APRI, spleen size ratio and spleen MRE values also correlated with varices grades. The AUC in predicting gastroesophageal varices was 0.844 at a cut-off of 0.65 for the APRI, and 0.844 at a cut-off of 9.9 kPa for spleen MRE values.
Spleen MRE values were useful for evaluating portal hypertension and gastroesophageal varices in biliary atresia patients after Kasai operation. At a cut-off of 9.9 kPa, spleen MRE values predicted gastroesophageal varices as well as the APRI and spleen size ratio. However, liver MRE values did not differ in patients with and without portal hypertension or gastroesophageal varices. Spleen MRE values may help screen out high-risk patients early and administer adequate interventions during follow up.
For biliary atresia patients after Kasai operation, spleen MRE values can be used to evaluate portal hypertension and gastroesophageal varices without invasive monitoring. The results of this study, needs to be verified by a large sample size study with multiple institutions.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Huang LY, Konishi H S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
| 1. | Serinet MO, Wildhaber BE, Broué P, Lachaux A, Sarles J, Jacquemin E, Gauthier F, Chardot C. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009;123:1280-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Chardot C, Buet C, Serinet MO, Golmard JL, Lachaux A, Roquelaure B, Gottrand F, Broué P, Dabadie A, Gauthier F, Jacquemin E. Improving outcomes of biliary atresia: French national series 1986-2009. J Hepatol. 2013;58:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 638] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 4. | Hadzić N, Davenport M, Tizzard S, Singer J, Howard ER, Mieli-Vergani G. Long-term survival following Kasai portoenterostomy: is chronic liver disease inevitable? J Pediatr Gastroenterol Nutr. 2003;37:430-433. [PubMed] |
| 5. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [PubMed] |
| 6. | Pariente D, Franchi-Abella S. Paediatric chronic liver diseases: how to investigate and follow up? Role of imaging in the diagnosis of fibrosis. Pediatr Radiol. 2010;40:906-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1398] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 8. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 9. | Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207-1213.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 771] [Cited by in RCA: 714] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 10. | Batheja M, Vargas H, Silva AM, Walker F, Chang YH, De Petris G, Silva AC. Magnetic resonance elastography (MRE) in assessing hepatic fibrosis: performance in a cohort of patients with histological data. Abdom Imaging. 2015;40:760-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Yoshimitsu K, Mitsufuji T, Shinagawa Y, Fujimitsu R, Morita A, Urakawa H, Hayashi H, Takano K. MR elastography of the liver at 3.0 T in diagnosing liver fibrosis grades; preliminary clinical experience. Eur Radiol. 2016;26:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Morisaka H, Motosugi U, Ichikawa S, Sano K, Ichikawa T, Enomoto N. Association of splenic MR elastographic findings with gastroesophageal varices in patients with chronic liver disease. J Magn Reson Imaging. 2015;41:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Talwalkar JA, Yin M, Venkatesh S, Rossman PJ, Grimm RC, Manduca A, Romano A, Kamath PS, Ehman RL. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR Am J Roentgenol. 2009;193:122-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Shin SU, Lee JM, Yu MH, Yoon JH, Han JK, Choi BI, Glaser KJ, Ehman RL. Prediction of esophageal varices in patients with cirrhosis: usefulness of three-dimensional MR elastography with echo-planar imaging technique. Radiology. 2014;272:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Nedredal GI, Yin M, McKenzie T, Lillegard J, Luebke-Wheeler J, Talwalkar J, Ehman R, Nyberg SL. Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging. 2011;34:79-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Yin M, Kolipaka A, Woodrum DA, Glaser KJ, Romano AJ, Manduca A, Talwalkar JA, Araoz PA, McGee KP, Anavekar NS, Ehman RL. Hepatic and splenic stiffness augmentation assessed with MR elastography in an in vivo porcine portal hypertension model. J Magn Reson Imaging. 2013;38:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Shin HJ, Kim HG, Kim MJ, Koh H, Kim HY, Roh YH, Lee MJ. Normal range of hepatic fat fraction on dual- and triple-echo fat quantification MR in children. PLoS One. 2015;10:e0117480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Binkovitz LA, El-Youssef M, Glaser KJ, Yin M, Binkovitz AK, Ehman RL. Pediatric MR elastography of hepatic fibrosis: principles, technique and early clinical experience. Pediatr Radiol. 2012;42:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Xanthakos SA, Podberesky DJ, Serai SD, Miles L, King EC, Balistreri WF, Kohli R. Use of magnetic resonance elastography to assess hepatic fibrosis in children with chronic liver disease. J Pediatr. 2014;164:186-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Alústiza Echeverría JM, Castiella A, Emparanza JI. Quantification of iron concentration in the liver by MRI. Insights Imaging. 2012;3:173-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Shneider BL, Abel B, Haber B, Karpen SJ, Magee JC, Romero R, Schwarz K, Bass LM, Kerkar N, Miethke AG, Rosenthal P, Turmelle Y, Robuck PR, Sokol RJ; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr. 2012;55:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3242] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 23. | North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 841] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 24. | Megremis SD, Vlachonikolis IG, Tsilimigaki AM. Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology. 2004;231:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Serai SD, Towbin AJ, Podberesky DJ. Pediatric liver MR elastography. Dig Dis Sci. 2012;57:2713-2719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Ma X, Wang L, Wu H, Feng Y, Han X, Bu H, Zhu Q. Spleen Stiffness Is Superior to Liver Stiffness for Predicting Esophageal Varices in Chronic Liver Disease: A Meta-Analysis. PLoS One. 2016;11:e0165786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Chongsrisawat V, Vejapipat P, Siripon N, Poovorawan Y. Transient elastography for predicting esophageal/gastric varices in children with biliary atresia. BMC Gastroenterol. 2011;11:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Shalaby A, Makin E, Davenport M. Portal venous pressure in biliary atresia. J Pediatr Surg. 2012;47:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Voutilainen S, Kivisaari R, Lohi J, Jalanko H, Pakarinen MP. A Prospective Comparison of Noninvasive Methods in the Assessment of Liver Fibrosis and Esophageal Varices in Pediatric Chronic Liver Diseases. J Clin Gastroenterol. 2016;50:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Shin NY, Kim MJ, Lee MJ, Han SJ, Koh H, Namgung R, Park YN. Transient elastography and sonography for prediction of liver fibrosis in infants with biliary atresia. J Ultrasound Med. 2014;33:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Leschied JR, Dillman JR, Bilhartz J, Heider A, Smith EA, Lopez MJ. Shear wave elastography helps differentiate biliary atresia from other neonatal/infantile liver diseases. Pediatr Radiol. 2015;45:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Shima H, Igarashi G, Wakisaka M, Hamano S, Nagae H, Koyama M, Kitagawa H. Noninvasive acoustic radiation force impulse (ARFI) elastography for assessing the severity of fibrosis in the post-operative patients with biliary atresia. Pediatr Surg Int. 2012;28:869-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Goldschmidt I, Streckenbach C, Dingemann C, Pfister ED, di Nanni A, Zapf A, Baumann U. Application and limitations of transient liver elastography in children. J Pediatr Gastroenterol Nutr. 2013;57:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Yin M, Glaser KJ, Talwalkar JA, Chen J, Manduca A, Ehman RL. Hepatic MR Elastography: Clinical Performance in a Series of 1377 Consecutive Examinations. Radiology. 2016;278:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 35. | Huwart L, Sempoux C, Salameh N, Jamart J, Annet L, Sinkus R, Peeters F, ter Beek LC, Horsmans Y, Van Beers BE. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007;245:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 36. | Trout AT, Sheridan RM, Serai SD, Xanthakos SA, Su W, Zhang B, Wallihan DB. Diagnostic Performance of MR Elastography for Liver Fibrosis in Children and Young Adults with a Spectrum of Liver Diseases. Radiology. 2018;287:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Lim JK, Groszmann RJ. Transient elastography for diagnosis of portal hypertension in liver cirrhosis: is there still a role for hepatic venous pressure gradient measurement? Hepatology. 2007;45:1087-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Mannelli L, Godfrey E, Joubert I, Patterson AJ, Graves MJ, Gallagher FA, Lomas DJ. MR elastography: Spleen stiffness measurements in healthy volunteers--preliminary experience. AJR Am J Roentgenol. 2010;195:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |