Published online Jan 21, 2019. doi: 10.3748/wjg.v25.i3.330
Peer-review started: October 18, 2018
First decision: November 29, 2018
Revised: December 21, 2018
Accepted: December 21, 2018
Article in press: December 21, 2018
Published online: January 21, 2019
Processing time: 95 Days and 22.5 Hours
Atrophic gastritis is characterized by loss of appropriate glands and reduction in gastric secretory function due to chronic inflammatory processes in gastric mucosa. Moreover, atrophic gastritis is considered as a precancerous condition of gastric cancer. However, little is known about the molecular mechanism underlying gastric mucosal atrophy and its contribution to gastric carcinogenesis. Thus, we hypothesized that transcription factor NKX6.3 might be involved in maintaining gastric epithelial homeostasis by regulating amyloid β (Aβ) production.
To determine whether NKX6.3 might protect against gastric mucosal atrophy by regulating Aβ production.
We identified NKX6.3 depletion induced cell death by cell count and Western blot assay. Production and mechanism of Aβ oligomer were analyzed by enzyme-linked immunosorbent assay, Western blot, immunoprecipitation, real-time quantitative polymerase chain reaction and immunofluorescence analysis. We further validated the correlation between expression of NKX6.3, Helicobacter pylori CagA, Aβ oligomer, apolipoprotein E (ApoE), and β-secretase 1 (Bace1) in 55 gastric mucosae.
NKX6.3 depletion increased both adherent and floating cell populations in HFE-145 cells. Expression levels of cleaved caspase-3, -9, and poly ADP ribose polymerase were elevated in floating HFE-145shNKX6.3 cells. NKX6.3 depletion produced Aβ peptide oligomers, and increased expression of ApoE, amyloid precursor protein, Aβ, Bace1, low-density lipoprotein receptor, nicastrin, high mobility group box1, and receptor for advanced glycosylation end product proteins. In immunoprecipitation assay, γ-secretase complex was stably formed only in HFE-145shNKX6.3 cells. In gastric mucosae with atrophy, expression of Aβ peptide oligomer, ApoE, and Bace1 was detected and inversely correlated with NKX6.3 expression. Treatment with recombinant Aβ 1-42 produced Aβ oligomeric forms and decreased cell viability in HFE-145shNKX6.3 cells. Additionally, NKX6.3 depletion increased expression of inflammatory cytokines and cyclooxygenase-2.
NKX6.3 inhibits gastric mucosal atrophy by regulating Aβ accumulation and inflammatory reaction in gastric epithelial cells.
Core tip: In human gastric epithelial cells, NKX6.3 depletion induced production of amyloid β (Aβ) oligomers, and also increased expression of apolipoprotein E (ApoE), Aβ, β-secretase 1 (Bace1), nicastrin, and receptor for advanced glycosylation end product proteins. Moreover, NKX6.3 depletion leads to stably formed of γ-secretase complex and binding to Bace1 protein. In gastric mucosae with atrophy, expression of Aβ oligomer, ApoE, and Bace1 was detected and inversely correlated with NKX6.3 expression. Additionally, treatment with recombinant Aβ 1-42 produced oligomeric forms of Aβ and significantly decreased cell viability in NKX6.3 depleting cells. These observations provide evidences that NKX6.3 can inhibit gastric mucosal atrophy by regulating Aβ peptide accumulation and inflammatory reaction in gastric epithelial cells.
- Citation: Yoon JH, Lee YS, Kim O, Ashktorab H, Smoot DT, Nam SW, Park WS. NKX6.3 protects against gastric mucosal atrophy by downregulating β-amyloid production. World J Gastroenterol 2019; 25(3): 330-345
- URL: https://www.wjgnet.com/1007-9327/full/v25/i3/330.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i3.330
Atrophic gastritis is characterized by loss of appropriate glands and reduction in gastric secretory function due to chronic inflammatory processes in gastric mucosa[1]. In South Korea, the prevalence of atrophic gastritis is relatively high. It is 59.4% in people older than 60 years[2], and the crude incidence for cancer is 1.7% in atrophic gastritis[3]. It has been widely accepted that chronic inflammation of the stomach can initiate histopathologic progression from chronic gastritis to gastric atrophy, intestinal metaplasia, dysplasia and finally gastric cancer[4-6]. Thus, atrophic gastritis is considered as a precancerous condition of gastric cancer. However, little is known about the molecular mechanism underlying gastric mucosal atrophy and its contribution to gastric carcinogenesis.
Amyloid β peptide (Aβ) plays a key role in pathogenesis of Alzheimer’s disease. It is a 4-kDa metalloprotein with 39- to 43-amino acids derived from proteolytic cleavage of amyloid precursor protein (APP) by β- and γ-secretase[7]. In contrast, α-secretase ADAM10, a metalloprotease, cleaves APP within the Aβ domain, thus preventing Aβ generation[8]. It has been reported that β-site amyloid precursor protein cleaving enzyme (BACE) is a novel transmembrane aspartic protease that exhibits properties of β-secretase[9]. γ-secretase is a high molecular weight complex minimally composed of four components: presenilin (PS), nicastrin (NCT), anterior pharynx-defective-1 (APH-1), and presenilin enhancer-2 (PEN2)[10-13]. BACE1 cleaves APP not processed by α-secretase to generate carboxy-terminal fragments of 99 amino acids. This is further processed by γ-secretase to Aβ40 and Aβ42 that are transported to the cell surface which they are secreted via recycling vesicle[14]. In addition, receptor for advanced glycation end products (RAGE) is one of receptors that medicate Aβ effects on neurons and microglia[15] and is implicated in a wide spectrum of pathological responses, including inflammation and cancer[16]. Apolipoprotein E (ApoE) increases oligomerization of Aβ peptide in an isoform-dependent manner[17] and major ApoE receptors belong to low-density lipoprotein (LDL) receptor family[18]. It has been proposed that accumulated Aβ proteins can generate oligomers and induce synaptic dysfunction and death of neurons[19,20].
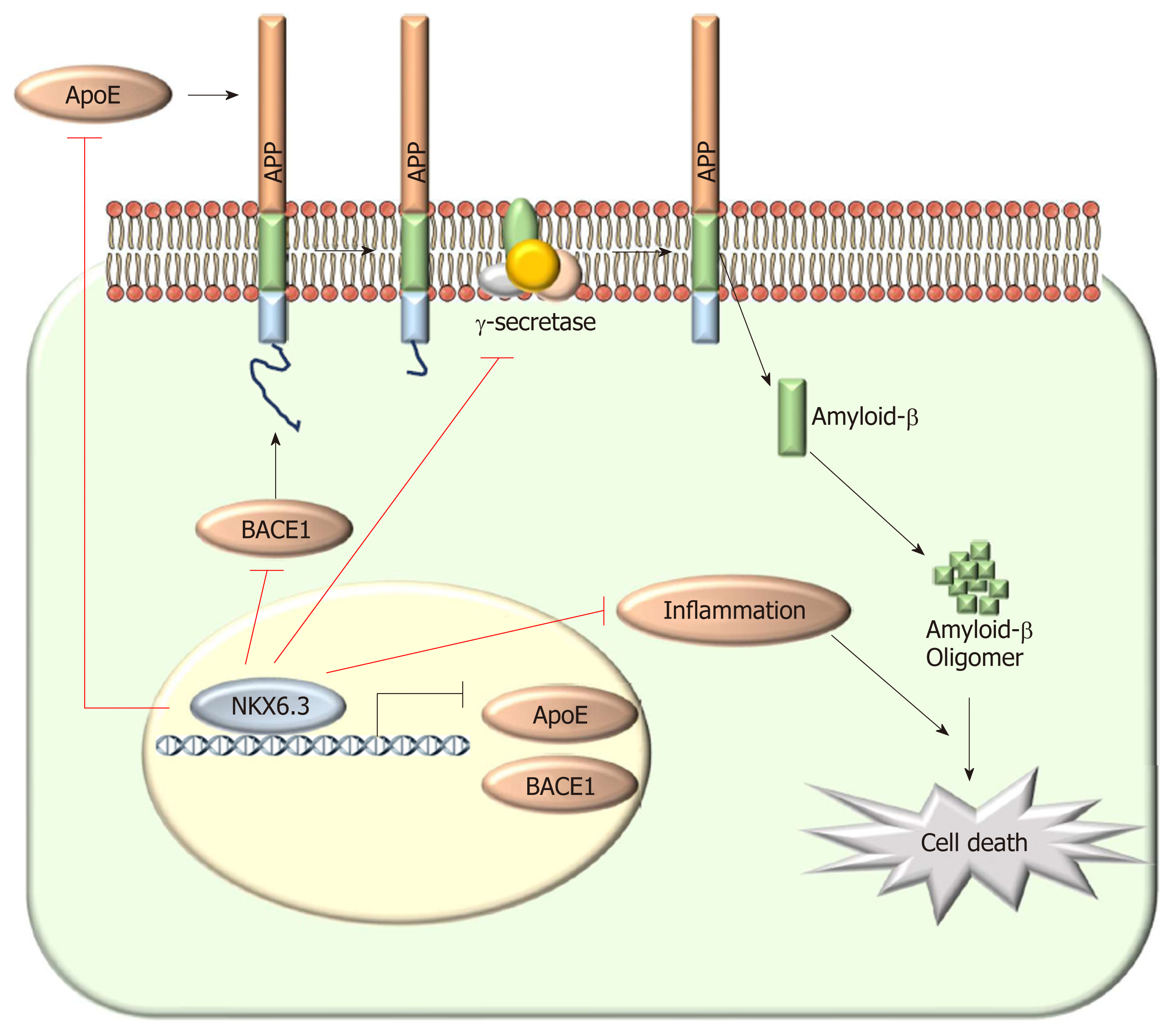
NKX family of homeodomain transcription factors are involved in a variety of developmental processes, and the NKX6.3 member is expressed in epithelium of the most distal stomach[21,22]. Previously, we have reported that NKX6.3 functions as a master regulator of gastric differentiation by modulating SOX2 and CDX2 expression and as a tumor suppressor by inhibiting cell proliferation and inducing apoptosis[23,24]. Interestingly, gastric tumor suppressor gastrokine 1 (GKN1), a downstream target of NKX6.3, interacts with APP and inhibits polymerization of Aβ[25,26]. Thus, we hypothesized that transcription factor NKX6.3 might be involved in maintaining gastric epithelial homeostasis by regulating Aβ production. Here, we provide the first evidence that NKX6.3 may protect gastric mucosal epithelial cells from atrophy by inhibiting Aβ production and polymerization.
A total of 55 patients with sporadic gastric cancer who underwent a gastrectomy at Chonnam National University Hwasun Hospital were included. Fresh-frozen non-neoplastic gastric mucosae remote (≥ 5 cm) from the tumor were used in this study. In addition, gastric mucosal tissues adjacent to each frozen specimen were fixed in formalin and stained with hematoxylin-eosin. Patients with a history of familial gastric cancer were excluded. Two expert gastrointestinal pathologists independently assessed the histologic specimens according to the updated Sydney system and the reached a consensus for all specimens[27]. Atrophy was defined as loss of appropriate glands and a periodic acid Schiff staining was used to identify intestinal metaplasia. Gastric mucosae with atrophy and intestinal metaplasia were considered as atrophic gastritis. The presence of Helicobacter pylori (H. pylori) CagA was determined by Western blot analysis[28]. This study was approved by the Institutional Review Board (IRB) of The Catholic University of Korea, College of Medicine (approval number: MC16SISI0130).
HFE-145 immortalized gastric epithelial cell line expressing NKX6.3 was cultured at 37 °C and 5% CO2 in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum. shNKX6.3 was cloned into pSilencer 3.1 H1-neo (Invitrogen, Carlsbad, CA, United States). We generated stable shNKX6.3 transfectants of HFE-145 cells (HFE-145shNKX6.3 cells), stably silencing human NKX6.3 expression, as well as shControl transfectants of HFE-145shCtrl cells as described previously[24]. Expression levels of NKX6.3 in HFE-145shCtrl and HFE-145shNKX6.3 cells were confirmed by Western blot analysis. The CagA gene of H. pylori was cloned into a pSP65SRalpha vector containing a hemagglutinin (HA) tag, and the HFE-145 cells were transfected with CagA gene, as described previously[24]. The CagA construct was kindly provided by Dr. Hatakeyama (Tokyo University, Tokyo, Japan).
HFE-145shCtrl and HFE-145shNKX6.3 cells in complete medium were seeded onto 12-well plates at a density of 1 × 104 cells per well. Floating and adherent cells were harvested after 48 h of culture and counted using a hemocytometer.
For cell viability analysis, MTT assay were performed for HFE-145 immortalized gastric epithelial cells at 24, 48, 72, and 96 h after treatment with recombinant Aβ (1 μg/mL, rAβ, Sigma, St. Louis, MO, United States). Absorbance at 540 nm was measured using a spectrophotometer and cell viability was expressed relative to non-treated cells.
To analyze the effect of NKX6.3 on apoptosis, caspase-3 and -7 activities were examined using an Apo-One Homogeneous caspase 3/7 assay kit (Promega Corporation, Madison, WI, United States) as described previously[28].
Expression levels of NKX6.3, ApoE, and Bace1 mRNA transcripts were examined in 55 gastric mucosal tissues by real-time RT-PCR. In addition, to investigate whether ablation of NKX6.3 might contributed to inflammatory cytokine expression, the expression of IL-1β, IL-6, IL-8, and COX-2 mRNAs in HFE-145shCtrl and HFE-145shNKX6.3 cells were analyzed by real-time RT-PCR. The effects of ApoE silencing with siApoE on expression levels of inflammatory cytokines were also examined. After quantification of total RNA extracted from 55 frozen gastric mucosal tissues, HFE-145shCtrl, and HFE-145shNKX6.3 cells, RT-PCR was carried out using SYBR Green Q-PCR Master Mix (Bio-Rad, Hercules, CA, United States) according to the manufacturer’s protocol. The mRNA expression of these genes was quantified by quantitative real-time reverse transcription PCR (QRT-PCR) and normalized to mRNA level of β-actin. Primer sequences are presented in Supplementary Table 1. Data are reported as relative quantities according to an internal calibrator using a 2-ΔΔCT method[23]. The standard curve method was used for quantification of the relative amounts of gene expression products. This method provides unit-less normalized expression values that can be used for direct comparison of the relative amount of RNA in different samples.
Primary anti-Aβ antibody (Merck-millipore, Darmstadt, Germany), anti-BACE1 (Abcam, Cambridge, United Kingdom) and secondary anti-alexa-488 conjugated goat anti-mouse IgG antibody (Invitrogen, CA, United States) were used to visualize immunoreactivity. The specificity of reactions was tested by incubation with non-immune mouse serum (Invitrogen).
We examined expression levels of NKX6.3 and Aβ-related genes in cell lysates by Western blot analysis. We separated equal amounts of cell lysates by 12.5% SDS-PAGE and transferred them to Hybond-polyvinylidene difluoride transfer membranes (Amersham Biosciences, NJ, United States). After blocking with 0.5% skim milk, we blotted the membranes with the primary antibodies and then incubated them with horseradish peroxidase-conjugated secondary antibodies. We detected the protein bands using westernsure ECL substrate (LI-COR Biosciences, NE, United States) and visualized the intensity of bands using a LAS 4000 image analyzer (Fuji Film, Japan). The antibody list is described in Supplementary Table 2.
For assessing the NKX6.3 binding activity to the promoter regions of ApoE and Bace1, chromatin immunoprecipitation (ChIP) assays were performed using the Thermo Scientific Pierce Agarose ChIP kit (Thermo Scientific Pierce, Rockford, IL, United States), as previously described[24]. Briefly, HFE-145shCtrl and HFE-145shNKX6.3 cells were cultured in a 10-cm dish for 4 d. The cells were fixed with 1% formaldehyde in PBS for 10 min, washed twice with ice-cold PBS and re-suspended in lysis buffer. Nuclei were recovered by centrifugation and MNase digestion was carried out at 37 °C for 15 min. Nuclei were lysed and the extracts were immunoprecipitated with 4 μg of antibody against NKX6.3 at 4 °C overnight. Normal rabbit IgG was used as a negative control. Protein-bound DNA was recovered using affinity chromatography purification columns according to the manufacturer’s protocol (Thermo Scientific), and 5 μL of lysed nuclei were also purified under the same procedure and used as input. DNA amplification was performed by PCR using primers for the ApoE and Bace1 promoters described in Supplementary Table 1. Amplification products were separated on a 2% agarose gel.
ELISAs for measuring Aβ42 peptide were performed using commercial kits (Invitrogen) following the manufacturer’s instructions. Briefly, Aβ42 ELISAs were performed using 6E10 as a capture antibody and anti-Aβ42 HRP-conjugated antibodies (Covance, Dedham, MA, United States) as detection antibodies. Synthetic Aβ42 were used to generate a standard curve for each experiment. The plates were developed using TMB substrate kit (Pierce, Rockford, IL, United States) and the reaction was stopped by addition of equal volume of 1 mol/L HCl. The results were read using a Spectramax colorimetric plate reader (Molecular Devices, Sunnyvale, CA, United States).
For coimmunoprecipitation experiments, cells were harvested in PBS, centrifuged at 800 g for 10 min, and lysed in 125 mmol/L NaCl, 50 mmol/L Hepes, pH 7.4 (supplemented with 1% Triton X-100 or CHAPS and Complete protease inhibitors) for 30 min at 4 °C. After centrifugation at 16000 g for 15 min, cleared cell extracts were incubated overnight at 4 °C with protein A/G-agarose (Santa Cruz Biotechnology, Santa Cruz, CA, United States) and anti-PSN1 or anti-rabbit IgG beads. Immunoprecipitated proteins were resolved on 12% SDS-polyacrylamide gels and transferred to PVDF membranes (Bio-Rad, Richmond, CA, United States). The membranes were blocked for 1 h in PBS containing 0.1% Tween 20 (PBS-T) and 5% non-fat dry milk (Sigma) and reacted with antibodies against PSN1, each diluted 1:1000. The membranes were washed with PBS-T, then incubated for 1 h at room temperature with horseradish peroxidase-conjugated anti-rabbit IgG antibody (Sigma), diluted 1:5000, and developed with westernsure ECL substrate (LI-COR Biosciences). Immunoreactive bands were identified by co-migration of prestained protein size markers (Fermentas, Glen Burnie, MD, United States). To confirm equivalent protein loading and transfer, the blots were stripped and re-probed for GAPDH (Santa Cruz Biotechnology).
Pearson and Student’s t-tests were used to analyze the correlation between expression of NKX6.3, GKN1, Aβ peptide oligomer, ApoE, and BACE1. We performed all experiments in duplicate to verify the reproducibility of the findings. Data are expressed as means ± SD from two independent experiments. A P-value < 0.05 was considered to be the limit of statistical significance.
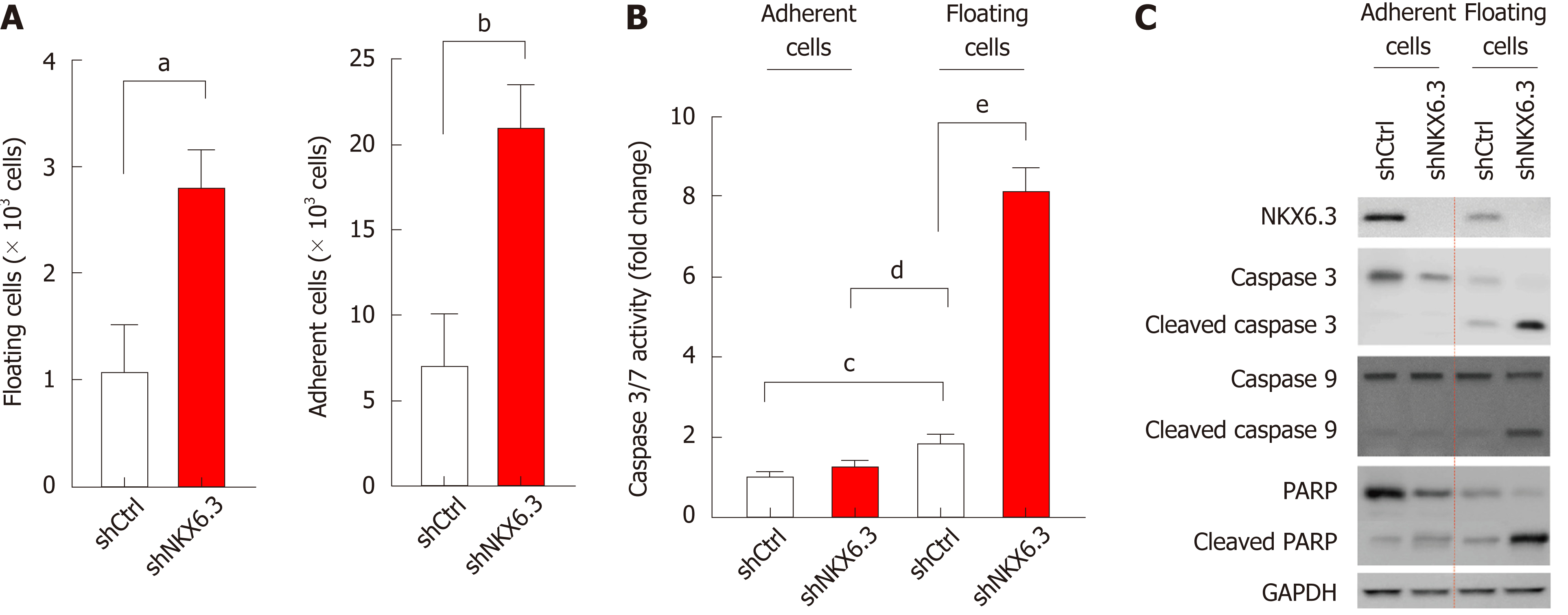
During cell culture, NKX6.3 depletion in HFE-145shNKX6.3 cells increased populations of both adherent and floating cells compared to HFE-145shCtrl cells (Figure 1A). Since previously we have reported that NKX6.3 depletion increases cell population of HFE-145 cells[24], here we focused on the increase of floating HFE-145shNKX6.3 cells. When we examined the effects of NKX6.3 depletion on cell death, caspase 3/7 activity was significantly increased only in floating HFE-145shNKX6.3 cells (Figure 1B). Interestingly, NKX6.3 depletion did not affect expression of apoptosis markers including caspase-3, -9, and PARP in attached cells. However, expression levels of cleaved forms of caspase-3, -9, and PARP were increased in floating HFE-145shNKX6.3 cells compared with those in floating HFE-145shCtrl cells (Figure 1C). Thus, it is likely that NKX6.3 may inhibit both cell proliferation and apoptotic cell death in gastric epithelial cells.
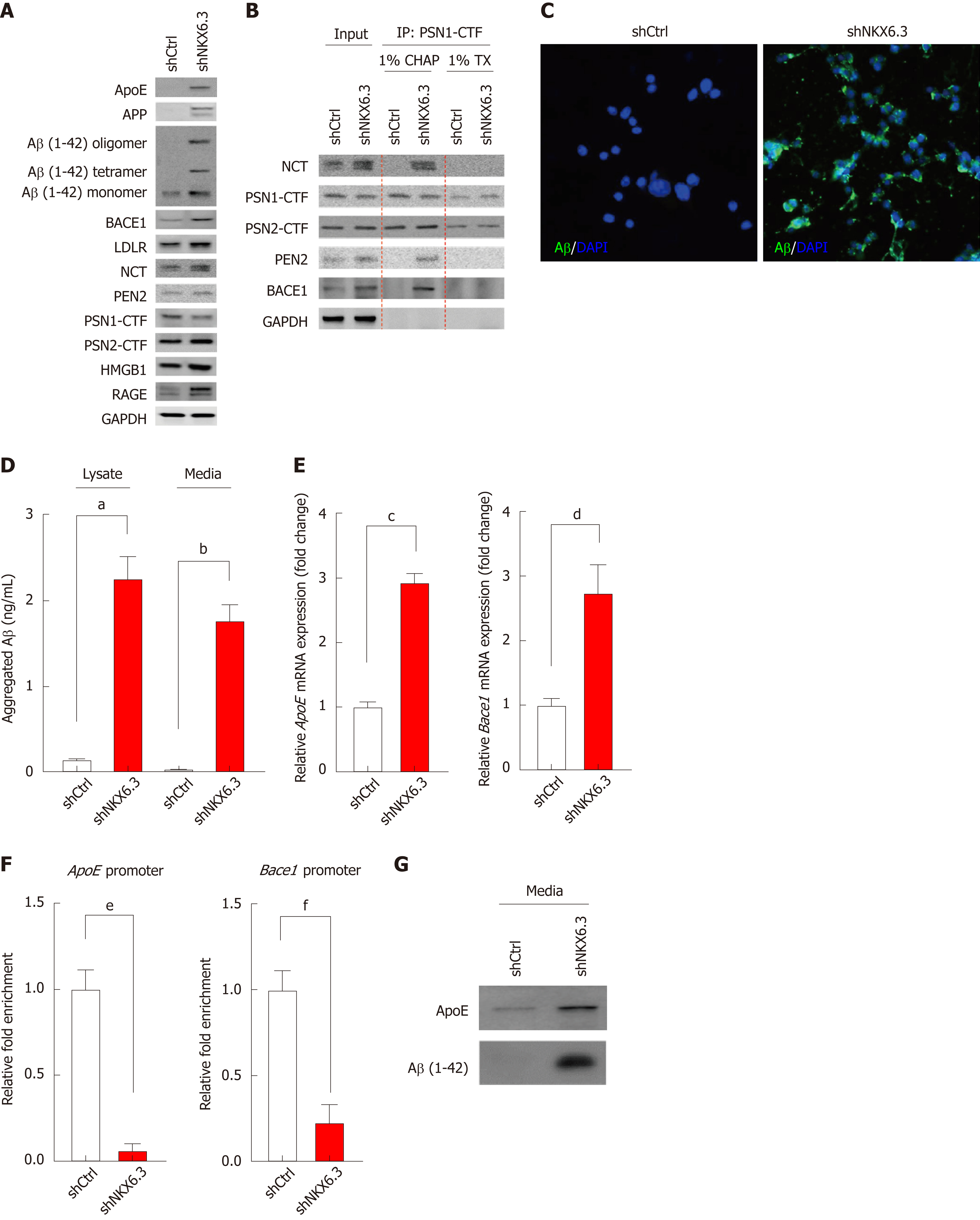
Next, we examined whether NKX6.3 was involved in Aβ production in HFE-145 cells. Interestingly, NKX6.3 depletion markedly induced expression of ApoE, APP, Aβ peptide, BACE1, LDLR, NCT, high-mobility group box 1 (HMGB1), and RAGE proteins but decreased expression of presenilin1 (PSN1) protein in HFE-145 cells (Figure 2A). There are three distinct pools of Aβ species: monomers, soluble oligomers, and insoluble fibrils[29]. Interestingly, HFE-145shCtrl cells only expressed Aβ monomer, while NKX6.3 depleted HFE-145shNKX6.3 cells induced production of Aβ oligomers and increased expression of Aβ monomer (Figure 2A). When we examined the effect of NKX6.3 on γ-secretase assembly by immunoprecipitation assay, γ-secretase complex, including PSN1, PSN2, NCT, and PEN2 was stably formed and binding to BACE1 protein only in NKX6.3 depleted HFE-145shNKX6.3 cells (Figure 2B). In immunofluorescence analysis, strong expression of Aβ peptides were detected in the cytoplasm of NKX6.3 depleted HFE-145shNKX6.3 cells (Figure 2C). Notably, aggregated Aβ peptide was not detected in HFE-145shCtrl cells expressing NKX6.3 whereas NKX6.3 depletion significantly increased concentrations of the aggregated Aβ peptide in both cell lysate and culture media (Figure 2D). In NKX6.3 depleted HFE-145shNKX6.3 cells, mRNA expression of ApoE and Bace1 genes were significantly increased (Figure 2E) while binding capacity of NKX6.3 to promoter regions of ApoE and Bace1 genes was dramatically decreased in ChIP assay (Figure 2F), suggesting that NKX6.3 might be a transcriptional repressor of ApoE and Bace1 genes. When we treated HFE-145shCtrl and HFE-145shNKX6.3 cells with recombinant Aβ 1-42 (rAβ 1-42), ApoE and oligomeric forms of Aβ were detected in NKX6.3 depleted HFE-145shNKX6.3 cells (Figure 2G). Taken together, these results suggest that NKX6.3 may inhibit accumulation and oligomerization of Aβ peptide in gastric epithelial cells.
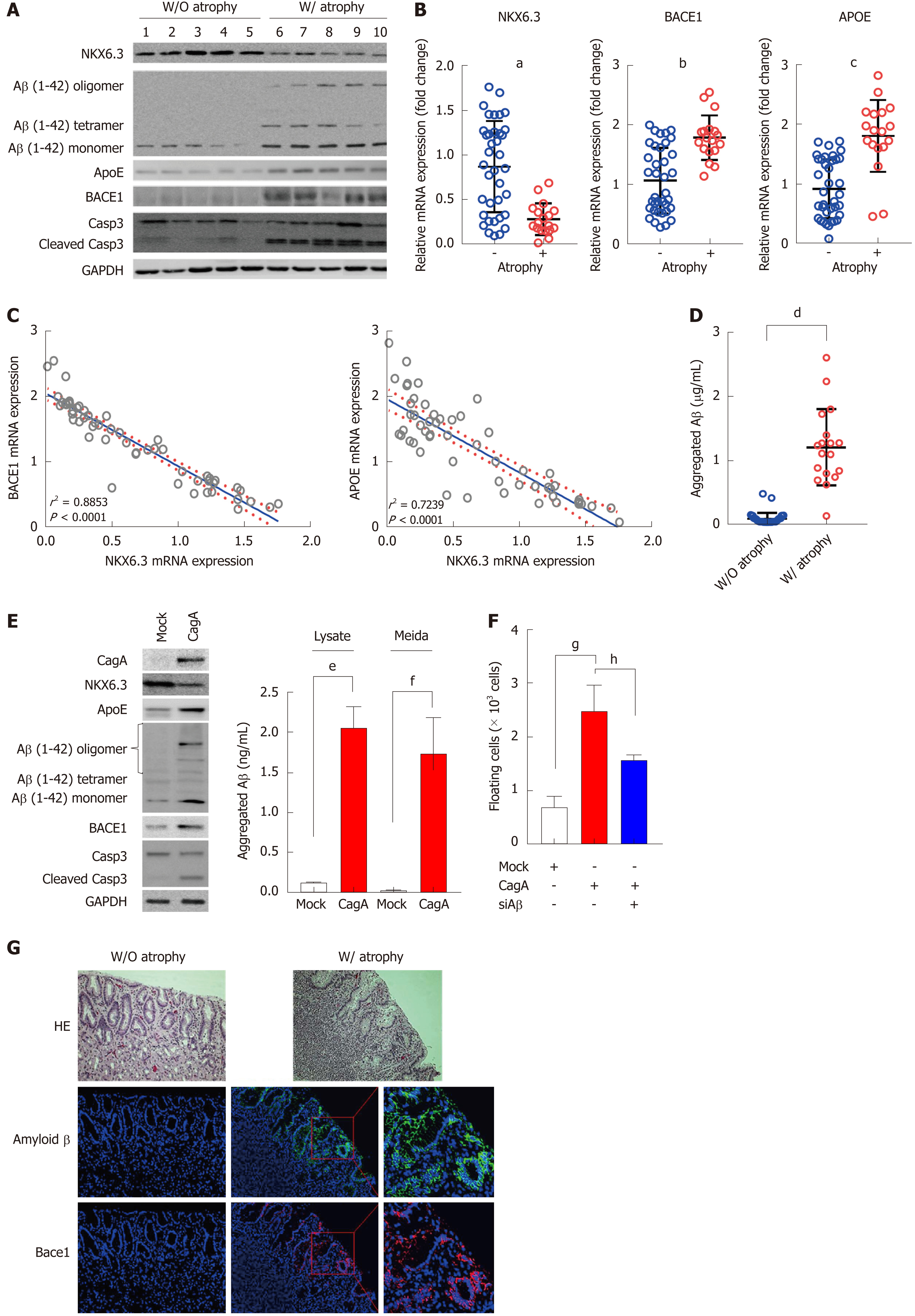
Histologically, gastric mucosal atrophy was found in 18 (32.7%) of 55 gastric mucosae. In Western blot analysis, Aβ oligomers, including tetramer, were mainly expressed in gastric mucosae with atrophy. In addition, expression levels of ApoE, Bace1, and cleaved form of caspase-3 were increased in atrophic gastric mucosae along with reduced NKX6.3 expression (Figure 3A). To further confirm the effect of NKX6.3 on ApoE and Bace1 expression, we compared expression levels of NKX6.3 with ApoE and Bace1 mRNA expression levels in 55 non-neoplastic gastric mucosae by real-time RT-PCR. Previously, NKX6.3 expression has been found to be significantly reduced in the cases with atrophy[23]. As expected, mRNA expression levels of ApoE and Bace1 were significantly higher in gastric mucosae with atrophy (Figure 3B), showing inverse correlations with NKX6.3 expression (P = 0.0001) (Figure 3C). In addition, reduced expression of NKX6.3 protein, expression of Aβ oligomer, Bace1, ApoE, and cleaved caspase-3 proteins, and CagA were significantly associated with gastric mucosal atrophy. The expression of Aβ oligomer was positively correlated with expression of Bace1, ApoE, cleaved caspase-3, and CagA (Figure 3D). We also measured aggregated Aβ peptide concentrations in 55 gastric mucosae by ELISA and found significantly higher Aβ peptide concentrations in gastric mucosae with atrophy (1.20 ± 0.60 μg/mL) than those in gastric mucosae without atrophy (0.09 ± 0.09 μg/mL; Figure 3E). When we transfected HFE-145 cells with CagA, ectopic expression of CagA reduced the expression of NKX6.3 but increased Aβ oligomerization and expression of ApoE, Bace1, and cleaved caspase-3 (Figure 3F). Next, we examined Aβ oligomer expression in 6 non-neoplastic gastric mucosae with atrophy (n = 3) and without atrophy (n = 3) using specific antibody against Aβ oligomer by immunofluorescent assay. Consistent with above result, expression of Aβ oligomer was detected in gastric mucosae with atrophy in immunofluorescent assay (Figure 3G). Interestingly, Aβ oligomer was present only in gastric mucosal epithelial cells, but not in extracellular matrix including inflammatory cells (Figure 3G).
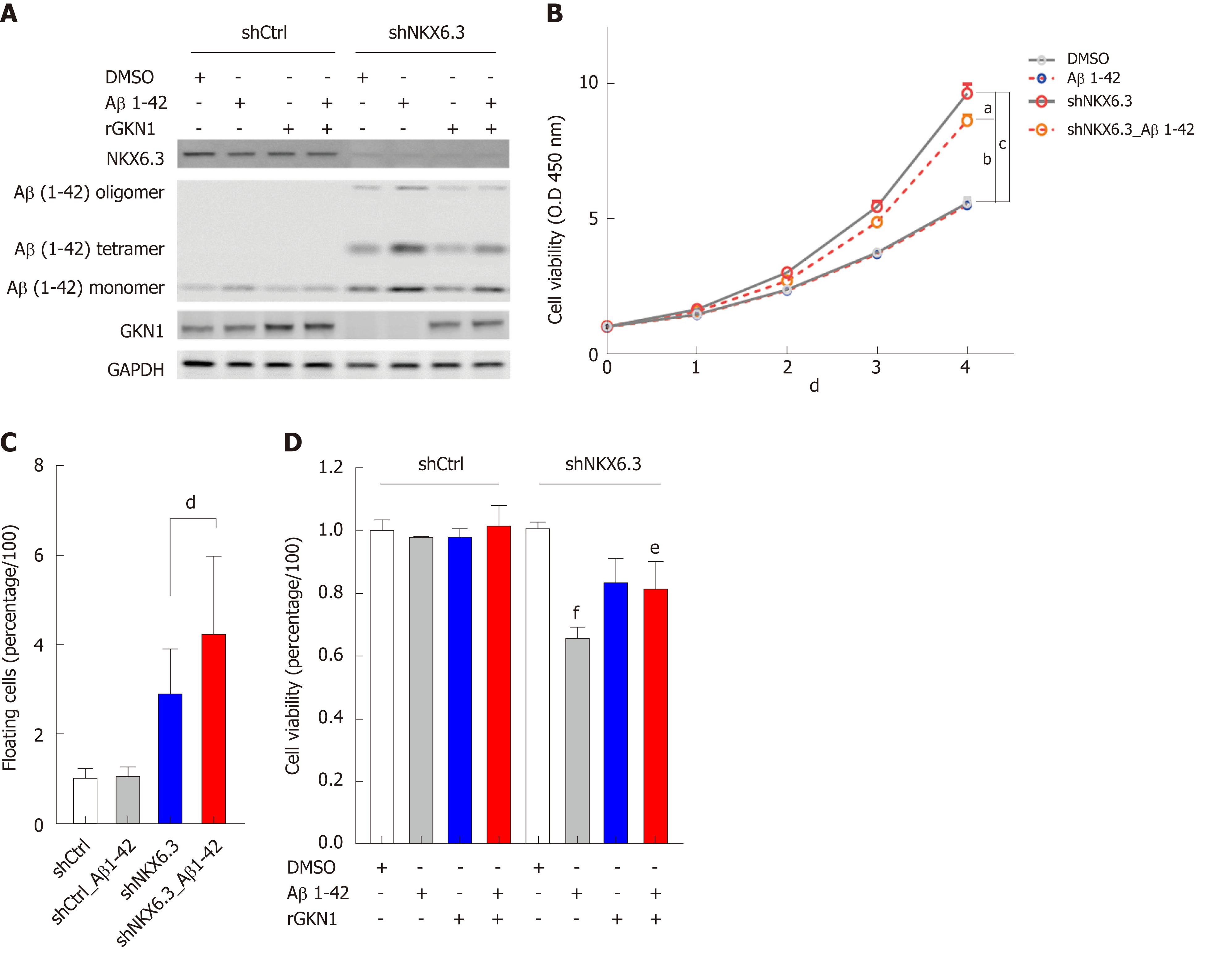
Next, to investigate whether oligomeric forms of Aβ were associated with gastric mucosal atrophy, we analyzed cell viability of gastric epithelial cells after treatment with rAβ 1-42. Because we found 1.18 ± 0.57 μg/mL of aggregated Aβ peptide in gastric mucosae with atrophy (Figure 3), we treated HFE-145shCtrl and HFE-145shNKX6.3 cells with 1 μg/mL of rAβ for 4 d. As shown in Figure 4A, treatment with rAβ 1-42 produced oligomeric forms of Aβ only in HFE-145shNKX6.3 cells, but not in HFE-145shCtrl cells (Figure 4A). In addition, recombinant GKN1 (rGKN1) partially reduced expression of Aβ oligomers (Figure 4A). In HFE-145shNKX6.3 cells, treatment with rAβ 1-42 significantly reduced cell viability but markedly increased floating cell population in HFE-145shNKX6.3 cells (Figure 4B and C). Notably, rGKN1 partially revoked the effect of rAβ on cell viability (Figure 4D). However, treatment with rAβ 1-42 did not affect cell viability of HFE-145shCtrl cells (Figure 4B). These results suggest that Aβ oligomerization prompted by NKX6.3 depletion may induce cell death in gastric epithelial cells.
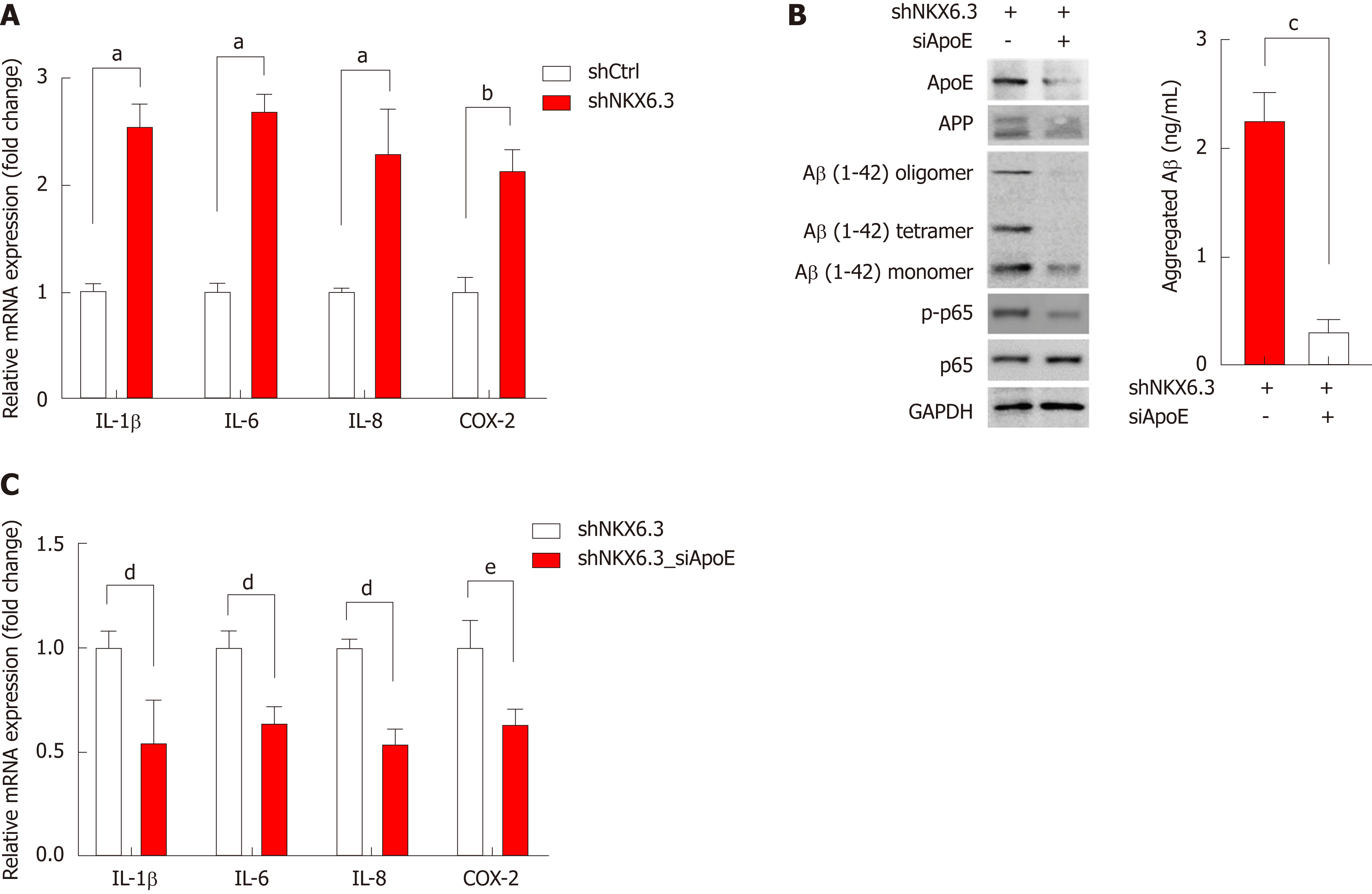
It is known that Aβ accumulation in human nerve cells leads to synthesis of proinflammatory cytokines and activation of inflammatory pathways[30]. Thus, we further examined the expression of inflammatory markers including IL-1β, -6, -8, and COX-2 by real-time RT-PCR. Expectedly, NKX6.3 depletion in HFE-145shNKX6.3 cells dramatically increased the expression of these inflammatory cytokines and COX-2 (Figure 5A). In HFE-145shNKX6.3 cells, silencing of ApoE with siApoE markedly inhibited oligomerization and aggregation of Aβ peptide. It also suppressed expression of NF-κB p-p65 and inflammatory cytokines (Figure 5B and C). This suggests that ApoE plays an important role in Aβ oligomerization and synthesis of inflammatory cytokines.
Inflammatory disorders have been well recognized as key risk factor for many types of cancers. Stages of precancerous cascade for gastric adenocarcinoma are a series of histologically discernible changes in gastric mucosa with the following sequence: non-atrophic gastritis, multifocal atrophic gastritis, intestinal metaplasia (IM), and dysplasia[31]. H. pylori infection has been identified as the causative agent of chronic gastric inflammation, such as atrophic gastritis and metaplastic gastritis[32]. However, molecular mechanisms underlying gastric mucosal atrophy still remain to be determined for the prevention and early diagnosis of gastric cancer. Since intracellular generation of the Aβ protein is known to lead to neuronal death[19] while NKX6.3 expression is confined to the gut and caudal hindbrain[33]. Thus, we hypothesized that NKX6.3 could protect gastric mucosa from atrophic changes by inhibiting Aβ accumulation.
We first examined whether NKX6.3 was involved in proliferation and death of gastric mucosa. As shown in Figure 1A, NKX6.3 depletion in HFE-145shNKX6.3 cells significantly increased proportions of both adherent and floating cells. In addition, increased caspase 3/7 activity was found in floating HFE-145shNKX6.3 cells (Figure 1B). In Western blot analysis, NKX6.3 did not affect expression of apoptosis markers, including PARP, caspase-3, or caspase-9 in adherent HFE-145shNKX6.3 cells, but NKX6.3 depletion induced expression of these apoptotic markers (Figure 1C), providing an intriguing scenario where NKX6.3 might be a key regulator of gastric mucosal homeostasis by inhibiting both cell proliferation and death. Here, we focused on the inhibitory activity of NKX6.3 on death of gastric mucosal epithelial cells.
In general, regulation of proteolysis plays a crucial role in many processes of development, cell proliferation and death. Dysregulation of proteolytic system can destroy cellular homeostasis by accumulation of specific proteins, thus contributing to disease pathogenesis. Amyloid aggregates have been found to be associated with disruption of several cellular functions, including mitochondrial activity[34], oxidative stress[35,36], and apoptosis[37]. Aβ acts as a neurotoxin that directly induces oxidative stress whereas RAGE mediates Aβ-induced oxidative stress and inflammatory response. Increased expression of RAGE has been implicated in the pathogenesis of neuronal dysfunction and death[15]. In the present study, we hypothesized that Aβ peptide accumulation caused by NKX6.3 depletion could induce gastric epithelial cell death which subsequently could contribute to gastric mucosal atrophy. Interestingly, depletion of NKX6.3 induced Aβ peptide accumulation in the cytoplasm of HFE-145shNKX6.3 cells and increased expression of Aβ in cell lysate and culture medium (Figure 2). These results suggest that NKX6.3 may inhibit accumulation and oligomerization of Aβ peptide in gastric epithelial cells.
Cleavage of APP by Bace1 produces a large soluble ectodomain form and a membrane-bound 99-amino acid C-terminal fragment. Subsequently, the γ-secretase protein complex will cleave the C-terminus of C99 to produce a 40- to 42-amino acid Aβ-peptide[38]. After sequential cleavage of APP by β- and γ-secretases[38,39], Aβ monomer can aggregate to form oligomers, protofibrils, and fibrils that deposit as amyloid plaque[40]. RAGE and HMGB1 are involved in activating NF-κB[41], and the interaction of RAGE and HMGB1 can be lined to necrosis and a proinflammatory response in cells[42]. Here, we found that NKX6.3 depletion induced expression of ApoE, APP, Aβ, Bace1, LDLR, NCT, HMGB1, and RAGE proteins in HFE-145shNKX6.3 cells (Figure 2A). In addition, NKX6.3 inhibited the γ-secretase complex assembly (Figure 2B) and down-regulated expression of the APOE and Bace1 genes by acting as a transcriptional repressor (Figure 2D and E). In gastric mucosae with atrophy, oligomeric forms of Aβ and Bace1 were detected by immunofluorescent and Western blot analyses (Figure 3A and G), and the increased expression of APOE and Bace1 was inversely correlated with NKX6.3 expression (Figure 3B and C). Interestingly, expression of Aβ oligomer, Bace1, ApoE, cleaved caspase-3, and H. pylori CagA was closely associated with gastric mucosal atrophy and inversely correlated with NKX6.3 expression (P < 0.0001, Table 1). Furthermore, Aβ peptide concentrations were significantly higher in gastric mucosae with atrophy (1.18 ± 0.57 μg/ml) than those in gastric mucosae without atrophy (0.07 ± 0.04 μg/ml; Figure 3D). In HFE-145 cells, H. pylori CagA increased the expression of ApoE, Bace1, and cleaved caspase-3 and induced oligomerization and aggregation of Aβ peptide (Figure 3E). Notably, siAβ markedly inhibited CagA effect of cell viability (Figure 3F), indicating that Aβ be involved in CagA-induced cell death in non-neoplastic gastric epithelial cells. Taken together, these results suggest that NKX6.3 may prevent gastric mucosal atrophy by regulating Aβ degradation or clearance pathway.
| Atrophy | NKX6.3 | Aβ oligomer | Bace1 | ApoE | Cleaved Casp3 | ||||||||||||||
| + | - | P value | + | - | P value | + | - | P value | + | - | P value | + | - | P value | + | - | P value | ||
| NKX6.3 | + | 3 | 33 | 0.0001 | |||||||||||||||
| - | 15 | 4 | |||||||||||||||||
| Aβ oligomer | + | 17 | 2 | 0.0001 | 3 | 16 | 0.0001 | ||||||||||||
| - | 1 | 35 | 33 | 3 | |||||||||||||||
| Bace1 | + | 16 | 5 | 0.0001 | 2 | 19 | 0.0001 | 18 | 3 | 0.0001 | |||||||||
| - | 2 | 32 | 34 | 0 | 1 | 33 | |||||||||||||
| ApoE | + | 18 | 5 | 0.0001 | 4 | 19 | 0.0001 | 19 | 4 | 0.0001 | 21 | 2 | 0.0001 | ||||||
| - | 0 | 32 | 32 | 0 | 0 | 32 | 0 | 32 | |||||||||||
| Cleaved Casp3 | + | 16 | 8 | 0.0001 | 5 | 19 | 0.0001 | 19 | 5 | 0.0001 | 21 | 3 | 0.0001 | 22 | 2 | 0.0001 | |||
| - | 2 | 29 | 31 | 0 | 0 | 31 | 0 | 31 | 1 | 30 | |||||||||
| CagA | + | 14 | 12 | 0.0041 | 9 | 17 | 0.0001 | 16 | 10 | 0.0002 | 19 | 7 | 0.0001 | 19 | 7 | 0.0001 | 21 | 5 | 0.0001 |
| - | 4 | 25 | 27 | 2 | 3 | 26 | 2 | 27 | 4 | 25 | 3 | 26 | |||||||
Recent studies have shown that soluble oligomeric species of Aβ have direct adverse effects, whereas fibrillar or monomeric Aβ seems to be less harmful in vitro and in animal models[43-45]. Soluble Aβ oligomers can produce cognitive deficits in the absence of plaques[46] while blocking the Aβ oligomerization can protect cells from toxicity[47], suggesting that oligomerization of Aβ peptide may be a key event in the development of cerebral atrophy. In addition, it has been reported that Aβ oligomers can induce inflammation through RAGE receptor[48] and inhibition of RAGE has therapeutic potential to prevent Aβ-induced inflammatory damage to the brain[49]. NF-κB, a key regulator of inflammation, is activated by Aβ which then transcriptionally regulates IL-6 and IL-8[50]. HMGB1 binds to RAGE receptor to activate a multitude of proinflammatory genes[51]. Recent studies have shown that GKN1 can prevent amyloid aggregation and fibril formation and block the access of γ-secretase to APP in vitro[26,52]. Experimental studies showed that partial atrophy of the gastric mucosa in aging rat is not related to the inflammation and gastric mucosa of aging rat has increased susceptibility to injury by a variety of damaging agents[53,54]. Previously, we have reported that NKX6.3 transcriptionally suppresses reactive oxygen production and NF-κB activation which is required for expression of IL-6 and IL-8[55]. Here, we found that treatment with rAβ 1-42 produced oligomeric forms of Aβ and significantly decreased cell viability of HFE-145shNKX6.3 cells (Figure 4A and B). In addition, NKX6.3 depletion dramatically increased expression of inflammatory cytokines and COX-2 by upregulating ApoE (Figure 5). These results are consistent with our previous report showing that NKX6.3 transcriptionally suppresses reactive oxygen production and NF-κB activation which is required for expression of IL-6 and IL-8[55]. Thus, upregulation of ApoE prompted by NKX6.3 depletion might play an important role in Aβ oligomerization and gastric mucosal inflammation. These findings suggest that depletion of NKX6.3 may account for increased susceptibility of gastric epithelial cells to Aβ-induced cytotoxicity and contribute to gastric mucosal atrophy.
In conclusion, NKX6.3 is a key regulator of gastric mucosal homeostasis via inhibiting both cell proliferation and apoptotic cell death. Aβ accumulation was detected in the cytoplasm of HFE-145shNKX6.3 cells and gastric mucosae with atrophy. NKX6.3 inhibited accumulation and oligomerization of Aβ peptide in gastric epithelial cells by regulating Aβ degradation or clearance pathways. Finally, NKX6.3 suppressed gastric mucosal inflammation by modulating ApoE-induced NF-κB and expression of cytokines. These observations provide evidence that NKX6.3 can restrain gastric mucosal atrophy by regulating Aβ accumulation and inflammatory reaction in gastric epithelial cells (Figure 6).
Atrophic gastritis is characterized by loss of appropriate glands and considered as a precancerous condition of gastric cancer. However, little is known about the molecular mechanism underlying gastric mucosal atrophy. NKX6.3 plays a key role in the maintaining gastric epithelial homeostasis. Amyloid β (Aβ) acts as a neurotoxin that directly induces oxidative stress and receptor for advanced glycation end products (RAGE) mediates Aβ-induced oxidative stress and inflammatory response. Increased expression of RAGE has been implicated in the pathogenesis of neuronal cell death. Interestingly, gastrokine 1, a downstream target of NKX6.3, interacts with amyloid precursor protein (APP) and inhibits polymerization of Aβ.
A better understanding of molecular mechanism underlying gastric mucosal atrophy could protect against gastric mucosal atrophy and gastric carcinogenesis.
To investigate whether NKX6.3 might play a critical role in the development of gastric mucosal atrophy by regulating Aβ production.
We examined whether NKX6.3 depletion induces cell death by cell count and Western blot assay. Production and mechanism of Aβ oligomer were analyzed by enzyme-linked immunosorbent assay, Western blot, immunoprecipitation, real-time quantitative polymerase chain reaction and immunofluorescence analysis in HFE-145 non-neoplastic gastric epithelial cells and gastric mucosal tissues. We further validated the correlation between expression of NKX6.3, Helicobacter pylori CagA, Aβ oligomer, apolipoprotein E (ApoE), and β-secretase 1 (Bace1) in gastric mucosae.
We found that NKX6.3 depletion increased floating cell populations in HFE-145 cells and induced production of Aβ peptide oligomers. In addition, NKX6.3 depletion increased expression of APP, Aβ, and RAGE proteins. In gastric mucosae with atrophy, expression of Aβ peptide oligomer, ApoE, and Bace1 was detected and inversely correlated with NKX6.3 expression. Treatment with rAβ 1-42 produced oligomeric forms of Aβ and significantly decreased cell viability only in HFE-145shNKX6.3 cells. Furthermore, NKX6.3 depletion in HFE-145shNKX6.3 cells increased expression of inflammatory cytokines and cyclooxygenase-2.
These data strongly suggest that NKX6.3 inhibit gastric mucosal atrophy by regulating Aβ accumulation and inflammatory reaction in gastric epithelial cells.
Additional studies are needed to validate the results obtained. Identification of molecular mechanism underlying gastric mucosal atrophy could contribute to the prevention of atrophic gastritis and gastric cancer.
We thank Dr. SeongYeob Ryu, Department of Gastroenterologic Surgery, Chonnam National University Hwasun Hospital, 160, Ilsim-ri, Hwasun-eup, Hwasun-gun, Jeollanam-do, 519-809, Korea, for providing the gastric cancer samples with clinical information.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Slomiany BL, Tarnawski AS S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
| 1. | Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, Miwa H, Lim KJ, Das KM. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. 2014;20:5461-5473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (5)] |
| 2. | Joo YE, Park HK, Myung DS, Baik GH, Shin JE, Seo GS, Kim GH, Kim HU, Kim HY, Cho SI, Kim N. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia: a nationwide multicenter prospective study in Korea. Gut Liver. 2013;7:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Song JH, Kim SG, Jin EH, Lim JH, Yang SY. Risk Factors for Gastric Tumorigenesis in Underlying Gastric Mucosal Atrophy. Gut Liver. 2017;11:612-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3182] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 5. | Fox JG, Wang TC. Helicobacter pylori--not a good bug after all. N Engl J Med. 2001;345:829-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H, Shimizu Y, Takeshita T, Mohara O, Ichinose M. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 373] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 7. | Cappai R, White AR. Amyloid beta. Int J Biochem Cell Biol. 1999;31:885-889. [PubMed] |
| 8. | Lichtenthaler SF. Alpha-secretase cleavage of the amyloid precursor protein: proteolysis regulated by signaling pathways and protein trafficking. Curr Alzheimer Res. 2012;9:165-177. [PubMed] |
| 9. | Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735-741. [PubMed] |
| 10. | Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer LS, Sorbi S, Bruni A, Fraser P, St George-Hyslop P. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature. 2000;407:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 707] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 11. | Goutte C, Tsunozaki M, Hale VA, Priess JR. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc Natl Acad Sci USA. 2002;99:775-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 324] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 717] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 13. | Ahn K, Shelton CC, Tian Y, Zhang X, Gilchrist ML, Sisodia SS, Li YM. Activation and intrinsic gamma-secretase activity of presenilin 1. Proc Natl Acad Sci USA. 2010;107:21435-21440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Baranello RJ, Bharani KL, Padmaraju V, Chopra N, Lahiri DK, Greig NH, Pappolla MA, Sambamurti K. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Curr Alzheimer Res. 2015;12:32-46. [PubMed] |
| 15. | Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1543] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 16. | Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 405] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 17. | Hashimoto T, Serrano-Pozo A, Hori Y, Adams KW, Takeda S, Banerji AO, Mitani A, Joyner D, Thyssen DH, Bacskai BJ, Frosch MP, Spires-Jones TL, Finn MB, Holtzman DM, Hyman BT. Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid β peptide. J Neurosci. 2012;32:15181-15192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 18. | Kanekiyo T, Xu H, Bu G. ApoE and Aβ in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81:740-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 467] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 19. | LaFerla FM, Hall CK, Ngo L, Jay G. Extracellular deposition of beta-amyloid upon p53-dependent neuronal cell death in transgenic mice. J Clin Invest. 1996;98:1626-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Manczak M, Reddy PH. Abnormal interaction of oligomeric amyloid-β with phosphorylated tau: implications to synaptic dysfunction and neuronal damage. J Alzheimers Dis. 2013;36:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Alanentalo T, Chatonnet F, Karlen M, Sulniute R, Ericson J, Andersson E, Ahlgren U. Cloning and analysis of Nkx6.3 during CNS and gastrointestinal development. Gene Expr Patterns. 2006;6:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Choi MY, Romer AI, Wang Y, Wu MP, Ito S, Leiter AB, Shivdasani RA. Requirement of the tissue-restricted homeodomain transcription factor Nkx6.3 in differentiation of gastrin-producing G cells in the stomach antrum. Mol Cell Biol. 2008;28:3208-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Yoon JH, Choi SS, Kim O, Choi WS, Park YK, Nam SW, Lee JY, Park WS. Inactivation of NKX6.3 in the stomach leads to abnormal expression of CDX2 and SOX2 required for gastric-to-intestinal transdifferentiation. Mod Pathol. 2016;29:194-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Yoon JH, Choi WS, Kim O, Choi SS, Lee EK, Nam SW, Lee JY, Park WS. NKX6.3 controls gastric differentiation and tumorigenesis. Oncotarget. 2015;6:28425-28439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Yoon JH, Choi YJ, Choi WS, Ashktorab H, Smoot DT, Nam SW, Lee JY, Park WS. GKN1-miR-185-DNMT1 axis suppresses gastric carcinogenesis through regulation of epigenetic alteration and cell cycle. Clin Cancer Res. 2013;19:4599-4610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Di Stadio CS, Altieri F, Minopoli G, Miselli G, Rippa E, Arcari P. Role of human GKN1 on APP processing in gastric cancer. Biochimie. 2017;135:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Choi WS, Seo HS, Song KY, Yoon JH, Kim O, Nam SW, Lee JY, Park WS. Gastrokine 1 expression in the human gastric mucosa is closely associated with the degree of gastritis and DNA methylation. J Gastric Cancer. 2013;13:232-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Yoon JH, Seo HS, Choi SS, Chae HS, Choi WS, Kim O, Ashktorab H, Smoot DT, Nam SW, Lee JY, Park WS. Gastrokine 1 inhibits the carcinogenic potentials of Helicobacter pylori CagA. Carcinogenesis. 2014;35:2619-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Goure WF, Krafft GA, Jerecic J, Hefti F. Targeting the proper amyloid-beta neuronal toxins: a path forward for Alzheimer’s disease immunotherapeutics. Alzheimers Res Ther. 2014;6:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 30. | Currais A, Quehenberger O, M Armando A, Daugherty D, Maher P, Schubert D. Amyloid proteotoxicity initiates an inflammatory response blocked by cannabinoids. NPJ Aging Mech Dis. 2016;2:16012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 32. | Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 410] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 33. | Nelson SB, Janiesch C, Sander M. Expression of Nkx6 genes in the hindbrain and gut of the developing mouse. J Histochem Cytochem. 2005;53:787-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Palmblad M, Westlind-Danielsson A, Bergquist J. Oxidation of methionine 35 attenuates formation of amyloid beta -peptide 1-40 oligomers. J Biol Chem. 2002;277:19506-19510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548-554. [PubMed] |
| 36. | Martins RN, Harper CG, Stokes GB, Masters CL. Increased cerebral glucose-6-phosphate dehydrogenase activity in Alzheimer’s disease may reflect oxidative stress. J Neurochem. 1986;46:1042-1045. [PubMed] |
| 37. | Wei W, Norton DD, Wang X, Kusiak JW. Abeta 17-42 in Alzheimer’s disease activates JNK and caspase-8 leading to neuronal apoptosis. Brain. 2002;125:2036-2043. [PubMed] |
| 38. | Sathya M, Premkumar P, Karthick C, Moorthi P, Jayachandran KS, Anusuyadevi M. BACE1 in Alzheimer’s disease. Clin Chim Acta. 2012;414:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med. 2010;12:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 480] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 40. | Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9775] [Cited by in RCA: 10217] [Article Influence: 444.2] [Reference Citation Analysis (0)] |
| 41. | Luan ZG, Zhang H, Yang PT, Ma XC, Zhang C, Guo RX. HMGB1 activates nuclear factor-κB signaling by RAGE and increases the production of TNF-α in human umbilical vein endothelial cells. Immunobiology. 2010;215:956-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 42. | Li G, Liang X, Lotze MT. HMGB1: The Central Cytokine for All Lymphoid Cells. Front Immunol. 2013;4:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 43. | Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3192] [Cited by in RCA: 3244] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 44. | Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3235] [Cited by in RCA: 3340] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 45. | Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2994] [Cited by in RCA: 2921] [Article Influence: 171.8] [Reference Citation Analysis (0)] |
| 46. | Gandy S, Simon AJ, Steele JW, Lublin AL, Lah JJ, Walker LC, Levey AI, Krafft GA, Levy E, Checler F, Glabe C, Bilker WB, Abel T, Schmeidler J, Ehrlich ME. Days to criterion as an indicator of toxicity associated with human Alzheimer amyloid-beta oligomers. Ann Neurol. 2010;68:220-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | De Felice FG, Vieira MN, Saraiva LM, Figueroa-Villar JD, Garcia-Abreu J, Liu R, Chang L, Klein WL, Ferreira ST. Targeting the neurotoxic species in Alzheimer’s disease: inhibitors of Abeta oligomerization. FASEB J. 2004;18:1366-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Minter MR, Taylor JM, Crack PJ. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J Neurochem. 2016;136:457-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 326] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 49. | Sengupta U, Nilson AN, Kayed R. The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy. EBioMedicine. 2016;6:42-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 551] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 50. | Park SY, Jin ML, Kim YH, Kim Y, Lee SJ. Anti-inflammatory effects of aromatic-turmerone through blocking of NF-κB, JNK, and p38 MAPK signaling pathways in amyloid β-stimulated microglia. Int Immunopharmacol. 2012;14:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 1016] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 52. | Pavone LM, Del Vecchio P, Mallardo P, Altieri F, De Pasquale V, Rea S, Martucci NM, Di Stadio CS, Pucci P, Flagiello A, Masullo M, Arcari P, Rippa E. Structural characterization and biological properties of human gastrokine 1. Mol Biosyst. 2013;9:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Tarnawski A, Pai R, Deng X, Ahluwalia A, Khomenko T, Tanigawa T, Akahoshi T, Sandor Z, Szabo S. Aging gastropathy-novel mechanisms: hypoxia, up-regulation of multifunctional phosphatase PTEN, and proapoptotic factors. Gastroenterology. 2007;133:1938-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Tarnawski AS, Ahluwalia A, Jones MK. Increased susceptibility of aging gastric mucosa to injury: the mechanisms and clinical implications. World J Gastroenterol. 2014;20:4467-4482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (4)] |
| 55. | Yoon JH, Kim O, Nam SW, Lee JY, Park WS. NKX6.3 Regulates Reactive Oxygen Species Production by Suppressing NF-kB and DNMT1 Activities in Gastric Epithelial Cells. Sci Rep. 2017;7:2807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |