Published online Jan 21, 2019. doi: 10.3748/wjg.v25.i3.282
Peer-review started: August 29, 2018
First decision: October 9, 2018
Revised: October 14, 2018
Accepted: October 21, 2018
Article in press: October 21, 2018
Published online: January 21, 2019
Processing time: 145 Days and 22.4 Hours
Long-term nucleos(t)ide analogue therapy in chronic hepatitis B virus (HBV) infection is effective in suppressing viral replication and reducing liver-related complications. However, HBV-related liver events can still occur in different patient sub-groups. There is emerging evidence that, similar to chronic hepatitis C virus infection, metabolic risk factors may play a role in the disease process of chronic HBV. While the mechanistic nature of metabolic-HBV interactions remains uncertain, studies in different HBV-infected populations have demonstrated that hepatic steatosis, increased body-mass index, diabetes, or a combination of different metabolic risk factors are associated with an increased risk of hepatocellular carcinoma and cirrhosis. The impact of metabolic risk factors is especially prominent in patients with quiescent virological activity, including on-treatment patients with effective viral suppression. As the proportion of on-treatment chronic HBV patients increases worldwide, longitudinal studies determining the relative risks of different metabolic parameters with respect to clinical outcomes are needed. Future studies should also determine if metabolic-directed interventions can improve disease outcomes in chronic HBV.
Core tip: Metabolic risk factors, including hepatic steatosis, increased body-mass index and diabetes, may be associated with worsened disease outcomes and reduced treatment response in chronic hepatitis B. Their effect may be most pronounced in patients with quiescent viral activity, including patients on long-term nucleos(t)ide analogue therapy.
- Citation: Seto WK. Chronic hepatitis B and metabolic risk factors: A call for rigorous longitudinal studies. World J Gastroenterol 2019; 25(3): 282-286
- URL: https://www.wjgnet.com/1007-9327/full/v25/i3/282.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i3.282
Affecting 248 million individuals worldwide, chronic hepatitis B virus (HBV) infection is a leading cause of liver-related morbidity and mortality[1]. Current nucleos(t)ide analogues, when taken long-term, can effectively suppress viral replication, improve liver histology, and reduce liver-related complications[2]. Yet nucleos(t)ide analogue therapy is never a “magic bullet” that can eliminate and prevent all HBV-related complications, with the benefit of therapy mitigated in certain patient sub-groups. For example, in an Asian population-based study, nucleoside analogue failed to significantly reduce liver cancer incidence in elderly chronic HBV patients[3].
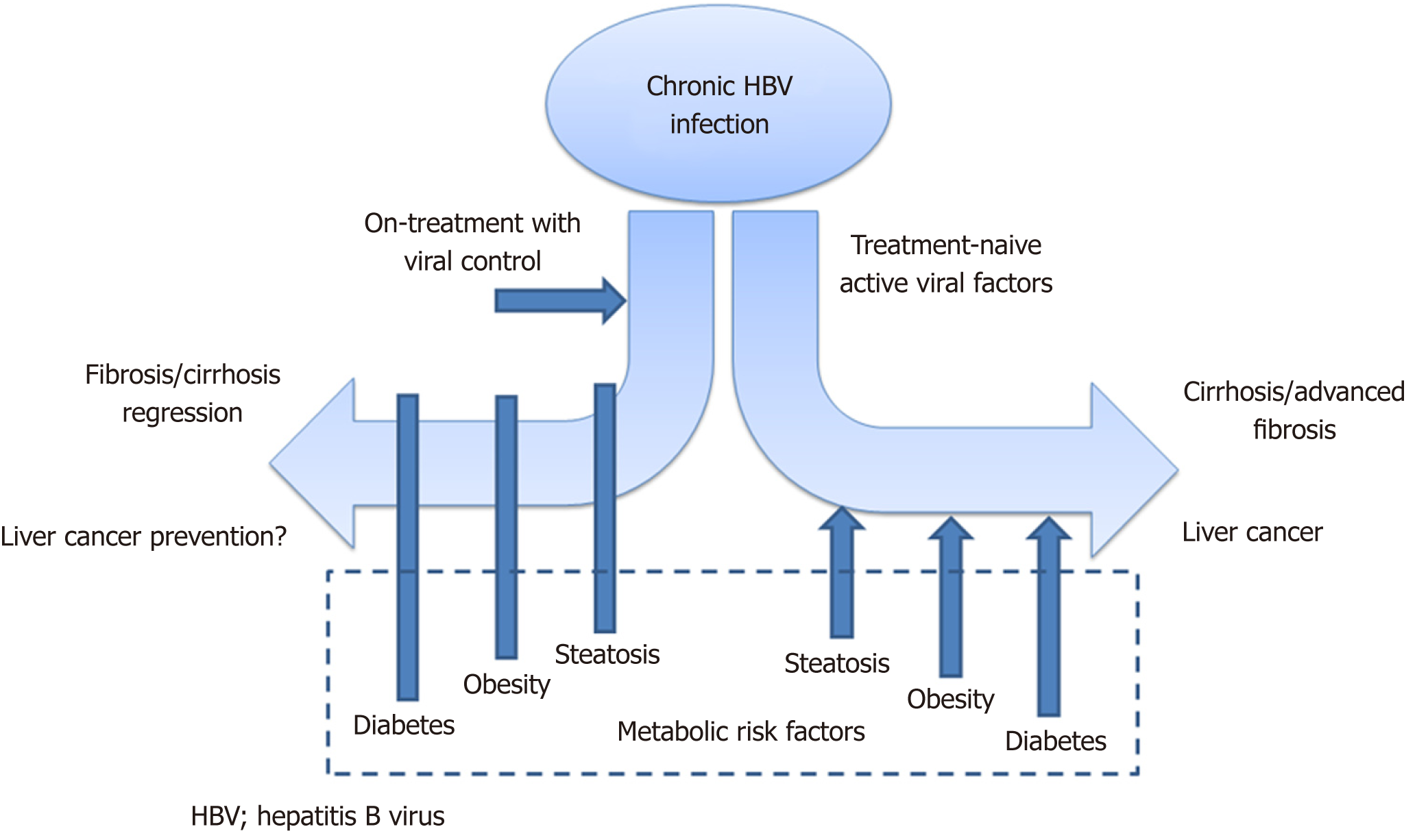
Metabolic parameters have been demonstrated to play a prominent role in the disease course of chronic hepatitis C virus infection[4]. The interaction of metabolic factors with chronic HBV has been less extensively studied. Now, there is emerging evidence on how metabolic risk factors may influence the natural history and treatment response of chronic HBV (Figure 1), and these will be described in detail in this editorial.
The impact of liver fat on the disease course of HBV is controversial. There are studies indicating that steatosis may actually be protective. A cohort study from Taiwan involving 83339 participants showed hepatitis B surface antigen (HBsAg)-positive patients to have a lower risk of non-alcoholic fatty liver disease (NAFLD) development compared to HBsAg-negative individuals[5]. Another study demonstrated that a treatment-naïve chronic HBV patient with co-existing NAFLD had significantly lower serum HBV DNA levels compared to chronic HBV without steatosis, after adjusting for potential confounders[6].
Paradoxically, there is evidence that co-existing hepatic steatosis may contribute to the chronic HBV disease process. A study employed the noninvasive quantification of steatosis using controlled attenuation parameter (commonly known as CAP) measurements, with standardized cut-off values used to categorize steatosis severity[7]. Severe steatosis (CAP ≥ 280 dB/m) was found to be independently associated with increased liver fibrosis in both treatment-naïve patients and on-treatment patients achieving long-term virological suppression. The results suggest that even during quiescent viral activity, fibrogenesis can still develop in the presence of hepatic steatosis[8].
The above findings will require longitudinal validation in the clinical setting, as well as mechanistic studies for any HBV-steatosis interaction. Nonetheless, the management implications can be potentially huge, since both chronic HBV and NAFLD are common diseases. In Asia, the prevalence of NAFLD is greater than 30%[9], while more than a quarter of chronic HBV patients have concomitant NAFLD[10].
An increased body-mass index (BMI) has been demonstrated to worsen the disease outcome of chronic HBV. In a population-based study involving 2903 HBsAg-positive men after a mean follow-up of 14.7 years, obesity (BMI ≥ 30 kg/m2) was associated with increased risk of both hepatocellular carcinoma (HCC) and liver-related mortality[11]. Obesity also diminishes treatment response by lessening fibrosis regression during long-term nucleos(t)ide analogue therapy. In a study with paired liver biopsies over a course of five years, increased BMI (≥ 25 kg/m2) in HBV-infected patients of majority European descent was associated with persistent severe fibrosis or cirrhosis during treatment when compared to patients with normal BMI[2]. Results were also similar in another study involving Asian on-treatment patients with paired transient elastography over a median duration of 87.5 mo[12].
Obesity is associated with adipokine dysregulation, including reduced adiponectin and increased leptin production, which leads to enhanced liver fibrogenesis[13]. However, it remains unclear whether this adipokine dysfunction has any mechanistic interaction with HBV. With the prevalence of obesity in HBV-endemic regions increasing[14], studies specifically concentrating on the obese HBV population will be needed.
Diabetes has a synergistic impact on the disease course of chronic HBV. While the exact mechanism remains unclear, hyperglycemia activates oxidative stress[15], which can be linked with the severity of liver disease in chronic HBV infection[16]. Diabetic chronic HBV patients have an increased chance of alanine aminotransferase elevation and liver damage compared to non-diabetic patients[17]. Diabetes also increases the risk of HBV-related cirrhotic decompensation[18] and liver-related mortality[19].
Large-population cohort studies have further established the association of diabetes with liver-related clinical outcomes. A cohort study involving 23,820 Taiwan residents and a mean follow-up duration of 14 years found that diabetes independently increased the risk of HCC in HBsAg-positive individuals[20]. In addition, in a recent study involving 512,891 Chinese adults (both HBsAg-positive and -negative) with a median follow-up duration of 10.1 years, the presence of diabetes significantly increased the risk of HCC and cirrhosis (adjusted hazards ratios of 1.49 and 1.87, respectively). In addition, in patients without previously diagnosed diabetes, an increase of plasma glucose levels by 1 mmol/L, even in the non-diabetic range, significantly increased the probability of HCC and cirrhosis (adjusted hazards ratios of 1.04 and 1.07, respectively)[21].
The combination of different metabolic risk factors in HBV-infected patients can increase the risk of liver-related events. Metabolic syndrome, which is a combination of increased waist circumference or obesity with the presence of different metabolic risk factors (hyperglycemia, hyperlipidemia, hypertriglyceridemia or hypertension) is a known risk factor for the development of HBV-related fibrosis progression[22]. More recently, a Taiwanese study followed up with 1690 men with chronic HBV infection for a median duration of 19 years. Having three or more metabolic risk factors (including diabetes, obesity, hypertriglyceridemia, hypercholesterolemia or hypertension) increased the risk of HCC and liver-related death (hazards ratios of 2.32 and 2.72, respectively). Notably, in patients with available virological data, the risk of HCC among patients with three or more metabolic risk factors was especially accentuated when serum HBV DNA was less than 2000 IU/mL (hazards ratio of 14.38)[23].
Despite the emerging evidence of metabolic risk factors being associated with HBV-related outcomes, one fundamental question remains unanswered: are HBV and metabolic-related liver injury synergistic, or simply two unrelated and different disease processes? Mechanistic studies to investigate their interaction are technically difficult, mainly due to the limitations of current HBV animal models which are unable to support the full HBV life cycle, restricting the study of host-viral interactions[24].
Nonetheless, the more important clinical question will be the magnitude of impact of different metabolic risk factors on HBV-related clinical outcomes. From available evidence, this impact is especially prominent in quiescent HBV disease[8,12,23], either in treatment-naïve patients with intrinsically low HBV DNA levels or in nucleos(t)ide analogue-treated patients with effective virological suppression. The proportion of patients receiving long-term treatment is increasing worldwide[25], while at the same time, the HBV patient cohort is ageing, suggesting that the concomitant presence of metabolic risk factors will increase. Taken together, these data suggest that the metabolic impact on the disease course of HBV will become more and more predominant. Finally, well-designed longitudinal studies will be needed to determine whether interventions directed at metabolic risk factors e.g., glycemic control in diabetes or weight loss, can improve HBV-related outcomes. Clinical data on this metabolic-HBV interaction will prove useful if we are to meet the World Health Organization’s objective in removing HBV as a public health threat by 2030.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Raghow R, Schuurman HJ S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1995] [Article Influence: 199.5] [Reference Citation Analysis (4)] |
| 2. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1368] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 3. | Seto WK, Lau EH, Wu JT, Hung IF, Leung WK, Cheung KS, Fung J, Lai CL, Yuen MF. Effects of nucleoside analogue prescription for hepatitis B on the incidence of liver cancer in Hong Kong: a territory-wide ecological study. Aliment Pharmacol Ther. 2017;45:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Serfaty L. Metabolic Manifestations of Hepatitis C Virus: Diabetes Mellitus, Dyslipidemia. Clin Liver Dis. 2017;21:475-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: A cohort study. Hepatology. 2017;65:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Hui RWH, Seto WK, Cheung KS, Mak LY, Liu KSH, Fung J, Wong DK, Lai CL, Yuen MF. Inverse relationship between hepatic steatosis and hepatitis B viremia: Results of a large case-control study. J Viral Hepat. 2018;25:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 836] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 8. | Seto WK, Hui RWH, Mak LY, Fung J, Cheung KS, Liu KSH, Wong DK, Lai CL, Yuen MF. Association Between Hepatic Steatosis, Measured by Controlled Attenuation Parameter, and Fibrosis Burden in Chronic Hepatitis B. Clin Gastroenterol Hepatol. 2018;16:575-583.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Seto WK, Yuen MF. Nonalcoholic fatty liver disease in Asia: emerging perspectives. J Gastroenterol. 2017;52:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Yu MW, Shih WL, Lin CL, Liu CJ, Jian JW, Tsai KS, Chen CJ. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol. 2008;26:5576-5582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Seto WK, Fung J, Cheung KS, Mak LY, Hui RW, Liu KS, Lai CL, Yuen MF. Body-mass index is associated with fibrosis regression during long-term nucleoside analogue therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2016;44:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Saxena NK, Anania FA. Adipocytokines and hepatic fibrosis. Trends Endocrinol Metab. 2015;26:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7951] [Cited by in RCA: 7995] [Article Influence: 726.8] [Reference Citation Analysis (0)] |
| 15. | Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1689] [Cited by in RCA: 1794] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 16. | Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Khalili M, Lombardero M, Chung RT, Terrault NA, Ghany MG, Kim WR, Lau D, Lisker-Melman M, Sanyal A, Lok AS; HBRN. Diabetes and prediabetes in patients with hepatitis B residing in North America. Hepatology. 2015;62:1364-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Huang YW, Wang TC, Lin SC, Chang HY, Chen DS, Hu JT, Yang SS, Kao JH. Increased risk of cirrhosis and its decompensation in chronic hepatitis B patients with newly diagnosed diabetes: a nationwide cohort study. Clin Infect Dis. 2013;57:1695-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Hsiang JC, Gane EJ, Bai WW, Gerred SJ. Type 2 diabetes: a risk factor for liver mortality and complications in hepatitis B cirrhosis patients. J Gastroenterol Hepatol. 2015;30:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, Chen CJ. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 21. | Pang Y, Kartsonaki C, Turnbull I, Guo Y, Clarke R, Chen Y, Bragg F, Yang L, Bian Z, Millwood IY, Hao J, Han X, Zang Y, Chen J, Li L, Holmes MV, Chen Z. Diabetes, Plasma Glucose, and Incidence of Fatty Liver, Cirrhosis, and Liver Cancer: A Prospective Study of 0.5 Million People. Hepatology. 2018;68:1308-1318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 22. | Wong GL, Chan HL, Yu Z, Chan AW, Choi PC, Chim AM, Chan HY, Tse CH, Wong VW. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B--a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-Related Death in Men With Chronic Hepatitis B: A Large Cohort Study. Gastroenterology. 2017;153:1006-1017.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Protzer U. Viral hepatitis: The bumpy road to animal models for HBV infection. Nat Rev Gastroenterol Hepatol. 2017;14:327-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Spradling PR, Xing J, Rupp LB, Moorman AC, Gordon SC, Teshale ET, Lu M, Boscarino JA, Schmidt MA, Trinacty CM, Holmberg SD; Chronic Hepatitis Cohort Study Investigators. Distribution of disease phase, treatment prescription and severe liver disease among 1598 patients with chronic hepatitis B in the Chronic Hepatitis Cohort Study, 2006-2013. Aliment Pharmacol Ther. 2016;44:1080-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (2)] |