Published online Aug 7, 2019. doi: 10.3748/wjg.v25.i29.3941
Peer-review started: March 29, 2019
First decision: June 10, 2019
Revised: June 21, 2019
Accepted: July 5, 2019
Article in press: July 5, 2019
Published online: August 7, 2019
Processing time: 131 Days and 22.6 Hours
Cholangiocarcinoma or biliary tract cancer has a high mortality rate resulting from late presentation and ineffective treatment strategy. Since immunotherapy by dendritic cells (DC) may be beneficial for cholangiocarcinoma treatment but their efficacy against cholangiocarcinoma was low. We suggest how such anti-tumor activity can be increased using cell lysates derived from an honokiol-treated cholangiocarcinoma cell line (KKU-213L5).
To increase antitumour activity of DCs pulsed with cell lysates derived from honokiol-treated cholangiocarcinoma cell line (KKU-213L5).
The effect of honokiol, a phenolic compound isolated from Magnolia officinalis, on choangiocarcinoma cells was investigated in terms of the cytotoxicity and the expression of damage-associated molecular patterns (DAMPs). DCs were loaded with tumour cell lysates derived from honokiol-treated cholangiocarcinoma cells their efficacy including induction of T lymphocyte proliferation, proinflammatory cytokine production and cytotoxicity effect on target cholangiocarcinoma cells were evaluated.
Honokiol can effectively activate cholangiocarcinoma apoptosis and increase the release of damage-associated molecular patterns. DCs loaded with cell lysates derived from honokiol-treated tumour cells enhanced priming and stimulated T lymphocyte proliferation and type I cytokine production. T lymphocytes stimulated with DCs pulsed with cell lysates of honokiol-treated tumour cells significantly increased specific killing of human cholangiocarcinoma cells compared to those associated with DCs pulsed with cell lysates of untreated cholangiocarcinoma cells.
The present findings suggested that honokiol was able to enhance the immunogenicity of cholangiocarcinoma cells associated with increased effectiveness of DC-based vaccine formulation. Treatment of tumour cells with honokiol offers a promising approach as an ex vivo DC-based anticancer vaccine.
Core tip: We constructed dendritic cells (DCs) loaded with cell lysates derived from honokiol-treated cholangiocarcinoma cells, with the aim of eliciting apoptosis in tumour cells and creating a broad array of tumour associated antigents in the form of dead and dying cells. Our data demonstrated that DCs primed with tumour cell lysates derived from honokiol-treated cholangiocarcinoma cells could improve the fuction of effector T lymphocytes in killing of the cancer cells. This suggested that honokiol enhanced the immunogenicity of cholangiocarcinoma antigens with increased effectiveness of DC-based vaccine formulation.
- Citation: Jiraviriyakul A, Songjang W, Kaewthet P, Tanawatkitichai P, Bayan P, Pongcharoen S. Honokiol-enhanced cytotoxic T lymphocyte activity against cholangiocarcinoma cells mediated by dendritic cells pulsed with damage-associated molecular patterns. World J Gastroenterol 2019; 25(29): 3941-3955
- URL: https://www.wjgnet.com/1007-9327/full/v25/i29/3941.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i29.3941
Cholangiocarcinoma (CCA) is the most common biliary tract tumour and second commonest primary hepatic malignancy[1]. High incidence of CCA in Southeast Asia is strongly associated with liver fluke Opisthorchis viverrini infection, while numbers of cases in Europe and North America have significantly increased in recent decades. CCA has poor prognosis with high mortality rates since patients with early stages of cancer are often asymptomatic and no specific biomarkers for clinical diagnosis currently exist[2,3]. Unfortunately, surgical resection is also limited by advanced cancer metastasis and chemotherapeutic drugs have shown unsatisfactory outcome for survival in inoperable patients. Therefore, a new therapeutic strategy for CCA treatment and prevention should be urgently addressed.
Dendritic cells (DCs) are potent inducers of antitumour responses and they are often used as tumour antigen delivery vehicles in cancer therapy. DC cancer vaccines are aimed to stimulate anticancer immunity in patients through their capacity to activate tumour-specific T cells[4]. Incubating DCs with whole tumour lysates or killed cancer cells generates a broad array of tumour-associated antigens (TAAs) on DCs. Previous preclinical and clinical studies indicated that DCs loaded with tumour cell lysates exhibit antitumour activity and can induce tumour regression in various cancers such as colon cancer[5], breast cancer[6], hepatocellular carcinoma[7] and CCA[8]. The efficacy of DCs loaded with whole CCA cell lysates has been argued in terms of tumour antigen properties and antitumour treatment[8]. Therefore, an improvement of tumour preparation protocol to enhance CCA immunogenicity for a putative DC cancer vaccine approach is urgently required.
Honokiol is a bioactive, biophenolic phytochemical compound extracted from Magnolia officinalis that has shown multiple pharmacological anti-inflammatory, anti-oxidant, anti-anxiety, anti-depressant, anti-stress and anti-tumour effects[9]. Previous studies have shown that honokiol can inhibit tumour growth both in vitro and in animal models by induction of cell apoptosis in many types of colon, breast, glioblastoma and liver cancers[9]. Interestingly, one recent study demonstrated that herbal-derived compounds can enhance the antitumour response of DCs loaded with tumour cell lysates by induction of cancer cell apoptosis and expression of damage-associated molecular patterns (DAMPs)[10]. Pulsing of DCs with DAMP components results in full activation of MyD88 signaling of DCs and activation of CD8+ lymphocytes leading to subsequent antitumour immune response[11]. Moreover, honokiol potentially suppresses the immunoresistant ability of glioblastoma without disrupting T lymphocyte function and may be recommended for combined immu-notherapy[12].
Taken together, the efficacy of DC cancer vaccines against CCA requires improvement but untill now there have been no reports on the effect of pulsing DCs with tumour antigen generated by honokiol. Hence, here, we constructed DCs loaded with cell lysates derived from honokiol-treated CCA tumour cells, with the aim of eliciting apoptosis in tumour cells and creating a broad array of TAAs in the form of dead and dying cells. Effects of honokiol on the CCA cell line associated with Opisthorchis viverrini, the Southeast Asian liver fluke, were studied in terms of cell cytotoxicity and apoptosis inducer. Furthermore, CCA cell lysates were used as tumour antigens for loading into DCs grown ex vivo and the DCs were then characterised for their phenotypic features. Moreover, the efficacy of DCs pulsed with tumour cell lysates derived from honokiol-treated CCA cells was investigated in terms of stimulating T lymphocyte proliferation, type I cytokine production and cytotoxic activity. Our model improved cancer vaccine efficacy against CCA based on DCs and demonstrated the use of honokiol as a herbal-derived compound in combination with tumour antigen pulsed DCs to stimulate cytotoxic antitumour T lymphocytes.
Well differentiated human CCA cell line, KKU-213L5 was obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). The immortalized cholangiocyte, MMNK1 cell line was a gift from Prof. Naoya Kobayashi. The cell lines were maintained in Dulbecco’s modified Eagle’s medium (Gibco, Thermo Fisher Scientific, MA, United States), supplemented with 5% fetal bovine serum, 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B. Cell grown in a humidified incubator at 37 °C with 5% CO2.
CCA cell line was seeded at a density of 5 × 103 cells/well in 96-well plate. After cultivation for 12 h, 0-100 μM honokiol were added at different concentrations. The cells were then further incubated for 24 and 48 h. Subsequently, 0.5 mg/mL of MTT reagent was added and incubated for another 4 h. After that, the formazan product was dissolved by DMSO and the light absorbance was read at 540 nm using microplate spectrophotometer (PerkinElmer, MA, United States). The percentage of cell viability was calculated following the formula [(honokiol treated Abs540)/(control Abs540)] × 100 (%).
Cell apoptosis was determined using the Muse™ Cell Analyzer from Millipore (MA, United States) following manufacturer’s instruction. Briefly, honokiol treated cells were washed with phosphate buffered saline (PBS) and resuspended using the Annexin V and Dead Cell Reagent (7-AAD, Millipore, MA, United States). This was incubated for 20 min before assessment. The results were presented as the percentage of live cell, apoptotic cell and dead cell.
KKU-213L5 cells were incubated with honokiol at indicated concentrations for 20 h. For the analysis of intracellular proteins, treated cells were washed with ice-cold PBS before cell lysis using RIPA lysis buffer plus protease inhibitor cocktail (AMRESCO, OH, United States). Then, protein lysates were collected by centrifugation and the total protein concentration was qualified by using Bradford assay. In addition, the secreted protein was collected from conditioned medium, which was concentrated using Amicon® Ultra-2 Centrifugal Filter units (Millipore, MA, United States) following the manufacturer’s instruction for 20× final concentration. The protein was then separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto the polyvinylidene fluoride membranes. After that, the non-specific binding was blocked with 5% skim milk buffer for 1 h before washing with TBST buffer. The membranes were then incubated with each primary antibody, anti-caspase 3 (Cell Signaling, MA, United States), anti-HMGB1 (ELabScience, TX, United States) and anti-HSP90 (Merck, Darmstadt, Germany) antibodies with gentle shaking at 4 °C overnight. Then, membranes were washed with TBST and incubated with horseradish peroxidase-linked anti-rabbit antibody (Cell Signaling, MA, United States) for 1 h at room temperature, and washed again before incubated with detection reagent. The image was developed by Chimidoc™ XRS (Bio-rad, CA, United States) and analyzed by Image Lab (Bio-rad, CA, United States).
Peripheral blood monocytes were isolated from healthy donors by gradient centrifugation using Ficoll-Hypaque and Percoll (GE Healthcare, Freiburg, Germany). The use of human blood with informed consent was approved by the ethics committee of Naresuan University (protocol No.0846/60). The monocyte fraction was resuspended in RPMI 1640 medium containing 10% foetal bovine serum (FBS) and 2 mM L-glutamine (Gibco, Thermo Fisher Scientific, MA, United States) in cell culture flask for 2 h. The non-adherent cells were gently removed before washing with PBS for 3 times. The adherent cells were then cultured in RPMI 1640 complete medium supplemented with 100 ng/mL of human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF, Miltenyi Biotec, Bergisch Gladbach, Germany) and 50 ng/mL of human recombinant interleukin-4 (IL-4, Miltenyi Biotec, Bergisch Gladbach, Germany) for 6 d. The medium was replaced every 3 d with fresh medium containing the same concentration of GM-CSF and IL-4.
Flow cytometric analysis was carried out using the following antibodies: antihuman CD11c antibody-PE (eBioscience, CA, United States) and antihuman CD14 antibody-FITC (Abcam, Cambridge, United Kindom). Monocytes and DCs were harvested and washed with PBS containing 3% FBS before staining with fluorescent-conjugated antibodies for 45 min. After that cells were washed with PBS containing 3% FBS and suspended in FACs buffer (PBS containing 10% FBS). Stained cells were analyzed on Cytomics FC 500 using CXP software (Beckman Coulter, IN, United States).
The CCA cell line were stained with CellTracker™ Red CMPTX (Thermo Fisher Scientific, MA, United States). Briefly, 6 × 106 cells were washed with PBS solution before incubation with fluorescent dye for 15 min. After that, the stained cells were washed twice with PBS and seeded at 6 × 106 cells per 100 mm dish. Then, 50 µM honokiol was add into the culture and incubated for 24 h. The honokiol treated cells were harvested and resuspended with 500 µL RPMI1640 serum free medium. Cell suspension was frozen in liquid nitrogen for 1.5 min and thawed in 37 °C water-bath for 5 min. This step of freezing and thawing was repeated for 3 times. Then, cell debris was removed by centrifugation at 5000 rpm for 10 min before collecting cell supernatant. In addition, the conditioned medium of honokiol treated tumor cells was collected and concentrated using Amicon® Ultra-2 Centrifugal Filter units (Millipore, MA, United States) for 20X final concentration. The protein concentration of tumor cell lysates and conditioned medium were measured using Bradford assay. These protein preparations were then used as honokiol derived-tumor cell lysates plus secreted protein.
Immature DCs were harvested from induction medium and stained with CellTracker™ Green CMFDA (Thermo Fisher Scientific, MA, United States) following the protocol described above. After that, DCs were suspended in RPMI1640 complete medium, supplemented with tumor cell lysates derived from honokiol treated tumor cells at the amount of 2 × 105 DCs per 100 µg of tumor cell lysate and 20 µg of secreted protein. The DCs alone and DCs cocultured with tumor cell lysates were studied as control and comparative groups, respectively. After 24 h, the DCs were maturated by adding 50 ng/mL of tumor necrosis factor alpha and 50 ng/mL of interferon gamma (IFN-γ) (ImmunoTool, Friesoythe, Germany) for another 24 h.
DCs-loaded with tumor cell lysates derived from honokiol-treated tumor cells were washed twice with PBS before they were seeded onto chamber slide and incubated for 12 h to allow cell adhesion. Then, adherent cells were fixed with 2% formadehyde for 20 min and mounted with prolong gold antifade reagent with DAPI (Invitrogen, CA, United States). Co-expression of green and red fluorescent was observed using EVOS fluorescent system (Invitrogen, CA, United States).
To activate the effector cell T lymphocytes, autologous T lymphocytes were isolated using Ficoll-Hypaque and Percoll gradient centrifugation as described above. For T lymphocyte enrichment, lymphocyte fraction was resuspended in RMPI1640 medium and incubated with nylon wool column for 1 h. Non-adhered cells were collected by gently eluting with RMPI1640 medium. The samples of autologous T lymphocytes with CD3-positive cells of more than 70% as analyzed by flow cytometry were used in T lymphocyte activation study.
After loading of tumor cell lysates into DCs, different groups of DCs (unpulsed, pulsed with tumor cell lysates and pulsed with honokiol derived tumor cell lysates) were harvested as stimulator cells. The stimulator cells were then cocultured with autologous T lymphocytes in a 96 well culture plate at a ratio of 1:10. They were continually cultured for 5 d. The lymphocyte culture alone was set as a control. The proliferation of activated of T lymphocytes was measured using direct counting by trypan blue exclusion and MTT assays. The absorption (A) at 540 nm was used to calculate relative T lymphocyte proliferation rate as: A experiment/A control.
During stimulation of effector T lymphocytes, the conditioned medium of different DCs (unpulsed, pulsed with tumor cell lysates and pulsed with honokiol derived tumor cell lysates) was collected at day 1, 3 and 5 for measurement of cytokines. IFN-γ and IL-12 concentrations in supernatants were measured by specific sandwich ELISA (PeproTech, NJ, United States) according to the manufacturer’s instruction.
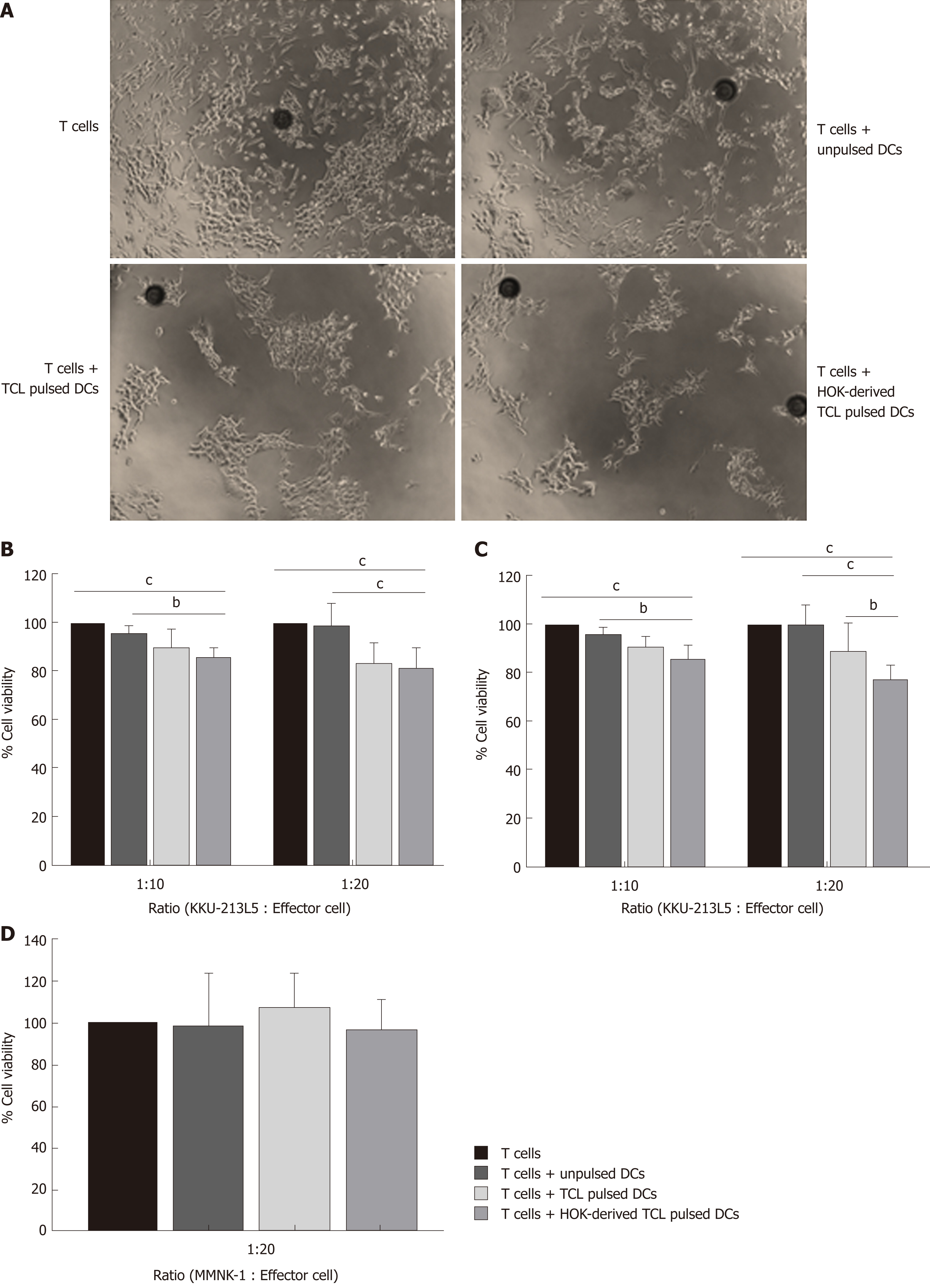
DCs (unpulsed, pulsed with tumor cell lysates and pulsed with honokiol derived tumor cell lysates) were harvested as stimulator cells and cocultured with autologous T lymphocytes at a ratio of 1:10 for 5 d. Then, differently treated effector T cells were added to the target KKU-213L5 and MMNK-1 cells at ratios ranking from 1:10 to 1:20 and they were cultured for 24 and 48 h. The unbound cells were washed with PBS and the cells were photographed under microscopy. The viability of target cell was measured using MTT assay. The absorbance at 540 nm of effector cells only was set as control, and the absorption of different groups relative to control was calculated as the percentage of cell viability.
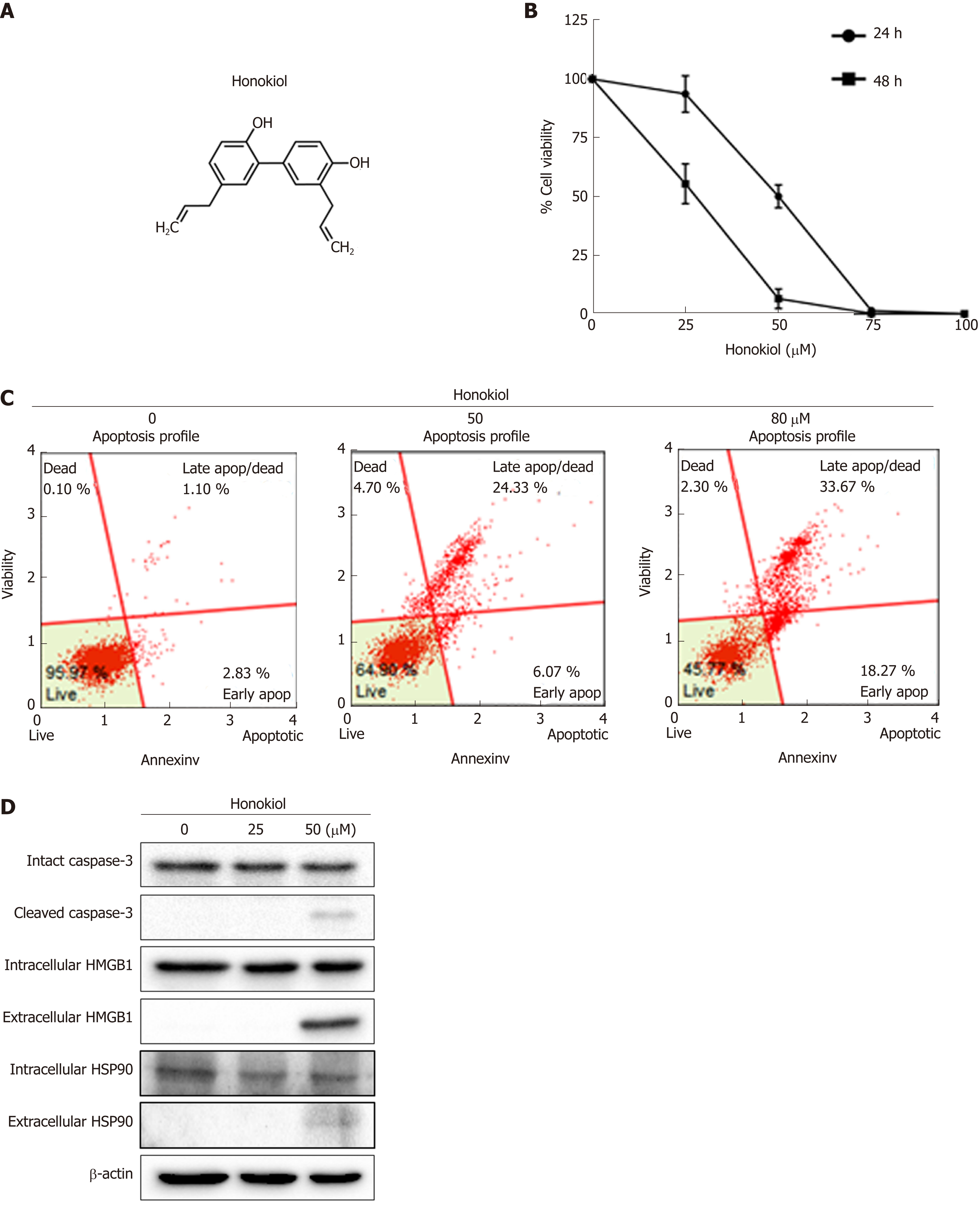
Results showed that honokiol significantly caused cell death in a dose- and time-dependent manner (Figure 1A and B). The IC50 of this compound at 24 and 48 h was 49.99 and 26.31 µM respectively with the underlying mechanism of cell death investigated using annexin V/PI staining. Treatment of honokiol for 24 h induced apoptosis of KKU-213L5 cells with significant increase in apoptotic cells in a dose-dependent manner (control = 3.93%, 50 µM honokiol = 30.4% and 70 µM honokiol = 52%) (Figure 1C). Increased apoptosis was confirmed by decrease of intact caspase-3, whereas cleaved caspase-3 increased (Figure 1D). Results suggested that honokiol was capable of inducing CCA death via cell apoptosis.
We investigated both intracellular and secreted protein expression of two DAMPs as the high mobility group box 1 (HMGB1) and heat shock protein90 (HSP90) molecules[13]. Results showed that treatment with honokiol at 50 µM concentration induced release of HMGB1 and HSP90 proteins in the conditioned medium. However, levels of intracellular HMGB1 and HSP90 did not change (Figure 1D). Data suggested that honokiol was able to induce CCA apoptosis with secretion of DAMPs.
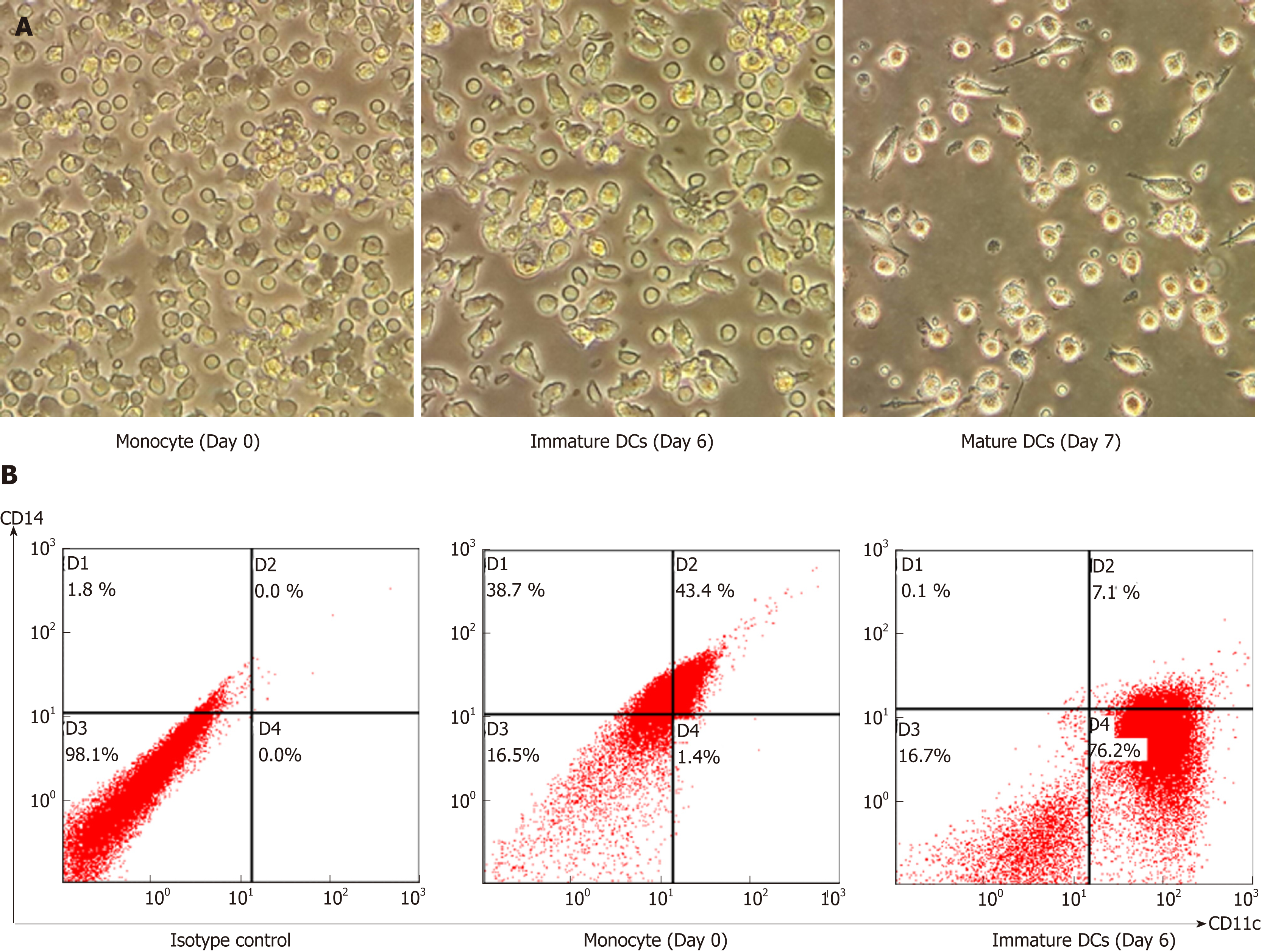
This study used monocyte-derived DCs as a model to construct DCs loaded with tumour cell lysates. Peripheral blood monocytes were induced to become mature DCs and the differentiation was indicated by changing cell morphology from spherical to large dendritic shape and a more expanded shape of mature DCs (Figure 2A). The cellular phenotype was confirmed by the expression of DC marker CD11c that dramatically increased from day 0 to day 6, whereas expression of the monocyte marker CD14 markedly decreased (Figure 2B). These results indicated that mature DCs were successfully generated in vitro from human peripheral blood.
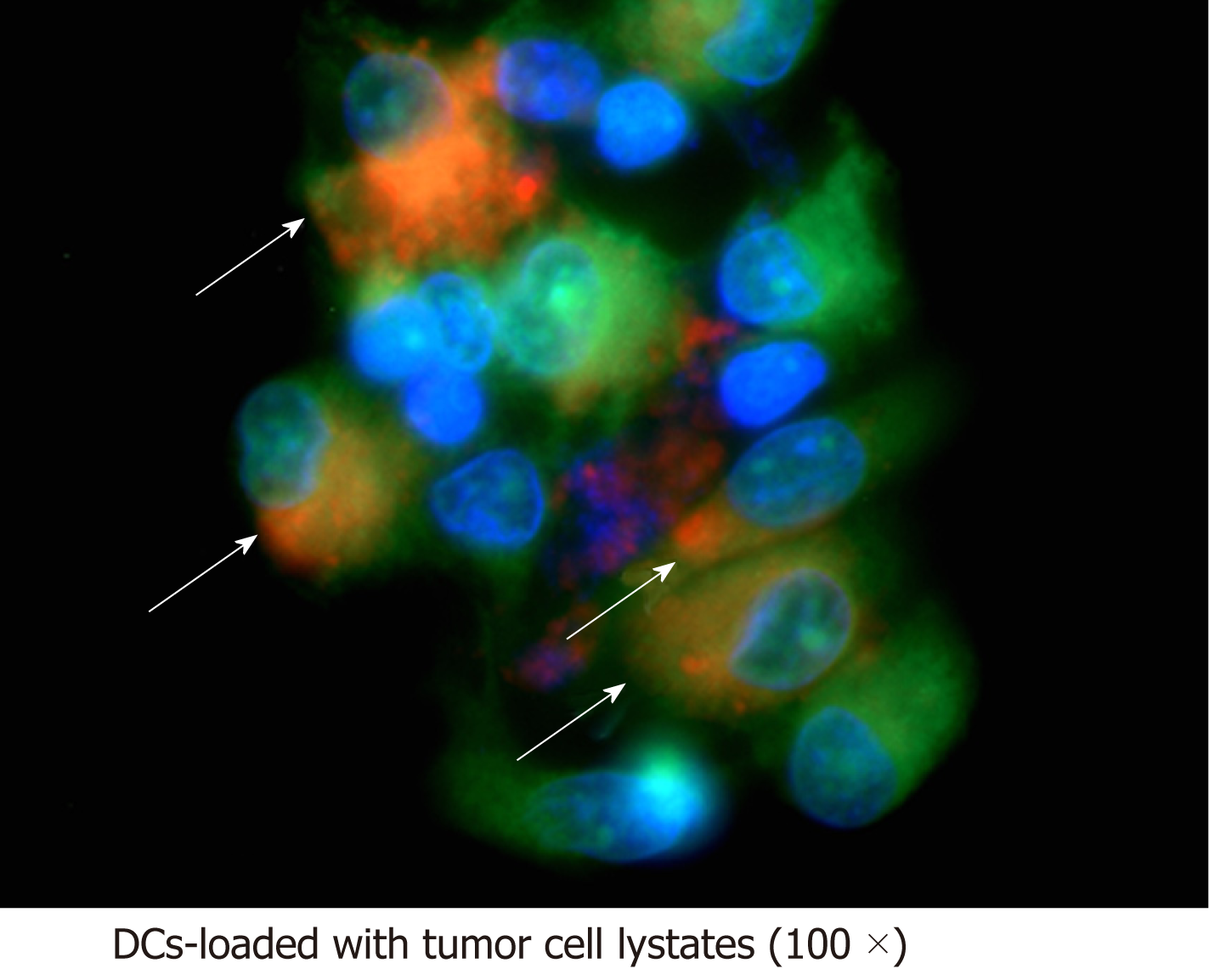
The ability of DCs to uptake honokiol-derived CCA tumour cell lysates was confirmed using fluorescence microscopy. Both immature DCs and tumour cell lysates were fluorescently labelled and cocultured for 24 h. Image analysis revealed co-localisation of green and red fluorescence in DCs pulsed with honokiol-derived tumour cell lysates (Figure 3), whereas green and red fluorescence appeared separately in DCs and honokiol-derived CCA tumour cell lysates only groups (Figure S1). Localisation of tumour antigen was indicated by 100× magnification which showed cytoplasmic localisation of tumour antigen on DCs, suggesting that generated immature DCs had phagocytic activity and were able to uptake honokiol-derived CCA tumour cell lysates.
To determine the effect of DCs loaded with honokiol-derived CCA tumour cell lysates on T lymphocyte proliferation, autologous T lymphocytes were cocultured with various types of DCs including unpulsed DCs, DCs pulsed with tumour cell lysates and DCs pulsed with honokiol-derived tumour cell lysates. After 5 d, lymphocyte number was reflected. Results showed that DCs loaded with honokiol-derived tumour cell lysates tended to increase T lymphocyte number compared with unpulsed DCs and DCs loaded with tumour cell lysates (Figure 4A). As well as relative lymphocyte proliferation (Figure 4B), DCs pulsed with honokiol-derived tumour cell lysates induced significantly higher T lymphocyte proliferation than untreated CCA antigen, suggesting that tumour cell lysates derived from honokiol-treated CCA cells may differentially activate DCs and mediate T lymphocyte proliferation.
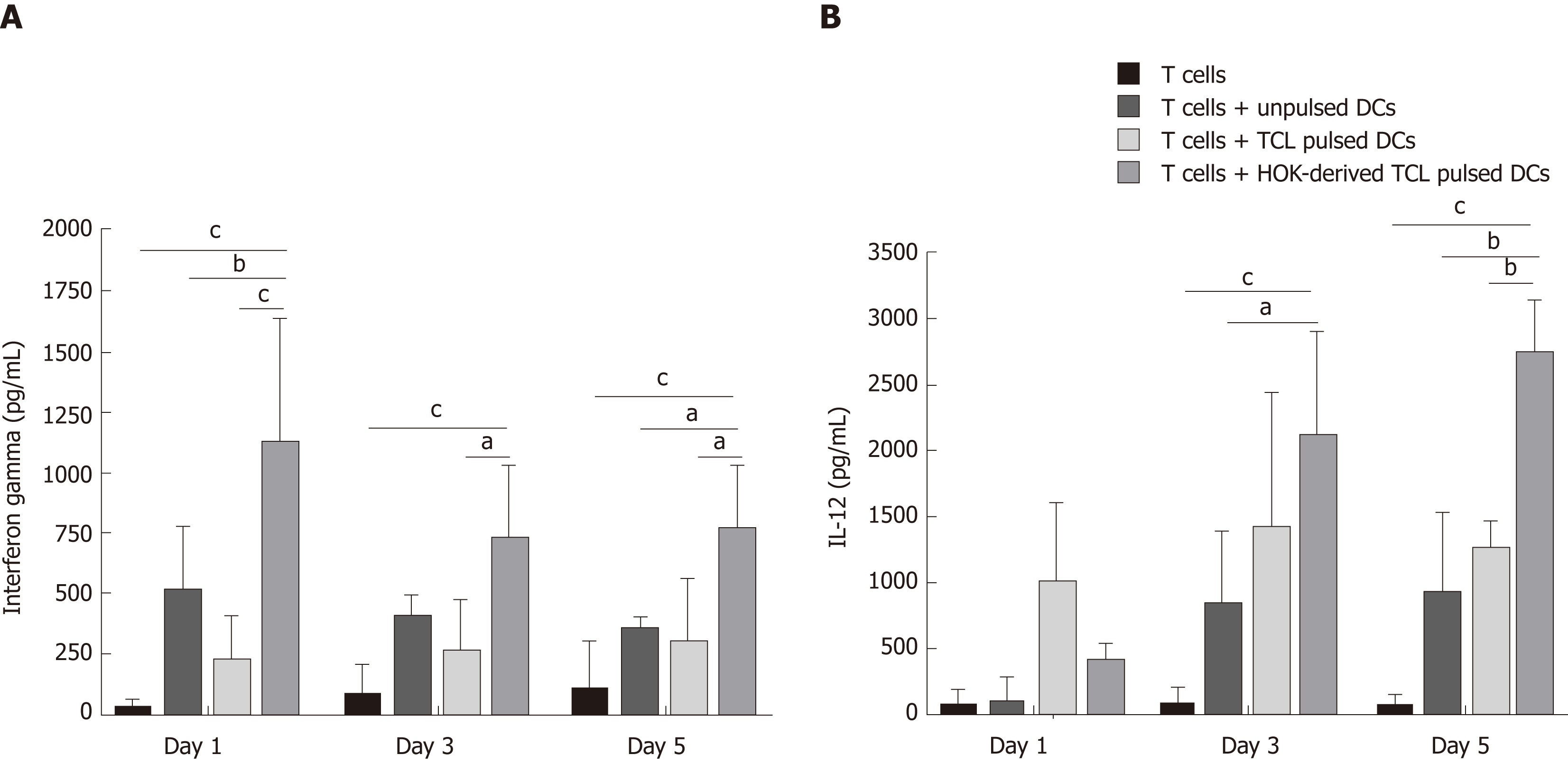
IFN-γ and IL-12 production were measured in supernatants collected from a culture system containing autologous T lymphocytes and DCs unloaded or loaded with tumour cell lysates and or tumour cell lysates derived from honokiol-treated CCA cells. Figure 5 shows that production of IFN-γ by DCs loaded with tumour cell lysates derived from honokiol-treated CCA cells significantly increased at day 1, 3 and 5 compared with control and unloaded DC groups (Figure 5A). Moreover, IL-12 production in similar conditions also significantly increased at day 3 and 5 (Figure 5B). Interestingly, between DCs-loaded with tumour cell lysates groups, production of both cytokines by DCs loaded with tumour cell lysates derived from honokiol-treated CCA cells was significantly higher than DCs loaded with tumour cell lysates. Results indicated that tumour cell lysates derived from honokiol-treated CCA cells enhanced cytokine production by DCs and activated T lymphocytes.
We further investigated the effect of DCs loaded with tumour cell lysates derived from honokiol-treated CCA cells on specific T lymphocyte killing effect of CCA cells. Autologous T lymphocytes were stimulated with different groups of DCs before collecting as effector cells and continually cocultured with KKU-213L5 at indicated ratios. After coculture, numbers of remaining target cells in the honokiol-treated group markedly decreased compared to unloaded DCs and DCs loaded with tumour cell lysates (Figure 6A). Moreover, results of specific killing effect measured using MTT assay showed that DCs primed with honokiol-derived tumour cell lysates and tumour cell lysates gave significantly more enhanced killing activity on target cells than naïve T lymphocytes, unloaded DCs and DCs loaded with tumour cell lysates (Figure 6B and C). Moreover, this specific killing of CCA cells by effector T lymphocytes activated by DCs loaded with CCA KKU-213L5 cell lysates was confirmed because coculturing of these T cells with the human cholangiocyte cell line (MMNK1) for 48 h did not significantly increase MMNK-1 cell death (Figure 6D). These findings suggested that DCs primed with cell lysates from honokiol-treated CCA cells specifically enhanced cytotoxic activity of effector T lymphocytes.
In the last decade, incidence of CCA has globally increased. Advanced metastatic stages of CCA cannot be treated by surgery. Moreover, palliative treatment by chemotherapy is generally unsuccessful because extreme chemoresistance leads to poor prognosis and high mortality rates[3]. Immunotherapy is used in cancer clinical trials. For CCA, DC cancer vaccines have been studied in non-Opisthorchis viverrini-associated CCA, including loading of DCs with synthetic peptide antigens[14] and tumour lysate-pulsed DCs plus ex vivo adoptive transfer T cells[15]. Recently, DCs loaded with pooled mRNA and tumour cell lysates of Opisthorchis viverrini-associated CCA were shown to effectively kill human CCA in vitro[8]. DCs loaded with whole tumour antigen could probably activate polyclonal effector immune cells since the broad array of tumour antigens would effectively eliminate the heterogeneous tumour. In particular, use of tumour cell lysates would be most feasible because the preparation process is easy to manipulate and inexpensive compared with other procedures. However, the efficacy of DCs loaded with tumour cell lysates is limited by antigen processing and presentation, mostly mediated by MHC class II to CD4+ T cells[16]. Here, using tumour cell lysates from honokiol-treated CCA cells to prime DCs, we demonstrated specific T lymphocyte killing enhancement of CCA cells mediated by primed DCs. Honokiol is known to have diverse pharmacological effects, including antitumour activities[9]. It also has immunoadjuvant activity. One previous study reported that honokiol activates cancer cell apoptosis either by receptor- or mitochondria-mediated mechanisms[17]. Moreover, honokiol could avtivate cancer cells death by other mechanism such as autophagy and necrosis[18,19]. We demonstrated that honokiol exhibited a cytotoxic effect on CCA that is likely to be mediated by activation of caspase-3. Moreover, the IC50 of honokiol on cholangiocyte and human derived-macrophage is higher than KKU-213L5, meaning that this compound shows less cytotoxicity on normal cells compared with cancer cells (manuscript in preparation). Immunogenic cell death is mainly mediated by expression of DAMPs which release or expose molecules of injured, damaged and apoptotic cells[13]. We examined the expression of two members of DAMPs as secreted HMGB1 and HSP90. The results indicated that both molecules were expressed when CCA cells were treated with honokiol at sub IC50, the same concentration that caused cell apoptosis. Therefore, we concluded that honokiol exhibited cytotoxicity against CCA cells by induction of cell apoptosis and caused DAMP expression in these cells. HMGB1 is a non-histone nuclear protein that responds to damage signals by translocation from the nucleus to extracellular space which then activates the immune system[20]. HMGB1 proficiently interacts with pattern recognition receptors including advanced glycosylation end product-specific receptor and toll-like receptor 4 (TLR4)[21]. Binding of extracellular HMGB1 with TLR4 on the surface of DCs can stimulate the MYD88-dependent signalling pathway that leads to optimal antigen processing[22]. Moreover, loading of tumour cell lysates plus immunogenic cell death molecules including HMGB1 can enhance DC maturation and antitumour activities in DC-based anticancer vaccine[10]. HSP90 is a molecular chaperone and an important driver for the posttranslational modification process. High expression of HSP90 is associated with poor prognosis in CCA patients[23]. Recently, a DC vaccine based on immunogenic cell death molecules including HMGB1 and HSP90 was shown to elicit danger signals and T cell activation, resulting in rejection of high-grade glioma in an animal model[11]. These data suggest that DAMPs plus tumour cell lysates may provide maximal efficacy of DC-based cancer vaccines.
To study the significance of honokiol-derived tumour cell lysates, DCs were pulsed with tumour cell lysates from KKU-213L5 cell line derived from Opisthorchis viverrini-associated CCA of a Thai patient. Peptide loading procedure is an important parameter for DC-based cancer vaccines and coculturing with tumour cell lysates or peptide antigens is the most commonly used strategy in clinical trials[24]. In coculture systems, a tumour antigen is recognised by phagocytic receptors, resulting in phagocytosis and subsequent processing and presentation on the MHC molecule[25]. Here, we demonstrated the localisation of tumour cell lysates in the cytoplasmic area of DCs. KKU-213L5 cell lysates might be engulfed by DCs; we were successful in constructing DCs loaded with honokiol-derived tumour cell lysates. Although we focus only on one CCA cell line, KKU-213L5, this cell is the representative of highly metastasis CCA cells that mimic the characteristic of lung metastatic CCA cells in CCA patients[26].
Presentation of tumour antigen either on class I or class II MHC molecules triggers the activation of T lymphocyte receptors and co-stimulatory molecules. DAMPs-associated tumour cell lysates can enhance effector T cell activation that mediates fully mature DCs loaded with tumour cell lysates[27]. We showed that autologous T lymphocytes were efficiently activated after coculture with DCs loaded with honokiol-derived tumour cell lysates. These activated T lymphocytes increased proliferation and production of type I cytokines. Interestingly, DCs loaded with tumour cell lysates from honokiol-treated CCA cells activate T lymphocytes better than DCs loaded with tumour cell lysates from untreated cells. Triggering DCs with HMGB1 through TLR-4 leads to full expression of co-stimulatory receptor molecules including CD80 and CD86 and increased production of proinflammatory cytokines[10,20]. In this study, we demonstrated that honokiol-derived tumour cell lysates enhanced IFN-γ and IL-12 secretion from DCs and effector T cells. Secretion of IL-12 from stimulated DCs preferentially drives Th1 effector T cell development, leading to high IFN-γ production[28]. Moreover, IL-12 is a key cytokine for activation of CD8+ T cells and crucial for the change of Th17 to Th1-like phenotype that can be armed to destroy cancer cells[29,30]. Therefore, we concluded that cell lysates from honokiol-treated CCA cells may modulate maturation of DCs that were then able to effectively activate T lymphocytes to differentiate and become effector Th1 and CD8+ T cells.
When the antitumour activity of effector immune cells was examined, T cells stimulated by honokiol-derived tumour cell lysate-primed DCs showed greater efficiency in killing KKU-213L5 cells compared with those stimulated by DCs primed with tumour cell lysates of untreated cells. Moreover, cytotoxicity against human cholangiocytes was not significantly changed, indicating this antitumour cytotoxicity as specific on CCA cells. Although the HLA typing was not performed in this study, KKU213L5 cell line was established from Thai CCA patients and the immune cells were also separated from Thai healthy donors, of which the chance for their compatibility would be high as being HLA-A2[8]. Patients with multiple myeloma have an impaired DC function when loaded with tumour cell lysates. This may be associated with abnormality of STAT3 and the NF-kappaB signalling pathway[31]. On the other hand, DAMPs function to trigger DC maturation via TLR4/2, which involves p38 MAPK and NF-kappaB downstream signalling pathways[32]. Moreover, honokiol could down-regulate the expression of CRT mediated by ER-stress and inhibit gastics tumour growth through reduction of epithelial-to-mesenchymal transition mechanisms[33]. This would explain why the antitumour response of effector T cells mediated by DCs loaded with honokiol-derived tumour cell lysates is superior to those mediated by DCs loaded with tumour cell lysates from cells not exposed to honokiol.
Although we focused only on HMGB1 and HSP90 molecules, several other proteins responsible for damage or danger signals include calreticulin, adenosine triphosphate and other members of the heat shock protein family. Interestingly, DAMPs could activate innate immune cells via many types of receptors as either membrane bound (e.g., TLR4) or intracellular (e.g., TLR3, TLR79, all NOD-like receptors and RIG-I-like receptors)[34]. For example, previous studies reported that the HSP family could activate the TLR-4 signalling pathway leading to facilitation of optimal tumour antigen processing that subsequently elicits antitumour immune response[35,36]. Moreover, treatment with anthracyclines on some cancer cell lines including prostate cancer, ovarian cancer and acute lymphoblastic leukemia cells could induce nuclear translocation of calreticulin, HSP70 and HSP90 as well as the release of HMGB1, causing maturation of DCs. These DCs could then stimulate tumour-specific IFN-γ-producing T cells[37]. Antitumour activity facilitated by the function of DCs loaded with honokiol-derived tumour cell lysates may, however, not involve only HMGB1 and HSP90 and roles of other molecules cannot be excluded. DAMPs may contribute to cancer progression and promote resistance to anticancer treatments[38]. Further in vivo study is required to ensure the effectiveness of this treatment and to differentiate the double-edged sword potential of DAMPs.
In summary, we demonstrated the immunoadjuvant effect of honokiol-derived CCA tumour antigens on a DC-based cancer vaccine approach, which enhanced tumour specific T lymphocyte responses including cell proliferation, cytokine production and cytotoxicity against human CCA cells. Antitumour T cell immunity might be mediated by induced expression of DAMPs in honokiol-treated KKU-213L5 cells. Therefore, in vitro and in vivo studies are urgently needed to assess for the use of honokiol in tumour antigen preparation as one promising approach to discover an effective DC-based vaccine against CCA.
Cholangiocarcinoma (CCA) is a biliary tract malignancy. As no specific biomarkers are available, CCA patients frequently present with a disseminated tumour too late for curative treatment. Honokiol is a hydroxylated biphenyl compound isolated from Magnolia offinalis. Many studies have reported that honokiol has anti-tumour properties on various types of cancer by induction of cell apoptosis. A dendritic cell (DC)-based cancer vaccine is a vaccine that aims to stimulate anticancer immunity in patients through the capacity to activate tumour-specific T cells. However, pulsing DCs with whole tumour cell lysates have shown low efficacy against CCA cells in vitro.
Evidence suggests that the efficacy of DC-based cancer vaccines on CCA is low, especially DCs loaded with tumour cell lysates strategy. In addition, the anti-tumour activity of honokiol could be due to the induction of cancer cell apoptosis. This effect may be associated with the release of damage-associated molecular patterns (DAMPs) from cancer cells, which increases the immunogenicity of tumour antigens. Therefore, the authors of this study were interested in the construction of DCs loaded with cell lysates derived from honokiol-treated CCA tumour cells with the aim of eliciting apoptosis in tumour cells as well as creating a broad array of TTAs in the form of dead and dying cells.
The aim of this study was to maximise the anti-tumour activities of DCs loaded with cell lysates from honokiol-treated CCA cells.
Anti-tumour activity of honokiol was studied, including the cytotoxicity and cell apoptosis assay. The effects of honokiol on DAMPs expression from CCA cells were also investigated. Then, CCA cells with or without honokiol treatments were derived to obtain tumour cell lysates used to pulse the DC cells, after which the latter were used to further stimulate T cells. Finally, the stimulated T cells were exposed to CCA cells and the killing of CCA cells by T cells was determined.
The data showed that honokiol was cytotoxic to human CCA cells KKU-213L5 via intrinsic or extrinsic apoptotic pathways. Interestingly, the induction of cell apoptosis by honokiol was associated with DAMPs release, including HMGB1 and HSP90. DCs loaded with tumour lysates derived from honokiol-treated KKU-213L5 cells enhanced priming and stimulated T lymphocyte proliferation as well as type I cytokine production. Importantly, T lymphocytes stimulated with DCs pulsed with cell lysates of honokiol-treated tumour cells, which significantly increased the specific killing of human CCA cells compared to those associated with DCs pulsed with cell lysates of untreated CCA cells.
These findings provide new evidence that honokiol may have anticancer properties against CCA cells. Further, honokiol may possess the potential to enhance DC-based cancer vaccines, most probably by enhancing the immunogenicity of CCA, which further promotes DCs and T cell stimulation.
Our model showed the improvement of cancer vaccine efficacy against CCA based on DCs and demonstrated the use of honokiol as a herbal-derived compound in combination with tumour antigen pulsed DCs to maximise the antitumour response of cytotoxic antitumour T lymphocytes.
We are grateful to Prof. Sopit Wongkham, Liver Fluke and Cholangiocarcinoma Research Center, Khon Kaen University, for kindly providing us with the CCA cell lines used in this study. Thank also goes to Asst. Prof. Sarawut Kumphune and Assoc. Prof. Kanchana Usuwanthim for their suggestion and for providing T lymphocyte isolation protocol.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Guo ZS, Hibberd AD, Ju SQ, Rajcani J, Uhlmann D S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
| 1. | Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2053] [Article Influence: 205.3] [Reference Citation Analysis (0)] |
| 2. | Blechacz B, Gores GJ. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 536] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 3. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 967] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 4. | Saxena M, Bhardwaj N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer. 2018;4:119-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | Wu YG, Wu GZ, Wang L, Zhang YY, Li Z, Li DC. Tumor cell lysate-pulsed dendritic cells induce a T cell response against colon cancer in vitro and in vivo. Med Oncol. 2010;27:736-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Lin M, Liang S, Jiang F, Xu J, Zhu W, Qian W, Hu Y, Zhou Z, Chen J, Niu L, Xu K, Lv Y. 2003-2013, a valuable study: Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in stage IV breast cancer. Immunol Lett. 2017;183:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Wang Q, Luan W, Warren L, Kadri H, Kim KW, Goz V, Blank S, Isabel Fiel M, Hiotis SP. Autologous Tumor Cell Lysate-Loaded Dendritic Cell Vaccine Inhibited Tumor Progression in an Orthotopic Murine Model for Hepatocellular Carcinoma. Ann Surg Oncol. 2016;23:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Junking M, Grainok J, Thepmalee C, Wongkham S, Yenchitsomanus PT. Enhanced cytotoxic activity of effector T-cells against cholangiocarcinoma by dendritic cells pulsed with pooled mRNA. Tumour Biol. 2017;39:1010428317733367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Lee YJ, Lee YM, Lee CK, Jung JK, Han SB, Hong JT. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther. 2011;130:157-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 342] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 10. | Chen HM, Wang PH, Chen SS, Wen CC, Chen YH, Yang WC, Yang NS. Shikonin induces immunogenic cell death in tumor cells and enhances dendritic cell-based cancer vaccine. Cancer Immunol Immunother. 2012;61:1989-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW, Agostinis P. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med. 2016;8:328ra27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 12. | Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, Simko JP, Waldman FM, Pieper RO, Parsa AT. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1434] [Cited by in RCA: 2235] [Article Influence: 171.9] [Reference Citation Analysis (0)] |
| 14. | Kobayashi M, Sakabe T, Abe H, Tanii M, Takahashi H, Chiba A, Yanagida E, Shibamoto Y, Ogasawara M, Tsujitani S, Koido S, Nagai K, Shimodaira S, Okamoto M, Yonemitsu Y, Suzuki N, Nagaya M; DC-vaccine study group at the Japan Society of Innovative Cell Therapy (J-SICT). Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg. 2013;17:1609-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Shimizu K, Kotera Y, Aruga A, Takeshita N, Takasaki K, Yamamoto M. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 17. | Lu CH, Chen SH, Chang YS, Liu YW, Wu JY, Lim YP, Yu HI, Lee YR. Honokiol, a potential therapeutic agent, induces cell cycle arrest and program cell death in vitro and in vivo in human thyroid cancer cells. Pharmacol Res. 2017;115:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Lin CJ, Chen TL, Tseng YY, Wu GJ, Hsieh MH, Lin YW, Chen RM. Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol Appl Pharmacol. 2016;304:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Li L, Han W, Gu Y, Qiu S, Lu Q, Jin J, Luo J, Hu X. Honokiol induces a necrotic cell death through the mitochondrial permeability transition pore. Cancer Res. 2007;67:4894-4903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | VanPatten S, Al-Abed Y. High Mobility Group Box-1 (HMGb1): Current Wisdom and Advancement as a Potential Drug Target. J Med Chem. 2018;61:5093-5107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Bianchi ME. HMGB1 loves company. J Leukoc Biol. 2009;86:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 22. | Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, André F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2084] [Cited by in RCA: 2416] [Article Influence: 134.2] [Reference Citation Analysis (0)] |
| 23. | Shirota T, Ojima H, Hiraoka N, Shimada K, Rokutan H, Arai Y, Kanai Y, Miyagawa S, Shibata T. Heat Shock Protein 90 Is a Potential Therapeutic Target in Cholangiocarcinoma. Mol Cancer Ther. 2015;14:1985-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Constantino J, Gomes C, Falcão A, Cruz MT, Neves BM. Antitumor dendritic cell-based vaccines: Lessons from 20 years of clinical trials and future perspectives. Transl Res. 2016;168:74-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4895] [Cited by in RCA: 4819] [Article Influence: 192.8] [Reference Citation Analysis (0)] |
| 26. | Uthaisar K, Vaeteewoottacharn K, Seubwai W, Talabnin C, Sawanyawisuth K, Obchoei S, Kraiklang R, Okada S, Wongkham S. Establishment and characterization of a novel human cholangiocarcinoma cell line with high metastatic activity. Oncol Rep. 2016;36:1435-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Lin TJ, Lin HT, Chang WT, Mitapalli S P, Hsiao PW, Yin SY, Yang NS. Shikonin-enhanced cell immunogenicity of tumor vaccine is mediated by the differential effects of DAMP components. Mol Cancer. 2015;14:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 413] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 29. | Henry CJ, Ornelles DA, Mitchell LM, Brzoza-Lewis KL, Hiltbold EM. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J Immunol. 2008;181:8576-8584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 30. | Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 857] [Cited by in RCA: 832] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 31. | Yang DH, Park JS, Jin CJ, Kang HK, Nam JH, Rhee JH, Kim YK, Chung SY, Choi SJ, Kim HJ, Chung IJ, Lee JJ. The dysfunction and abnormal signaling pathway of dendritic cells loaded by tumor antigen can be overcome by neutralizing VEGF in multiple myeloma. Leuk Res. 2009;33:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Saenz R, Futalan D, Leutenez L, Eekhout F, Fecteau JF, Sundelius S, Sundqvist S, Larsson M, Hayashi T, Minev B, Carson D, Esener S, Messmer B, Messmer D. TLR4-dependent activation of dendritic cells by an HMGB1-derived peptide adjuvant. J Transl Med. 2014;12:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Liu SH, Lee WJ, Lai DW, Wu SM, Liu CY, Tien HR, Chiu CS, Peng YC, Jan YJ, Chao TH, Pan HC, Sheu ML. Honokiol confers immunogenicity by dictating calreticulin exposure, activating ER stress and inhibiting epithelial-to-mesenchymal transition. Mol Oncol. 2015;9:834-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Rubartelli A, Lotze MT. Inside, outside, upside down: Damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 458] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 35. | Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1194] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 36. | Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 37. | Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spísek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821-4833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 327] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 38. | Krysko O, Løve Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013;4:e631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |