Published online Jul 21, 2019. doi: 10.3748/wjg.v25.i27.3607
Peer-review started: March 19, 2019
First decision: April 30, 2019
Revised: May 5, 2019
Accepted: June 25, 2019
Article in press: June 26, 2019
Published online: July 21, 2019
Processing time: 122 Days and 10.6 Hours
Hepatocellular carcinoma (HCC) represents the sixteenth most frequent cancer in Argentina. The rise of new therapeutic modalities in intermediate-advanced HCC opens up a new paradigm for the treatment of HCC.
To describe real-life treatments performed in patients with intermediate-advanced HCC before the approval of new systemic options.
This longitudinal observational cohort study was conducted between 2009 and 2016 in 14 different regional hospitals from Argentina. Included subjects had intermediate-advanced Barcelona Clinic Liver Cancer (BCLC) HCC stages (BCLC B to D). Primary end point analyzed was survival, which was assessed for each BCLC stage from the date of treatment until last patient follow-up or death. Kaplan Meier survival curves and Cox regression analysis were performed, with hazard ratios (HR) calculations and 95% confidence intervals (95%CI).
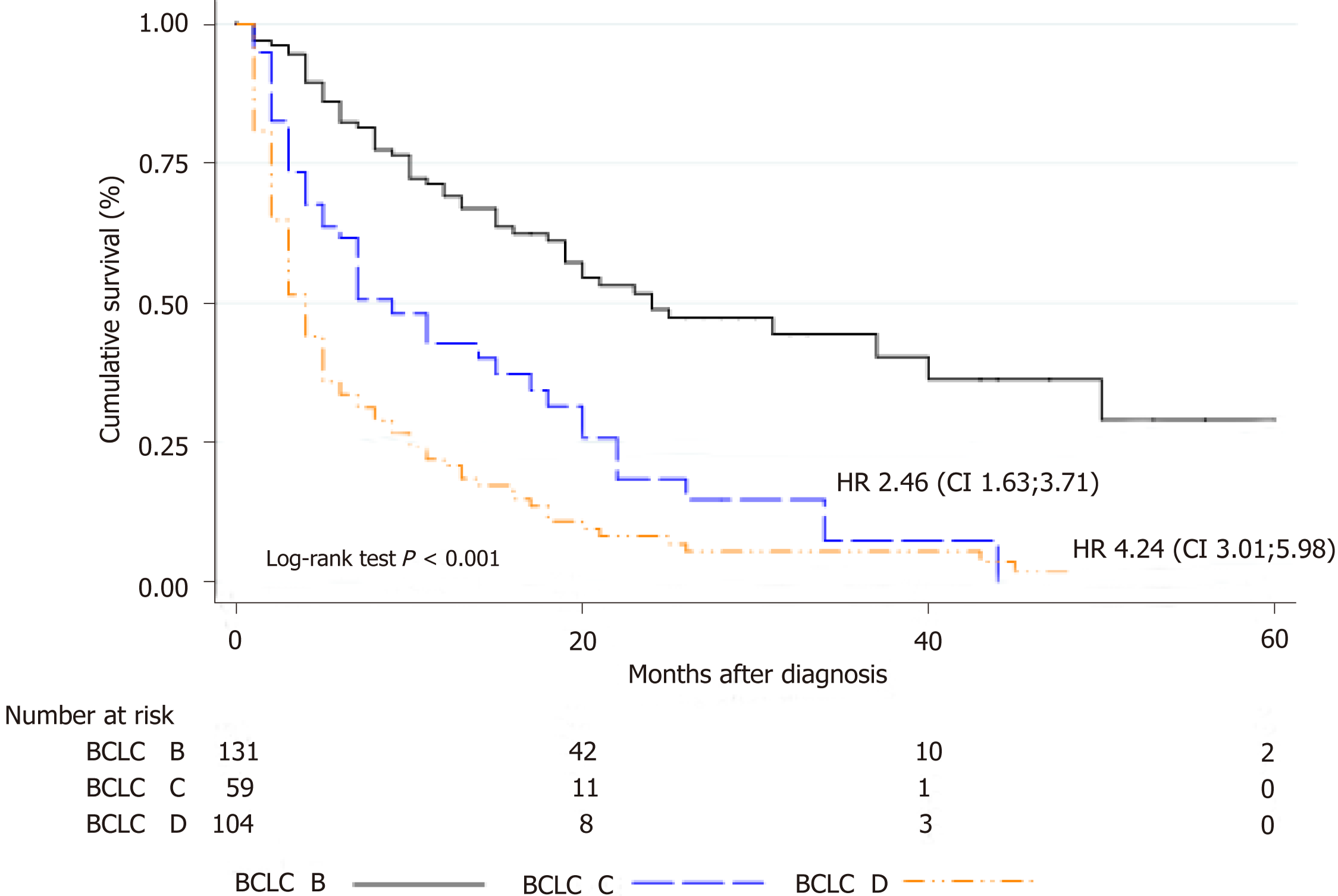
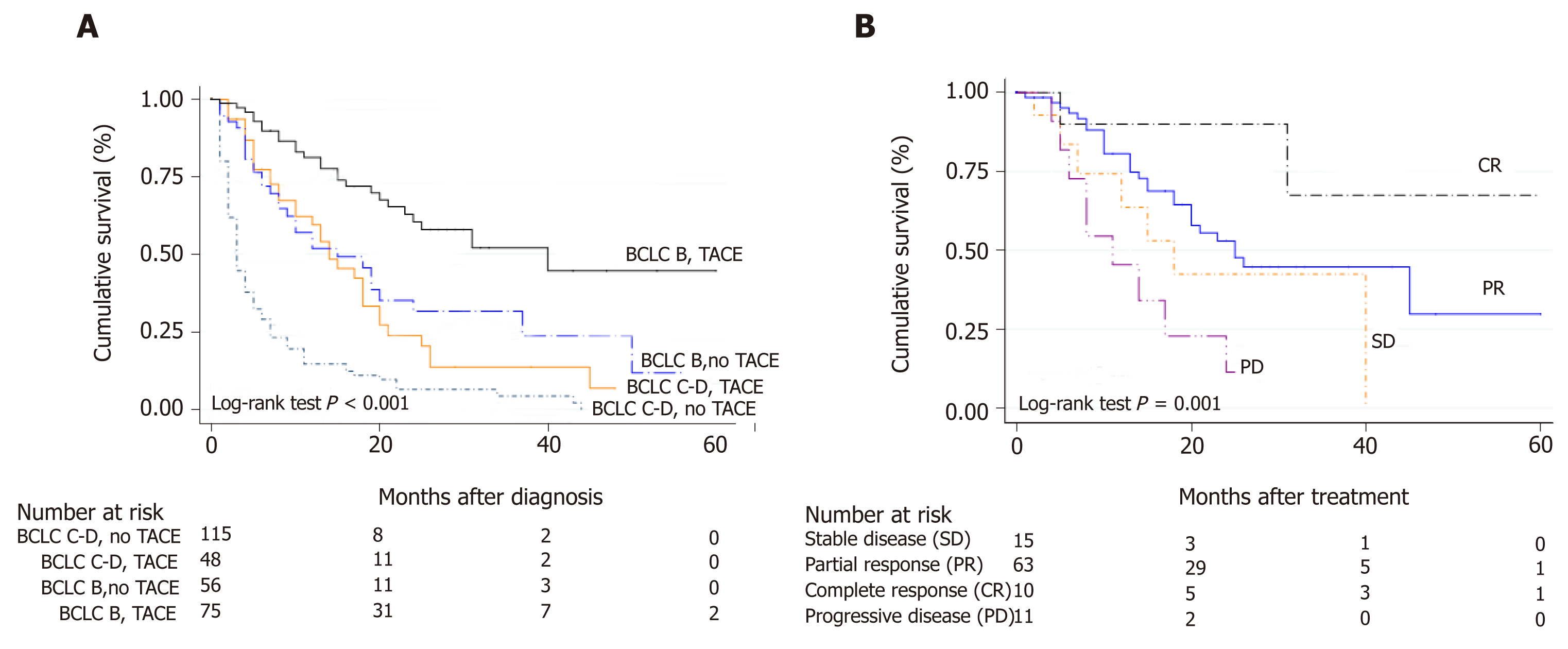
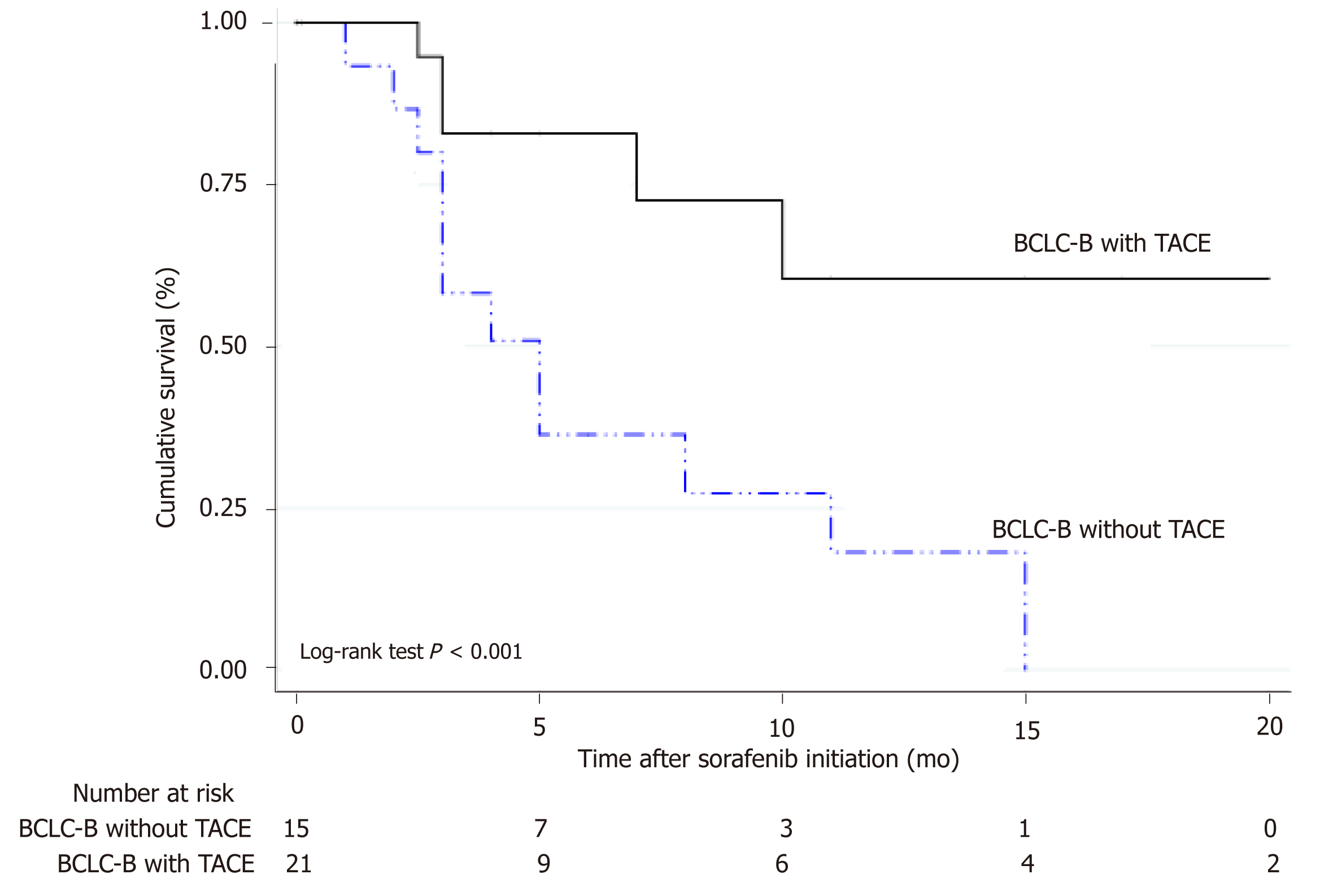
From 327 HCC patients, 41% were BCLC stage B, 20% stage C and 39% stage D. Corresponding median survival were 15 mo (IQR 5-26 mo), 5 mo (IQR 2-13 mo) and 3 mo (IQR 1-13 mo) (P < 0.0001), respectively. Among BCLC-B patients (n = 135), 57% received TACE with a median number of 2 sessions (IQR 1-3 sessions). Survival was significantly better in BCLC-B patients treated with TACE HR = 0.29 (CI: 0.21-0.40) than those without TACE. After tumor reassessment by RECIST 1.1 criteria following the first TACE, patients with complete response achieved longer survival [HR = 0.15 (CI: 0.04-0.56, P = 0.005)]. Eighty-two patients were treated with sorafenib, mostly BCLC-B and C (87.8%). However, 12.2% were BCLC-D. Median survival with sorafenib was 4.5 mo (IQR 2.3-11.7 mo); which was lower among BCLC-D patients 3.2 mo (IQR 2.0-14.1 mo). A total of 36 BCLC-B patients presented tumor progression after TACE. In these patients, treatment with sorafenib presented better survival when compared to those patients who received sorafenib without prior TACE [HR = 0.26 (CI: 0.09-0.71); P = 0.013].
In this real setting, our results were lower than expected. This highlights unmet needs in Argentina, prior to the introduction of new treatments for HCC.
Core tip: Trans-arterial chemoembolization and systemic treatment with sorafenib or lenvatinib are the standards of treatment for patients with intermediate and advanced stage hepatocellular carcinoma (HCC). The rise of new current therapeutic modalities such as radioembolization, the combination of antiangiogenic agents with locoregional therapies and other first and second line systemic options, open up a new paradigm for the treatment of HCC. In this dual cohort study, we describe the treatments performed in the real life setting before the approval of these new systemic options. Our real-data outcomes, lower than expected, highlight unmet needs and improvement areas in the daily practice prior to the introduction of new treatments for HCC.
- Citation: Piñero F, Marciano S, Fernández N, Silva J, Anders M, Zerega A, Ridruejo E, Romero G, Ameigeiras B, D’Amico C, Gaite L, Bermúdez C, Reggiardo V, Colombato L, Gadano A, Silva M. Intermediate-advanced hepatocellular carcinoma in Argentina: Treatment and survival analysis. World J Gastroenterol 2019; 25(27): 3607-3618
- URL: https://www.wjgnet.com/1007-9327/full/v25/i27/3607.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i27.3607
According to the latest estimates made by the International Agency for Research on Cancer [IARC (http://gco.iarc.fr)] for the year 2018, Argentina has an incidence rate of 212 cases per 100000 inhabitants[1]. This figure places it within the countries of the world with medium-high incidence of cancer (range 177 to 245.6 per 100000 inhabitants) and in seventh place in Latin America. Although liver cancer or hepa-tocellular carcinoma (HCC) is currently the 5th most common cancer and the 2nd cause of death from cancer worldwide, in Argentina represents the sixteenth most frequent cancer (http://www.argentina.gob.ar/salud/instituto-nacional-del-cancer)[1].
Given that in more than 90% of the cases this tumor develops in patients with cirrhosis or chronic infection with hepatitis B or C virus, the diagnostic, staging and therapeutic management in our country is mainly done by hepatologists or hepato-biliary surgeons, rather than clinical oncologists[2].
Transarterial chemoembolization (TACE) and systemic treatment with sorafenib or lenvatinib are the standard treatments for patients with intermediate and advanced stage HCC[3-5]. The rise of new therapeutic modalities such as radioembolization, the combination of antiangiogenic agents with locoregional therapies and other first and second line systemic options, open up a new paradigm for the treatment of HCC.
In this dual cohort study, we aimed to describe treatments performed in the real life setting before the approval of these new systemic options. It is of interest to know the real life context, in order to evaluate the therapeutic management in these patients and gaps that should be explored more thoroughly as areas of public health impro-vement.
This longitudinal observational cohort study was conducted in 14 different regional hospitals from Argentina. Two cohorts of consecutive adult patients (> 17 years of age) with newly diagnosed HCC were included. Between January 1 2009 and September 1 2014, a retrospective cohort was followed-up until death or last ambulatory visit until January 1 2016 (Cohort 1). A second prospective cohort was included from September 2 2014, followed until January 1 2016 (Cohort 2). Parti-cipating centers appointed a study coordinator responsible for data collection. Sites were instructed to enroll all eligible patients on a sequential basis and to record data from medical charts into a web-based electronic system. In cases of conflicting or missing data, central revision and resubmission was requested.
Patients with intermediate (BCLC-B) or advanced-end stage (BCLC C-D) HCC were included[6,7]. Criteria for inclusion required patients to be adult recipients with newly diagnosed HCC either by pathological criteria or imaging evaluation as recomm-ended by international Western guidelines[6,7]. Intermediate stage or BCLC B includes patients with preserved liver function with multifocal tumors, in the absence of cancer related symptoms, vascular invasion or extrahepatic spread. In these patients the recommended treatment is TACE. Advanced-stage HCC (BCLC C) comprises patients with preserved liver function, good performance status or ECOG 1-2, with extrahepatic spread (lymph node involvement or metastases) or vascular invasion. In this subgroup, sorafenib or lenvatinib are the recommended treatments. As lenvatinib has been recently approved in our country (March 2019), this cohort includes patients treated under sorafenib. Best supportive care (BSC) or symptomatic treatment is recommended for patients with unpreserved liver function (Child Pugh C) or ECOG > 2 or cancer related symptoms[8]. Patients were excluded if (1) clinical baseline data was missing; (2) BCLC stage was either 0 or A; and (3) patients with BCLC-B-D who underwent liver transplantation.
Baseline characteristics at HCC diagnosis included patients demographics, performance status (ECOG grade 0-4), grade of liver fibrosis (I-IV) assessed by liver biopsy or elastography or other non-invasive measurements or by clinical data (including imaging data, presence of gastro-esophageal varices or ascites or spleno-megaly > 120 mm diameter, or other complications related to portal hypertension), Child Pugh score; selected laboratory variables, serum alpha-fetoprotein (AFP) levels and tumor characteristics at diagnosis, as well as treatments performed. Computed tomography (CT) or magnetic resonance images (MRI) were evaluated considering tumor number and diameter, vascular invasion and extrahepatic or lymph node metastasis.
Tumor treatment after HCC diagnosis was reviewed, namely: Liver resection (LR), radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), trans-arterial chemoembolization (TACE), trans-arterial radioembolization (TARE), sorafenib and best supportive care (BSC). Each treatment was discussed at each center on a case-by-case basis. Imaging tumor reassessment after treatments were done according to RECIST 1.1 criteria as recommended by international Western guidelines[6,7].
Primary end point analyzed was survival, which was assessed for each BCLC stage from the date of treatment until last patient follow-up or death. Secondary objectives were to (1) describe treatments performed in each BCLC stage; (2) to evaluate the sequential treatment of TACE-sorafenib in BCLC-B patients; and (3) to evaluate adverse events and tolerability of sorafenib in the daily practice.
All procedures followed were in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement[9]. This study was approved by the Austral University School of Medicine and by each center; complied with the ethical standards (institutional and national) and with Helsinki Declaration of 1975, as revised in 2008.
Statistical significance is expressed as P < 0.05. Categorical data were compared using Fisher’s exact test or Chi-Square test. Continuous variables were compared with Student’s T test or Mann-Whitney U test according to their distribution, respectively. Multiple comparisons for continuous data were done according to its distribution with ANOVA or Kruskal Wallis tests as appropriate. Dummies for ordinal variables were assessed. For survival analysis, Cox regression analysis estimating hazard ratios (HR) and 95%CI for baseline variables related with mortality was performed. Proportional hazards through graphic and statistical evaluation (Schoenfeld residual test) were done. Kaplan Meier survival curves were compared using the log-rank test (Mantel-Cox) Collected data was analyzed using STATA 13.0.
From a total of 721 consecutive adult patients with HCC during the study period, 327 patients with newly diagnosed intermediate and advanced HCC were included. Patients who received a liver transplant in BCLC-B (n = 16), BCLC-C (n = 2) and BCLC-D (n = 28) were excluded.
Table 1 describes the main baseline patient characteristics. Overall, 41.3% of the patients were in BCLC stage B (n = 135), 19.9% in stage C (n = 65) and 38.8% in stage D (n = 127). Treatments performed during the whole follow-up period were LR (n = 36), RFA or PEI (n = 19), TACE (n = 126), TARE (n = 6), sorafenib (n = 82) and BSC (n = 146).
| Variable | Values |
| Age, yr (± SD) | 63 ± 10 |
| Male gender, n (%) | 265 (81.3) |
| Non-cirrhotic liver, n (%) | 41 (12.5) |
| Child Pugh A/B/C, n (%) | 137 (42)/98 (30)/92 (28) |
| Etiology of liver disease, n (%) | |
| Hepatitis C virus | 99 (30.3) |
| Alcohol | 77 (23.5) |
| NASH | 35 (10.7) |
| Cryptogenic | 37 (11.3) |
| Hepatitis B virus | 22 (6.7) |
| Cholestatic1 | 4 (1.2) |
| Autoimmune | - |
| Hemochromatosis | 6 (1.8) |
| Miscellaneous | 36 (11.0) |
| Comorbidities, n (%) | 141 (43.1) |
| Diabetes mellitus, n (%) | 84 (25.7) |
| Ascites, n (%) | |
| Mild | 76 (23.3) |
| Moderate-severe | 77 (23.5) |
| Encephalopathy, n (%) | |
| Grade I-II | 78 (23.8) |
| Grade III-IV | 6 (1.8) |
| CSPH, n (%) | 212 (64.8) |
| ECOG 0-2, n (%) | 262 (80.1) |
Outcomes were assessed in all patients during follow-up with a median survival of 12.0 mo (IQR 4.0-27.0 mo). Corresponding median survival for BCLC stages were as follows: stage B 15 mo (IQR 5-26 mo), stage C 5 mo (IQR 2-13 mo) and stage D 3 mo (IQR 1-13 mo)(Figure 1).
TACE was performed in 126 patients (38.5%); 77 were BCLC-B, 22 were BCLC-C and 27 patients were BCLC-D. According to the type of endovascular treatment, 43.6% of the patients were treated with conventional TACE (cTACE), 45.2% with TACE with drug eluting beads (TACE-DCbeads) and 11.2% with transarterial embolization (TAE).
Among BCLC-B patients (n = 135), 57% received TACE (n = 77) whereas 43% did not (Table 2). Median number of TACEs sessions was 2 (IQR 1-3 sessions); 40%, 26% and 34% of these patients received 1, 2 and 3 or more sessions, respectively. Other treatments than TACE were performed in BCLC-B patients, as follows: RFA or PEI in 7 patients, liver resection in 21 patients, sorafenib in 15 patients and BSC in 5 patients.
| Variable | BCLC stage B overall (n = 135) | BCLC stage B with TACE (n = 77) | BCLC stage B without TACE (n = 58) | P value |
| Age, yr (± SD) | 65 ± 10 | 65 ± 8 | 65 ± 11 | 0.86 |
| Male gender, n (%) | 111 (82.2) | 68 (88.3) | 43 (74.1) | 0.03 |
| Non-cirrhotic liver, n (%) | 23 (17.0) | 7 (9.1) | 16 (27.6) | 0.006 |
| Etiology, n (%) | 0.11 | |||
| HCV | 42 (31.1) | 25 (32.5) | 17 (29.3) | |
| HBV | 8 (5.9) | 5 (6.5) | 3 (5.2) | |
| Alcohol | 29 (21.5) | 21 (27.3) | 8 (13.8) | |
| Etiology, nNASH | 14 (10.4) | 6 (7.8) | 8 (13.8) | |
| Etiology, nOthers | 42 (31.1) | 20 (25.9) | 22 (37.9) | |
| Child Pugh A/B, n (%) | 88 (65.2)/47 (34.8) | 48 (62.3)/29 (37.7) | 40 (69.0)/18 (31.0) | 0.42 |
| CSPH1, n (%) | 67 (49.6) | 45 (58.4) | 22 (37.9) | 0.018 |
| Median nº HCC nodules (IQR) | 2 (2-3) | 2 (1-3) | 2 (1-4) | 0.39 |
| Median largest HCC diameter, mm, (IQR) | 65 (43-100) | 60 (43-88) | 69.5 (45-114.5) | 0.11 |
| Bilobar involvement, n (%) | 53 (39.3) | 30 (39.0) | 23 (39.7) | 0.72 |
| Diffuse HCC pattern, n (%) | 5 (3.7) | 2 (2.6) | 3 (5.2) | 0.72 |
| Median AFP, ng/mL (IQR) | 26.7 (4.7-248.5) | 27.5 (5.1-202.85) | 24.4 (4.3-285) | 0.91 |
| AFP > 200 ng/mL, n (%) | 36 (27.3) | 19 (25.0) | 17 (30.4) | 0.49 |
| AFP > 400 ng/mL, n (%) | 30 (22.7) | 17 (22.4) | 13 (23.2) | 0.91 |
| AFP > 1000 ng/mL, n (%) | 18 (13.6) | 11 (14.5) | 7 (12.5) | 0.74 |
| Vascular invasion, n (%) | - | |||
| Extrahepatic disease, n (%) | - |
Of the 22 BCLC-C patients who were treated with TACE, 13 had non-main portal trunk vascular invasion and 12 patients had extrahepatic disease (lymph node metastasis in 5, bone metastasis in 3 and 4 patients with lung involvement). Sorafenib was the following treatment performed in 7 patients. Among BCLC-D, 27 patients received TACE, 19 were Child Pugh C, 10 patients presented performance status ECOG 3-4, 2 patients presented non-main portal trunk vascular invasion and 1 had extrahepatic disease (lymph node metastasis). Best supportive care following TACE was done in all patients except for 1 who received sorafenib in this latter group.
Survival was significantly better in BCLC-B patients treated with TACE HR 0.29 (CI: 0.21-0.40) with a median survival of 15 mo (IQR 7-25 mo), when compared with BCLC-B without TACE and BCLC-C or D patients treated with TACE (Figure 2A). According to tumor reassessment after the first TACE, patients with complete response (CR) achieved a better overall survival with a HR of 0.15 (CI: 0.04-0.56. P = 0.005) (Figure 2B).
Table 3 describes baseline patient characteristic treated with sorafenib (n = 82). Of these, 43.9% were BCLC-B, 43.9% BCLC-C and 12.2% BCLC-D. Among BCLC-B, 15 were TACE naïve and 21 received a median number of 3 TACE sessions (IQR 2-4 sessions) until disease progression (n = 7) or no response (n = 14). Among BCLC-C patients (n = 65), 55.4% were treated with sorafenib, 21 received BSC and 8 patients received other treatments (4 patients TACE, 1 TARE and patients 3 LR).
| Variable | Overall (n = 82) | BCLC stage B (n = 36) | BCLC stage C (n = 36) | BCLC stage D (n = 10) | P value |
| Age, yr (± SD) | 63 ± 9 | 63 ± 8 | 62 ± 10 | 63 ± 8 | 0.86 |
| Male gender, n (%) | 68 (82.9) | 29 (80.6) | 31 (86.1) | 8 (80.0) | 0.88 |
| Non-cirrhotic liver, n (%) | 9 (11.0) | 3 (8.3) | 5 (13.9) | 1 (10.0) | 0.78 |
| Etiology, n (%) | 0.11 | ||||
| HCV | 28 (34.1) | 16 (44.4) | 11 (30.6) | 1 (10.0) | |
| HBV | 4 (4.9) | 2 (5.6) | 1 (2.8) | 1 (10.0) | |
| Alcohol | 18 (21.9) | 9 (25.0) | 8 (22.2) | 1 (10.0) | |
| NASH | 10 (12.2) | 4 (11.1) | 4 (11.1) | 2 (20.0) | |
| Others | 22 (26.8) | 5 (13.9) | 12 (33.3) | 5 (50.0) | |
| Child Pugh A/B/C, n (%) | 48 (58)/30 (37)/4 (5) | 25 (69)/11 (31)/- | 21 (58)/15 (42)/- | 2 (20)/4 (40)/4 (40) | |
| CSPH1, n (%) | 45 (54.9) | 21 (58.3) | 18 (50.0) | 6 (60.0) | 0.66 |
| Median nº HCC nodules (IQR)2 | 2 (1-4) | 2 (2-4) | 2 (1-4) | 1.5 (1-2) | 0.25 |
| Median largest HCC diameter, mm, (IQR)2 | 70 (47-100) | 65 (46-90) | 87 (48.5-130) | 57.5 (39-121) | 0.56 |
| Bilobar involvement, n (%) | 30 (37.0) | 10 (27.8) | 18 (50.0) | 3 (30.0) | 0.33 |
| Diffuse HCC pattern, n (%) | 6 (7.4) | 2 (5.6) | 3 (8.3) | 1 (10.0) | 0.35 |
| Median AFP, ng/mL (IQR) | 103 (7.0-1069) | 30 (7.2-739) | 150 (6.3-1210) | 649 (16-2198) | 0.26 |
| AFP > 200 ng/mL, n (%) | 35 (43.7) | 12 (34.3) | 16 (45.7) | 7 (70) | 0.13 |
| AFP > 400 ng/mL, n (%) | 30 (37.5) | 10 (28.6) | 13 (37.1) | 7 (70) | 0.06 |
| AFP > 1000 ng/mL, n (%) | 21 (25.9) | 5 (14.3) | 12 (33.3) | 4 (40) | 0.13 |
| Vascular invasion, n (%) | 27 (33.3) | - | 25 (69.4) | 3 (30.0) | < 0.0001 |
| Extrahepatic disease, n (%) | 19 (23.5) | - | 18 (50.0) | 1 (10.0) | < 0.0001 |
Median sorafenib treatment duration was 4.0 mo (IQR 2-11 mo). The most frequent sorafenib starting dose was 400 mg/d in 41% of the patients, followed by 800 mg/d in 32%. During follow-up, 55% of the patients achieved 800 mg full-dose of treatment, 35.4% had dose reductions (n = 29) of which in 21 patients dose-reduction were associated with drug-related adverse events. Most frequent adverse events (AEs) were fatigue (n = 27), diarrhea (n = 16), dermatologic events (n = 5), hand-foot-skin reaction (n = 3), and hypertension (n = 1). Permanent treatment discontinuation was observed in 12.2% of the patients secondary to treatment AEs (n = 10), tumor progression in 26.8%, (n = 22) and death in the rest of the patients. In 37 out of 82 patients in which radiologic evaluation after sorafenib initiation was performed, complete and partial responses were observed in 1.2% (n = 1) and 2.4% (n = 2), respectively. In these subgroup, median time to progression since sorafenib initiation was 7.3 mo (IQR 2.1-10.7 mo).
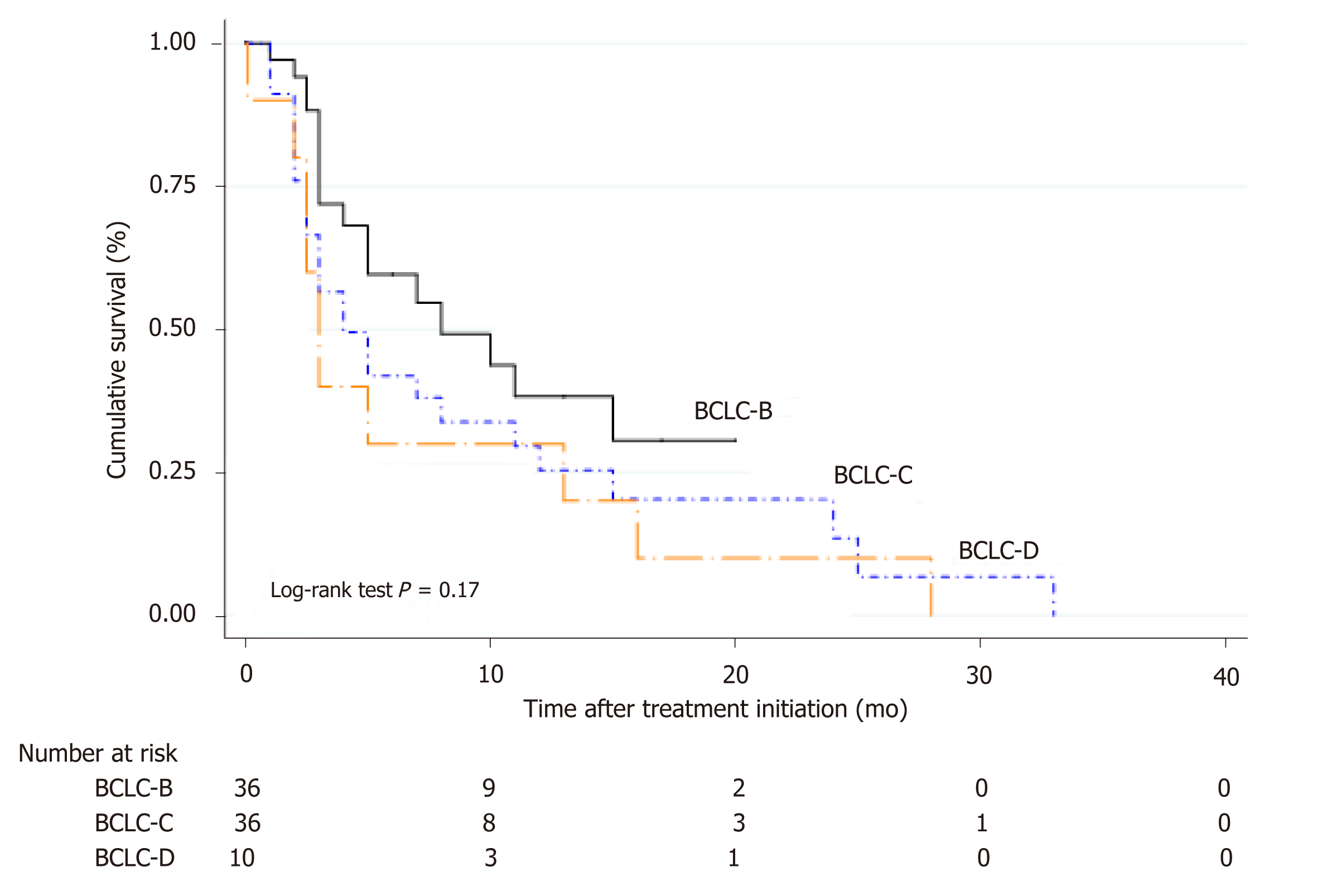
Corresponding median survival in all patients treated with sorafenib was 4.5 mo (IQR 2.3-11.7 mo); 5.2 mo (IQR 3.7-12.6 mo) in BCLC-B, 3.8 mo (IQR 1.9-9.9 mo) in BCLC-C and 3.2 mo (IQR 2.0-14.1 mo) in BCLC-D (Figure 3). When comparing BCLC-B and C vs BCLC-D treated patients, although it did not reach statistical significance, a better survival curve was observed in BCLC-B/C patients with a HR of 0.63 (CI: 0.31-1.27; P = 0.19).
Imaging evaluation after the first TACE in BCLC-B patients was registered in 64 out of 77 patients in median time from TACE to evaluation of 5 wk (IQR 4-6 wk). According to RECIST 1.1 criteria tumor response was as follows: partial response in 62.5% (n = 40), stable disease 15.6% (n = 10), complete response in 12.5% (n = 8), and disease progression in 9.3% (n = 6). Thus, overall objective response (ORR) and disease control rates (DCR) were 75% and 90.7% after first TACE, respectively.
In BCLC-B patients treated with sorafenib after progression (n = 36), the sequential treatment of sorafenib following TACE presented better survival when compared to those patients who received sorafenib without prior treatment with TACE [HR = 0.26 (CI: 0.09-0.71); P = 0.013] (Figure 4). Median number of TACEs in these patients prior to systemic treatment was 3 sessions (IQR 2-4 sessions). Among those patients not treated with TACE prior to sorafenib initiation, prior treatments were RFA/PEI (n = 4) and LR (n = 5).
This is the first observational study of treatments performed in the real life setting from Argentina in patients with intermediate to advanced stage HCC and one of the only ones to report post-treatment survival in Latin America. Knowing the real life treatment patterns is of interest to highlight unmet needs in the daily practice prior to the introduction of new treatments for HCC.
In this cohort we observed that in the majority of patients in intermediate stage, the most frequent treatment in daily practice was TACE. The effect on survival was beneficial in these patients in particular when treatment was established in accor-dance with Western clinical practice recommendations[6,7]. In patients with unpreserved liver function or BCLC-C, TACE was performed in a smaller proportion with heterogeneous effect on survival. On the other hand, those patients in BCLC-B stage with complete tumor response after TACE showed a better survival. Likewise, a non-negligible proportion of BCLC-B patients started sorafenib in the absence of prior TACE as a decision of “treatment stage migration”[10]. In the era of sequential treatment recommendation, in those BCLC-B patients with tumor progression after TACE[11], a better survival with sorafenib was observed with respect to those patients without prior TACE.
Knowing the therapeutic decisions in the daily practice is important because it reflects the gaps between interventional studies evaluating efficacy in ideal situations and those in the real-life setting. The BRIDGE study is an example, among others, of how therapeutic decisions in patients with HCC are complex, demanding a fine knowledge not only of tumor extension, but also of liver disease and its complica-tions[12]. That is why the role of hepatologists is of utmost importance in the treatment of these patients. In our cohort, most of the patients were screened, diagnosed and treated by hepatologists, both in referral or local centers.
Treatment with TACE has been established as the gold standard for intermediate stage HCC since more than 10 years ago[3,4]. Two randomized, placebo-controlled trials have shown its survival benefit[3,4]; results further underlined in a meta-analysis[13]. However, clinical and tumor heterogeneity, which are characteristic of BCLC-B patients, results in a diversity of established treatments[12]. In our analysis, we excluded BCLC-B patients who underwent transplantation, given that we considered performing a pure analysis in this stage. The same went for BCLC-D patients. In the original trials of TACE, a median survival was close to eighteen months[3,4,14] whereas in more recent observational studies, median survival of forty months has been reported[15]. In our study, median survival in BCLC-B patients treated with TACE was fifteen months. Survival was significantly better in BCLC-B patients treated with TACE with a 71% relative risk reduction of death when compared with BCLC-B without TACE and BCLC-C or D patients treated with TACE. According to tumor reassessment by RECIST 1.1 criteria after the first TACE, patients with complete response had the highest survival benefit, as previously reported elsewhere[16].
Systemic treatment of HCC is remarkably changing given the introduction of alternative therapies in first line such as lenvatinib[17] and second-line including regorafenib[18], cabozantinib[19] and ramucirumab[20]. In our country, as in many others from Latin America, approval of these new treatments usually takes between 12 and 24 mo later than other developed regions of the world. In 2009 and 2017, sorafenib and regorafenib were approved in our country, respectively. Recently, the use of lenvatinib has also been approved, not yet included in the daily practice. Treatment with immunotherapy, either with nivolumab[21] or pembrolizumab[22], has not been approved by the National Regulatory Agency in our country (ANMAT).
Argentina is a South American country with a wide extension, a great socio-cultural heterogeneity with a large variety in health care systems. In many cases, the main barrier for the access to health system is the authorization by insurances to carry out diagnostic studies or therapies due to costs or other barriers. This problem is common in Latin America[23]. In this study, the use of sorafenib slightly exceeded half of BCLC-C patients, presenting better survival when compared with those patients in the same stage but without systemic treatment. We observed that in our cohort, median survival with the use of sorafenib was strikingly low, being no more than 5 months. This lower than expected outcome can be explained, in part, by the delay in starting treatment, due to a wide range of authorizations and complex administrative processes. This might have leaded to a significant slowness in the initiation of systemic treatment. Moreover, most of the patients were initially treated with half dose rather than full dose.
It is noteworthy of mention that sorafenib tolerance was similar to that reported from first (SHARP and Asia-Pacific)[5,24] and second line (RESORCE)[18] clinical trials, with a rate of definitive treatment discontinuation due to related adverse events of 12.2%. On the other hand, in those patients in whom radiological response was evaluated, median time to progression under treatment with sorafenib was similar than that previously reported[18]. Finally, we observed that there was an inadequate use of sorafenib in patients with unpreserved liver function or BCLC-D that was associated with a poor prognosis, demonstrating an inadequate and inefficiency use of resources.
Our study has limitations. In particular, given that it was mainly a retrospective cohort study, exposed to different selection and information biases. Specifically, neither radiological evaluation assessing time to progression was homogeneous nor there was a centrally blinded evaluation through all participating centers. However, we enrolled a group of centers presenting similar decision making processes trying to homogenize the sample.
In conclusion, in this dual cohort study from Argentina, we described the treatme-nts performed in the real life setting before the approval of new systemic options. Knowing this life context is of interest, in order to assess the most common therap-eutic decision making processes and management in these patients. In this real setting, our results highlights unmet needs and improvement areas in public health among developing regions, particularly to promote early and correct treatments in each stage, prior to the introduction of new treatments for HCC.
Although liver cancer or hepatocellular carcinoma (HCC) is currently the 5th most common cancer and the 2nd cause of death from cancer worldwide, in Argentina represents the sixteenth most frequent cancer. Transarterial chemoembolization (TACE) and systemic treatment with sorafenib are the standards of treatment for patients with intermediate and advanced stage HCC.
The rise of new therapeutic modalities such as radioembolization, the combination of antian-giogenic agents with locoregional therapies and other first and second line systemic options, open up a new paradigm for the treatment of HCC.
Our aim was to describe the treatments performed in the real life setting before the approval of these new systemic options.
This longitudinal observational cohort study was conducted between in 14 different regional hospitals from Argentina between 2009 and 2016. Study data were registered into a web-based electronic system. Patients with intermediate (BCLC-B) or advanced (BCLC C-D) HCC were included. Patients were excluded if (1) clinical baseline data was missing; (2) BCLC stage was either 0 or A, in which potentially curative treatments are recommended such as liver resection (LR), percutaneous ethanol injection (PEI)/radiofrequency ablation (RFA) or liver transpla-ntation (LT); and (3) patients with BCLC-B-D who underwent liver transplantation. Baseline tumor and patients characteristics at HCC diagnosis, as well as treatments performed were registered. Each treatment was discussed at each center on a case-by-case basis. Imaging tumor reassessment after treatments were done according to RECIST 1.1 criteria as recommended by international Western guidelines. Median survival was assessed for each BCLC stage from the date of treatment until last patient follow-up or death. For survival analysis, Cox regression analysis estimating hazard ratios (HR) and 95%CI for baseline variables related with mortality was performed. Kaplan Meier survival curves were compared using the log-rank test (Mantel-Cox).
A total of 327 consecutive adult patients with intermediate and advanced HCC were included, of which 41.3% of the patients were in BCLC stage B (n = 135), 19.9% in stage C (n = 65) and 38.8% in stage D (n = 127). Corresponding median survival for BCLC stages were as follows: Stage B 15 mo (IQR 5-26 mo), stage C 5 mo (IQR 2-13 mo) and stage D 3 mo (IQR 1-13 mo)(Figure 1). TACE was performed in 126 patients (38.5%); 77 were BCLC-B, 22 were BCLC-C and 27 patients were BCLC-C. Among BCLC-B patients (n = 135), 57% received TACE (n = 77) whereas 43% did not (Table 2). Median number of TACEs sessions was 2 (IQR 1-3 sessions). Survival was significantly better in BCLC-B patients treated with TACE HR 0.29 (CI: 0.21-0.40) with a median survival of 15 mo (IQR 7-25 mo), when compared with BCLC-B without TACE and BCLC-C or D patients treated with TACE. According to tumor reassessment after the first TACE by RECIST 1.1 criteria, patients with complete response (CR) achieved a better overall survival with a HR of 0.15 (CI: 0.04-0.56, P = 0.005). Table 3 describes baseline patient characteristic treated with sorafenib (n = 82). Of these, 43.9% were BCLC-B, 43.9% BCLC-C and 12.2% BCLC-D. Among BCLC-B patients who received sorafenib, 15 were TACE naïve and 21 received a median number of TACEs of 3 (IQR 2-4) until disease progression (n = 7) or no response or un-TACE-able (n = 14). Among BCLC-C patients (n = 65), 55.4% were treated with sorafenib and those not treated with sorafenib received BSC (n = 21) and other treatments (4 patients TACE, 1 TARE and patients 3 LR). Corresponding median survival in all patients treated with sorafenib was 4.5 mo (IQR 2.3-11.7 mo); 5.2 mo (IQR 3.7-12.6 mo) in BCLC-B, 3.8 mo (IQR 1.9-9.9 mo) in BCLC-C and 3.2 mo (IQR 2.0-14.1 mo) in BCLC-D. In BCLC-B patients treated with sorafenib after progression (n = 36), the sequential treatment of sorafenib following TACE presented better survival since systemic treatment when compared to those patients who received sorafenib without prior treatment with TACE [HR = 0.26 (CI: 0.09-0.71); P = 0.013].
In conclusion, in this dual cohort study from Argentina, we describe the treatments performed in the real life setting before the approval of new systemic options.
Knowing the real life setting is of interest, in order to assess the most common therapeutic decision making processes and management in these patients. Our results highlights unmet needs and improvement areas in public health among developing regions such as Argentina, particularly to promote early and correct treatments in each stage, prior to the introduction of new treatments for HCC.
On behalf of the Latin American Liver Research, Education and Awareness Network (LALREAN).
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hoyos S-Editor: Ma RY L-Editor: A E-Editor: Wang J
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Piñero F, Marciano S, Fernández N, Silva J, Zambelo Y, Cobos M, Zerega A, Ridruejo E, Miguez C, Ameigeiras B, D'Amico C, Gaite L, Coronel M, Bermúdez C, Rosales C, Romero G, McCormack L, Reggiardo V, Colombato L, Gadano A, Rubinstein F, Silva M; Argentinean Association for the Study of Liver Diseases (A. A.E.E.H). Adherence to Barcelona Clinic Liver Cancer therapeutic algorithm for hepatocellular carcinoma in the daily practice: a multicenter cohort study from Argentina. Eur J Gastroenterol Hepatol. 2018;30:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 4. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10268] [Article Influence: 604.0] [Reference Citation Analysis (2)] |
| 6. | European Association for the Study of the Liver; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6059] [Article Influence: 865.6] [Reference Citation Analysis (3)] |
| 7. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3028] [Article Influence: 432.6] [Reference Citation Analysis (3)] |
| 8. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1275] [Article Influence: 141.7] [Reference Citation Analysis (2)] |
| 9. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5754] [Cited by in RCA: 9944] [Article Influence: 584.9] [Reference Citation Analysis (0)] |
| 10. | Reig M, Darnell A, Forner A, Rimola J, Ayuso C, Bruix J. Systemic therapy for hepatocellular carcinoma: the issue of treatment stage migration and registration of progression using the BCLC-refined RECIST. Semin Liver Dis. 2014;34:444-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Hucke F, Sieghart W, Pinter M, Graziadei I, Vogel W, Müller C, Heinzl H, Waneck F, Trauner M, Peck-Radosavljevic M. The ART-strategy: sequential assessment of the ART score predicts outcome of patients with hepatocellular carcinoma re-treated with TACE. J Hepatol. 2014;60:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 944] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 13. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2271] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 14. | Dunning JJ, Mullins PA, Scott JP, Solis E, Schofield PM, Large SR, Wallwork JW. Cardiac transplantation and cancer. Am J Cardiol. 1991;67:327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 15. | Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 16. | Choi J, Shim JH, Shin YM, Kim KM, Lim YS, Lee HC. Clinical significance of the best response during repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Hepatol. 2014;60:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3826] [Article Influence: 546.6] [Reference Citation Analysis (1)] |
| 18. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2712] [Article Influence: 339.0] [Reference Citation Analysis (0)] |
| 19. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1768] [Article Influence: 252.6] [Reference Citation Analysis (0)] |
| 20. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1248] [Article Influence: 208.0] [Reference Citation Analysis (0)] |
| 21. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3310] [Article Influence: 413.8] [Reference Citation Analysis (1)] |
| 22. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1897] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 23. | Piñero F, Poniachik J, Ridruejo E, Silva M. Hepatocellular carcinoma in Latin America: Diagnosis and treatment challenges. World J Gastroenterol. 2018;24:4224-4229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4650] [Article Influence: 273.5] [Reference Citation Analysis (0)] |