Published online Jul 21, 2019. doi: 10.3748/wjg.v25.i27.3590
Peer-review started: March 26, 2019
First decision: May 16, 2019
Revised: May 30, 2019
Accepted: June 8, 2019
Article in press: June 8, 2019
Published online: July 21, 2019
Processing time: 116 Days and 9.1 Hours
Obesity is a major risk factor for a variety of diseases such as diabetes, nonalcoholic fatty liver disease, and cardiovascular diseases. Restricting energy intake, or caloric restriction (CR), can reduce body weight and improve metabolic parameters in overweight or obese patients. We previously found that Lingguizhugan decoction (LZD) in combination with CR can effectively lower plasma lipid levels in patients with metabolic syndrome. However, the mechanism underlying CR and LZD treatment is still unclear.
To investigate whether CR and LZD improve metabolic parameters by modulating gut microbiota.
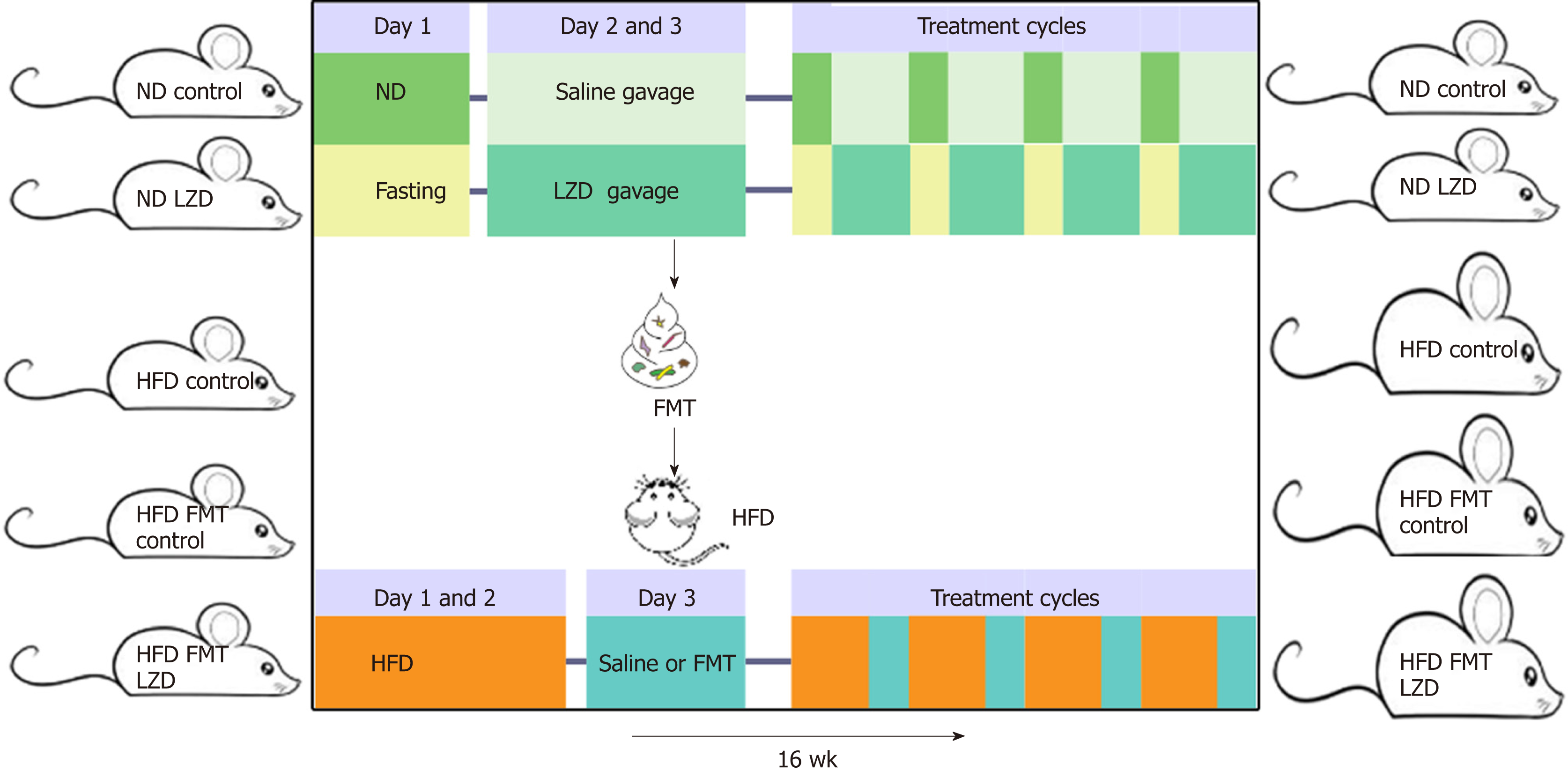
We extracted the water-soluble components out of raw materials and dried as LZD extracts. Eight-week old male C57BL/6 mice were treated with a 3-d treatment regime that included 24 h-fasting followed by gavage of LZD extracts for 2 consecutive days, followed by a normal diet (ND) ad libitum for 16 wk. To test the effects of gut microbiota on diet-induced obesity, 8-wk old male C57BL/6 mice received fecal microbiota transplantation (FMT) from CR and LZD-treated mice every 3 d and were fed with high-fat diet (HFD) ad libitum for 16 wk. Control mice received either saline gavage or FMT from ND-fed mice receiving saline gavage as mentioned above. Body weight was monitored bi-weekly. Food consumption of each cage hosting five mice was recorded weekly. To monitor blood glucose, total cholesterol, and total triglycerides, blood samples were collected via submandibular bleeding after 6 h fasting. Oxygen consumption rate was monitored with metabolic cages. Feces were collected, and fecal DNA was extracted. Profiles of gut microbiota were mapped by metagenomic sequencing.
We found that CR and LZD treatment significantly reduced the body weight of mice fed with ND (28.71 ± 0.29 vs 28.05 ± 0.15, P < 0.05), but did not affect plasma total cholesterol or total triglyceride levels. We then transplanted the fecal microbiota collected from CR and LZD-treated mice under ND feeding to HFD-fed mice. Intriguingly, transplanting the mice with fecal microbiota from CR and LZD-treated mice potently reduced body weight (44.95 ± 1.02 vs 40.53 ± 0.97, P < 0.001). FMT also reduced HFD-induced hepatosteatosis, in addition to improved glycemic control. Mechanistic studies found that FMT increased OCR of the mice and suppressed the expression and protein abundance of lipogenic genes in the liver. Metagenomic analysis revealed that HFD drastically altered the profile of gut microbiota, and FMT modified the profile of the gut microbiota.
Our study suggests that CR and LZD improve metabolic parameters by modulating gut microbiota.
Core tip: This study shows that caloric restriction (CR) together with Lingguizhugan decoction (LZD) only slightly reduce body weight and blood glucose levels of normal diet (ND)-fed mice. Yet, transplanting the fecal microbiota of these mice into high-fat diet (HFD)-fed mice potently attenuated diet-induced obesity, hepatic steatosis, and hyperglycemia. Moreover, we found that fecal microbiota transplantation increases oxygen consumption rate of the mice and suppresses hepatic lipid biosynthesis. Using metagenomic sequencing, we further discovered that CR and LZD treatment alters the profile of ND-fed mice, and fecal microbiota transplantation alters HFD-induced changes in gut microbiota. Taken together, our study highlights that CR and LZD treatment exerts its metabolic improving effects via modulating gut microbiota.
- Citation: Liu MT, Huang YJ, Zhang TY, Tan LB, Lu XF, Qin J. Lingguizhugan decoction attenuates diet-induced obesity and hepatosteatosis via gut microbiota. World J Gastroenterol 2019; 25(27): 3590-3606
- URL: https://www.wjgnet.com/1007-9327/full/v25/i27/3590.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i27.3590
The prevalence of obesity has drastically increased in recent decades and has become a major health issue worldwide. Excessive body weight or obesity is a major risk factor for a variety of diseases, such as insulin resistance, type 2 diabetes, dys-lipidemia, hypertension, non-alcoholic fatty liver disease, cardiovascular disease, and metabolic syndrome (MetS)[1-4]. However, there is no specific medical treatment for obesity, and patients are often treated for their metabolic complications such as hyperlipidemia, diabetes and hypertension. The most effective way to reduce body weight is perhaps adapting a more active lifestyle, that is, limiting food intake and increasing physical activity. Patients with severe obesity can be treated by gastric bypass surgery, but there are potential side effects of the operation, such as vitamin or iron deficiency, malnutrition, and hernia, limiting its application as a treatment for overweight or less obese patients[5]. Caloric restriction (CR) has recently been shown to improve metabolic parameters of obese patients with and without MetS[6,7]. Additionally, we have found that Lingguizhugan decoction (LZD), a traditional Chinese medicine, can be used to treat obesity and non-alcoholic fatty liver disease[8], as it potently decreases plasma total triglycerides (TG) and total cholesterol (TC) levels and blood glucose levels in patients with MetS, on top of the reductions induced by CR[7]. However, the underlying mechanism is still unclear. In the past decade, the gut microbiota has been found to play an important role in the development of obesity and its related diseases[9-13]. Interestingly, metformin, a commonly used medicine to treat diabetes, has been found to improve glycemic control in type 2 diabetes patients via modulating the gut microbiota[14]. We thus wondered whether CR in combination with LZD exerted its effects by modulating gut microbiota.
To this end, we first explored if CR in combination with LZD affects energy and lipid metabolism in mice without any disturbance in metabolism, that is, mice fed a normal diet (ND). We found that combined treatment of CR + LZD reduced body weight and blood glucose levels of ND-fed mice. To further clarify whether such effects were mediated by microbiota, we transplanted the fecal microbiota from CR + LZD treated ND-fed mice to high fat diet (HFD)-fed mice and studied its effects on metabolic parameters. Interestingly, we found that fecal microbiota transplantation (FMT) protected mice from diet-induced obesity and hepatosteatosis and lowered plasma lipid level by promoting fatty acid (FA) oxidation and inhibiting hepatic lipid biosynthesis. Our study thus highlights a novel pharmacological mechanism of combined treatment with CR + LZD in treating obesity and MetS.
LZD, which is a combination of 12 commonly used herbal medicines in China, has been widely used in clinical practice to treat patients with MetS[7]. To test the effects of LZD on mice, we extracted the water-soluble components out of the raw materials (Kangmei Pharmaceutical Co., Ltd). In short, to prepare the herbal extracts, 50.5 g Radix Codonopsis, 40.4 g Cortex Poria, 50.5 g Rhizoma Atractyldis Macrocephalae, 30.3 g Ramulus Cinnamomi, 101 g Radix Astragali, 101 g Rhizoma Dioscoreae, 20.2 g Pericarpium Citri Reticulatae, 30.3 g Rhizoma Pinelliae, 67.3 g Semen Coicis, 40.4 g Radix Morindae Officinalis, 40.4 g Epimedium Folium, and 20.2 g Radix Glycyrrhizae were briefly washed three times with sterilized ultrapure water, and then submerged into 2 L 100 °C sterilized ultrapure water and incubated for 1 h at 100 °C in a water bath. The supernatant was then filtered with a 300-mesh filter to remove any large debris, then centrifuged at 100× g for 30 min to remove any small debris. Prepared supernatant was then freeze-dried using a vacuum freezing-dryer (Christ Inc, Germany), and 122.8 g dried powder was obtained and stored at -80 °C until use. For medicine administration, dried LZD extracts were re-suspended with sterilized ultrapure water and dosed at 4.14 g/kg body weight. This dose is comparable to the dose used for human subjects described elsewhere[7].
C57BL/6 mice were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China) and were housed at a 12-h light/12-h dark cycle. To test the effects of CR in combination with LZD extracts, hereby denoted as CR + LZD treatment, 8-wk old male C57BL/6 mice were treated with a 3-d treatment cycle, which includes 24 h-fasting and LZD extracts gavage for 2 consecutive days, and fed with ND (Research Diet, United States) ad libitum for 16 wk. Control mice did not undergo 24 h-fasting and were given saline gavage instead of LZD extracts gavage. To test the effects of gut microbiota on diet-induced obesity, 8-wk old male C57BL/6 mice received FMT from CR + LZD treated mice every 3 d and were fed with HFD (60% kcal fat, Research Diet) ad libitum for 16 wk. Control mice received either saline gavage or FMT from ND-fed mice receiving saline gavage as above-mentioned. Experimental schemes are summarized in Figure 1. Body weight was monitored bi-weekly. Food consumption of each cage hosting five mice was recorded weekly. To monitor blood glucose, TC, and triglycerides, blood samples were collected via submandibular bleeding after 6 h fasting. Experimental procedures were approved by the local animal ethnic committee (2018-057) and met the requirements of National Institutes of Health guide for the care and use of laboratory animals.
To collect feces, mice were housed individually in regular cages without any padding for 1-2 h, and feces were collected manually and chilled immediately with ice until use. For each group, 1 g feces was weighed, homogenized with 2.5 mL sterilized phosphate buffered saline using a micro-tube homogenizer, and filtered with a 40 μm cell strainer (Thermo Scientific) to remove any large debris. Prepared fecal homogenates were stored on ice and used within 2 h. For FMT, 200 μL fecal homogenates were given to mice by oral gavage every 3 d.
One week prior to the end of the study, an intraperitoneal glucose tolerance test was performed as previously described[15]. Briefly, after 16 h fasting, 2 g/kg glucose was injected intraperitoneally to mice. Blood samples were collected from tail vain at 0, 15, 30, 60 and 120 min after glucose injection, and blood glucose levels were measured using a glucometer (Johnson and Johnson). Glucose levels were compared at each time point, and overall differences in glycemic control were compared using area under the curve.
Metabolic and physical activity of the mice were measured using an indirect open-circuit calorimeter (Oxylet, Panlab)[16]. In short, mice were housed individually in metabolic chambers and O2 consumption, CO2 production, and physical activity were recorded at 30 min intervals for 60 consecutive hours. For accuracy, the recorded data from the first 12 h after the mice have been housed in the chamber were not analyzed. Respiratory quotient (RQ) was calculated as the ratio between CO2 production and O2 consumption.
Hematoxylin and eosin (H and E) and oil red O (ORO) staining were performed as described previously (Ref). In short, H and E was performed on 5 μm liver sections that were Bouin’s-fixed and paraffin-embedded. For ORO staining, which was used to detected neutral lipids, fresh liver tissues embedded in optimal-cutting-temperature compound were cryosectioned for 7 μm in thickness. After fixation with 4% paraformaldehyde, sections were stained with 0.3% ORO following standard procedures and counterstained with hematoxylin. Images were scanned using Cytation 5 Cell Imaging Multi-mode reader (BioTek Instruments, United States).
To measure plasma levels of ALT, AST, BUN, and insulin, blood samples were collected after 6-h fasting, at the end of the study, and pre-cleared by centrifugation at 3000× g for 5 min. Insulin levels were measured with an ELISA kit from ALPCO (catalog nr. 80-INSMSU-E01) according to the manufacturer’s protocol. Plasma ALT and AST activities and BUN levels were measured using a colorimetric kit from Biosino (China), following the manufacturer’s standard protocol.
Liver samples were homogenized with Trizol (Thermo Fisher Scientific), and total RNA was extracted using Direct-zol RNA Miniprep kit (ZYMO Research). One microgram of total RNA was reverse transcribed with PrimeScriptTM RT Master Mix (TaKaRa). SYBR Green real-time quantitative PCR assays were performed on a qTOWER apparatus (Analytic Jena) using SYBR® Premix Ex TaqTM II kit (TaKaRa). The expression of sterol regulatory element-binding protein (SREBP)-1c, acetyl-CoA carboxylase (ACC) α, fatty acid synthase (FASN), stearoyl-CoA desaturase (SCD) 1, and peroxisome proliferator-activated receptor (PPAR) γ were measured. SREBP1-c is a membrane-bound transcription factor that plays an important role in regulating lipogenic gene expression[17]. PPARγ plays an important role in HFD-induced upregulation of lipogenic gene expression and hepatic lipid biosynthesis[18,19]. ACC catalyzes acetyl-CoA to form malonyl-CoA, an essential substrate for synthesizing FA and an inhibitor of FA oxidation[20]. Thus, ACC levels are crucial in determining lipid metabolism and overall energy metabolism[21]. FASN is a large multipeptide enzyme whose major function is synthesizing palmitate, a long-chain saturated FA[22]. SCD1 is a key enzyme in catalyzing the synthesis of unsaturated FA oleic acid. Primers used in the study were as follow: ACCα (Forward: 5’-atgggcggaatggtctctttc-3’; Reverse: 5’-tggggaccttgtcttcatcat-3’), PPARγ (Forward: 5’-gaaagacaacggacaaatcacc-3’; Reverse: 5’-gggggtgatatgtttgaacttg-3’), SCD1 (Forward: 5’ttccctcctgcaagctctac-3’; Reverse: 5’-cagagcgctggtcatgtagt-3’), SREBP-1c (Forward: 5’-ggttttgaacgacatcgaaga-3’; Reverse: 5’-cgggaagtcactgtcttggt-3’), FASN (Forward: 5’-gctgctgttggaagtcagc-3’; Reverse: 5’-agtgttcgttcctcggagtg-3’), and 36B4 (Forward: 5’-actggtctaggacccgagaag-3’; Reverse: 5’-ctcccaccttgtctccagtc-3’).
For immunoblotting, 50 mg mouse liver samples were homogenized in RIPA buffer (Beyotime, China) with a protease inhibitor cocktail (Roche) using a TissueLyzer (Jingxin, China). Homogenates were precleared by centrifugation at 10000× g for 10 min at 4 °C. Protein concentrations were determined using BCA assay (Pierce). Equal amounts of proteins (20-30 mg) were loaded and separated on 4%-12% Bis-Tris gels (GenScript) and transferred to PVDF membranes using iBlot® 2 Dry Blotting System (Thermo Fisher Scientific). The blots were then probed with the following antibodies: Anti-ACC (1:1000, Cell Signaling Technology), anti-PPARγ (1:1000, Proteintech), anti-SCD1 (1:500, Abcam), anti-SREBP-1c (1:1000, Proteintech), anti-FASN (1:1000, Proteintech), anti-β-actin (1:5000, Proteintech), anti-mouse IgG (1:5000, Jackson ImmunoResearch), anti-rabbit IgG (1:5000, Jacson ImmunoResearch), and then detected by ClarityTM Western Substrate (Bio-Rad). Band intensities were analyzed using ImageJ.
To measure lipid contents in the liver, Folch’s method was used to extract hepatic lipids[23]. In short, 50 mg liver samples were homogenized using TissueLyzer (60 Hz, 30 s) in chloroform and methanol mixture (2:1) followed by adding methanol, chloroform and ultrapure water. Extracted lipids were dried with N2 gas and dissolved with 200 µL PBS containing 1% Triton X-100. Cholesterol and triglycerides were measured using colorimetric kits (Wako).
Metagenomic sequencing and analysis were performed by Novagen (Beijing, China). In short, 40 mg feces from each mouse were pooled, and DNA was extracted using an automated DNA extractor (Chemagic360, PelkinElmer). DNA concentration was measured using Qubit® dsDNA Assay Kit in Qubit® 2.0 Fluorometer (Life Technologies, CA, United States), and DNA quality was checked using Labchip GX Touch 24 and HT DNA extended range Labchip kit. For library construction, 1 μg DNA per sample was used as input material. Sequencing libraries were generated using NEBNext® Ultra™ DNA Library Prep Kit for Illumina (NEB, United States) following the manufacturer’s recommendations. Index codes were added to attribute sequences to each sample. Briefly, the DNA sample was fragmented by sonication to a size of 350 bp, then DNA fragments were end-polished, A-tailed, and ligated with the full-length adaptor for Illumina sequencing with further PCR amplification. At last, PCR products were purified (AMPure XP system), and libraries were analyzed for size distribution by Agilent2100 Bioanalyzer and quantified using real-time PCR.
For data processing and analysis, raw data obtained from the Illumina HiSeq sequencing platform were first preprocessed using Readfq (version 8, ht tps://github.com/cjfields/readfq) to acquire clean data for downstream analysis. The criteria for processing the raw data were as follows: (1) Reads that contain low quality bases (default quality threshold value ≤ 38) above a certain portion (default length of 40 bp) were removed; (2) Reads in which the N base has reached a certain percentage (default length of 10 bp) were removed; (3) Reads that shared an overlap above a certain portion with adapter (default length of 15 bp) were removed. Obtained clean data were further blasted to the host (mouse) database using Bowtie (version 2.2.4, http://bowtiebio.sourceforge.net/bowtie2/index.shtml) to remove any genes that were of host origin. To assemble metagenomes from the acquired clean data, SOAPdenovo software (version 2.04, http://soap.genomics.org.cn/soapdenovo.html) was used. For gene prediction and abundance analysis, MetaGeneMark (version 2.10, http://topaz.gatech.edu/GeneMark) and CD-HIT (version 4.5.8, http://www.bioinformatics.org/cd-hit) were used. For taxonomy prediction, DIAMOND (version 0.9.9, https://github.com/bbuchfink/diamond), NR database (version 2018-01-02, https://http://www.ncbi.nlm.nih.gov), MEGAN software, R vegan package (version 2.15.3), and LEfSe software were used. Detailed parameters used in the analysis are available upon request.
All values were presented as mean ± SE. For experiments with n number ≥ 8/group, a D'Agostino-Pearson omnibus test was performed to test normality. For experiments with a lower n number, a Kolmogorov-Smirnov test was performed for normality test. After passing the normality test, one-way ANOVA followed by the Bonferroni test was performed for comparison of more than two groups. A student’s t-test was used to compare the differences between two groups. P values of < 0.05 were considered significant. The statistical methods of this study were reviewed by Dr. Huachun Zhou from the Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-Sen University.
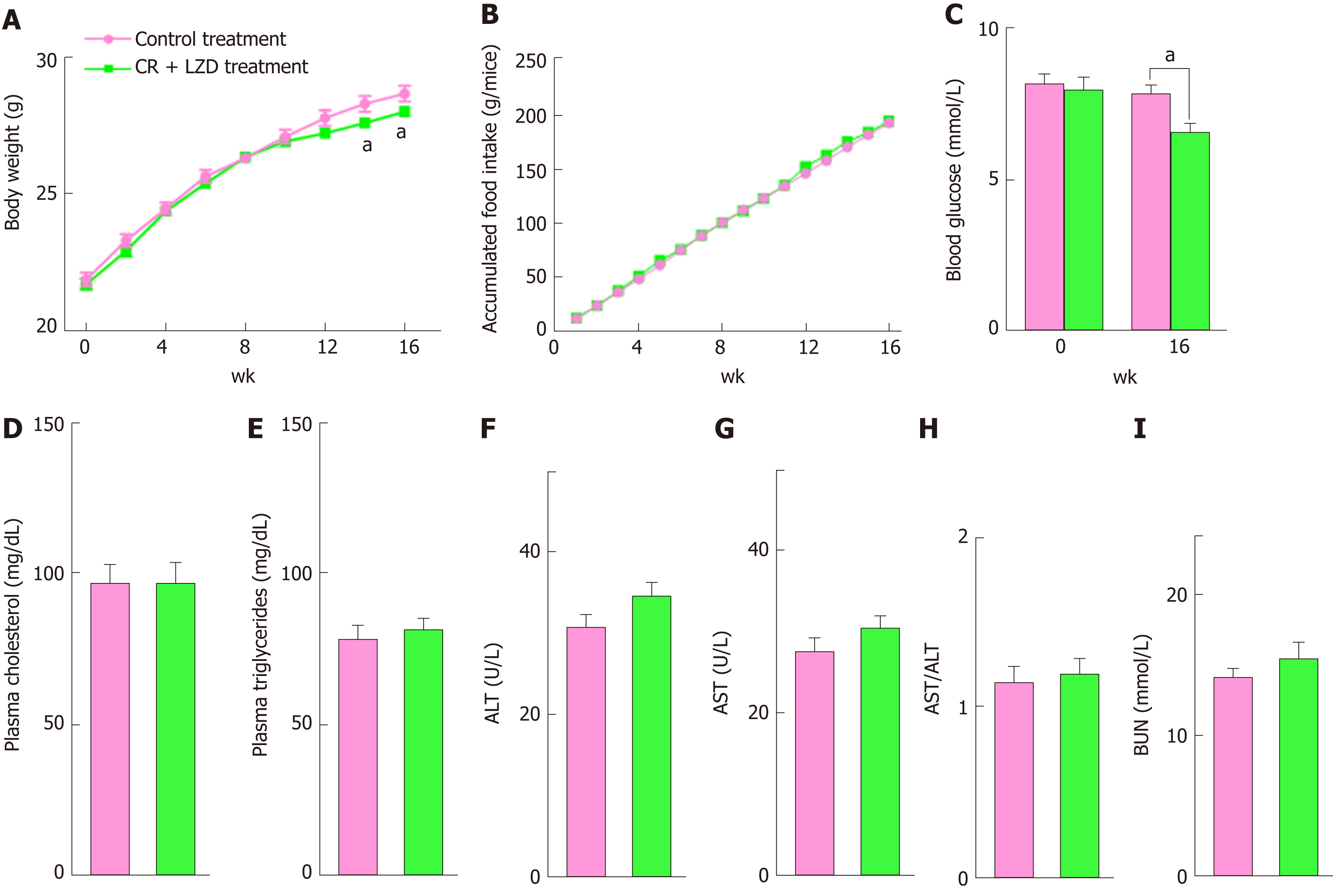
We first tested the effect of CR + LZD on ND-fed mice. To limit energy intake without causing any detrimental effect, the mice were fasted for 24 h every 3 d, and they received LZD by oral gavage. This treatment, in line with previous reports, reduced body weight (Figure 2A) without affecting accumulated food intake during the 16-wk experimental period (Figure 2B). It is likely that fasted mice consumed more food after fasting, thus leveling up the total food intake. Likely as a consequence of reduced body weight, blood glucose levels were also reduced approximately 20% by CR + LZD treatment, as compared to the levels of control treated mice (Figure 2C). Plasma levels of TG and TC were not affected by the treatment (Figure 2D and E), likely because these mice had normal baseline plasma lipid levels. To exclude the possibility that reduced body weight is not a result of liver injury induced by CR + LZD treatment, we measured plasma ALT and AST activities of the mice and found no changes in their activities and ratios (Figure 2F-H). This suggests that CR + LZD treatment did not impair liver functions. Additionally, we found that plasma levels of blood urea nitrogen, an indicator of renal damage, was unaltered by the treatment (Figure 2I), suggesting that the treatment did not cause renal damage. Taken together, these findings suggest that CR + LZD treatment is also effective in modulating metabolism in mice without any metabolic disorders.
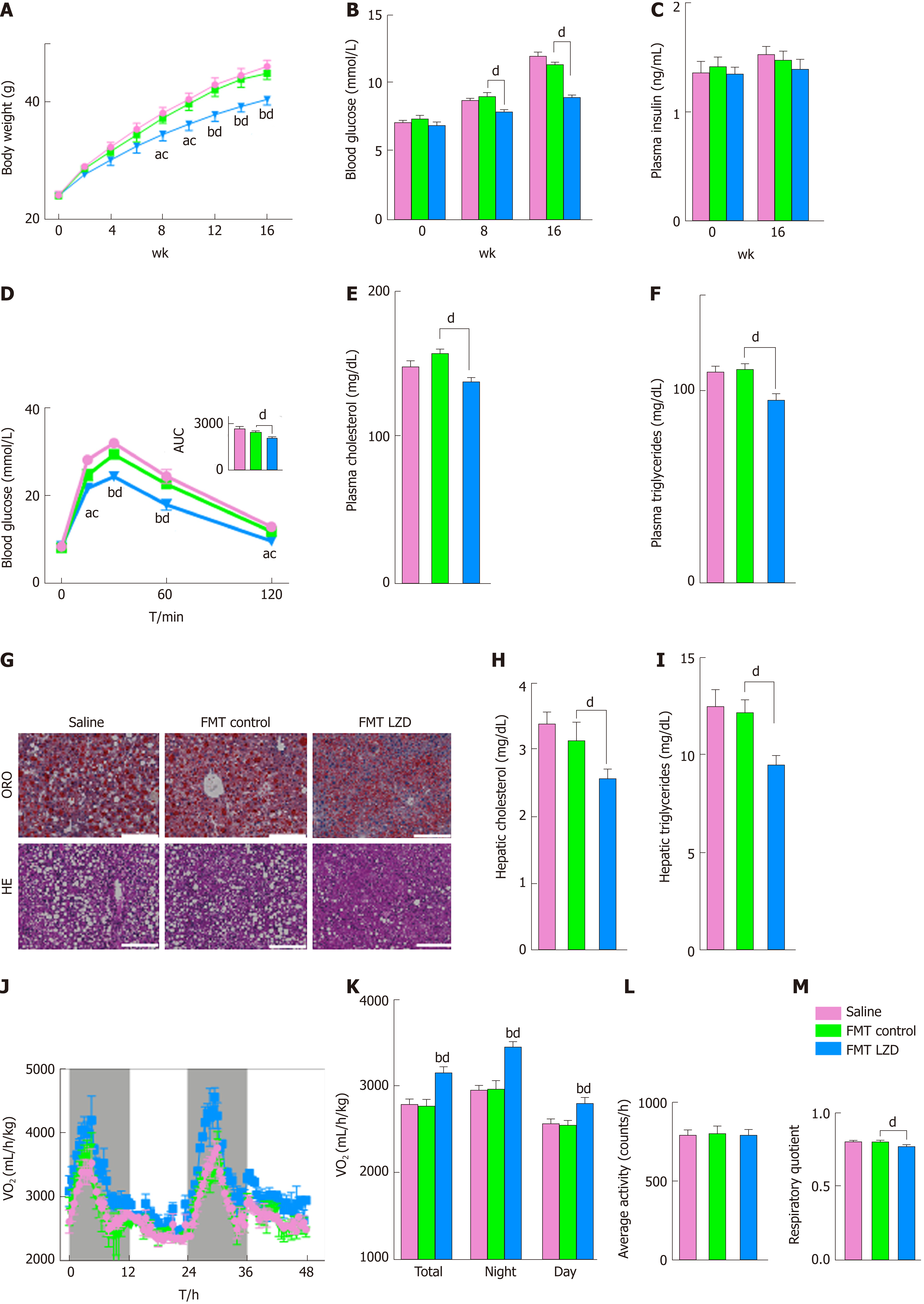
We speculated whether FMT from CR + LZD-treated mice would provide beneficial effects against diet-induced obesity in C57BL/6 mice. We collected feces from ND-fed mice receiving control treatment or CR + LZD treatment and transplanted the gut microbiota by oral gavage of fecal homogenates. Intriguingly, FMT from CR + LZD-treated mice attenuated HFD-induced body weight gain in C57BL/6 mice (Figure 3A). In accordance with reduced body weight, HFD-induced increase in blood glucose levels was also attenuated by FMT from CR + LZD-treated mice (Figure 3B). However, plasma insulin levels remained unaltered (Figure 3C), suggesting that treatment improved insulin sensitivity. Glucose tolerance was also improved by FMT from CR + LZD-treated mice, but not from control mice (Figure 3D). Similar to the effects in ND-fed mice, FMT from CR + LZD-treated mice lowered plasma TG and TC levels (Figure 3E and F). However, reduced plasma lipid levels could be caused by reduced hepatic lipid output, which could in turn cause lipid accumulation in the liver. To exclude this possibility, we examined hepatic lipid content by ORO staining. Strikingly, hepatic lipid staining was also reduced by FMT (Figure 3G). We further extracted lipid contents from the liver. When we measured lipid levels, we found that in line with ORO staining, hepatic TG and TC levels were both reduced in mice that received FMT from CR + LZD-treated ND-fed mice (Figure 3H and I). These findings suggest that FMT either promoted lipid oxidation or inhibited lipid biosynthesis to reduce plasma and hepatic lipid levels. To gain a better understanding, we measured oxygen consumption rate (OCR) and physical activity of the mice. FMT from mice treated with CR + LZD, but not from control mice, drastically increased OCR (Figure 3J and K) without affecting physical activity (Figure 3L), suggesting that these mice were more active in metabolism. Additionally, RQ, a reflector for fuels being oxidized, was reduced by FMT (Figure 3M), implying that FA oxidation is likely increased. Taken together, these findings imply that FMT from CR + LZD-treated mice promotes lipid oxidation, thus reducing body weight and hepatic lipid contents.
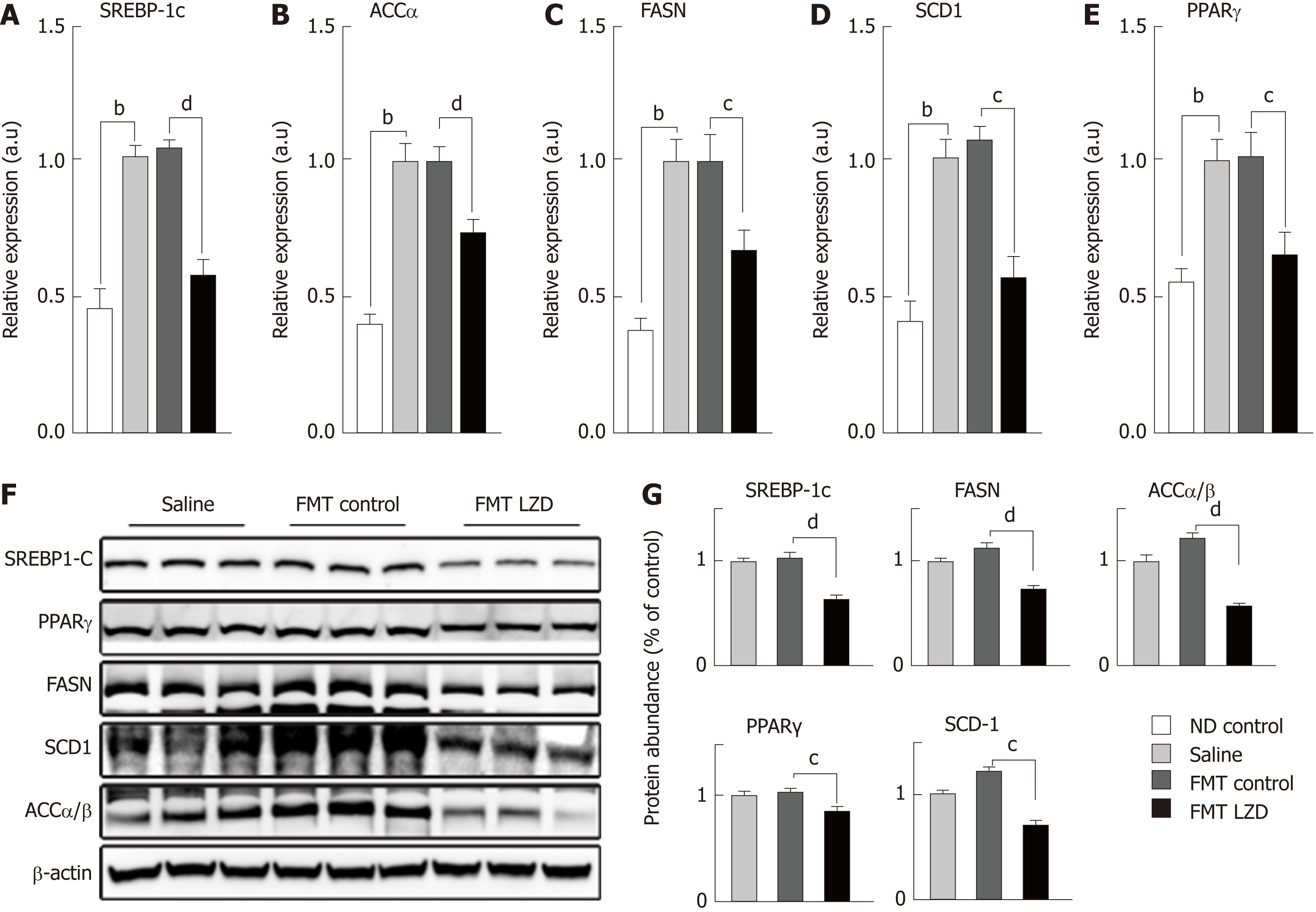
It is unclear whether FMT reduced hepatic lipid levels solely by increasing lipid oxidation. Thus, we measured the hepatic expression of key genes involved in lipid biosynthesis. We found that HFD increased expression of SREBP-1c, ACCα, FASN, SCD1, and PPARγ in the liver[24], as compared to the expression levels in the liver of ND-fed mice (Figure 4A-E). Thus, increased expression of these lipogenic genes explains the increases in lipid deposition in the liver and adipose tissues. Intriguingly, FMT from CR + LZD-treated mice, but not from control mice, reduced hepatic expression of these lipogenic genes (Figure 4A-E). Furthermore, we measured protein abundance of these key factors by immunoblotting. In line with gene expression profiles, SREBP-1c, ACCα, FASN, SCD-1 and PPARγ proteins were all reduced by FMT (Figure 4F and G). Taken together, these data suggest that FMT also suppresses lipid biosynthesis in the liver, further explaining the observation that hepatic lipid content was reduced by FMT.
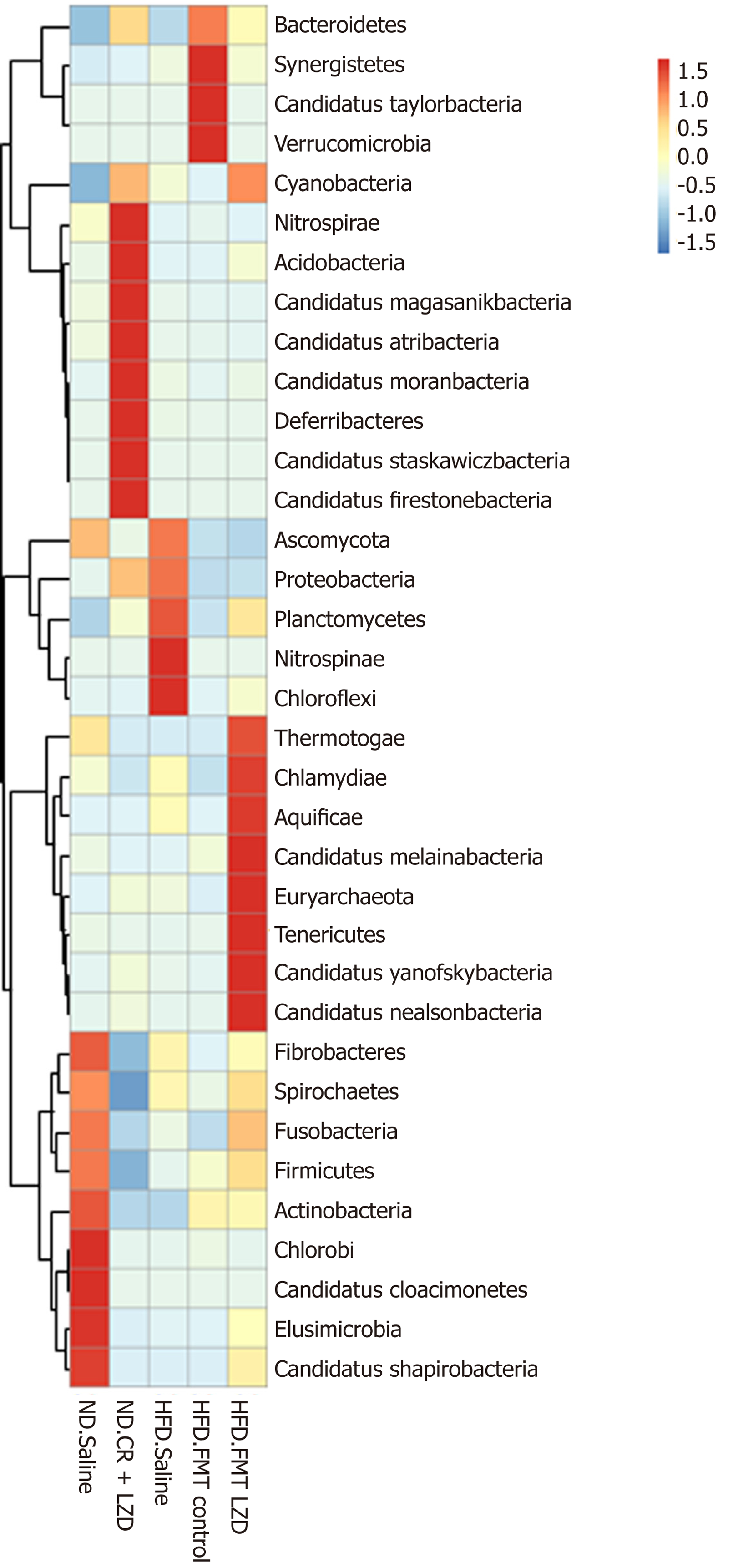
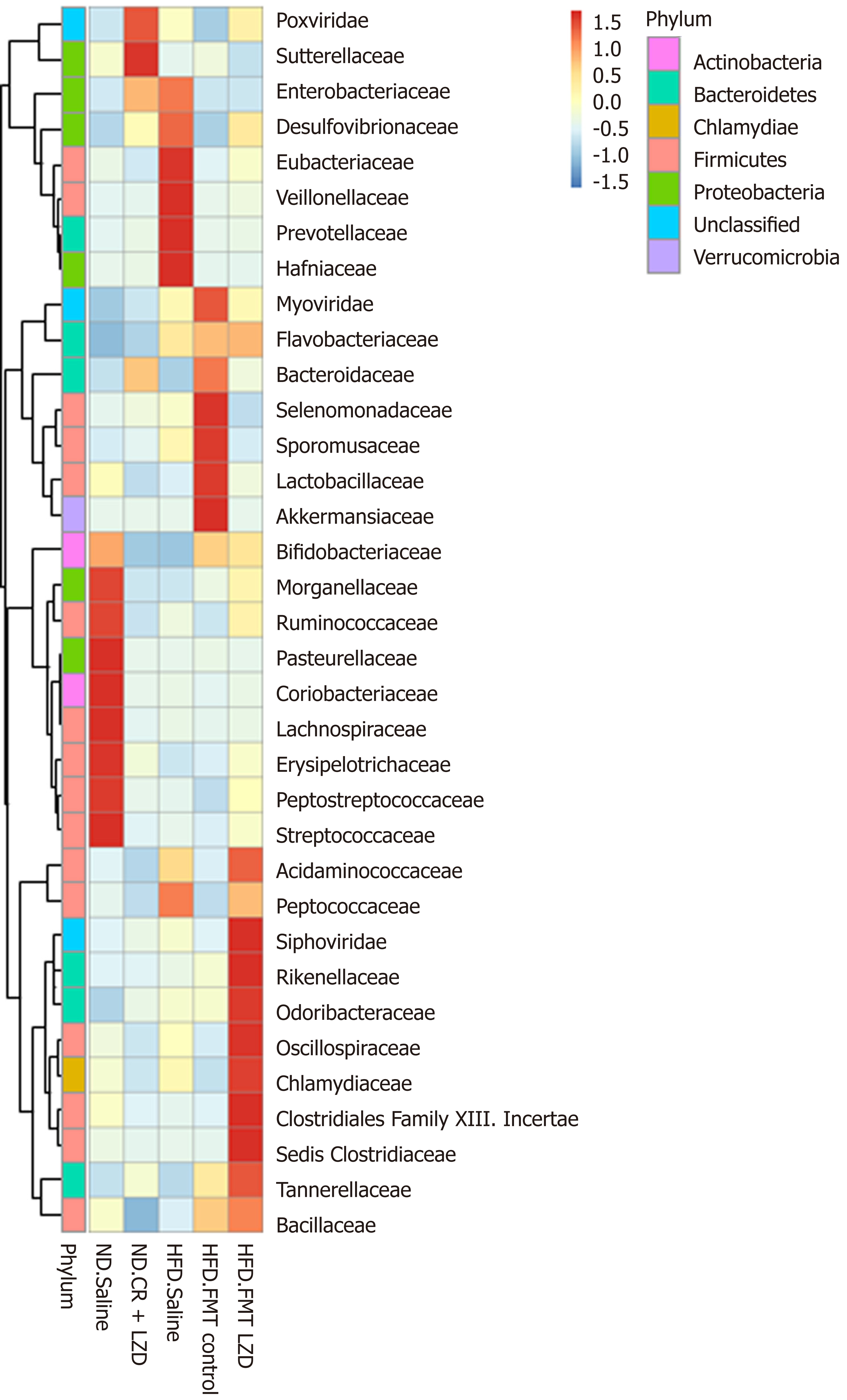
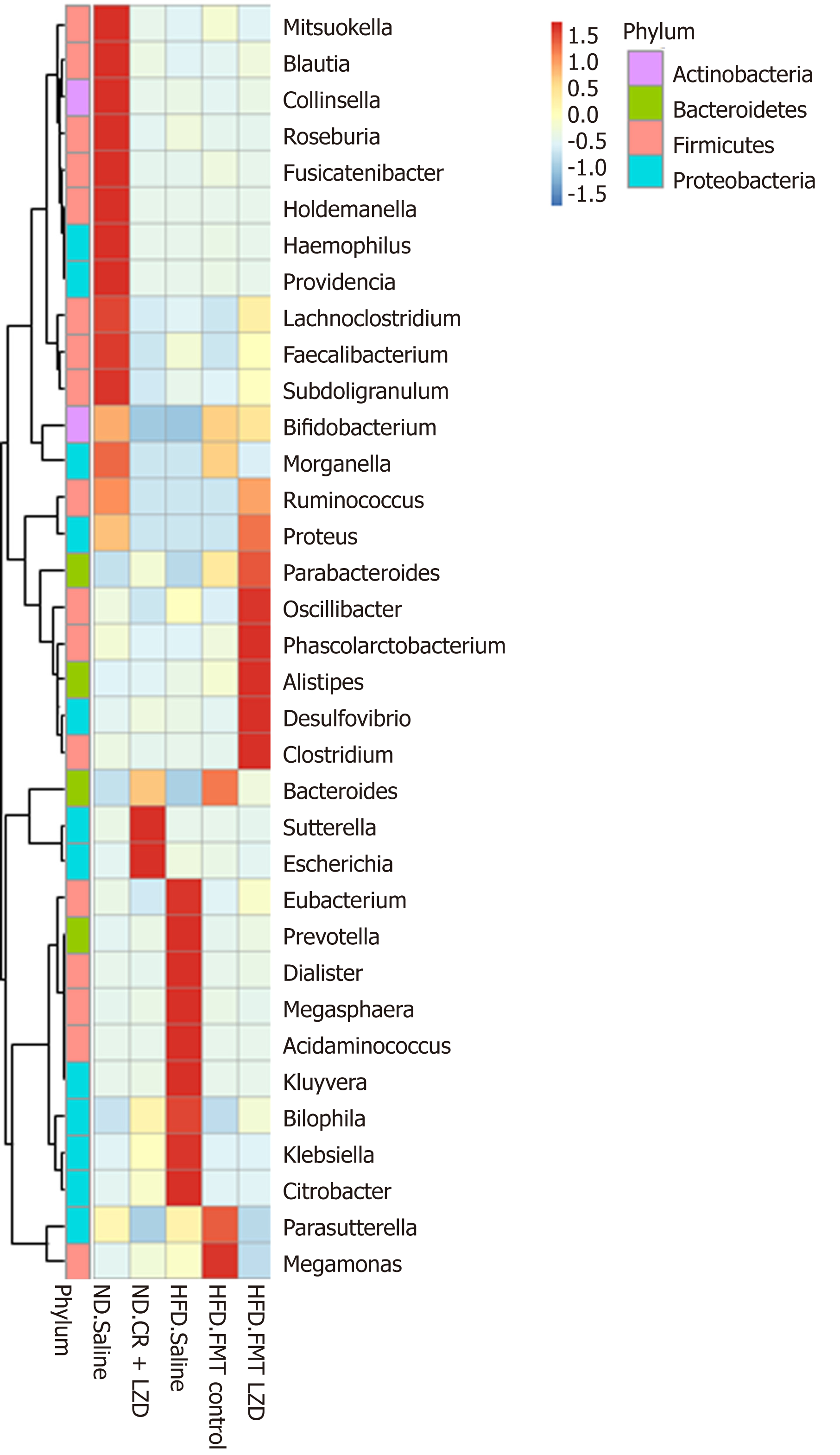
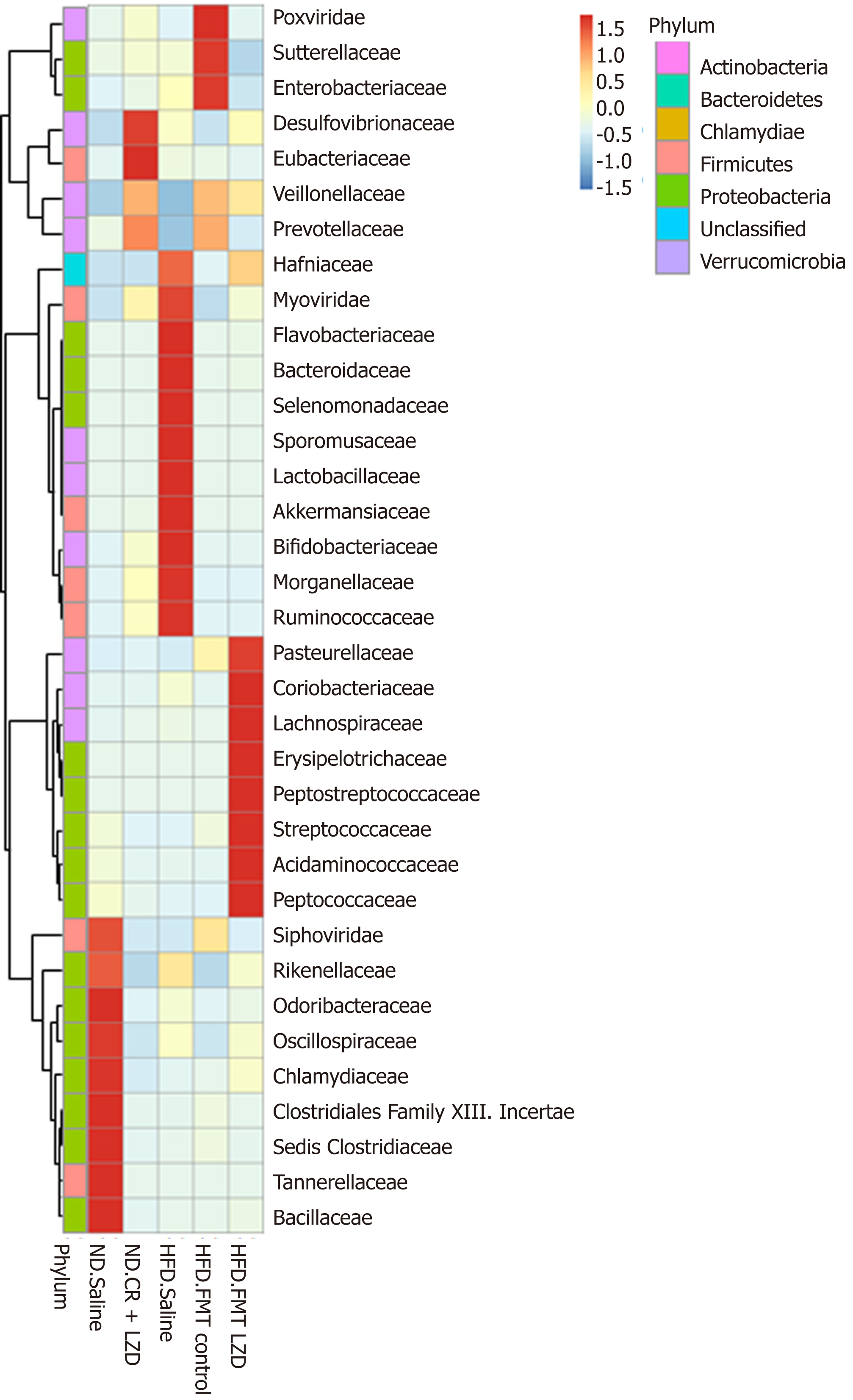
To gain a better understanding of FMT-induced changes in metabolic profiles, we mapped the gut microbiota by metagenome sequencing. Gut microbiota profile of the mice fed an ND was completely different from that of mice fed an HFD (Figures 5-8). At the phylum level, Fibrobacteres, Spirochaetes, Fusobacteria, Firmicutes, Actinobacteria, Chlorobi, Candidatus, Cloacimonets, Elusimicrobia, and Candidatus Shapirobacteria were abundantly detected in the feces of ND-fed mice, while they were less abundantly detected in the feces of HFD-fed mice (Figure 5). FMT from CR + LZD-treated mice into HFD-fed mice increased the abundance of the phyla Fusobacteria, Actinobacteria, Elusimicrobia and Candidatus Shapirobacteria, suggesting that alterations in gut microbiota induced by HFD were partially reversed by FMT. Additionally, the abundance of the phyla Thermotogae, Chlamydiae, Aquificae, Candidatus Melainabacteria, Euryarchaeota, Tenericutes, Candidatus Yanofskybacteria, and Candidatus Nealsonbacteria was increased by FMT from CR + LZD-treated mice (Figure 5). At the family level, FMT from CR + LZD-treated mice into HFD-fed mice increased the abundance of Siphoviridae, Rikenellaceae, Odoribacteraceae, Oscillospiraceae, Chlamydiaceae, Clostridiales Family XIII, Incertae Sedis, Clostridiceae, Tannerellaceae and Bcillaceae compared to ND-fed control mice and HFD-fed mice receiving saline gavage (Figure 6). At the genus level, FMT from CR + LZD-treated mice into HFD-fed mice strongly increased the abundance of Rumiococcus, Proteus, Parabacteroides, Oscillibacter, Phascolarctobacterium, Alistipes, Desulfovibrio, and Clostridium (Figure 7). At the species level, FMT from CR + LZD-treated mice into HFD-fed mice increased the abundance of Alistipes finegoldii, Alistipes puredinis, Bacteroides coprophilus, Clostridium sp. CAG: 389, Clostridium sp. CAG: 343, Phascolarctobacterium sp. CAG: 207, and uncultured Ruminococcus sp. (Figure 8).
Regular or prolonged CR is effective in treating metabolic disorders, such as obesity, diabetes, hypertension and hyperlipidemia[25,26]. CR + LZD treatment can effectively lower plasma TC and TG levels in patients with MetS[7]. Similarly, CR + LZD attenuates HFD-induced obesity and hyperlipidemia in rats[27]. Despite its effectiveness, little is known about its underlying mechanism. In the current study, we demonstrated that CR + LZD treatment is also capable of lowering blood glucose levels and body weight in ND-fed C57BL/6 mice. Yet, it is not clear how LZD reduces body weight. Perhaps, as illustrated in HFD-fed mice receiving FMT of CR + LZD treated mice, CR + LZD may increase OCR of the mice by promoting FA oxidation. However, unlike previous findings, plasma TC and TG levels were not affected by the treatment. It is possible that CR + LZD treatment only attenuates dyslipidemia induced by excessive fat intake because under ND feeding, C57BL/6 mice have normal plasma lipid levels. This implies that CR + LZD treatment is more likely correcting disturbed lipid metabolism rather than directly modulating plasma lipid metabolism. We found that the gut microbiota profile of C57BL/6 mice is drastically changed by HFD feeding. Recent studies have highlighted the importance of gut microbiota in the development of metabolic diseases, such as obesity and diabetes. It is thus possible that CR + LZD can modulate gut microbiota to improve metabolism. Indeed, we found that FMT from CR + LZD-treated mice attenuated HFD-induced obesity, hyperglycemia, and hepatosteatosis. FMT improves metabolic parameters to a similar degree as those achieved by CR + LZD treatment in HFD-fed rats[27], highlighting that the gut microbiota is the primary, if not the only, target of CR and LZD in modulating metabolism. Recent studies have also revealed that traditionally used herbal medicines, such as Ganoderma lucidum and Hirsutella sinensis, exert their effects by modulating gut microbiota[28,29]. These findings, including ours, present a new aspect in understanding the pharmacological effects of herbal plants. Yet, it is still not clear how these herbal medicines affect the composition of gut microbiota. It is possible that components of the medical plants inhibit the growth of certain bacteria while promoting the growth of others. Extract of Glycyrrhiza glabra has been shown to inhibit the growth of Salmonella, Shigella and enterotoxigenic Escherichia coli in vivo[30]. It is worth noting that Radix Glycyrrhizae, a component of LZD, contains dried roots and rhizomes of Glycyrrhiza glabra. Another possibility is that the components of the medical plants can be transformed into other metabolites that facilitate the growth of certain types of microbes or directly affect the metabolism of the host, as reviewed elsewhere[31,32].
It must be noted that the gut microbiota profile of CR + LZD-treated and ND-fed mice did not overlap with that of HFD-fed mice that received FMT. The exact reason for this is not clear. Possibly, the most abundant microbes present in the donor feces did not necessarily well inhabit the gut of the recipient mice. Recent studies have reported that HFD increases the abundance of the phyla Firmicutes and Pro-teobacteria and decreases the abundance of Bacteroidetes[33,34]. In the current study, we found that HFD decreased the abundance of phyla Firmicutes and Bacteroidetes, but increased the abundance of Proteobacteria, differing with previous report. It is possible that the origin of the mice and the housing environment may have had an impact on the gut microbiota profile. Yet, we found that FMT from CR + LZD-treated mice increased the abundance of species Alistipes finegoldii, Alistipes putredinis and Bacteroides coprophilus, which belong to the phyla Bacteroidetes. Of note, a decreased abundance of Bacteroides coprophilus has been reported in MetS patients[35]. Alistipes putredinis has recently been identified as a butyrate producer in the gut[36]. Butyrate is a short-chain FA and plays an important role in maintaining colon health. The main butyrate-producing bacteria are within the Firmicutes phylum, but members of the Actinobacteria, Bacteroidetes, Fusobacteria, Proteobacteria, Spirochaetes and Thermotogae phyla also have butyrate-synthesis pathways[36]. It is worth noting that FMT from mice treated with CR + LZD strongly increased the abundance of Thermotogae. Dietary butyrate supplementation can attenuate diet-induced obesity, insulin resistance, and hyperlipidemia in mice by activating mitochondrial functions[37,38]. Additionally, butyrate is also capable of reducing lipid secretion and lipoprotein biosynthesis in hepatic cells[39]. Interestingly, butyrate also increases OCR of epithelial cells[40]. Taken together, FMT from CR + LZD-treated mice could increase FA oxidation and limit lipid biosynthesis by increasing the abundance of butyrate-producing bacteria in the gut. In conclusion, we report that CR in combination with LZD attenuates diet-induced obesity and hepatosteatosis by modulating the gut microbiota.
Lingguizhugan decoction (LZD) in combination with food restriction is widely used in clinical practice to treat patients with metabolic disorders, such as obesity, diabetes, high plasma lipid levels, and non-alcoholic fatty liver disease. Despite its wide application and effectiveness, little is known about its mechanism.
Although it is well accepted to use LZD in clinic to treat patients with metabolic disorders, lacking the knowledge on its mechanism has limited its use. Clarifying the pharmacological mechanism will provide scientific evidence for its clinic usage. Recent studies have revealed that traditional Chinese herbal medicines exert their effects by modulating gut microbiota, which has been previously unrecognized.
To investigate whether LZD combined with food restriction improves metabolic parameters via modulating gut microbiota.
To answer this question, we administered LZD gavage in addition to food restriction to mice fed a normal diet, and monitored body weight, blood glucose, and plasma lipid levels. At the same time, we collected the feces of these mice and homogenized with saline. We gave these fecal homogenates, which contain microbes, to mice fed a high fat diet. As high fat diet increases body weight, it causes increases in plasma lipid levels and blood glucose levels and induces abnormal lipid accumulation in the liver. Thus, we studied the effects of giving fecal homogenates from LZD treated and food-restricted mice on diet-induced metabolic abnormalities.
We found that LZD together with food restriction slightly reduced body weight and blood glucose levels but did not affect plasma lipid levels. However, giving the fecal homogenates collected from LZD and food-restricted mice greatly reduced body weight, plasma lipid levels, hepatic lipid contents, and blood glucose levels of mice on a high-fat-diet. We also found that giving the mice fecal homogenates significantly promoted fat oxidation and inhibited fat synthesis. Using DNA sequencing techniques, we found that LZD together with food restriction significantly changed the composition of bacteria in the gut.
We found that a widely used traditional Chinese medicine can change the bacteria composition of the gut. Transferring these gut bacteria into high-fat-diet fed mice can reduce diet-induced increase in blood glucose, plasma lipid levels, hepatic lipid contents and body weight gain. Thus, gut microbes are the most likely primary target of LZD and food restriction treatment.
Our study highlights the possibility of using bacteria to treat metabolic disorders such as obesity in the future. Using metagenomics, metatranscriptomic sequencing, and fecal metabolomics, it is possible to identify the most important bacteria and metabolites underlying the treatment of LZD and food restriction. This will make it possible to culture the identified bacteria in vivo and treat them with LZD extracts. Then giving the patients with such cultured and treated bacteria would provide similar effects as LZD treatment, thus reducing any potential toxic effects of the herbal medicine.
The authors thank the members of the laboratory of Dr. Xi-Feng Lu for their technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barzilay J, Hassan AI, Muriel P, Zhao JB S-Editor: Yan JP L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity-associated liver disease. J Clin Endocrinol Metab. 2008;93:S74-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 2. | Abbasi F, Brown BW, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 372] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 541] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 4. | Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis. 2016;5:2048004016633371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 245] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 5. | Duvoisin C, Favre L, Allemann P, Fournier P, Demartines N, Suter M. Roux-en-Y Gastric Bypass: Ten-year Results in a Cohort of 658 Patients. Ann Surg. 2018;268:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Ghachem A, Prud'homme D, Rabasa-Lhoret R, Brochu M. Effects of a 6-month caloric restriction induced-weight loss program in obese postmenopausal women with and without the metabolic syndrome: A MONET study. Menopause. 2017;24:908-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Yang Y, Li Q, Chen S, Ke B, Huang Y, Qin J. Effects of modified lingguizhugan decoction combined with weekend fasting on metabolic syndrome. J Tradit Chin Med. 2014;34:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Zhu M, Hao S, Liu T, Yang L, Zheng P, Zhang L, Ji G. Lingguizhugan decoction improves non-alcoholic fatty liver disease by altering insulin resistance and lipid metabolism related genes: A whole trancriptome study by RNA-Seq. Oncotarget. 2017;8:82621-82631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Baothman OA, Zamzami MA, Taher I, Abubaker J, Abu-Farha M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016;15:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 339] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 10. | Sun L, Ma L, Ma Y, Zhang F, Zhao C, Nie Y. Insights into the role of gut microbiota in obesity: Pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell. 2018;9:397-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 11. | Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 602] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 12. | Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3074] [Article Influence: 236.5] [Reference Citation Analysis (0)] |
| 13. | Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 945] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 14. | Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 1133] [Article Influence: 141.6] [Reference Citation Analysis (0)] |
| 15. | Ren L, Sun Y, Lu H, Ye D, Han L, Wang N, Daugherty A, Li F, Wang M, Su F, Tao W, Sun J, Zelcer N, Mullick AE, Danser AHJ, Jiang Y, He Y, Ruan X, Lu X. (Pro)renin Receptor Inhibition Reprograms Hepatic Lipid Metabolism and Protects Mice From Diet-Induced Obesity and Hepatosteatosis. Circ Res. 2018;122:730-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Franckhauser S, Muñoz S, Elias I, Ferre T, Bosch F. Adipose overexpression of phosphoenolpyruvate carboxykinase leads to high susceptibility to diet-induced insulin resistance and obesity. Diabetes. 2006;55:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Brown MS, Goldstein JL. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2786] [Cited by in RCA: 2896] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 18. | Inoue M, Ohtake T, Motomura W, Takahashi N, Hosoki Y, Miyoshi S, Suzuki Y, Saito H, Kohgo Y, Okumura T. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005;336:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 311] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 19. | Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268-34276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 637] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 20. | Foster DW. Malonyl-CoA: The regulator of fatty acid synthesis and oxidation. J Clin Invest. 2012;122:1958-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 21. | Tong L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell Mol Life Sci. 2005;62:1784-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 357] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Röhrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1099] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 23. | Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497-509. [PubMed] |
| 24. | Mei S, Yang X, Guo H, Gu H, Zha L, Cai J, Li X, Liu Z, Bennett BJ, He L, Cao W. A small amount of dietary carbohydrate can promote the HFD-induced insulin resistance to a maximal level. PLoS One. 2014;9:e100875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Normandin E, Chmelo E, Lyles MF, Marsh AP, Nicklas BJ. Effect of Resistance Training and Caloric Restriction on the Metabolic Syndrome. Med Sci Sports Exerc. 2017;49:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Nicoll R, Henein MY. Caloric Restriction and Its Effect on Blood Pressure, Heart Rate Variability and Arterial Stiffness and Dilatation: A Review of the Evidence. Int J Mol Sci. 2018;19:pii: E751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Yang Y, Qin J, Ke B, Zhang J, Shi L, Li Q. Effect of Linguizhugan decoction on hyperlipidemia rats with intermittent fasting. J Tradit Chin Med. 2013;33:250-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng SF, Wu TR, Chen YY, Young JD, Lai HC. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 669] [Cited by in RCA: 939] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 29. | Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, Ojcius DM, Lu CC, Young JD, Lai HC. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68:248-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 543] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 30. | Shirazi MH, Ranjbar R, Eshraghi S, Sadeghi G, Jonaidi N, Bazzaz N, Izadi M, Sadeghifard N. An Evaluation of Antibacterial Activity of Glycyrrhiza glabra Extract on the Growth of Salmonella, Shigella and ETEC E. coli. J Biol Sci. 2007;7:827-829. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Xu J, Chen HB, Li SL. Understanding the Molecular Mechanisms of the Interplay Between Herbal Medicines and Gut Microbiota. Med Res Rev. 2017;37:1140-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 32. | Feng W, Ao H, Peng C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front Pharmacol. 2018;9:1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 33. | Zhang C, Zhang M, Pang X, Zhao Y, Wang L, Zhao L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012;6:1848-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 34. | Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716-24.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1234] [Cited by in RCA: 1157] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 35. | Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim SW, Morita H, Hattori M, Yamamura T. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One. 2015;10:e0137429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 567] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 36. | Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio. 2014;5:e00889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 822] [Article Influence: 74.7] [Reference Citation Analysis (1)] |
| 37. | Hong J, Jia Y, Pan S, Jia L, Li H, Han Z, Cai D, Zhao R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget. 2016;7:56071-56082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 38. | Mollica MP, Mattace Raso G, Cavaliere G, Trinchese G, De Filippo C, Aceto S, Prisco M, Pirozzi C, Di Guida F, Lama A, Crispino M, Tronino D, Di Vaio P, Berni Canani R, Calignano A, Meli R. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes. 2017;66:1405-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 39. | Marcil V, Delvin E, Seidman E, Poitras L, Zoltowska M, Garofalo C, Levy E. Modulation of lipid synthesis, apolipoprotein biogenesis, and lipoprotein assembly by butyrate. Am J Physiol Gastrointest Liver Physiol. 2002;283:G340-G346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 1187] [Article Influence: 118.7] [Reference Citation Analysis (0)] |