Published online Jul 21, 2019. doi: 10.3748/wjg.v25.i27.3503
Peer-review started: March 6, 2019
First decision: May 16, 2019
Revised: May 22, 2019
Accepted: May 31, 2019
Article in press: June 1, 2019
Published online: July 21, 2019
Processing time: 136 Days and 17.7 Hours
Eosinophils are currently regarded as versatile mobile cells controlling and regulating multiple biological pathways and responses in health and disease. These cells store in their specific granules numerous biologically active substances (cytotoxic cationic proteins, cytokines, growth factors, chemokines, enzymes) ready for rapid release. The human gut is the main destination of eosinophils that are produced and matured in the bone marrow and then transferred to target tissues through the circulation. In health the most important functions of gut-residing eosinophils comprise their participation in the maintenance of the protective mucosal barrier and interactions with other immune cells in providing immunity to microbiota of the gut lumen. Eosinophils are closely involved in the development of inflammatory bowel disease (IBD), when their cytotoxic granule proteins cause damage to host tissues. However, their roles in Crohn’s disease and ulcerative colitis appear to follow different immune response patterns. Eosinophils in IBD are especially important in altering the structure and protective functions of the mucosal barrier and modulating massive neutrophil influx to the lamina propria followed by transepithelial migration to colorectal mucus. IBD-associated inflammatory process involving eosinophils then appears to expand to the mucus overlaying the internal gut surface. The author hypothesises that immune responses within colorectal mucus as well as ETosis exerted by both neutrophils and eosinophils on the both sides of the colonic epithelial barrier act as additional pathogenetic factors in IBD. Literature analysis also shows an association between elevated eosinophil levels and better colorectal cancer (CRC) prognosis, but mechanisms behind this effect remain to be elucidated. In conclusion, the author emphasises the importance of investigating colorectal mucus in IBD and CRC patients as a previously unexplored milieu of disease-related inflammatory responses.
Core tip: Eosinophils are multifunctional granulocytes possessing readily releasable stores of cytotoxic proteins, regulatory cytokines and chemokines in their specific granules. In health eosinophils reside in the gut, exerting homeostatic functions including protective mucosal barrier integrity maintenance and contribution to gut-associated immunity. Eosinophils are important players in inflammatory bowel disease pathogenesis (both Crohn’s disease and ulcerative colitis). These cells are also associated with a favourable prognosis in colorectal cancer, however mechanisms of this association remain obscure. The author presents a comprehensive analysis of the current literature on eosinophils in the gut and highlights the importance of poorly investigated immune responses occurring within colorectal mucus.
- Citation: Loktionov A. Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders. World J Gastroenterol 2019; 25(27): 3503-3526
- URL: https://www.wjgnet.com/1007-9327/full/v25/i27/3503.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i27.3503
The eosinophil was first described in the middle of XIX century when these granul-ocytes displaying a strong affinity to acidophilic dyes producing red staining of their granules were first identified. The honour of discovering and characterising eosinophils belongs to Paul Ehrlich, who also made first assumptions regarding their biological significance[1]. For many decades eosinophils were regarded as terminally differentiated end-stage cells of innate immunity exerting cytotoxic effector defence against parasitic helminths, but also capable of causing damage to host tissues in allergic conditions[2-5]. That simplistic paradigm had to be fundamentally revised once recent research advances revealed numerous previously unknown eosinophil functions, comprising inflammation control, epithelial barrier maintenance, participation in tissue remodelling and linking innate and adaptive immunity[2-5]. Nonetheless, the whole range of roles played by these granulocytes in health and disease remains to be elucidated.
This review focuses on eosinophil action in the distal gastrointestinal tract, both in the normal physiological conditions and in pathology, especially in the context of inflammatory bowel disease (IBD) and colorectal cancer (CRC). The declared task looks timely since eosinophils, despite being recognised as important players in both gut homeostasis maintenance and colorectal disorder pathogenesis, are often overlooked when disease mechanisms are considered. It is remarkable that none of recent comprehensive reviews on the pathogenesis of either IBD[6-10] or CRC[11-15] even mentions possible eosinophil involvement in these complex processes. The author decided to focus on analysing literature describing research in humans, but experimental modelling, especially the use of genetically modified mice is one of the key sources of new information in the field. For this reason, selected experimental studies are considered as well, but caution is always required when results obtained in murine models are extrapolated to humans.
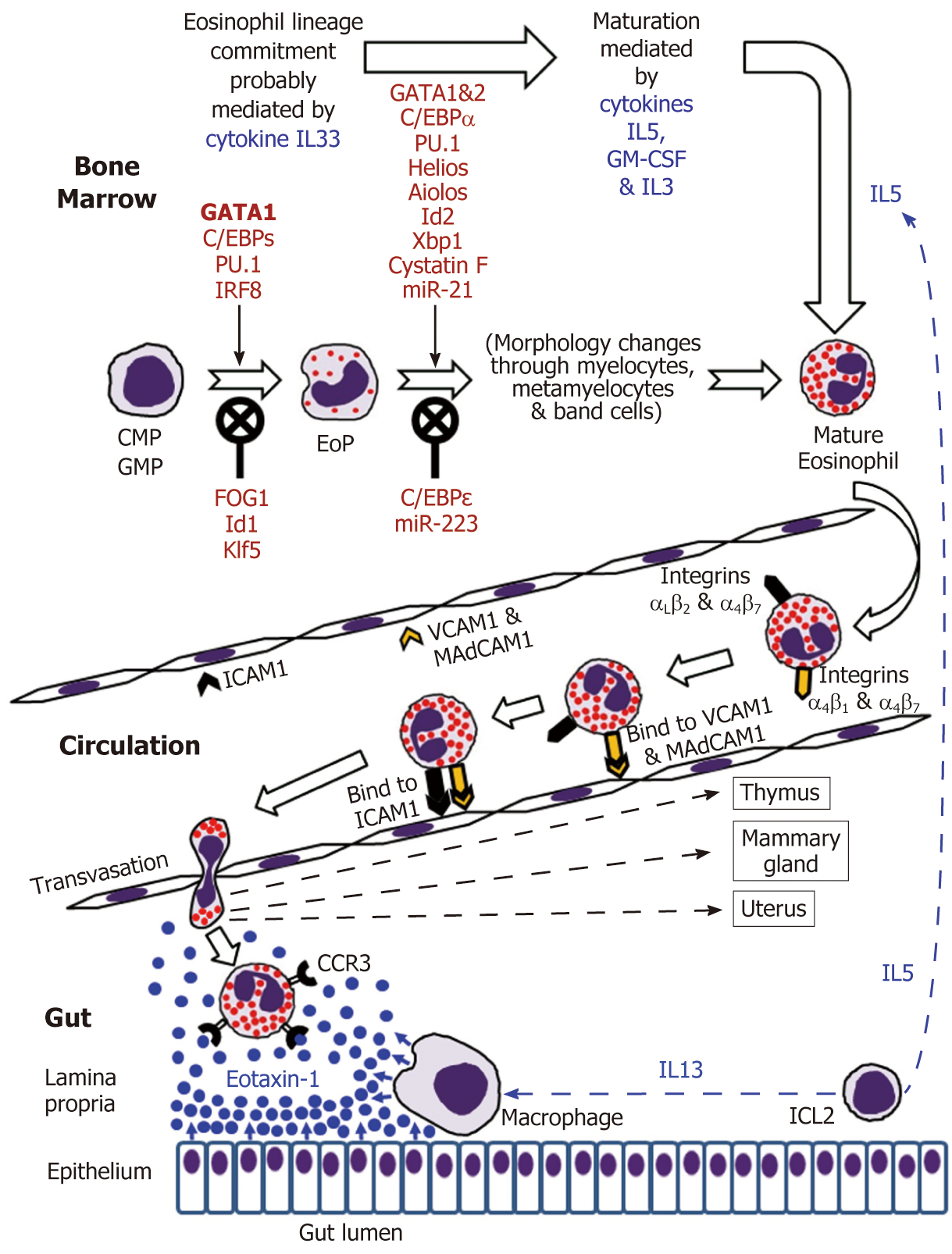
Eosinophils are continuously generated in the bone marrow from pluripotent CD34+ stem cells, and it was presumed that their development proceeds through the granulocyte/macrophage progenitor (GMP) in mice and the common myeloid progenitor (CMP) in humans[2,16-19]. However, using a mouse model, Drissen et al[20] have recently identified a distinct myeloid differentiation pathway characterised by GATA1 expression and giving rise to eosinophils, mast cells, megakaryocytes and erythroid cells, but not to monocytes, neutrophils and lymphocytes. In any case, it is generally accepted that the control of eosinophilopoiesis is exerted on the one hand by transcription factors produced internally by the developing cells of the eosinophil lineage, but on the other hand by cytokines secreted predominantly by other immune cells (Figure 1).
The analysis of cell transcriptome changes during eosinophil lineage commitment followed by maturation has revealed a marked development-related increase in the number of expressed genes[21]. Early stages of this process appear to be regulated by cytokine IL33[22] and are driven at the cellular level by a few transcription factors, GATA1 being essential for eosinophil lineage commitment. Figure 1 illustrates the necessity of the interplay between pro-differentiation transcription factors including GATA1 (the key element), C/EBPs, PU.1 and IRF-8[16-19] and inhibitory influences of FOG1, Id1 and Klf5 in generating eosinophil-restricted precursor (EoP) cells[16-19,23,24]. Importantly, the acquisition of surface receptor IL5Rα occurs at the EoP stage[25], thus making EoP cells responsive to the external signalling by IL5, an eosinophil-targeting cytokine secreted by type 2 innate immune cells (ILC2)[26]. Indeed, IL5, IL3 and GM-CSF are recognised as the three cytokines regulating eosinophil proliferation, maturation and functional activities in homeostasis and pathology[2,16,18]. Their stimulating effect during eosinophil maturation is modulated by influences of several transcription factors as well as protease activity regulator cystatin F and regulatory miRNAs (Figure 1)[16-19,21,23,27,28]. It is notable that terminally differentiated mature eosinophils produced in the bone marrow have fully formed specific granules essential for the functions of these cells (see below).
Mature human eosinophils can be easily morphologically distinguished by their bilobed nuclei and the presence of specific cytoplasmic granules stained red by eosin. These specific (also called crystalloid, secretory or “secondary”) granules are unique organelles that consist of a dense central crystalloid core and an outer matrix surrounded by a trilaminar membrane[4,29,30]. Although earlier publications sometimes mention the existence of both large “primary” granules lacking the crystalloid core and rich in Charcot-Leyden crystal protein and “small” granules, recent studies suggest that the coreless large granules are simply immature specific granules, whereas the “small” granules are membrane-bound vesiculotubular structures associated with eosinophil secretory activity[31]. Thus, there is only one granule type present in human eosinophils, and these specific granules store pre-formed biologically active substances comprising cytotoxic cationic proteins, cytokines, growth factors, chemokines and enzymes[4,32,33]. Other eosinophil-specific organelles are lipid bodies[34] charged with the synthesis of lipid mediators of inflammation (cysteinyl leukotrienes, thromboxanes and prostaglandins) and pleiomorphic vesiculotubular carriers (eosinophil sombrero vesicles)[35]. Like all somatic cells, eosinophils also have mitochondria, endoplasmic reticulum and Golgi bodies.
Specific granule formation and maturation associated with the accumulation of cationic granule proteins is essential for eosinophil development, function and survival[4,5,32,33]. Table 1 presents key biomolecules secreted by human eosinophils, and it is evident that, in addition to the well-known cationic proteins, these cells contain pre-formed stores of numerous regulatory molecules including cytokines, chemo-kines, growth factors and enzymes. Studies in murine models also demonstrated that parallel de novo synthesis of cytokines occurs in mature eosinophils[40-42], however the relationship between pre-formed and de novo synthesised regulatory factors remains to be elucidated.
| Substance | Characteristics | Pre-prodiced or de novo synthesysed | Main functions | Ref. |
| Located within the core of specific (crystalloid) granules | ||||
| Major basic proteins 1 and 2 (MBP1 and MBP2) | Small highly cationic proteins (MBP2 is less basic) | Pre-produced | Highly cytotoxic to host cells, antihelminthic, antibacterial (MBP1 is more potent and associated with EETosis) | [2,4,32,36] |
| Located within the matrix of specific (crystalloid) granules | ||||
| Eosinophil cationic protein (ECP) | Small highly cationic protein with a weak ribonuclease activity (ribonuclease 3) | Pre-produced | Highly cytotoxic to host cells, neurotoxic, antihelminthic, antibacterial, antiviral, associated with EETosis | [2,4,32,36] |
| Eosinophil-derived neurotoxin | Small basic (less cationic than MBP1 and ECP) protein with a ribonuclease activity (ribonuclease 2) | Pre-produced | Cytotoxic to host cells, neurotoxic, strongly antiviral, antibacterial, | [2,4,32,36] |
| Eosinophil peroxidase | Highly cationic heme-containing haloperoxidase | Pre-produced | Generates ROS exerting potent antibacterial and antihelminthic effects | [2,4,32,36] |
| Charcot-Leyden crystal protein (CLC, galectin-10) | Small slightly acidic protein | Probably pre-produced | Unclear, but involvement EETosis and a role in interactions between eosinophils and T-cells are suggested | [31,32,36-38] |
| Most likely stored within specific granules, but some may be synthesised de novo in the cytoplasm | ||||
| IL1β, IL2, IL3, IL4, IL5, IL6, IL10, IL11, IL13, IL16, IL18, IL25 (IL17E), IFNγ, GM-CSF, TGFα, TNFα, TNFβ, leukaemia inhibitory factor | Cytokines | Mostly pre-produced; some may be de novo synthesised | A wide range of signaling and regulatory functions | [4,5,32,39] |
| HB-EGF-LBP, NGF, PDGF, SCF, EGF, VEGF, APRIL | Growth factors | Pre-produced or de novo synthesised | Signalling functions related to cell proliferation and differentiation | [4,5,32] |
| CCL3, CCL5, CCL6, CCL7, CCL8, CCL9, CCL11 (eotaxin-1), CCL13, CXCL1, CXCL8, CXCL10, CXCL12 | Chemokines | Pre-produced or de novo synthesised | Cell migration regulation | [4,5,32,39] |
| Matrix metalloproteinases MMP9 and MMP17, acid phosphatase, collagenase, arylsulfatase B, histaminase, phospholipase D, catalase, non-specific esterases | Enzymes | Pre-produced or de novo synthesised | Inflammation-related effector functions including cytotoxicity, extracellular matrix modification and phagocytosis | [4,5,32] |
| Produced within the lipid bodies | ||||
| Leukotrienes C4, D4 and E4, Thromboxane B2, Prostaglandins E1 and E2, 15-hydroxyeicosatetranoeic acid, platelet-activating factor | Lipid signalling factors, mostly eicosanoids | De novo synthesised | A broad range of effects in inflammatory and allergic responses | [4,5,34] |
In addition to secretory substances, eosinophils express a considerable number of surface markers comprising receptors for adhesion molecules, cytokines, chemokines, growth factors, lipid mediators as well as pattern recognition receptors (PRRs) and Fc receptors. Table 2 lists hitherto identified eosinophil surface markers. Notably, stores of receptor molecules were also identified within the specific granules[55], and membrane receptors for cytokines and chemokines located on the surface of these granules enable them to act as receptor-mediated secretory organelles even extracellularly[56,57]. The abundance of receptors and cell surface-associated molecules defines eosinophil ability of participating in an extremely wide range of physiological and pathological processes. More details on eosinophil surface markers can be found in a few recent reviews (Rosenberg et al[5], Ravin et al[43] and Gangwar et al[44]).
| Group of surface markers | Markers identified for eosinophils | Ref. |
| Cytokine and growth factor receptors | IL-2R, IL-3R, IL-4R, IL-5R, IL-9R, IL-10R, IL-13R, IL-17R, IL-23R, IL-27-R, IL-31R, IL-33R (ST2), TSLPR, GM-CSFR, KIT, IFNγR, TGFβR | [5,43-45] |
| Chemokine and chemoattractant receptors | CCR1, CCR3, CCR4, CCR5, CCR6, CCR8, CCR9, CXCR2, CXCR3, CXCR4, FPR1, FPR2, C3aR, C5aR | [5,43,44,46] |
| Lipid mediator receptors | Platelet-activating factor receptor, DP1 prostaglandin receptor, DP2 prostaglandin receptor (CRTH2), EP4 prostaglandin receptor, E2 prostaglandin receptor, Leukotriene B4 receptor, Lysophosphatidylserine receptor P2Y10, S1P receptors | [5,43,44,47] |
| Fc receptors | FcαR, FcγRII, FcγRIII, FcεRI, FcεRII | [5,43,44] |
| Adhesion molecule receptors | Integrin αLβ2 (LFA1), Integrin αMβ2 (CR3), Integrin αXβ2 (CR4), Integrin α4β1 (VLA4), Integrin α6β1, Integrin αDβ2, Integrin α4β7 (LPAM), cell surface adhesion receptor CD44, CD62L (L-selectin), PSGL1 (P-selectin lipoprotein ligand), CD34, CD244 (2B4) | [5,43,44,48,49] |
| Pattern recognition receptors | TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR9, TLR10, NOD1, NOD2, RIG-1, RAGE | [5,43,44] |
| Other receptors and surface markers | PIRB, SIGLECs, SIRPα (shown for mice), LAIR1, Cannabinoid receptor CB2, Kinin B1 and B2 receptors, Histamine receptors, PAR1 or PAR2, CD80 or CD86, CD48, CD300 receptors (a and f), MHC class II | [5,43,44,50-53] |
The current view on the versatility of eosinophil action in health and disease was concisely formulated by Lee et al[3] in their LIAR (Local Immunity And/or Remo-delling/Repair) hypothesis. Expanding this notion, eosinophil functions can be classified into the following four categories: (1) Terminal effector functions; (2) Main-taining homeostasis and supporting tissue repair/remodelling; (3) Immunomo-dulatory role; and (4) Cooperative interactions with other immune cells[4]. Essentially, all functional activities of eosinophils in both normal and pathological conditions are exerted through the secretion of their specific products. Although classical or compound exocytosis implying granule fusion with the plasma membrane followed by extracellular release of entire granule contents[58] may be occasionally employed by eosinophils attacking multicellular helminths[33,59], this secretory mechanism is rarely observed during inflammatory and allergic responses, when immediate but selective release of pre-formed granule-stored factors is required. Extensive investigation of eosinophil degranulation modes has shown that either piecemeal degranulation (PMD) or cytolysis followed by whole granule release clearly prevail in most situations associated with human disease[33,59,60].
PMD is characterised by stimulus-dependent (receptor-mediated) differential packaging of selected granule-derived proteins into secretory vesicles that are then transported to the cell surface and expelled through it. During this process specific granules are partially emptied but otherwise remain intact[60]. Being highly selective and rapid, PMD is typically employed for cytokine secretion by eosinophils. It is the most common secretion type observed in the context of inflammation and allergy in humans[5,33,59,60].
Recent reports imply that cytolysis or primary lysis of human eosinophils[61] is the second most frequently observed degranulation mode in inflammatory and allergic conditions[33,60,61]. Cytolysis is now regarded as a regulated mechanism of rapid cell death (distinct from apoptosis and necrosis) that is characterised by chromatin de-condensation and dissolution of nuclear and plasma membranes as well as the release of intact specific granules retaining their functionality[33,56,57,59]. It was later demonstrated that eosinophil cytolysis is often accompanied by the formation of “extracellular traps”, i.e., web-like nets composed of extruded histone-coated strands of DNA and specific granules released from the same lysed cells[62-64]. The latter phenomenon is similar to the formation of bactericidal NETs (neutrophil extracellular traps) by neutrophils first described in 2004 by Brinkmann et al[65] and often defined as a unique form of cell death initially called NETosis[66]. It is, however, more appropriate to call this phenomenon ETosis since it is not neutrophil-specific and was observed in eosinophils[62-64], mast cells[67], basophils[68], monocytes[69], macrophages[70] and even B and T lymphocytes[71]. Moreover, an alternative, “non-lethal”, mechanism of extracellular DNA trap generation by eosinophils was described by Yousefi et al[72], who observed a catapult-like release of mitochondrial DNA from eosinophils remaining alive. The released DNA formed extracellular traps that also contained eosinophil granule proteins apparently secreted through PMD[72]. In the context of eosinophil effector functions, the association of released (by either mechanism) DNA fibres and functional specific granules (or their proteins) certainly provides a formidable defensive weapon that can be effectively used against infective agents (bacteria, viruses, fungi) or multicellular parasites (helminths)[64]. However, extracellular traps generated by eosinophils are highly cytotoxic and can damage host tissues. Both defensive and host-damaging effects of eosinophils are especially important for barrier tissues including the gut and will be discussed in more detail in further sections of this review.
According to generally accepted views, upon maturation in the bone marrow eosinophils enter the circulation and migrate to the gastrointestinal tract, their accumulation in the gut mucosa commencing during embryonal development[73,74].
IL5 and possibly GM-CSF stimulate the release of eosinophils from the bone marrow into the circulation[2,16,32]. Mouse eosinophils can stay in circulation for up to 36 h[75], and this estimate has later been shown to be close to their average 25-h intravascular presence in humans[76]. Eosinophil trafficking from the circulation to the gut and other peripheral target tissues (such as thymus, uterus and mammary gland in the normal conditions) is a complex multi-step sequence of events. As for all leukocytes, it comprises tethering, rolling, adhesion and transendothelial migration followed by polarisation and amoeboid movement in the interstitial space[73,77-81]. Alongside chemokine and cytokine signalling, the interaction between adhesion molecule receptors expressed by eosinophils and corresponding adhesion proteins of endothelial cells is crucially important for efficient eosinophil trafficking. Although leukocyte extravasation is better studied in inflammatory conditions, it can be assumed that in health the initial adherence (tethering) of eosinophils to endothelial cells and the initiation of rolling motion are selectin-dependent, whereas further rolling, firm adhesion and transendothelial migration are mediated by integrins[77-79]. In the context of eosinophil homing in the intestinal lamina propria (schematically shown in Figure 1), binding of the integrins α4β7 and α4β1 expressed by eosinophils to endothelial adhesion molecules MAdCAM1 and VCAM1 respectively is especially important, and interactions of αLβ2 and αMβ2 with ICAM1 appear to lead to the eventual transmigration from capillaries[48,82,83]. Further interstitial migration of eosinophils to their destination in the lamina propria is believed to be governed mostly by chemokine eotaxin-1 interacting with its CCR3 receptor on the surface of eosinophils[2,74]. Eotaxin-2 and eotaxin-3 may also contribute to the chemotaxis, but appear to be less eosinophil-specific[2,74]. In the human colon eotaxin-1 concentration gradient directing eosinophil migration depends on the secretion of this chemokine by intestinal macrophages and epithelial cells[84-86], while IL13 produced by ILC2 cells[26] stimulates macrophage eotaxin-1 expression (Figure 1).
Mouse experiments demonstrated that eosinophil homing in the gut occurs in the foetal life, i.e., independently of later intestinal colonisation by microbiota[73]. In the normal human gastrointestinal tract the presence of eosinophils increases in the distal direction (oesophagus < stomach < small intestine < colon) reaching its peak in the caecum and ascending colon[87-89]. As already mentioned, in the normal gut eosinophils are primarily located in the lamina propria of the mucosa rather than in the surface epithelium[82,90].
Unfortunately, functions of human gastrointestinal eosinophils in health remain poorly investigated, mouse models being the main source of available information. Still, recent progress in this complex field allows making some generalisations.
Eosinophil presence in the gut was previously believed to simply constitute an effector element of the innate host defensive barrier, but it is now becoming clear that resident intestinal eosinophils continuously monitor and modulate complex immune responses and tissue remodelling throughout the huge surface of the gastrointestinal tract[3]. It is remarkable that, unlike their circulating counterparts, gastrointestinal eosinophils exhibit an activated phenotype suggesting permanent functional activity[4,91]. Participation in the following physiological mechanisms is currently attributed to these gut-dwelling cells: (1) Maintenance of gastrointestinal mucosal barrier function; (2) Provision of immunity to pathogens present in the gut lumen; (3) Interactions with the enteric nervous system; (4) Linking innate and adaptive immunity[4]. These functions are discussed in more detail hereafter.
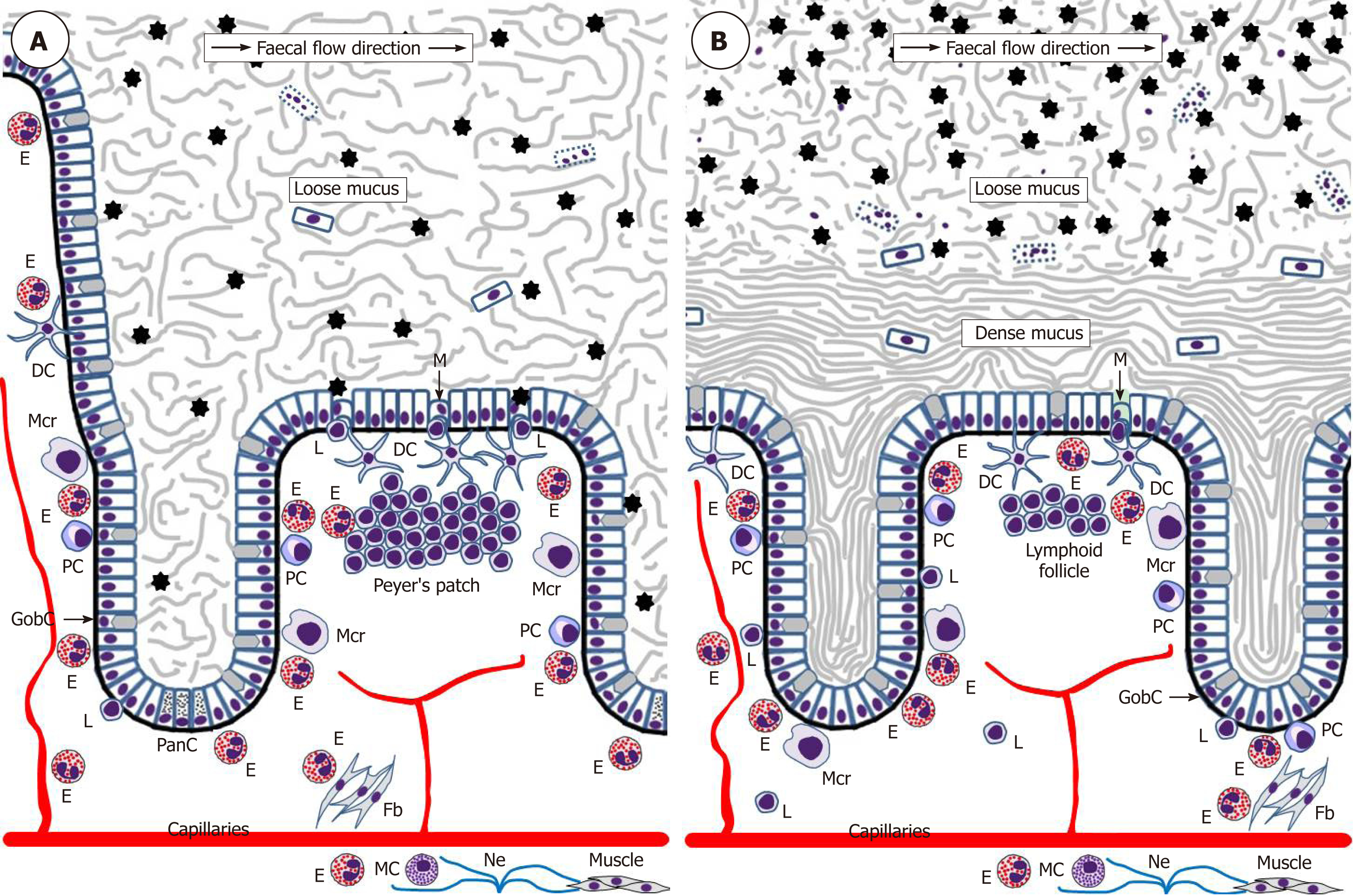
The intestinal epithelium acts as a uniquely important body interface with the environment. In addition to its physical barrier function that prevents underlying tissues from contacting harmful microbiota and dietary antigens of the gut lumen, this epithelium regulates the selective absorption of nutrients, water and electrolytes[92]. The epithelial surface throughout the gut is covered by the glycocalyx of enterocytes and colonocytes[93] and abundant mucus rich in mucin 2 (MUC2) produced by goblet cells. The utmost importance of this mucus became evident only recently, and it is now established that the structure of the mucus layer throughout the intestine is not uniform. The small intestine has a single and relatively loose layer of mucus populated by microbiota, whereas the colon has a two-layered system with a dense inner layer firmly attached to the mucosal surface and an outer layer permeable for bacteria (Figure 2)[94,95]. It is remarkable that this structure essentially excludes any direct contact of bacteria with the colonic epithelium. The protective role of the mucus is further enhanced by the presence of antibacterial substances, such as α-defensins and lysozyme secreted by Paneth cells of small intestinal crypts[96] and IgA produced by plasma cells of the lamina propria[97,98].
Interestingly, it has recently been shown that intestinal mucus layer maintenance depends on eosinophil presence in the lamina propria since eosinophil-deficient mice had significantly decreased numbers of mucus-secreting goblet cells in the small intestine[98]. This is not surprising since eosinophils are known to directly induce mucin production in airway epithelial cells by activating EGFR cascade[99]. Moreover, although possible interactions between eosinophils and Paneth cells remain obscure, the transcription factor Xbp1 is important for the development of the both cell types[27], and, like Paneth cells, human eosinophils were demonstrated to produce α-defensins[100]. It was also reported that eosinophils are required for maintaining mucosal IgA production by plasma cells in the lamina propria[97,98,101], probably through mechanisms involving IL1β[98] and TGFβ[97,100] signalling.
Taken together, this information suggests that eosinophils are intimately involved in the maintenance of the protective intestinal mucus in the normal conditions.
The immune system of the gut is an extremely complex entity, analysis of which is beyond the scope of this review, but eosinophil impacts are highlighted herein. It is obvious that in the normal homeostatic conditions immune surveillance focused on sampling and assessing luminal antigens occasionally contacting the mucosal surface is of utmost importance. The gut-associated lymphoid tissue (GALT) is believed to be responsible for luminal antigen sampling. In the small intestine this function is primarily associated with microfold (M) cells[102-104] located in the epithelium overlaying Peyer’s patches (Figure 2) and capable of communicating with immune cells comprising B lymphocytes, T lymphocytes, macrophages and dendritic cells. M-cells are also believed to be present in the epithelium overlaying lymphoid follicles in the colon[103], but their location and functions in the normal human colon remain very poorly investigated. M-cells exert luminal antigen transcytosis followed by antigen transfer to the relevant immune cells of the lamina propria, but alternative antigen capture mechanisms appear to exist as well. Dendritic cells were shown to sample bacterial antigens by extending their dendrites to penetrate the epithelium and reach the lumen[105,106]. In addition, goblet cells can deliver low molecular weight intestinal antigens to the underlying dendritic cells of the lamina propria[107]. The latter mechanism shown for the normal small intestine[107] may be especially important for the colon, where it becomes activated only when mucus barrier, luminal microbiota balance or microbial sensing by goblet cells are disturbed[108]. Given that the stratified mucus layer securely protects colonic epithelium from any contact with gut contents in the normal conditions, it is likely that immune cells located in the colonic lamina propria may be completely unaware of luminal antigens until the protective barrier is damaged[108]. Although the role of eosinophils in luminal antigen recognition and presentation at baseline remains poorly investigated, it should be noted that these granulocytes promote the development of Peyer’s patches[98] and are known to be closely involved in regulating dendritic cell activation and migration[109]. In addition, intestinal eosinophils can express antigen presentation-associated markers, including MHCII and CD80[91] as well as activating receptor FcγRIII[110]. Their possible antigen-presenting function has not been demonstrated in the normal gut and remains to be investigated.
Eosinophil influences on the nervous system were extensively studied in the context of asthma and allergic respiratory inflammation[111,112]. It is also known that human eosinophils can produce nerve growth factor in abundance[113]. Eosinophil-nerve interactions in the gut were demonstrated experimentally[114], and accumulating evidence indicates that eosinophil impact on the enteric nervous system may often be exerted through mast cells residing in the lamina propria[115]. Indeed, eosinophils and mast cells are often found in close proximity to each other. Physical interaction between them can induce a hyperactivation state accompanied by soluble mediator release[116,117]. Mast cells and eosinophils in the gut are often located near sensory nerve fibres and are known to be involved in the pathogenesis of functional gastrointestinal disorders accompanied by motility changes, hyperalgesia and diarrhoea[115,118]. Concerted action of eosinophils and mast cells can produce multiple effects in addition to interactions with the nervous system, being among major factors in allergy and responses to infections[119], which are beyond the scope of this review.
It is now recognised that eosinophils can modulate T cell-mediated immune responses, owing to their ability to rapidly produce cytokines, chemokines and growth factors[3-5], but little is known about their regulatory functions in the normal intestine. Although eosinophils are able to secrete cytokines associated with both Th1 and Th2 cells[86], in the gut they appear to be primarily associated with Th2 immunity, being producers of Th2-inducing cytokines IL4 and IL13[120]. It is remarkable that IL13 was detectable in a considerable fraction of these cells in the normal human duodenal lamina propria[121]. Recent demonstration of significantly increased Th1 cell presence in the gut of eosinophil-deficient transgenic mice corroborates organ-specific Th1 immunity suppression by these granulocytes[122].
It is assumed that eosinophils may regulate the magnitude and Th2 polarisation of immune responses through interactions with B and T lymphocytes[86]. However, there are many unanswered questions regarding types of cell-mediated immune responses and corresponding cytokine profiles, and a view advocating the existence of three rather than two types of effector immunity has recently emerged[123]. Only further intense research in this dynamic area will lead to better understanding of eosinophil interactions with other immune cells in the human gut.
Clearly, the four topics highlighted above do not entirely cover all activities of intestinal eosinophils. For instance, experimental studies have revealed eosinophil influences on smooth muscle cells[74,124], fibroblasts[74,125] and capillary endothelium[74], but these roles remain to be investigated for the normal human gut.
The author opted not to address eosinophil-associated gastrointestinal disorders (EGID) comprising eosinophilic oesophagitis, eosinophilic gastritis, eosinophilic enteritis and eosinophilic colitis as well as gastrointestinal manifestations of hypereosinophilic syndromes. These relatively rare conditions were extensively reviewed elsewhere[83,86,126,127]. The concluding part of this review is focused on two major colorectal diseases: IBD and CRC.
IBD is represented mainly by two major conditions: ulcerative colitis (UC), which affects exclusively colonic mucosa[10], and Crohn’s disease (CD), a transmural asymmetrical inflammation that can involve the entire gastrointestinal tract[9]. These diseases are highly prevalent all over the world[128-131]. Both UC and CD are chronic relapsing disorders with complex and not entirely understood mechanisms of development. IBD etiopathogenesis is, however, believed to include genetic predisposition, environmental/microbial impacts, intestinal barrier dysfunction and dysregulation of the mucosal immune system of the gut[6-10,132]. The latter two pathogenetic components can certainly be influenced by eosinophils, the abundance of which, correlating with disease severity, is well documented in the gut mucosa of patients with UC[133-140] and some CD cases[135,137,141,142]. The increased presence of eosinophils in the mucosa of these patients apparently results from an enhanced production of eotaxin 1 in the lamina propria[84,143-145] by colonocytes[84,136], macro-phages[84,145] or B lymphocytes[144]. Furthermore, Manousou et al[146] described an elevated expression of eotaxin receptor CCR3 in colonic biopsy samples from UC rather than CD patients. Although the authors interpreted this finding as a sign of the accumulation of CCR3-expressing T cells, it is likely that lamina propria eosinophils expressing the same receptor could be at least equally responsible for this UC-associated change. Conversely, serum eotaxin levels were reported to surge in active IBD[141,147,148], but the increase looked more pronounced in CD patients compared to UC cases[147,148]. The contrast between UC and CD becomes even more evident in view of different patterns of colonic eosinophil activation described by Lampinen et al[84,136,137,145,170,186,188] and indicating that in disease remission activated eosinophils persisted in the lamina propria of UC, but not CD patients[136,137].
The observed differences in colonic eosinophil presence and activity between the two IBD types are not surprising because it is traditionally accepted that CD pathogenesis, which has a stronger genetic component[6], is dominated by Th1 cytokine profile (combined with Th17 influences)[149-152], whereas Th2 immune response tends to characterise UC[150-153]. Given recent advances in the understanding of immune response types, involved cell populations and cytokine profiles[123,154,155], this straightforward division may now look simplistic, but the association of eosinophils with Th2 immunity is well proven[120]. Therefore, their role in UC pathogenesis looks more evident and easier to explain.
Before further discussing the role of eosinophils in IBD development it will be useful to briefly address gut barrier-related aspects of disease onset. Although numerous factors admittedly contribute to IBD pathogenesis[6-8,156], the precise mechanism of disease initiation remains elusive. Multiple lines of evidence indicate that in most cases IBD can be triggered by an initial contact of the gut microbiota with the mucosal immune cells followed by the development of inadequate immune responses. It is probable that preconditions for this initial contact are associated with functional deficiencies of the protective barrier of the gut mucosa, and they are likely to differ between CD and UC. Indeed, the loose mucus layer of the small intestine may be easily penetrated by microorganisms that then directly contact the epithelium, particularly mucosal M-cells[102-104] of the ileum. Such events can be facilitated by α-defensin deficiency caused by Paneth cell dysfunction that is frequently observed in CD patients, including those genetically predisposed to the disease[157,158]. In contrast, M-cells in the normal colonic mucosa can hardly be reached by microbiota since two layers of mucus, especially the dense inner one[93-95], exclude any bacterial contact with the epithelium (Figure 2B). The protective role of the inner mucus layer is well illustrated by spontaneous colitis development in MUC2-deficient mice[159,160]. Bacterial penetration through the inner mucus layer was observed in UC patients[161], but it is not entirely clear what triggers the initial change of inner mucus layer properties. Mouse models suggest MUC2 secretion deficiency[159,160] or goblet cell depletion[162] as probable causes, and goblet cell numbers in UC patients, indeed, tend to be reduced[163]. Some authors believe that unresolved endoplasmic reticulum stress and the unfolded protein response are early events leading to goblet cell disfunction and mucus layer impairment in UC[164,165]. Altered eosinophil behaviour may well be associated with these phenomena, but this possibility remains to be investigated. Alternatively, the inner colonic mucus layer can be primarily damaged by mucus-degrading bacteria of the gut lumen[166]. The latter process may potentially be modulated by dietary factors since it was experimentally demonstrated that dietary fibre deficiency leads to switching of the gut microbiota on using mucus glycoproteins as a nutrient source and eventual erosion of the mucus barrier[167]. All the pathogenetic components discussed above may contribute to IBD initiation in humans, but further research in the area is obviously needed.
Whatever scenario causes IBD initiation, there is little doubt that bacterial antigen interaction with the gut-associated lymphoid tissue triggers complex cascades of inadequate immune responses leading to disease development. In these circum-stances, activated eosinophils present in the lamina propria predominantly start acting as effector cells, excessive protective response of which can cause serious damage to the host through several mechanisms. Activated eosinophils accumulating in the gut of IBD patients[133-142] have an extended lifespan[168], and their degranulation leads to a massive release of both cytotoxic granule proteins and pro-inflammatory cytokines. Gut epithelium is one of the key targets of cytotoxic eosinophil proteins as it was demonstrated that MBP alters colonic epithelium barrier function[169]. Another mechanism of eosinophil contribution to colonic barrier dysfunction in UC involves muscarinic receptors expressed by these granulocytes. Wallon et al[170] showed that cholinergic signals received by the muscarinic receptors caused corticotropin-releasing factor (CRF) production by eosinophils, and CRF induced degranulation of neighbouring mast cells that led to an increase in mucosal barrier permeability. Concerted action of eosinophils and mast cells may have multiple endpoints in IBD as the both types of cells are important sources of cytokines comprising TNFα that, intriguingly, induces M-cell appearance in the mouse colon during inflammation[171]. Moreover, cooperation between eosinophils and mast cells was demonstrated in CD-associated fibrosis development at later stages of the disease[172]. Inflammation intensity can also be aggravated by human gut eosinophils through blocking anti-inflammatory interleukin 22 (IL22)[173] by overproducing IL22-binding protein in both CD and UC patients[174].
Interactions of gut eosinophils with other immune cells, especially lymphocytes, are very complex and poorly investigated in IBD patients. There is no room for discussing all of them here, but crosstalk between eosinophils and neutrophils deserves to be mentioned. Neutrophils are not present in the lamina propria in the normal conditions but are rapidly attracted there when inflammatory response develops (Figure 3). It is believed that chemokines, especially CXCL8, produced by gut epithelium in inflammation trigger neutrophil chemotaxis[175]. Interestingly, eosinophil impact in this process is now becoming evident since they can synthesise CXCL8[176]. Also, eosinophils were demonstrated to cooperate with colonic epithelium in producing a wider range of neutrophil chemoattractants[177]. In conclusion of discussing the role of lamina propria eosinophils in IBD it needs to be noted that the presented facts are mostly related to human disease. There is a considerable body of additional information obtained in murine models which has been reviewed elsewhere[178].
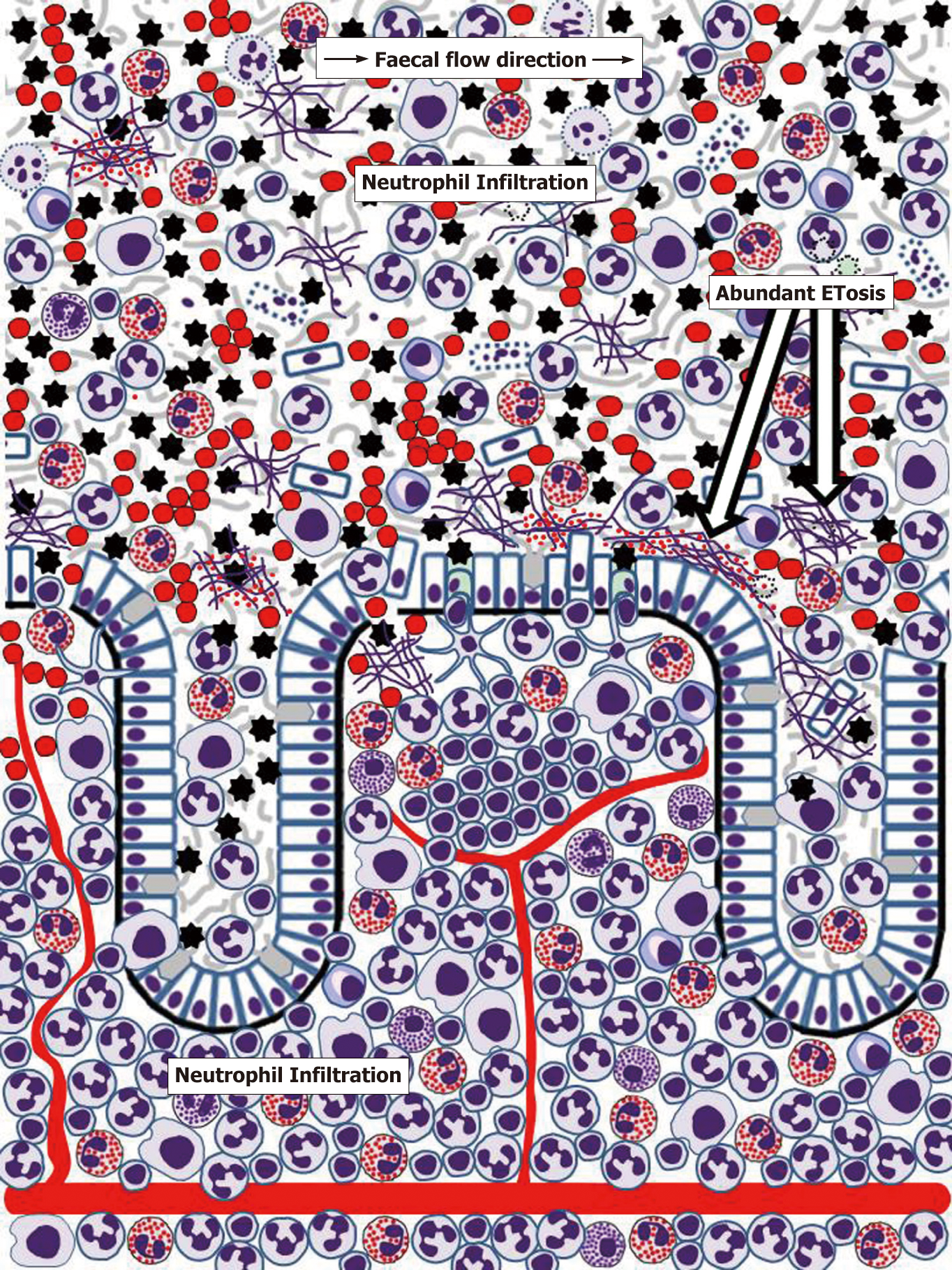
Until recently, pathogenetic mechanisms of IBD were considered only at the level of events occurring within the gut wall. However, it is now becoming evident that gut mucus layer presents another, poorly investigated but potentially highly important, battlefield for innate immune responses. Although massive neutrophil influx to the mucosa, often leading to crypt abscess formation, is recognised as a hallmark feature of IBD, the importance and mechanisms of immune cell migration through mucosal epithelia remain poorly understood and insufficiently investigated[179,180]. Transepithelial neutrophil migration involving a chain of molecular events that include initial attachment to the basal surface of epithelial cells, movement through paracellular space (passing by desmosomes, adherens junctions and tight junctions) and eventual contact with the apical membrane of the epithelium is relatively well understood[179,180]. Moreover, it is known that following transmigration neutrophils release MMP9-rich microparticles that disrupt epithelial junctions facilitating further transmigration[182]. Rapid eosinophil migration through tracheobronchial epithelium in experimental conditions was also reported[181], but remains unexplored in the gut. The process is, thus, well-defined, but the final destination of cells crossing the epithelial barrier remains obscure, being often indicated simply as “gut lumen”[179,180]. Neutrophil-associated markers of inflammation are, indeed, easily detectable in faeces of most patients with active UC and CD, as the popularity of faecal diagnostic tests, particularly stool calprotectin, proves[183,184]. Similarly, the presence of eosinophil markers in stool or colorectal perfusion fluid of IBD patients was repeatedly reported[184-188]. It is, however, apparent that any cells or biomolecules leaving gut epithelium surface should first enter mucus barrier already discussed above, and only its occasionally separated fragments can be incorporated into the faecal matter. The author of the present review previously hypothesised that colorectal mucus retains these highly informative cells and molecules and can be conveniently used for diagnosing colorectal diseases[189]. Studies of our group convincingly demonstrated the abundance of inflammatory cells, predominantly neutrophils, in colorectal mucus collected from IBD patients either intrarectally[190,191] or non-invasively[192,193]. Common eosinophil presence in this material was also noted[191,193], especially in UC patients[193], and dramatically increased EDN levels were determined[191,194], typically with higher values in UC compared to CD[194]. Detailed cytological analysis of these samples demonstrated that colorectal mucus from patients with active IBD commonly contains not only huge amounts of neutrophils, but also eosinophils, macrophages, erythrocytes as well as occasional plasma cells, lymphocytes and basophils[193]. Most of these cells are viable and functionally active as our frequent observations of phagocytosis by neutrophils and macrophages indicate[193]. Furthermore, it appears that signs of ETosis were also present in colorectal mucus from IBD patients[193]. These findings allow hypothesising that gut mucus acts in IBD as a unique additional milieu, where immune responses expand from the mucosa. It is apparent that the abundance of active cells, especially granulocytes releasing contents of their granules, considerably loosens the inner mucus layer in the colon, thus making it both permeable for gut microbiota and facilitating further immune cell transmigration and movement through the mucus. These circumstances should favour antibacterial activity of the effector cells, but extensive collateral damage of host epithelium is highly likely. ETosis exerted by neutrophils, eosinophils and other immune cells[62-72] may be especially important in this context. Figure 3 reflects the author’s opinion on the extent of inflammatory process in the human gut.
Protection from invading microbiota appears to be the main biological aim of ETosis since histones enveloping released DNA are antibacterial[195], and the addition of DNA strands may increase mucus viscosity, thus mechanically compensating for MUC2 degradation. Further antibacterial action is provided by granule proteins of both neutrophils[196,197] and eosinophils (Table 1). However, ETosis in the mucus, especially combined with the release of intact eosinophil granules, can seriously damage enterocytes or colonocytes. Eosinophil-derived extracellular DNA traps have already been shown to injure airway epithelium in chronic obstructive pulmonary disease[198] and chronic rhinosinusitis[199], where MBPs released from specific granules were especially toxic[200]. This phenomenon is still poorly investigated in relation to UC and CD, but interest in IBD-associated ETosis is emerging. In addition to our results discussed above, NET presence has been demonstrated in biopsy samples from IBD patients[201-203], notably within crypt abscesses[201], i.e., beyond the mucosa. Although the significance of immune responses occurring within colorectal mucus remains to be elucidated, it is impossible to exclude that eosinophil-generated extracellular DNA threads, loaded with entrapped specific granules that release cytotoxic cationic proteins, can cause a continuing colonocyte damage leading to sustained ulceration. This so far unexplored mechanism may constitute an important pathogenetic factor in UC and, to some extent, in colonic CD. On the other hand, it should not be forgotten that transepithelial migration of immune cells is a major factor in mucosal disease resolution, as demonstrated for airway diseases[204,205]. Indeed, the presence of both inflammatory cells and biomarkers associated with them significantly decreased in colorectal mucus samples from successfully treated IBD patients[194]. It is, therefore, probable that gradual distal movement of colorectal mucus[189] creates favourable conditions for eliminating dead or obsolete immune cells from the surface of colonic epithelium if inflammation is successfully resolved.
The presented analysis of literature on eosinophil impact in IBD pathogenesis reveals that eosinophils are closely involved in this process through regulatory activities, interactions with other cells and tissues and effector functions that can often be excessive and damage the host. The impact of eosinophils appears to be especially important in altering the structure and protective functions of the mucosal barrier. The author also tried highlighting an interesting new research direction related to exploring poorly investigated immune responses occurring in the protective mucus layer of the gut. It is, however, apparent that our understanding of IBD pathogenesis, including eosinophil participation in it, remains fragmentary and needs further thorough investigation. For this reason, it would be premature to speculate on possible therapeutic interventions targeting eosinophils in IBD.
CRC is one of the most frequent oncological conditions with estimated global figures of 1801000 new CRC cases and 861700 deaths due to this disease in 2018[206]. Although there are many good reviews addressing various aspects of CRC pathogenesis[11-15], possible role of eosinophils in this process is usually overlooked despite the existence of reports deserving attention and briefly discussed below.
Eosinophil infiltration is often observed in malignancies, but for different tumours it was reported as either prognostically favourable or unfavourable[3,4,207-210]. Nevertheless, the presence of eosinophils in CRC patients is strongly linked with a decreased disease risk, better prognosis and extended patient survival. Indeed, elevated blood eosinophil counts were associated with a decreased CRC development risk[211] as well as better prognosis[212-214]. Eosinophil infiltration of colorectal tumours is a common phenomenon, and higher numbers of infiltrating eosinophils detected both in the tumour tissue[215-217] and peritumourally[218-220] were repeatedly shown to be prognostically favourable. Despite these seemingly cogent findings, the quoted descriptive clinical studies could not provide any direct evidence of anti-cancer eosinophil action, and possible mechanisms of CRC growth inhibition by eosinophils remain poorly understood.
Tumour-associated inflammation is currently recognised among hallmarks of cancer[11]. Eosinophil accumulation accompanying inflammation-related cancer cell death and proliferation in CRC is one of its components that probably reflects Th2 immune responses enhanced at the expense of Th1 immunity[3]. Besides, eosinophils are likely to stimulate tissue remodelling and tumour-related angiogenesis[3,221]. The latter MBP-modulated effect may in theory promote tumour growth, however only non-cytotoxic MBP concentrations enhanced angiogenesis in vitro[221], and significant eosinophil infiltration in CRC is likely to produce high MBP concentrations. Ellyard et al[222] argued that some components of Th2-driven inflammation in cancer can be associated with anti-tumour activity of CD4+ Th2 cells collaborating with tumour-infiltrating granulocytes, especially eosinophils that exert regulatory functions. In any case, it is now becoming clear that there are several mechanisms driving eosinophil attraction to tumours and defining their influence on malignant tissue. In particular, it was demonstrated in vitro that eosinophil chemotaxis could be induced by necrotic, but not viable cells of neoplastic intestinal epithelium[223]. Experiments in a xenograft mouse model indicated that eosinophil infiltration developed rapidly, involved mostly tumour necrotic areas or capsule regions and was not associated with the presence of CD4+ T cells[224], thus suggesting that eosinophil chemotaxis depended on tumour-derived factors, such as damage-associated molecular pattern molecules (DAMPs) including the nuclear protein high mobility group box 1 (HMGB1)[225]. Notably, the presence of cell-free cytotoxic MBP was confined to the necrotic areas of tumours[224]. There are also reports describing expression of eosinophil attractant ecalectin (variant of galectin-9) by human colorectal carcinoma cell lines[226] and of eotaxin 1 in tumours resected from CRC patients[227]. The latter phenomenon was recently investigated further in a mouse model by Hollande et al[228], who found that IL33 expressed by tumour cells induced eotaxin 1 production that led to eosinophil recruitment and degranulation-dependent suppression of tumour growth[228]. Interestingly, eotaxin 1 concentration is negatively regulated by serine protease DPP4, and treatment with DPP4 inhibitor sitagliptin was shown to result in an increase in eotaxin 1 level[228]. Sitagliptin is a drug already approved by the US FDA for hyperglycaemia treatment, and it may potentially be re-purposed as a new anti-tumour agent promoting tumoricidal action of eosinophils[228,229]. Coming back to IL33, its role as an influential modulator of early stages of eosinophil development[22], was noted in the beginning of this review, but it is also a potent eosinophil activator stimulating their degranulation[230]. Moreover, it was reported that IL33-deficient mice had gut microbiota dysbiosis and were highly susceptible to both colitis and colitis-associated cancer[231], which could also be related to impaired eosinophil-driven responses. Hence, multiple parallel pathways are likely to be involved in generating eosinophil infiltration of colorectal tumours and direct killing of malignant cells, a phenomenon already proven experimentally. In vitro studies by French investigators assessing tumoricidal activity of eosinophils against Colo-205, a human colon carcinoma cell line, have shown that this effect was mediated by eosinophil-produced ECP, TNFα and proteolytic granzyme A[232]. The same group later reported that eosinophil attachment to Colo-205 cells depended on interaction between adhesion molecules LFA-1 and ICAM-1 that was upregulated by IL18[233]. Direct tumoricidal effect of tumour-infiltrating degranulating eosinophils was also observed in model experiments using genetically modified mice[234]. It is, however, obvious that the tumoricidal action of eosinophils in vivo may involve multiple interactions with other immune cells. Notably, there is evidence that eosinophils can promote either Th2 or Th1 immune responses, depending on varying cytokine profiles[86,235]. Carretero et al[236] have recently shown in experiments with xenograft-bearing mice that tumour-homing eosinophils secreted chemoattractants that guided CD8+ effector T cells to tumours, eventually causing tumour rejection. Thus, it is apparent that different immune response scenarios can be involved in anti-CRC action of eosinophils.
Completing this final section of the review, the author is tempted to briefly mention already discussed ETosis as another possible, but hitherto poorly investigated factor in CRC. One interesting link here is provided by recently published results implicating inflammation-induced ETosis in extracellular matrix remodelling and awakening of dormant cancer cells[237]. As tumour-associated inflammation is a characteristic feature of CRC, and significantly increased extracellular trap formation in these tumours is now proven[238,239], it looks probable that previous reports of CRC-associated increase in the amount of DNA in stool[240,241] or on the surface of colorectal mucosa[190,242] at least partially reflected abundant ETosis occurring within mucus layers contacting tumour surface. In essence, cellular presence in the mucus overlaying colorectal tumour surface is quite similar to that depicted by Figure 3, the abundance of exfoliated malignant cells being the only major difference[190]. Although today there is no published evidence of eosinophil contribution in CRC-related ETosis, this evidence is very likely to emerge soon. In contrast to anti-cancer effects of eosinophils in fully developed tumours, which were discussed above, it is impossible to exclude carcinogenicity of eosinophil-derived DNA traps loaded with highly cytotoxic released granules damaging colonic mucosa. Such a carcinogenic action could be involved at early stages of CRC development, especially in IBD-associated context[243,244]. Conversely, eosinophil-driven ETosis may prevent further tumour expansion at later stages of advanced tumour growth.
The presented analysis of literature establishes a link between eosinophil presence and favourable CRC prognosis, but functional versatility of these multifaceted cells may comprise both anti-cancer and tumour-promoting features. Only several experimental studies addressing eosinophil roles in cancer could be highlighted, however it already becomes transparent that alternative mechanisms involving both direct effector action of eosinophils and complex cooperation with other immune cells, especially T lymphocytes, can be engaged in different circumstances. This fascinating area still poses numerous unanswered questions requiring further intense investigation.
Upon the presented analysis of the current literature it can be concluded that eosinophils are now regarded as multifunctional mobile cells routinely involved in controlling and regulating a range of biological pathways and responses in both health and disease. The versatility of eosinophils largely depends on the availability of numerous biologically active substances (cytotoxic cationic proteins, cytokines, growth factors, chemokines, enzymes) stored in their specific granules and ready for rapid release. The lamina propria of the human gut is one of the main destinations of eosinophils produced and matured in the bone marrow and transferred through the circulation. In the normal physiological conditions, the most important functions of gut-residing eosinophils appear to be their participation in the maintenance of the protective mucosal barrier and interactions with other immune cells, particularly in providing immunity to microbiota inhabiting the lumen of the gut. In health the latter regulatory role may be more important in the small intestine compared to the colon, mostly due to structural differences of mucus layers covering epithelial surfaces of these two segments of the intestine. Eosinophils are proven to be closely involved in the development of inflammation in IBD, when their uncontrolled cytotoxic effector functions cause damage to host tissues. However, their roles in CD and UC differ. While eosinophil impact in UC pathogenesis largely corresponds to Th2 immune response pattern, the involvement of these cells in CD pathogenesis is less clear. Eosinophils in IBD appear to be especially important in altering the structure and protective functions of the mucosal barrier and modulating massive neutrophil influx to the lamina propria followed by transepithelial migration to colorectal mucus. The author believes that accumulating evidence suggests that IBD-associated inflammatory process expands to the mucus overlaying the internal gut surface, and the presence of eosinophils in this mucus is well documented. It can be hypothesised that colorectal mucus presents a previously unexplored unique milieu for disease-related inadequate immune responses in the gut. These responses involving cytotoxic effects and ETosis exerted by both neutrophils and eosinophils on the both sides of the colonic epithelial barrier act as additional pathogenetic factors leading to colonic epithelium ulceration in IBD. However, further research is needed for testing the proposed hypothesis. Literature analysis also highlights an association between elevated eosinophil levels and better CRC prognosis. Mechanisms behind this link may involve both direct anti-tumour action of eosinophils and complex cooperation of eosinophils with T cells but remain to be elucidated. This challenging area still presents an important goal for future research.
It should be admitted that addressing all interesting topics related to eosinophil role in the gut was impossible for obvious reasons. A huge amount of literature covering gut immunity in health and disease exists, and certain selectivity was inevitable. Nonetheless, the author would like to specifically emphasise his opinion on the importance of investigating colorectal mucus in the context of major colorectal diseases including IBD and CRC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong KY, Perse M, Vagholkar KR S-Editor: Ma RY L-Editor: A E-Editor: Liu JH
| 1. | Kay AB. The early history of the eosinophil. Clin Exp Allergy. 2015;45:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1119] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 3. | Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 4. | Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343:57-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 679] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 6. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1534] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 7. | Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62:1653-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1114] [Article Influence: 123.8] [Reference Citation Analysis (1)] |
| 9. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1788] [Article Influence: 223.5] [Reference Citation Analysis (111)] |
| 10. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2464] [Article Influence: 308.0] [Reference Citation Analysis (2)] |
| 11. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47006] [Article Influence: 3357.6] [Reference Citation Analysis (5)] |
| 12. | Pernot S, Terme M, Voron T, Colussi O, Marcheteau E, Tartour E, Taieb J. Colorectal cancer and immunity: what we know and perspectives. World J Gastroenterol. 2014;20:3738-3750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1117] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 14. | Grady WM, Markowitz SD. The molecular pathogenesis of colorectal cancer and its potential application to colorectal cancer screening. Dig Dis Sci. 2015;60:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. 2017;32:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 16. | Ackerman SJ, Bochner BS. Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders. Immunol Allergy Clin North Am. 2007;27:357-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Uhm TG, Kim BS, Chung IY. Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol Res. 2012;4:68-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Willebrand R, Voehringer D. Regulation of eosinophil development and survival. Curr Opin Hematol. 2017;24:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Fulkerson PC. Transcription factors in fosinophil development and as therapeutic targets. Front Med (Lausanne). 2017;4:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Drissen R, Buza-Vidas N, Woll P, Thongjuea S, Gambardella A, Giustacchini A, Mancini E, Zriwil A, Lutteropp M, Grover A, Mead A, Sitnicka E, Jacobsen SEW, Nerlov C. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat Immunol. 2016;17:666-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 21. | Bouffi C, Kartashov AV, Schollaert KL, Chen X, Bacon WC, Weirauch MT, Barski A, Fulkerson PC. Transcription factor repertoire of homeostatic eosinophilopoiesis. J Immunol. 2015;195:2683-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, Berdnikovs S, Bryce PJ. IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol. 2016;197:3445-3453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 23. | Buitenhuis M, van Deutekom HW, Verhagen LP, Castor A, Jacobsen SE, Lammers JW, Koenderman L, Coffer PJ. Differential regulation of granulopoiesis by the basic helix-loop-helix transcriptional inhibitors Id1 and Id2. Blood. 2005;105:4272-4281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Shahrin NH, Diakiw S, Dent LA, Brown AL, D'Andrea RJ. Conditional knockout mice demonstrate function of Klf5 as a myeloid transcription factor. Blood. 2016;128:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Iwasaki H, Mizuno S, Mayfield R, Shigematsu H, Arinobu Y, Seed B, Gurish MF, Takatsu K, Akashi K. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J Exp Med. 2005;201:1891-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 692] [Cited by in RCA: 856] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 27. | Bettigole SE, Lis R, Adoro S, Lee AH, Spencer LA, Weller PF, Glimcher LH. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat Immunol. 2015;16:829-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 28. | Matthews SP, McMillan SJ, Colbert JD, Lawrence RA, Watts C. Cystatin F ensures eosinophil survival by regulating granule biogenesis. Immunity. 2016;44:795-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Melo RC, Dvorak AM, Weller PF. Contributions of electron microscopy to understand secretion of immune mediators by human eosinophils. Microsc Microanal. 2010;16:653-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Muniz VS, Weller PF, Neves JS. Eosinophil crystalloid granules: structure, function, and beyond. J Leukoc Biol. 2012;92:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Melo RCN, Weller PF. Contemporary understanding of the secretory granules in human eosinophils. J Leukoc Biol. 2018;104:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 573] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 33. | Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017;17:746-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 341] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 34. | Melo RC, Weller PF. Unraveling the complexity of lipid body organelles in human eosinophils. J Leukoc Biol. 2014;96:703-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Melo RC, Spencer LA, Dvorak AM, Weller PF. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J Leukoc Biol. 2008;83:229-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406-17415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 367] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 37. | Ueki S, Tokunaga T, Melo RCN, Saito H, Honda K, Fukuchi M, Konno Y, Takeda M, Yamamoto Y, Hirokawa M, Fujieda S, Spencer LA, Weller PF. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood. 2018;132:2183-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 38. | Lingblom C, Andersson J, Andersson K, Wennerås C. Regulatory eosinophils suppress T cells partly through galectin-10. J Immunol. 2017;198:4672-4681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Melo RC, Liu L, Xenakis JJ, Spencer LA. Eosinophil-derived cytokines in health and disease: unraveling novel mechanisms of selective secretion. Allergy. 2013;68:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Fulkerson PC, Schollaert KL, Bouffi C, Rothenberg ME. IL-5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J Immunol. 2014;193:4043-4052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M, Schleich FN, Radermecker M, Thielemans K, Gillet L, Thiry M, Belvisi MG, Louis R, Desmet C, Marichal T, Bureau F. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279-3295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 411] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 42. | Grace JO, Malik A, Reichman H, Munitz A, Barski A, Fulkerson PC. Reuse of public, genome-wide, murine eosinophil expression data for hypotheses development. J Leukoc Biol. 2018;104:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2016;50:214-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 44. | Gangwar RS, Landolina N, Arpinati L, Levi-Schaffer F. Mast cell and eosinophil surface receptors as targets for anti-allergic therapy. Pharmacol Ther. 2017;170:37-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Mitchell PD, Salter BM, Oliveria JP, El-Gammal A, Tworek D, Smith SG, Sehmi R, Gauvreau GM, O Apos Byrne PM. IL-33 and its receptor ST2 after inhaled allergen challenge in allergic asthmatics. Int Arch Allergy Immunol. 2018;176:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Sun J, Dahlén B, Agerberth B, Haeggström JZ. The antimicrobial peptide LL-37 induces synthesis and release of cysteinyl leukotrienes from human eosinophils--implications for asthma. Allergy. 2013;68:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Hwang SM, Kim HJ, Kim SM, Jung Y, Park SW, Chung IY. Lysophosphatidylserine receptor P2Y10: A G protein-coupled receptor that mediates eosinophil degranulation. Clin Exp Allergy. 2018;48:990-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Barthel SR, Johansson MW, McNamee DM, Mosher DF. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol. 2008;83:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | El-Shazly AE, Henket M, Lefebvre PP, Louis R. 2B4 (CD244) is involved in eosinophil adhesion and chemotaxis, and its surface expression is increased in allergic rhinitis after challenge. Int J Immunopathol Pharmacol. 2011;24:949-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Verjan Garcia N, Umemoto E, Saito Y, Yamasaki M, Hata E, Matozaki T, Murakami M, Jung YJ, Woo SY, Seoh JY, Jang MH, Aozasa K, Miyasaka M. SIRPα/CD172a regulates eosinophil homeostasis. J Immunol. 2011;187:2268-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Verbrugge A, de Ruiter T, Geest C, Coffer PJ, Meyaard L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J Leukoc Biol. 2006;79:828-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Oka S, Ikeda S, Kishimoto S, Gokoh M, Yanagimoto S, Waku K, Sugiura T. 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J Leukoc Biol. 2004;76:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Bertram CM, Misso NL, Fogel-Petrovic M, Figueroa CD, Foster PS, Thompson PJ, Bhoola KD. Expression of kinin receptors on eosinophils: comparison of asthmatic patients and healthy subjects. J Leukoc Biol. 2009;85:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Rozenberg P, Reichman H, Moshkovits I, Munitz A. CD300 family receptors regulate eosinophil survival, chemotaxis, and effector functions. J Leukoc Biol. 2018;104:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Spencer LA, Melo RC, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci USA. 2006;103:3333-3338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Neves JS, Perez SA, Spencer LA, Melo RC, Reynolds L, Ghiran I, Mahmudi-Azer S, Odemuyiwa SO, Dvorak AM, Moqbel R, Weller PF. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci USA. 2008;105:18478-18483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Neves JS, Weller PF. Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr Opin Immunol. 2009;21:694-699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Stow JL, Murray RZ. Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 2013;24:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 59. | Spencer LA, Bonjour K, Melo RC, Weller PF. Eosinophil secretion of granule-derived cytokines. Front Immunol. 2014;5:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 60. | Melo RC, Weller PF. Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol Histopathol. 2010;25:1341-1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 61. | Persson C, Uller L. Theirs but to die and do: primary lysis of eosinophils and free eosinophil granules in asthma. Am J Respir Crit Care Med. 2014;189:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 62. | Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121:2074-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 63. | Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, Honda K, Ohta N, Yamamoto S, Takagi Y, Wada A, Weller PF. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016;137:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 64. | Mukherjee M, Lacy P, Ueki S. Eosinophil extracellular traps and inflammatory pathologies - untangling the web! Front Immunol. 2018;9:2763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 65. | Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5773] [Cited by in RCA: 7214] [Article Influence: 343.5] [Reference Citation Analysis (0)] |
| 66. | Sollberger G, Tilley DO, Zychlinsky A. Neutrophil extracellular traps: The biology of chromatin externalization. Dev Cell. 2018;44:542-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 67. | von Köckritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070-3080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 421] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 68. | Morshed M, Hlushchuk R, Simon D, Walls AF, Obata-Ninomiya K, Karasuyama H, Djonov V, Eggel A, Kaufmann T, Simon HU, Yousefi S. NADPH oxidase-independent formation of extracellular DNA traps by basophils. J Immunol. 2014;192:5314-5323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 69. | Webster SJ, Daigneault M, Bewley MA, Preston JA, Marriott HM, Walmsley SR, Read RC, Whyte MK, Dockrell DH. Distinct cell death programs in monocytes regulate innate responses following challenge with common causes of invasive bacterial disease. J Immunol. 2010;185:2968-2979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Doster RS, Rogers LM, Gaddy JA, Aronoff DM. Macrophage extracellular traps: A scoping review. J Innate Immun. 2018;10:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 71. | Rocha Arrieta YC, Rojas M, Vasquez G, Lopez J. The lymphocytes stimulation induced DNA release, a phenomenon similar to NETosis. Scand J Immunol. 2017;86:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 728] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 73. | Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103:1719-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 298] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 74. | Woodruff SA, Masterson JC, Fillon S, Robinson ZD, Furuta GT. Role of eosinophils in inflammatory bowel and gastrointestinal diseases. J Pediatr Gastroenterol Nutr. 2011;52:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Ohnmacht C, Pullner A, van Rooijen N, Voehringer D. Analysis of eosinophil turnover in vivo reveals their active recruitment to and prolonged survival in the peritoneal cavity. J Immunol. 2007;179:4766-4774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Farahi N, Singh NR, Heard S, Loutsios C, Summers C, Solanki CK, Solanki K, Balan KK, Ruparelia P, Peters AM, Condliffe AM, Chilvers ER. Use of 111-Indium-labeled autologous eosinophils to establish the in vivo kinetics of human eosinophils in healthy subjects. Blood. 2012;120:4068-4071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3129] [Article Influence: 173.8] [Reference Citation Analysis (1)] |
| 78. | Weninger W, Biro M, Jain R. Leukocyte migration in the interstitial space of non-lymphoid organs. Nat Rev Immunol. 2014;14:232-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 79. | Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 833] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 80. | Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 425] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 81. | Lämmermann T, Germain RN. The multiple faces of leukocyte interstitial migration. Semin Immunopathol. 2014;36:227-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 82. | Fulkerson PC, Rothenberg ME. Origin, regulation and physiological function of intestinal oeosinophils. Best Pract Res Clin Gastroenterol. 2008;22:411-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Rothenberg ME. Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology. 2015;148:1143-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 84. | Ahrens R, Waddell A, Seidu L, Blanchard C, Carey R, Forbes E, Lampinen M, Wilson T, Cohen E, Stringer K, Ballard E, Munitz A, Xu H, Lee N, Lee JJ, Rothenberg ME, Denson L, Hogan SP. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J Immunol. 2008;181:7390-7399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 85. | Adar T, Shteingart S, Ben Ya'acov A, Bar-Gil Shitrit A, Goldin E. From airway inflammation to inflammatory bowel disease: eotaxin-1, a key regulator of intestinal inflammation. Clin Immunol. 2014;153:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015;8:464-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 87. | DeBrosse CW, Case JW, Putnam PE, Collins MH, Rothenberg ME. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 88. | Matsushita T, Maruyama R, Ishikawa N, Harada Y, Araki A, Chen D, Tauchi-Nishi P, Yuki T, Kinoshita Y. The number and distribution of eosinophils in the adult human gastrointestinal tract: a study and comparison of racial and environmental factors. Am J Surg Pathol. 2015;39:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 89. | Kiss Z, Tél B, Farkas N, Garami A, Vincze Á, Bajor J, Sarlós P, Márta K, Erős A, Mikó A, Szakács Z, Pécsi D, Mátrai P, Hegyi P, Veres G. Eosinophil counts in the cmall intestine and colon of children without apparent gastrointestinal disease: A meta-analysis. J Pediatr Gastroenterol Nutr. 2018;67:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 90. | Yantiss RK. Eosinophils in the GI tract: how many is too many and what do they mean? Mod Pathol. 2015;28 Suppl 1:S7-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 91. | Xenakis JJ, Howard ED, Smith KM, Olbrich CL, Huang Y, Anketell D, Maldonado S, Cornwell EW, Spencer LA. Resident intestinal eosinophils constitutively express antigen presentation markers and include two phenotypically distinct subsets of eosinophils. Immunology. 2018;154:298-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 92. | Luissint AC, Parkos CA, Nusrat A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology. 2016;151:616-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 395] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 93. | Johansson ME, Ambort D, Pelaseyed T, Schütte A, Gustafsson JK, Ermund A, Subramani DB, Holmén-Larsson JM, Thomsson KA, Bergström JH, van der Post S, Rodriguez-Piñeiro AM, Sjövall H, Bäckström M, Hansson GC. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68:3635-3641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 384] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 94. | Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4659-4665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 1014] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 95. | Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GM, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nyström EE, Wising C, Johansson ME, Hansson GC. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 916] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 96. | Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 490] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 97. | Chu VT, Beller A, Rausch S, Strandmark J, Zänker M, Arbach O, Kruglov A, Berek C. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40:582-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 98. | Jung Y, Wen T, Mingler MK, Caldwell JM, Wang YH, Chaplin DD, Lee EH, Jang MH, Woo SY, Seoh JY, Miyasaka M, Rothenberg ME. IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015;8:930-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 99. | Burgel PR, Lazarus SC, Tam DC, Ueki IF, Atabai K, Birch M, Nadel JA. Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation. J Immunol. 2001;167:5948-5954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 100. | Driss V, Legrand F, Hermann E, Loiseau S, Guerardel Y, Kremer L, Adam E, Woerly G, Dombrowicz D, Capron M. TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood. 2009;113:3235-3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Berek C. Eosinophils: important players in humoral immunity. Clin Exp Immunol. 2016;183:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 102. | Dahan S, Roth-Walter F, Arnaboldi P, Agarwal S, Mayer L. Epithelia: lymphocyte interactions in the gut. Immunol Rev. 2007;215:243-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 103. | Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 104. | Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013;34:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 105. | Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 1230] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 106. | Farache J, Koren I, Milo I, Gurevich I, Kim KW, Zigmond E, Furtado GC, Lira SA, Shakhar G. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 346] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 107. | McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 597] [Cited by in RCA: 708] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 108. | Knoop KA, McDonald KG, McCrate S, McDole JR, Newberry RD. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 2015;8:198-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 109. | Chu DK, Jimenez-Saiz R, Verschoor CP, Walker TD, Goncharova S, Llop-Guevara A, Shen P, Gordon ME, Barra NG, Bassett JD, Kong J, Fattouh R, McCoy KD, Bowdish DM, Erjefält JS, Pabst O, Humbles AA, Kolbeck R, Waserman S, Jordana M. Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J Exp Med. 2014;211:1657-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 110. | Smith KM, Rahman RS, Spencer LA. Humoral immunity provides resident intestinal eosinophils access to luminal antigen via eosinophil-expressed low-affinity Fcγ receptors. J Immunol. 2016;197:3716-3724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 111. | Drake MG, Lebold KM, Roth-Carter QR, Pincus AB, Blum ED, Proskocil BJ, Jacoby DB, Fryer AD, Nie Z. Eosinophil and airway nerve interactions in asthma. J Leukoc Biol. 2018;104:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 112. | Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120:3882-3890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 113. | Kobayashi H, Gleich GJ, Butterfield JH, Kita H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99:2214-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 114. | O'Brien LM, Fitzpatrick E, Baird AW, Campion DP. Eosinophil-nerve interactions and neuronal plasticity in rat gut associated lymphoid tissue (GALT) in response to enteric parasitism. J Neuroimmunol. 2008;197:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 115. | Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 116. | Minai-Fleminger Y, Elishmereni M, Vita F, Soranzo MR, Mankuta D, Zabucchi G, Levi-Schaffer F. Ultrastructural evidence for human mast cell-eosinophil interactions in vitro. Cell Tissue Res. 2010;341:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 117. | Elishmereni M, Alenius HT, Bradding P, Mizrahi S, Shikotra A, Minai-Fleminger Y, Mankuta D, Eliashar R, Zabucchi G, Levi-Schaffer F. Physical interactions between mast cells and eosinophils: a novel mechanism enhancing eosinophil survival in vitro. Allergy. 2011;66:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 118. | Walker MM, Talley NJ, Prabhakar M, Pennaneac'h CJ, Aro P, Ronkainen J, Storskrubb T, Harmsen WS, Zinsmeister AR, Agreus L. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 119. | Robida PA, Puzzovio PG, Pahima H, Levi-Schaffer F, Bochner BS. Human eosinophils and mast cells: Birds of a feather flock together. Immunol Rev. 2018;282:151-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 120. | Spencer LA, Weller PF. Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol. 2010;88:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 121. | Straumann A, Kristl J, Conus S, Vassina E, Spichtin HP, Beglinger C, Simon HU. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm Bowel Dis. 2005;11:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 122. | Arnold IC, Artola-Borán M, Tallón de Lara P, Kyburz A, Taube C, Ottemann K, van den Broek M, Yousefi S, Simon HU, Müller A. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J Exp Med. 2018;215:2055-2072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 123. | Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 558] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 124. | Motomura Y, Khan WI, El-Sharkawy RT, Verma-Gandhu M, Grencis RK, Collins SM. Mechanisms underlying gut dysfunction in a murine model of chronic parasitic infection. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1354-G1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 125. | Takemura N, Kurashima Y, Mori Y, Okada K, Ogino T, Osawa H, Matsuno H, Aayam L, Kaneto S, Park EJ, Sato S, Matsunaga K, Tamura Y, Ouchi Y, Kumagai Y, Kobayashi D, Suzuki Y, Yoshioka Y, Nishimura J, Mori M, Ishii KJ, Rothenberg ME, Kiyono H, Akira S, Uematsu S. Eosinophil depletion suppresses radiation-induced small intestinal fibrosis. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 126. | Zuo L, Rothenberg ME. Gastrointestinal eosinophilia. Immunol Allergy Clin North Am. 2007;27:443-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 127. | Nanagas VC, Kovalszki A. Gastrointestinal manifestations of hypereosinophilic syndromes and mast cell disorders: a comprehensive review. Clin Rev Allergy Immunol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1557] [Article Influence: 111.2] [Reference Citation Analysis (1)] |
| 129. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3522] [Article Influence: 270.9] [Reference Citation Analysis (5)] |
| 130. | Burisch J, Jess T, Martinato M, Lakatos PL; ECCO -EpiCom. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 737] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 131. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4074] [Article Influence: 509.3] [Reference Citation Analysis (110)] |
| 132. | Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 799] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 133. | Nishitani H, Okabayashi M, Satomi M, Shimoyama T, Dohi Y. Infiltration of peroxidase-producing eosinophils into the lamina propria of patients with ulcerative colitis. J Gastroenterol. 1998;33:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 134. | Bischoff SC, Mayer J, Nguyen QT, Stolte M, Manns MP. Immunnohistological assessment of intestinal eosinophil activation in patients with eosinophilic gastroenteritis and inflammatory bowel disease. Am J Gastroenterol. 1999;94:3521-3529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 135. | Jeziorska M, Haboubi N, Schofield P, Woolley DE. Distribution and activation of eosinophils in inflammatory bowel disease using an improved immunohistochemical technique. J Pathol. 2001;194:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 136. | Lampinen M, Rönnblom A, Amin K, Kristjansson G, Rorsman F, Sangfelt P, Säfsten B, Wagner M, Wanders A, Winqvist O, Carlson M. Eosinophil granulocytes are activated during the remission phase of ulcerative colitis. Gut. 2005;54:1714-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 137. | Lampinen M, Backman M, Winqvist O, Rorsman F, Rönnblom A, Sangfelt P, Carlson M. Different regulation of eosinophil activity in Crohn's disease compared with ulcerative colitis. J Leukoc Biol. 2008;84:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 138. | Zezos P, Patsiaoura K, Nakos A, Mpoumponaris A, Vassiliadis T, Giouleme O, Pitiakoudis M, Kouklakis G, Evgenidis N. Severe eosinophilic infiltration in colonic biopsies predicts patients with ulcerative colitis not responding to medical therapy. Colorectal Dis. 2014;16:O420-O430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 139. | Park S, Abdi T, Gentry M, Laine L. Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2016;111:1692-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 140. | Leoncini G, Villanacci V, Marin MG, Crisafulli V, Cadei M, Antonelli E, Leoci C, Bassotti G. Colonic hypereosinophilia in ulcerative colitis may help to predict the failure of steroid therapy. Tech Coloproctol. 2018;22:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 141. | Masterson JC, Capocelli KE, Hosford L, Biette K, McNamee EN, de Zoeten EF, Harris R, Fernando SD, Jedlicka P, Protheroe C, Lee JJ, Furuta GT. Eosinophils and IL-33 perpetuate chronic inflammation and fibrosis in a pediatric population with stricturing Crohn's ileitis. Inflamm Bowel Dis. 2015;21:2429-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 142. | Brennan GT, Melton SD, Spechler SJ, Feagins LA. Clinical implications of histologic abnormalities in ileocolonic biopsies of patients with Crohn's disease in remission. J Clin Gastroenterol. 2017;51:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 143. | Coburn LA, Horst SN, Chaturvedi R, Brown CT, Allaman MM, Scull BP, Singh K, Piazuelo MB, Chitnavis MV, Hodges ME, Rosen MJ, Williams CS, Slaughter JC, Beaulieu DB, Schwartz DA, Wilson KT. High-throughput multi-analyte Luminex profiling implicates eotaxin-1 in ulcerative colitis. PLoS One. 2013;8:e82300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 144. | Rehman MQ, Beal D, Liang Y, Noronha A, Winter H, Farraye FA, Ganley-Leal L. B cells secrete eotaxin-1 in human inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 145. | Lampinen M, Waddell A, Ahrens R, Carlson M, Hogan SP. CD14+CD33+ myeloid cell-CCL11-eosinophil signature in ulcerative colitis. J Leukoc Biol. 2013;94:1061-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 146. | Manousou P, Kolios G, Valatas V, Drygiannakis I, Bourikas L, Pyrovolaki K, Koutroubakis I, Papadaki HA, Kouroumalis E. Increased expression of chemokine receptor CCR3 and its ligands in ulcerative colitis: the role of colonic epithelial cells in in vitro studies. Clin Exp Immunol. 2010;162:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 147. | Chen W, Paulus B, Shu D, Wilson, Chadwick V. Increased serum levels of eotaxin in patients with inflammatory bowel disease. Scand J Gastroenterol. 2001;36:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 148. | Mir A, Minguez M, Tatay J, Pascual I, Peña A, Sanchiz V, Almela P, Mora F, Benages A. Elevated serum eotaxin levels in patients with inflammatory bowel disease. Am J Gastroenterol. 2002;97:1452-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 149. | Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 519] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 150. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 887] [Cited by in RCA: 852] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 151. | Li J, Ueno A, Fort Gasia M, Luider J, Wang T, Hirota C, Jijon HB, Deane M, Tom M, Chan R, Barkema HW, Beck PL, Kaplan GG, Panaccione R, Qian J, Iacucci M, Gui X, Ghosh S. Profiles of lamina propria T helper cell subsets discriminate between ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 2016;22:1779-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 152. | Li J, Ueno A, Iacucci M, Fort Gasia M, Jijon HB, Panaccione R, Kaplan GG, Beck PL, Luider J, Barkema HW, Qian J, Gui X, Ghosh S. Crossover subsets of CD4+ T lymphocytes in the intestinal lamina propria of patients with Crohn's disease and ulcerative colitis. Dig Dis Sci. 2017;62:2357-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 153. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 895] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 154. | Ebbo M, Crinier A, Vély F, Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. 2017;17:665-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 155. | Lloyd CM, Snelgrove RJ. Type 2 immunity: Expanding our view. Sci Immunol. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 156. | Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, Vavricka SR, Fiocchi C. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 627] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 157. | Koslowski MJ, Beisner J, Stange EF, Wehkamp J. Innate antimicrobial host defense in small intestinal Crohn's disease. Int J Med Microbiol. 2010;300:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 158. | Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1443] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 159. | Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1216] [Article Influence: 64.0] [Reference Citation Analysis (1)] |
| 160. | Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064-15069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1528] [Article Influence: 89.9] [Reference Citation Analysis (1)] |
| 161. | Johansson ME, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 731] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 162. | Irie A, Imamura T, Michibata Y, Kubo T, Takeda N, Shibuya I, Sogo S, Araki K, Nishimura Y. Accumulation of HLA-DR4 in colonic epithelial cells causes severe colitis in homozygous HLA-DR4 transgenic mice. Inflamm Bowel Dis. 2017;23:2121-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 163. | Alipour M, Zaidi D, Valcheva R, Jovel J, Martínez I, Sergi C, Walter J, Mason AL, Wong GK, Dieleman LA, Carroll MW, Huynh HQ, Wine E. Mucosal barrier depletion and loss of bacterial diversity are primary abnormalities in paediatric ulcerative colitis. J Crohns Colitis. 2016;10:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 164. | McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 461] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 165. | Antoni L, Nuding S, Wehkamp J, Stange EF. Intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2014;20:1165-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 248] [Cited by in RCA: 299] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 166. | Lennon G, Balfe A, Earley H, Devane LA, Lavelle A, Winter DC, Coffey JC, O'Connell PR. Influences of the colonic microbiome on the mucous gel layer in ulcerative colitis. Gut Microbes. 2014;5:277-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 167. | Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Núñez G, Martens EC. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339-1353.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1285] [Cited by in RCA: 1902] [Article Influence: 237.8] [Reference Citation Analysis (0)] |
| 168. | Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol. 2013;34:398-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 169. | Furuta GT, Nieuwenhuis EE, Karhausen J, Gleich G, Blumberg RS, Lee JJ, Ackerman SJ. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289:G890-G897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 170. | Wallon C, Persborn M, Jönsson M, Wang A, Phan V, Lampinen M, Vicario M, Santos J, Sherman PM, Carlson M, Ericson AC, McKay DM, Söderholm JD. Eosinophils express muscarinic receptors and corticotropin-releasing factor to disrupt the mucosal barrier in ulcerative colitis. Gastroenterology. 2011;140:1597-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 171. | Xu X, Rivkind A, Pikarsky A, Pappo O, Bischoff SC, Levi-Schaffer F. Mast cells and eosinophils have a potential profibrogenic role in Crohn disease. Scand J Gastroenterol. 2004;39:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 172. | Bennett KM, Parnell EA, Sanscartier C, Parks S, Chen G, Nair MG, Lo DD. Induction of colonic M cells during intestinal inflammation. Am J Pathol. 2016;186:1166-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 173. | Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 821] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 174. | Martin JC, Bériou G, Heslan M, Bossard C, Jarry A, Abidi A, Hulin P, Ménoret S, Thinard R, Anegon I, Jacqueline C, Lardeux B, Halary F, Renauld JC, Bourreille A, Josien R. IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol. 2016;9:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 175. | Kucharzik T, Hudson JT, Lügering A, Abbas JA, Bettini M, Lake JG, Evans ME, Ziegler TR, Merlin D, Madara JL, Williams IR. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54:1565-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 176. | Yamashita N, Koizumi H, Murata M, Mano K, Ohta K. Nuclear factor kappa B mediates interleukin-8 production in eosinophils. Int Arch Allergy Immunol. 1999;120:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 177. | Dent G, Loweth SC, Hasan AM, Leslie FM. Synergic production of neutrophil chemotactic activity by colonic epithelial cells and eosinophils. Immunobiology. 2014;219:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 178. | Jung Y, Rothenberg ME. Roles and regulation of gastrointestinal eosinophils in immunity and disease. J Immunol. 2014;193:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 179. | Matthews JD, Weight CM, Parkos CA. Leukocyte-epithelial interactions and mucosal homeostasis. Toxicol Pathol. 2014;42:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 180. | Brazil JC, Parkos CA. Pathobiology of neutrophil-epithelial interactions. Immunol Rev. 2016;273:94-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 181. | Erjefält JS, Uller L, Malm-Erjefält M, Persson CG. Rapid and efficient clearance of airway tissue granulocytes through transepithelial migration. Thorax. 2004;59:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 182. | Butin-Israeli V, Houser MC, Feng M, Thorp EB, Nusrat A, Parkos CA, Sumagin R. Deposition of microparticles by neutrophils onto inflamed epithelium: a new mechanism to disrupt epithelial intercellular adhesions and promote transepithelial migration. FASEB J. 2016;30:4007-4020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 183. | Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. 2015;149:1275-1285.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 184. | Di Ruscio M, Vernia F, Ciccone A, Frieri G, Latella G. Surrogate fecal biomarkers in inflammatory bowel disease: rivals or complementary tools of fecal calprotectin? Inflamm Bowel Dis. 2017;24:78-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 185. | Carlson M, Raab Y, Peterson C, Hällgren R, Venge P. Increased intraluminal release of eosinophil granule proteins EPO, ECP, EPX, and cytokines in ulcerative colitis and proctitis in segmental perfusion. Am J Gastroenterol. 1999;94:1876-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 186. | Wagner M, Peterson CG, Stolt I, Sangfelt P, Agnarsdottir M, Lampinen M, Carlson M. Fecal eosinophil cationic protein as a marker of active disease and treatment outcome in collagenous colitis: a pilot study. Scand J Gastroenterol. 2011;46:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 187. | Li Z, Long Y, Bai M, Li J, Feng Z. Neutrophil and eosinophil granule proteins as potential biomarkers of assessing disease activity and severity in patients with ulcerative colitis. J Clin Lab Anal. 2016;30:776-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 188. | Peterson CG, Lampinen M, Hansson T, Lidén M, Hällgren R, Carlson M. Evaluation of biomarkers for ulcerative colitis comparing two sampling methods: fecal markers reflect colorectal inflammation both macroscopically and on a cellular level. Scand J Clin Lab Invest. 2016;76:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 189. | Loktionov A. Cell exfoliation in the human colon: myth, reality and implications for colorectal cancer screening. Int J Cancer. 2007;120:2281-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 190. | Loktionov A, Bandaletova T, Llewelyn AH, Dion C, Lywood HG, Lywood RC, Rockall TA, Stebbing JF, Broughton M, Caffarey S, Marks CG. Colorectal cancer detection by measuring DNA from exfoliated colonocytes obtained by direct contact with rectal mucosa. Int J Oncol. 2009;34:301-311. [PubMed] |
| 191. | Anderson N, Suliman I, Bandaletova T, Obichere A, Lywood R, Loktionov A. Protein biomarkers in exfoliated cells collected from the human rectal mucosa: implications for colorectal disease detection and monitoring. Int J Colorectal Dis. 2011;26:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 192. | Loktionov A, Chhaya V, Bandaletova T, Poullis A. Assessment of cytology and mucin 2 in colorectal mucus collected from patients with inflammatory bowel disease: Results of a pilot trial. J Gastroenterol Hepatol. 2016;31:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 193. | Bandaletova T, Chhaya V, Poullis A, Loktionov A. Colorectal mucus non-invasively collected from patients with inflammatory bowel disease and its suitability for diagnostic cytology. APMIS. 2016;124:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 194. | Loktionov A, Chhaya V, Bandaletova T, Poullis A. Inflammatory bowel disease detection and monitoring by measuring biomarkers in non-invasively collected colorectal mucus. J Gastroenterol Hepatol. 2017;32:992-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 195. | von Köckritz-Blickwede M, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J Mol Med (Berl). 2009;87:775-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 196. | Jena P, Mohanty S, Mohanty T, Kallert S, Morgelin M, Lindstrøm T, Borregaard N, Stenger S, Sonawane A, Sørensen OE. Azurophil granule proteins constitute the major mycobactericidal proteins in human neutrophils and enhance the killing of mycobacteria in macrophages. PLoS One. 2012;7:e50345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 197. | Green JN, Chapman ALP, Bishop CJ, Winterbourn CC, Kettle AJ. Neutrophil granule proteins generate bactericidal ammonia chloramine on reaction with hydrogen peroxide. Free Radic Biol Med. 2017;113:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 198. | Uribe Echevarría L, Leimgruber C, García González J, Nevado A, Álvarez R, García LN, Quintar AA, Maldonado CA. Evidence of eosinophil extracellular trap cell death in COPD: does it represent the trigger that switches on the disease? Int J Chron Obstruct Pulmon Dis. 2017;12:885-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 199. | Ueki S, Tokunaga T, Fujieda S, Honda K, Hirokawa M, Spencer LA, Weller PF. Eosinophil ETosis and DNA traps: a new look at eosinophilic inflammation. Curr Allergy Asthma Rep. 2016;16:54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 200. | Ponikau JU, Sherris DA, Kephart GM, Kern EB, Congdon DJ, Adolphson CR, Springett MJ, Gleich GJ, Kita H. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005;116:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 201. | Savchenko AS, Inoue A, Ohashi R, Jiang S, Hasegawa G, Tanaka T, Hamakubo T, Kodama T, Aoyagi Y, Ushiki T, Naito M. Long pentraxin 3 (PTX3) expression and release by neutrophils in vitro and in ulcerative colitis. Pathol Int. 2011;61:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 202. | Bennike TB, Carlsen TG, Ellingsen T, Bonderup OK, Glerup H, Bøgsted M, Christiansen G, Birkelund S, Stensballe A, Andersen V. Neutrophil extracellular traps in ulcerative colitis: a proteome analysis of intestinal biopsies. Inflamm Bowel Dis. 2015;21:2052-2067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 203. | Gottlieb Y, Elhasid R, Berger-Achituv S, Brazowski E, Yerushalmy-Feler A, Cohen S. Neutrophil extracellular traps in pediatric inflammatory bowel disease. Pathol Int. 2018;68:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 204. | Uller L, Persson CG, Erjefält JS. Resolution of airway disease: removal of inflammatory cells through apoptosis, egression or both? Trends Pharmacol Sci. 2006;27:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 205. | Persson C, Uller L. Resolution of leucocyte-mediated mucosal diseases. A novel in vivo paradigm for drug development. Br J Pharmacol. 2012;165:2100-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 206. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4879] [Article Influence: 697.0] [Reference Citation Analysis (1)] |
| 207. | Gatault S, Legrand F, Delbeke M, Loiseau S, Capron M. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. 2012;61:1527-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 208. | Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 209. | Sakkal S, Miller S, Apostolopoulos V, Nurgali K. Eosinophils in cancer: favourable or unfavourable? Curr Med Chem. 2016;23:650-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 210. | Varricchi G, Galdiero MR, Loffredo S, Lucarini V, Marone G, Mattei F, Marone G, Schiavoni G. Eosinophils: The unsung heroes in cancer? Oncoimmunology. 2017;7:e1393134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 211. | Prizment AE, Anderson KE, Visvanathan K, Folsom AR. Inverse association of eosinophil count with colorectal cancer incidence: atherosclerosis risk in communities study. Cancer Epidemiol Biomarkers Prev. 2011;20:1861-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 212. | Yalcin AD, Kargi A, Gumuslu S. Blood eosinophil and platelet levels, proteomics patterns of trail and CXCL8 correlated with survival in bevacizumab treated metastatic colon cancers. Clin Lab. 2014;60:339-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 213. | Wei Y, Zhang X, Wang G, Zhou Y, Luo M, Wang S, Hong C. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage I-III colorectal cancer. Asia Pac J Clin Oncol. 2018;14:e243-e251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 214. | Wu J, Ge XX, Zhu W, Zhi Q, Xu MD, Duan W, Chen K, Gong FR, Tao M, Shou LM, Wu MY, Wang WJ. Values of applying white blood cell counts in the prognostic evaluation of resectable colorectal cancer. Mol Med Rep. 2019;19:2330-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 215. | Moezzi J, Gopalswamy N, Haas RJ, Markert RJ, Suryaprasad S, Bhutani MS. Stromal eosinophilia in colonic epithelial neoplasms. Am J Gastroenterol. 2000;95:520-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 216. | Fernández-Aceñero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88:1544-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 217. | Prizment AE, Vierkant RA, Smyrk TC, Tillmans LS, Lee JJ, Sriramarao P, Nelson HH, Lynch CF, Thibodeau SN, Church TR, Cerhan JR, Anderson KE, Limburg PJ. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women's Health Study. Mod Pathol. 2016;29:516-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 218. | Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brünner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 219. | Nagtegaal ID, Marijnen CA, Kranenbarg EK, Mulder-Stapel A, Hermans J, van de Velde CJ, van Krieken JH. Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect--a histopathological and immunohistochemical study. BMC Cancer. 2001;1:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 220. | Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Bokemeyer C, Langner C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod Pathol. 2015;28:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 221. | Puxeddu I, Berkman N, Nissim Ben Efraim AH, Davies DE, Ribatti D, Gleich GJ, Levi-Schaffer F. The role of eosinophil major basic protein in angiogenesis. Allergy. 2009;64:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 222. | Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 223. | Stenfeldt AL, Wennerås C. Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunology. 2004;112:605-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 224. | Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O'Neill K, Colbert D, Lombari TR, Constant S, McGarry MP, Lee JJ, Lee NA. Pivotal advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 225. | Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 226. | Lahm H, Hoeflich A, Andre S, Sordat B, Kaltner H, Wolf E, Gabius HJ. Gene expression of galectin-9/ecalectin, a potent eosinophil chemoattractant, and/or the insertional isoform in human colorectal carcinoma cell lines and detection of frame-shift mutations for protein sequence truncations in the second functional lectin domain. Int J Oncol. 2000;17:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 227. | Wågsäter D, Löfgren S, Hugander A, Dienus O, Dimberg J. Analysis of single nucleotide polymorphism in the promoter and protein expression of the chemokine eotaxin-1 in colorectal cancer patients. World J Surg Oncol. 2007;5:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 228. | Hollande C, Boussier J, Ziai J, Nozawa T, Bondet V, Phung W, Lu B, Duffy D, Paradis V, Mallet V, Eberl G, Sandoval W, Schartner JM, Pol S, Barreira da Silva R, Albert ML. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat Immunol. 2019;20:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 229. | Munitz A, Hogan SP. Alarming eosinophils to combat tumors. Nat Immunol. 2019;20:250-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 230. | Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 402] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 231. | Malik A, Sharma D, Zhu Q, Karki R, Guy CS, Vogel P, Kanneganti TD. IL-33 regulates the IgA-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis. J Clin Invest. 2016;126:4469-4481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 232. | Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, Capron M. Human eosinophils exert TNF-α and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol. 2010;185:7443-7451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 233. | Gatault S, Delbeke M, Driss V, Sarazin A, Dendooven A, Kahn JE, Lefèvre G, Capron M. IL-18 is involved in eosinophil-mediated tumoricidal activity against a colon carcinoma cell line by upregulating LFA-1 and ICAM-1. J Immunol. 2015;195:2483-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 234. | Reichman H, Itan M, Rozenberg P, Yarmolovski T, Brazowski E, Varol C, Gluck N, Shapira S, Arber N, Qimron U, Karo-Atar D, Lee JJ, Munitz A. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol Res. 2019;7:388-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 235. | Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol. 2007;179:4840-4848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 236. | Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hämmerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 387] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 237. | Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, Bružas E, Maiorino L, Bautista C, Carmona EM, Gimotty PA, Fearon DT, Chang K, Lyons SK, Pinkerton KE, Trotman LC, Goldberg MS, Yeh JT, Egeblad M. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1018] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 238. | Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, Mitsios A, Kotsianidis I, Skendros P, Sivridis E, Maroulakou I, Giatromanolaki A, Ritis K. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS One. 2016;11:e0154484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 239. | Richardson JJR, Hendrickse C, Gao-Smith F, Thickett DR. Neutrophil extracellular trap production in patients with colorectal cancer in vitro. Int J Inflam. 2017;2017:4915062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 240. | Calistri D, Rengucci C, Molinari C, Ricci E, Cavargini E, Scarpi E, Milandri GL, Fabbri C, Ravaioli A, Russo A, Amadori D, Silvestrini R. Quantitative fluorescence determination of long-fragment DNA in stool as a marker for the early detection of colorectal cancer. Cell Oncol. 2009;31:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 241. | Teixeira Y, Lima JM, Souza ML, Aguiar P, Silva TD, Forones NM. Human DNA quantification in the stools of patients with colorectal cancer. Arq Gastroenterol. 2015;52:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 242. | Loktionov A, Ferrett CG, Gibson JJS, Bandaletova T, Dion C, Llewelyn AH, Lywood HGG, Lywood RCG, George BD, Mortensen NJ. A case-control study of colorectal cancer detection by quantification of DNA isolated from directly collected exfoliated colonocytes. Int J Cancer. 2010;126:1910-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 243. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 853] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 244. | Choi CR, Bakir IA, Hart AL, Graham TA. Clonal evolution of colorectal cancer in IBD. Nat Rev Gastroenterol Hepatol. 2017;14:218-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |