Published online Jul 7, 2019. doi: 10.3748/wjg.v25.i25.3268
Peer-review started: March 14, 2019
First decision: April 11, 2019
Revised: May 5, 2019
Accepted: May 31, 2019
Article in press: June 1, 2019
Published online: July 7, 2019
Processing time: 115 Days and 16.3 Hours
A large proportion of patients with Hirschsprung disease experience persistent obstructive symptoms after corrective surgery. Persistent obstructive symptoms may result in faecal stasis that can develop into Hirschsprung-associated enterocolitis, a potential life-threatening condition. Important treatment to improve faecal passage is internal anal sphincter relaxation using botulinum toxin injections.
To give an overview of all empirical evidence on the effectiveness of botulinum toxin injections in patients with Hirschsprung disease.
A systematic review and meta-analysis was done by searching PubMed, EMBASE and the Cochrane Library, using entry terms related to: (1) Hirschsprung disease; and (2) Botulinum toxin injections. 14 studies representing 278 patients met eligibility criteria. Data that were extracted were proportion of patients with improvement of obstructive symptoms or less enterocolitis after injection, proportion of patients with adverse effects and data on type botulinum toxin, mean dose, average age at first injection and patients with associated syndromes. Random-effects meta-analysis was used to aggregate effects and random-effects meta-regression was used to test for possible confounding factors.
Botulinum toxin injections are effective in treating obstructive symptoms in on average 66% of patients [event rate (ER) = 0.66, P = 0.004, I2 = 49.5, n = 278 patients]. Type of botulinum toxin, average dose, average age at first injections and proportion of patients with associated syndromes were not predictive for this effect. Mean 7 duration of improvement after one botulinum toxin injections was 6.4 mo and patients needed on average 2.6 procedures. There was a significant higher response rate within one month after botulinum toxin injections compared to more than one month after Botulinum toxin injections (ER = 0.79, vs ER = 0.46, Q = 19.37, P < 0.001). Botulinum toxin injections were not effective in treating enterocolitis (ER 0.58, P = 0.65, I2 = 71.0, n = 52 patients). There were adverse effects in on average 17% of patients (ER = 0.17, P < 0.001, I2 = 52.1, n = 187 patients), varying from temporary incontinence to mild anal pain.
Findings from this systematic review and meta-analysis indicate that botulinum toxin injections are effective in treating obstructive symptoms and that adverse effects were present, but mild and temporary.
Core tip: Patients with obstructive symptoms after surgery for Hirschsprung disease can benefit from intra-sphincteric botulinum toxin injections. This study indicates that Botulinum toxin injections are effective in treating obstructive symptoms after surgery for Hirschsprung disease and were associated with mild and temporary adverse effects. The proportion of patients that benefited from botulinum toxin injections was significantly higher within one month after administration (79%) compared to longer follow-up (46%). Beneficial effect was temporary and lasted on average 6 months, therefore requiring on average 2-3 injections per patient before satisfactory clinical improvement was achieved.
- Citation: Roorda D, Abeln ZA, Oosterlaan J, van Heurn LW, Derikx JP. Botulinum toxin injections after surgery for Hirschsprung disease: Systematic review and meta-analysis. World J Gastroenterol 2019; 25(25): 3268-3280
- URL: https://www.wjgnet.com/1007-9327/full/v25/i25/3268.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i25.3268
Hirschsprung disease is a congenital absence of ganglions of the distal gut, causing neonatal bowel obstruction. Treatment of Hirschsprung disease consists of surgical resection of the affected aganglionic bowel segment. Despite removal of the affected aganglionic bowel segment, about 8%-30% of patients experience persistent ob-structive symptoms after corrective surgery, varying from mild constipation to ileus[1]. Causes of obstructive symptoms include: (1) Mechanical obstruction, such as anastomotic stricture or adhesions; (2) Residual aganglionosis; (3) Stool-holding behavior; (4) General motility disorders of the bowel; and (5) Anal outlet obstruction[1,2], caused by the absence of the recto-anal inhibition reflex or anal sphincter defects[1]. When treated inadequately, persistent obstructive symptoms may result in faecal stasis that can develop into Hirschsprung-associated enterocolitis, a potential life-threatening condition that occurs in 25% to 37% of patients after surgery[3]. Therefore, it is important to achieve adequate bowel passage in patients with Hirschsprung disease.
Many patients with Hirschsprung disease use dietary adaptations, laxatives or rectal irrigation to manage bowel passage after surgery. When these conservative measures are not enough, a mechanical obstruction or residual aganglionosis needs to be ruled out, according to current consensus-based practice[1]. Current practice describes administration of intra-sphincteric botulinum toxin (BT) injections as a second step in treatment of obstructive symptoms after surgery, in order to obtain temporary relaxation of the internal anal sphincter, which subsequently improves faecal passage. We know from patients with childhood constipation and chronic anal fissures, that BT can be beneficial in treating constipation, regardless of sphincter dynamics[4], suggesting comparable beneficial effects for patients with Hirschsprung disease. Langer was the first to introduce treatment with BT injections for patients with Hirschsprung disease in 1997, as an alternative to myotomy of the anal sphincter and to use it as a predictive tool to assess necessity of sphincter myotomy[5].
Current consensus-based practice advises administration of 60-100 units BT diluted in 1.0 mL of saline with a maximum concentration of 100 IU/mL, given circum-ferentially at the level of the dentate line where the internal anal sphincter is located. The guideline also states that, BT injections need to be repeated every 3-6 mo as many times as necessary upon clinical improvement, as symptoms often will improve over time and BT injections generally become unnecessary at age older than five years. Alternatively, topical application of nitroglycerin or nifedipine cream or myotomy of the internal anal sphincter may be considered as treatment for post-operative obstructive symptoms. Non-operative management however is recommended, given the risk of faecal soiling after myotomy[1]. All these recommendations however, are consensus-based and are not substantiated by empirical evidence.
A meta-analysis on different treatment strategies for obstructive symptoms showed short-term improvement after BT injections in 77% of patients and decreased to 43% of patients in the long-term[6]. However, that meta-analysis did neither draw conclusions on effects on the prevalence of enterocolitis, nor on the complication rate and adverse events after BT injections. In addition, that meta-analyses did not assess potential predictors of effectiveness. Better knowledge is clearly needed and would benefit indication for treatment with BT injections and management of expectations of patients and their parents.
The current systematic review and meta-analysis aims to provide a comprehensive overview of all empirical evidence on: (1) Effects of treatment with BT injections on obstructive symptoms after surgery for Hirschsprung disease and factors moderating this effect (type of BT used, dose, age and the presence of associated syndromes); (2) Effects of treatment with BT injections on occurrence of post-operative Hirschsprung-associated enterocolitis; and (3) Complication rate and adverse effects after BT injections in patients after surgery for Hirschsprung disease.
The search strategy combined two groups of search terms and their equivalents: “Hirschsprung disease” AND “Botulinum toxin injections”. The search was performed in the electronic databases PubMed, EMBASE, Web of Science and the Cochrane Database using both simple search terms and hierarchical family forms (e.g., Medical Subject Headings, Thesaurus, Emtree). The reference lists of eligible articles were also screened for additional articles. The last search was conducted in December 2018.
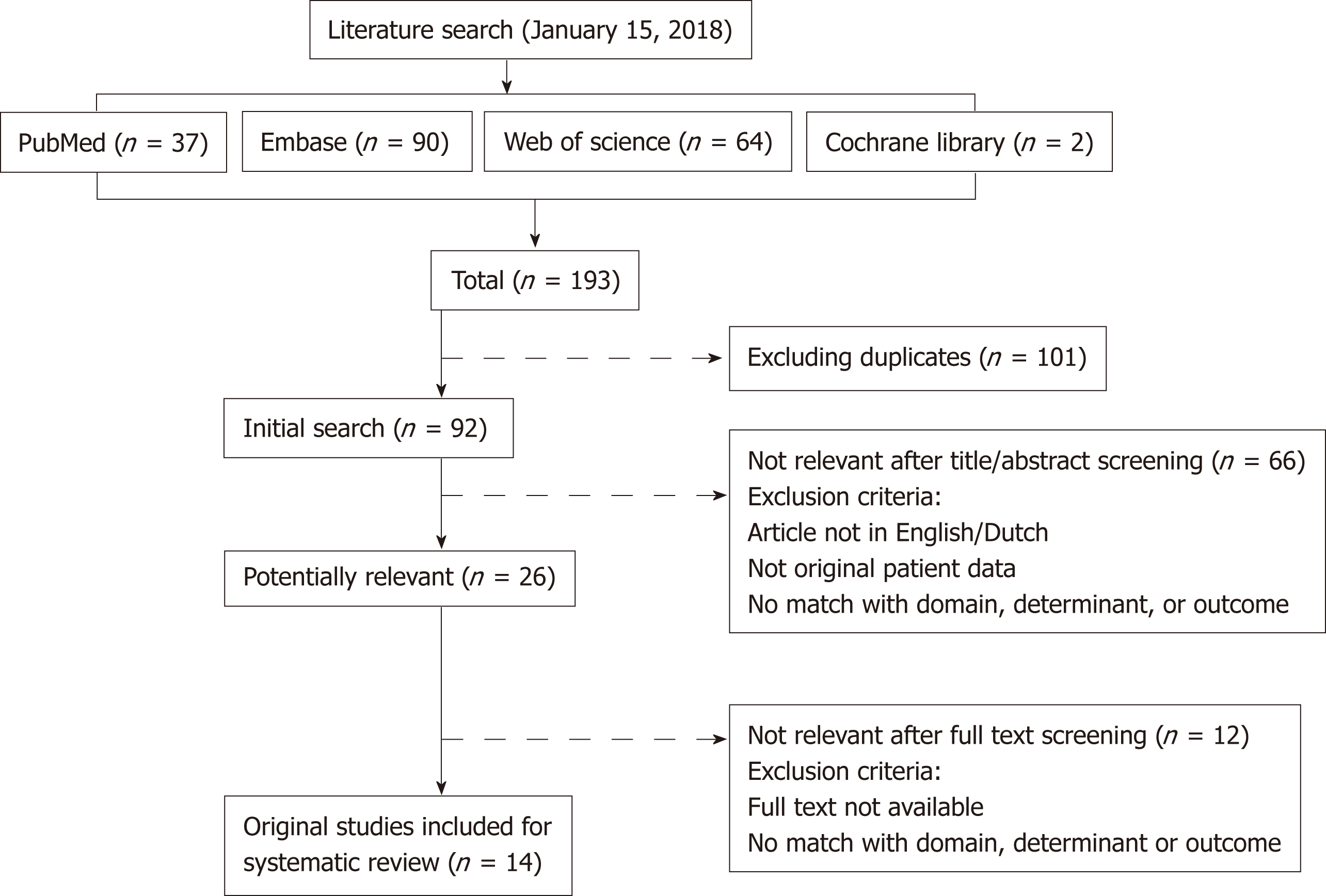
A flow diagram of the study search and selection is provided in Figure 1. A total of 193 records were identified corresponding to 92 unique articles. Two authors (DR and ZAMA) independently assessed each article for eligibility. Conflicts in the selection process were solved by either consensus or by consulting a third reviewer (JD). Studies were included in this systematic review and meta-analysis if they: (1) Contained original patient data; (2) Described patients with Hirschsprung disease with post-operative obstructive symptoms or enterocolitis; (3) Described treatment of these patients with BT injections in the internal anal sphincter; (4) Described outcomes in terms of occurrence of obstructive symptoms and/or enterocolitis at follow-up; in (5) Were published in a peer-reviewed journal; and (6) Written in the English language. In case multiple articles reported on (partly) overlapping cohorts, we in-cluded the article that had the largest sample size to maximize generalizability of the sample and statistical power of our meta-analysis. In case articles reported patients with obstructive defecation problems that consisted of patients with Hirschsprung disease and Internal Sphincter Achalasia and data about patients with Hirschsprung disease could not be extracted separately, the study was still included and the data were extracted for the total sample. 14 studies were included in both the systematic review as in the meta-analysis[5,7-17].
Primary outcome was effectiveness of BT injections in treating obstructive symptoms in patients after surgery for Hirschsprung disease, expressed as the proportion of patients with clinical improvement as reported in the various studies, the mean duration of improvement in months and the average number of BT needed to obtain satisfactory clinical improvement. Secondary outcomes were: (1) The proportion of patients that previously suffered from at least one episode of Hirschsprung-associated enterocolitis and were free of enterocolitis after treatment with BT injection; and (2) The proportion of patients with complications and/or adverse effects after BT injection. Primary and secondary outcome measures with the accompanying samples sizes were extracted from the included articles by two authors (DR and ZAMA) from the articles. In addition, possible confounding factors of effectiveness of BT injections in treating obstructive symptoms or Hirschsprung-associated enterocolitis (e.g., type of BT, average dose, average age at first BT injection, proportion of patients with an associated syndrome and proportion of male patients) were extracted from the articles. In case when studies reported medians, these measures were considered as the best approximation of means.
The quality of the included studies was evaluated using the Newcastle-Ottowa Quality Assessment Scale (NOS)[18]. This scale assesses study quality based on: (1) Selection procedure (4 points); (2) Comparability of controls (2 points); and (3) Outcome measurement (3 points). Thus, nine points can maximally be assigned to each study. In accordance with the Agency for Healthcare Research and Quality standards, quality of studies was considered “good” (Selection 3-4 AND Comparability 1-2 AND Outcome 2-3 points), “fair” (Selection 2 AND Comparability 1-2 AND Outcome 2-3 points) or “poor” (Selection 0-1 OR Comparability 0 OR Outcome 0 points). Quality assessment was conducted by two independent reviewers (ZA and DN) and in case of conflict, resolved with consensus.
Analyses were performed using Comprehensive Meta-Analysis Software (version 3.0, Biostat)[19]. For primary and secondary outcomes, the proportion of patients with clinical improvement on obstructive symptoms and enterocolitis, or with adverse effects was calculated for each study and expressed as the event rate (ER). The individual study’s effect sizes were subsequently aggregated across studies into meta-analytic effect sizes using the random model to account for heterogeneity introduced by the included range of outcome definitions, differences in follow-up duration and methods for administering BT injections. Mean duration of improvement and mean number of BT injections needed were calculated by averaging these measurements from the studies. Post-hoc analysis tested differences between the meta-analytic findings pertaining to: Outcomes assessed within one month after administration vs outcomes assessed in follow-up longer than one month. If a meta-analytic effect size was built up by a minimum of 10 individual studies’ effect sizes, we explored possible moderation effects on the outcomes of: (1) Type of BT; (2) Average dose; (3) Average age at first BT injection; (4) Proportion of patients with associated syndromes; and (5) Proportion of males. Only significant univariate moderators were further analyzed using multivariate meta-regression. Furthermore, post-hoc analysis were performed to test for possible differences between the different types of BT used for the injection, by aggregating effect sizes of observations for each type of BT. Sensitivity analyses were performed by repeating analyses after excluding studies that consisted of both patients with Hirschsprung disease and internal sphincter achalasia. ERs significantly higher than 0.50 are suggestive to be found not by chance and were arbitrarily considered to be clinically relevant. Heterogeneity was interpreted as small (I2 ≤ 0.25), medium (I2 = 0.25-0.50) or strong (I2 ≥ 0.50), according to Higgins et al[20]. The possibility of publication bias was assessed by calculating Funnel plot asymmetry expressed as the Eggers regression intercept t[21], fail-safe n (fail-safe n values > 5 k+10 where considered robust)[22] and by calculating the moderating effect of samples sizes on effect sizes. A P value of 0.05 was considered statistically significant.
In this systematic review and meta-analysis, 14 studies (representing 278 patients) met eligibility criteria and were included. Table 1 describes study characteristics of included studies. Length of follow-up after BT injections ranged from 6 to 126 mo. Dysport® (used in 4 of 14 studies) was administered with an average dose of 200 IU per procedure, whereas Botox® (used in 6 of 14 studies) was administered with an average mean dose of 95 IU per procedure, ranging from 60 to 120 IU per procedure. In the other four studies no details on the type of BT was provided. Ultrasonography was used to identify the internal anal sphincter in two studies, whereas in six studies palpation was used. The six other studies did not elaborate on their methods of identifying the internal anal sphincter. Mean age at administration of first BT injection was 4.5 years (SD 1.0 years). Proportion of patients with an associated syndrome was reported in seven studies and was on average 16% (ranging from 0-33%). The proportion of males in the included studies was on average 71% (SD 10%).
| Study | Study design | Inclusion period | Sample size, n | Male (%) | Syndromal patients (%) | Total follow-up in months | Number of BT injections needed per patient | Age at first BT injection (yr) | Type BT | Mean dose (IU/injection) | Guidance at BT injection | Definition of outcomes | Improvement in obstructive symptoms < 1 mo | Prolonged improvement in obstructive symtpoms | Improvement of enterocolitis | Complications/adverse effects | Decrease in mean resting pressure on anorectal manometry (mmHG) |
| Basson (2014) | Retrospective | 2010-2014 | 11 | 67 | NR | 12-72 | 1pt: 1BTI5pt: 2BTI4pt: 3BTI1pt: 4BTI | 5 | Dysport | 200 | Palpation | Successful-Improvement-FailedFavorable: successful/improvement | 10/11 (91%) | 5/11 (45%) | NR | 1 (transient soiling) | NR |
| Chumpitazi (2008) | Retrospective | 1998-2007 | 30 | 80 | 10 | 41.2 ± 4.9 | 2.7 ± 0.2 | 5 | Botox | NR | Palpation | Poor-Fair-Good-Excellent Favorable: Excellent/good | 27/30 (90%) | 11/30 (37%) | NR | 8 (7 transients soiling, 1 anal pain) | NR |
| Chumpitazi (2011) | Retrospective | 1998-2016 | 37 | 80 | 23 | 41.4 ± 4.5 | 2.8 ± 0.3 | NR | Botox | 100 | NR | Poor-Fair-Good-Excellent Favorable: Excellent/good | 33/37 (90%) | NA | NR | NR | NR |
| Church (2016) | Retrospective | 2010-2015 | 30 | NR | NR | 20 | 87% in total:With US: 2Without US: 1 | 3.1 | NR | 40 | US-guided | Improvement of symptoms | NA | NR | 3/4 (75%) | NR | NR |
| Han-Geurts (2014) | Retrospective | 2002-2013 | 33 | 79 | 0 | 7.3 yr (1-24) | 2 (1-5) | 3.6 | Dysport | 200 | NR | Poor-Fair-Good-Excellent Favorable: Excellent/good | 25/33 (76%) | 17/33 (52%) | 7/19 (37%) | 2 | NR |
| Hemanshoo Thakkar (2017) | Retrospective | 2002-2014 | 6 | NR | NR | 6 yr (1-12) | 3 | Dysport | 200 | US-guided | Short-term: postoperative complications < 30 dLong-term: Rintala Bowel Function Score (BFS) | 1/6 (17%) | NR | NR | NR | NR | |
| Hosseini (2008) | Prospective | 2002-2006 | 16 | 62 | NR | 8 pt: 1-3 | NR | Dysport | NR | NR | Improvement in Constipation score (good/recurrence/non-responders) | 14/16 (88%) | 8/16 (50%) | NR | NR | NR | |
| Jiang (2009) | Prospective | 2000-2008 | 23 | 65 | NR | 12 | NR | Botox | 120 | Palpation | Poor-Moderate-Excellent Favorable: Moderate/Excellent | NR | 21/23 (91%) | NR | 9 (anal pain) | 8-30 | |
| Koisuvalo (2009) | Retrospective | 2005-2008 | 16 * | 62 | 12.5 | 19 (3-43) | 2 (1-4) | 4 | NR | 100 | NR | No effect-Little effect-Significant effect-Symptoms dissapearedFavorable: Significant effect/symptoms dissapeared | 10/16 (63%) | 3/8 (38%) | 1/4 (25%) | 4 (increased soiling) | 28-31 (2 w); 8-24 (8 m) |
| Langer (1997) | Prospective | NR | 4 | 50 | 25 | 3 pt: 1, 1 pt 2 | 6 | Botox | NR | Palpation | Improvement of obstructive symptoms, presence of incontinence | 3/4 (75%) | 1/4 (25%) | NR | 1 (transient incontinence) | NR | |
| Langer (2004) | Retrospective | NR | 14 | NR | NR | 24 | 4 pt: 1, 9pt: 1-4 | 4 | NR | 150 | NR | Improvement of obstructive symptoms, presence of incontinence | 9/14 (64%) | 4/14 (29%) | NR | NR | NR |
| Minkes (2000) | Prospective | NR | 18 | 78 | NR | 8: 1; 10: 2-6 | NR | Botox | 60 | Palpation | No response-Significant response | 12/18 (67%) | 5/18 (28%) | NR | 4 (transient incontinence) | 35-37 | |
| Patrus (2010) | Retrospective | 1998-2008 | 22 | 78 | 5 | 5.0 ± 2.9 yr (0-10) | 2 (1-23) | 8.4 | NR | 120 | NR | Improvement of obstructive symptoms, presence of incontinence | 18/22 (81%) | 6/22 (27%) | NR | 0 | NR |
| Wester (2015) | Retrospective | 2007-2014 | 18 | 83 | 33 | 3, 8 yr (0, 1-8, 3) | 3 (1-13) | 2.4 | Botox | 100 | Palpation | Good-Insufficient | NR | 13/18 (72%) | NR | NR | NR |
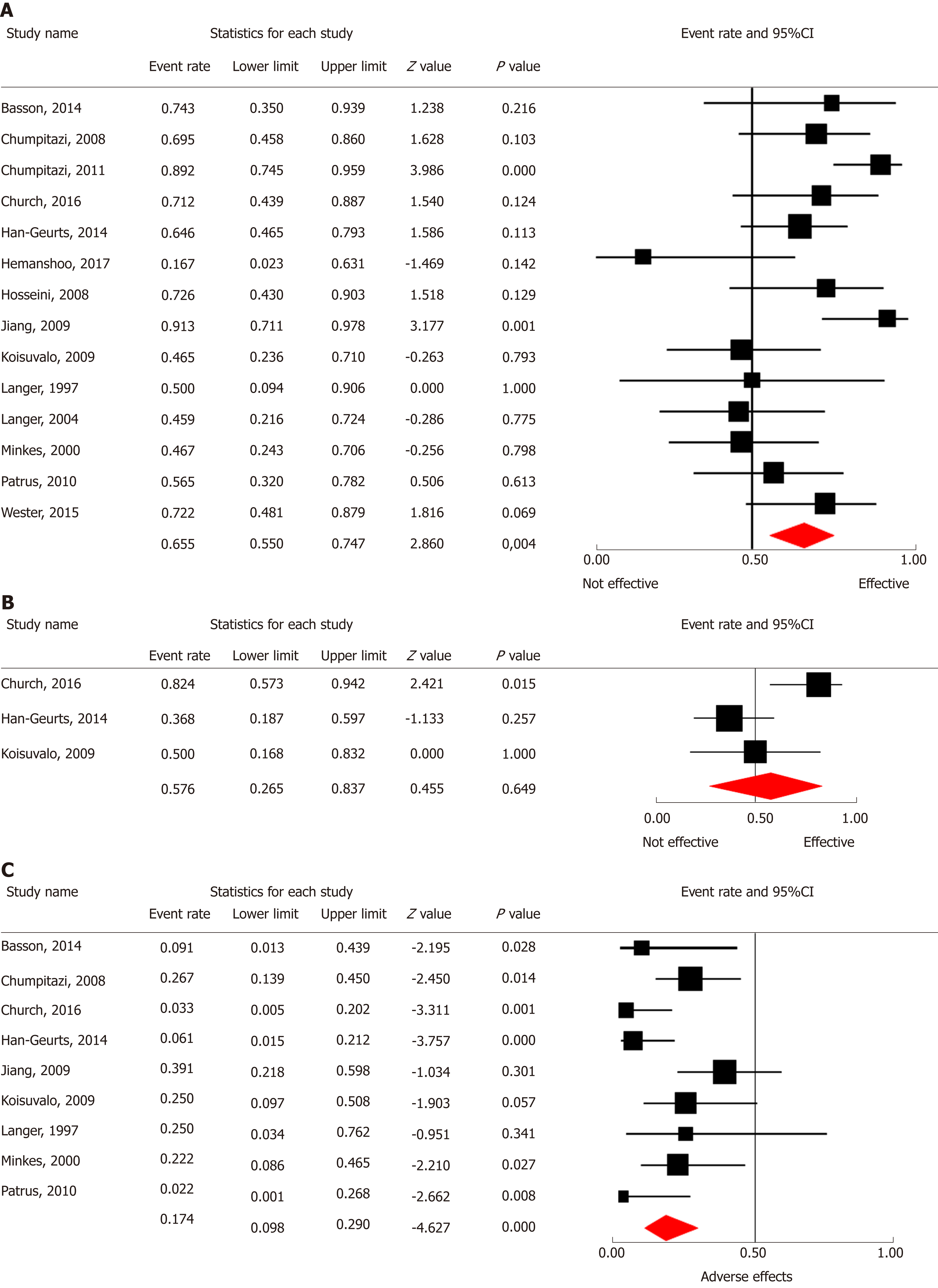
Primary outcome was reported in all 14 studies including 278 patients. Figure 2A shows a forest plot of the proportion of patients showing overall clinical improvement in each study and the aggregated ER of improvement of obstructive symptoms. Two of the 14 studies showed significant improvement of obstructive symptoms. The other 12 studies found no significant effect of treatment with BT injections. Meta-analytic aggregation of the effect sizes of all 14 studies showed significant effectiveness of BT injections, with improvement of obstructive symptoms in on average 66% of the patients [ER = 0.66, P = 0.004; 95% confidence interval (CI): 0.55-0.75, I2 = 49.5%] (Table 2). There was a significant higher response rate within one month after BT injections (ER = 0.79, P < 0.001; 95%CI: 0.71-0.85, I2 = 24.4%, n = 201 patients), compared to more than one month after BT injections (ER = 0.46, P = 0.50; 95%CI 0.34-0.58, I2 = 61.8%, n = 241 patients) (Q = 19.37, P < 0.001). None of the tested moderators had a significant predictive value for the magnitude of studies’ effect sizes in univariate analysis (i.e., mean dose, n = 228, P = 0.28; mean age at first BT injections, n = 184, P = 0.81; proportion of patients with an associated syndrome, n = 160, P = 0.10; sex of patients, n = 201, P = 0.94). Subgroup comparison showed no significant differences in improvement of obstructive symptoms after administration of Botox® (ER = 0.72, 95%CI: 0.58-0.83, n = 8 studies) compared to Dysport® (ER = 0.57, 95%CI: 0.33-0.77, n = 4 studies) (Q = 0.46, P = 0.49, n = 242 patients). Mean duration of improvement after one BT injections was 6.4 mo, ranging from 1 to 60 mo (n = 97). Patients needed on average 2.6 procedures of BT injection (ranging from 1 to a maximum of 23 procedures per patient) before clinical improvement was obtained.
| Effect | No. of studies | Event rate (95%CI) | Heterogeneity (I2) | Significant predictors | Eggers intercept | Fail safe, n |
| Improvement of obstructive symptoms | 14 | ER = 0.66 95%CI: 0.55-0.75a | 49.5% | None | -0.42 | 43 |
| Decreasing enterocolitis incidence | 3 | ER = 0.58, 95%CI: 0.27-0.84b | 71.0% | NA | 3.27 | 0 |
| Adverse effects | 9 | ER = 0.17, 95%CI: 0.10-0.29c | 52.1% | NA | -2.78 b | 101 |
In the meta-analysis effectiveness of BT injections in treating enterocolitis three studies representing 52 patients were included. Figure 2B shows that none of the three studies showed significant effectiveness of BT injections. Meta-analytic aggregation of the effect sizes of all three studies showed non-significant effectiveness of BT injections, with improvement in on average 58% of the patients (ER = 0.58, P = 0.65; 95%CI: 0.27-0.84, I2 = 71.0%) (Table 2). The number of studies describing effects on enterocolitis did not allow for assessment of confounding factors.
In the meta-analysis on complications and adverse events after administration BT injections as shown in Figure 2C, nine studies representing 187 patients were included. Meta-analytic aggregation of the effect sizes of all nine studies showed significant occurrence of complications or adverse events in on average 17% of the patients (ER = 0.17, P < 0.001; 95%CI: 0.10-0.29, I2 = 52.1%) (Table 2). The number of studies describing adverse effects of treatment with BT injections did not allow for assessment of confounding factors. Adverse events that were described in the studies were mild and included: (1) Transient soiling or incontinence in a total of 17 patients; (2) Anal pain in nine patients; and (3) Muscle fatigue of the lower extremities in two patients. In two other patients adverse effects were present but not described in detail.
Overall judgement of quality of included studies, as well as scores on each domain of the NOS are presented in Table 3. All 14 studies were of poor quality, because of the observational uncontrolled study design. Funnel plot asymmetry as expressed by Eggers regression intercept was significant for findings on adverse effects (P = 0.01), but non-significant for other findings (P values ranged from 0.69 to 0.78). This indicates that there was a low risk op publication bias for findings on improvement of obstructive symptoms and enterocolitis. The latter observation was further supported by a significant positive correlation between sample size and ERs (t = 0.07, P = 0.01), suggesting there was a low risk of a publication bias. Fail safe n’s ranged from 0 to 101, suggesting that only our findings on adverse effects were robust to the influence of publication bias. Results of the risk of bias analysis for every separate finding are presented in Table 2. Main effects were not significantly altered by excluding studies that included both patients with internal sphincter achalasia and Hirschsprung disease.
| Study | Selection | Comparibility | Outcome | Total |
| Basson (2014) | 2 | 0 | 3 | Poor |
| Chumpitazi (2008) | 3 | 0 | 3 | Poor |
| Chumpitazi (2011) | 3 | 0 | 3 | Poor |
| Church (2016) | 2 | 0 | 2 | Poor |
| Han-Geurts (2014) | 2 | 0 | 3 | Poor |
| Hemanshoo Takkar (2017) | 3 | 0 | 3 | Poor |
| Hosseini (2008) | 3 | 0 | 2 | Poor |
| Jiang (2009) | 3 | 0 | 3 | Poor |
| Koisuvalo (2009) | 3 | 0 | 3 | Poor |
| Langer (1997) | 3 | 0 | 3 | Poor |
| Langer (2004) | 3 | 0 | 2 | Poor |
| Minkes (2000) | 3 | 0 | 2 | Poor |
| Patrus (2010) | 3 | 0 | 3 | Poor |
| Wester (2015) | 3 | 0 | 3 | Poor |
This systematic review and meta-analysis aimed to provide a comprehensive overview of all empirical evidence on: (1) Effectiveness of treatment with BT injections for obstructive symptoms; (2) Effectiveness of treatment with BT injections for enterocolitis; and (3) Complications and adverse event after BT injections in patients that underwent surgery for Hirschsprung disease. Our findings indicate that BT injections improve obstructive symptoms in most patients (66%), although the proportion of patients that benefits is significantly higher within one month after administration (79%) compared to the proportion that still benefits after one month of follow-up or longer (46%). This underlines the transient effect of BT injections and explains that most patients will need multiple injections before satisfactory clinical improvement of obstructive symptoms is achieved. Our results further show that current evidence on whether BT injections are effective in reducing Hirschsprung-associated enterocolitis is inconclusive. Our analysis lacked the power to make a very specific point estimate of effectiveness, as shown by the broad CI ranging from effectiveness of 27% to 84%. BT injections were associated with adverse effects in on average 17% of patients, with adverse effects varying from transient incontinence to anal pain and muscle fatigue.
Our results indicated that duration of improvement of obstructive symptoms was on average six months and that most patients need on average two to three injections. This in line with previous meta-analytic findings and with evidence on the effectiveness of BT injections in other patient groups, including chronic constipation, anal fissures and internal sphincter achalasia[4,6,23]. In addition, evidence from three studies (all included in our meta-analysis), indicated that short term response was predictive for long-term response, although studies used different cut-off points for defining short- and long term response[7-9].
Our analyses showed that differences between studies in the proportion of patients showing clinical improvement, could not be explained by differences in average dose and type of BT used or by patient characteristics. There was large heterogeneity between studies in the dosage administered, which suggests there is no current consensus on optimal dose. Dysport® was on average administered in higher dosages than Botox®. However, we could not test the unique contribution of dosage and type in multivariate analyses, because only ten studies described both type of BT as well as average dose used. We hypothesize that our findings do not reflect this difference in dosage, as neither type of BT nor average dose used correlated significantly to rates of clinical improvement in univariate analysis. Furthermore, our findings are in line with findings in patients with chronic anal fissures, in whom dose and type of BT were not predictive of clinical improvement[24,25].
With regard to age at first BT injection, our findings indicated the age at which the BT injection was administered was not correlated to the proportion of patients showing clinical improvement, suggesting that BT injections can be used at all ages. The proportion of patients with an associated syndrome was not correlated to the effectiveness of BT injection in the treatment of obstructive symptoms, suggesting that our results were not over- or underestimated by patients with an associated syndrome. Moreover, the average amount of patients with an associated syndrome in our study was comparable to what we know from the general Hirschsprung population[26].
Because of lack of power caused by the limited number of studies available, the small sample sizes and heterogeneity in outcome definitions, our meta-analysis could not assess the predictive value of a number of possible interesting predictors of treatment effectiveness, including length of aganglionosis (only six studies), type of reconstruction that was done, findings on anorectal manometry (three studies) and specific procedural aspects of BT injections. Individual studies included in this meta-analyses suggest that short-segment disease is associated with better responsiveness to BT injections than long-segment disease[14,15]. Contrarily one study found no difference between short-segment and long-segment disease[16]. Three studies suggested that mean resting pressure decreases significantly after BT injections[11,12,14], but the degree of decrease in pressure was not predictive for clinical improvement[12]. One study by Church further suggests that US-guided BT injections decreases the amount of injections necessary compared to identifying the internal anal sphincter by palpation, although US-guided BT injections were not associated with higher response rates[17]. The study by Church also suggested that BT injection in the external anal sphincter is associated with higher response rates compared to injections in the internal anal sphincter[17]. In the majority of studies included in our systematic review (8/14 studies) residual aganglionosis or a mechanical obstruction was excluded as a cause of obstruction by barium enema and rectal biopsy before BT was administered. The other six studies did not exclude patients with these causes of obstruction from the study, but did not specifically report the cause of obstruction in individual patients prior to BT injections. Therefore, we could not compare differences in effectiveness of BT injections between different reasons of obstructive symptoms.
Another limitation of our study is the large heterogeneity between studies in outcome definitions and procedural aspects, including the position of the patients (lithotomy vs lateral decubitus position) during BT injection, the amount of injections administered and the number of sites in which BT was injected. This shows the lack of a standardized approach for BT injections.
Quality of evidence on the effects of BT injections was poor in all studies because of the lack of the use of randomized and controlled designs. Two studies assessed a combined sample of both patients with internal sphincter achalasia and Hirschsprung disease. This could account for a selection bias, resulting in an overestimation of effects, although sensitivity analyses in which these two studies were excluded showed no significant alteration of main effects. It may be hypothesized that the effects of BT injections are larger in patients with internal sphincter achalasia, as in these patients the absent rectoanal inhibitory reflex is the only explanatory factor for obstruction. The present systematic review and meta-analysis also carries the risk of assessment bias due to large variety of outcome definitions used in the included studies. There is only a risk of publication bias for our findings on complications and adverse effects, but our findings were robust to this influence. For other findings no evidence for risk of publication bias was found.
In this systematic review and meta-analysis we found evidence for improvement of obstructive symptoms after BT injections in patients that underwent surgery for Hirschsprung disease, although this effect was often transient and most patients needed multiple injections. Future studies using a standardized procedural approach and outcome definitions would be useful to determine dose-response effects and identify optimal dosages. Furthermore, better insight in predictors of clinical response would optimize treatment. Future studies should also assess factors that predict improvement of obstructive symptoms and enterocolitis incidence after BT injection, including length of aganglionosis and functional parameters such as mean resting pressures of the anal canal.
Patients with Hirschsprungs disease often suffer from persistent obstructive complaints after surgery. Improving faecal passage is important in these patients in order to prevent Hirsch-sprung-associated enterocolitis. Relaxation of the internal anal sphincter with botulinum toxin (BT) injections can be used to improve faecal passage.
BT injections are increasingly used to treat obstructive symptoms but an overview of the current evidence describing effectiveness of this treatment is lacking.
The objective of this study was to give a comprehensive overview of all evidence on effectiveness of intra-sphincteric BT injections to treat obstructive symptoms and enterocolitis in patients after surgery for Hirschsprungs disease, and to summarize evidence on its adverse effects.
A systematic review and meta-analysis according to the PRISMA Guidelines was conducted, searching PubMed, EMBASE, Web of Science and Cochrane library using simple and hier-archical entry terms including “botulinum toxin injections” and “Hirschsprungs disease”. Predefined predictors of effectiveness that were analysed were age at injection, sex, associated syndromes, dosage and type of BT used.
Data of 14 studies representing 278 patients were analysed. BT injections were effective in treating obstructive symptoms in 66% of patients, ranging from 79% in the first month of follow-up to 46% in follow-up longer than month. This was regardless of age at injection, sex, associated syndromes, dosage and type of BT used. Enterocolitis incidence was reduced in 57%, but the meta-analysis lacked power to draw conclusions. Mild adverse effects were present in 17%, which mainly consisted of temporary faecal incontinence or anal pain.
Our systematic review and meta-analysis shows that BT injections effectively treat obstructive symptoms in patients after surgery for Hirschsprungs disease, regardless of age at injection, sex, associated syndromes, dosage and type of BT used. Furthermore, the data suggests that BT injections are associated with mild adverse effects. Evidence on effectiveness of BT injections in treating enterocolitis is limited and lacked power to draw conclusions. Our findings show that BT injections are a useful treatment modality in clinical practice.
Future studies should further elucidate what factors predict good response to BT injections and subsequently if we can predict which patients can and cannot benefit from BT injections.
This research was not funded. The authors want to acknowledge the patient association of Hirschsprungs disease in the Netherlands for their support and cooperation in this research project.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tam PHK, Tang ST S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
| 1. | Langer JC, Rollins MD, Levitt M, Gosain A, Torre L, Kapur RP, Cowles RA, Horton J, Rothstein DH, Goldstein AM; American Pediatric Surgical Association Hirschsprung Disease Interest Group. Guidelines for the management of postoperative obstructive symptoms in children with Hirschsprung disease. Pediatr Surg Int. 2017;33:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Bjørnland K, Pakarinen MP, Stenstrøm P, Stensrud KJ, Neuvonen M, Granström AL, Graneli C, Pripp AH, Arnbjörnsson E, Emblem R, Wester T, Rintala RJ; Nordic Pediatric Surgery Study Consortium. A Nordic multicenter survey of long-term bowel function after transanal endorectal pull-through in 200 patients with rectosigmoid Hirschsprung disease. J Pediatr Surg. 2017;52:1458-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Gosain A, Frykman PK, Cowles RA, Horton J, Levitt M, Rothstein DH, Langer JC, Goldstein AM; American Pediatric Surgical Association Hirschsprung Disease Interest Group. Guidelines for the diagnosis and management of Hirschsprung-associated enterocolitis. Pediatr Surg Int. 2017;33:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 4. | Zar-Kessler C, Kuo B, Belkind-Gerson J. Botulinum toxin injection for childhood constipation is safe and can be effective regardless of anal sphincter dynamics. J Pediatr Surg. 2018;53:693-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Langer JC, Birnbaum E. Preliminary experience with intrasphincteric botulinum toxin for persistent constipation after pull-through for Hirschsprung's disease. J Pediatr Surg. 1997;32:1059-61; discussion 1061-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Soh HJ, Nataraja RM, Pacilli M. Prevention and management of recurrent postoperative Hirschsprung's disease obstructive symptoms and enterocolitis: Systematic review and meta-analysis. J Pediatr Surg. 2018;53:2423-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Basson S, Charlesworth P, Healy C, Phelps S, Cleeve S. Botulinum toxin use in paediatric colorectal surgery. Pediatr Surg Int. 2014;30:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Chumpitazi BP, Fishman SJ, Nurko S. Long-term clinical outcome after botulinum toxin injection in children with nonrelaxing internal anal sphincter. Am J Gastroenterol. 2009;104:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Han-Geurts IJ, Hendrix VC, de Blaauw I, Wijnen MH, van Heurn EL. Outcome after anal intrasphincteric Botox injection in children with surgically treated Hirschsprung disease. J Pediatr Gastroenterol Nutr. 2014;59:604-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Thakkar HS, Bassett C, Hsu A, Manuele R, Kufeji D, Richards CA, Agrawal M, Keshtgar AS. Functional outcomes in Hirschsprung disease: A single institution's 12-year experience. J Pediatr Surg. 2017;52:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Hosseini SM, Foroutan HR, Bahador A, Khosravi MB, Geramizadeh B, Sabet B, Zeraatian S, Razmi T, Banani SJ. Role of rectal biopsy in predicting response to intrasphincteric botulinum toxin injection for obstructive symptoms after a pullthrough operation. Indian J Gastroenterol. 2008;27:99-102. [PubMed] |
| 12. | Jiang da P, Xu CQ, Wu B, Li ZZ, Zhang YB, Han FY. Effects of botulinum toxin injection on anal achalasia after pull-through operations for Hirschsprung's disease: A 1-year follow-up study. Int J Colorectal Dis. 2009;24:597-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Langer JC. Persistent obstructive symptoms after surgery for Hirschsprung's disease: Development of a diagnostic and therapeutic algorithm. J Pediatr Surg. 2004;39:1458-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Minkes RK, Langer JC. A prospective study of botulinum toxin for internal anal sphincter hypertonicity in children with Hirschsprung's disease. J Pediatr Surg. 2000;35:1733-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Patrus B, Nasr A, Langer JC, Gerstle JT. Intrasphincteric botulinum toxin decreases the rate of hospitalization for postoperative obstructive symptoms in children with Hirschsprung disease. J Pediatr Surg. 2011;46:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Wester T, Granström AL. Botulinum toxin is efficient to treat obstructive symptoms in children with Hirschsprung disease. Pediatr Surg Int. 2015;31:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Church JT, Gadepalli SK, Talishinsky T, Teitelbaum DH, Jarboe MD. Ultrasound-guided intrasphincteric botulinum toxin injection relieves obstructive defecation due to Hirschsprung's disease and internal anal sphincter achalasia. J Pediatr Surg. 2017;52:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 18. | Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 25 Apr. 2019; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 19. | Borenstein M, Hedges, L, Higgins, J, Rothstein, H. Comprehensive Meta-Analysis Version 3, Biostat, Englewood, NJ 2013. Available from: https://www.meta-analysis.com/. |
| 20. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24741] [Article Influence: 1767.2] [Reference Citation Analysis (3)] |
| 21. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40404] [Article Influence: 1443.0] [Reference Citation Analysis (2)] |
| 22. | Rosenthal R. Writing meta-analytic reviews. Psychol Bull. 1995;118:183-192. [RCA] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 671] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 23. | Friedenberg F, Gollamudi S, Parkman HP. The use of botulinum toxin for the treatment of gastrointestinal motility disorders. Dig Dis Sci. 2004;49:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Bobkiewicz A, Francuzik W, Krokowicz L, Studniarek A, Ledwosiński W, Paszkowski J, Drews M, Banasiewicz T. Botulinum Toxin Injection for Treatment of Chronic Anal Fissure: Is There Any Dose-Dependent Efficiency? A Meta-Analysis. World J Surg. 2016;40:3064-3072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Gui D, Rossi S, Runfola M, Magalini SC. Review article: Botulinum toxin in the therapy of gastrointestinal motility disorders. Aliment Pharmacol Ther. 2003;18:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Moore SW. Chromosomal and related Mendelian syndromes associated with Hirschsprung's disease. Pediatr Surg Int. 2012;28:1045-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |