Published online Jul 7, 2019. doi: 10.3748/wjg.v25.i25.3207
Peer-review started: March 7, 2019
First decision: April 8, 2019
Revised: May 14, 2019
Accepted: June 7, 2019
Article in press: June 8, 2019
Published online: July 7, 2019
Processing time: 121 Days and 22.5 Hours
Stent insertion can effective alleviate the symptoms of benign esophageal strictures (BES). Magnesium alloy stents are a good candidate because of biological safety, but show a poor corrosion resistance and a quick loss of mechanical support in vivo.
To test the therapeutic and adverse effects of a silicone-covered magnesium alloy biodegradable esophageal stent.
Fifteen rabbits underwent silicone-covered biodegradable magnesium stent insertion into the benign esophageal stricture under fluoroscopic guidance (stent group). The wall reconstruction and tissue reaction of stenotic esophagus in the stent group were compared with those of six esophageal stricture models (control group). Esophagography was performed at 1, 2, and 3 weeks. Four, six, and five rabbits in the stent group and two rabbits in the control groups were euthanized, respectively, at each time point for histological examination.
All stent insertions were well tolerated. The esophageal diameters at immediately, 1, 2 and 3 wk were 9.8 ± 0.3 mm, 9.7 ± 0.7 mm, 9.4 ± 0.8 mm, and 9.2 ± 0.5 mm, respectively (vs 4.9 ± 0.3 mm before stent insertion; P < 0.05). Magnesium stents migrated in eight rabbits [one at 1 wk (1/15), three at 2 wk (3/11), and four at 3 wk (4/5)]. Esophageal wall remodeling (thinner epithelial and smooth muscle layers) was found significantly thinner in the stent group than in the control group (P < 0.05). Esophageal injury and collagen deposition following stent insertion were similar and did not differ compared to rabbits with esophageal stricture and normal rabbits (P > 0.05).
Esophageal silicone-covered biodegradable magnesium stent insertion is feasible for BES without causing severe injury or tissue reaction. Our study suggests that insertion of silicone-covered magnesium esophageal stent is a promising approach for treating BES.
Core tip: Stent insertion can be a safe, easy, and effective way to alleviate the symptoms of benign esophageal strictures (BES). However, metallic stent implantation is associated with some severe complications, such as migration, tissue ingrowth, and in-stent restenosis. Biodegradable stent has been used as an effective and accepted method to treat BES. We fabricated a silicone-covered biodegradable magnesium stent, and evaluated technical feasibility, tissue reaction, and stent degradation for treating benign esophageal stricture in a rabbit model. We found that implantation of silicone-covered magnesium stent provided reliable support for at least two weeks, suggesting that it is a promising strategy to treat benign esophageal stricture.
- Citation: Yang K, Cao J, Yuan TW, Zhu YQ, Zhou B, Cheng YS. Silicone-covered biodegradable magnesium stent for treating benign esophageal stricture in a rabbit model. World J Gastroenterol 2019; 25(25): 3207-3217
- URL: https://www.wjgnet.com/1007-9327/full/v25/i25/3207.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i25.3207
Esophageal stricture is the abnormally stenotic segment of the esophagus, and benign esophageal stricture (BES) indicates a narrowing or tightening of the esophagus caused by non-cancerous reasons[1,2]. BES can be caused by non-operative factors like reflux, radiation, infection, sclerotherapy, and corrosion, as well as operative factors including surgical anastomosis and minimally invasive surgery for early esophageal neoplasms[3,4]. As one of the most common gastrointestinal conditions impacting patients on a day-to-day basis, BES can seriously degrade the quality of life and result in many problems, such as dysphagia, malnutrition, weight loss, aspiration, and respiratory failure[5-7]. Therefore, the development of effective therapeutic strategies for BES is a critical medical need.
Esophageal stenosis can be alleviated through esophageal stent insertion, which has been widely used as an effective means to improve the quality of life for patients with BES[8-11]. Implantation of stents fabricated from many materials can be applied for treating BES. However, metallic stent implantation is associated with some severe complications, such as migration, tissue ingrowth, and in-stent restenosis[8-11], which significantly limits the use of metallic stents in BES. In addition, the temporary recyclable stent represents a simple and feasible approach to provide sufficient support in 10-14 days for treating BES, but it needs to be removed after use and frequently causes pain, significant foreign body reaction, as well as potential risks including perforation and bleeding[11]. With the advancements in biological medical material technology, stents made from biodegradable alloy or polymer are extensively improved to be able to provide enough force to tear the BES and reduce complications caused by stents[8,12,13].
Recently, biodegradable stents (BDS) have been used as an effective and accepted method to treat BES patients to improve their quality of life[14,15]. For example, polymer polylactic L-acid (PLLA) BDS exhibited a low complication rate in BES patients, but the high rate (77%) of early stent migration greatly limited its wider clinical application[16]. PLLA esophageal BDS manufactured with polydioxanone can reduce stent migration risk, but results in significant hyperblastosis than PLLA-BDS[17]. Therefore, esophageal BDS with prominent therapeutic effects and minimal adverse effects is still the great challenge in the field. More recently, Yuan et al[18] used a poly (ε-caprolactone) (PCL) and poly (trimethylene carbonate) (PTMC)-covered magnesium alloy stent to treat BES in a rabbit model. The stent can provide sufficient support for at least 4 wk, and did not result in damage or collagen loss in the esophageal wall[18]. With acceptable stent migration rates, the stent covered with biodegradable PCL-PTMC significantly prevented serious corrosion of magnesium alloy in the corrosive environment[18].
In this study, we integrated the biocompatibility of silicone membrane[19] and corrosion resistant property of magnesium alloy[20] into the design of an esophageal stent, and applied the silicone-covered magnesium stent into a rabbit model of BES. We determined the feasibility of this stent, and monitored the in vivo tissue reaction and stent degradation after stent insertion. Our study suggests that silicone-covered magnesium alloy is an ideal biodegradable material for fabricating esophageal stents to treat patients with BES.
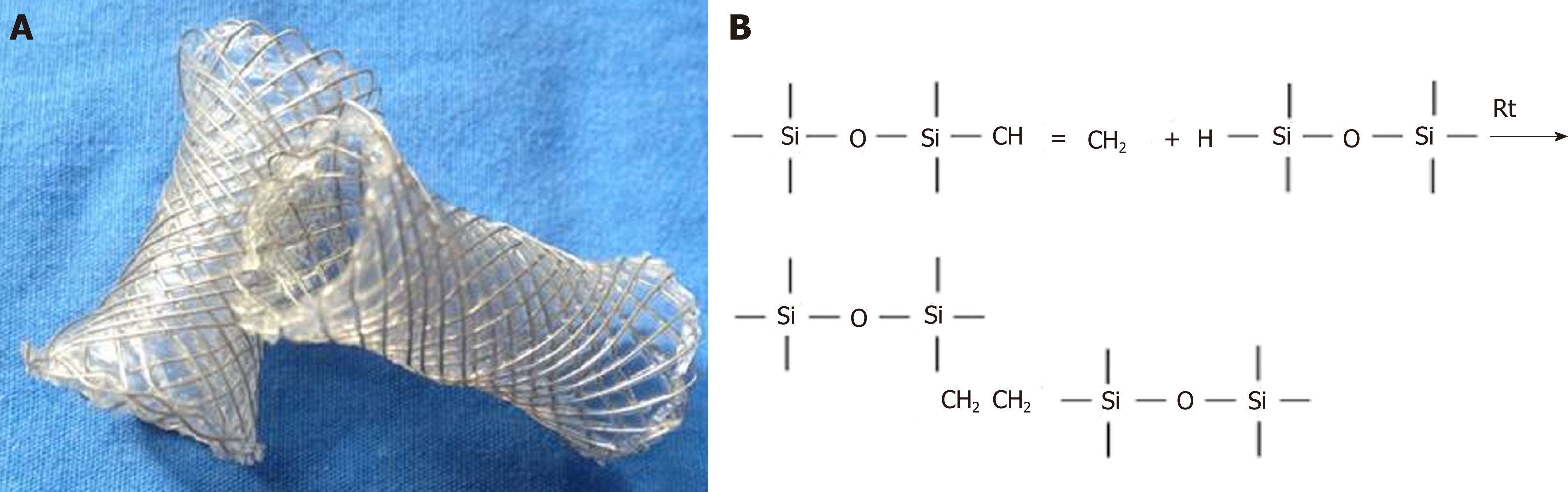
Silicone-covered magnesium stents are magnesium stents coated with a silicone membrane. The commercial magnesium alloy was purchased from Sanming Biomedical Company (Yangzhou, China). The bare stent was constructed with 0.20 mm magnesium alloy, as previously described[18,21]. The skeleton of the stent is cylindrical, which was made of the magnesium alloy wires through cross-linked mesh. The diameter and length of the stent were 10 mm and 30 mm in the entire expansion state, respectively. The stents had 5 mm cydariform and tubiform shapes at both ends to prevent their migration (Figure 1).
The surface of the entire magnesium stent was covered with a silicon membrane through the dipping and spinning method developed in our laboratory[18,21]. Briefly, silicone rubber A and rubber B in the same amount (Shanghai Yanchen Industrial Company, Shanghai, China) were thoroughly mixed with n-octane (Shanghai Aladdin Biochemical Technology Company, Shanghai, China). The mixed silicone was impregnated on the stent mold and solidified for 6 h at 80 °C for drying. The molds were cooled in ambient conditions, and the magnesium stents were peeled off. Since the magnesium alloy and the silicon film were transparent and not developed under the X-ray, marks on both ends of the stents were placed to facilitate their positioning under fluoroscopy. The silicone-covered magnesium stents were compressed and loaded through a 6-mm-wide (18 French) delivery system.
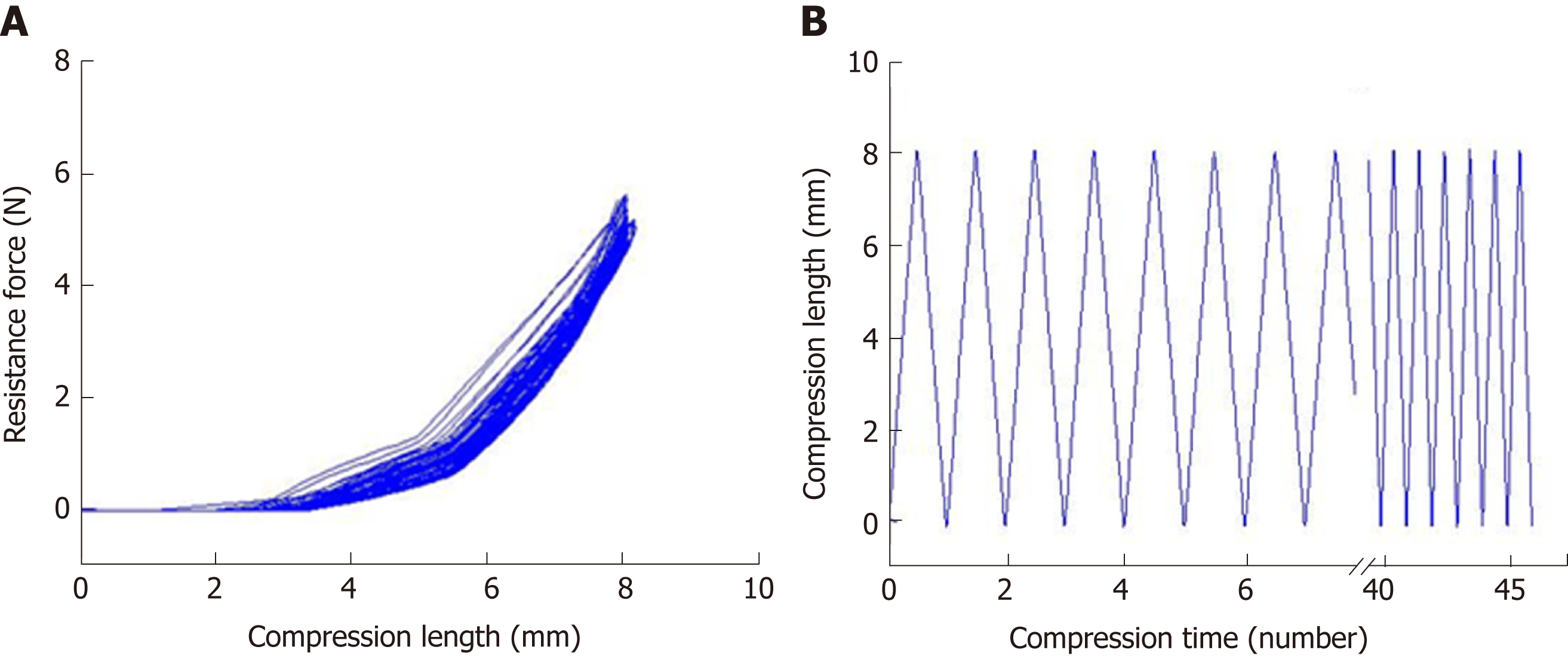
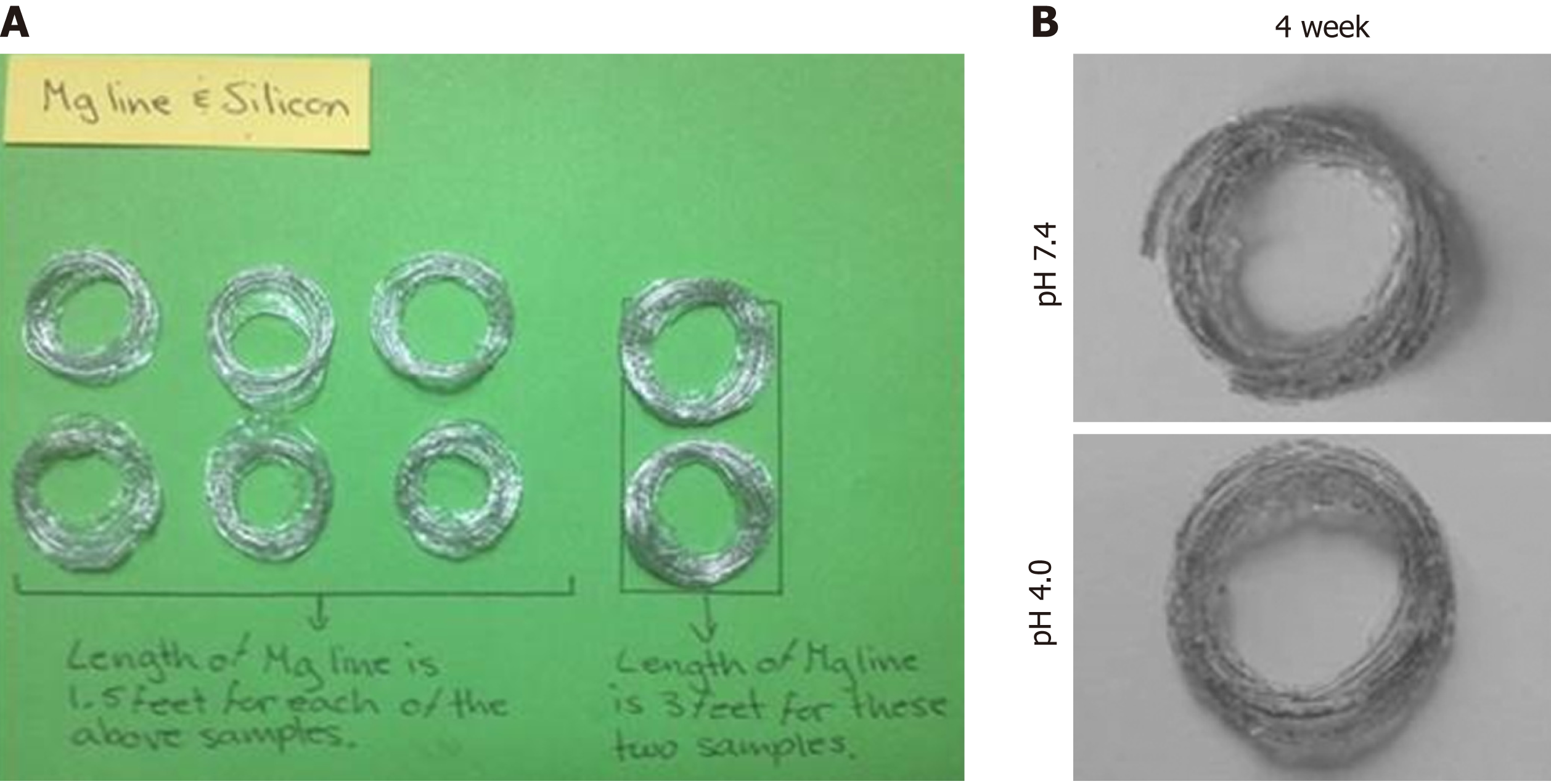
The mechanical properties of the silicone-covered magnesium stents were tested by the mechanical compression curve analysis and the tensile stress (46 compressions), as previously described[18,21]. The degradation behaviors of the silicone-covered magnesium stents in terms of the magnesium mass lost were determined by incubating in two phosphate-buffered solutions with pH values of 7.4 and 4.0, as previously described[18,21].
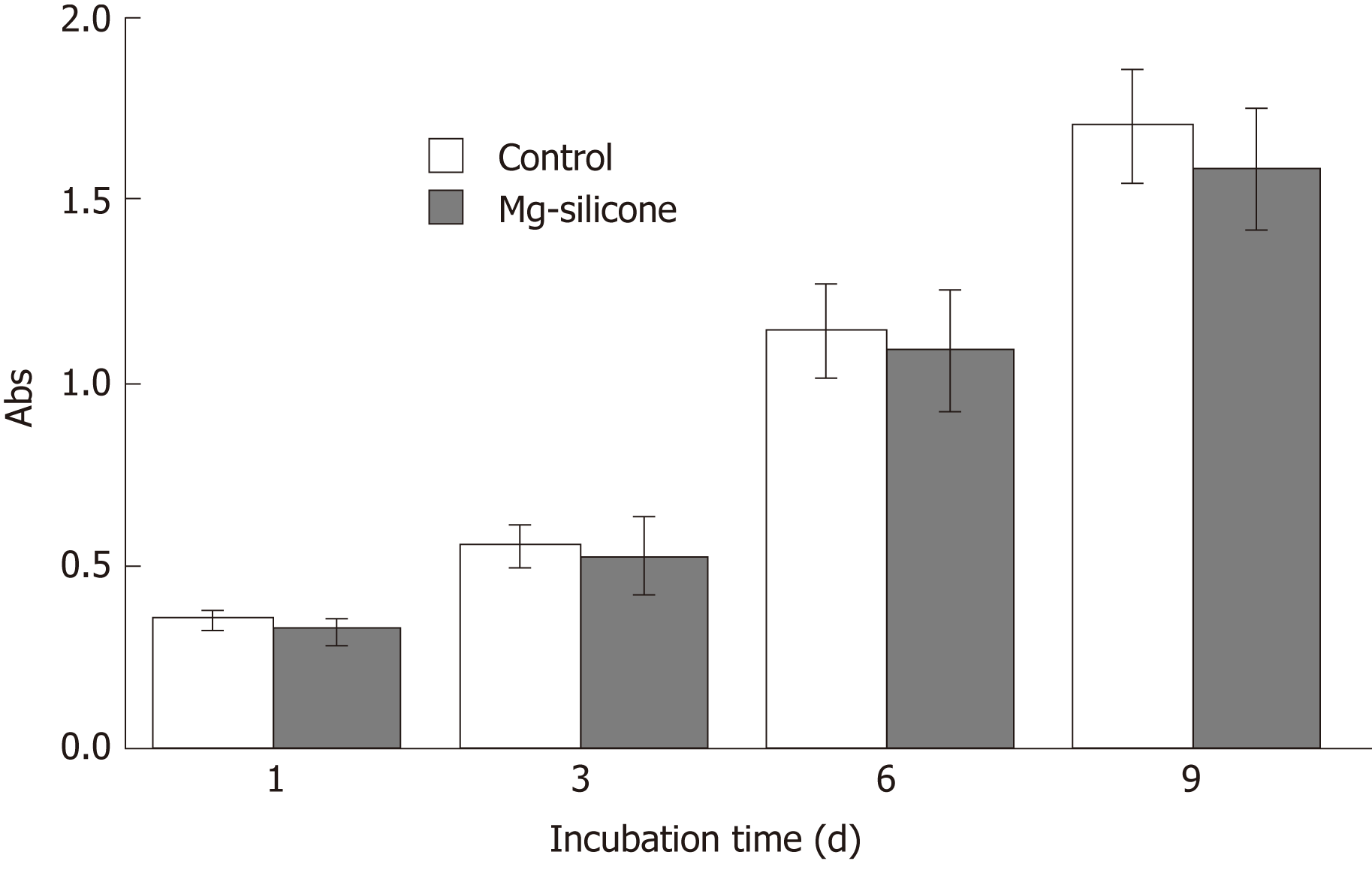
The biological safety of silicone-covered magnesium stent was evaluated by testing its impact on the proliferation of human smooth muscle cell line HITC6 that was purchased from the Cell Bank of Type Culture Collection Committee of the Chinese Academy of Sciences, Shanghai, China. Cells were maintained in RPMI 1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, United States) supplemented with 10% fetal bovine serum (TransGen Biotech, Inc., Beijing, China), 100 units/mL of penicillin, and 100 µg/mL of streptomycin (Gibco; Thermo Fisher Scientific, Waltham, MA, United States), and grown in a humidified atmosphere with 5% CO2 at 37 ˚C. Cells were seeded in 96 well-plates at a density of 5 × 103/well and incubated with or without silicone-coated magnesium stents. On days 1, 3, 6 and 9, cell proliferation was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)] assay[22].
All experimental protocols were approved by the Animal Research Council of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University and followed the guidelines of the International Committee of Animal Care (US National Institutes of Health and European Commission).
New Zealand rabbits were provided by the Experimental Animals of the Public Health Center of Shanghai Jiao Tong University, Shanghai, China. The rabbits were housed in the Experimental Animal Center of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University. Rabbits were kept in cages with free access to food in an animal room with a relative humidity of 40%-50%, temperature of 22-25 °C, and 12 h/12 h light and dark alternates. Healthy New Zealand rabbits (weight, 2.3-3.8 kg and age, 10-11 wk) were used for constructing a rabbit esophageal stenosis model using the esophageal sewing method, similarly as previously described[23].
After anesthesia with 5% pentobarbital via the ear vein, rabbits were fixed in the supine position. A longitudinal incision about 3 cm away to the thoracic entrance was made to separate the subcutaneous tissue and muscle and expose the trachea. From the left side of the trachea, the esophagus was truncated about 3 cm, the distal segment of the esophagus was connected with an infusion tube, and the esophagus was further separated from the thorax about 3 cm. The 4.0 operation suture was applied to suture the esophageal lumen, and the position of stitches was adjusted to control the stenosis of esophagus by 50% to 60% under digital subtraction angiography (DSA; GE Medical Systems, United States).
There were 15 rabbits in the stent group, while 5 rabbits in the control group did not receive any intervention. The silicone-covered magnesium stent was implanted into the esophageal stricture of the rabbits under DSA guidance, as previously described[18,21]. After the rabbits were anesthetized with 5% pentobarbital via the ear vein, a stiff wire (0.035-inch, 260-cm-long, Terumo, Tokyo, Japan) was implanted through the mouth to the stomach under DSA guidance, and used as a guidewire to deliver the silicone-coated magnesium stent into the esophageal stricture. After the release of the silicone-coated magnesium stent, a balloon catheter (10 mm × 40 mm, Changhong Medical Instrument Co., Ltd., Changzhou, China) was inflated within the silicone-covered magnesium stent to make it fully expanded. The expansion time of balloon was 20-30 s to ensure complete expansion of the stent. The diet and water intake in the experiment and control groups were indiscriminate.
Esophageal angiography was performed at 1, 2, and 3 wk after the stent implantation. The migration, patency of the stents, and the diameter of the esophagus between the stent group and the control group was compared.
Two rabbits each time in the control group, and 4, 6, and 5 rabbits in the stent group at the indicated time points (at 1, 2, and 3 wk after the stent implantation) were euthanized to evaluate the response and reconstruction of the esophagus wall. The silicone-covered magnesium stent was removed from the esophagus or gastrointestinal tract. The degradation rate of silicone-covered magnesium stents was assessed by calculating the percentage of destroyed mesh. If one of its four edges broke down under a microscope, a mesh was considered degraded. Mild, moderate, and severe degradations were defined as below 25%, 25%-50%, and over 50% in degradation rate.
Histology evaluation of the samples was performed as previously described[18,21]. Hematoxylin and eosin (HE) staining was used to assess the inflammation responses to obtain an inflammatory score. Evaluation of submucosal collagen deposition was conducted by Mason’s trichrome staining. The Elivision immunohistochemical technique was used to stain the esophageal samples. Mouse anti-proliferating cell nuclear antigen (PCNA) antibody (1:100 dilution; NeoMarkers, Thermo Fisher Scientific Inc., Fremont, CA, United States) and α-smooth muscle actin (α-SMA) antibody (1:50 dilution; Santa Cruz Biotechnology Inc., Dallas, TX, United States) were used as primary antibodies. Specimens were evaluated by two pathologists independently in a blinded manner.
GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, United States) was used for data analyses. One-way or two-way analysis of variance (ANOVA) was used to compare the overall changes in esophageal diameter, PCNA proliferation index, and collagen area between the control and stent groups at 1, 2, and 3 wk after stent insertion. The Shapiro-Wilk test was used to evaluate the variance and normal distribution of the dependent variables before one-way ANOVA. Statistical significance was set at P < 0.05.
The mechanical properties of the silicone-covered magnesium stent were tested by the tensile stress and strain (Figure 2). The silicone membrane was tightly wrapped and fixed to the cross-linked, knitted, bare magnesium mesh tube (Figure 1), which could maintain its size and morphology because of its prominent elasticity and flexibility. The silicone-covered magnesium stent showed good elastic deformation properties with no tearing or breakdown, thus was able to provide enough support against in vivo lesion compression. The magnesium mass lost was used to determine the degradation of the silicone-covered magnesium stent in buffered solutions with pH values of 4.0 and 7.4. As shown in Figure 3, the silicone-covered magnesium stent showed excellent biodegradable ability and maintained the main structure even at 4 wk after incubation in the acidic environment. Therefore, the silicone-covered magnesium stent had a retention time as long as 4 weeks. The biological safety of magnesium-silicone gel was evaluated by its impact on the proliferation of smooth muscle cells. As shown in Figure 4, the cells demonstrated similar proliferation ratios at the tested time points in the presence and absence of magnesium-silicone gel, which suggested that the silicone-covered magnesium stent had no cellular cytotoxicity against the growth of host cells.
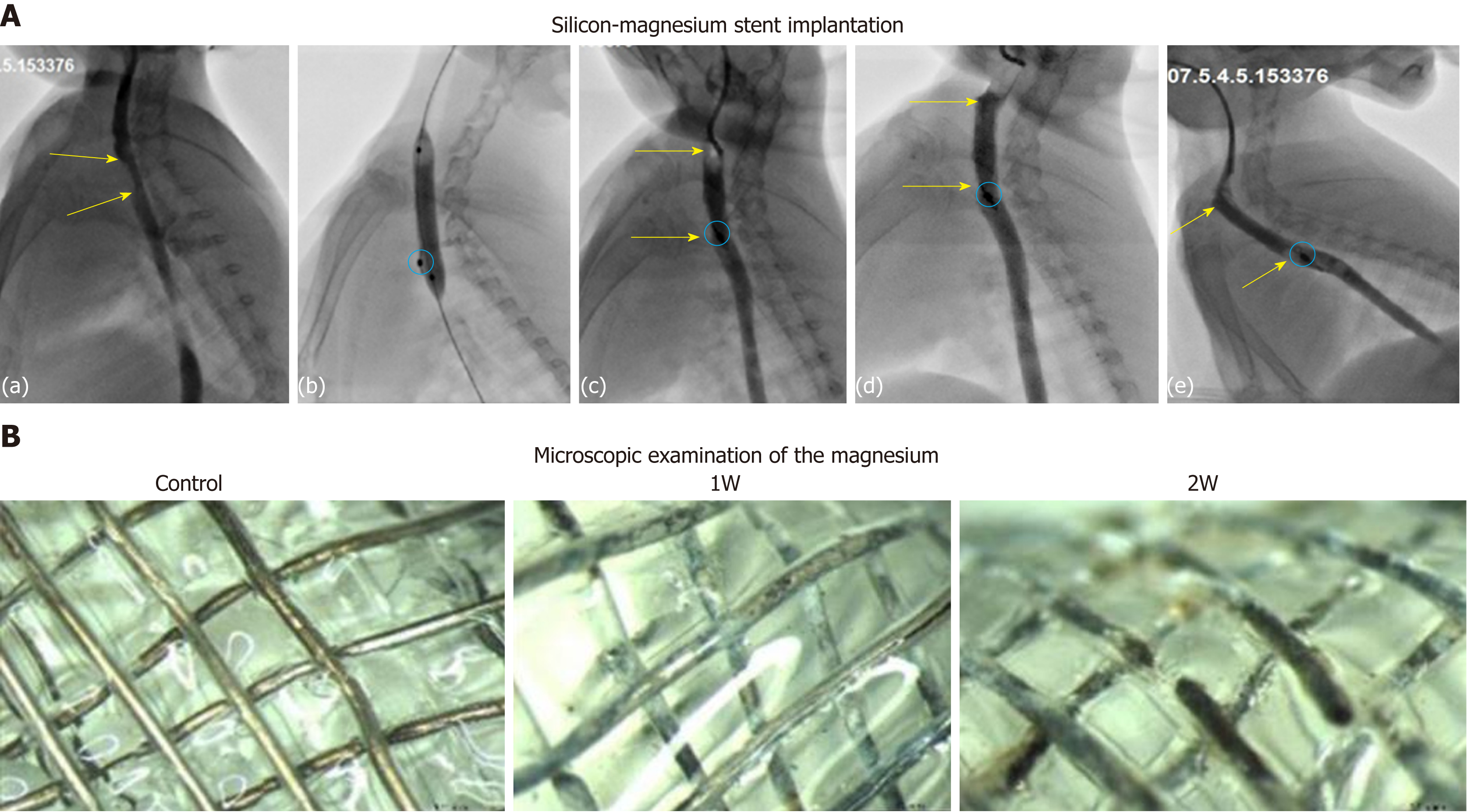
Esophageal angiography was performed to verify the stent expansion and the absence of esophageal perforation. No obvious aspiration, asphyxia, or death occurred during the procedures of stent implantation in rabbits. No stent migration occurred during the procedure period. Stent-related complications, such as perforation and bleeding of the esophagus, did not occur during the process or follow-up period. Esophagography demonstrated that stent expansion was good and the contrast agent can pass the stented esophagus smoothly [Figure 5A (a-c)].
After successful modeling, a total of 15 rabbits were implanted with silicone-covered magnesium stents, while 6 rabbits were left untreated. All of these rabbits underwent regular esophagography during follow-up and no animal died. At 1, 2, and 3 wk after stent implantation, 4, 6, and 5 rabbits in the stent group, respectively, were euthanized for examining the migration and location of silicone-covered magnesium stents. Stent migration was observed in 1 (25%) of 4 rabbits at 1 wk, 3 (50%) of 6 rabbits at 2 wk, and 4 (80%) of 5 rabbits at 3 wk after therapy. The esophageal diameter was 4.9 ± 0.3 mm before silicone-covered magnesium stent insertion and 9.8 ± 0.3 mm right after the stent implantation. The diameter was measured as 9.7 ± 0.7 mm, 9.8 ± 0.8 mm, and 9.2 ± 0.5 mm after 1, 2, and 3 wk, respectively (P < 0.05). In the stent group, in-stent stenosis did not occur in the follow-up period [Figure 5A (d-e)]. The weight was measured as 3.56 ± 0.3 kg before stent insertion and 3.48 ± 0.4 kg, 3.23 ± 0.3 kg, and 2.89 ± 0.2 kg after 1, 2, and 3 wk, while the weight of controls was 3.53 ± 0.3 kg.
Under microscopic examination, the degradation rates of silicone-covered magnesium stents, in terms of the number of degraded mesh units, in the rabbits without stent migration were 5.23% (minor degradation; 5.0%, 5.2%, and 5.5% for three individual rabbits) at 1 wk, 17.06% (minor degradation; 15.1%, 18.6%, and 17.5% for three individual rabbits) at 2 wk, and 88.0% for 1 rabbit at 3 wk after stent implantation (Figure 5B). Three stents (1 found at the first week and 2 at the second week) were in the stomach with partial degradation. These stents had about 40% remaining and a large amount of food residue in the cavity. Three stents (1 found in the second week and 2 in the third week) were located in the stomach, and they were almost completely degraded with only 1.5% ± 2.3% residual identified. In addition, two stents were excreted in the third week after stent insertion.
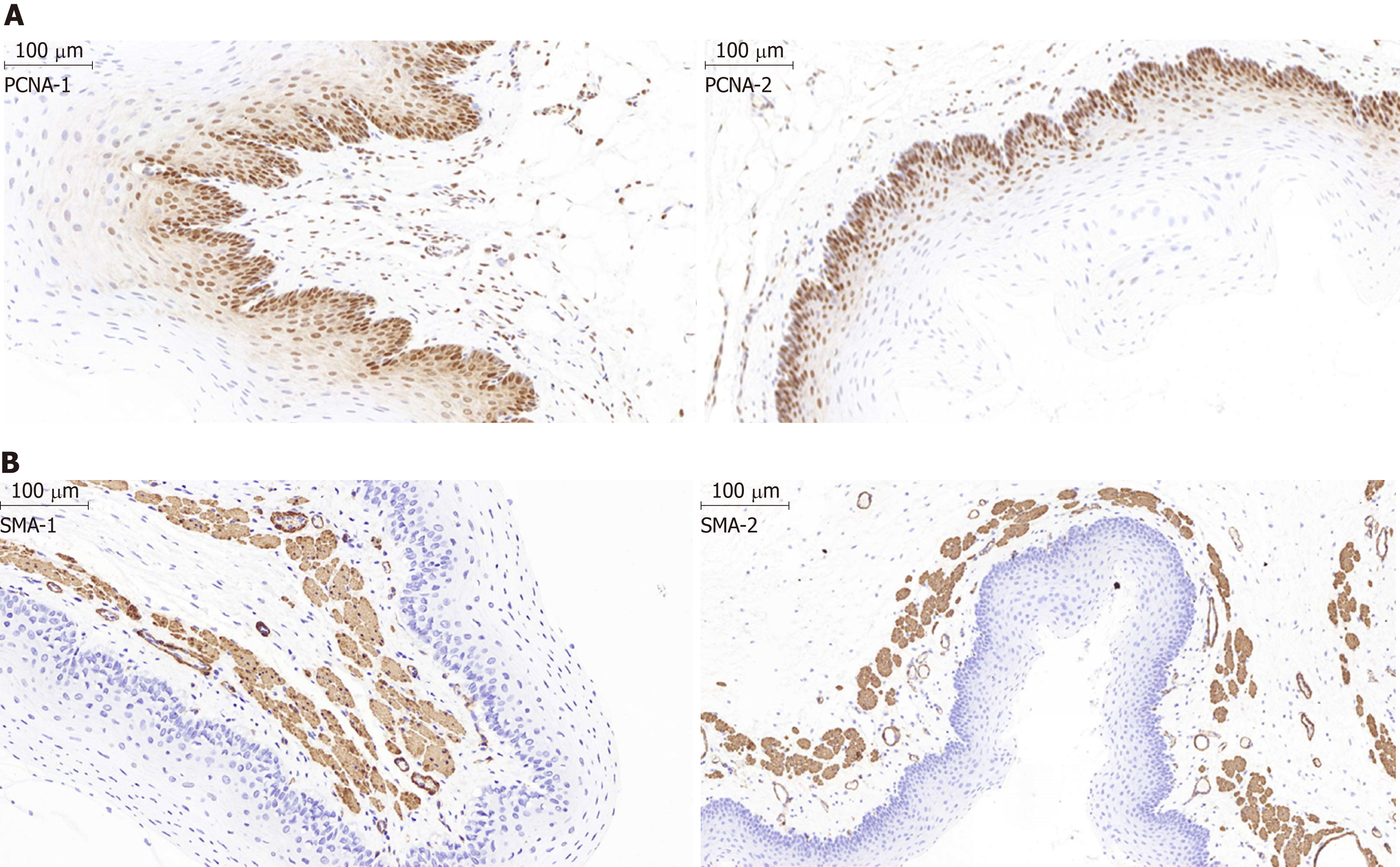
The inflammation scores were 0.25 ± 0.4, 0.40 ± 0.5, and 0.22 ± 0.4 at 1, 2, and 3 wk after stent implantation, respectively, in the stent group, and no difference was identified between the stent group and the normal control group (P > 0.05). The proliferation index by quantitative analysis of the PCNA-positive cells revealed a significant difference between the two groups (P < 0.05). As indicated by the distribution of PCNA-positive cells (Figure 6A), the epithelial layer in the stent group was obviously thinner than that in the normal control group (141.2 ± 30.5 μm vs 261.5 ± 17.2 μm; P < 0.05). As revealed by immunostaining, the SMA layer in the muscle layer was slightly thicker than that in the control group (129.0 ± 9.5 μm vs 90.5 ± 17.0 μm; P > 0.05) (Figure 6B). The thickness of the epithelial and SMA layers at 1, 2, and 3 wk after stent insertion in the stent group had no difference during the follow-up period (P > 0.05), which indicated that the reconstruction of the esophageal wall was completed within 1 week. Moreover, the amount of collagen did not display differences between these two groups at 1, 2, and 3 wk during follow-up (P > 0.05; data not shown), indicating the absence of adverse tissue responses caused by stent dilatation injury and degradation.
Currently, two types of degradable stents, biodegradable metal stent and high polymer stent, are widely used in clinical practice. Due to the absence of chronic inflammation and SMC hyperplasia after complete biodegradation, biodegradable stents have already been used as an effective and accepted method to treat BES patients to improve their quality of life[8,15,24,25]. However, there are no ideal stents that can provide enough and long-lasting force to tear the benign stricture of the esophagus as well as completely reduce complications caused by stent implantation. Here, we determined the feasibility of silicone-covered magnesium alloy for fabricating esophageal stents, and analyzed the mechanical property, degradation behaviors, and biological safety of silicone-covered magnesium stents. Through in vivo study using a rabbit model of BES, we found that the inserted silicone-covered magnesium stents can provide reliable support for at least two weeks, and did not cause severe injury or collagen deposition in rabbits.
Magnesium alloy is a fully biodegradable and ideal material for in vivo stents. It provides sufficient radial support force for moderate or severe stenosis and reduces tissue hyperplasia[26,27]. However, the rapid degradation rate of magnesium alloy makes it very difficult to maintain radial support force as long as 4 wk after its insertion for treating BES[21,28,29]. Silicone has numerous advantages like excellent elasticity, prominent coating performance, and remarkable resistance to degradation. We designed a new biodegradable esophageal stent with bare magnesium wire stent coated with a silicone membrane, which was named silicone-covered magnesium stent. This stent was proven to bear many advantages including outstanding degradability, elasticity, flexibility, and biocompatibility, which meets the general needs in clinical practice.
The silicone-covered magnesium stent in our study can provide a reliable radial force. After 46 compressions, the silicone-covered magnesium stent could still maintain sufficient effective mechanical compression performance. This demonstrates that silicone can stabilize the mechanism of magnesium alloy tube and maintain its structural stability, thus providing a sufficient support radial force during the expected time in vivo. In addition, the silicone membrane isolated magnesium alloy in direct contact with esophageal digestive juice, and significantly reduced the degradation rate of magnesium alloy. Our in vitro degradation experiments demonstrated that silicone membrane significantly reduced the degradation rate of magnesium wire. The degradation rates were 5.2% and 16.1% after 1 and 2 wk in the environment with a low pH value of 4.0, respectively. This suggests that the silicone-covered magnesium stent could provide sufficient support within 2 weeks in the esophagus stenosis.
The basic concept of BES treatment is that the stent could tear the hyperplastic smooth muscle layer and provide sufficient support during esophageal repair[12]. In our model, silicone-covered magnesium stent implantation was successful in all the rabbits with esophagus stenosis, and early complications like esophageal perforation, bleeding, and stent migration did not occur. Through pathological examination, we found that the reconstruction of the esophageal wall was completed in 1 and 2 wk after stent insertion. Analyses of the inflammation scores, PCNA-positive cells in the epithelial and SMA layers demonstrated that silicone-covered magnesium stent did not induce severe injury, but caused a very slight inflammatory reaction to the esophageal wall. Some studies showed that the best time of temporary stenting for achalasia was usually 1-2 wk[30,31]. In our experiment, the effective support time could last 2 weeks in the BES model, and esophageal wall reconstruction can be completed successfully during this period. Therefore, our in vivo experiments confirmed that the silicone-coated magnesium stent exhibited satisfactory therapeutic effects for treating BES in rabbits. Moreover, the silicone-covered magnesium stent displayed reliable biocompatibility without adverse effects on the growth of SMCs, while the excretion of silicone membrane through the digestive tract further supports its biological safety. Taken together, our study on the silicone-covered magnesium stent provides a theoretical basis for the treatment of BES, and also inspires new ideas to treat other stenoses.
Although the experimental results are encouraging, there are still some limitations in this study. For example, there is still room for extending the in vivo retention time of the stent, as the effective support time is 2 wk. Further study is needed to reduce the biodegradation rate and prolong the support time. In addition, a longer follow-up study is required for determining the efficacy, optimal insertion time, and tissue responses.
In conclusion, the silicone-covered magnesium stent designed in this study can meet the requirements for clinical esophageal stents, in terms of tensile strength, biological safety, and complications. The silicone-covered magnesium esophageal stent exhibited good therapeutic effects in a rabbit model of BES. Injury to the esophagus and stent migration during sustained strength expansion did not occur after the insertion of the silicone-covered magnesium stent in rabbits. As a simple, controllable approach, implantation of the biodegradable silicone-covered magnesium stent is a promising strategy in treating BES.
Stent insertion has been widely used as an effective alternative to improve the quality of life of patients with benign esophageal strictures (BES). However, the metallic stents implantation is associated with some severe complications, such as migration, tissue ingrowth, and instent restenosis. Stents made from biodegradable alloy or polymer are able to provide enough force to tear the benign stricture of the esophagus, as well as reduce complications caused by stents.
Magnesium alloy stents are a good candidate because of biological safety, but they show a poor corrosion resistance and a quick loss of mechanical support in vivo. Silicone coating could prolong degradation time of magnesium alloy and enhance the support force of magnesium alloy stents.
The aim of the present study was to evaluate the technique feasibility and therapeutic effect of and tissue response to silicone-covered bio-degradable magnesium stent insertion into the benign esophageal stricture in rabbits.
The silicone-covered magnesium stent was made of the magnesium alloy wires through cross-linked mesh, and was fabricated by covering with a silicone membrane. The mechanical testing demonstrated that silicone-covered magnesium stent possessed good flexibility and elasticity, and could provide adequate support in vivo. Fifteen rabbits underwent silicone-covered biodegradable magnesium stent insertion into the benign esophageal stricture under fluo-roscopic guidance (stent group). The wall reconstruction and tissue reaction of stenotic esophagus were compared with those of six stenosis esophagus models (control group). Esoph-agography was performed at 1, 2, and 3 wk after stent insertion.
Histological examination revealed that the inflammation scores at 4 wk in the BES rabbits with stent implantation (stent group) were similar to those in the control rabbits (control group). Both the epithelial and smooth muscle cell layers were significantly thinner in the stent group than in the control group. The smooth muscle actin layer in the muscle layer was thinner in the stent group than in the control group. Without causing severe injury or collagen deposition, im-plantation of silicone-covered magnesium stent provided reliable support for at least 2 wk in rabbits.
The present study demonstrated that insertion of silicone-covered magnesium esophageal stent is a promising approach for treating benign esophageal stricture without causing severe injury or tissue reaction.
The silicone-covered magnesium stent can provide reliable support for at least 1 wk, however, reliable support of the silicone-covered biodegradable magnesium for 2 wk is not enough and associated with high migration rates. There are still some limitations in this study. Further study is needed to reduce the biodegradation rate and prolong the support time. Longer follow-up study is required for determining the efficacy, optimal insertion time, and tissue responses.
We thank Wen-Guo Cui and his team at the Research Laboratory Center, the Animal Experiment Center of Shanghai Sixth People's Hospital for providing experiment support.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garbuzenko DV, Su CC, Uygun I S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Ravich WJ. Endoscopic Management of Benign Esophageal Strictures. Curr Gastroenterol Rep. 2017;19:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Poincloux L, Rouquette O, Abergel A. Endoscopic treatment of benign esophageal strictures: a literature review. Expert Rev Gastroenterol Hepatol. 2017;11:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Samanta J, Dhaka N, Sinha SK, Kochhar R. Endoscopic incisional therapy for benign esophageal strictures: Technique and results. World J Gastrointest Endosc. 2015;7:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Kim JH, Song HY, Choi EK, Kim KR, Shin JH, Lim JO. Temporary metallic stent placement in the treatment of refractory benign esophageal strictures: results and factors associated with outcome in 55 patients. Eur Radiol. 2009;19:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Gambardella C, Allaria A, Siciliano G, Mauriello C, Patrone R, Avenia N, Polistena A, Sanguinetti A, Napolitano S, Conzo G. Recurrent esophageal stricture from previous caustic ingestion treated with 40-year self-dilation: case report and review of literature. BMC Gastroenterol. 2018;18:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Vermeulen BD, Siersema PD. Esophageal Stenting in Clinical Practice: an Overview. Curr Treat Options Gastroenterol. 2018;16:260-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Zhang YW, Wei FX, Qi XP, Liu Z, Xu XD, Zhang YC. Efficacy and Safety of Endoscopic Intralesional Triamcinolone Injection for Benign Esophageal Strictures. Gastroenterol Res Pract. 2018;2018:7619298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Yang K, Ling C, Yuan T, Zhu Y, Cheng Y, Cui W. Polymeric Biodegradable Stent Insertion in the Esophagus. Polymers (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Zhu Y, Edmonds L, Zhao X, Chen X, Hu C, Cheng Y, Cui W. In vitro and in vivo evaluation of Rapamycin-eluting nanofibers coated on cardia stents. RSC Adv. 2014;4:34405-34411. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Yuan T, Zheng R, Yu J, Edmonds L, Wu W, Cao J, Gao F, Zhu Y, Cheng Y, Cui W. Fabrication and evaluation of polymer-based esophageal stents for benign esophagus stricture insertion. RSC Adv. 2016;6:16891-16898. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Zhu YQ, Cheng YS, Tang GY, Li MH, Zhao JG, Li F. Comparison of temporary stent insertion with pneumatic dilation of the same diameter in the treatment of achalasia patients: a retrospective study. J Gastroenterol Hepatol. 2010;25:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Hindy P, Hong J, Lam-Tsai Y, Gress F. A comprehensive review of esophageal stents. Gastroenterol Hepatol (NY). 2012;8:526-534. [PubMed] |
| 13. | Zhu Y, Yang K, Cheng R, Xiang Y, Yuan T, Cheng Y, Sarmento B, Cui W. The current status of biodegradable stent to treat benign luminal disease. Materials Today. 2017;20:516-529. [RCA] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Sigounas DE, Siddhi S, Plevris JN. Biodegradable esophageal stents in benign and malignant strictures - a single center experience. Endosc Int Open. 2016;4:E618-E623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Repici A, Vleggaar FP, Hassan C, van Boeckel PG, Romeo F, Pagano N, Malesci A, Siersema PD. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc. 2010;72:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Tanaka T, Takahashi M, Nitta N, Furukawa A, Andoh A, Saito Y, Fujiyama Y, Murata K. Newly developed biodegradable stents for benign gastrointestinal tract stenoses: a preliminary clinical trial. Digestion. 2006;74:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Sabharwal T, Gulati MS, Fotiadis N, Dourado R, Botha A, Mason R, Adam A. Randomised comparison of the FerX Ella antireflux stent and the ultraflex stent: proton pump inhibitor combination for prevention of post-stent reflux in patients with esophageal carcinoma involving the esophago-gastric junction. J Gastroenterol Hepatol. 2008;23:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Yuan T, Yu J, Cao J, Gao F, Zhu Y, Cheng Y, Cui W. Fabrication of a Delaying Biodegradable Magnesium Alloy-Based Esophageal Stent via Coating Elastic Polymer. Materials (Basel). 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Improving the biocompatibility of silicone: Surface Engineering. Materials Today 2003;6:19. . [DOI] [Full Text] |
| 20. | Esmaily M, Svensson JE, Fajardo S, Birbilis N, Frankel GS, Virtanen S, Arrabal R, Thomas S, Johansson LG. Fundamentals and advances in magnesium alloy corrosion. Pro Mater Sci. 2017;89:92-193. [DOI] [Full Text] |
| 21. | Zhu YQ, Yang K, Edmonds L, Wei LM, Zheng R, Cheng RY, Cui WG, Cheng YS. Silicone-covered biodegradable magnesium-stent insertion in the esophagus: a comparison with plastic stents. Therap Adv Gastroenterol. 2017;10:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Stepanenko AA, Dmitrenko VV. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene. 2015;574:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 210] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 23. | Hwang JC, Jin B, Kim JH, Lim SG, Yang MJ, Kim SS, Shin SJ, Lee KM, Kim JH. Esophageal stricture induced by an ultraslim upper endoscope in a novel rabbit model of corrosive injury. Scand J Gastroenterol. 2014;49:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Hair CS, Devonshire DA. Severe hyperplastic tissue stenosis of a novel biodegradable esophageal stent and subsequent successful management with high-pressure balloon dilation. Endoscopy. 2010;42 Suppl 2:E132-E133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Hirdes MM, Siersema PD, van Boeckel PG, Vleggaar FP. Single and sequential biodegradable stent placement for refractory benign esophageal strictures: a prospective follow-up study. Endoscopy. 2012;44:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Gu X, Zheng Y, Cheng Y, Zhong S, Xi T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials. 2009;30:484-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 532] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 27. | Li X, Chu CL, Liu L, Liu XK, Bai J, Guo C, Xue F, Lin PH, Chu PK. Biodegradable poly-lactic acid based-composite reinforced unidirectionally with high-strength magnesium alloy wires. Biomaterials. 2015;49:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Wong HM, Yeung KW, Lam KO, Tam V, Chu PK, Luk KD, Cheung KM. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials. 2010;31:2084-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 273] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 29. | Wu Q, Zhu S, Wang L, Liu Q, Yue G, Wang J, Guan S. The microstructure and properties of cyclic extrusion compression treated Mg-Zn-Y-Nd alloy for vascular stent application. J Mech Behav Biomed Mater. 2012;8:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Zhu YQ, Cheng YS, Li F, Li MH, Zhao JG, Chen NW. Application of the newly developed stents in the treatment of benign cardia stricture: an experimental comparative study. Gastrointest Endosc. 2011;73:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Zhu YQ, Cui WG, Cheng YS, Chang J, Chen NW, Yan L. Evaluation of biodegradable paclitaxel-eluting nanofibre-covered metal stents for the treatment of benign cardia stricture in an experimental model. Br J Surg. 2013;100:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |