Published online Jul 7, 2019. doi: 10.3748/wjg.v25.i25.3151
Peer-review started: March 6, 2019
First decision: April 4, 2019
Revised: April 28, 2019
Accepted: May 18, 2019
Article in press: May 18, 2019
Published online: July 7, 2019
Processing time: 123 Days and 17.8 Hours
Liver cancer is one of the most common malignancies, and various pathogenic factors can lead to its occurrence and development. Among all primary liver cancers, hepatocellular carcinoma (HCC) is the most common. With extensive studies, an increasing number of molecular mechanisms that promote HCC are being discovered. Surgical resection is still the most effective treatment for patients with early HCC. However, early detection and treatment are difficult for most HCC patients, and the postoperative recurrence rate is high, resulting in poor clinical prognosis of HCC. Although immunotherapy takes longer than conventional chemotherapy to produce therapeutic effects, it persists for longer. In recent years, the emergence of many new immunotherapies, such as immune checkpoint blockade and chimeric antigen receptor T cell therapies, has given new hope for the treatment of HCC.
Core tip: Among all primary liver cancers, hepatocellular carcinoma (HCC) is the most common and accounts for 90% of cases. Mechanisms related to HCC progression and treatment strategies have been extensively reported. In this paper, we review the molecular mechanisms involved in HCC progression and the latest advancements in immunotherapy by combining the research progress and results from our laboratory in recent years.
- Citation: Jiang Y, Han QJ, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol 2019; 25(25): 3151-3167
- URL: https://www.wjgnet.com/1007-9327/full/v25/i25/3151.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i25.3151
Hepatocellular carcinoma (HCC) is predicted to be the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide in 2018, accounting for approximately 841,000 new cases and 782,000 deaths annually. Primary liver cancer includes HCC and intrahepatic cholangiocarcinoma as well as other rare types, with HCC accounting for 75%-85% of cases[1,2]. The main risk factors for HCC are chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), aflatoxin-contaminated foodstuffs, heavy alcohol intake and type 2 diabetes[2]. Studies on the mechanisms of HCC processes have confirmed that the inactivation of multiple tumor suppressor genes (such as p53), abnormal activation of oncogenes (K-ras, etc.,) and multiple signaling pathways (PI3K, MAPK, JAK/STAT, NF-κB, Wnt/β-catenin, etc), abnormal regulation of epigenetic events (such as microRNAs), and even exosomes that deliver a large number of protumorigenic molecules are all involved in HCC development and progression[3]. The liver also acts as a special immune organ. In addition to the above carcinogenic factors, the immunological microenvironment in the liver is associated with HCC occurrence and development. In recent years, the interaction between various immune cells and tumor cells has attracted extensive attention. Many molecular mechanisms associated with the biological characteristics of tumor cells during hepatocarcinogenesis also have important effects on the immune system. Although improvements have been made in surgery, radiofrequency ablation and chemotherapy for HCC, the prognosis of HCC patients remains unsatisfactory due to the high rates of recurrence and metastasis. The emergence of many new immunotherapies, such as immune checkpoint blockade and chimeric antigen receptor (CAR) T-cell therapies, has given new hope for the treatment of HCC. Here, we review the molecular mechanisms that influence HCC progression and the latest advancements in immunotherapy by combining the latest research progress and results from our laboratory.
Chronic hepatitis caused by HBV infection is one of the main causes of HCC. Numerous studies have confirmed that HBV can activate a variety of signals to promote viral replication and inflammation progression and to accelerate hepatocarcinogenesis[4-7].
HBx and HCC: We also found that HBV further promoted viral replication by activating signal transducer and activator of transcription 3 (STAT3) signaling in HBV+ HCC, while blocking STAT3 can inhibit HBV replication and proliferation and angiogenesis in HBV+ HCC[8]. The HBV genome encodes four proteins, including the envelope protein (S/Pre-S), the core protein (C/pre-C), the polymerase (P), and the X protein (HBx). Among them, the multifunctional HBx protein has attracted substantial attention. HBx can block p53-mediated apoptosis and activate numerous signal transduction cascades (STAT, NF-κB, AP-1, etc.,) associated with cell proliferation and survival, promoting HCC occurrence and development[9-13]. HBx mutations in HCC patients haven been shown to be important for the development of HCC[14-16].
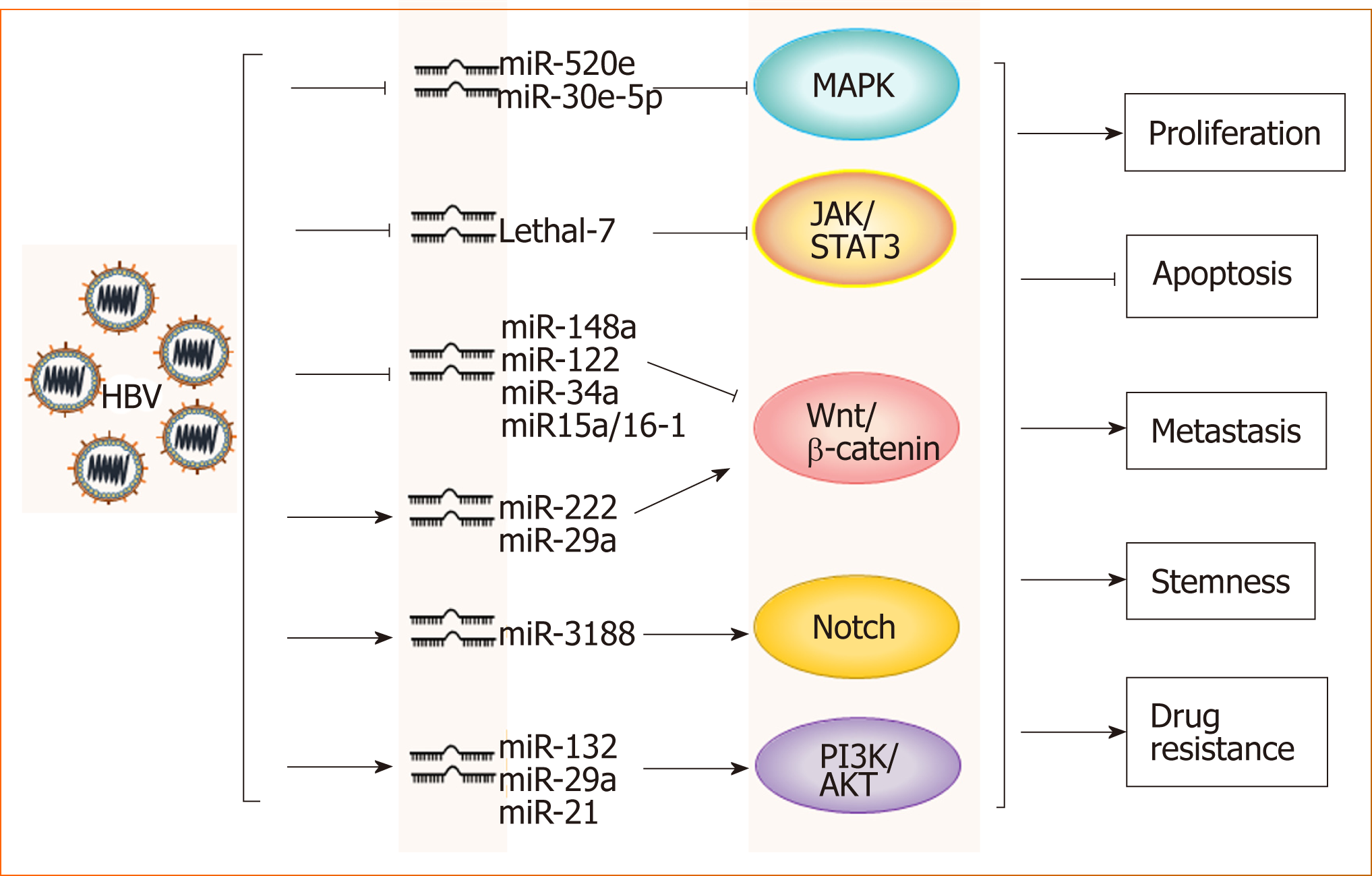
HBV and microRNAs: A large number of recent studies have shown that microRNAs play important roles in the occurrence and development of HBV-related HCC. Through the analysis of microRNA profiles, the expression levels of various micro-RNAs, such as miR-150 and miR-342-3p, were found to be changed in HBV-related HCC[17]. The analysis of a large number of clinical samples showed that microRNAs such as miR-375, miR-25, and let-7f are specific for HBV and have potential clinical value for the prediction and diagnosis of HBV+ HCC[18-20]. Further studies have demonstrated that HBV promotes HCC by intervening with Wnt, MAPK, Notch and other signaling pathways through different microRNAs[17,21-24] (Figure 1). However, miR-122 expression is inhibited in HBV+ HCC, which suggests that this microRNA likely plays an inhibitory role in HCC progression[25]. Mao et al[26] found that the tolerance of HBV+ HCC patients to sorafenib was significantly higher than that of non-HBV-infected HCC patients, which was related to activated Mcl-1-mediated inhibitory effects on miR-193b, and restoration of miR-193b expression could increase the sensitivity of HBV+ HCC to sorafenib. These phenomena indicate that the role of microRNAs in the progression of HBV-related HCC is complex and not simply promotive or suppressive.
HBV and immune tolerance: In addition to the above mechanisms, HBV-induced suppressive effects on innate and adaptive immune cells promote the evolution from inflammation to tumorigenesis. In patients with chronic HBV hepatitis, the activation and function of natural killer (NK) cells are significantly inhibited, and these impaired NK cells cannot effectively clear HBV, which further accelerates the progression of hepatitis to HCC[27,28]. The TGF-β-miR-34a-CCL22 signal induces regulatory T (Treg) cell infiltration and promotes the metastasis of HBV+ HCC[29], and the imbalance between helper T (Th)-17 and Treg cells is a risk factor for patients with HBV infection progressing to HCC[30]. In the course of chronic HBV infection, the expression of programmed death-1 (PD-1), cytotoxic T lymphocyte antigen-4 (CTLA-4), CD244 and other inhibitory receptors on virus-specific CD8+ T cells is increased, which mediates T cell depletion[27,31]. Recently, Zong et al[32] showed that in HBsAg-transgenic mice, the expression of TIGIT, a promising immune checkpoint in tumor immunotherapy, maintained the tolerance of CD8+ T cells to HBV, and disrupting this tolerance by TIGIT blockade or deficiency could induce chronic hepatitis in HBsAg-transgenic mice and eventually lead to HCC development, suggesting that immune checkpoint therapy in HBV carriers might increase the risk of chronic hepatitis and liver cancer.
To date, the role of HBV in HCC progression has been widely studied, providing new ideas for the effective prevention and treatment of HCC.
As an important member of the STAT family, STAT3 is constitutively activated in many tumor and immune cells in the tumor microenvironment. The abnormal activation of STAT3 signaling is closely related to the occurrence, proliferation, drug resistance and stemness of various tumors, including HCC. STAT3 also plays an important role in the regulation of the complex network formed in the tumor microenvironment[33-36].
STAT3 and microRNAs: STAT3 deficiency prevents hepatocarcinogenesis in the thioacetamide-induced liver injury model[37]. Constitutive activation of STAT3 is observed in HCC cells and tissues, and STAT3 decoy oligodeoxynucleotides (STAT3-decoy ODN) can specifically block the activation of STAT3 signaling in HCC, resulting in the inhibition of proliferation and apoptosis in tumor cells[38]. MicroRNAs such as miR-589-5p and miR-500a-3p maintain the drug resistance and stemness of HCC by activating STAT3 signaling[39-41]. However, some microRNAs have been found to inhibit tumor development by interfering with STAT3. MiR-345 upregulation has been shown to inhibit epithelial-mesenchymal transition (EMT) in HCC by targeting interferon regulatory factor 1 (IRF1)-mediated mTOR/STAT3/AKT signaling[42]. Furthermore, miR-451 may function as a potential suppressor of tumor angiogenesis in HCC by targeting IL-6R/STAT3/VEGF signaling, indicating a promising therapeutic strategy for HCC[43]. Although different microRNAs have different effects on HCC, STAT3 plays a key role in the regulation of microRNA signaling during hepatocarcinogenesis.
TLR4 and STAT3: The relationship between inflammation and tumors has been well established[44]. Approximately 70% of the blood supply to the liver comes from the outflow of intestinal veins, and the presence of the hepato-intestinal axis makes the liver the first line of defense against enterogenous antigens. Pathogen-associated molecular patterns (PAMPs) derived from the intestinal microbiota play a regulatory role in liver diseases by activating Toll-like receptors (TLRs). In a liver injury-cancer model induced by a combination of diethylnitrosamine (DEN) and the hepatotoxin carbon tetrachloride (CCl4), TLR4-/- mice showed a significant decrease in tumor number and volume formation compared to wild-type mice. Moreover, intestinal microbiota-derived lipopolysaccharides (LPS) can activate TLR4 signaling in hepatocytes to promote inflammation-induced hepatocarcinogenesis[45]. We also found that TLR4 was constitutively expressed in HCC, and further study demonstrated that TLR4 promoted HCC occurrence and progression depending on the activation of the Cox-2/PGE2/STAT3 axis and was associated with multiple drug resistance[46]. Significantly, sorafenib can inhibit HCC by blocking TLR4/STAT3/SUMO1 activation[47].
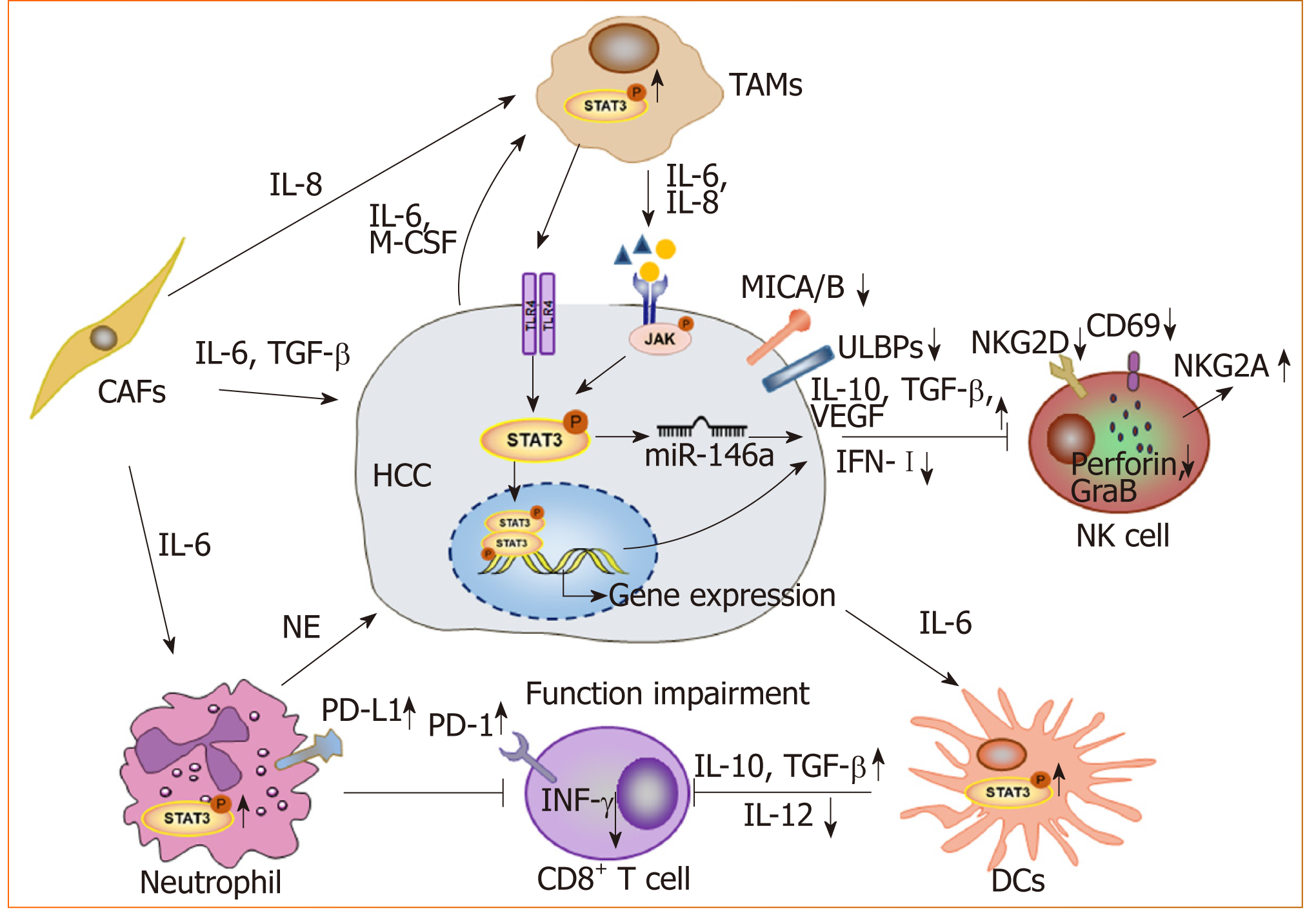
STAT3 and the tumor microenvironment: With advancements in research, the occurrence and development of tumors is thought to arise due to not only the deterioration and proliferation of tumor cells, but also the immunosuppressive tumor microenvironment. As a key transcription factor, STAT3 is constitutively activated in both tumor cells and immune cells in the microenvironment (Figure 2). We found that blocking STAT3 in HCC cells could effectively disrupt tumor-induced immune tolerance and induce an antitumor reaction in tumor-bearing mice, which might be related to the downregulation of transforming growth factor-beta (TGF-β) and interleukin(IL)-10 and the upregulation of type I interferons (IFNs)[48,49]. Alternatively, STAT3 could directly regulate miR-146a expression to upregulate the expression of TGF-β, IL-17 and VEGF and downregulate the expression of type I IFNs, mediating the inhibitory effect of NK cells on HCC[50]. More importantly, STAT3 could inhibit the Th1 immune response and promote the formation of an immunosuppressive micro-environment[51,52]. Studies have shown that tumor-associated macrophages (TAMs) promote HCC progression by secreting cytokines such as IL-8 and IL-6 to activate STAT3 in HCC[53,54]. HCC-associated fibroblasts induce the activation of STAT3 pathways in neutrophils and dendritic cells (DCs), and these STAT3-over-activated neutrophils and DCs display protumorigenic roles[55,56]. Blocking STAT3 activation in immune cells such as TAMs and DCs can inhibit HCC progression[56,57]. Meanwhile, blocking STAT3 not only inhibits HCC proliferation but also upregulates NKG2D ligand expression, such as ULBPs and MICA/B, in HCC cells to increase the sensitivity of HCC to NK cell-mediated cytolysis and enhance the anti-HCC activity of NK cells[48,49].
In summary, STAT3 signaling can interact with multiple pathways to promote HCC. STAT3 plays an important role in both the maintenance of HCC malignancy and the suppression of the immune microenvironment, which makes STAT3 an ideal target for HCC treatment. The development of STAT3 inhibitors used for clinical application is an attractive research topic. STAT3 inhibitors, including AZD9150 and TTI-101, have entered the clinical trial phase for HCC treatment (ClinicalTrials.gov identifier: NCT03195699 and NCT01839604, respectively). Several drugs, which were not initially applied for tumor treatment, have also been found to exert anticancer effects by blocking STAT3[58-60]. Thus, the identification of new STAT3-targeted inhibitors is still an important direction for drug development.
Homeobox genes were first discovered in the fruit fly Drosophila, which are divided into many subfamilies (Hox, PAX, NKX, etc.,) on the basis of the level of similarity among them[61]. Homologous homeobox genes have been found in mammals. Most homeobox genes are involved in regulating the expression of genes related to embryonic development and cell differentiation. Mutations in these genes can lead to abnormal organ development in eukaryotes[61-64]. In addition, in recent years, a variety of homeobox genes have been found to be involved in the occurrence and development of tumors[64,65], and different homeobox genes have been shown to play different roles in the progression of cancer such as HCC.
Hox and HCC: The Hox gene, an important member of the homeobox family, is abnormally expressed in multiple malignant solid tumors[65]. The role of Hox has also been studied in HCC over the years. Among HOX genes, HOXA13 has been reported to be the most deregulated in HCC. HOXA13 overexpression in HCC cell lines results in increased colony formation and migration but reduced sensitivity to sorafenib[66-68]. Knockdown of endogenous HOXA7 results in decreased proliferation of HCC cells by inhibiting cyclin E1/cyclin-dependent kinase-2[69]. HOXB7 can promote EMT and stemness formation by upregulating the expression of c-Myc and Slug in HCC[70].
HMBOX1 and HCC: Homeobox containing 1 (HMBOX1), a novel human homeobox gene, was first isolated from the human pancreatic cDNA library. HMBOX1 belongs to the HNF homeobox class of the homeobox family[63,71]. The expression of HMBOX1 is reported to be up- or downregulated in some tumors[72-75]. Our previous study revealed that the expression level of HMBOX1 in liver cancer was lower than that in adjacent non‑cancerous tissues[74]. Further study showed that HMBOX1 expression was negatively correlated with the differentiation and clinical stage of HCC, and HMBOX1 overexpression could inhibit HCC by promoting autophagy, inhibiting the cancer stem cell phenotype and increasing tumor cell sensitivity to NK cell-mediated cytolysis. The underlying mechanisms may be related to changes in the expression of Fas and programmed cell death ligand 1 (PD-L1) in HCC cells mediated by HMBOX1 overexpression[76]. Additionally, HMBOX1 expression in hepatocytes has been shown to prevent inflammation and liver injury by reducing macrophage infiltration and acti-vation, thereby blocking inflammation-related tumor development[77].
Other homeobox genes and HCC: Prospero-related homeobox 1 (PROX1) is closely related to proliferation, differentiation and prognosis in HCC. PROX1 can upregulate IL-8 expression and activate NF-κB and β-catenin signals to promote HCC angiogenesis and sorafenib resistance[78-80]. NK3 homeobox 1 (NKX3.1) was initially found to play an important role in the regulation of prostate development and tumorigenesis[81]. Recently, NKX3.1 expression was shown to be decreased in HCC tissues, and NKX3.1 overexpression induced G1/S phase arrest in HCC cells through up-regulation of FOXO1[82]. HLXB9 is highly expressed in poorly differentiated HCC samples[83].
From the above, different homeobox genes exhibit different functions via related mechanisms to promote or inhibit HCC progression (Table 1). Because the homeobox family has a large number of genes, its role in HCC and other tumors remains to be further explored.
| Homeobox genes | Involvement in HCC process | Target genes | Ref. |
| HOXA13 | Colony formation (+), migration (+), Drug resistance (+) | — | [65,67] |
| HOXA7 | Proliferation (-) | Cyclin e1/cdk2 | [68] |
| HOXB7 | Stemness (+), EMT (+) | c-Myc, slug | [69] |
| HMBOX1 | Autophagy (+), Stemness (-), Immunosuppression (-) | PD-L1, Fas | [75] |
| PROX1 | Drug resistance (+), angiogenesis (+) | IL-8, NF-κB, β-catenin | [77-79] |
| NKX3.1 | Proliferation (-) | FOXO1 | [80] |
As a highly conserved signaling pathway during biological evolution, the Wnt signaling pathway plays an important role in a variety of physiological and pathological processes[84]. With advancements in research, the regulatory role of the Wnt signaling pathway in cancer progression and the emergence of stemness has attracted widespread attention. According to the different mechanisms of signal tran-sduction, the Wnt pathway is generally divided into the canonical Wnt/β-catenin and non-canonical β-catenin-independent pathways.
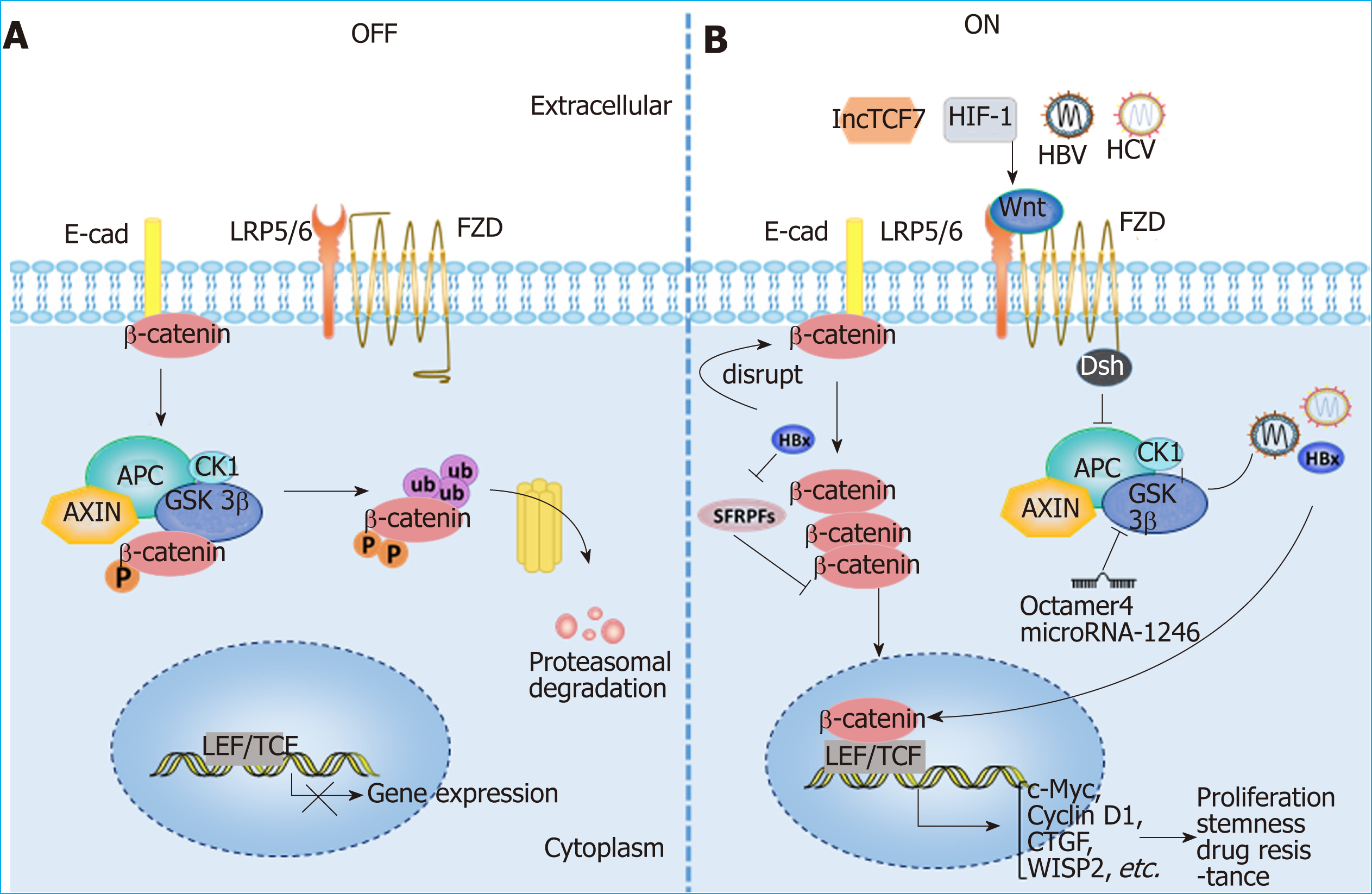
Wnt signaling and HCC: Aberrant activation of the Wnt/β-catenin signaling path-way has been observed in HCC patients, and various molecules, such as the protein components of HBV and HCV, as well as hypoxia-induced factor (commonly known as HIF), can activate the Wnt/β-catenin pathway in HCC[5,85-88]. The activation of Wnt/β-catenin signaling is closely related to the occurrence and development of HCC, the formation of stemness and drug resistance[79,89-91] (Figure 3). In addition, Wnt signals regulate HCC progression by interacting with Hippo and Notch signaling pathways[92-94]. In addition to mutations in CTNNB1, AXIN1 and other related genes[95-98], epigenetic regulation is involved in the aberrant activation of the Wnt signaling pathway. For instance, the long noncoding RNA lncTCF7 promotes stemness and dissemination in HCC by activating Wnt signaling[99]. In HBV-related HCC, HBx silences secreted frizzled-related proteins (SFRPs) by mediating DNA methylation to activate Wnt signaling[100]. Many microRNAs regulate the activation of Wnt signaling at the posttranscriptional level to affect HCC progression. For example, miR-542-3p can target the frizzled 7/Wnt signaling pathway to inhibit HCC[101], while Octamer 4/miR-1246 promotes stemness by inhibiting AXIN2 and GSK3 and thereby activating Wnt/β-catenin signaling in HCC[102].
Wnt signaling and the tumor microenvironment: In addition to the effect on tumor cells themselves, Wnt signaling has recently been found to play an important role in the formation of a tumor immunosuppressive microenvironment. β-catenin activation in DCs can inhibits the process of antigen cross presentation at CD8+ T cells[103,104] and participates in the differentiation and activation of Treg cells[105]. Wnt/β-catenin activation in TAMs facilitates M2 polarization, which promotes HCC[106]. TAMs also activate β-catenin by secreting CCL17 to promote EMT in HCC[107]. Although many studies have demonstrated that Wnt activation plays an immunosuppressive role, the mechanism of Wnt activation in the tumor microenvironment remains to be further explored. A better understanding of the role of Wnt signaling in HCC progression is thus essential for the prevention and treatment of HCC.
Exosomes can be released by all cell types, including cancer cells and immune cells, and play important roles in intercellular communication[108]. As carriers and transporters, exosomes deliver a variety of biological molecules, including proteins, lipids, and nucleic acids. Exosomes have been shown to play important roles in most cancer-associated processes.
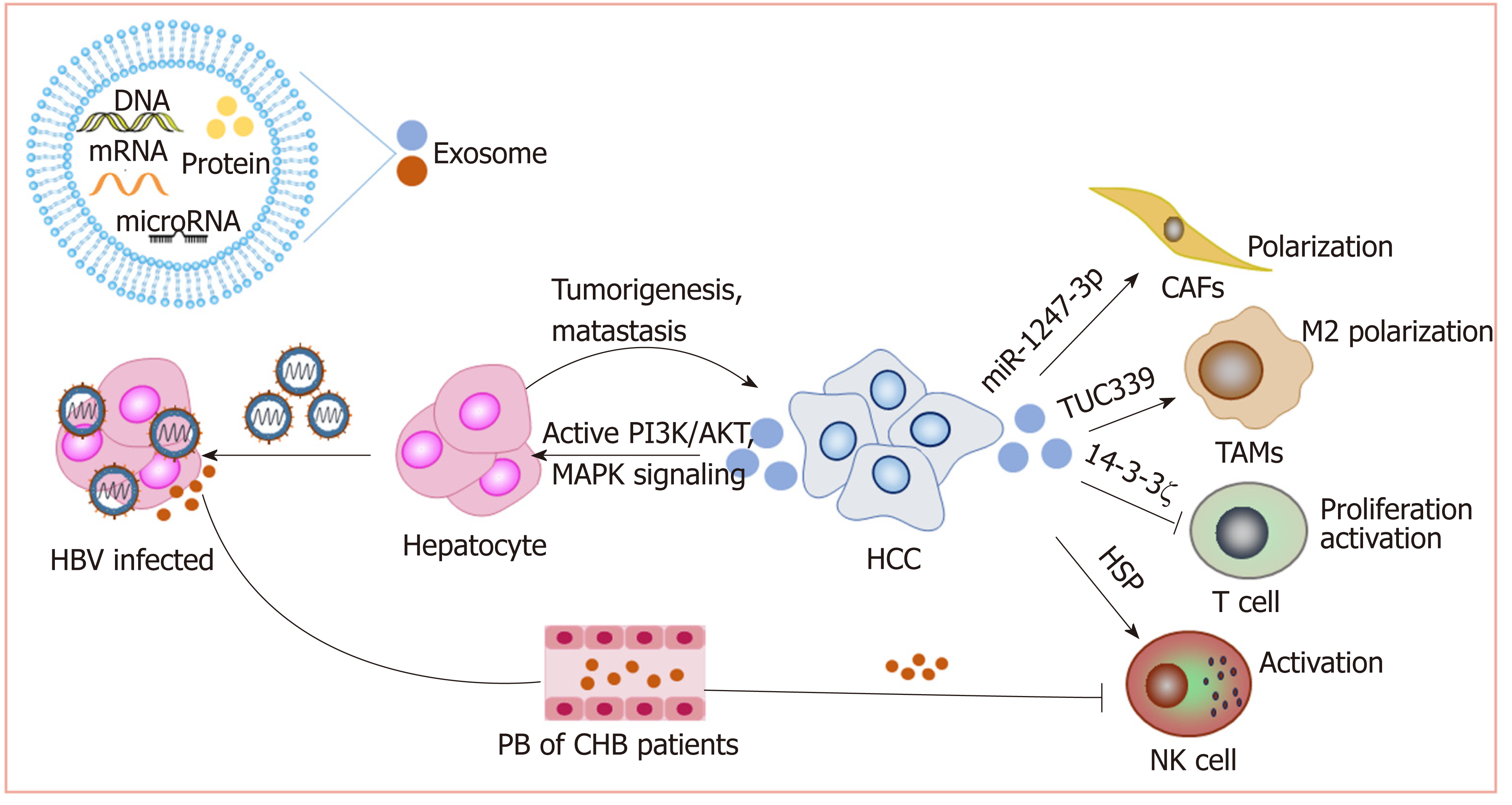
Exosomes promote HCC: We have reported that exosomes present in the sera of chronic hepatitis B patients contain both HBV-derived nucleic acids and HBV proteins and can transfer HBV to hepatocytes in an active manner. Moreover, exosomes mediate the transmission of HBV into NK cells, resulting in the impairment of NK cell functions[109]. This may contribute to the progression of chronic HBV infection to HCC. Exosomes derived from metastatic HCC cell lines carry a large number of protumorigenic RNAs and proteins, such as MET protooncogenes and S100 family members. These exosomes promote metastasis by triggering the PI3K/AKT and MAPK signaling pathways in hepatocytes and increasing the secretion of active matrix metalloproteinase (MMP) 2 and MMP-9[110].
Exosomes and chemoresistance: HCC is highly resistant to chemotherapy. Qu et al[111] found that exosomes derived from HCC cells can induce sorafenib resistance by activating the HGF/c-Met/Akt signaling pathway. Moreover, exosomes derived from highly invasive HCC cells have greater efficacy than exosomes derived from less invasive cells[111]. Exosomal miR-32-5p can induce multidrug resistance in HCC by activating the PI3K/Akt pathway[112]. Therefore, HCC cell-derived exosomes might be an important target for reversing chemoresistance.
Exosomes and the tumor microenvironment: As carriers, exosomes play an im-portant role in cell-cell interactions in the tumor microenvironment. Anticancer drugs induce the release of exosomes containing heat shock proteins (HSPs) from HCC cells, which elicit effective NK cell-mediated antitumor responses[113]. However, 14-3-3ζ proteins delivered by exosomes can be transmitted from HCC cells to tumor-infiltrating T cells, impairing the functions, proliferation and activation of T cells[114]. Additionally, HCC-derived exosomes containing lncRNA TUC339 can be taken up by macrophages to play important roles in macrophage activation and regulation of M1/M2 polarization[115]. Additionally, highly metastatic HCC cell-secreted exosomal miR-1247-3p directly targets B4GALT3, leading to the activation of β1-integrin-NF-κB signaling in fibroblasts, which converts normal fibroblasts to cancer-associated fibroblasts. Activated cancer-associated fibroblasts further promote cancer progression. Clinical data also show that high serum exosomal miR-1247-3p levels are correlated with lung metastasis in HCC patients[116].
Therefore, exosomes play important roles in the development of HCC (Figure 4). Nevertheless, more studies on how exosomes mediate HCC progression are still needed to promote the clinical utilization of exosomes.
Clinical treatment of HCC includes liver transplantation, surgical resection, chemotherapy, radiotherapy, interventional therapy and immunotherapy. Liver transplantation is the only treatment option for HCC patients with unresectable tumors or cirrhosis[117]. The 5-year survival rates can reach 60%–70% in patients with HCC after liver transplantation[118]. However, the number of patients that need liver transplantation exceeds the number of available donor organs[119]. Therefore, HCC patients must be selected very carefully in terms of tumor size and number of tumor nodules[120]. Surgical resection of early HCC is still the first choice for treatment, but the recurrence rate within five years is as high as 70%[2]. The multikinase inhibitor sorafenib is recognized as the most effective molecular targeted drug for the treatment of advanced HCC worldwide. Despite advancements in molecular therapy with the multikinase inhibitor sorafenib, the prognosis of advanced HCC cases remains poor, with five-year survival rates of 3%-11%[121]. The immune system plays a key role in controlling and eradicating cancer. Therefore, immunotherapy has received much attention in recent years. Additionally, although immunotherapy takes longer than conventional chemotherapy to produce a therapeutic effect, it persists for longer. Earlier immunotherapy mainly included cytokine-mediated immunotherapy, oncolytic virus therapy, TLR agonist therapy and DC vaccine. In recent years, emerging immunotherapies, such as immune checkpoint blockade and CAR T cell therapies, have shown better therapeutic effects on some tumors, thus giving us new hope for the treatment of HCC.
Checkpoint blockade, currently the top candidate in immunotherapy, has been shown to be effective for the treatment of many cancers, especially for chemotherapy resistant malignant tumors. Among the available immune checkpoint inhibitors, CTLA-4 and PD-1 display the most pronounced effects and have shown remarkable efficacy in the treatment of malignant melanoma[122,123]. Nivolumab, pembrolizumab (PD-1 inhibitor) and tremelimumab (CTLA-4 inhibitor) have been demonstrated to be safe and effective in clinical trials[124-126], and nivolumab has been approved by the U.S. Food and Drug Administration (commonly known as the FDA) as a second-line treatment for HCC[127].
Although good results have been achieved in the treatment of HCC with checkpoint blockade, the response rate to the treatment is relatively low due to the formation of an immunosuppressive microenvironment. The combination of checkpoint blockade inhibitors and other methods may enhance the efficacy[128,129]. Recently, Zhou et al[130] found that the combined use of checkpoint inhibitors and myeloid-derived suppressor cell infiltration blockers can augment the therapeutic effect of anti-PD-L1 antibody in HCC. The use of tremelimumab combined with radio-frequency ablation to treat advanced HCC promoted the accumulation of CD8+ T cells in tumor tissues, suggesting that this combined treatment might be a new therapeutic method[131]. The combined use of anti-CTLA-4 antibodies, anti-PD-L1 antibodies and the histone deacetylase inhibitor belinostat completely eliminated tumor load in HCC tumor-bearing mice[132]. An increasing number of researchers are investigating the therapeutic effect of combination therapy when immune checkpoint blockade alone is not effective. Thus, combined therapy may be a new strategy for the future application of checkpoint blockade in solid tumors, including liver cancer, but large amounts of clinical trial data are still needed to support the development of specific treatment strategies.
Adoptive cell transfer is the most representative tumor immunotherapy at present, and it is mediated by cytolytic activity against tumor cells by the transfer of lymphocytes from the patient themselves or from donors. Before the emergence of CAR T cells, adoptive transfer therapy in HCC mainly focused on tumor-infiltrating lymphocytes and cytokine induced killer cells[133-135]. With the recent discovery of CAR T cells and their amazing therapeutic effect on hematological tumors[136], the effect of CAR T cell therapy in solid tumors such as HCC has attracted increasing attention.
CAR T cells: CARs provide T cells with the ability to directly recognize tumor antigens independent of the human leukocyte antigen. This allows CAR engineered T cells to recognize a wider range of targets than natural T cells. At present, the most widely used CAR structure consists of a single-chain antibody extracellular domain that recognizes and binds specific antigens, an extracellular hinge region, a transmembrane region, and an intracellular domain that provides proliferation and activation signals. Throughout the entirety of the immunotherapy process, the design and integration of CARs into T cells to generate CAR T cells are the most critical steps[137]. Multiple studies have demonstrated that glypican 3 (GPC3) is an attractive liver cancer-specific target, because its expression is high in HCC tissues but limited in normal tissues[138]. GPC3-specific CAR T cell therapy for HCC exhibits a strong killing effect on GPC3-positive HCC cells both in vivo and in vitro[139]. Furthermore, a relevant phase 1 clinical trial study (ClinicalTrials.gov identifier: NCT02395250) showed that autologous T cells bearing GPC3-specific CARs were safe and effective in patients with relapsed or refractory HCC. Meanwhile, another phase 1 clinical trial (ClinicalTrials.gov identifier: NCT02541370) involving CD133-directed CAR T cells for advanced HCC demonstrated feasibility, controllable toxicities and effectiveness[140]. However, immunosuppressive microenvironments can hinder the infiltration of CAR T cells into tumor tissues, thereby reducing CAR T cell-mediated antitumor effects[141]. Interestingly, Guo et al[142] showed that further inhibition of PD-1 expression in GPC3-specific CAR T cells can enhance the killing effect of CAR T cells on HCC cells.
Treatment of HCC based on NK cells: Similar to T cells, NK cells can be modified with CARs that recognize antigens expressed by tumors and combine with signaling components that enhance NK cell activity. At present, clinical studies on CAR NK cells mostly focus on the treatment of lymphoma and hematological tumors, and only a few studies exist regarding the treatment of solid tumors such as HCC. Yu et al[143] found that GPC3-specific CAR NK cells constructed with NK-92 cells could effectively inhibit proliferation and promote apoptosis in HCC cells. Furthermore, CAR NK cells display lower toxicity than CAR T cells and do not need patient matching, which makes CAR NK cells more promising for cancer treatment[144]. Previously, we constructed gene-modified NK cells to augment NK cell activity and found that IL-15- or IFN-α-gene modification increased the production of TNF-α and IFN-γ by NK-92 or NKL cells, promoting apoptosis in HCC cells by upregulating the expression of NKG2D ligands and Fas on HCC cells. These NK cells also exerted enhanced antitumor effects in vivo[145-147].
The role of TLR agonists as a vaccine adjuvant and tumor immunotherapeutic agent has been recently noted[148-150]. As a vaccine adjuvant, TLR agonists trigger antigen presentation by promoting the maturation of DCs. Multiple TLRs, such as TLR3 and TLR9, have been confirmed to be expressed on HCC cells[151-153], and the role of TLR agonists in tumor therapy has received much attention. The TLR2/4 agonist OM-174 has potential roles in the prevention of invasion and metastasis in HCC[154]. We also found that both TLR3 agonist poly (I:C) and TLR9 agonist ODN M362 can exert antitumor effects on HCC cells. Surprisingly, we found that simultaneous transfection of poly (I:C) and ODN M362 exhibits a lower proapoptotic effect on HCC than transfection of poly (I:C) alone. Further investigation demonstrated that ODN M362 blocks the entrance of poly (I:C) when simultaneously used to treat HCC cells and then decreases the activation of poly (I:C)-triggered cellular apoptosis; however, poly (I:C)-mediated proapoptotic effects could be enhanced by pretreating HCC cells with CpG ODN[155]. TLR agonists can also work as adjuvants to stimulate the immune system during tumor treatment, but their effects on tumor cells cannot be ignored. These therapeutic effects may thus be the overall outcome of various mechanisms.
DCs are central regulators of the adaptive immune response and are thus necessary for T-cell-mediated antitumor immunity. DC vaccines have the characteristics of low complication rates and good tolerance, and DC-based tumor vaccines have been used for a variety of solid tumors[156]. Currently, there are many clinical studies on the use of DC vaccines for HCC treatment. The injection of DCs prestimulated by HCC-specific antigens can increase the number of CD8+ T cells, promote antitumor immune responses and improve liver function[157-159]. The combined use of DC vaccines and other treatments, such as radiotherapy, can induce immunogenic death of tumor cells, which can prolong the overall survival of patients[160].
Exosomes display an array of HCC antigens. Rao et al[161] demonstrated that tumor cell-derived exosomes could trigger a stronger DC-mediated immune response than cell lysates and improve the HCC tumor microenvironment. Exosomes derived from α-fetoprotein (AFP)-expressing DCs (DEXAFP) elicited strong antigen-specific immune responses, resulting in significantly delayed tumor growth and prolonged survival rates in mice with HCC tumors[162]. Therefore, DEXAFP might be a promising vaccine for HCC immunotherapy. AFP, a carcinoembryonic antigen, is highly expressed in HCC and serves as an important marker in the diagnosis of HCC, as well as a potential immunotherapy target for HCC. Zhang et al[163] prepared a mouse AFP recom-binant vaccine by genetic engineering and found that it could induce cellular and humoral immune responses in tumor-bearing mice and show obvious antitumor effects. Additionally, with the use of HCC cell lysates derived from STAT3-inhibited HCC cells to immunize healthy mice, we found that a variety of immune cells, such as T cells and NK cells, were significantly activated after challenge with murine HCC cells in these immunized mice, showing effective inhibition characteristics against the transplanted tumor and resulting in the formation of immune memory[164]. Therefore, as a target for HCC treatment and prevention, blocking STAT3 not only prevents tumor growth but also exerts an important effect on immune system activation.
Although immunotherapy for HCC has made significant progress, the clinical efficacy still needs to be further improved. Finding new targets for the treatment of HCC is still the direction of scientific researchers in the next few years. In recent years, studies about the roles of epigenetics and metabolomics on HCC progression have also become hot spots. Related drug development is also ongoing. A single treatment may not bring satisfactory therapeutic effect. Individual differences need to be more considered. Combined therapy and individualized therapy may be a promising option in HCC treatment.
The development of HCC results from the accumulation of many factors and the interaction among many mechanisms. Exploring the molecular mechanisms underlying the occurrence and development of HCC is important for us to obtain a more comprehensive understanding of the disease process and to identify more effective therapeutic targets and strategies. With continuous breakthroughs in research, in addition to traditional therapies, immunotherapies have shown good efficacy for HCC in both preclinical and clinical trials, offering hope for curing this disease. Thus, the combination of drugs acting on various pathways, targets and treatment methods might be effective strategies to achieve greater clinical benefits for the treatment of HCC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Gayyar M, Mikulic D S-Editor: Ma RY L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 2. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1873] [Article Influence: 208.1] [Reference Citation Analysis (4)] |
| 3. | Aravalli RN, Cressman EN, Steer CJ. Cellular and molecular mechanisms of hepatocellular carcinoma: an update. Arch Toxicol. 2013;87:227-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Wang M, Xi D, Ning Q. Virus-induced hepatocellular carcinoma with special emphasis on HBV. Hepatol Int. 2017;11:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Daud M, Rana MA, Husnain T, Ijaz B. Modulation of Wnt signaling pathway by hepatitis B virus. Arch Virol. 2017;162:2937-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Gao J, Xiong Y, Wang Y, Wang Y, Zheng G, Xu H. Hepatitis B virus X protein activates Notch signaling by its effects on Notch1 and Notch4 in human hepatocellular carcinoma. Int J Oncol. 2016;48:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Yoneda M, Hyun J, Jakubski S, Saito S, Nakajima A, Schiff ER, Thomas E. Hepatitis B Virus and DNA Stimulation Trigger a Rapid Innate Immune Response through NF-κB. J Immunol. 2016;197:630-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Yang Y, Zheng B, Han Q, Zhang C, Tian Z, Zhang J. Targeting blockage of STAT3 inhibits hepatitis B virus-related hepatocellular carcinoma. Cancer Biol Ther. 2016;17:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Arbuthnot P, Capovilla A, Kew M. Putative role of hepatitis B virus X protein in hepatocarcinogenesis: effects on apoptosis, DNA repair, mitogen-activated protein kinase and JAK/STAT pathways. J Gastroenterol Hepatol. 2000;15:357-368. [PubMed] |
| 10. | Diao J, Khine AA, Sarangi F, Hsu E, Iorio C, Tibbles LA, Woodgett JR, Penninger J, Richardson CD. X protein of hepatitis B virus inhibits Fas-mediated apoptosis and is associated with up-regulation of the SAPK/JNK pathway. J Biol Chem. 2001;276:8328-8340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Martin-Lluesma S, Schaeffer C, Robert EI, van Breugel PC, Leupin O, Hantz O, Strubin M. Hepatitis B virus X protein affects S phase progression leading to chromosome segregation defects by binding to damaged DNA binding protein 1. Hepatology. 2008;48:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Diao J, Garces R, Richardson CD. X protein of hepatitis B virus modulates cytokine and growth factor related signal transduction pathways during the course of viral infections and hepatocarcinogenesis. Cytokine Growth Factor Rev. 2001;12:189-205. [PubMed] |
| 13. | Geng M, Xin X, Bi LQ, Zhou LT, Liu XH. Molecular mechanism of hepatitis B virus X protein function in hepatocarcinogenesis. World J Gastroenterol. 2015;21:10732-10738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Chen GG, Li MY, Ho RL, Chak EC, Lau WY, Lai PB. Identification of hepatitis B virus X gene mutation in Hong Kong patients with hepatocellular carcinoma. J Clin Virol. 2005;34:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Kim JK, Chang HY, Lee JM, Baatarkhuu O, Yoon YJ, Park JY, Kim DY, Han KH, Chon CY, Ahn SH. Specific mutations in the enhancer II/core promoter/precore regions of hepatitis B virus subgenotype C2 in Korean patients with hepatocellular carcinoma. J Med Virol. 2009;81:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Benhenda S, Cougot D, Buendia MA, Neuveut C. Hepatitis B virus X protein molecular functions and its role in virus life cycle and pathogenesis. Adv Cancer Res. 2009;103:75-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Wang G, Dong F, Xu Z, Sharma S, Hu X, Chen D, Zhang L, Zhang J, Dong Q. MicroRNA profile in HBV-induced infection and hepatocellular carcinoma. BMC Cancer. 2017;17:805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Zhu HT, Liu RB, Liang YY, Hasan AME, Wang HY, Shao Q, Zhang ZC, Wang J, He CY, Wang F, Shao JY. Serum microRNA profiles as diagnostic biomarkers for HBV-positive hepatocellular carcinoma. Liver Int. 2017;37:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Chen S, Chen H, Gao S, Qiu S, Zhou H, Yu M, Tu J. Differential expression of plasma microRNA-125b in hepatitis B virus-related liver diseases and diagnostic potential for hepatitis B virus-induced hepatocellular carcinoma. Hepatol Res. 2017;47:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, Shen HB, Zhang CY, Zen K. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798-9807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 378] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 21. | Tian JH, Liu WD, Zhang ZY, Tang LH, Li D, Tian ZJ, Lin SW, Li YJ. Influence of miR-520e-mediated MAPK signalling pathway on HBV replication and regulation of hepatocellular carcinoma cells via targeting EphA2. J Viral Hepat. 2019;26:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Qin X, Li C, Guo T, Chen J, Wang HT, Wang YT, Xiao YS, Li J, Liu P, Liu ZS, Liu QY. Upregulation of DARS2 by HBV promotes hepatocarcinogenesis through the miR-30e-5p/MAPK/NFAT5 pathway. J Exp Clin Cancer Res. 2017;36:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Zhou SJ, Deng YL, Liang HF, Jaoude JC, Liu FY. Hepatitis B virus X protein promotes CREB-mediated activation of miR-3188 and Notch signaling in hepatocellular carcinoma. Cell Death Differ. 2017;24:1577-1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Xie KL, Zhang YG, Liu J, Zeng Y, Wu H. MicroRNAs associated with HBV infection and HBV-related HCC. Theranostics. 2014;4:1176-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Liang HW, Wang N, Wang Y, Wang F, Fu Z, Yan X, Zhu H, Diao W, Ding Y, Chen X, Zhang CY, Zen K. Hepatitis B virus-human chimeric transcript HBx-LINE1 promotes hepatic injury via sequestering cellular microRNA-122. J Hepatol. 2016;64:278-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Mao K, Zhang J, He C, Xu K, Liu J, Sun J, Wu G, Tan C, Zeng Y, Wang J, Xiao Z. Restoration of miR-193b sensitizes Hepatitis B virus-associated hepatocellular carcinoma to sorafenib. Cancer Lett. 2014;352:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol. 2011;54:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 28. | Sun C, Sun H, Zhang C, Tian Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol. 2015;12:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 29. | Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, Deng Y, Zhao J, Jiang S, Yuan Y, Wang HY, Cheng SQ, Xie D, Wang XF. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 451] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 30. | Li K, Liu H, Guo T. Th17/Treg imbalance is an indicator of liver cirrhosis process and a risk factor for HCC occurrence in HBV patients. Clin Res Hepatol Gastroenterol. 2017;41:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Raziorrouh B, Schraut W, Gerlach T, Nowack D, Grüner NH, Ulsenheimer A, Zachoval R, Wächtler M, Spannagl M, Haas J, Diepolder HM, Jung MC. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology. 2010;52:1934-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 32. | Zong L, Peng H, Sun C, Li F, Zheng M, Chen Y, Wei H, Sun R, Tian Z. Breakdown of adaptive immunotolerance induces hepatocellular carcinoma in HBsAg-tg mice. Nat Commun. 2019;10:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1338] [Cited by in RCA: 1661] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 34. | Wörmann SM, Song L, Ai J, Diakopoulos KN, Kurkowski MU, Görgülü K, Ruess D, Campbell A, Doglioni C, Jodrell D, Neesse A, Demir IE, Karpathaki AP, Barenboim M, Hagemann T, Rose-John S, Sansom O, Schmid RM, Protti MP, Lesina M, Algül H. Loss of P53 Function Activates JAK2-STAT3 Signaling to Promote Pancreatic Tumor Growth, Stroma Modification, and Gemcitabine Resistance in Mice and Is Associated With Patient Survival. Gastroenterology. 2016;151:180-193.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 35. | Jones LM, Broz ML, Ranger JJ, Ozcelik J, Ahn R, Zuo D, Ursini-Siegel J, Hallett MT, Krummel M, Muller WJ. STAT3 Establishes an Immunosuppressive Microenvironment during the Early Stages of Breast Carcinogenesis to Promote Tumor Growth and Metastasis. Cancer Res. 2016;76:1416-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1429] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 37. | Abe M, Yoshida T, Akiba J, Ikezono Y, Wada F, Masuda A, Sakaue T, Tanaka T, Iwamoto H, Nakamura T, Sata M, Koga H, Yoshimura A, Torimura T. STAT3 deficiency prevents hepatocarcinogenesis and promotes biliary proliferation in thioacetamide-induced liver injury. World J Gastroenterol. 2017;23:6833-6844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Sun X, Zhang J, Wang L, Tian Z. Growth inhibition of human hepatocellular carcinoma cells by blocking STAT3 activation with decoy-ODN. Cancer Lett. 2008;262:201-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Long J, Jiang C, Liu B, Dai Q, Hua R, Chen C, Zhang B, Li H. Maintenance of stemness by miR-589-5p in hepatocellular carcinoma cells promotes chemoresistance via STAT3 signaling. Cancer Lett. 2018;423:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Jiang C, Long J, Liu B, Xu M, Wang W, Xie X, Wang X, Kuang M. miR-500a-3p promotes cancer stem cells properties via STAT3 pathway in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 41. | Li T, Li M, Hu S, Cheng X, Gao Y, Jiang S, Yu Q, Zhang C, Sun P, Xian W, Song Z, Zhang Y, Zheng Q. MiR-221 mediates the epithelial-mesenchymal transition of hepatocellular carcinoma by targeting AdipoR1. Int J Biol Macromol. 2017;103:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Yu M, Xue H, Wang Y, Shen Q, Jiang Q, Zhang X, Li K, Jia M, Jia J, Xu J, Tian Y. miR-345 inhibits tumor metastasis and EMT by targeting IRF1-mediated mTOR/STAT3/AKT pathway in hepatocellular carcinoma. Int J Oncol. 2017;50:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Liu X, Zhang A, Xiang J, Lv Y, Zhang X. miR-451 acts as a suppressor of angiogenesis in hepatocellular carcinoma by targeting the IL-6R-STAT3 pathway. Oncol Rep. 2016;36:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 44. | Morrison WB. Inflammation and cancer: a comparative view. J Vet Intern Med. 2012;26:18-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 1029] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 46. | Lin A, Wang G, Zhao H, Zhang Y, Han Q, Zhang C, Tian Z, Zhang J. TLR4 signaling promotes a COX-2/PGE2/STAT3 positive feedback loop in hepatocellular carcinoma (HCC) cells. Oncoimmunology. 2015;5:e1074376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Li J, Zhou Y, Liu Y, Dai B, Zhang YH, Zhang PF, Shi XL. Sorafenib inhibits caspase-1 expression through suppressing TLR4/stat3/SUMO1 pathway in hepatocellular carcinoma. Cancer Biol Ther. 2018;1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Sun X, Sui Q, Zhang C, Tian Z, Zhang J. Targeting blockage of STAT3 in hepatocellular carcinoma cells augments NK cell functions via reverse hepatocellular carcinoma-induced immune suppression. Mol Cancer Ther. 2013;12:2885-2896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Sui Q, Zhang J, Sun X, Zhang C, Han Q, Tian Z. NK cells are the crucial antitumor mediators when STAT3-mediated immunosuppression is blocked in hepatocellular carcinoma. J Immunol. 2014;193:2016-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Sun X, Zhang J, Hou Z, Han Q, Zhang C, Tian Z. miR-146a is directly regulated by STAT3 in human hepatocellular carcinoma cells and involved in anti-tumor immune suppression. Cell Cycle. 2015;14:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39-49. [PubMed] |
| 52. | Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mulé J, Kerr WG, Jove R, Pardoll D, Yu H. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 797] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 53. | Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ, Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, Fan J. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol. 2015;46:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 54. | Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 531] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 55. | Cheng Y, Li H, Deng Y, Tai Y, Zeng K, Zhang Y, Liu W, Zhang Q, Yang Y. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 290] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 56. | Cheng JT, Deng YN, Yi HM, Wang GY, Fu BS, Chen WJ, Liu W, Tai Y, Peng YW, Zhang Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 57. | Yin Z, Ma T, Lin Y, Lu X, Zhang C, Chen S, Jian Z. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J Cell Biochem. 2018;119:9419-9432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 58. | Zhao T, Jia H, Cheng Q, Xiao Y, Li M, Ren W, Li C, Feng Y, Feng Z, Wang H, Zheng J. Nifuroxazide prompts antitumor immune response of TCL-loaded DC in mice with orthotopically-implanted hepatocarcinoma. Oncol Rep. 2017;37:3405-3414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Ma H, Yan D, Wang Y, Shi W, Liu T, Zhao C, Huo S, Duan J, Tao J, Zhai M, Luo P, Guo J, Tian L, Mageta L, Jou D, Zhang C, Li C, Lin J, Lv J, Li S, Lin L. Bazedoxifene exhibits growth suppressive activity by targeting interleukin-6/glycoprotein 130/signal transducer and activator of transcription 3 signaling in hepatocellular carcinoma. Cancer Sci. 2019;110:950-961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Sharma D, Wang J, Fu PP, Sharma S, Nagalingam A, Mells J, Handy J, Page AJ, Cohen C, Anania FA, Saxena NK. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology. 2010;52:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 61. | Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 472] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 62. | Bienz M. Homeotic genes and positional signalling in the Drosophila viscera. Trends Genet. 1994;10:22-26. [PubMed] |
| 63. | Holland PW, Booth HA, Bruford EA. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007;5:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 64. | Samuel S, Naora H. Homeobox gene expression in cancer: insights from developmental regulation and deregulation. Eur J Cancer. 2005;41:2428-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | Bhatlekar S, Fields JZ, Boman BM. HOX genes and their role in the development of human cancers. J Mol Med (Berl). 2014;92:811-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 66. | Quagliata L, Matter MS, Piscuoglio S, Arabi L, Ruiz C, Procino A, Kovac M, Moretti F, Makowska Z, Boldanova T, Andersen JB, Hämmerle M, Tornillo L, Heim MH, Diederichs S, Cillo C, Terracciano LM. Long noncoding RNA HOTTIP/HOXA13 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Hepatology. 2014;59:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 362] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 67. | Pan TT, Jia WD, Yao QY, Sun QK, Ren WH, Huang M, Ma J, Li JS, Ma JL, Yu JH, Ge YS, Liu WB, Zhang CH, Xu GL. Overexpression of HOXA13 as a potential marker for diagnosis and poor prognosis of hepatocellular carcinoma. Tohoku J Exp Med. 2014;234:209-219. [PubMed] |
| 68. | Quagliata L, Quintavalle C, Lanzafame M, Matter MS, Novello C, di Tommaso L, Pressiani T, Rimassa L, Tornillo L, Roncalli M, Cillo C, Pallante P, Piscuoglio S, Ng CK, Terracciano LM. High expression of HOXA13 correlates with poorly differentiated hepatocellular carcinomas and modulates sorafenib response in in vitro models. Lab Invest. 2018;98:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 69. | Li Y, Yang XH, Fang SJ, Qin CF, Sun RL, Liu ZY, Jiang BY, Wu X, Li G. HOXA7 stimulates human hepatocellular carcinoma proliferation through cyclin E1/CDK2. Oncol Rep. 2015;33:990-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Huan HB, Yang DP, Wen XD, Chen XJ, Zhang L, Wu LL, Bie P, Xia F. HOXB7 accelerates the malignant progression of hepatocellular carcinoma by promoting stemness and epithelial-mesenchymal transition. J Exp Clin Cancer Res. 2017;36:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Chen S, Saiyin H, Zeng X, Xi J, Liu X, Li X, Yu L. Isolation and functional analysis of human HMBOX1, a homeobox containing protein with transcriptional repressor activity. Cytogenet Genome Res. 2006;114:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Yu YL, Diao NN, Li YZ, Meng XH, Jiao WL, Feng JB, Liu ZP, Lu N. Low expression level of HMBOX1 in high-grade serous ovarian cancer accelerates cell proliferation by inhibiting cell apoptosis. Biochem Biophys Res Commun. 2018;501:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Gong J, Liu R, Zhuang R, Zhang Y, Fang L, Xu Z, Jin L, Wang T, Song C, Yang K, Wei Y, Yang A, Jin B, Chen L. miR-30c-1* promotes natural killer cell cytotoxicity against human hepatoma cells by targeting the transcription factor HMBOX1. Cancer Sci. 2012;103:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Dai J, Wu L, Zhang C, Zheng X, Tian Z, Zhang J. Recombinant expression of a novel human transcriptional repressor HMBOX1 and preparation of anti-HMBOX1 monoclonal antibody. Cell Mol Immunol. 2009;6:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Zhang P, Liu Q, Yan S, Yuan G, Shen J, Li G. Homeoboxcontaining protein 1 loss is associated with clinicopathological performance in glioma. Mol Med Rep. 2017;16:4101-4106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Zhao H, Jia H, Han Q, Zhang J. Homeobox containing 1 inhibits liver cancer progression by promoting autophagy as well as inhibiting stemness and immune escape. Oncol Rep. 2018;40:1657-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Zhao H, Han Q, Lu N, Xu D, Tian Z, Zhang J. HMBOX1 in hepatocytes attenuates LPS/D-GalN-induced liver injury by inhibiting macrophage infiltration and activation. Mol Immunol. 2018;101:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Liu Y, Zhang Y, Wang S, Dong QZ, Shen Z, Wang W, Tao S, Gu C, Liu J, Xie Y, Qin LX. Prospero-related homeobox 1 drives angiogenesis of hepatocellular carcinoma through selectively activating interleukin-8 expression. Hepatology. 2017;66:1894-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Liu Y, Ye X, Zhang JB, Ouyang H, Shen Z, Wu Y, Wang W, Wu J, Tao S, Yang X, Qiao K, Zhang J, Liu J, Fu Q, Xie Y. PROX1 promotes hepatocellular carcinoma proliferation and sorafenib resistance by enhancing β-catenin expression and nuclear translocation. Oncogene. 2015;34:5524-5535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 80. | Shimoda M, Takahashi M, Yoshimoto T, Kono T, Ikai I, Kubo H. A homeobox protein, prox1, is involved in the differentiation, proliferation, and prognosis in hepatocellular carcinoma. Clin Cancer Res. 2006;12:6005-6011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, Abate-Shen C, Shen MM. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999;13:966-977. [PubMed] |
| 82. | Jiang J, Liu Z, Ge C, Chen C, Zhao F, Li H, Chen T, Yao M, Li J. NK3 homeobox 1 (NKX3.1) up-regulates forkhead box O1 expression in hepatocellular carcinoma and thereby suppresses tumor proliferation and invasion. J Biol Chem. 2017;292:19146-19159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Wilkens L, Jaggi R, Hammer C, Inderbitzin D, Giger O, von Neuhoff N. The homeobox gene HLXB9 is upregulated in a morphological subset of poorly differentiated hepatocellular carcinoma. Virchows Arch. 2011;458:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3740] [Cited by in RCA: 4400] [Article Influence: 338.5] [Reference Citation Analysis (0)] |
| 85. | Aicher S, Kakkanas A, Cohen L, Blumen B, Oprisan G, Njouom R, Meurs EF, Mavromara P, Martin A. Differential regulation of the Wnt/β-catenin pathway by hepatitis C virus recombinants expressing core from various genotypes. Sci Rep. 2018;8:11185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Wang W, Pan Q, Fuhler GM, Smits R, Peppelenbosch MP. Action and function of Wnt/β-catenin signaling in the progression from chronic hepatitis C to hepatocellular carcinoma. J Gastroenterol. 2017;52:419-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 87. | Xu W, Zhou W, Cheng M, Wang J, Liu Z, He S, Luo X, Huang W, Chen T, Yan W, Xiao J. Hypoxia activates Wnt/β-catenin signaling by regulating the expression of BCL9 in human hepatocellular carcinoma. Sci Rep. 2017;7:40446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 88. | Chen Z, Tang J, Cai X, Huang Y, Gao Q, Liang L, Tian L, Yang Y, Zheng Y, Hu Y, Tang N. HBx mutations promote hepatoma cell migration through the Wnt/β-catenin signaling pathway. Cancer Sci. 2016;107:1380-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67:10831-10839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 360] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 90. | Mokkapati S, Niopek K, Huang L, Cunniff KJ, Ruteshouser EC, deCaestecker M, Finegold MJ, Huff V. β-catenin activation in a novel liver progenitor cell type is sufficient to cause hepatocellular carcinoma and hepatoblastoma. Cancer Res. 2014;74:4515-4525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 91. | Huang M, Chen C, Geng J, Han D, Wang T, Xie T, Wang L, Wang Y, Wang C, Lei Z, Chu X. Targeting KDM1A attenuates Wnt/β-catenin signaling pathway to eliminate sorafenib-resistant stem-like cells in hepatocellular carcinoma. Cancer Lett. 2017;398:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 92. | Kim W, Khan SK, Gvozdenovic-Jeremic J, Kim Y, Dahlman J, Kim H, Park O, Ishitani T, Jho EH, Gao B, Yang Y. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest. 2017;127:137-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 93. | Wang R, Sun Q, Wang P, Liu M, Xiong S, Luo J, Huang H, Du Q, Geller DA, Cheng B. Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget. 2016;7:5754-5768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 94. | Kim W, Khan SK, Yang Y. Interacting network of Hippo, Wnt/β-catenin and Notch signaling represses liver tumor formation. BMB Rep. 2017;50:1-2. [PubMed] |
| 95. | Monga SP. β-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology. 2015;148:1294-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 96. | Amaddeo G, Cao Q, Ladeiro Y, Imbeaud S, Nault JC, Jaoui D, Gaston Mathe Y, Laurent C, Laurent A, Bioulac-Sage P, Calderaro J, Zucman-Rossi J. Integration of tumour and viral genomic characterizations in HBV-related hepatocellular carcinomas. Gut. 2015;64:820-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 97. | Perugorria MJ, Olaizola P, Labiano I, Esparza-Baquer A, Marzioni M, Marin JJG, Bujanda L, Banales JM. Wnt-β-catenin signalling in liver development, health and disease. Nat Rev Gastroenterol Hepatol. 2019;16:121-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 98. | Abitbol S, Dahmani R, Coulouarn C, Ragazzon B, Mlecnik B, Senni N, Savall M, Bossard P, Sohier P, Drouet V, Tournier E, Dumont F, Sanson R, Calderaro J, Zucman-Rossi J, Vasseur-Cognet M, Just PA, Terris B, Perret C, Gilgenkrantz H. AXIN deficiency in human and mouse hepatocytes induces hepatocellular carcinoma in the absence of β-catenin activation. J Hepatol. 2018;68:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 99. | Wang Y, He L, Du Y, Zhu P, Huang G, Luo J, Yan X, Ye B, Li C, Xia P, Zhang G, Tian Y, Chen R, Fan Z. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16:413-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 514] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 100. | Xie Q, Chen L, Shan X, Shan X, Tang J, Zhou F, Chen Q, Quan H, Nie D, Zhang W, Huang AL, Tang N. Epigenetic silencing of SFRP1 and SFRP5 by hepatitis B virus X protein enhances hepatoma cell tumorigenicity through Wnt signaling pathway. Int J Cancer. 2014;135:635-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 101. | Wu W, Dang S, Feng Q, Liang J, Wang Y, Fan N. MicroRNA-542-3p inhibits the growth of hepatocellular carcinoma cells by targeting FZD7/Wnt signaling pathway. Biochem Biophys Res Commun. 2017;482:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 102. | Chai S, Ng KY, Tong M, Lau EY, Lee TK, Chan KW, Yuan YF, Cheung TT, Cheung ST, Wang XQ, Wong N, Lo CM, Man K, Guan XY, Ma S. Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology. 2016;64:2062-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 103. | Fu C, Liang X, Cui W, Ober-Blöbaum JL, Vazzana J, Shrikant PA, Lee KP, Clausen BE, Mellman I, Jiang A. β-Catenin in dendritic cells exerts opposite functions in cross-priming and maintenance of CD8+ T cells through regulation of IL-10. Proc Natl Acad Sci USA. 2015;112:2823-2828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 104. | Liang X, Fu C, Cui W, Ober-Blöbaum JL, Zahner SP, Shrikant PA, Clausen BE, Flavell RA, Mellman I, Jiang A. β-catenin mediates tumor-induced immunosuppression by inhibiting cross-priming of CD8⁺ T cells. J Leukoc Biol. 2014;95:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 105. | Hong Y, Manoharan I, Suryawanshi A, Majumdar T, Angus-Hill ML, Koni PA, Manicassamy B, Mellor AL, Munn DH, Manicassamy S. β-catenin promotes regulatory T-cell responses in tumors by inducing vitamin A metabolism in dendritic cells. Cancer Res. 2015;75:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 106. | Yang Y, Ye YC, Chen Y, Zhao JL, Gao CC, Han H, Liu WC, Qin HY. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9:793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 107. | Zhu F, Li X, Chen S, Zeng Q, Zhao Y, Luo F. Tumor-associated macrophage or chemokine ligand CCL17 positively regulates the tumorigenesis of hepatocellular carcinoma. Med Oncol. 2016;33:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 108. | Ruivo CF, Adem B, Silva M, Melo SA. The Biology of Cancer Exosomes: Insights and New Perspectives. Cancer Res. 2017;77:6480-6488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 418] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 109. | Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. 2017;14:465-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 110. | He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, Ngai SM, Chan TF, Wong N. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36:1008-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 210] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 111. | Qu Z, Wu J, Wu J, Luo D, Jiang C, Ding Y. Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. J Exp Clin Cancer Res. 2016;35:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 112. | Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, Wen H, Yang Y, Wang S, Wang J, Nan K, Yao Y, Tian T. Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 113. | Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287:15874-15885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 353] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 114. | Wang X, Shen H, Zhangyuan G, Huang R, Zhang W, He Q, Jin K, Zhuo H, Zhang Z, Wang J, Sun B, Lu X. 14-3-3ζ delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis. 2018;9:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 115. | Li X, Lei Y, Wu M, Li N. Regulation of Macrophage Activation and Polarization by HCC-Derived Exosomal lncRNA TUC339. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 116. | Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S, Cao D, Tang L, Tang S, Wu M, Yang W, Wang H. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 750] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 117. | Mazzola A, Costantino A, Petta S, Bartolotta TV, Raineri M, Sacco R, Brancatelli G, Cammà C, Cabibbo G. Recurrence of hepatocellular carcinoma after liver transplantation: an update. Future Oncol. 2015;11:2923-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 118. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10453] [Article Influence: 696.9] [Reference Citation Analysis (0)] |
| 119. | Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 333] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 120. | Kollmann D, Selzner N, Selzner M. Bridging to liver transplantation in HCC patients. Langenbecks Arch Surg. 2017;402:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 121. | Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 392] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 122. | Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11786] [Article Influence: 785.7] [Reference Citation Analysis (0)] |
| 123. | Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 534] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 124. | El-Khoueiry AB. Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3314] [Article Influence: 414.3] [Reference Citation Analysis (1)] |
| 125. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Pérez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 751] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 126. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1900] [Article Influence: 271.4] [Reference Citation Analysis (0)] |
| 127. | Kudo M. Systemic Therapy for Hepatocellular Carcinoma: 2017 Update. Oncology. 2017;93 Suppl 1:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 128. | Greten TF, Wang XW, Korangy F. Current concepts of immune based treatments for patients with HCC: from basic science to novel treatment approaches. Gut. 2015;64:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 129. | Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 451] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 130. | Zhou J, Liu M, Sun H, Feng Y, Xu L, Chan AWH, Tong JH, Wong J, Chong CCN, Lai PBS, Wang HK, Tsang SW, Goodwin T, Liu R, Huang L, Chen Z, Sung JJ, Chow KL, To KF, Cheng AS. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut. 2018;67:931-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 131. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 132. | Llopiz D, Ruiz M, Villanueva L, Iglesias T, Silva L, Egea J, Lasarte JJ, Pivette P, Trochon-Joseph V, Vasseur B, Dixon G, Sangro B, Sarobe P. Enhanced anti-tumor efficacy of checkpoint inhibitors in combination with the histone deacetylase inhibitor Belinostat in a murine hepatocellular carcinoma model. Cancer Immunol Immunother. 2019;68:379-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 133. | Pan K, Li YQ, Wang W, Xu L, Zhang YJ, Zheng HX, Zhao JJ, Qiu HJ, Weng DS, Li JJ, Wang QJ, Huang LX, He J, Chen SP, Ke ML, Wu PH, Chen MS, Li SP, Xia JC, Zeng YX. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol. 2013;20:4305-4311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 134. | Jiang SS, Tang Y, Zhang YJ, Weng DS, Zhou ZG, Pan K, Pan QZ, Wang QJ, Liu Q, He J, Zhao JJ, Li J, Chen MS, Chang AE, Li Q, Xia JC. A phase I clinical trial utilizing autologous tumor-infiltrating lymphocytes in patients with primary hepatocellular carcinoma. Oncotarget. 2015;6:41339-41349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 135. | Yu X, Zhao H, Liu L, Cao S, Ren B, Zhang N, An X, Yu J, Li H, Ren X. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J Clin Immunol. 2014;34:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 136. | Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, Sadelain M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1402] [Cited by in RCA: 1630] [Article Influence: 135.8] [Reference Citation Analysis (0)] |
| 137. | June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 2066] [Article Influence: 295.1] [Reference Citation Analysis (0)] |
| 138. | Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 139. | Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, Kong J, Wang H, Yang S, Gu J, Li Z. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20:6418-6428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 140. | Wang Y, Chen M, Wu Z, Tong C, Dai H, Guo Y, Liu Y, Huang J, Lv H, Luo C, Feng KC, Yang QM, Li XL, Han W. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology. 2018;7:e1440169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 141. | Bagley SJ, Desai AS, Linette GP, June CH, O'Rourke DM. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 2018;20:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 142. | Guo X, Jiang H, Shi B, Zhou M, Zhang H, Shi Z, Du G, Luo H, Wu X, Wang Y, Sun R, Li Z. Disruption of PD-1 Enhanced the Anti-tumor Activity of Chimeric Antigen Receptor T Cells Against Hepatocellular Carcinoma. Front Pharmacol. 2018;9:1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 143. | Yu M, Luo H, Fan M, Wu X, Shi B, Di S, Liu Y, Pan Z, Jiang H, Li Z. Development of GPC3-Specific Chimeric Antigen Receptor-Engineered Natural Killer Cells for the Treatment of Hepatocellular Carcinoma. Mol Ther. 2018;26:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 144. | Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell. 2018;23:181-192.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 716] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 145. | Jiang W, Zhang C, Tian Z, Zhang J. hIFN-α gene modification augments human natural killer cell line anti-human hepatocellular carcinoma function. Gene Ther. 2013;20:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 146. | Jiang W, Zhang C, Tian Z, Zhang J. hIL-15 gene-modified human natural killer cells (NKL-IL15) augments the anti-human hepatocellular carcinoma effect in vivo. Immunobiology. 2014;219:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 147. | Jiang W, Zhang J, Tian Z. Functional characterization of interleukin-15 gene transduction into the human natural killer cell line NKL. Cytotherapy. 2008;10:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 148. | Dubensky TW, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 149. | Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1553] [Cited by in RCA: 1419] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 150. | Xu A, Zhang L, Yuan J, Babikr F, Freywald A, Chibbar R, Moser M, Zhang W, Zhang B, Fu Z, Xiang J. TLR9 agonist enhances radiofrequency ablation-induced CTL responses, leading to the potent inhibition of primary tumor growth and lung metastasis. Cell Mol Immunol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 151. | Preiss S, Thompson A, Chen X, Rodgers S, Markovska V, Desmond P, Visvanathan K, Li K, Locarnini S, Revill P. Characterization of the innate immune signalling pathways in hepatocyte cell lines. J Viral Hepat. 2008;15:888-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 152. | Zhang Y, Lin A, Zhang C, Tian Z, Zhang J. Phosphorothioate-modified CpG oligodeoxynucleotide (CpG ODN) induces apoptosis of human hepatocellular carcinoma cells independent of TLR9. Cancer Immunol Immunother. 2014;63:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 153. | Khvalevsky E, Rivkin L, Rachmilewitz J, Galun E, Giladi H. TLR3 signaling in a hepatoma cell line is skewed towards apoptosis. J Cell Biochem. 2007;100:1301-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 154. | Yu P, Cheng X, Guo J, Wang X. Toll-like receptors 2/4 agonists: a potential strategy for preventing invasion and metastasis of hepatocellular carcinoma. Gut. 2010;59:1447-8; author reply 1448-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 155. | Zhang Y, Lin A, Sui Q, Zhang C, Tian Z, Zhang J. Phosphorothioate modification of the TLR9 ligand CpG ODN inhibits poly(I:C)-induced apoptosis of hepatocellular carcinoma by entry blockade. Cancer Lett. 2014;355:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 156. | Gardner A, Ruffell B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016;37:855-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 652] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 157. | Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter DM, McBride WH, Finn R, Glaspy JA, Economou JS. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817-2825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 158. | Tada F, Abe M, Hirooka M, Ikeda Y, Hiasa Y, Lee Y, Jung NC, Lee WB, Lee HS, Bae YS, Onji M. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol. 2012;41:1601-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 159. | El Ansary M, Mogawer S, Elhamid SA, Alwakil S, Aboelkasem F, Sabaawy HE, Abdelhalim O. Immunotherapy by autologous dendritic cell vaccine in patients with advanced HCC. J Cancer Res Clin Oncol. 2013;139:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 160. | Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y, Hsieh SL. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005;28:129-135. [PubMed] |
| 161. | Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du Z, Yin H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64:456-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 162. | Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z, Qi H, Guo H, Yin H. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 309] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 163. | Zhang W, Liu J, Wu Y, Xiao F, Wang Y, Wang R, Yang H, Wang G, Yang J, Deng H, Li J, Wen Y, Wei Y. Immunotherapy of hepatocellular carcinoma with a vaccine based on xenogeneic homologous alpha fetoprotein in mice. Biochem Biophys Res Commun. 2008;376:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 164. | Han Q, Wang Y, Pang M, Zhang J. STAT3-blocked whole-cell hepatoma vaccine induces cellular and humoral immune response against HCC. J Exp Clin Cancer Res. 2017;36:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |