Published online Jun 28, 2019. doi: 10.3748/wjg.v25.i24.3021
Peer-review started: March 14, 2019
First decision: April 30, 2019
Revised: May 7, 2019
Accepted: May 31, 2019
Article in press: May 31, 2019
Published online: June 28, 2019
Processing time: 107 Days and 21.6 Hours
Esophageal cancer is known as one of the malignant cancers with poor prognosis. To improve the outcome, combined multimodality treatment is attempted. On the other hand, advances in genomics and other “omic” technologies are paving way to the patient-oriented treatment called “personalized” or “precision” medicine. Recent advancements of imaging techniques such as functional imaging make it possible to use imaging features as biomarker for diagnosis, treatment response, and prognosis in cancer treatment. In this review, we will discuss how we can use imaging derived tumor features as biomarker for the treatment of esophageal cancer.
Core tip: Advances in imaging techniques enable us to assess tumor biology such as viability, vascular physiology, heterogeneity, or metabolism, which can be new approaches to investigate biomarker for cancer treatment. In this review, we will discuss various functional imaging techniques including computed tomography/magnetic resonance perfusion, texture analysis, diffusion-weighted imaging, and positron emission tomography in terms of prediction of treatment response or prognosis in esophageal cancer.
- Citation: Hayano K, Ohira G, Hirata A, Aoyagi T, Imanishi S, Tochigi T, Hanaoka T, Shuto K, Matsubara H. Imaging biomarkers for the treatment of esophageal cancer. World J Gastroenterol 2019; 25(24): 3021-3029
- URL: https://www.wjgnet.com/1007-9327/full/v25/i24/3021.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i24.3021
Esophageal cancer is the seventh most common cancer, and sixth leading cause of death in the world[1]. Surgical resection is the only curative method for esophageal cancer, but is limited to early stage disease, and the recurrence rate after radical resection of esophageal cancer is reported to be approximately 50%, and most cases of recurrence occur within two years after surgery[2-4].
In this context, personalized or precision medicine, which enables the best choice of treatment based on certain biomarkers, is highly desirable, preventing side-effect and extra expenses, leading to more effective multidisciplinary treatments. Angiogenesis, tumor stroma, hypoxia, heterogeneity, and metabolism are known as typical biological features of malignancies, and these have been investigated to be biomarkers for diagnosis, prognosis, and treatment response. These biological features are usually investigated by the cell and molecular biology, but recent advances in imaging technique enable non-invasive assessment of various tumor functions, which have been investigated their biomarker value in malignancies. Imaging derived markers have the advantage of being non-invasive, spatially resolved and repeatable, compared to bio-specimen biomarkers which are obtained by removing a sample from a patient. Recent increasing interests in “Radiomics”, which is an emerging field that converts imaging data into a high dimensional mineable feature space using a large number of automatically extracted data-characterization algorithms, makes imaging derived biomarkers more valuable. In these contexts, this review will discuss how we can use various imaging derived biomarkers including perfusion analysis using computed tomography (CT) or magnetic resonance imaging (MRI), texture analysis, diffusion-weighted imaging (DWI), and positron emission tomography (PET) in terms of prediction of treatment response or prognosis to improve outcome of esophageal cancer patients.
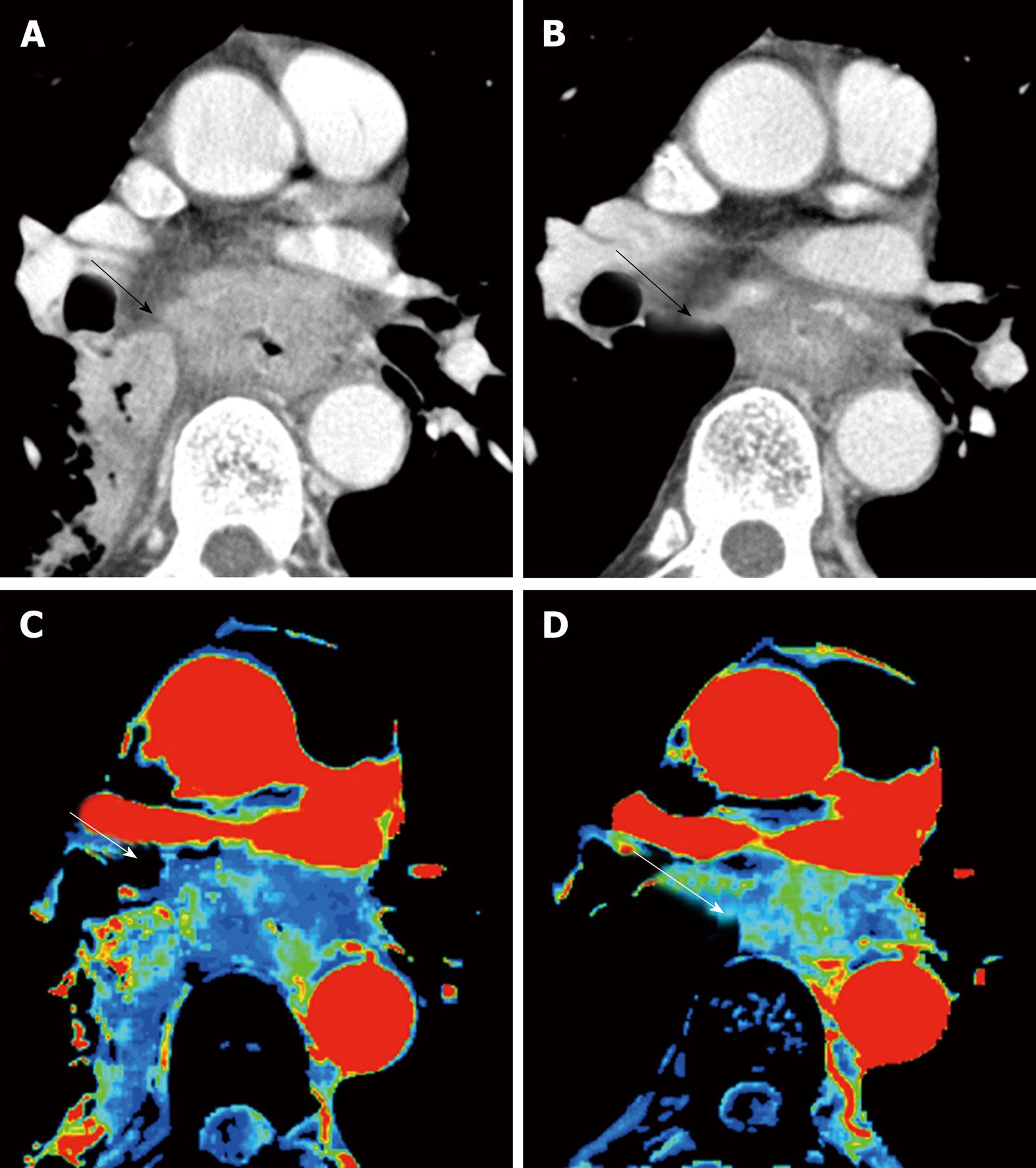
Perfusion analysis using dynamic contrast-enhanced CT (DCE-CT/CT perfusion) and DCE-MRI (MR perfusion) can quantify tissue hemodynamics by measuring the temporal changes in tissue attenuation after administration of intravenous contrast media[5-7]. Since “angiogenesis” plays an important role in almost all types of cancer progression[8-10], quantification of vascular physiology using CT or MR perfusion techniques may reflect tumor angiogenesis, and has a potential to be a biomarker in cancer treatment[11]. These perfusion techniques are readily incorporated into the existing CT and MRI protocol, and enables in vivo quantification of tissue hemodynamics using the modeling tracer kinetics on the imaging workstation (Figure 1)[5,6]. In esophageal cancer, several previous papers reported associations of CT or MR perfusion parameters with treatment response to chemotherapy or chemo-radiotherapy (Table 1). In MR perfusion studies, it was reported that higher Ktrans value (a parameter related to vessel permeability and tissue blood flow) before chemoradiation therapy (CRT) was associated with better treatment response[12,13]. In CT perfusion studies, Hayano et al[14] reported that higher blood flow of the tumor before CRT was associated with better treatment response and overall survival in esophageal squamous cell carcinoma patients. Makari et al[15] also reported that high tumor BF measure by CT perfusion might predict good response to neoadjuvant chemotherapy and CRT. Interestingly, all these reports suggested that a higher blood flow/perfusion of the tumor was associated with a better outcome of CRT or chemotherapy. It is reasonable because a higher tumor blood flow/perfusion leads to a better drug delivery and a higher oxygenation, resulting in better chemo- and radio-sensitivity. There are a few paper reporting perfusion change in esophageal cancer during chemotherapy or CRT. Sun et al[12] demonstrated that the complete response group showed a significant decrease in Ktrans. Similarly, Hayano et al[16] reported that a significant decrease of blood flow in the tumor was observed from CT perfusion study of esophageal cancer, and they reported that patients with a greater reduction in tumor blood flow during CRT survived significantly longer than those with lower tumor blood flow reduction. It is speculated that the tissue fibrosis due to CRT leads to compression of tumor capillaries and increased flow resistance, results in decrease of blood flow/perfusion after CRT. In fact, it was reported that patients who achieved pathological complete response (pCR) after neoadjuvant CRT had tumors with lower blood flow than non-pCR[17].
| Year | Patients | Biomarker candidate | Prediction of treatment outcome | |
| Hayano et al[14] | 2007 | 31 | Baseline BF (CT perfusion) | High baseline BF associated with good response and OS after CRT (31) |
| Makari et al[15] | 2007 | 46 | Baseline BF (CT perfusion) | High baseline BF associated with good response and OS after chemotherapy (36) and CRT (10) |
| Djuric-Stefanovic et al[17] | 2015 | 40 | Post-therapeutic BF (CT perfusion) | Post-therapeutic BF < 30 mL/min/100 g can predict pCR with 100% of sensitivity and specificity |
| Lei et al[13] | 2015 | 25 | Baseline Ktrans (MR perfusion) | Ktrans was significantly different between CR and non-CR after CRT |
| Sun et al[12] | 2018 | 59 | Change of Ktrans (MR perfusion) | Change in Ktrans was the best parameter to assess treatment response |
Regarding relationship between CT/MR perfusion and angiogenesis, published results are controversial. For example, Chen et al[18] demonstrated that tumor blood volume measured by CT perfusion was significantly correlated with micro-vessel density in esophageal cancer, while Sato et al[19] reported that there was no significant correlation of tumor blood flow with the micro-vessel density in CT perfusion study of gastric cancer. Sato et al[19] speculated that blood flow assessed with perfusion imaging reflected only the functional vessels with a lumen, and not the functionless tumor vascularity; and therefore, micro-vessel density studied immuno-histochemically in vitro using surgical specimens might be inadequate for “in vivo tumor vascular physiology. These factors may affect controversial results on relationship of CT/MR perfusion with immunohistochemically evaluated angiogenesis.
This perfusion technique using CT and MRI is very interesting and exciting technique with a potential to be a useful biomarker, but this technique is still considered a research tool in the realm of oncology. A consensus and standardization of data acquisition and analysis methods have yet to be established. The definition of the tumor region of interest (ROI) is subject to similar consideration, because the method used to draw the ROI clearly influences the perfusion parameters. Relatively high radiation dose and complicated procedure should be improved to be more common examination in clinical practice of esophageal cancer.
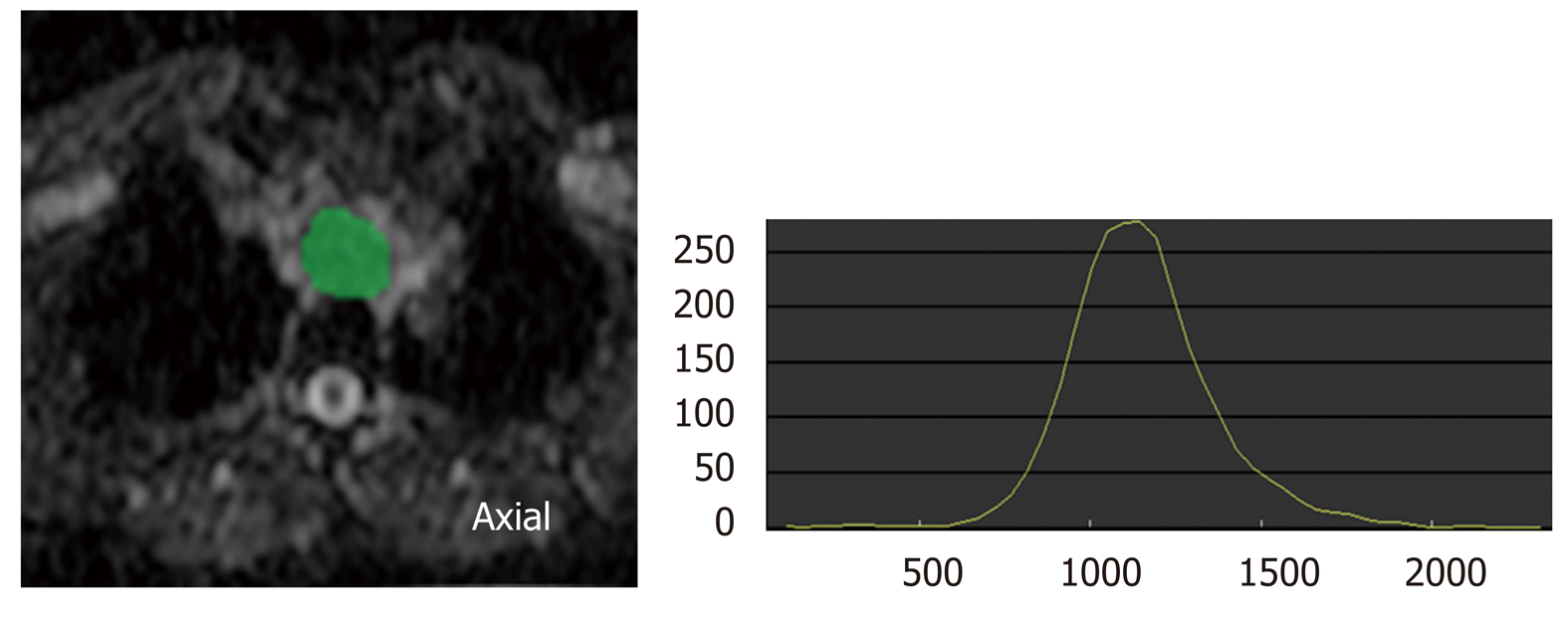
Analysis of texture within tumor on medical imaging such as CT, MRI, and PET, which reflects structural abnormality or heterogeneity in the tumor, is emerging as a potential biomarker to predict prognosis and treatment response in patients with cancer[20], because most malignant tumors show a striking amount of intratumor heterogeneity, which has implications for diagnosis, treatment efficacy, and the identification of drug targets[21]. There are various methods including statistical-, model-, and transform-based methods with various texture parameters[22]. Common texture parameters are entropy (a measure of irregularity), uniformity (a measure of uniform distribution of grey-levels), skewness (a measure of asymmetry of the histogram), kurtosis (a measure of peakedness and tailedness), and fractal dimension (a measure of complexity)[23-25]. Ganeshan et al[26] reported that tumor heterogeneity (uniformity) assessed on unenhanced CT was correlated with 18F-fluorodeoxyglucose (18F-FDG) uptake, and was an independent predictor of survival in 21 patients with esophageal cancers. Yip et al[27] reported that post-treatment uniformity and entropy of the tumor measured on contrast-enhanced CT were correlated with overall survival in esophageal cancer patients treated with CRT. In fractal analysis of PET imaging, Tochigi et al[28] reported that the low fractal dimension of tumor 18F-FDG uptake was associated with favorable survival, and they concluded that metabolic heterogeneity measured by fractal analysis can be a novel imaging biomarker for survival in patients with esophageal squamous cell carcinoma.
These texture analysis is a post-processing mathematical technique, which can apply to any medical imaging with no additional radiation exposure, special protocol, and cost, and maximizes the information obtained from current standard medical imaging (Figure 2). This technique still needs further investigation and standardization to be used in clinical practice, but has a potential to be a valuable clinical tool in the management of esophageal cancer.
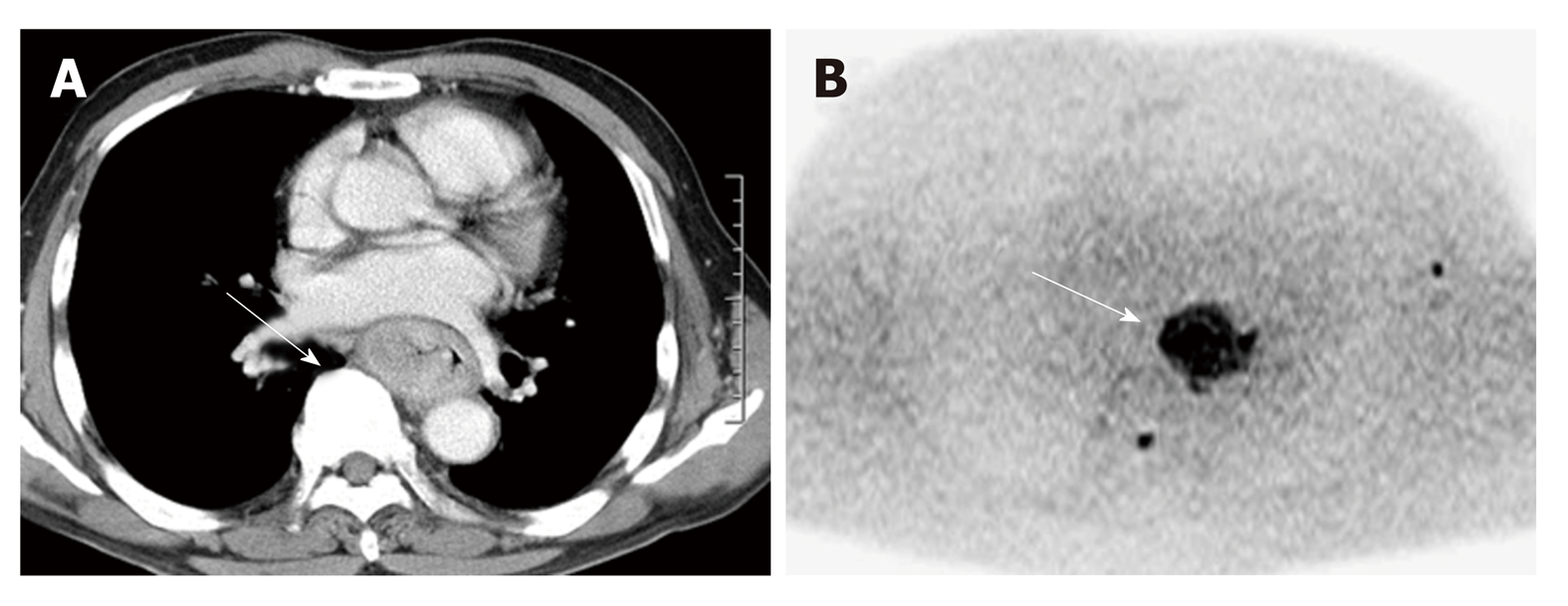
In 1905, Einstein described molecular diffusion or Brownian motion formally on the basis of the random translational motion of molecules[29]. Recent advances in magnetic resonance gradient technology have allowed acquisition of the apparent diffusion coefficient (ADC) value, which can be calculated by the DWI measurements acquired with a different gradient duration and amplitude (b-values)[30]. DWI has been discussed in terms of its biomarker value for cancer treatment in a consensus meeting, and a publication on consensus and recommendations for DWI as a cancer biomarker has been published highlighting the potential of this technique in the management of cancer patients (Figure 3)[31].
In esophageal cancer, there are seven papers evaluating DWI for prediction of CRT response and prognosis[32], but the results are controversial (Table 2). In 2011, Aoyagi et al[33] reported that higher baseline tumor ADC was associated with better survival. Another study also suggested that high baseline tumor ADC was associated with good response to CRT[34] , while De Cobelli et al[35] reported that the high baseline tumor ADC was associated with poor response to CRT. Because of these conflicting results, it is still unclear whether pre-therapeutic tumor ADC can really predict response or survival after CRT. Cheng et al[32] performed meta-analysis, and reported that change of ADC and post-therapeutic ADC of the tumor were promising reliable and valuable predictor for the response to CRT, rather than pre-therapeutic ADC. Interestingly, Imanishi et al[36] reported that early increase of tumor ADC (> 15% after 20 Gy) could predict treatment response with 85% of accuracy and 100% of positive predictive value. Similarly, three studies suggested importance of post-CRT ADC and the change of ADC after 2-3weeks of CRT in terms of prediction of response to CRT in esophageal cancer[37-39].
| Year | Patients | Biomarker candidate | Prediction of treatment outcome | |
| Aoyagi et al[33] | 2011 | 80 | Baseline ADC | High baseline ADC associated with favorable survival after CRT |
| Imanishi et al[36] | 2013 | 27 | Early change of ADC, post-CRT ADC | Increase of ADC/high post-CRT ADC associated with good response to CRT |
| De Cobelli et al[35] | 2013 | 32 | Baseline ADC | High baseline ADC associated with poor response to CRT |
| Van Rossum et al[39] | 2015 | 20 | Early change of ADC | Increase of ADC associated with good response to CRT |
| Wang et al[37] | 2016 | 38 | Early change of ADC, post-CRT ADC | Increase of ADC/high post-CRT ADC associated with good response to CRT |
| Li et al[38] | 2017 | 28 | Early change of ADC, post-CRT ADC | Increase of ADC/high post-CRT ADC associated with good response to CRT |
| Cong et al[34] | 2019 | 52 | Baseline ADC | High baseline ADC associated with good response to RT |
Because DWI does not need radiation exposure and contrast agents, it can be an ideal biomarker. However, standardization of data acquisition and analysis methods have yet to be established for DWI. Low spatial resolution, especially in high b-value image, should be improved for accurate detection and quantification of the tumor.
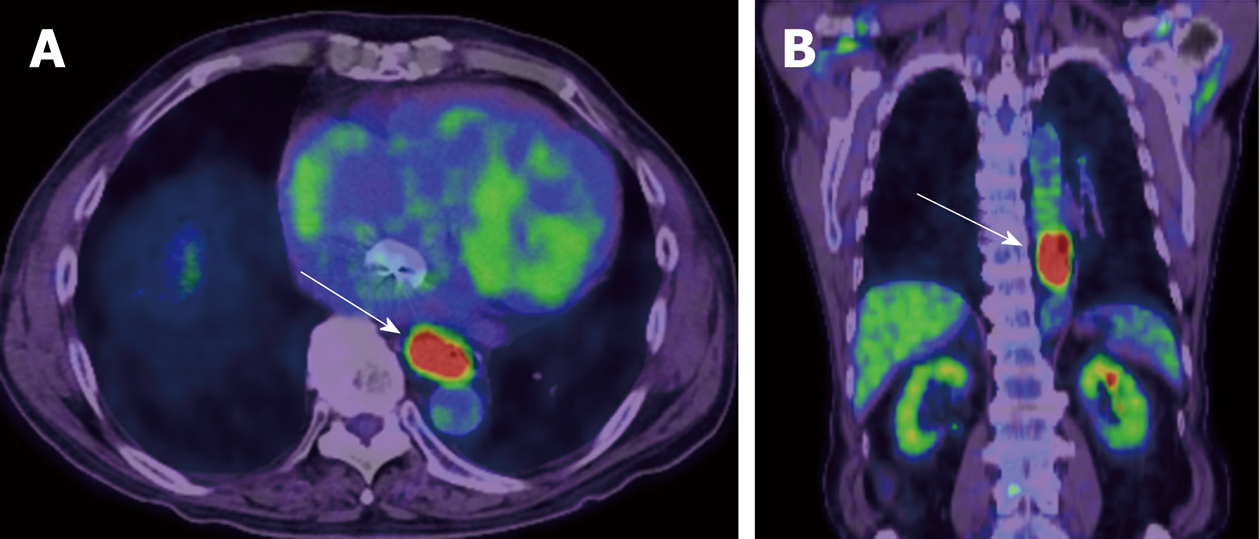
PET is a quantitative imaging technique with use of various types of tracers. 18F-FDG, which can quantify glucose metabolism of the tissue, is the most common clinical PET tracer. Theoretically, malignant tumor cells exhibit strongly enhanced energy consumption, and lead to increased 18F-FDG uptake due to the increased number of glucose transporters and the increased hexokinase activity (Figure 4). The standardized uptake value (SUV) is generally used to quantify the tissue glucose metabolism, which has been reported its biomarker value in the treatment response and prognosis in various types of malignancies.
Regarding the biomarker value of pre-therapeutic PET in surgically treated esophageal cancer, Fukunaga et al[40] reported that a high SUV of the tumor before surgery had a poorer prognosis compared with those with low FDG uptake in esophageal cancer patients who received curative surgery without neoadjuvant therapy in 1998. After this paper had been published, seven papers on this subject were published[41-47], and all those papers suggested that high tumor SUV before surgery was associated with poor survival in surgically treated esophageal cancer (no neoadjuvant therapy)[48]. On the other hand, interestingly, pretherapeutic tumor SUV may not associate with survival in patients who received neoadjuvant chemotherapy or CRT[49,50], while the tumor SUV after neoadjuvant therapy can be a biomarker for survival. Swisher et al. reported that the tumor SUV after CRT is the most accurate test to predict survival in esophageal cancer patients (87% is adenocarcinoma) who were treated CRT followed by curative surgery[51]. Higuchi et al[52] also demonstrated that post-CRT SUV uptake in the tumor (cut-off 2.5) was the preoperative prognostic factor in esophageal squamous cell carcinoma patients who were treated neoadjuvant CRT or chemotherapy. Regarding change in tumor SUV during neoadjuvant therapy, early decrease (after 2 wk of neoadjuvant therapy) in FDG uptake is reported to be a predictive marker for response and survival[53-59]. However, some studies included patients with a wide range of disease (adenocarcinomas and squamous cell carcinomas, stage I through IV), and studies used different neoadjuvant treatment regimens.
Nevertheless, FDG-PET seems to be served as a useful biomarker for treatment response and prognosis in various types of treatments, and we need further investigation with a large multicenter prospective trial to confirm usefulness of FDG-PET in the management of esophageal cancer.
Ideal biomarker should be simple, non-invasive, reproducible, and widely available. Given the wide availability and the less invasiveness, imaging has a big potential to be an ideal biomarker. As we reviewed, various imaging biomarkers showed interesting results, and some of them are ready to use in clinical practice of esophageal cancer patients, which would provide patients more personalized and effective treatment, leading better outcome.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bagheri Lankarani K, Kakaei F, Liu DL S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 2. | Su XD, Zhang DK, Zhang X, Lin P, Long H, Rong TH. Prognostic factors in patients with recurrence after complete resection of esophageal squamous cell carcinoma. J Thorac Dis. 2014;6:949-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 3. | Chen G, Wang Z, Liu XY, Liu FY. Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomy. World J Surg. 2007;31:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Axel L. Cerebral blood flow determination by rapid-sequence computed tomography: Theoretical analysis. Radiology. 1980;137:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 395] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Miles KA, Hayball M, Dixon AK. Colour perfusion imaging: A new application of computed tomography. Lancet. 1991;337:643-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Padhani AR. Functional MRI for anticancer therapy assessment. Eur J Cancer. 2002;38:2116-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823-1832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6490] [Article Influence: 259.6] [Reference Citation Analysis (0)] |
| 10. | Jain RK, Carmeliet P. SnapShot: Tumor angiogenesis. Cell. 2012;149:1408-1408.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Miles KA. Perfusion CT for the assessment of tumour vascularity: Which protocol? Br J Radiol. 2003;76 Spec No 1:S36-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 231] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Sun NN, Liu C, Ge XL, Wang J. Dynamic contrast-enhanced MRI for advanced esophageal cancer response assessment after concurrent chemoradiotherapy. Diagn Interv Radiol. 2018;24:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Lei J, Han Q, Zhu S, Shi D, Dou S, Su Z, Xu X. Assessment of esophageal carcinoma undergoing concurrent chemoradiotherapy with quantitative dynamic contrast-enhanced magnetic resonance imaging. Oncol Lett. 2015;10:3607-3612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Hayano K, Okazumi S, Shuto K, Matsubara H, Shimada H, Nabeya Y, Kazama T, Yanagawa N, Ochiai T. Perfusion CT can predict the response to chemoradiation therapy and survival in esophageal squamous cell carcinoma: Initial clinical results. Oncol Rep. 2007;18:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Makari Y, Yasuda T, Doki Y, Miyata H, Fujiwara Y, Takiguchi S, Matsuyama J, Yamasaki M, Hirao T, Koyama MK, Nakamuara H, Monden M. Correlation between tumor blood flow assessed by perfusion CT and effect of neoadjuvant therapy in advanced esophageal cancers. J Surg Oncol. 2007;96:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Hayano K, Shuto K, Satoh A, Aoyagi T, Narushima K, Gunji H, Kono T, Yanagawa N, Okazumi S, Matsubara H. Tumor blood flow change measured by CT perfusion during chemoradiation therapy (CRT) for monitoring response and predicting survival in patients with esophageal cancer. Esophagus. 2014;11:72-79. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Djuric-Stefanovic A, Micev M, Stojanovic-Rundic S, Pesko P, Saranovic Dj. Absolute CT perfusion parameter values after the neoadjuvant chemoradiotherapy of the squamous cell esophageal carcinoma correlate with the histopathologic tumor regression grade. Eur J Radiol. 2015;84:2477-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Chen TW, Yang ZG, Wang QL, Li Y, Qian LL, Chen HJ. Whole tumour quantitative measurement of first-pass perfusion of oesophageal squamous cell carcinoma using 64-row multidetector computed tomography: Correlation with microvessel density. Eur J Radiol. 2011;79:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Satoh A, Shuto K, Okazumi S, Ohira G, Natsume T, Hayano K, Narushima K, Saito H, Ohta T, Nabeya Y, Yanagawa N, Matsubara H. Role of perfusion CT in assessing tumor blood flow and malignancy level of gastric cancer. Dig Surg. 2010;27:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Ganeshan B, Miles KA. Quantifying tumour heterogeneity with CT. Cancer Imaging. 2013;13:140-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 21. | Durrett R, Foo J, Leder K, Mayberry J, Michor F. Intratumor heterogeneity in evolutionary models of tumor progression. Genetics. 2011;188:461-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, Ganeshan B, Miles KA, Cook GJ, Goh V. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 695] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 23. | Miles KA, Ganeshan B, Hayball MP. CT texture analysis using the filtration-histogram method: What do the measurements mean? Cancer Imaging. 2013;13:400-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 24. | Hayano K, Yoshida H, Zhu AX, Sahani DV. Fractal analysis of contrast-enhanced CT images to predict survival of patients with hepatocellular carcinoma treated with sunitinib. Dig Dis Sci. 2014;59:1996-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Hayano K, Lee SH, Yoshida H, Zhu AX, Sahani DV. Fractal analysis of CT perfusion images for evaluation of antiangiogenic treatment and survival in hepatocellular carcinoma. Acad Radiol. 2014;21:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles K. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: Preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol. 2012;67:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 27. | Yip C, Landau D, Kozarski R, Ganeshan B, Thomas R, Michaelidou A, Goh V. Primary esophageal cancer: Heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology. 2014;270:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Tochigi T, Shuto K, Kono T, Ohira G, Tohma T, Gunji H, Hayano K, Narushima K, Fujishiro T, Hanaoka T, Akutsu Y, Okazumi S, Matsubara H. Heterogeneity of Glucose Metabolism in Esophageal Cancer Measured by Fractal Analysis of Fluorodeoxyglucose Positron Emission Tomography Image: Correlation between Metabolic Heterogeneity and Survival. Dig Surg. 2017;34:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Einstein A. Investigations on the theory of the brownian movement. 1st ed. New York: Dover Publications, 1956. |
| 30. | Koh DM, Padhani AR. Diffusion-weighted MRI: A new functional clinical technique for tumour imaging. Br J Radiol. 2006;79:633-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D, Hammoud DA, Rustin GJ, Taouli B, Choyke PL. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia. 2009;11:102-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1553] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 32. | Cheng B, Yu J. Predictive value of diffusion-weighted MR imaging in early response to chemoradiotherapy of esophageal cancer: A meta-analysis. Dis Esophagus. 2019;32:pii: doy065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Aoyagi T, Shuto K, Okazumi S, Shimada H, Kazama T, Matsubara H. Apparent diffusion coefficient values measured by diffusion-weighted imaging predict chemoradiotherapeutic effect for advanced esophageal cancer. Dig Surg. 2011;28:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Cong Q, Li G, Wang Y, Zhang S, Zhang H. DW-MRI for esophageal squamous cell carcinoma, correlations between ADC values with histologic differentiation and VEGF expression: A retrospective study. Oncol Lett. 2019;17:2770-2776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | De Cobelli F, Giganti F, Orsenigo E, Cellina M, Esposito A, Agostini G, Albarello L, Mazza E, Ambrosi A, Socci C, Staudacher C, Del Maschio A. Apparent diffusion coefficient modifications in assessing gastro-oesophageal cancer response to neoadjuvant treatment: Comparison with tumour regression grade at histology. Eur Radiol. 2013;23:2165-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | Imanishi S, Shuto K, Aoyagi T, Kono T, Saito H, Matsubara H. Diffusion-weighted magnetic resonance imaging for predicting and detecting the early response to chemoradiotherapy of advanced esophageal squamous cell carcinoma. Dig Surg. 2013;30:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Wang L, Liu L, Han C, Liu S, Tian H, Li Z, Ren X, Shi G, Wang Q, Wang G. The diffusion-weighted magnetic resonance imaging (DWI) predicts the early response of esophageal squamous cell carcinoma to concurrent chemoradiotherapy. Radiother Oncol. 2016;121:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Li QW, Qiu B, Wang B, Wang DL, Yin SH, Yang H, Liu JL, Fu JH, Liu MZ, Xie CM, Liu H. Prediction of pathologic responders to neoadjuvant chemoradiotherapy by diffusion-weighted magnetic resonance imaging in locally advanced esophageal squamous cell carcinoma: A prospective study. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | van Rossum PS, van Lier AL, van Vulpen M, Reerink O, Lagendijk JJ, Lin SH, van Hillegersberg R, Ruurda JP, Meijer GJ, Lips IM. Diffusion-weighted magnetic resonance imaging for the prediction of pathologic response to neoadjuvant chemoradiotherapy in esophageal cancer. Radiother Oncol. 2015;115:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 40. | Fukunaga T, Okazumi S, Koide Y, Isono K, Imazeki K. Evaluation of esophageal cancers using fluorine-18-fluorodeoxyglucose PET. J Nucl Med. 1998;39:1002-1007. [PubMed] |
| 41. | Choi JY, Jang HJ, Shim YM, Kim K, Lee KS, Lee KH, Choi Y, Choe YS, Kim BT. 18F-FDG PET in patients with esophageal squamous cell carcinoma undergoing curative surgery: Prognostic implications. J Nucl Med. 2004;45:1843-1850. [PubMed] |
| 42. | Choi JY, Jang KT, Shim YM, Kim K, Ahn G, Lee KH, Choi Y, Choe YS, Kim BT. Prognostic significance of vascular endothelial growth factor expression and microvessel density in esophageal squamous cell carcinoma: Comparison with positron emission tomography. Ann Surg Oncol. 2006;13:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Kato H, Kuwano H, Nakajima M, Miyazaki T, Yoshikawa M, Ojima H, Tsukada K, Oriuchi N, Inoue T, Endo K. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer. 2002;94:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Kato H, Takita J, Miyazaki T, Nakajima M, Fukai Y, Masuda N, Fukuchi M, Manda R, Ojima H, Tsukada K, Kuwano H, Oriuchi N, Endo K. Correlation of 18-F-fluorodeoxyglucose (FDG) accumulation with glucose transporter (Glut-1) expression in esophageal squamous cell carcinoma. Anticancer Res. 2003;23:3263-3272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Rizk N, Downey RJ, Akhurst T, Gonen M, Bains MS, Larson S, Rusch V. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography standardized uptake values predict survival after esophageal adenocarcinoma resection. Ann Thorac Surg. 2006;81:1076-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | van Westreenen HL, Plukker JT, Cobben DC, Verhoogt CJ, Groen H, Jager PL. Prognostic value of the standardized uptake value in esophageal cancer. AJR Am J Roentgenol. 2005;185:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Omloo JM, Sloof GW, Boellaard R, Hoekstra OS, Jager PL, van Dullemen HM, Fockens P, Plukker JT, van Lanschot JJ. Importance of fluorodeoxyglucose-positron emission tomography (FDG-PET) and endoscopic ultrasonography parameters in predicting survival following surgery for esophageal cancer. Endoscopy. 2008;40:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Omloo JM, van Heijl M, Hoekstra OS, van Berge Henegouwen MI, van Lanschot JJ, Sloof GW. FDG-PET parameters as prognostic factor in esophageal cancer patients: A review. Ann Surg Oncol. 2011;18:3338-3352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Chatterton BE, Ho Shon I, Baldey A, Lenzo N, Patrikeos A, Kelley B, Wong D, Ramshaw JE, Scott AM. Positron emission tomography changes management and prognostic stratification in patients with oesophageal cancer: Results of a multicentre prospective study. Eur J Nucl Med Mol Imaging. 2009;36:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Hong D, Lunagomez S, Kim EE, Lee JH, Bresalier RS, Swisher SG, Wu TT, Morris J, Liao Z, Komaki R, Ajani JA. Value of baseline positron emission tomography for predicting overall survival in patient with nonmetastatic esophageal or gastroesophageal junction carcinoma. Cancer. 2005;104:1620-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Swisher SG, Maish M, Erasmus JJ, Correa AM, Ajani JA, Bresalier R, Komaki R, Macapinlac H, Munden RF, Putnam JB, Rice D, Smythe WR, Vaporciyan AA, Walsh GL, Wu TT, Roth JA. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152-60; discussion 1152-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 52. | Higuchi I, Yasuda T, Yano M, Doki Y, Miyata H, Tatsumi M, Fukunaga H, Takiguchi S, Fujiwara Y, Hatazawa J, Monden M. Lack of fludeoxyglucose F 18 uptake in posttreatment positron emission tomography as a significant predictor of survival after subsequent surgery in multimodality treatment for patients with locally advanced esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2008;136:205-212, 212.e1-212.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, Lorenzen S, Schuster T, Wieder H, Herrmann K, Bredenkamp R, Höfler H, Fink U, Peschel C, Schwaiger M, Siewert JR. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol. 2007;8:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 579] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 54. | Westerterp M, Omloo JM, Sloof GW, Hulshof MC, Hoekstra OS, Crezee H, Boellaard R, Vervenne WL, ten Kate FJ, van Lanschot JJ. Monitoring of response to pre-operative chemoradiation in combination with hyperthermia in oesophageal cancer by FDG-PET. Int J Hyperthermia. 2006;22:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Ott K, Weber WA, Lordick F, Becker K, Busch R, Herrmann K, Wieder H, Fink U, Schwaiger M, Siewert JR. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2006;24:4692-4698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 354] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 56. | Port JL, Lee PC, Korst RJ, Liss Y, Meherally D, Christos P, Mazumdar M, Altorki NK. Positron emission tomographic scanning predicts survival after induction chemotherapy for esophageal carcinoma. Ann Thorac Surg. 2007;84:393-400; discussion 400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE, Meisetschläger G, Busch R, Siewert JR, Schwaiger M, Fink U. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 493] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 58. | Wieder HA, Brücher BL, Zimmermann F, Becker K, Lordick F, Beer A, Schwaiger M, Fink U, Siewert JR, Stein HJ, Weber WA. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 336] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 59. | Wieder HA, Ott K, Lordick F, Becker K, Stahl A, Herrmann K, Fink U, Siewert JR, Schwaiger M, Weber WA. Prediction of tumor response by FDG-PET: Comparison of the accuracy of single and sequential studies in patients with adenocarcinomas of the esophagogastric junction. Eur J Nucl Med Mol Imaging. 2007;34:1925-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |