Published online Jun 21, 2019. doi: 10.3748/wjg.v25.i23.2887
Peer-review started: March 20, 2019
First decision: April 4, 2019
Revised: May 7, 2019
Accepted: May 8, 2019
Article in press: May 8, 2019
Published online: June 21, 2019
Processing time: 93 Days and 18.4 Hours
Through the implementation of national bowel cancer screening programmes we have seen a three-fold increase in early pT1 colorectal cancers, but how these lesions should be managed is currently unclear. Local excision can be an attractive option, especially for fragile patients with multiple comorbidities, but it is only safe from an oncological point of view in the absence of lymph node metastasis. Patient risk stratification through careful analysis of histopathological features in local excision or polypectomy specimens should be performed according to national guidelines to avoid under- or over-treatment. Currently national guidelines vary in their recommendations as to which factors should be routinely reported and there is no established multivariate risk stratification model to determine which patients should be offered major resectional surgery. Conventional histopathological parameters such as tumour grading or lymphovascular invasion have been shown to be predictive of lymph node metastasis in a number of studies but the inter- and intra-observer variation in reporting is high. Newer parameters including tumour budding and poorly differentiated clusters have been shown to have great potential, but again some improvement in the inter-observer variation is required. With the implementation of digital pathology into clinical practice, quantitative parameters like depth/area of submucosal invasion and proportion of stroma can be routinely assessed. In this review we present the various histopathological risk factors for predicting systemic spread in pT1 colorectal cancer and introduce potential novel quantitative variables and multivariable risk models that could be used to better define the optimal treatment of this increasingly common disease.
Core tip: Since the implementation of national bowel cancer screening programmes we have seen a three-fold increase in early colorectal cancers but how these lesion should surgical managed is currently unclear. Conventional histopathological parameters such as tumour grading or lymphovascular invasion have been shown to be predictive of lymph node metastasis but the inter- and intra-observer variation in reporting is significant. This review present the various conventional histopathological risk factors for predicting systemic spread in pT1 colorectal cancer and introduces novel quantitative variables and multivariable risk models that could be used to better define the optimal treatment of this increasingly common disease.
- Citation: Brockmoeller SF, West NP. Predicting systemic spread in early colorectal cancer: Can we do better? World J Gastroenterol 2019; 25(23): 2887-2897
- URL: https://www.wjgnet.com/1007-9327/full/v25/i23/2887.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i23.2887
Colorectal cancer (CRC) is one of the most common cancers worldwide[1,2]. It is the third most common cause of cancer death in the United Kingdom and the United States in both females and males[1]. Through the implementation of national population screening[3] like the United Kingdom National Health Service Bowel Cancer Screening Programme (NHS BCSP) we have observed a three-fold increase in early CRC (stage pT1) from 5% to 17%[4]. Early CRC is defined as the “invasion of neoplastic glandular epithelial cells through the muscularis mucosae into the sub-mucosa of the bowel wall but not beyond”[5].
Currently it is unclear as to how pT1 CRC should be optimally managed. Major bowel resection can be performed, however, this is associated with a significant risk of post-operative mortality, especially in elderly patients, and also morbidity including permanent colostomy formation, sexual and genitourinary problems, and low anterior resection syndrome. Internationally, postoperative mortality rates vary markedly, largely depending on background comorbidity in the population. Local excision of the tumour and avoidance of major surgery is an attractive option for patients with rectal cancer or significant comorbidity, but this is only safe from an oncological viewpoint in the absence of lymph node metastasis (LNM)[6,7]. It is therefore important that when deciding whether major bowel surgery or local excision should be performed, the postoperative mortality and lymph node metastasis risk are accurately estimated to inform the decision. Approximately 10%-15% of all pT1 CRC have LNM at the time of primary diagnosis with pedunculated pT1 CRC having an even lower risk (3% to 7% in the Asian population). Despite this major bowel resection rates in pT1 CRC can be as high as 76%, meaning that many patients are potentially exposed to unnecessary risk[8-11].
When a local excision (including polypectomy) is performed for pT1 CRC, patient risk stratification is undertaken by histopathologists through careful analysis of the specimen to determine the risk of LNM. The detailed macroscopic and microscopic evaluation of the specimen produces a large amount of information to guide further treatment. Routine information that is generally collected internationally includes the type of tumour, differentiation grade, TNM stage, level of invasion, number of lymph nodes involved, lymphatic invasion status, venous invasion status, perineural invasion status and resection margin status[12]. Currently various national guidelines differ in their recommendations as to which histopathological factors should be reported and used to determine the risk of LNM and therefore use of major surgery, and there is no established multivariate risk stratification model. The classification of some factors is also not performed according to an international standard with various systems in use. This review will present and discuss the various histo-pathological risk factors that can be used to predict systemic spread in in pT1 CRC and introduce potential novel quantitative variables and multivariable risk models that could be used to better define the optimal treatment of this increasingly common disease.
Literature searching was performed in PubMed (https://www.ncbi.nlm.nih.gov /pubmed) for the following keywords: “lymph nodes”, “lymph node metastasis”, “T1” and “pT1” combined with “colorectal cancer”. Articles published between July 2004 and January 2019 were reviewed. In addition, manual cross-referencing was performed and further relevant papers published before 2007 identified through review articles. Inclusion criteria for studies included publication in English, use of at least 100 patients and availability of LNM status. Studies were excluded if neo-adjuvant therapy was used due to the potential effect on tumour staging.
The majority of CRCs are adenocarcinoma but specific histological variants including cribriform or micropapillary adenocarcinoma have been reported in case series to have a higher rate of LNM in early CRC[13], and may be used to indicate further treatment in margin negative local excisions[14]. Mucinous adenocarcinomas (> 50% of the tumour area composed of extracellular mucin), signet ring cell adenocarcinomas (> 50% of the area composed of signet ring cells) and medullary carcinomas are associated with deficient mismatch repair (dMMR), which has a better prognosis when compared to cases with proficient mismatch repair. Yet in the absence of deficient mismatch repair, these histological subtypes are associated with a poorer prognosis than conventional CRC and further treatment may be indicated. The importance of routine mismatch repair immunostaining is detailed below.
Poor tumour differentiation has been shown in numerous studies, including meta-analyses, to be significantly associated with poorer survival[15-17] and prediction of LNM in early CRC[7,18]. Poor differentiation is primarily based on the architecture of the tumour and the hallmark is the absence of any tubular formation or irregularly folded, distorted and often small tubules. Tumour differentiation is a subjective parameter and grading may vary between assessors. Currently the WHO grading system proposes a four tier classification including: Grade 1 (well differentiated), grade 2 (moderately differentiated), grade 3 (poorly differentiated) and grade 4 (undifferentiated)[5]. An improvement in inter-observer agreement can be achieved through compressing this system into two grades, i.e., well/moderate differentiation vs poor differentiation[19]. This has been routinely adopted in the United Kingdom on the basis that poor differentiation is an important high-risk feature in early CRC. With the implementation of digital pathology into routine clinical practice, tumour differentiation grading could be further improved by the implementation of auto-mated algorithms to reduce the subjectivity of pathologist assessment[20-22].
For local excision specimens, there is currently uncertainty as to whether grading should be based on the predominant or worst area of differentiation, as most pub-lished studies have not specified how poor differentiation was defined. To avoid the risk of under-treatment in early CRC, the Royal College of Pathologists re-commend grading on the worst area in local excision specimens until further data are avai-lable[19]. In contrast, a major resection specimen is graded based on the predominant area of the tumour.
Whilst poor differentiation is generally accepted to be a poor prognostic feature, an exception in which it is associated with a more favourable stage-adjusted prognosis is in patients with dMMR, seen in 12%-15% of all cases[23,24]. Most dMMR cases are due to somatic epigenetic silencing of the MLH1 gene but a minority of cases are due to Lynch syndrome. From the current literature it is unclear if patients with poorly differentiated pT1 dMMR CRCs have a lower risk of LNM than poorly differentiated pT1 CRCs without dMMR. However, given the rarity of metastatic disease in dMMR CRC it is likely that poor differentiation is only an adverse factor in cases with proficient mismatch repair. Further evidence is required to confirm this hypothesis. Mismatch repair immunohistochemistry or alternative technologies including microsatellite instability testing should therefore be considered mandatory when determining LNM risk in local excision specimens.
The presence of submucosal lymphatic invasion[7,17,25,26] [relative risk (RR) = 5.2, 95% confidence interval (CI): 4.0-6.8[7] ] and to a much lesser extent venous invasion (RR = 2.2; 95%CI: 1.4 -3.2[7]) and perineural invasion[27] have been shown to be some of the strongest predictors of LNM in early CRC. It is therefore important to carefully assess for their presence and report these factors separately rather than stating the presence “lymphovascular invasion” for example. The location of the deepest point of involvement (either intramural or extramural) should be specified as this is also prognostic[28]. In the context of a local excision, the deepest point visible will usually be intramural. Lymphatic invasion is well recognised to be subjective with significant rates of inter-observer variation[29]. This can be caused by difficulties distinguishing lymphatics from venules, retraction artefacts, tumour budding and poorly di-fferentiated clusters[29,30]. In cases of doubt, D2-40 immunohistochemistry can be helpful to confirm the presence of a lymphatic channel and elastin stains can be helpful to identify veins[25]. The use of such ancillary stains has been shown to significantly improve the inter-observer agreement[31].
In polypectomy/local excision specimens, the status of the resection margin in conjunction with other high risk histopathology factors determines the risk of local recurrence. Tumours which are present at the resection margin or within the dia-thermised zone should be considered for further treatment regardless of high risk factors. In cases where the invasive tumour extends to the peripheral resection margin only, a repeat endoscopy and further local excision should be considered[19].
There is currently significant controversy about the degree of risk in cases where a tumour extends close to the deep resection margin (1 mm or less) but does not directly involve it. Within the NHS BCSP, the recently revised pathological reporting guidance has maintained 1 mm as the optimal cut-off to define margin involvement in order to reduce the risk of incomplete resection, despite the risk that this strategy will lead to a higher rate of major bowel surgery. Despite this guidance, in the absence of any other high-risk histopathology features, local re-excision may be a reasonable treatment option[11,32,33].
Depending on the shape of the lesion (pedunculated or non-pedunculated), and the status of the muscularis mucosa (identifiable or non-identifiable), different classification systems may be used to define the level of submucosal invasion. A qualitative assessment of sessile lesions was initially proposed by Kudo et al[34] which separated the submucosa into thirds: sm1 (superficial); sm2 (middle); and sm3 (deep). Invasion into sm3 has been associated with a higher risk of LNM when compared to invasion confined to sm1/sm2 (RR = 3.6, 95%CI: 1.3-9.8)[7]. This method was further subsequently modified into a semi-quantitative system by Kikuchi (sm1: Invasion up to 0.2-0.3 mm; sm2: Intermediate invasion; sm3: Invasion near the muscularis propria)[35]. A third quantitative measurement with a clear cut-off defining the levels as sm1: Up to 0.5 mm; sm2: 0.5 -1.0 mm; sm3: Beyond 1.0 mm[36]. A second classification system was proposed by Haggitt for pT1 CRC with a polypoid shape, which assesses the depth of invasion into four levels, with level four invasion described as an adverse factor[37].
Several issues arise when attempting to apply these two classification systems in a routine clinical setting. Firstly, the received sample can become fragmented or suboptimally orientated on histological sections meaning that accurate assessment is not possible. To apply the Kudo system, the muscularis propria needs to be visible which is not usually present in local excisions (with the exception of full thickness transanal resection specimens). Without the muscularis propria indicating that the full thickness of the submucosa is included, accurate division of the submucosa into thirds is impossible. Haggitt classification can also be difficult to apply in poorly orientated specimens or polyps that are lacking a clear stalk. These limitations and difficulties show the need for better alternative measures that reply on quantitative parameters.
Studies, in particular of Japanese populations, have identified the absolute depth of submucosal invasion as an important quantitative factor for predicting lymph node metastasis[38,39]. Ueno et al[11] proposed that the absolute depth of invasion beyond the muscularis mucosa and the width of the invasive tumour are more objective para-meters than the conventional factors described above[11,17]. On univariate analysis, submucosal invasion ≥ 1 mm was predictive for LNM in pT1 CRC (RR = 5.2, 95%CI: 1.8-15.4)[7].
The current guidelines from the Japanese Society for Cancer of the Colon and Rectum recommend major resection if the cancer has an involved deep margin or if one of the following high risk factors is present: poor differentiation, signet-ring cell carcinoma, mucinous carcinoma, depth of invasion > 1000 µm, vascular invasion or budding G2/G3[14]. Interestingly a study in the Japanese population found that submucosal invasion depth > 1000 µm alone would lead to approximately 80% of malignant polyps being treated with laparotomy[39]. This approach has not been routinely adopted in western populations as it would cause a significant increase in the major resection rate in patients with a significantly higher risk of post-operative mortality. In addition, there is only limited data on the prediction of LNM according to the absolute depth of submucosal invasion in western populations[40].
Three dimensional histological reconstructions of the large intestinal submucosa has demonstrated that the number and size of blood and lymphatic vessels does not increase towards the base of the submucosa as expected[41,42]. There are a greater number of vessels in sm1 and the vessels are largest in sm2, suggesting that the absolute depth of submucosal invasion may not be the most important parameter. Pilot studies in a western populations have suggested that the width of the cancer (cut-off 11.5 mm; P = 0.001) and the area of submucosal invasion (cut-off 35 mm2; P < 0.001) were significantly associated with the risk of LNM and showed superior prediction when compared to the depth of invasion[40]. Taken together, it is highly likely that the absolute area of submucosal invasion in sm1/sm2 where the majority of vessels are located is the best predictor of LNM. With the routine adoption of digital pathology, measuring the area of submucosal invasion is now readily feasible (Figure 1).
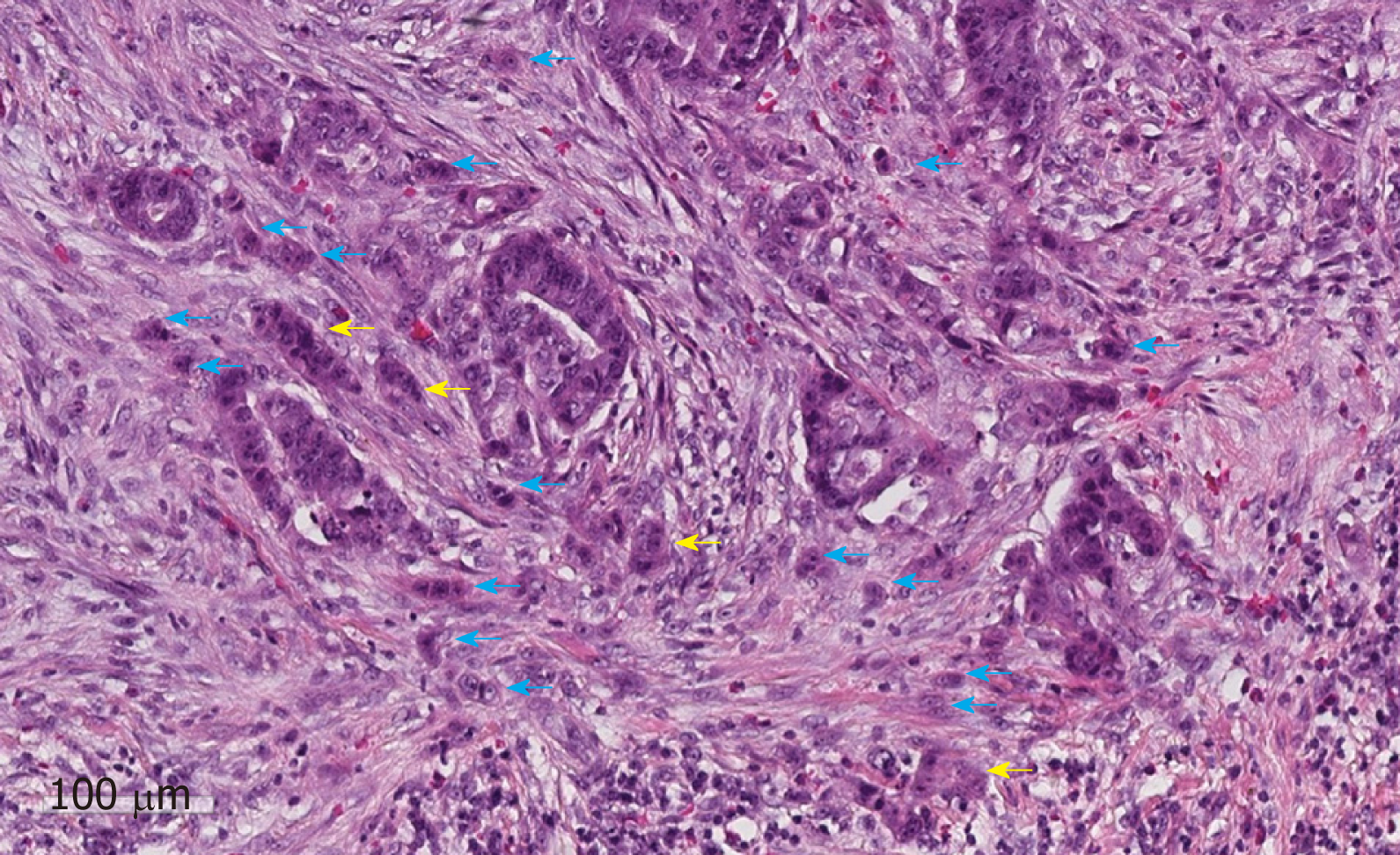
The predictive value of tumour budding for LNM has been demonstrated for various cancers[43-46], but implementation into routine guidelines has been hindered by a lack of practical guidance on assessment. Multiple different systems are described in the literature leading to confusion over which system should be used[47]. Recently an international consensus group agreed the definition of budding as “a single cancer cell or a cell cluster of up to four tumour cells”[48] as well as detailing the practical steps that should be taken to evaluate this marker (Figure 2). This led to the im-plementation of tumour budding as an additional prognostic factor in the eighth edition of the Union for International Cancer Control's (UICC's) TNM classification. Tumour budding has been included in the College of American Pathologists (CAP) guidelines but not yet as a core factor in the UK guidelines until sufficient evidence of reproducibility and its role in patient risk stratification exists[19]. Nevertheless some studies have shown that the inter-observer variation in tumour budding is improved by the international consensus definition, although further studies in western populations are needed given that most of the large studies to date have been per-formed in Asian populations.
A new emerging risk factor for LNM in pT1 CRC is the presence of poorly di-fferentiated clusters (PDC), which are defined as “malignant clusters with five or more cells lacking glandular differentiation”[49,50] (Figure 2). Studies have shown that the presence of PDC is a strong predictor of LNM with greater reproducibility than tumour differentiation or budding in a study of 3,556 pT1 CRC [OR = 3.3 (95%CI: 2.6-4.1) P < 0.0001]. Further validation work is now required to confirm the importance of PDC in early CRC and define the optimal risk cut-offs in addition to clear practical guidance on the method of assessment[49].
An emerging quantitative prognostic factor is the proportion of stroma within the overall tumour area[51]. It has been shown in a number of different cancers, including CRC, that a greater proportion of stroma is a poor prognostic factor[52-55]. The pro-portion of stroma can be subjectively estimated by the tumour stroma ratio[56] or accurately quantitated by cell density measurements[54]. It is thought that in CRC the high stroma group correlates with CMS4 consensus molecular subtyping, with both groups accounting for around 25% of cases with the poorest prognosis[57-59]. The prediction of LNM in pT1 CRC according to the proportion of stroma is as yet un-known but studies are ongoing.
The quantitation of the total number of tumour infiltrating lymphocytes has been shown to correlate with prognosis in CRC and other cancer types[60-62]. A study of 29 early stage CRC (stage I and II) showed that the combination of CD8(+) plus CD45RO(+) cells could be predictive for tumour recurrence and survival[63]. An increase in specific lymphocyte populations has been shown to strongly predict prognosis in CRC. The best-reported system in the recent literature is the Im-munoscore®, which is generated on the basis of CD3 and CD8 expression in the tumour[64-66]. Automated algorithms to assess the number of lymphocytes are currently being explored. Again, the prediction of LNM in pT1 CRC according to tumour im-munology is as yet unknown.
A meta-analysis from 2011 included 17 studies with a total of 3621 patients and showed that the presence of lymphatic invasion (RR = 5.2, 95%CI: 4.0-6.8), high tumour budding (RR = 5.1, 95%CI: 3.6-7.3), submucosal invasion ≥ 1 mm (RR = 5.2, 95%CI: 1.8-15.4), and poor differentiation (RR = 4.8, 95%CI: 3.3-6.9) were associated with a higher risk of LNM in pT1 CRC on univariate analysis[7]. Another fixed-effects meta-analysis included 76 studies and showed that lymphatic invasion (OR = 8.62) was the strongest factor, followed by tumour depth (pT2 vs pT1; OR = 2.62) and tumour differentiation (OR = 2.38) in predicting LNM[26]. In a subset analysis, poor differentiation at the invasive front (OR = 6.08) and tumour budding (OR = 5.82) were the most predictive in rectal cancer[26]. A meta-analysis from 2013 included 23 studies with 4510 patients and demonstrated similar findings, with a greater risk of LNM in pT1 CRC with a depth of submucosal invasion of > 1 mm (OR = 3.87, 95%CI: 1.50-10.00, P = 0.005), lymphovascular invasion (OR = 4.81, 95%CI: 3.14-7.37, P < 0.00001), poor differentiation (OR = 5.60, 95%CI: 2.90-10.82, P < 0.00001) or tumour budding (OR = 7.74, 95%CI: 4.47-13.39, P < 0.001)[17]. Finally, a meta-analysis in early CRC included 41 studies with 10137 patients and showed a strong association between the presence of tumour budding and risk of LNM in pT1 CRC (OR = 6.44; 95%CI: 5.26-7.87; P < 0.0001)[67].
The univariate analyses described above have identified a number of conventional and novel histopathological risk factors for LNM in pT1 CRC. To date, very few studies have proposed multivariate risk prediction models and currently there is no single risk stratification model used internationally. Ueno et al[11] demonstrated that the best risk multivariate prediction model in a cohort of 251 cases included poor differentiation (OR = 2.9; 95%CI: 1.2-7.4; P = 0.023), vascular invasion (OR = 2.7; 95%CI: 1.1-7.0; P = 0.039), tumour budding (OR = 3.7; 95%CI: 1.4-9.9; P = 0.008) and width of submucosal invasion (≥ 4000 μm)[11]. Another study of 140 cases used a logistic regression analysis and built an algorithm based on lymphatic invasion (OR = 1.45, P < 0.05), absence of lymphocyte infiltration (OR = 16.6, P = 0.016), cribriform-type structural atypia (OR = 3.7; P < 0.05), venous invasion (OR = 3.26, P < 0.05), and depth of invasion (cut-off > 2 mm; OR = 1.45, P < 0.05)[68]. Lymphocyte infiltration was investigated in the invasive area of submucosal carcinoma and classified as either negative (no or little infiltration) or positive (follicular structures or infiltration by more lymphocytes than the number of tumour cells).
A larger retrospective study of 806 cases showed that independent predictors of LNM in multivariate analysis were: depth of submucosal invasion ≥ 1000 µm (OR = 5.56; 95%CI: 2.14-19.10) and high-grade budding (OR = 3.14; 95%CI: 1.91-5.21). High-grade budding was defined as five or more buds per high power field (0.95 mm2). The study proposed a three-tier risk classification system based on the depth of submucosal invasion and budding: high-risk with a depth of submucosal invasion ≥ 1000 µm and high-grade budding, intermediate-risk with a depth of submucosal invasion ≥ 1000 µm and low-grade budding, and low-risk with a depth of submucosal invasion < 1000 µm. Additional factors for used to subclassify the intermediate risk group further were lymphovascular invasion and differentiation grade[69].
A recently published study proposed a risk stratification model for pedunculated pT1 CRC (3% to 7% LNM rate in Asian populations)[9,10,39,69] and investigated the following six factors: tumour differentiation; submucosal invasion depth by Haggitt classification; lymphovascular invasion; tumour budding; PDCs; and the condition of the muscularis mucosae[70]. Tumour budding and PDC were defined and graded as above but assessed in a 0.785 mm2 field (grade 1: 0-4; Grade 2: 5-9; Grade 3: 10 or more). The status of the muscularis mucosa was classified as Type A: Shattered but aligned muscularis mucosa or Type B: Incompletely or completely disrupted mu-scularis mucosae. The authors ultimately recommended a new model including the following four risk factors: lymphovascular invasion; Haggitt level 4 invasion; muscularis mucosae type B (incompletely or completely disrupted); high grade PDCs/tumour budding (both grade 2-3). High-grade tumour budding and high-grade PDC were grouped together as “any kind of positive budding”. The model had a sensitivity of 83.8% and specificity of 70.3% for predicting LNM (area under the curve value of 0.83), and would classify 32% of all cases in the high-risk group, 68% in the low risk group and miss 1.3% of all LNM.
Unfortunately the majority of these studies include retrospective series with small numbers of patients. Due to the 10%-15% rate of LNM in pT1 CRC, only a limited number of events are present leading to limited data in both Western and Asian populations. Well-designed prospective cohort studies are urgently needed to define robust international standards[49,69]. Ultimately a validated multivariate risk stra-tification model including both clinical and histopathological factors could be used to define prognosis in a similar way to that already developed for breast cancer.
In this review we have summarised the main conventional histopathological risk factors for LNM in pT1 CRC in addition to a number of novel factors that have recently been proposed. It is well recognised that parameters including lym-phovascular invasion, poor differentiation, and depth of submucosal invasion are significantly correlated with an increased risk of LNM. Unfortunately, lymph-ovascular invasion and tumour differentiation grading show high levels of inter-observer variation, which limits their clinical usefulness. Univariate markers for LNM in pT1 CRC should therefore be interpreted with caution when deciding whether to proceed with major bowel resection after local excision due to the significant risks of major morbidity and mortality.
With the routine implementation of digital pathology into clinical diagnostics[20-22], novel histopathological markers including the absolute depth of invasion, area of submucosal invasion and proportion of stroma can now be easily measured. Due to the quantitative nature of these parameters, it is likely that they are considerably more reproducible than the traditional subjective parameters. However, their evaluation can take a considerable amount of time to perform manually, hence there is a clear role for artificial intelligence, which is also likely to improve the reproducibility of con-ventional and novel risk factors.
Multivariate risk stratification models need to be developed and validated to optimise the management of pT1 CRC. In the future, artificial intelligence on digital pathology slides could be applied to compare different multivariate risk stratification models to identify the optimal way to accurately predict LNM[71]. This will should reduce the inaccuracy associated with relying on individual subjective markers and has the potential to further refine the group at highest risk of LNM and therefore reduce the overall number of patients exposed to the risks of major bowel resection. By combining the histopathological data with molecular data, patient data and treat-ment data, a personalised risk stratification model could be created with the aim of determining the optimal treatment pathway for individual patients[49].
In this review we present the various histopathological risk factors for predicting systemic spread in pT1 colorectal cancer and introduce potential novel quantitative variables and multivariable risk models that could be used to better define the op-timal treatment of this increasingly common disease.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Merkel S, Tandon RK S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13141] [Article Influence: 1877.3] [Reference Citation Analysis (4)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55638] [Article Influence: 7948.3] [Reference Citation Analysis (131)] |
| 3. | Amri R, Bordeianou LG, Sylla P, Berger DL. Impact of screening colonoscopy on outcomes in colon cancer surgery. JAMA Surg. 2013;148:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Morris EJ, Penegar S, Whitehouse LE, Quirke P, Finan P, Bishop DT, Wilkinson J, Houlston RS. A retrospective observational study of the relationship between family history and survival from colorectal cancer. Br J Cancer. 2013;108:1502-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | The International Agency for Research on Cancer. WHO Classification of Tumours of the Digestive System. Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. World health organization international histological classification of tumours, 4th ed. 2010;3. |
| 6. | Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T, Takao M, Shinohara T, Murakami Y, Fujimori T, Kaneko K, Saito Y. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551-559; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 7. | Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Hassan C, Zullo A, Risio M, Rossini FP, Morini S. Histologic risk factors and clinical outcome in colorectal malignant polyp: a pooled-data analysis. Dis Colon Rectum. 2005;48:1588-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Matsuda T, Fukuzawa M, Uraoka T, Nishi M, Yamaguchi Y, Kobayashi N, Ikematsu H, Saito Y, Nakajima T, Fujii T, Murakami Y, Shimoda T, Kushima R, Fujimori T. Risk of lymph node metastasis in patients with pedunculated type early invasive colorectal cancer: a retrospective multicenter study. Cancer Sci. 2011;102:1693-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Asayama N, Oka S, Tanaka S, Nagata S, Furudoi A, Kuwai T, Onogawa S, Tamura T, Kanao H, Hiraga Y, Okanobu H, Kuwabara T, Kunihiro M, Mukai S, Goto E, Shimamoto F, Chayama K. Long-term outcomes after treatment for pedunculated-type T1 colorectal carcinoma: a multicenter retrospective cohort study. J Gastroenterol. 2016;51:702-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H, Ozawa K, Yoshimura K, Bekku S. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385-394. [PubMed] |
| 12. | Marks KM, West NP, Morris E, Quirke P. Clinicopathological, genomic and immunological factors in colorectal cancer prognosis. Br J Surg. 2018;105:e99-e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Xu F, Xu J, Lou Z, Di M, Wang F, Hu H, Lai M. Micropapillary component in colorectal carcinoma is associated with lymph node metastasis in T1 and T2 Stages and decreased survival time in TNM stages I and II. Am J Surg Pathol. 2009;33:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 603] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 15. | Halvorsen TB, Seim E. Degree of differentiation in colorectal adenocarcinomas: a multivariate analysis of the influence on survival. J Clin Pathol. 1988;41:532-537. [PubMed] |
| 16. | Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003;16:376-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 277] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis. 2013;15:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 18. | Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol. 2010;23:1068-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Dataset for histopathological reporting of colorectal cancer. September 2018 G049. . |
| 20. | Williams BJ, Bottoms D, Treanor D. Future-proofing pathology: the case for clinical adoption of digital pathology. J Clin Pathol. 2017;70:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Williams BJ, Bottoms D, Clark D, Treanor D. Future-proofing pathology part 2: building a business case for digital pathology. J Clin Pathol. 2019;72:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Williams BJ, Hanby A, Millican-Slater R, Nijhawan A, Verghese E, Treanor D. Digital pathology for the primary diagnosis of breast histopathological specimens: an innovative validation and concordance study on digital pathology validation and training. Histopathology. 2018;72:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 290] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 24. | Ryan E, Sheahan K, Creavin B, Mohan HM, Winter DC. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Wada H, Shiozawa M, Katayama K, Okamoto N, Miyagi Y, Rino Y, Masuda M, Akaike M. Systematic review and meta-analysis of histopathological predictive factors for lymph node metastasis in T1 colorectal cancer. J Gastroenterol. 2015;50:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Glasgow SC, Bleier JI, Burgart LJ, Finne CO, Lowry AC. Meta-analysis of histopathological features of primary colorectal cancers that predict lymph node metastases. J Gastrointest Surg. 2012;16:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, Berger DH, Albo D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131-5137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 367] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 28. | Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak P, Vieth M, Hoefler G, Langner C. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer. 2012;118:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 29. | Ishii M, Ota M, Saito S, Kinugasa Y, Akamoto S, Ito I. Lymphatic vessel invasion detected by monoclonal antibody D2-40 as a predictor of lymph node metastasis in T1 colorectal cancer. Int J Colorectal Dis. 2009;24:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Wada H, Shiozawa M, Sugano N, Morinaga S, Rino Y, Masuda M, Akaike M, Miyagi Y. Lymphatic invasion identified with D2-40 immunostaining as a risk factor of nodal metastasis in T1 colorectal cancer. Int J Clin Oncol. 2013;18:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Suzuki A, Togashi K, Nokubi M, Koinuma K, Miyakura Y, Horie H, Lefor AT, Yasuda Y. Evaluation of venous invasion by Elastica van Gieson stain and tumor budding predicts local and distant metastases in patients with T1 stage colorectal cancer. Am J Surg Pathol. 2009;33:1601-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Brown IS, Bettington ML, Bettington A, Miller G, Rosty C. Adverse histological features in malignant colorectal polyps: a contemporary series of 239 cases. J Clin Pathol. 2016;69:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Gill MD, Rutter MD, Holtham SJ. Management and short-term outcome of malignant colorectal polyps in the north of England(1). Colorectal Dis. 2013;15:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 553] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 35. | Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, Fujiyoshi T, Uchida Y. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995;38:1286-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 439] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 36. | Yamamoto S, Watanabe M, Hasegawa H, Baba H, Yoshinare K, Shiraishi J, Kitajima M. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004;51:998-1000. [PubMed] |
| 37. | Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985;89:328-336. [PubMed] |
| 38. | Kawachi H, Eishi Y, Ueno H, Nemoto T, Fujimori T, Iwashita A, Ajioka Y, Ochiai A, Ishiguro S, Shimoda T, Mochizuki H, Kato Y, Watanabe H, Koike M, Sugihara K. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod Pathol. 2015;28:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, Iwashita A, Ajioka Y, Watanabe H, Watanabe T, Muto T, Nagasako K. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 484] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 40. | Toh EW, Brown P, Morris E, Botterill I, Quirke P. Area of submucosal invasion and width of invasion predicts lymph node metastasis in pT1 colorectal cancers. Dis Colon Rectum. 2015;58:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Roberts N, Magee D, Song Y, Brabazon K, Shires M, Crellin D, Orsi NM, Quirke R, Quirke P, Treanor D. Toward routine use of 3D histopathology as a research tool. Am J Pathol. 2012;180:1835-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 42. | Brown PJ, Toh EW, Smith KJ, Jones P, Treanor D, Magee D, Burke D, Quirke P. New insights into the lymphovascular microanatomy of the colon and the risk of metastases in pT1 colorectal cancer obtained with quantitative methods and three-dimensional digital reconstruction. Histopathology. 2015;67:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Almangush A, Bello IO, Keski-Säntti H, Mäkinen LK, Kauppila JH, Pukkila M, Hagström J, Laranne J, Tommola S, Nieminen O, Soini Y, Kosma VM, Koivunen P, Grénman R, Leivo I, Salo T. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36:811-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 44. | Almangush A, Salo T, Hagström J, Leivo I. Tumour budding in head and neck squamous cell carcinoma - a systematic review. Histopathology. 2014;65:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | Masuda R, Kijima H, Imamura N, Aruga N, Nakamura Y, Masuda D, Takeichi H, Kato N, Nakagawa T, Tanaka M, Inokuchi S, Iwazaki M. Tumor budding is a significant indicator of a poor prognosis in lung squamous cell carcinoma patients. Mol Med Rep. 2012;6:937-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Jesinghaus M, Strehl J, Boxberg M, Brühl F, Wenzel A, Konukiewitz B, Schlitter AM, Steiger K, Warth A, Schnelzer A, Kiechle M, Beckmann MW, Noske A, Hartmann A, Mehlhorn G, Koch MC, Weichert W. Introducing a novel highly prognostic grading scheme based on tumour budding and cell nest size for squamous cell carcinoma of the uterine cervix. J Pathol Clin Res. 2018;4:93-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol. 2012;25:1315-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 48. | Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 716] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 49. | Ueno H, Hase K, Hashiguchi Y, Shimazaki H, Yoshii S, Kudo SE, Tanaka M, Akagi Y, Suto T, Nagata S, Matsuda K, Komori K, Yoshimatsu K, Tomita Y, Yokoyama S, Shinto E, Nakamura T, Sugihara K. Novel risk factors for lymph node metastasis in early invasive colorectal cancer: a multi-institution pathology review. J Gastroenterol. 2014;49:1314-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, Maekawa K, Katsurada Y, Nakamura T, Mochizuki H, Yamamoto J, Hase K. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. 2012;36:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 51. | West RB, van de Rijn M. Experimental approaches to the study of cancer-stroma interactions: recent findings suggest a pivotal role for stroma in carcinogenesis. Lab Invest. 2007;87:967-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Hutchins GGA, Treanor D, Wright A, Handley K, Magill L, Tinkler-Hundal E, Southward K, Seymour M, Kerr D, Gray R, Quirke P; QUASAR trial collaborators and the UK National Cancer Research Institute Colorectal Cancer Clinical Studies Group. Intratumoral stromal morphometry predicts disease recurrence but not response to 5-fluorouracil-results from the QUASAR trial of colorectal cancer. Histopathology. 2018;72:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Wright AI, Coe A, Dattani M, Toh E, Hutchins G, West N, Grabsch H, Magee D, Quirke P, Treanor D. Automatic Image Analysis to Calculate the Cancer: Stroma Ratio in Colorectal Cancer. J Pathol. 2012;228:S41-S41. |
| 54. | West NP, Dattani M, McShane P, Hutchins G, Grabsch J, Mueller W, Treanor D, Quirke P, Grabsch H. The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br J Cancer. 2010;102:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 55. | Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 56. | van Pelt GW, Sandberg TP, Morreau H, Gelderblom H, van Krieken JHJM, Tollenaar RAEM, Mesker WE. The tumour-stroma ratio in colon cancer: the biological role and its prognostic impact. Histopathology. 2018;73:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 57. | Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3408] [Cited by in RCA: 3518] [Article Influence: 351.8] [Reference Citation Analysis (0)] |
| 58. | Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 588] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 59. | Sveen A, Bruun J, Eide PW, Eilertsen IA, Ramirez L, Murumägi A, Arjama M, Danielsen SA, Kryeziu K, Elez E, Tabernero J, Guinney J, Palmer HG, Nesbakken A, Kallioniemi O, Dienstmann R, Lothe RA. Colorectal Cancer Consensus Molecular Subtypes Translated to Preclinical Models Uncover Potentially Targetable Cancer Cell Dependencies. Clin Cancer Res. 2018;24:794-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 60. | Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer JU, Karn T, Pfitzner BM, Kümmel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 1423] [Article Influence: 177.9] [Reference Citation Analysis (0)] |
| 61. | Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, Loi S. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13:228-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 639] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 62. | Jass JR, Ajioka Y, Allen JP, Chan YF, Cohen RJ, Nixon JM, Radojkovic M, Restall AP, Stables SR, Zwi LJ. Assessment of invasive growth pattern and lymphocytic infiltration in colorectal cancer. Histopathology. 1996;28:543-548. [PubMed] |
| 63. | Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944-5951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 738] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 64. | Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, Chouchane L, Delrio P, Arndt H, Asslaber M, Maio M, Masucci GV, Mihm M, Vidal-Vanaclocha F, Allison JP, Gnjatic S, Hakansson L, Huber C, Singh-Jasuja H, Ottensmeier C, Zwierzina H, Laghi L, Grizzi F, Ohashi PS, Shaw PA, Clarke BA, Wouters BG, Kawakami Y, Hazama S, Okuno K, Wang E, O'Donnell-Tormey J, Lagorce C, Pawelec G, Nishimura MI, Hawkins R, Lapointe R, Lundqvist A, Khleif SN, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Palmqvist R, Nagtegaal ID, Wang Y, D'Arrigo C, Kopetz S, Sinicrope FA, Trinchieri G, Gajewski TF, Ascierto PA, Fox BA. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 632] [Cited by in RCA: 632] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 65. | Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, Sasso M, Bilocq AM, Kirilovsky A, Obenauf AC, Hamieh M, Berger A, Bruneval P, Tuech JJ, Sabourin JC, Le Pessot F, Mauillon J, Rafii A, Laurent-Puig P, Speicher MR, Trajanoski Z, Michel P, Sesboüe R, Frebourg T, Pagès F, Valge-Archer V, Latouche JB, Galon J. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 754] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 66. | Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1470] [Article Influence: 210.0] [Reference Citation Analysis (0)] |
| 67. | Cappellesso R, Luchini C, Veronese N, Lo Mele M, Rosa-Rizzotto E, Guido E, De Lazzari F, Pilati P, Farinati F, Realdon S, Solmi M, Fassan M, Rugge M. Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: a meta-analysis. Hum Pathol. 2017;65:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Egashira Y, Yoshida T, Hirata I, Hamamoto N, Akutagawa H, Takeshita A, Noda N, Kurisu Y, Shibayama Y. Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol. 2004;17:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 69. | Kobayashi H, Higuchi T, Uetake H, Iida S, Ishikawa T, Ishiguro M, Sugihara K. Resection with en bloc removal of regional lymph node after endoscopic resection for T1 colorectal cancer. Ann Surg Oncol. 2012;19:4161-4167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Backes Y, Elias SG, Groen JN, Schwartz MP, Wolfhagen FHJ, Geesing JMJ, Ter Borg F, van Bergeijk J, Spanier BWM, de Vos Tot Nederveen Cappel WH, Kessels K, Seldenrijk CA, Raicu MG, Drillenburg P, Milne AN, Kerkhof M, Seerden TCJ, Siersema PD, Vleggaar FP, Offerhaus GJA, Lacle MM, Moons LMG; Dutch T1 CRC Working Group. Histologic Factors Associated With Need for Surgery in Patients With Pedunculated T1 Colorectal Carcinomas. Gastroenterology. 2018;154:1647-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 71. | Ichimasa K, Kudo S, Mori Y, Misawa M, Fumio I. Artificial Intelligence Can Accurately Predict the Presence of Lymph Node Metastasis in Pt1 Colorectal Cancers. Gastrointestinal Endoscopy. 2017;85:Ab377-Ab377. |