Published online Jun 14, 2019. doi: 10.3748/wjg.v25.i22.2809
Peer-review started: March 14, 2019
First decision: April 10, 2019
Revised: April 30, 2019
Accepted: May 8, 2019
Article in press: May 8, 2019
Published online: June 14, 2019
Processing time: 93 Days and 19.6 Hours
Autoimmune hepatitis (AIH) is a rare chronic inflammatory liver disease with a high risk of progression to liver cirrhosis. The initial treatment for AIH usually includes a steroid, with or without azathioprine. AIH can present at any age; however, the most effective and safe induction treatment for AIH in the elderly remains unclear.
To systematically review available data on both effectiveness and safety of AIH treatments in elderly subjects.
To identify studies on AIH induction treatment in elderly patients (≥ 60 years of age), an electronic research was performed (PubMed, EMBASE and Cochrane Library databases) until February 2019. Eligible studies were selected through screening of titles and abstracts, followed by full-text critical evaluation. After risk of bias assessment, data on study designs, interventions, and outcomes were extracted and reviewed.
Among the 1736 retrieved papers, 15 studies were selected. Out of them, eight studies were excluded because of a critical risk of bias. The remaining seven studies included 789 patients and out of them 239 subjects were elders. First-line treatment was a steroid either alone or in combination with azathioprine in most patients (87.6%) and only one study investigated the effect of combined steroid and mycophenolate mofetil therapy. Standard therapy was effective in inducing remission in the elderly. Moreover, treatment failure and relapses occurred less often in the elderly compared to younger people.
Treatment of AIH is challenging in elderly patients. This systematic review confirms the efficacy and safety of standard induction treatment for AIH in the elderly. Available evidence is insufficient to draw any conclusion on the effect of novel AIH treatments in elderly subjects.
Core tip: Autoimmune hepatitis (AIH) is a severe liver disease that affects patients worldwide. Conventional treatment with a steroid and azathioprine is the mainstay of treatment. Although elderly patients have a relatively high incidence of AIH, data on its treatment in the elderly are limited. We focused on this subgroup of patients and systematically reviewed studies testing both efficacy and safety of AIH treatments in old patients. Available data support the use of conventional treatment, while the effect of other drugs has only been tested in small case series.
- Citation: Durazzo M, Lupi G, Scandella M, Ferro A, Gruden G. Autoimmune hepatitis treatment in the elderly: A systematic review. World J Gastroenterol 2019; 25(22): 2809-2818
- URL: https://www.wjgnet.com/1007-9327/full/v25/i22/2809.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i22.2809
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease, potentially leading to liver cirrhosis and hepatic failure. AIH has a variety of clinical presentation patterns, ranging from asymptomatic course to acute severe liver disease[1]. It is characterized by the presence of interface hepatitis on histological examination, elevated aminotransferases, hyper-gammaglobulinemia, and circulating auto-antibodies, such as antinuclear antibodies, smooth muscle autoantibodies, liver kidney microsomial antibodies type 1, and liver cytosol specific antibody type 1. AIH of type 1 is the most frequent subtype (90%), while AIH of type 2 is relatively rare (10%) and occurs mainly in childhood.
A diagnostic scoring system was developed in 1993 by the International Autoimmune Hepatitis Group (IAIHG) and revised in 1999[2,3]. A simplified scoring system based on autoantibody titres, serum IgG levels, liver histology, and exclusion of viral hepatitis was proposed in 2008 to ease clinical application[4]. AIH is relatively rare with a prevalence ranging from 16 to 18 cases per 100000 inhabitants in Europe. However, prevalence is higher in some population groups, as Alaska natives (42 cases per 100000 inhabitants)[5]. Although initially thought to be particularly prevalent in young women and children, the disease can affect all age groups and in the last decade several studies reported a relatively high incidence in the elderly[6,7].
Untreated AIH has a poor prognosis; however, immunosuppressive therapy shows a high response rate and significantly improves prognosis[8]. Therapy with a steroid, with or without azathioprine (AZA), is the mainstay of treatment, but the most effective and safe therapy in elderly patients remains undetermined as old people are often excluded from clinical trials[9]. Moreover, both comorbidities and polypharmacy make treatment choice very challenging in this age group. To clarify the efficacy of AIH treatments specifically in the elderly, we performed a systematic review.
The study was carried out according to the Preferred Items for Reporting of Systematic Reviews and Meta-Analyses (PRISMA) guidelines[10]. The study protocol was not registered.
We searched electronic databases (PubMed National Library of Medicine, EMBASE, and Cochrane Library) to identify all published studies on AIH treatment in the elderly up to 20th of February 2019. The research terms (keywords, medical subject headings, free-terms) “autoimmune hepatitis/therapy”, “autoimmune hepatitis/ treatment”, ‘‘elderly”, “80 and over”, “older”, “aged” were used in combination (AND or OR operators) (Appendix 1). Reference lists were also screened to identify additional relevant studies. The search strategy was limited to English language publications, while no timeline restriction was used.
We included both randomized and non-randomized studies, whereas case-reports, case series, and letters to the Editor were excluded. Eligible studies had to assess the effect of induction treatment of newly diagnosed AIH in either elderly patients or a well-defined subgroup of old patients (threshold: ≥ 60 years of age). Studies on elderly patients with overlap syndromes, primary biliary cirrhosis, primary sclerosing cholangitis, chronic viral hepatitis, and drug-induced AIH were excluded.
Three review authors independently screened titles and abstracts of eligible studies. Disagreements were resolved through consensus by discussion. Full text of each study was then read and critically assessed by two review authors, who in-dependently agreed on selection. A third review author resolved discrepancies.
All selected studies were non-randomized studies of interventions; therefore, we assessed study quality using the Risk of Bias In Non-Randomized Studies of Interventions (ROBINS-I). This is a new tool for evaluating the risk of bias in estimates of the comparative effectiveness of interventions from studies that did not use randomization to allocate units to comparison groups. The tool includes seven bias domains: three pre-intervention domains (confounding, selection of participants into the study, classification of interventions) and four post-intervention domains (deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results)[11]. Two review authors (G.L., M.S.) independently assessed the risk of bias across the seven domains for each included study and a third review author (A.F.) acted as an arbitrator (Table 1). We excluded all the studies that were at critical risk of bias in at least one domain.
| Biasdomains | Schram et al[6], 2001 | Granito et al[21], 2005 | Al-Chalabi et al[23], 2006 | Czaja et al[9], 2006 | Zhang et al[22], 2012 | Zachou et al[20], 2016 | Morii et al[24], 2017 |
| Confounding | Moderate | Serious | Moderate | Moderate | Serious | Moderate | Moderate |
| Selection of participants into study | Serious | Low | Low | Moderate | Moderate | Serious | Moderate |
| Classification of interventions | Serious | Moderate | Moderate | Moderate | Serious | Moderate | Moderate |
| Deviation from intended interventions | No information | Low | No information | No information | No information | Low | Moderate |
| Missing data | Moderate | Moderate | Moderate | Serious | Serious | Serious | Serious |
| Measurement of outcomes | Low | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Selection of the reported results | Moderate | Moderate | Moderate | Moderate | Moderate | Serious | Moderate |
| Overall | Serious | Serious | Moderate | Serious | Serious | Serious | Serious |
We extracted the following data from included studies: (1) Characteristics of study participants (age, AIH type); (2) Type of intervention [steroid, steroid plus AZA, steroid plus mycophenolate mofetil (MMF), ursodeoxycholic acid (UDCA), and other treatments] in the elderly compared to younger subjects; and (3) Type of outcomes (both partial and complete remission, relapses, treatment failure, side effects/allergy).
For each study, data on first author surname, publication year, country of the first author, study design, total sample size, number of elderly patients enrolled, and follow-up duration were also collected. We used the definition of remission given by the Authors in the paper and we did not request study data where they were missing from the articles.
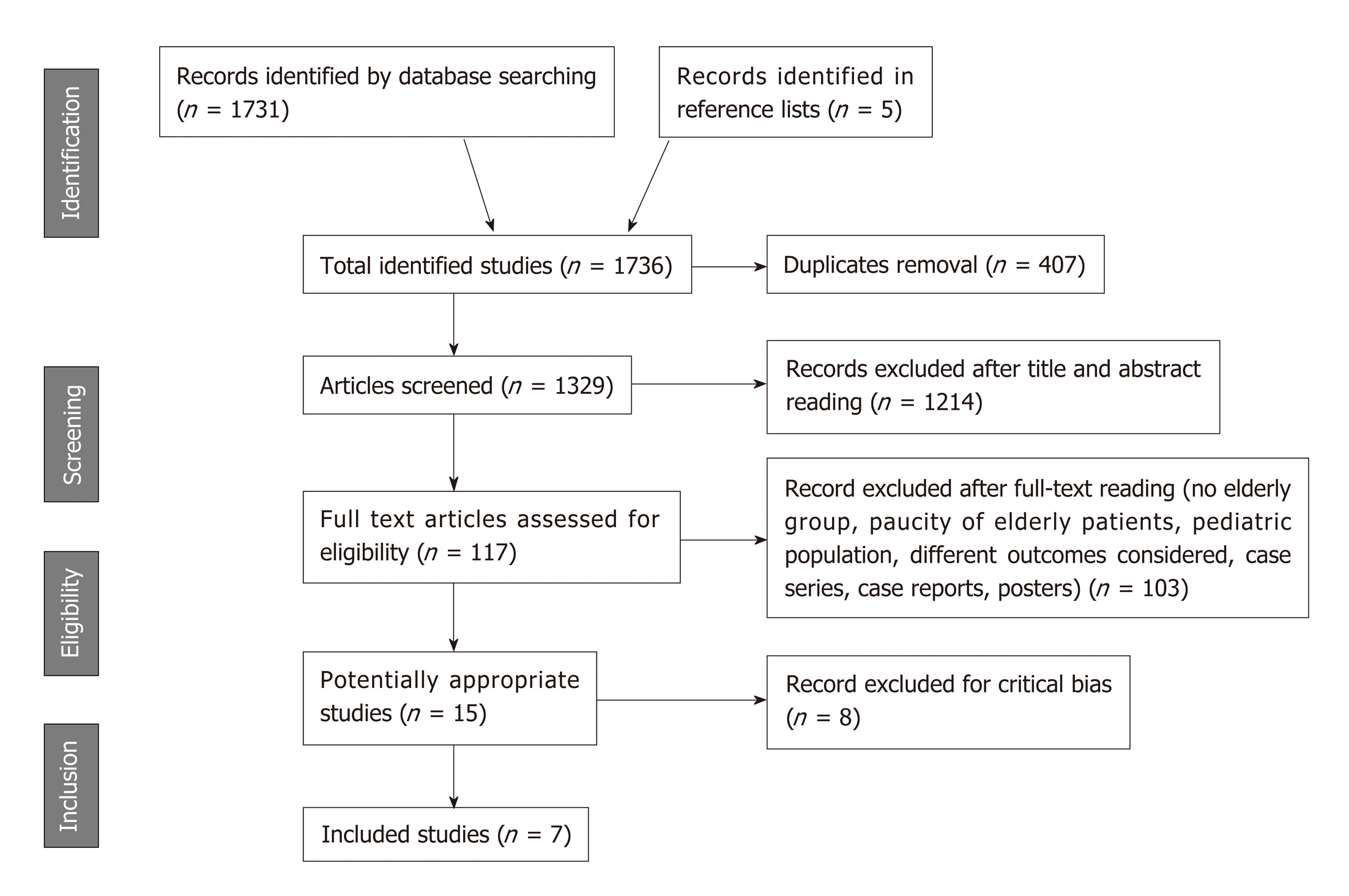
A PRISMA flow diagram of study selection is shown in Figure 1. Database search retrieved 1736 references. After exclusion of 407 duplicates, 1329 records were screened. Primary selection by title and abstract assessment led to the exclusion of further 1214 articles. Among the remaining 115 papers, 100 studies were excluded after full-text reading (e.g., case series and case reports, posters, insufficient number of elderly patients in the sample, no sub-analysis in the elderly subgroup, studies assessing clinical presentation, prevalence, and economic issues in elderly AIH patients rather than therapy, AIH in pediatric population). Out of the 15 remaining studies, eight were excluded for critical risk of bias as assessed by ROBINS-I[12-19].
Characteristics of the seven included studies are summarised in Table 2[6,9,20-24]. They were comparative cohort studies, which were performed in seven different countries (North America, Europe and Asia) and published between 2001 and 2017. A total of 789 patients (640 F, 149 M) with new-onset AIH were studied. The clinical presentation was heterogeneous with both acute and chronic onset. Among AIH patients, 239 subjects (203 F, 36 M) were elders (≥ 60 years of age)[6,9,20-24]. Because different age threshold (≥ 60, ≥ 65, ≥ 70) were used to define elderly patients in the seven studies, the age range also differ in the comparator group. Moreover, three studied included in the comparator group only patients with pre-defined age threshold (≤ 30, ≤ 32, and ≤ 50 years of age, respectively)[6,9,22]. Diagnostic criteria for AIH differed in the included studies. Specifically, five studies used the revised IAIHG criteria, one study the simplified IAIHG criteria[20], and the study by Zhang et al[22] both diagnostic scoring systems.
| Author(year) | Methods | No. of patients (F:M) | Diagnostic criteria | Country | Interventions | Median FU | Outcomes | Side effects |
| Elderly:Youn-ger | ||||||||
| Schramm et al[6], 2001 | Comparative Cohort Study | 40 (32:8) | IAIHG (1999) | Germany | -P alone | 4-164 mo | CR, relapse, all causes and liver-related death | GI symptoms, cholestasis, skin rush (AZA). VZ infection, pulmonary TB reactivation |
| -P + AZA | (40 mo in elderly group) | |||||||
| 20 (≥ 65 yr, 50%):20 | -No treatment | |||||||
| Granito et al[21], 2005 | Comparative Cohort Study | 76 (64:12) | IAIHG (1999) | Italy | -M alone | 1-16 yr (5 yr) in the elderly | CR, relapse, death | None in elderly group |
| 20 (≥ 65 yr, 26%):56 | -M + AZA | |||||||
| -No treatment | ||||||||
| Al-Chalabi et al[23], 2006 | Comparative Cohort Study | 164 (128:36) | IAIHG (1999) | UK | -P alone | 1-28 yr (9 yr) | CR, PR, TF, relapse, deaths/OLT | Cushingoid faces, osteoporosis, T2DM, hypertension, psychoses. Cytopenia (AZA) |
| -P + AZA | ||||||||
| 43 (≥ 60 yr, 26%):121 | -(P + Cyclo | |||||||
| and P + D-P in young only) | ||||||||
| Czaja et al[9], 2006 | Comparative Cohort Study | 205 (175:30) | IAIHG (1999) | USA | -Pp alone | (77 mo) | Remission, SR, TF, relapse, death or OLT | Not reported |
| -Pp + AZA | ||||||||
| 47 (≥ 60 yr, 23%):158 | -P + AZA | |||||||
| -No treatment | ||||||||
| Zhang et al[22], 2012 | Comparative Cohort Study | 75 (71:4) | IAIHG (1999) | China | -P alone | 6 mo-8 yr | Remission, SR, TF, relapse, death | Not reported |
| 36 (≥ 60 yr, 48%):39 | ||||||||
| + IAIHG (2008) | ||||||||
| Zachou et al[20], 2016 | Comparative Cohort Study | 158 (114:44) | IAIHG (2008) | Greece | -P + MMF | 3-168 mo (72) in MMF group | CR, PR, TF, relapse, liver-related death, progression during FU, OLT | Sepsis, airway infections, VZ, mild GI symptoms, cytopenia (MMF) |
| 45 (> 60 yr, 28%):113 | -P alone | |||||||
| -P + AZA | ||||||||
| Morii et al[24], 2017 | Comparative Cohort Study | 71 (56:15) | IAIHG (1999) | Japan | -Pp alone | 2-69 mo (31 mo) | Remission, relapses | Not reported |
| 28 (≥ 70 yr, 39%):43 | -UDCA |
Studies differed for types of treatments and drug dosages (Table 3). However, in all studies except one, elderly patients were treated with a steroid either alone or in combination with AZA (170 out of 194 patients)[6,9,21-24]. The study by Zachou et al[20] assessed the performance of an alternative first-line treatment with prednisolone and MMF. Moreover, the study by Morii et al[24] also included a sub-study on the potential benefit of UDCA treatment in 13 elderly AIH patients with chronic disease onset. Fifty-nine patients were excluded from the final analysis because they were not eligible for immunosuppressive treatment (27 patients)[20], they did not satisfy the criteria for treatment or received experimental therapies (26 patients)[9], or they did not receive any treatment (6 patients)[6,21,22]. Follow-up duration varied widely from two months to 33 years.
| Author (year) | Treatments |
| Schramm et al[6], 2001 | P 1 mg/kg/die |
| P 1 mg/kg/die + AZA 1-1.5 mg/kg/die | |
| No treatment | |
| Granito et al[21], 2005 | M 1 mg/kg/die |
| M 30 mg/die + AZA 50 mg/die | |
| No treatment | |
| Al-Chalabi et al[23], 2006 | P 20-40 mg/die |
| P 20-40 mg/die + AZA (1 mg/kg/die) | |
| P 20-40 mg/die + Cyclo (only in the younger group) | |
| P 20-40 mg/die + D-P (only in the younger group) | |
| Czaja et al[9], 2006 | Pp (doses not specified) |
| Pp + AZA (doses not specified) | |
| Investigational therapies (drugs not specified) | |
| No Treatment | |
| Zhang et al[22], 2012 | P (doses not specified) |
| P + AZA (doses not specified) | |
| No treatment | |
| Zachou et al[20], 2016 | P 0,5-1 mg/kg/die + MMF 1.5-2g/die |
| P 0,5-1 mg/kg/die | |
| P 0.5-1 mg/kg/die + AZA 1.5-2 mg/kg/die | |
| Morii et al[24], 2017 | Pp 30-40 mg/die |
| UDCA (dose not specified) |
Definitions of remission, treatment failure, and relapse are reported in Table 4. In the elderly, standard therapy with a steroid either alone or in combination with AZA induced remission in 119 out of 151 patients. In the studies by Granito et al[21] and Al-Chalabi et al[23], the remission rate was of 90% and 95%, respectively. The likelihood of remission in the elderly was similar to that observed in the other age groups. Treatment failure occurred less often in old compared to younger patients (Czaja et al[9]: 9.1% vs 28.2%, P = 0.041; Zhang et al[22]: 5% vs 24%, P = 0.03). In the study by Zachou et al[20], treatment with a steroid combined with MMF achieved a greater complete response rate than standard treatment with a steroid combined with AZA, independently of age.
| Author (year) | Remission | Treatment failure | Relapse |
| Schramm et al[6], 2001 | IAIHG revised criteria (1999) | - | IAIHG revised criteria (1999) |
| Granito et al[21], 2005 | IAIHG revised criteria (1999) | - | IAIHG revised criteria (1999) |
| Al-Chalabi et al[23], 2006 | IAIHG revised criteria (1999) | IAIHG original criteria (1993) | IAIHG revised criteria (1999) |
| PR: IAIHG original criteria (1993) | |||
| Czaja et al[9], 2006 | Symptoms: Absent | Worsening of clinical, laboratory and/or histological alterations despite compliance to therapy | Symptom recurrence and increased serum AST level (> three-fold the ULN) after drug withdrawal |
| AST level: Normal or near normal (< two-fold the UNL) | |||
| Histology: Minimal/no inflammation | |||
| SR: | |||
| Symptoms: Absent | |||
| Serum AST levels: Normal or below the relapse threshold | |||
| after drug withdrawal | |||
| Zhang et al[22], 2012 | Symptoms: Absent | Worsening of clinical, laboratory and/or histological alterations despite compliance to therapy | Symptom recurrence and increased serum AST level (> three-fold the ULN) after drug withdrawal |
| AST levels: Normal or near normal (< two-fold the UNL) | |||
| Histology: Minimal or no inflammation. | |||
| SR: | |||
| Symptoms: Absent | |||
| AST levels: Normal or below the relapse threshold after drug withdrawal | |||
| Zachou et al[20], 2016 | CR: | Persistently elevated AST and ALT (> three-fold the UNL) and/or increased IgG despite intensive immunosuppression and compliance to therapy | Rise in AST and ALT levels (> three-fold the UNL) and/or increased IgG (> 2000 mg/dL) during therapy with or without symptom recurrence after initial CR |
| Symptoms: Improved | |||
| AST, ALT, IgG levels: normal | |||
| Histology: Minimal/no inflammation | |||
| PR: | |||
| ALT or AST levels: Decreased (< two-fold ULN) without achieving complete normalization and inability to withdraw or taper prednisolone | |||
| Morii et al[24], 2017 | Normal serum ALT and IgG levels | - | Re-exacerbation not explicitly defined |
The response rate to therapy was similar in the Czaja et al[9] and the Zhang et al[22] studies despite different ethnicity of the studied populations (Caucasian vs Chinese patients). However, a higher rate of response was seen in the Caucasian patients enrolled in the Schramm et al[6] study. No information on patient ethnicity was provided by the other studies.
Only a few studies reported the relapse rate during steroid taper or soon after discontinuation. Relapse rate was higher in younger compared to elderly patients in the study by Al-Chalabi et al[23] (70% vs 42%, P = 0.002). A similar trend was observed in the studies by Granito et al[21] and Schramm et al[6], though it did not reach statistical significance. On the contrary, no difference in relapse rate between old and younger patients was observed in the study by Czaja et al[9]. In patients treated with UDCA, remission was observed in 17 out of 34 patients (50%), but data stratified for age were not reported[24].
Treatment side effects were described in four studies. In the paper by Granito et al[21], the elderly group did not report any side effect, while steroid-related adverse effects, such as osteoporosis, diabetes, and myopathy, occurred in the comparator group. In the study by Al-Chalabi et al[23], side effect frequency did not differ in the two age groups, except for Cushingoid facies and psychotic episodes that were reported in elderly group only (2 cases). Other side effects in elderly patients were osteoporosis (12%), hypertension (7%), type 2 diabetes (2%), and AZA-induced cytopenia (5%)[23]. In the paper by Schramm et al[6], five patients discontinued treatment with AZA because of gastrointestinal undesirable events, cholestasis, and skin rash. Moreover, one elderly patient had a varicella zoster infection and another one pulmonary tuberculosis reactivation[6]. In the study assessing the effect of MMF, treatment was well tolerated in all age groups, though few patients had septicaemia, mild gastrointestinal symptoms, or cytopenia[20].
AIH, a chronic inflammatory liver disease, may present at any age. However, there is relatively little information on AIH clinical features and management in older patients. In 2013, Chen et al[25] published a systematic review on AIH treatment in the elderly, supporting the use of a steroid combined with AZA to achieve long-term remission. Since then, alternative treatments, including 6-mercaptopurine, everolimus, tacrolimus, and methotrexate, were successfully used as first-line AIH therapy in adult patients[26]. However, efficacy was not tested in randomized clinical trials and available data in elderly patients are scarce.
Our systematic review results show that therapy with a steroid either alone or with AZA is still the most frequently used treatment in elderly patients. This therapeutic strategy is highly effective in achieving remission. Moreover, treatment failure occurs less frequently in the elderly than in younger patients and elderly people are more likely to maintain remission[6,9,21-24].
Treatments were well tolerated; however, included studies did not systematically report adverse effects. Notably, none of studies reported the use of calcium and vitamin D supplementations to prevent osteoporosis, though osteoporosis is one of the most common adverse effect in elderly people treated with steroids. Similarly, a multicentre study reported that only AIH patients with established osteoporosis were treated with bisphosphonates, calcium, and vitamin D supplements[27].
In elderly patients, other drugs can be used instead of standard therapy, particularly in subjects who are likely to experience relevant steroid- and AZA-related side effects. Nevertheless, the effect of novel treatments for AIH in the elderly was only described in case reports and small case series[28-33], which were not included in this systematic review. Among the included studies, only the paper of Zachou et al[20] tested an alternative first-line therapy with MMF.
Our systematic review has several limitations. The sample size of included studied was small and the study quality relatively low. In addition, studies were hetero-geneous in terms of AIH diagnostic criteria, outcome definition, cluster classification, disease severity, and comorbidities. Finally, we have no data on elderly patients of different age subgroups.
In conclusion, there is insufficient evidence to draw any conclusion regarding both efficacy and safety of novel AIH treatment approaches in the elderly. However, present data confirm that conventional AIH treatment is effective in elderly patients and that efficacy may be even greater than in younger subjects. Moreover, early treatment is recommended to achieve remission and avoid progression. Larger clinical studies are required to establish whether elderly patients may benefit from other therapeutic approaches.
Autoimmune hepatitis (AIH) is a rare chronic inflammatory disease potentially leading to severe liver damage. Untreated AIH has a poor prognosis, but the response rate to fist-line immunosuppressive therapy with a steroid either alone or in combination with azathioprine is very high. Moreover, novel immunosuppressive drugs have been recently proposed for AIH treatment in adult patients.
AIH also affects elderly patients; however, only a few intervention studies were performed in old patients. Whether standard treatment with a steroid, with or without azathioprine, is the best therapeutic strategy in the elderly is unclear.
To assess current evidence on AIH therapy in the elderly, we systematically reviewed studies testing both efficacy and safety of first-line pharmacotherapy for AIH in the elderly.
Electronic databases (PubMed National Library of Medicine, EMBASE, Cochrane Library) were searched to identify studies on AIH treatment in the elderly (≥ 60 years of age). Following study selection, eligible studies underwent risk of bias assessment. Data on study characteristics, interventions, and outcomes were then extracted. The work was carried out according to the PRISMA Guidelines.
Seven cohort studies, enrolling 789 AIH patients, were included. Elderly patients were 239 (30.3%). Six studies reported data on the efficacy of convention treatment with a steroid (alone or in combination with azathioprine) in elderly and younger AIH patients. Only one study compared the effect of another drug (mycophenolate mofetil). Overall remission rate was high and comparable in elderly and younger patients. Notably, both failure of treatment and relapses occurred less frequently in elderly patients. Adverse effects were rare in both groups. The quality of the evidence was low and the heterogeneity elevated among included studies.
Our results confirm the efficacy and safety of standard treatment with a steroid and azathioprine for AIH in the elderly. Data on other type of treatments were insufficient to draw final conclusion.
Available data on pharmacotherapy for AIH in the elderly are very limited and the effect of novel drugs poorly known. Moreover, results were obtained in small non-randomised studies that were heterogeneous for patient clinical characteristics, outcome assessment, and follow-up duration. Therefore, well-designed randomized clinical trials, comparing efficacy and safety of currently available first-line drugs for AIH in the elders, are needed. In addition, further studies are required to establish effectiveness of second-line treatments for elderly patients resistant to conventional therapy.
The authors thank Dr. Chiara Monagheddu for her valuable help and support in the statistical analysis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lei YC, Tang ZH, Zhu X S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Landeira G, Morise S, Fassio E, Ramonet M, Alvarez E, Caglio P, Longo C, Domínguez N. Effect of cirrhosis at baseline on the outcome of type 1 autoimmune hepatitis. Ann Hepatol. 2012;11:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 664] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 3. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston AL, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RN, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Büschenfelde KH, Zeniya M. International Autoimmune Hepatitis Group Report: Review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2003] [Cited by in RCA: 1986] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 4. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1253] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 5. | Hurlburt KJ, McMahon BJ, Deubner H, Hsu-Trawinski B, Williams JL, Kowdley KV. Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterol. 2002;97:2402-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Schramm C, Kanzler S, zum Büschenfelde KH, Galle PR, Lohse AW. Autoimmune hepatitis in the elderly. Am J Gastroenterol. 2001;96:1587-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 7. | Peng M, Li Y, Zhang M, Jiang Y, Xu Y, Tian Y, Peng F, Gong G. Clinical features in different age groups of patients with autoimmune hepatitis. Exp Ther Med. 2014;7:145-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Karkhanis J, Verna EC, Chang MS, Stravitz RT, Schilsky M, Lee WM, Brown RS; Acute Liver Failure Study Group. Steroid use in acute liver failure. Hepatology. 2014;59:612-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Czaja AJ, Carpenter HA. Distinctive clinical phenotype and treatment outcome of type 1 autoimmune hepatitis in the elderly. Hepatology. 2006;43:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 7640] [Article Influence: 477.5] [Reference Citation Analysis (1)] |
| 11. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10862] [Article Influence: 1206.9] [Reference Citation Analysis (2)] |
| 12. | Parker DR, Kingham JG. Type I autoimmune hepatitis is primarily a disease of later life. QJM. 1997;90:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Newton JL, Burt AD, Park JB, Mathew J, Bassendine MF, James OF. Autoimmune hepatitis in older patients. Age Ageing. 1997;26:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Verslype C, George C, Buchel E, Nevens F, van Steenbergen W, Fevery J. Diagnosis and treatment of autoimmune hepatitis at age 65 and older. Aliment Pharmacol Ther. 2005;21:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Miyake Y, Iwasaki Y, Takaki A, Kobashi H, Sakaguchi K, Shiratori Y. Clinical features of Japanese elderly patients with type 1 autoimmune hepatitis. Intern Med. 2007;46:1945-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Miyake T, Miyaoka H, Abe M, Furukawa S, Shigematsu S, Furukawa E, Ikeda R, Okita S, Okada T, Yoshida O, Murata Y, Akbar SM, Matsuura B, Michitaka K, Horiike N, Hiasa Y, Onji M. Clinical characteristics of autoimmune hepatitis in older aged patients. Hepatol Res. 2006;36:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Ngu JH, Gearry RB, Frampton CM, Stedman CA. Predictors of poor outcome in patients w ith autoimmune hepatitis: A population-based study. Hepatology. 2013;57:2399-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Wang Z, Sheng L, Yang Y, Yang F, Xiao X, Hua J, Guo C, Wei Y, Tang R, Miao Q, Zhang J, Li Y, Fang J, Qiu D, Krawitt EL, Bowlus CL, Gershwin ME, Wang Q, Ma X. The Management of Autoimmune Hepatitis Patients with Decompensated Cirrhosis: Real-World Experience and a Comprehensive Review. Clin Rev Allergy Immunol. 2017;52:424-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Baven-Pronk MAMC, Biewenga M, van Silfhout JJ, van den Berg AP, van Buuren HR, Verwer BJ, van Nieuwkerk CMJ, Bouma G, van Hoek B. Role of age in presentation, response to therapy and outcome of autoimmune hepatitis. Clin Transl Gastroenterol. 2018;9:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Zachou K, Gatselis NK, Arvaniti P, Gabeta S, Rigopoulou EI, Koukoulis GK, Dalekos GN. A real-world study focused on the long-term efficacy of mycophenolate mofetil as first-line treatment of autoimmune hepatitis. Aliment Pharmacol Ther. 2016;43:1035-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Granito A, Muratori L, Pappas G, Muratori P, Ferri S, Cassani F, Lenzi M, Bianchi FB. Clinical features of type 1 autoimmune hepatitis in elderly Italian patients. Aliment Pharmacol Ther. 2005;21:1273-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 22. | Zhang Y, Sun WL, Jin DL, Jing-Hua D. Clinical features of elderly Chinese patients with autoimmune hepatitis. Turk J Gastroenterol. 2013;24:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Al-Chalabi T, Boccato S, Portmann BC, McFarlane IG, Heneghan MA. Autoimmune hepatitis (AIH) in the elderly: A systematic retrospective analysis of a large group of consecutive patients with definite AIH followed at a tertiary referral centre. J Hepatol. 2006;45:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Morii K, Nagano Y, Yamamoto T, Nakamura S, Okushin H. Increasing incidence of elderly-onset autoimmune hepatitis. Geriatr Gerontol Int. 2017;17:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Chen J, Eslick GD, Weltman M. Systematic review with meta-analysis: Clinical manifestations and management of autoimmune hepatitis in the elderly. Aliment Pharmacol Ther. 2014;39:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J Gastroenterol. 2017;23:6030-6048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 27. | Floreani A, Niro G, Rosa Rizzotto E, Antoniazzi S, Ferrara F, Carderi I, Baldo V, Premoli A, Olivero F, Morello E, Durazzo M. Type I autoimmune hepatitis: Clinical course and outcome in an Italian multicentre study. Aliment Pharmacol Ther. 2006;24:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Hübener S, Oo YH, Than NN, Hübener P, Weiler-Normann C, Lohse AW, Schramm C. Efficacy of 6-Mercaptopurine as Second-Line Treatment for Patients With Autoimmune Hepatitis and Azathioprine Intolerance. Clin Gastroenterol Hepatol. 2016;14:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 29. | Rodrigues S, Lopes S, Magro F, Cardoso H, Horta e Vale AM, Marques M, Mariz E, Bernardes M, Lopes J, Carneiro F, Macedo G. Autoimmune hepatitis and anti-tumor necrosis factor alpha therapy: A single center report of 8 cases. World J Gastroenterol. 2015;21:7584-7588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Ytting H, Larsen FS. Everolimus treatment for patients with autoimmune hepatitis and poor response to standard therapy and drug alternatives in use. Scand J Gastroenterol. 2015;50:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Weiler-Normann C, Schramm C, Quaas A, Wiegard C, Glaubke C, Pannicke N, Möller S, Lohse AW. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol. 2013;58:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 32. | Than NN, Wiegard C, Weiler-Normann C, Füssel K, Mann J, Hodson J, Hirschfield GM, Lohse AW, Adams DH, Schramm C, Oo YH. Long-term follow-up of patients with difficult to treat type 1 autoimmune hepatitis on Tacrolimus therapy. Scand J Gastroenterol. 2016;51:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Devlin SM, Swain MG, Urbanski SJ, Burak KW. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory to standard therapy. Can J Gastroenterol. 2004;18:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |