Published online Jun 14, 2019. doi: 10.3748/wjg.v25.i22.2743
Peer-review started: March 13, 2019
First decision: March 27, 2019
Revised: March 29, 2019
Accepted: April 19, 2019
Article in press: April 19, 2019
Published online: June 14, 2019
Processing time: 95 Days and 0.6 Hours
Gastrectomy with radical lymph node dissection is the most promising treatment avenue for patients with gastric cancer. However, this procedure sometimes induces excessive intraoperative blood loss and requires perioperative allogeneic blood transfusion. There are lasting discussions and controversies about whether intraoperative blood loss or perioperative blood transfusion has adverse effects on the prognosis in patients with gastric cancer. We reviewed laboratory and clinical evidence of these associations in patients with gastric cancer. A large amount of clinical evidence supports the correlation between excessive intraoperative blood loss and adverse effects on the prognosis. The laboratory evidence revealed three possible causes of such adverse effects: anti-tumor immunosuppression, unfavorable postoperative conditions, and peritoneal recurrence by spillage of cancer cells into the pelvis. Several systematic reviews and meta-analyses have suggested the adverse effects of perioperative blood transfusions on prognostic parameters such as all-cause mortality, recurrence, and postoperative complications. There are two possible causes of adverse effects of blood transfusions on the prognosis: Anti-tumor immunosuppression and patient-related confounding factors (e.g., preoperative anemia). These factors are associated with a worse prognosis and higher requirement for perioperative blood transfusions. Surgeons should make efforts to minimize intraoperative blood loss and transfusions during gastric cancer surgery to improve patients’ prognosis.
Core tip: Whether perioperative blood loss or blood transfusion has adverse effects on the prognosis in patients with gastric cancer remains unclear. We reviewed laboratory and clinical evidence of this association in patients with gastric cancer. A large amount of clinical evidence revealed that excessive intraoperative blood loss and blood transfusion have adverse effects on the prognosis. The possible mechanisms underlying the association between intraoperative blood loss and a poor prognosis are immunosuppression, unfavorable postoperative conditions, and tumor cell spillage into the pelvis, and those underlying the association between blood transfusions and a poor prognosis are immunosuppression and preoperative anemia.
- Citation: Nakanishi K, Kanda M, Kodera Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J Gastroenterol 2019; 25(22): 2743-2751
- URL: https://www.wjgnet.com/1007-9327/full/v25/i22/2743.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i22.2743
Surgical resection is still the most promising avenue for patients with resectable gastric cancer. However, it sometimes induces excessive intraoperative blood loss (IBL) and requires perioperative allogeneic blood transfusion (BTF), especially when gastrectomy with systematic lymph node dissection is performed. Furthermore, as IBL becomes more excessive, the need for BTF further increases; thus, IBL is closely associated with BTF.
There are lasting discussions and controversies about whether IBL or BTF has adverse effects on the prognosis in patients with gastric cancer[1-3]. Patients requiring BTF often have severe illness, advanced cancer, a poor general condition, and a higher prevalence of comorbidities, and these confounding factors themselves induce postoperative complications, surgical death, and a worse prognosis[4-6]. The same is true of IBL. Thus, it is difficult to evaluate whether IBL or BTF itself has adverse effects on the prognosis. However, clinical trials that determine these effects in surgical treatment are not ethically permissible. We therefore reviewed clinical and laboratory evidence to explore the effects of IBL and BTF on the prognosis of patients with gastric cancer.
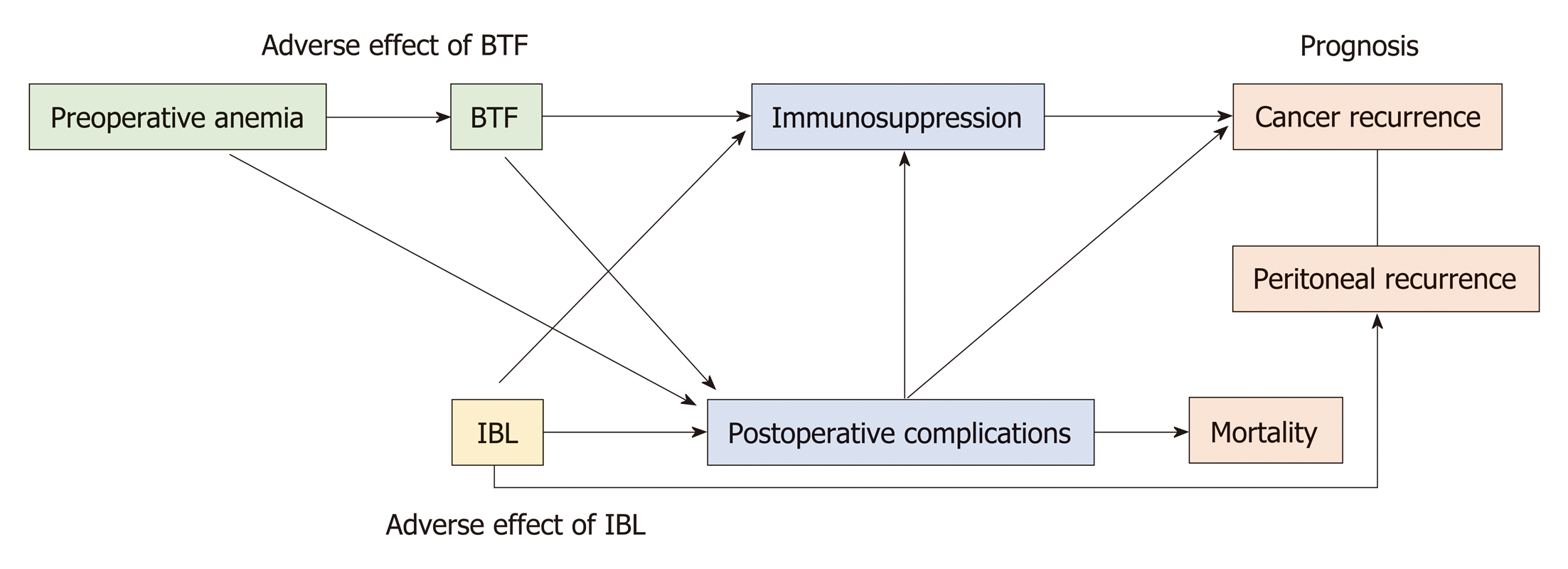
There are three possible causes of the adverse effect of IBL on the prognosis: Anti-tumor immunosuppression induced by IBL, an unfavorable postoperative condition induced by IBL, and spillage of microscopic cancer cells in the pelvic cavity via the blood lost during IBL. These mechanisms are summarized in Figure 1.
First, many studies have shown that the main cause of the adverse effect of excessive IBL is anti-tumor immunosuppression via the loss of plasma con-stituents[7-10]. In support of this concept, several studies have revealed that the prevalence of hematogenous recurrence, which is correlated with immuno-suppression, was significantly higher in patients with excessive IBL[9,10]. However, these studies did not demonstrate the mechanism. Bruns et al[11] reported that IBL of > 700 mL during gastrointestinal surgery was associated with a significant decrease in natural killer cell activity, leading to an unfavorable prognosis. Miki et al[12] reported that interleukin (IL)-6 and tumor growth factor triggered by IL-6 were increased in patients with colorectal cancer receiving BTF due to excessive IBL. Thus, the mechanism of immunosuppression in gastric cancer surgery is speculative, and laboratory evidence is lacking. A validation study is therefore needed.
Second, excessive IBL may lead to an unfavorable postoperative condition, such as the development of postoperative complications, thus adversely affecting the prognosis[9,13]. Postoperative complications occur may lead to severe tissue damage caused by local and generalized inflammatory reactions, resulting in more severe immunosuppression[13].
Third, Kamei et al[14] reported that excessive IBL is an independent risk factor for peritoneal recurrence after curative gastrectomy. They suggested the possibility that blood loss into the peritoneal cavity may promote tumor spillage during surgery, which may be specifically associated with peritoneal recurrence. Arita et al[15] further confirmed the association between IBL and peritoneal recurrence in the laboratory setting. Although this idea is very interesting, no other study to date has supported this hypothesis. Moreover, it is unclear whether this adverse effect remains when administering S-1 monotherapy which is one of the standard postoperative adjuvant chemotherapies that mainly suppresses peritoneal recurrence[16]. Therefore, further analysis in the clinical practice setting is needed.
Our investigation of the relationship between IBL and the prognosis was derived from that of patients with colorectal cancer. Heiss et al[17] first reported the possibility that IBL itself may be beneficial for survival of malignant cells in the host and also found a positive link with tumor recurrence and poor outcomes in patients with colorectal cancer. The adverse effect of IBL on the prognosis in patients with gastric cancer was first reported by Dhar et al[7] in 2000. We have summarized the studies reporting the effect of IBL on the prognosis in Table 1. Dhar et al[7] reported that IBL of > 500 mL was an independent predictor of survival in an analysis of 152 patients with transmural (T2N0–T3N2) gastric cancer. They hypothesized that IBL reduced the body’s immunity and its ability to fight cancer cells; this concept was quoted from the report by Bruns et al[11]. However, Dhar et al[7] provided no information on perioperative BTF, which is a strong confounding factor for the prognosis. Similar studies were subsequently reported. Kamei et al[14] reported that IBL of ≥ 475 mL was specifically associated with the development of peritoneal recurrence in 146 patients who underwent curative gastrectomy for advanced gastric cancer. They reported for the first time the relationship between IBL and the recurrence pattern. Liang et al[8] also reported that IBL of ≥ 200 mL was an independent prognostic factor in 845 patients who underwent curative gastrectomy. In their study, IBL of ≥ 200 mL was a prognostic factor even when patients who underwent BTF were excluded; however, BTF administration was not a prognostic factor. Mizuno et al[9] reported that IBL of ≥ 400 mL was a significant predictor of survival and cancer recurrence in 203 patients with stage II/III gastric cancer and was associated with the prevalence of hematogenous recurrence. Their study excluded patients who received BTF to eliminate a potential confounding bias caused by the adverse effects of BTF. Ito et al[10] reported that IBL of > 330 mL had an adverse effect on the long-term prognosis in 1013 patients with stage II/III gastric cancer. Their study also excluded patients who received BTF and was the largest-scale study, thoroughly eliminating complicated confounding factors. IBL is closely associated with BTF administration, and the prognostic significance of IBL might be masked by the adverse effect of BTF. From this viewpoint, three studies[8-10] excluded this confounding influence, indicating that IBL itself has an adverse effect on the long-term prognosis in patients with gastric cancer.
| Study | Period | Sample size | Selected group | Amount of IBL | Patients received BTF | Adverse effect of BTF on prognosis | Adverse effect of IBL on prognosis |
| Dhar et al[7], 2000 | 1979-1989 | 152 | T2N0–T3N2 | > 500 ml | Not specified | - | Yes (Survival) |
| Kamei et al[14], 2009 | 1992-2003 | 146 | Curative Gastrectomy | ≥ 475 mL | Included (13%) | No (Peritoneal recurrence) | Yes (Peritoneal recurrence) |
| Liang et al[8], 2013 | 2003-2007 | 845 | stage I-III | ≥ 200 ml | Included (25%) | No (Survival) | Yes (Survival) |
| Ishino et al[18], 2014 | 2001-2012 | 214 | Laparoscopic,stage I-II | ≥ 1% body weight | 0% | - | Yes (Survival) |
| Arita et al[15], 2015 | 1997-2012 | 540 | Curative Gastrectomy | > 326 ml | Not specified | - | Yes (Peritoneal recurrence) |
| Mizuno et al[9], 2016 | 1999-2015 | 203 | stage II/III | ≥ 400 mL | Not specified | - | Yes (Survival, Recurrence) |
| Ito et al[10], 2018 | 2010-2014 | 1013 | stage II/III | > 330 mL | Not specified | - | Yes (Recurrence) |
| Ojima et al[19], 2009 | 1991-2002 | 856 | Curative gastrectomy | ≥ 1000 mL | Included (18%) | Yes (Survival) | No (Survival) |
| Squires et al[20], 2015 | 2000-2012 | 765 | Curative gastrectomy | > 250 mL | Included (22%) | Yes (Survival, Recurrence) | No (Survival, Recurrence) |
| Kanda et al[6], 2016 | 1999-2014 | 250 | stage II/III | ≥ 800 mL | Included (23%) | Yes (Survival, Recurrence) | No (Survival) |
Evidence was also found in the field of laparoscopic surgery. Ishino et al[18] reported that IBL of ≥ 1% body weight was significantly correlated with postoperative complications and was an independent predictor of survival in 214 patients who underwent laparoscopy-assisted gastrectomy for gastric cancer. Conversely, several negative studies of the adverse effects of IBL on the prognosis have also been published (summarized in Table 1). Ojima et al[19] reported that BTF administration was an independent prognostic factor for survival in 856 patients who underwent curative gastrectomy but that IBL of ≥ 1000 mL was not a prognostic factor. Likewise, two studies showed that BTF administration was an independent prognostic factor for survival but that excessive IBL was not prognostic factor[6,20]. However, the threshold of the IBL volume in these reports was greatly different, and neither study excluded the confounding influence of BTF.
The accumulation of clinical evidence reveals that excessive IBL may have adverse effects on the prognosis in patients with gastric cancer by promoting anti-tumor immunosuppression, unfavorable postoperative conditions, and a specific association with peritoneal recurrence by spillage of cancer cells into the pelvic cavity during surgery. However, the laboratory evidence is weak and some issues remain unclear. IBL thresholds varied, and the results might differ depending on these thresholds. A higher threshold for the amount of IBL would introduce more confounding factors (e.g., BTF, postoperative anemia, and postoperative complications). Another issue is that only two studies have reported the relationship between IBL and peritoneal recurrence.
There are two possible causes of the adverse effect of BTF on the prognosis: anti-tumor immunosuppression induced by BTF and patient-related confounding factors (e.g., preoperative anemia and postoperative complications). These factors are associated with a worse prognosis and higher requirement for perioperative BTF. These mechanisms are summarized in Figure 1.
Gantt[21] was the first to report the possibility of promoting tumor growth by immunosuppression due to BTF in 1981. Numerous authors have since considered that BTF administration has profound adverse effects on the host’s immune system[22-25]. Mechanisms of inhibition of host immunity by BTF are diverse and include cytokine-mediated immune responses and suppression of cellular and humoral immunity against cancer cells. BTF-induced immunomodulatory effects drive the immune system to inhibit IL-2 production[25], decrease interferon gamma[23], suppress natural killer cell function[24], release immunosuppressive prostaglandins[25], decrease monocyte activity[25], and increase of regulatory T cells (suppressor T cells)[24-27].
BTF administration also promotes increases in IL-6[12], vascular endothelial growth factor[28], and hepatocyte growth factor[29], which play fundamental roles in tumor growth, malignant transformation, and invasion of tumor cells[30,31]. Additionally, in patients with gastric cancer, overexpression of these cytokines is reportedly correlated with a poor prognosis[12,32-34]. The immunosuppression caused by BTF creates favorable conditions for tumor growth; additionally, BTF increases the risk of postoperative complications[3], which also have adverse effects on the prognosis[13].
However, some issues remain unclear. The type of blood products received (e.g., red blood cells, leukodepleted blood, whole blood) was not constant among studies. The disorder observed after BTF administration is caused by the presence of leukocytes and their products, as mentioned above. Current BTF products are often leukodepleted, and filtered transfusion is routinely performed; thus, the conta-mination of cytokines is decreased and the effect is weakened. In contrast to this concept, no difference in the prognosis was found in comparative studies between leukocyte-depleted blood and non-leukocyte-depleted blood[35,36]. The roles of these cytokines and growth factors in current transfusion treatment remain unclear.
Preoperative anemia, which is a patient-related confounding factor, is another possible cause of a worse prognosis in patients with malignancy[17]. Gastrointestinal tumors sometimes bleed due to the passage of intestinal contents and the effect of digestive juices, and this bleeding may lead to anemia. In particular, once anemia has occurred in patients of advanced age, it persists because of these patients’ physiological decrease in hematopoietic cells in the bone marrow, decrease in hematopoietic stem cells, and reduced serum erythropoietin levels due to renal impairment[37]. Additionally, continuous anemia causes malnutrition, which also has adverse effects on the prognosis[38]. Numerous prospective and retrospective studies have shown that patients with preoperative anemia have a worse prognosis than patients without anemia[39]. Hence, preoperative anemia is a cause of the requirement for BTF, which itself also has adverse effects on the prognosis.
Although we have summarized the mechanisms of adverse effects of BTF, some unmeasurable and non-excludable confounding factors remain. Surgical damage is one such factor and can also lead to severe immunosuppression. The degree of surgical damage depends on the surgical organ. Surgical damage induced during colon cancer surgery is considered to be relatively mild, and there are many negative reports on the influence of BTF on the prognosis of such patients[40,41]. However, surgical damage induced during esophageal cancer surgery is considered to be relatively severe, and there are many positive reports on the influence of BTF on the prognosis of such patients[42,43]. In gastric cancer surgery, the degree of surgical damage varies greatly depending on the operation type; therefore, it may be more effective to investigate this issue according to the operation type (total gastrectomy vs distal gastrectomy, open surgery vs laparoscopic surgery).
Many studies have been performed to evaluate the adverse effect of perioperative BTF on the prognosis in patients with gastric cancer, and we have summarized these studies in Table 2. Kaneda et al[44] first reported that BTF administration had an adverse effect on survival in 231 patients who underwent curative gastrectomy. Ojima et al[19] subsequently reported that BTF administration was an independent prognostic factor for survival in 856 patients who underwent curative gastrectomy, even when the amount of transfused blood was small. Kanda et al[6] reported that BTF administration was an independent prognostic factor for survival and recurrence in patients with stage II/III gastric cancer, regardless of the volume of BTF. They also reported that the prognostic impact of BTF became less clear after introduction of adjuvant chemotherapy with S-1. Three systematic reviews and meta-analyses support the idea that BTF is associated with a worse prognosis, all-cause mortality, cancer-related mortality, and recurrence (summarized in Table 3).
| Study | Period | Sample size | Selected group | Rates of received BTF | Timing of transfusions | Adverse effect of BTF on prognosis |
| Kaneda et al[44], 1987 | 1976-1980 | 231 | Not specified | 51% | Not specified | Yes (Survival) |
| Ojima et al[19], 2009 | 1991-2002 | 856 | Curative gastrectomy | 18% | Pre + Intra + Post | Yes (Survival) |
| Squires et al[20], 2015 | 2000-2012 | 765 | Curative gastrectomy | 22% | Intra + Post | Yes (Survival, Recurrence) |
| Kanda et al[6], 2016 | 1999-2014 | 250 | stage II /III | 23% | Pre + Intra + Post | Yes (Survival, Recurrence) |
| Kampschoer et al[45], 1989 | 1976-1981 | 1000 | Curative gastrectomy | 37% | Pre + Intra + Post | No (Survival) |
| Kamei et al[14], 2009 | 1992-2003 | 146 | Curative gastrectomy | 13% | Not specified | No (Peritoneal recurrence) |
| Pacelli et al[46], 2011 | 1990-2005 | 927 | Curative gastrectomy | 35% | Pre + Intra + Post | No (Survival) |
| Liang et al[8], 2013 | 2003-2007 | 845 | stage I-III | 25% | Pre + Intra + Post | No (Survival) |
| Rausei et al[47], 2013 | 1995-2011 | 224 | stage I-III | 20% | Pre + Intra + Post | No (Survival) |
| Study | Rate of received | Adverse effect on OS | Adverse effect on CSS | Adverse effect on RFS | Adverse effect on PCs |
| BTF patients | |||||
| Sun et al[1], 2015 | 36.3% | Yes, | Yes, | Yes, | Not specified |
| HR, 2.17; | HR, 2.57; | HR, 1.52; | |||
| 95%CI, 1.86-2.37 | 95%CI, 1.24-5.34 | 95%CI, 1.08-2.15 | |||
| Li et al[2], 2015 | 44.8% | Yes, | Not specified | Yes, | Yes, |
| HR, 1.26; | |||||
| HR, 1.36; | HR, 3.33; | ||||
| 95 % CI, 1.21-1.31 | |||||
| 95%CI, 1.02-1.81 | 95%CI, 1.02-1.81 | ||||
| Agnes et al[3], 2018 | Not specified | Yes, | Yes, | Yes, | Yes, |
| HR, 1.34; | HR, 1.66; | HR, 1.48; | HR, 1.36; | ||
| 95%CI, 1.23-1.45 | 95%CI, 1.50-2.19 | 95%CI, 1.18-1.86 | 95%CI, 2.10-5.29 |
However, some studies have shown that BTF does not have an adverse effect on the prognosis (summarized in Table 2). Kampschöer et al[45] performed a large-scale retrospective study and found that the survival of patients who had undergone BTF was shorter than that of patients who had not undergone BTF; however, after stratifying patients into stages and applying proportional regression analyses, BTF administration did not appear to have any effect on the prognosis but was instead associated with other prognostic features. Pacelli et al[46] conducted a multicenter retrospective study and reported that BTF had a slight, but not significant, adverse effect on survival of 927 patients who underwent curative gastrectomy.
The adverse effects of BTF have been well verified by clinical and laboratory data; these adverse effects are caused by anti-tumor immunosuppression and patient-related confounding factors that lead to a requirement for BTF. However, this information may not be helpful in the clinical setting because BTF is still required in the event of massive bleeding during surgery or preoperative anemia. However, there may be room for consideration such as adjusting preoperative anemia and paying attention so as not to lead postoperative complications.
The adverse effects of IBL or BTF were previously ascertained by clinical evidence. However, continuous and untiring efforts to minimize IBL and surgical damage have been progressing (i.e., development of laparoscopic surgery, improvements in surgical techniques and devices, and enhanced recovery after surgery programs), and the amount of IBL and frequency of BTF administration have been decreasing. In addition, perioperative chemotherapy has been further developed, helping to prolong survival. Further accumulation of data and performance of high-quality studies are required to clarify whether IBL or BTF still have adverse effects on the prognosis.
IBL and BTF lead to adverse effects on the prognosis in patients with gastric cancer, and the main causes are anti-tumor immunosuppression and confounding factors such as postoperative complications and preoperative anemia. Surgeons should make efforts to minimize IBL and BTF to improve patients’ prognosis.
We thank Angela Morben, DVM, ELS from Edanz Group for editing a draft of this manuscript.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hu XT, Lee JI S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: Systematic review and meta-analysis. Int J Surg. 2015;13:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Li L, Zhu D, Chen X, Huang Y, Ouyang M, Zhang W. Perioperative Allogenenic Blood Transfusion is Associated With Worse Clinical Outcome for Patients Undergoing Gastric Carcinoma Surgery: A Meta-Analysis. Medicine (Baltimore). 2015;94:e1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Agnes A, Lirosi MC, Panunzi S, Santocchi P, Persiani R, D'Ugo D. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: A systematic review and meta-analysis of non-randomized, adjusted studies. Eur J Surg Oncol. 2018;44:404-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 4. | Sima CS, Jarnagin WR, Fong Y, Elkin E, Fischer M, Wuest D, D'Angelica M, DeMatteo RP, Blumgart LH, Gönen M. Predicting the risk of perioperative transfusion for patients undergoing elective hepatectomy. Ann Surg. 2009;250:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: A systematic review and meta-analysis. Ann Surg. 2012;256:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 6. | Kanda M, Kobayashi D, Tanaka C, Iwata N, Yamada S, Fujii T, Nakayama G, Sugimoto H, Koike M, Nomoto S, Murotani K, Fujiwara M, Kodera Y. Adverse prognostic impact of perioperative allogeneic transfusion on patients with stage II/III gastric cancer. Gastric Cancer. 2016;19:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Watanabe R, Kohno H, Nagasue N. Long-term survival of transmural advanced gastric carcinoma following curative resection: Multivariate analysis of prognostic factors. World J Surg. 2000;24:588-93; discussion 593-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Liang YX, Guo HH, Deng JY, Wang BG, Ding XW, Wang XN, Zhang L, Liang H. Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J Gastroenterol. 2013;19:5542-5550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Mizuno A, Kanda M, Kobayashi D, Tanaka C, Iwata N, Yamada S, Fujii T, Nakayama G, Sugimoto H, Koike M, Fujiwara M, Kodera Y. Adverse Effects of Intraoperative Blood Loss on Long-Term Outcomes after Curative Gastrectomy of Patients with Stage II/III Gastric Cancer. Dig Surg. 2016;33:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Ito Y, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, Tanaka C, Kobayashi D, Fujiwara M, Murotani K, Kodera Y. Intraoperative Blood Loss is Associated with Shortened Postoperative Survival of Patients with Stage II/III Gastric Cancer: Analysis of a Multi-institutional Dataset. World J Surg. 2019;43:870-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Bruns CJ, Schäfer H, Wolfgarten B, Engert A. [Effect of intraoperative blood loss on the function of natural killer cells in tumors of the upper gastrointestinal tract]. Langenbecks Arch Chir Suppl Kongressbd. 1996;113:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Miki C, Hiro J, Ojima E, Inoue Y, Mohri Y, Kusunoki M. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol (R Coll Radiol). 2006;18:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 14. | Kamei T, Kitayama J, Yamashita H, Nagawa H. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J Surg. 2009;33:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Hiramoto H, Hamada J, Shoda K, Kawaguchi T, Hirajima S, Nagata H, Fujiwara H, Okamoto K, Otsuji E. Increase in peritoneal recurrence induced by intraoperative hemorrhage in gastrectomy. Ann Surg Oncol. 2015;22:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 17. | Heiss MM, Mempel W, Delanoff C, Jauch KW, Gabka C, Mempel M, Dieterich HJ, Eissner HJ, Schildberg FW. Blood transfusion-modulated tumor recurrence: First results of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. J Clin Oncol. 1994;12:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 226] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Ishino Y, Saigusa S, Ohi M, Yasuda H, Tanaka K, Toiyama Y, Mohri Y, Kusunoki M. Preoperative C-reactive protein and operative blood loss predict poor prognosis in patients with gastric cancer after laparoscopy-assisted gastrectomy. Asian J Endosc Surg. 2014;7:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Ojima T, Iwahashi M, Nakamori M, Nakamura M, Naka T, Katsuda M, Iida T, Hayata K, Yamaue H. Association of allogeneic blood transfusions and long-term survival of patients with gastric cancer after curative gastrectomy. J Gastrointest Surg. 2009;13:1821-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Squires MH, Kooby DA, Poultsides GA, Weber SM, Bloomston M, Fields RC, Pawlik TM, Votanopoulos KI, Schmidt CR, Ejaz A, Acher AW, Worhunsky DJ, Saunders N, Levine EA, Jin LX, Cho CS, Winslow ER, Russell MC, Staley CA, Maithel SK. Effect of Perioperative Transfusion on Recurrence and Survival after Gastric Cancer Resection: A 7-Institution Analysis of 765 Patients from the US Gastric Cancer Collaborative. J Am Coll Surg. 2015;221:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Gantt CL. Red blood cells for cancer patients. Lancet. 1981;2:363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 131] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Sun CF, Hsieh YY, Ngan KW, Wang WT. Search for immunomodulatory effects of blood transfusion in gastric cancer patients: Flow cytometry of Th1/Th2 cells in peripheral blood. Ann Clin Lab Sci. 2001;31:171-178. [PubMed] |
| 23. | Chen G, Zhang FJ, Gong M, Yan M. Effect of perioperative autologous versus allogeneic blood transfusion on the immune system in gastric cancer patients. J Zhejiang Univ Sci B. 2007;8:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Wu HS, Little AG. Perioperative blood transfusions and cancer recurrence. J Clin Oncol. 1988;6:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 336] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 26. | Roelen D, Brand A, Claas FH. Pretransplant blood transfusions revisited: A role for CD(4+) regulatory T cells? Transplantation. 2004;77:S26-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Baumgartner JM, Silliman CC, Moore EE, Banerjee A, McCarter MD. Stored red blood cell transfusion induces regulatory T cells. J Am Coll Surg. 2009;208:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Nielsen HJ, Werther K, Mynster T, Brünner N. Soluble vascular endothelial growth factor in various blood transfusion components. Transfusion. 1999;39:1078-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Fukuura T, Miki C, Inoue T, Matsumoto K, Suzuki H. Serum hepatocyte growth factor as an index of disease status of patients with colorectal carcinoma. Br J Cancer. 1998;78:454-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1222] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 31. | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4848] [Cited by in RCA: 4796] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 32. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6954] [Article Influence: 316.1] [Reference Citation Analysis (0)] |
| 33. | Park DJ, Thomas NJ, Yoon C, Yoon SS. Vascular endothelial growth factor a inhibition in gastric cancer. Gastric Cancer. 2015;18:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Lordick F. Targeting the HGF/MET pathway in gastric cancer. Lancet Oncol. 2014;15:914-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | van Hilten JA, van de Watering LM, van Bockel JH, van de Velde CJ, Kievit J, Brand R, van den Hout WB, Geelkerken RH, Roumen RM, Wesselink RM, Koopman-van Gemert AW, Koning J, Brand A. Effects of transfusion with red cells filtered to remove leucocytes: Randomised controlled trial in patients undergoing major surgery. BMJ. 2004;328:1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Lange MM, van Hilten JA, van de Watering LM, Bijnen BA, Roumen RM, Putter H, Brand A, van de Velde CJ; cooperative clinical investigators of the Cancer Recurrence And Blood Transfusion (CRAB) study and the Transfusion Associated Complications = Transfusion Induced Complications? (TACTIC) study. Leucocyte depletion of perioperative blood transfusion does not affect long-term survival and recurrence in patients with gastrointestinal cancer. Br J Surg. 2009;96:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: A systematic review of the literature. Am J Med. 2004;116 Suppl 7A:3S-10S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 255] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 38. | Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 469] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 39. | Shander A, Van Aken H, Colomina MJ, Gombotz H, Hofmann A, Krauspe R, Lasocki S, Richards T, Slappendel R, Spahn DR. Patient blood management in Europe. Br J Anaesth. 2012;109:55-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 40. | McAlister FA, Clark HD, Wells PS, Laupacis A. Perioperative allogeneic blood transfusion does not cause adverse sequelae in patients with cancer: A meta-analysis of unconfounded studies. Br J Surg. 1998;85:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 374] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 42. | Christein JD, Hollinger EF, Millikan KW. Prognostic factors associated with resectable carcinoma of the esophagus. Am Surg. 2002;68:258-62; discussion 262-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Dresner SM, Lamb PJ, Shenfine J, Hayes N, Griffin SM. Prognostic significance of peri-operative blood transfusion following radical resection for oesophageal carcinoma. Eur J Surg Oncol. 2000;26:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Kaneda M, Horimi T, Ninomiya M, Nagae S, Mukai K, Takeda I, Shimoyama H, Chohno S, Okabayashi T, Kagawa S. Adverse affect of blood transfusions on survival of patients with gastric cancer. Transfusion. 1987;27:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Kampschöer GH, Maruyama K, Sasako M, Kinoshita T, van de Velde CJ. The effects of blood transfusion on the prognosis of patients with gastric cancer. World J Surg. 1989;13:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Pacelli F, Rosa F, Marrelli D, Pedrazzani C, Bossola M, Zoccali M, Marchet A, Di Cosmo M, Roata C, Graziosi L, Cavazzoni E, Covino M, D'Ugo D, Roviello F, Nitti D, Doglietto GB. Do perioperative blood transfusions influence prognosis of gastric cancer patients? Analysis of 927 patients and interactions with splenectomy. Ann Surg Oncol. 2011;18:1615-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Rausei S, Ruspi L, Galli F, Tirotta F, Inversini D, Frattini F, Chiappa C, Rovera F, Boni L, Dionigi G, Dionigi R. Peri-operative blood transfusion in gastric cancer surgery: Prognostic or confounding factor? Int J Surg. 2013;11 Suppl 1:S100-S103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |